Abstract
Helicobacter pylori was identified in human liver tissue by PCR, hybridization, and partial DNA sequencing. Liver biopsies were obtained from patients with primary sclerosing cholangitis (n = 12), primary biliary cirrhosis (n = 12), and noncholestatic liver cirrhosis (n = 13) and (as controls) normal livers (n = 10). PCR analyses were carried out using primers for the Helicobacter genus, Helicobacter pylori (the gene encoding a species-specific 26-kDa protein and the 16S rRNA), Helicobacter bilis, Helicobacter pullorum, and Helicobacter hepaticus. Samples from patients with primary biliary cirrhosis and primary sclerosing cholangitis (11 and 9 samples, respectively) were positive by PCR with Helicobacter genus-specific primers. Of these 20 samples, 8 were positive with the 16S rRNA primer and 9 were positive with the 26-kDa protein primer of H. pylori. These nine latter samples were also positive by Southern blot hybridization for the amplified 26-kDa fragment, and four of those were verified to be H. pylori by partial 16S rDNA sequencing. None of the samples reacted with primers for H. bilis, H. pullorum, or H. hepaticus. None of the normal livers had positive results in the Helicobacter genus PCR assay, and only one patient in the noncholestatic liver cirrhosis group, a young boy who at reexamination showed histological features suggesting primary sclerosing cholangitis, had a positive result in the same assay. Helicobacter positivity was thus significantly more common in patients with cholestatic diseases (20 of 24) than in patients with noncholestatic diseases and normal controls (1 of 23) (P = <0.00001). Patients positive for Helicobacter genus had significantly higher values of alkaline phosphatases and prothrombin complex than Helicobacter-negative patients (P = 0.0001 and P = 0.0003, respectively). Among primary sclerosing cholangitis patients, Helicobacter genus PCR positivity was weakly associated with ulcerative colitis (P = 0.05). Significant differences related to blood group or HLA status were not found.
During the past few years Helicobacter infections have been reported to be associated with certain diseases in the liver of some animal species such as Helicobacter canis in dogs (10), Helicobacter pullorum in poultry (31), and Helicobacter hepaticus (33) and Helicobacter bilis (12) in mice. These findings, in conjunction with the role of Helicobacter pylori as a major pathogenic factor of chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoma, and gastric cancer (7), demand further studies to explore the possibility of a relationship between Helicobacter infection and liver disease in humans.
Primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) are diseases affecting the human liver. The etiology of PSC is unknown (6). There is ample evidence that the disease, but not the course of it, is associated with specific HLA antigens (24). About 65% of PSC patients are positive for anti-neutrophil cytoplasma antibody in serum (1). Few studies have suggested that pathogens may cause PSC (29), but this was not confirmed (4). Clinical symptoms are jaundice, pruritus, right upper quadrant pain, fever, and fatigue (25). Complications involve bacterial cholangitis, hepatosplenomegaly, and gallbladder and biliary stones (6, 13). The disease is characterized by fibrosis of the extra- and/or intrahepatic bile ducts, biliary fibrosis and cirrhosis, portal hypertension, liver failure (6, 13), and cholangiocarcinoma (3). Diagnosis is based on the cholangiographic demonstration of multiple stenoses, dilatations of the biliary tree, and a cholestatic liver laboratory profile. PSC is correlated with ulcerative colitis (UC), Crohn's disease, and other forms of inflammatory bowel disease (6, 26).
PBC is an autoimmune disease characterized by destruction of the intrahepatic bile ducts and inflammation of the portal system, followed by tissue fibrosis and liver failure. Lethargy, pruritus, and jaundice are common symptoms (16), and PBC may be associated with inherited abnormalities of immunoregulation (16). Diagnosis is based on a cholestatic liver laboratory profile, the demonstration of serum antimitochondrial antibodies, and a characteristic histological picture.
The aim of this study was to investigate if Helicobacter gene sequences in general, and H. pylori, H. bilis, H. pullorum, or H. hepaticus in particular, could be detected in human liver samples from patients with PSC, a disease with many features suggestive of an infectious etiopathology. For comparison, we studied liver samples not only from patients with another cholestatic disease, namely, PBC, but also from patients with noncholestatic liver cirrhosis (NCLC) as well as from controls with normal livers.
(Part of this study was presented at the European Helicobacter pylori Study Group Workshop in Helsinki, Finland, in September 1999 [22a].)
MATERIALS AND METHODS
Patients and samples.
Liver specimens were collected from explanted livers from patients with PSC (n = 12), PBC (n = 12), NCLC (n = 13) and from autopsy livers with normal histology (n = 10) at the Sahlgrenska University hospital. The group of patients with NCLC comprised patients with alcoholic cirrhosis (n = 6), cirrhosis from chronic autoimmune hepatitis (n = 4), and cryptogenic cirrhosis (n = 3). Clinical and laboratory features are summarized in Table 1. The diagnosis of PSC and PBC was based on the criteria mentioned in the introduction. The samples were paraffin embedded prior to histological examination and deembedded by washing in xylene and ethanol. Biopsy samples (15 to 20 mg/specimen) were homogenized in 300 μl of phosphate-buffered saline (pH 7.2) by using a plastic microcentrifuge tube-adapted pestle.
TABLE 1.
Clinical features of the four patient groups and laboratory values at the time of liver transplantation in the PSC, PBC, and NCLC groups
| Characteristic | Value for group
|
|||
|---|---|---|---|---|
| PSC | PBC | NCLC | Normal | |
| Total patients | 12 | 12 | 13 | 10 |
| Mean age (yr) | 47 ± 7 | 55 ± 10 | 50 ± 12 | 57 ± 17 |
| Female/male | 8/4 | 10/2 | 5/8 | 6/4 |
| Mean bilirubin (μmol/liter) | 234 | 157 | 194 | NDa |
| Mean ALP (μkat/liter) | 23 | 22 | 4.7 | ND |
| Mean PTK (%) | 68 | 97 | 44 | ND |
ND, not determined.
DNA extraction.
The DNA extraction method has been described previously (21). Briefly, 5 to 50 μl of homogenized liver tissue was added to 100 μl of extraction buffer (75 mM KCl, 3 mM EDTA, 150 mM Tris-HCl [pH 8.0], 0.75% Tween 20), and the mixture was vortexed and incubated at 22°C for 15 min. The samples were heated at 90°C for 10 min and cooled on ice for 2 min. An ion-exchange resin (AG 51-X8, 20 to 50 mesh; Bio-Rad Laboratories, Hercules, Calif.) was added to a final concentration of 10% (wt/vol). Samples were vortexed and centrifuged for 10 min at 12,000 × g at 4°C. The upper phase, containing the DNA, was used as the template in the PCR.
Primer specificity.
The various primers were tested for amplification specificity using the following panel of Helicobacter and Flexispira strains: H. pylori CCUG 17874, H. hepaticus CCUG 33637, H. canis CCUG 33835, H. felis CCUG 28539, H. pullorum CCUG 33838, H. bilis CCUG 38995B, H. muridarum CCUG 29262, H. mustelae CCUG 23950, and F. rappini CCUG 28710. All Helicobacter strains and Flexispira rappini were obtained from the Culture Collection at the University of Gothenburg. A clinical isolate of F. rappini (H1) and F. rappini K0210, isolated from dog feces, were kindly provided by M.-L. Hänninen, Department of Food and Environmental Hygiene, University of Helsinki, Helsinki, Finland. Salmonella typhi, Proteus mirabilis, and Escherichia coli were clinical isolates from Lund University Hospital. DNA was extracted as described above. A range of bacterial strains was used previously to test the specificity of the different primers used in this study (28, 32).
PCR amplification.
PCR was performed as previously described (22), with minor modifications. Two units of Taq polymerase (MBI Fermentas, Vilnius, Lithuania) and 3 mM MgCl2 were used. Five to 10 μl of an extracted sample was added to the PCR reaction mixture. All primers were purchased from Scandinavian Gene Synthesis (Köping, Sweden). PCR was performed in a Techne Genius thermal cycler (Cambridge Ltd., Duxford, Cambridge, United Kingdom). The amplified products were analyzed with 1.5% (wt/vol) agarose (Bio-Rad Laboratories), gels, and the sizes of the PCR products were estimated by comparison with 100-bp DNA size markers (MBI Fermentas). At each amplification event a corresponding Helicobacter DNA extract was used as a positive control. For the Helicobacter genus PCR, H. pylori or H. bilis DNA was used as the positive control. Double-distilled water was used as the negative control.
E. coli 16S rRNA PCR.
PCR with E. coli 16S rRNA broad-range bacterial primers was performed as previously described (21). These primers yield an 881-bp product from several bacterial genomic DNAs (21). Bacterial DNA extracts from the PSC, PBC, and NCLC, patients were analyzed by this PCR assay.
PCR for Helicobacter genus.
Initially, samples were amplified by Helicobacter genus-specific 16S rRNA primers (designated HC) (9). The forward (C97) and the reverse (C98) primer amplified a product of approximately 400 bp. Amplification consisted of initial denaturation at 94°C for 4 min, followed by denaturation at 94°C for 1 min, primer annealing at 55°C for 1.5 min, and extension at 72°C for 2 min. The samples were amplified for 35 cycles, with a final extension step at 72°C for 10 min.
Species-specific PCR analyses.
Samples generating a positive result in Helicobacter genus PCR were subsequently analyzed with another five different sets of primers. A primer pair (designated HpD), amplifying a 298-bp product, based on the partial DNA sequence of a species-specific gene encoding a 26-kDa cell surface protein of H. pylori (The Institute for Genomic Research [TIGR] database locus HP1536, GenBank and EMBL database accession number M55507) was previously described (28). Primers based on a specific H. pylori 16S rRNA sequence (designated HpACT), amplifying a 537-bp product, were used in a second amplification protocol for H. pylori, as previously described (32). PCR primers based on 16S rRNA amplified H. hepaticus (2), H. bilis (using primers C62 and C12 [12]), and H. pullorum (31) according to published methods.
Southern blot hybridization.
Hybridization was performed using a probe generated by amplification of H. pylori strain CCUG 17874 with the species-specific HpD primers (see above), using the digoxygenin DNA labeling kit (Boehringer Mannheim) according to the manufacturer's instructions. Ten microliters of the PCR product was transferred to a nylon membrane (Amersham, Buckinghamshire, United Kingdom) by the capillary blotting technique. The membrane was prehybridized at 65°C for 4 h, freshly denatured probe was added, and hybridization performed at 67°C for 6 h before the membrane was washed and bound probes were detected by using the digoxygenin nucleic acid detection kit (Boehringer Mannheim) according to the manufacturer's instructions.
DNA sequencing.
PCR-amplified Helicobacter genus-specific PCR products were purified from agarose gels by the JETsorb DNA extraction kit (Genomed, GmbH, Bad Oeynhausen, Germany). Sequence analysis was performed with an Applied Biosystems DNA sequencer (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) by the protocols of the manufacturer, using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit. For sequencing, primer C97 or C98 (9) was used. Sequence comparison was carried out using the Blast program (Genetics Computer Group, Madison, Wis.) and the GenBank and EMBL databases.
Statistical analyses.
For comparison between continuous data we used the Mann-Whitney U test, and for comparison between numerical data, a contingency table was used. The level of significance was set to 5%. Helicobacter genus PCR positivity and negativity was compared using the chi-square test.
The study was approved by the Medical Ethics Committee in Gothenburg.
RESULTS
Specificity test.
Helicobacter genus specificity was examined using a panel of Helicobacter strains by PCR with the genus-specific primer sets designated HC. The HpACT (H. pylori 16S rRNA) and HpD (gene for the H. pylori 26-kDa protein) primer sets indicated PCR positivity only with H. pylori DNA. As previously described (28, 32), a wide range of Helicobacter strains were negative by PCR using the H. pylori 16S rRNA or 26-kDa protein primers. Primers for H. bilis, H. pullorum, and H. hepaticus were PCR positive only with the corresponding strain (data not shown). The bacterial DNA extracts from non-Helicobacter species did not react with any of the Helicobacter primers used in this study.
E. coli 16S rRNA PCR.
The results of the broad-range E. coli 16S rRNA PCR of the PSC, PBC, and NCLC patients are shown in Table 2. Generally, a high level of PCR positivity was found equally distributed among these patients.
TABLE 2.
Number of samples positive in the E. coli and various Helicobacter PCR assays
| Histological group (n) | No. of positive samples for the following primer set:
|
|||
|---|---|---|---|---|
| Eca | HCb | HpDc | HpACTd | |
| PSC (12) | 11 | 9 | 5 | 5 |
| PBC (12) | 12 | 11 | 4 | 3 |
| NCLC (13) | 12 | 1 | 0 | 0 |
| Normal (10) | NTe | 0 | NT | NT |
16S rRNA primers for E. coli.
16S rRNA primers for Helicobacter genus.
26-kDa protein primers for H. pylori.
16S rRNA primers for H. pylori.
NT, not tested.
PCR for Helicobacter genus.
Helicobacter genus-specific 16S rRNA primers (designated HC) identified Helicobacter species in 20 of 24 samples of the PSC and PBC patient groups. Under UV illumination the size of the PCR product corresponded to the expected 400 bp (Fig. 1). As shown in Table 2, a high level of PCR positivity was found in the PSC and PBC patient groups, whereas only one sample was positive in the NCLC group and among the normal liver controls. In comparing total Helicobacter genus positivity of the cholestatic liver disease patients (20 of 24) and the control groups (1 of 23) a high level of significance was found (P = <0.00001).
FIG. 1.
Analysis of Helicobacter genus-specific PCR products from liver samples of patients with PSC and PBC. The 400-bp fragments were analyzed by 2% agarose gel electrophoresis. Lanes 1 to 5, positive samples from PSC patients; lanes 6 to 11, positive samples from PBC patients; lane 12, negative control (double-distilled water); lane 13, H. pylori DNA; lane 14, H. bilis DNA; lane M, 100-bp DNA ladder size markers.
PCR for species identification.
The Helicobacter genus-positive samples from the PSC and PBC patients that were positive using primers for the H. pylori gene encoding the 26-kDa protein (HpD) and for H. pylori 16S rRNA (HpACT) are shown in Table 2. The sizes of the PCR fragments generated with the HpD primers (298 bp) and the HpACT primers (537 bp) corresponded to the respective expected sizes. Several samples positive by Helicobacter genus-specific PCR were negative using both sets of primers targeting H. pylori genes (Table 2). None of the 20 Helicobacter genus-positive PSC and PBC samples reacted in PCR assays using primers for 16S rRNA of H. bilis, H. pullorum, or H. hepaticus. The NCLC patient that was Helicobacter genus positive was negative in all species-specific PCR assays.
Southern blot hybridization.
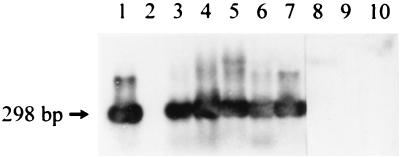
The liver samples that were positive by PCR using primers targeting the gene for the 26-kDa protein of H. pylori were all positive by Southern blot hybridization with a digoxygenin-labeled probe generated by PCR using the species-specific HpD primers. A representative Southern blot hybridization is shown in Fig. 2. The results of the hybridization confirm the presence of gene sequences of H. pylori in liver tissue samples obtained from patients with a chronic cholestatic liver disease.
FIG. 2.
Southern blot hybridization of PCR products generated by primers based on the gene encoding a 26-kDa surface protein of H. pylori. Hybridization was performed using a digoxygenin-labeled probe generated by amplification of DNA from H. pylori strain 17874 with the species-specific D primers. Lane 1, positive control (H. pylori DNA); lane 2, negative control (double-distilled water); lanes 3 and 4, positive samples from PSC patients; lanes 5 to 7, positive samples from PBC patients; lanes 8 and 9, Helicobacter-positive but H. pylori-negative samples from PSC patients; lane 10, Helicobacter-positive but H. pylori-negative sample from a PBC patient.
DNA sequencing.
Four 16S ribosomal DNA fragments, obtained by PCR using C97 and C98 primers, from Helicobacter genus-positive samples were sequenced. All were found to be at least 98% identical to H. pylori strain J99 and Helicobacter spp. liver 16S ribosomal DNA (GenBank accession number AF 142585). One of the Helicobacter species-positive but H. pylori-negative 16S rDNA fragments was also sequenced. Sequence comparison showed only low homology to Helicobacter spp. pig F8 16S rDNA (GenBank accession number AF 142151), H. suis (GenBank accession number AF 27028), and Helicobacter spp. liver 16S ribosomal DNA.
Clinical correlation to Helicobacter positivity.
Patients positive for Helicobacter genus had significantly higher values of alkaline phosphatases (ALP) and prothrombin complex (PTK; i.e., coagulation factors II, VII, and X) than patients negative for Helicobacter genus (Table 3). It is notable that the only patient in the NCLC group who was positive for Helicobacter genus had the highest ALP value (70% higher than the upper reference value) in that group. In fact, at microscopic reexamination, the pathologist found the microscopical pattern suggestive of PSC. We also found a significantly higher prevalence of Helicobacter genus positivity for patients with UC. It should be observed that UC was only present in PSC patients. The difference in prevalence of Helicobacter spp. positivity between PSC and PBC patients was not significant. We failed to demonstrate significant differences related to blood group or HLA status.
TABLE 3.
Clinical correlates of Helicobacter positivity in the PSC, PBC, and NCLC patient groups
| Characteristic | Value for groupa
|
P | |
|---|---|---|---|
| Positive | Negative | ||
| Bilirubin (μmol/liter) | 204 ± 175 | 184 ± 255 | NSb |
| ALP (μkat/liter) | 32 ± 12 | 7 ± 6 | 0.0001 |
| PTK (%) | 85 ± 29 | 49 ± 18 | 0.0003 |
| Present or previous UC/no UCc | 8/13 | 1/15 | 0.05d |
Sample groups were positive (n = 21) or negative (n = 16) by PCR with Helicobacter genus-specific primers.
NS, not significant.
Only found in PSC patients.
Statistical significance related to difference in prevalence of Helicobacter spp. positivity between patients with previous history of or present UC and those without UC.
DISCUSSION
The detection of gene sequences of Helicobacter species in liver tissue samples of patients with PSC and PBC (Table 2) is interesting, since some previous reports have suggested an association of Helicobacter and liver disease (5, 9, 20, 23, 30). In one study using PCR and subsequent sequencing of a part of the amplified ureA gene, H. pylori was detected in 3 of 7 human bile samples collected by percutaneous transhepatic cholangiodrainage from patients with pancreatic head tumors, suggesting that H. pylori may be associated with asymptomatic cholangitis (20). Another study using PCR and immunohistochemical staining observed a H. pylori-like organism in the gallbladder mucosa of a 41-year-old woman admitted to the hospital with fever and upper right quadrant pain (17). A high prevalence of antibodies to H. pylori in the serum of patients with liver diseases was also reported (30). These observations prompted us to explore a possible association of Helicobacter and chronic liver disease in Swedish patients.
Twenty of 24 liver samples from patients with PSC or PBC were positive by PCR analysis using Helicobacter genus-specific primers. Nine of these 20 samples were positive for H. pylori by PCR analysis. Lin et al. (20) detected H. pylori in bile samples with primers based on the ureA gene. We detected H. pylori by analysis with two independent PCR assays, based on the sequence of a gene encoding a species-specific 26-kDa surface protein and 16S rRNA, respectively, to avoid the possibility of cross-reaction with other Helicobacter species. Each liver biopsy was homogenized, extracted, and amplified on different occasions by different investigators. PCR results with Helicobacter genus-specific as well as H. pylori species-specific primers were reproduced very well. These precautions were taken to certify that laboratory contamination did not account for the positive PCR results. Moreover, reagent mixing, sample addition and thermocycling were performed separately. One sample, positive with the H. pylori 26-kDa protein primers, was negative using H. pylori 16S rRNA primers. An explanation for this one negative sample in the 16S rRNA PCR could be strain variation at one of the primer sites, especially one located in a variable region.
H. pylori has been shown to be sensitive in vitro to the major free bile acids in human bile, deoxycholic and chemodeoxycholic acid (15), arguing against H. pylori colonizing the liver. However, it is possible that H. pylori in vivo adapt to bile acids, as shown by studies recovering H. pylori in human feces (19). Moreover, under certain pathological conditions, such as bile duct obstruction, bile components inhibitory for the growth of H. pylori may change (34), and duodenogastric bile reflux does not seem to affect the growth of H. pylori in the antrum (18).
The predominant association with cholestatic liver disease is underlined by the significantly higher ALP and PTK levels in the Helicobacter positive patients. On the other hand, the lack of difference in bilirubin levels between Helicobacter-positive and -negative patients, and the significantly higher PTK levels in the Helicobacter-positive patients show that the Helicobacter positivity was not primarily related to severe liver failure. The fact that not all patients with the two cholestatic liver diseases were positive for Helicobacter should be considered against the fact that there is a considerable sampling variability as to histologic changes, especially for patients with PSC (27), but also for patients with PBC (14). Thus, the possibility remains that even more patients with these two diseases could be Helicobacter positive.
The large-duct involvement in PSC and the frequent occurrence of fever in PSC, in contrast to PBC, initiated the study in the PSC patients. Thus, PBC patients, who also suffer from a chronic cholestatic inflammatory disease, were primarily chosen as cholestatic controls. To our surprise, however, approximately equal proportions of positive results were obtained with the specimens from patients with PSC and PBC. This argues against a specific etiopathogenic role of Helicobacter in either of the diseases. Our findings do not exclude, however, the possibility that Helicobacter may have a triggering effect, where the response is modified by host factors.
Previous studies have found immunoglobulin G serum antibodies to H. pylori to be more common in cirrhotic compared with noncirrhotic patients (30). However, in a recent study, PBC patients with negative gastric biopsy colonization for H. pylori often had high antibody titers in an H. pylori enzyme immunoassay (8). The reason for this is unclear, but a past H. pylori infection or cross-reactivities of antibodies against other Helicobacter species are possible factors accounting for this (11). H. hepaticus and H. bilis have been shown to be possible causes of inflammatory disease in the liver of mice (11). Primary sclerosing cholangitis is often accompanied by inflammatory bowel disease in human patients, and Fox et al. (9) recently reported on H. bilis, H. pullorum, or H. rappini in gallbladder as well as bile samples of humans with chronic cholecystitis by cloning and sequencing of amplified 16S rRNA PCR products. In our present study, 9 of 20 samples found to be Helicobacter genus positive by PCR were identified as H. pylori, a finding which was verified for 4 of the 9 by sequence analysis. None of the Helicobacter genus-positive samples were positive in PCR assays targeting H. bilis, H. pullorum, or H. hepaticus. The samples not identified to the species level may represent other possible hepatic Helicobacter species. The sequence of one such 16S rDNA fragment was determined, and the result from sequence comparison showed only weak homology to some different Helicobacter spp. Further studies are needed to establish the role of H. pylori and Helicobacter species in PSC and PBC.
ACKNOWLEDGMENTS
This study was supported by a grant from the Swedish Medical Research Council (16×04723) and grants from the Lund Medical Faculty and Lund University Hospital.
REFERENCES
- 1.Bansi D S, Fleming K A, Chapman R V. Importance of antineutrofil cytoplasmic antibodies in primary sclerosing cholangitis and ulcerative colitis: prevalence, titre and IgG subclass. Gut. 1996;38:384–389. doi: 10.1136/gut.38.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battles J K, Williamson J C, Pike K M, Gorelick P L, Ward J M, Gonda M A. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol. 1995;33:1344–1347. doi: 10.1128/jcm.33.5.1344-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergqvist A, Glaumann H, Persson B, Bromé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311–316. doi: 10.1002/hep.510270201. [DOI] [PubMed] [Google Scholar]
- 4.Björnsson E, Kilander A, Olsson R. Bile duct bacterial isolates in primary sclerosing cholangitis and certain other forms of cholestasis: a study of bile cultures from ERCP. J Hepatol. 1999;28:426–432. [Google Scholar]
- 5.Calvet X, Navarro M, Gil M, Mas P, Rivero E, Sanfeliu I, Brullet E, Campo R, Dalmau B, Lafont A. Seroprevalence and epidemiology of Helicobacter pylori infection in patients with cirrhosis. J Hepatol. 1997;26:1249–1254. doi: 10.1016/s0168-8278(97)80459-x. [DOI] [PubMed] [Google Scholar]
- 6.Chapman R W, Arborgh B A, Summergield J M, Dick R, Schever P J, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography and hepatohistology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floreani A, Biagini M R, Zappala F, Farinati F, Plebani M, Rugge M, Surrenti C, Naccarato R. Chronic athrophic gastritis and Helicobacter pylori infection in primary biliary cirrhosis: a cross-sectional study with matching. Ital J Gastroenterol Hepatol. 1997;29:13–17. [PubMed] [Google Scholar]
- 9.Fox J G, Dewhirst F E, Shen Z, Feng Y, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 10.Fox J G, Drolet R, Higgins R, Messier S, Yan L, Coleman B E, Paster B J, Dewhirst F E. Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. J Clin Microbiol. 1996;34:2479–2482. doi: 10.1128/jcm.34.10.2479-2482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J G, Wang T C. Helicobacter and liver disease. Ital J Gastroenterol Hepatol. 1997;29:5–12. [PubMed] [Google Scholar]
- 12.Fox J G, Yan L L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galperin C, Gershwin M E. Immunopathogenesis of gastrointestinal and hepatobiliary diseases. JAMA. 1997;278:1946–1955. [PubMed] [Google Scholar]
- 14.Garrido M C, Hubscher S G. Accuracy of staging in primary biliary cirrhosis. J Clin Pathol. 1996;49:556–559. doi: 10.1136/jcp.49.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hänninen M-L. Sensitivity of Helicobacter pylori to different bile salts. Eur J Clin Microbiol Infect Dis. 1991;10:515–518. doi: 10.1007/BF01963941. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan I, Labigne A. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi M, Saito T, Ohno H, Midorskawa S, Sanji T, Handa Y, Morita S, Yosshida H, Tsurui M, Misaka R, Hirota T, Saito M, Minams K. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol. 1996;31:294–298. doi: 10.1007/BF02389534. [DOI] [PubMed] [Google Scholar]
- 18.Kellosalo J, Alavaikko M, Laitinen S. Effect of biliary tract procedures on duodenogastric reflux and the gastric mucosa. Scand J Gastroenterol. 1991;26:1272–1278. doi: 10.3109/00365529108998624. [DOI] [PubMed] [Google Scholar]
- 19.Kelly S M, Pitcher M C L, Farmery S M, Gibson G R. Isolation of Helicobacter pylori from feces of patients with dyspepsia in the United Kingdom. Gastroenterology. 1994;107:1671–1674. doi: 10.1016/0016-5085(94)90806-0. [DOI] [PubMed] [Google Scholar]
- 20.Lin T T, Yeh C T, Wu C S, Liaw Y F. Detection and partial sequences analysis of Helicobacter pylori DNA in the bile samples. Dig Dis Sci. 1995;40:2214–2219. doi: 10.1007/BF02209009. [DOI] [PubMed] [Google Scholar]
- 21.Mariani B D, Martin D S, Levine M J, Booth R E, Tuan R S. Polymerase chain reaction detection of bacterial infection in total knee arthoplasty. J Clin Microbiol. 1996;331:11–22. doi: 10.1097/00003086-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson H-O, Alejung P, Nilsson I, Tyszkiewicz T, Wadström T. Immunomagnetic bead enrichment and PCR for detection of Helicobacter pylori in human stools. J Microbiol Methods. 1996;27:73–79. [Google Scholar]
- 22a.Nilsson H-O, Taneera J, Castedal M, Olsson R, Wadström T. Detection of Helicobacter by PCR and DNA hybridization in human liver samples from patients with primary sclerosing cholangitis and primary biliary cirrhosis. Gut. 1999;45(Suppl. 3):A1. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson I, Lindgren S, Eriksson S, Wadström T. Analysis of antibodies to Helicobacter hepaticus in sera from patients with chronic liver disease. Gut. 1998;43(Suppl. 2):A117–A118. doi: 10.1136/gut.46.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olerup O, Olsson R, Hultcrantz R, Broomé U. HLA-DR and HLA-DQ are not markers for rapid disease progression in primary sclerosing cholangitis. Gastroenterology. 1995;108:870. doi: 10.1016/0016-5085(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 25.Olsson R, Broomé U, Danielsson Å, Hägerstrand I, Järnerot G, Lööf L, Prytz H, Rydén B-O. Spontaneous course of symptoms in primary sclerosing cholangitis: relationships with biochemical and histological features. Hepato-gastroenterology. 1999;46:136–141. [PubMed] [Google Scholar]
- 26.Olsson R, Danielsson Å, Järnerot G, Lindström E, Lööf L, Rolny P, Ryden B O, Tysk C, Wallerstedt S. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:13119–13123. [PubMed] [Google Scholar]
- 27.Olsson R, Hägerstrand I, Broomé U, Danielsson Å, Järnerot G, Lööf L, Prytz H, Rydén B O, Wallerstedt S. Sampling variability of percutaneous liver biopsy in primary sclerosing cholangitis. J Clin Pathol. 1995;48:933–935. doi: 10.1136/jcp.48.10.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole P W, Logan S M, Kostrzynska M, Wadström T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrett A D, Higgins G, Johnston H H, Massarella G R, Truelove S C, Wright R. The liver in ulcerative colitis. Q J Med. 1971;40:211–238. doi: 10.1093/oxfordjournals.qjmed.a067266. [DOI] [PubMed] [Google Scholar]
- 30.Siringo S, Vaira D, Menegatti M, Piscaglia F, Sofia S, Gaetani M, Miglioli M, Corinaldesi R, Bolondi L. High prevalence of Helicobacter pylori in liver cirrhosis. Dig Dis Sci. 1997;42:2024–2030. doi: 10.1023/a:1018849930107. [DOI] [PubMed] [Google Scholar]
- 31.Stanley J, Linton D, Burens A P, Dewhirst F E, On S L, Porter A, Owen R J, Costas M. Helicobacter pullorum sp. nov. genotype and phenotype of a new species isolated from poultry and from human with gastroenteritis. Microbiology. 1994;140:3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 32.Thoreson A C E, Borre M B, Anderson L P, Elsborg L, Holk S, Conway P, Henrichsen J, Vuust J, Krogfelt K A. Development of a PCR-based technique for detection of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995;10:325–334. doi: 10.1111/j.1574-695X.1995.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 33.Ward J M, Anver M R, Haines D C, Benvensite R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G R, Kirk C J, Goode A W. Changes in biliary lipid concentrations in bile duct obstruction: an experimental study. J R Soc Med. 1986;79:522–527. doi: 10.1177/014107688607900908. [DOI] [PMC free article] [PubMed] [Google Scholar]