Abstract
We describe a patient who developed bilateral oculomotor nerve palsy, ataxia, facial diplegia and lower limb weakness 2 weeks post-Oxford-AstraZeneca SARS-CoV2 vaccination, consistent with Miller-Fisher syndrome (MFS) and Guillain-Barre syndrome (GBS) overlap syndrome. Although some features of the patient’s presentation were typical of recently reported cases of a rare GBS variant post-Oxford-AstraZeneca vaccination, including severe facial weakness and a lack of respiratory involvement, to our knowledge this is the first reported case of MFS associated with SARS-CoV2 vaccination. While postvaccination GBS remains rare, it appears to have a favourable prognosis, and recognising this entity is therefore important for patient counselling and monitoring for potential complications.
Keywords: COVID-19, neurology, peripheral nerve disease, cranial nerves
Background
Guillain-Barre syndrome (GBS) is a clinically heterogeneous immune-mediated condition involving the peripheral nervous system. It is the most common form of acute neuropathy with a lifetime risk for an individual of under 1 in 1000, and an incidence of one to two cases per 100 000 worldwide.1 2 Antecedent infections, such as Campylobacter jejuni and cytomegalovirus are commonly associated with GBS.3 A proposed pathophysiological mechanism is that of molecular mimicry whereby a foreign antigen evokes an immune response which cross reacts with gangliosides on the peripheral nerves.3 Miller-Fisher syndrome (MFS) is a rare variant of GBS characterised by the triad of ataxia, areflexia and ophthalmoplegia. Cases in which MFS coexists with other features of GBS, such as limb weakness and facial weakness, are considered MFS-GBS overlap syndromes.4
An association between GBS and vaccination has previously been described.5 6A meta-analysis of adverse event data following mass influenza A (H1N1) vaccination in 2009 revealed a small increased risk of GBS (relative risk 2.35, 95% CI 1.42 to 4.01).6 Interestingly, two recent case series have documented striking examples of the rare bifacial weakness with limb paraesthesia (BFP) variant of GBS within 3 weeks post Oxford-AstraZeneca vaccination.7 8 These patients developed profound facial weakness and lower limb symptoms but had a good prognosis with no respiratory complications.7 8 Reports of postvaccination MFS are less frequent and, as yet, no cases of MFS have been reported post-SARS-CoV-2 vaccine.
Here, we report the first case of a patient presenting with MFS-GBS overlap syndrome following their first dose of Oxford-AstraZeneca vaccination. The patient avoided respiratory complications and had a good outcome, supporting the evidence for a favourable prognosis in cases of GBS following SARS-CoV-2 vaccination. This case also highlights the need for vigilance for a broad spectrum of SARS-CoV-2 vaccine-associated GBS variants.
Case presentation
A 63-year-old man presented 14 days following his first dose of Oxford-AstraZeneca vaccination. He experienced new-onset lower back pain 9 days postvaccination, and then 5 days later developed severe bilateral facial weakness, unsteadiness, lower limb weakness and paraesthesia over a 48-hour period. There were no urinary or bowel symptoms. He had no medical history and was not on any regular medications. He lived with his wife at home and was independent.
Neurological examination revealed profound sensory ataxia, facial diplegia involving the forehead, proximal lower limb weakness, bilateral lower limb areflexia and impaired distal lower limb proprioception. He was unable to walk without assistance and had incomplete eye closure bilaterally.
On his second day of admission, he reported diplopia on lateral gaze bilaterally. Examination revealed impaired adduction, restricted upward gaze and intorsion with down gaze bilaterally, consistent with partial cranial nerve III palsies. Direct and consensual pupillary responses were normal. His bedside forced vital capacity was 2.2 L. His GBS disability rating score was 3.9
Investigations
Testing for acquired causes of neuropathy was normal, including urea and electrolytes, liver function tests, glycated haemoglobin, B12/folate levels, thyroid function tests, serum protein electrophoresis and serum-free light chains, hepatitis B/C, HIV and syphilis serology, erythrocyte sedimentation rate, C reactive protein, antinuclear antibodies and antineutrophil cytoplasmic antibodies.
Cerebrospinal fluid (CSF) examination revealed albumin cytological dissociation with a markedly elevated protein of 2.99 g/L (normal range 0.15–0.45 g/L), 0 /µL erythrocytes, 4 /µL lymphocytes and 1 /µL polymorphonuclear leukocytes. Serum anti-GQ1b antibody was negative. Other potential causes for an elevated CSF protein were unrevealing with a negative CSF culture, normal CSF cytology and flow cytometry.
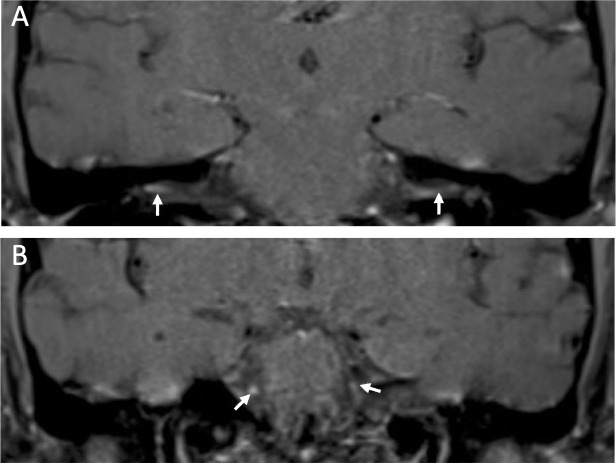
MRI of the brain with contrast demonstrated enhancement of the facial and oculomotor nerves bilaterally, consistent with the clinical examination findings of facial diplegia and partial bilateral cranial nerve III palsies (figure 1).
Figure 1.
T1 MRI brain postcontrast images. (A, top) bilateral oculomotor nerve enhancement. (B, bottom) Bilateral facial nerve enhancement.
Nerve conduction studies (NCS) and needle electromyography (EMG) showed evidence of a long-standing axonal neuropathy with reduced motor and sensory amplitudes and length-dependent chronic neurogenic changes on EMG, but no acute abnormalities. NCS and EMG may not show changes early in the course of GBS and may be normal in cases of MFS.10 11
Treatment
Given the clinical features and temporal progression of symptoms, the patient was treated with a 5-day course of intravenous immunoglobulin (2 g/kg). He also received supportive care with topical lubricating eye drops, eye patches and an indwelling catheter after developing urinary retention. He underwent 4 weeks of inpatient rehabilitation for persistent gait difficulties and lower limb weakness.
Outcome and follow-up
At 6 weeks after onset of symptoms, the patient’s ophthalmoplegia and ataxia have resolved and he has minimal residual bilateral facial weakness. He has persistent bilateral proximal lower limb weakness (Medical Research Council grade 4). The patient has returned home, is living independently and is ambulating with a four-wheel frame.
Discussion
To our knowledge, this case is the first to report the MFS-GBS overlap syndrome associated with the Oxford-AstraZeneca SARS-CoV2 vaccination. Recently, two case series have documented 12 patients who developed the rare BFP variant within 3 weeks following the first dose of the Oxford-AstraZeneca vaccination.7 8 Notably, patients in these series had a favourable prognosis, a lack of respiratory complications, and four had markedly elevated CSF protein (>1.9 g/L).7 8 Our case exhibited strikingly similar clinical characteristics, raising the possibility of a similar pathogenic mechanism, but also had classical features of MFS with ataxia, ophthalmoplegia and areflexia.
Several cases of MFS have also been documented following SARS-CoV-2 infection.12 Interestingly, although the GQ1b antibody is observed in 85% of MFS cases,13 all documented cases of MFS post SARS-CoV-2 infection have been anti-GQ1b negative,12 suggesting a novel immunopathogenic mechanism.12 14 The anti-GQ1b antibody was also negative in our case which may imply a shared mechanism to MFS post-SARS-CoV-2, perhaps via molecular mimicry of the SARS-CoV-2 spike protein.
We performed a literature review using PubMed, MEDLINE and Embase for all published postvaccination MFS cases up to 21 August 2021. The following keywords, [“Miller Fisher Syndrome”] AND [“vaccine” OR “post vaccination” OR “SARS-CoV-2” OR “COVID-19”], were used in the search strategy. There have been six prior case reports of postvaccination MFS: two following combined diphtheria, tetanus and pertussis vaccine (Tdap), one following seasonal influenza vaccine, one following combined Pneumovax and seasonal influenza vaccine, two following combined seasonal influenza and H1N1 vaccine, and one following H1N1 vaccine alone.11 15–20 All cases presented with symptoms from 5 days to 14 days postvaccination, except in one patient with HIV in which symptom onset was 40 days postseasonal influenza vaccine and 34 days post-H1N1 vaccine, respectively.17
All previous cases of post-vaccination MFS had raised CSF protein of under 1 g/L. Our case is distinct in this respect, with a CSF protein of 2.99 g/L, but consistent with cases of the BFP variant post-Oxford-AstraZeneca Vaccine.7 Three previous cases had positive anti-GQ1b antibodies, and in two cases, GQ1b status was not reported. All cases were treated with intravenous immunoglobulin, except one case post-Tdap vaccine reported by Garg and Moudgil, which was treated with plasmapheresis.15 All cases of MFS following Tdap vaccine and one case postseasonal influenza vaccine had complete resolution of symptoms; however, the prognosis of other cases may have been confounded by the variable duration of follow-up.
Our case highlights the wide clinical spectrum of GBS variants and the need for close surveillance for atypical complications of the SARS-CoV2 vaccination. Although a coincidental relationship with the Oxford-AstraZeneca vaccination cannot be excluded, we feel that the temporal onset of clinical symptoms, the presence of anti-GQ1b seronegativity and the coexistence of the rare BFP variant that has emerged as an associated clinical syndrome makes this unlikely. The patient avoided respiratory and autonomic complications and is making a promising recovery, supporting the existing evidence for a good prognosis in post-SARS-CoV2 vaccine GBS.
Patient’s perspective.
I initially presented to my local general practitioner (GP) with severe back pain and was prescribed painkillers. Two days later, I revisited my GP as the pain had not diminished and had spread to my legs. He advised me to present to the hospital, given the increasing severity of symptoms. Fortunately, the neurological team ran a battery of tests and were quickly able to confirm I had GBS with the Miller-Fisher variant. Within less than 24 hours of admission, I was receiving the first of five doses of IvIg treatments. I remained in hospital for a further week for observation then transferred to a rehabilitation facility for 3 weeks to work on the damage to my legs’ nerves, which is progressing well. During the whole process, I was kept informed of both what was being done and outcomes to expect, and this really took the stress out of the situation.
Learning points.
Guillain-Barre syndrome (GBS) is a heterogeneous autoimmune condition and can present with the overlap of rare clinical variants including bifacial weakness with limb paraesthesia and Miller-Fisher syndrome (MFS).
The SARS-CoV-2 vaccination may be associated with atypical forms of GBS, including MFS–GBS overlap syndrome.
SARS-CoV-2 vaccine-associated GBS may be associated with atypical investigation results, such as markedly elevated CSF protein and anti-GQ1B seronegativity, but appears to have a favourable prognosis.
Footnotes
Contributors: YLD and AB acquisition of case information, drafting of manuscript, preparation of figures.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Sejvar JJ, Baughman AL, Wise M, et al. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology 2011;36:123–33. 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet 2016;388:717–27. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 3.Yuki N, Hartung H-P. Guillain–Barré syndrome. N Engl J Med Overseas Ed 2012;366:2294–304. 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 4.Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011;29:599–612. 10.1016/j.vaccine.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 5.Prestel J, Volkers P, Mentzer D, et al. Risk of Guillain-Barré syndrome following pandemic influenza A(H1N1) 2009 vaccination in Germany. Pharmacoepidemiol Drug Saf 2014;23:1192–204. 10.1002/pds.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon DA, Proschan M, Forshee R, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet 2013;381:1461–8. 10.1016/S0140-6736(12)62189-8 [DOI] [PubMed] [Google Scholar]
- 7.Allen CM, Ramsamy S, Tarr AW, et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol 2021;90:315–8. 10.1002/ana.26144 [DOI] [PubMed] [Google Scholar]
- 8.Bonifacio GB, Patel D, Cook S, et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry 2021. 10.1136/jnnp-2021-327027. [Epub ahead of print: 14 Jul 2021]. [DOI] [PubMed] [Google Scholar]
- 9.van Koningsveld R, Steyerberg EW, Hughes RAC, et al. A clinical prognostic scoring system for Guillain-Barré syndrome. Lancet Neurol 2007;6:589–94. 10.1016/S1474-4422(07)70130-8 [DOI] [PubMed] [Google Scholar]
- 10.Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. plasma Exchange/Sandoglobulin Guillain-Barré syndrome trial group. Ann Neurol 1998;44:780–8. 10.1002/ana.410440512 [DOI] [PubMed] [Google Scholar]
- 11.Shaikh AG, Termsarasab P, Nwankwo C, et al. Atypical forms of Guillain-Barré syndrome and H1N1-influenza vaccination. Vaccine 2012;30:3251–4. 10.1016/j.vaccine.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Senel M, Abu-Rumeileh S, Michel D, et al. Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur J Neurol 2020;27:2378–80. 10.1111/ene.14473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimoto Y, Odaka M, Hirata K, et al. Usefulness of anti-GQ1b IgG antibody testing in Fisher syndrome compared with cerebrospinal fluid examination. J Neuroimmunol 2004;148:200–5. 10.1016/j.jneuroim.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 14.Kajani S, Kajani R, Huang C-W, et al. Miller fisher syndrome in the COVID-19 Era - a novel target antigen calls for novel treatment. Cureus 2021;13:e12424. 10.7759/cureus.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg R, Moudgil SS. Miller Fisher syndrome following tetanus, diphtheria, and pertussis (Tdap) vaccine. Neurol India 2019;67:1122–3. 10.4103/0028-3886.266262 [DOI] [PubMed] [Google Scholar]
- 16.Krämer HH, Niemöller U, Döring K, et al. Postvaccination Miller Fisher syndrome after combined pertussis, diphtheria and tetanus toxoid vaccine. J Infect 2013;66:460–1. 10.1016/j.jinf.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 17.Annunziata P, Carnicelli N, Galluzzi P, et al. Miller-Fisher syndrome following vaccination against influenza virus A/H1N1 in an AIDS patient. Infection 2012;40:97–9. 10.1007/s15010-011-0184-7 [DOI] [PubMed] [Google Scholar]
- 18.Shoamanesh A, Chapman K, Traboulsee A. Postvaccination Miller Fisher syndrome. Arch Neurol 2011;68:1327–7. 10.1001/archneurol.2011.236 [DOI] [PubMed] [Google Scholar]
- 19.Blanco-Marchite CI, Buznego-Suárez L, Fagúndez-Vargas MA, et al. Síndrome de Miller Fisher, oftalmoplejía interna Y externa tras vacunación antigripal. Arch Soc Esp Oftalmol 2008;83:433–5. 10.4321/S0365-66912008000700008 [DOI] [PubMed] [Google Scholar]
- 20.Thaler A. Miller Fisher syndrome in a 66-year-old female after flu and pneumovax vaccinations. J Am Med Dir Assoc 2008;9:283–4. 10.1016/j.jamda.2008.01.013 [DOI] [PubMed] [Google Scholar]