ABSTRACT
The consumption of dietary phytochemicals has been associated with several health benefits and relevant biological activities. It is postulated that biotransformations of these compounds regulated by the microbiota, Phase I/II reactions, transport proteins, and deconjugating enzymes contribute not only to their metabolic clearance but also, in some cases, to their bioactivation. A number of factors (age, genetics, sex, physiopathological conditions, and the interplay with other dietary phytochemicals) modulating metabolic activities are important sources and contributors to the interindividual variability observed in clinical studies evaluating the biological activities of phytochemicals. In this review, we discuss all the processes that can affect the bioaccessibility and beneficial effects of these bioactive compounds. Herein, we argue that the role of these factors must be further studied to correctly understand and predict the effects observed following the intake of phytochemicals. This is, in particular, with regard to in vitro investigations, which have shown great inconsistency with preclinical and clinical studies. The complexity of in vivo metabolic activity and biotransformation should therefore be considered in the interpretation of results in vitro and their translation to human physiopathology.
Keywords: phytochemicals, phenolic compounds, microbiota, drug-metabolizing enzymes, transport proteins, phytochemicals metabolism, interindividual variability
Statement of significance: This is the first review article focused on the contribution of the microbiota, drug-metabolizing enzymes, and transport proteins in the biological activity of dietary phytochemical metabolites.
Introduction
Healthy dietary patterns in humans are characterized by the regular consumption of fruits and vegetables. The contribution of plant foods to prevent or slow down chronic disease (cardiovascular, cancer, cognitive decline) has been discussed in several recent review articles in Advances in Nutrition (1, 2). Plant foods are rich in a number of nonnutrient chemicals also known as phytochemicals (because of their plant origin) or polyphenols (based on their chemical structure). Many are also designated as antioxidants due to their in vitro and ex vivo free-radical-scavenging properties. These antioxidant activities are relevant in plant physiology and in the human gut, where they may reach concentrations large enough to display this biological activity. Furthermore, in general terms, dietary ingestion of plant polyphenols modulates several other biological activities of interest to human health that are not explained simply by their scavenging capacity such as the modulation of signaling pathways (3).
The bioactivity of dietary phytochemicals has been investigated mainly in vitro and in some instances in animal models. Nevertheless, most of these observed biological effects are not substantiated when tested in human nutrition randomized controlled trials. Several factors may contribute to the unsuccessful translation of the biological effects observed in preclinical studies to human studies. The present review will not discuss all these contributing factors but will focus on the fate of dietary phytochemicals in the human body.
Firstly, regarding dose, one should distinguish between doses compatible with human diet and supra dietary or pharmacological doses. A well-known dietary phytochemical, resveratrol, mainly present in wine, was the topic of a review entitled “How Much Wine Do You Have to Drink to Stay Healthy?” (4), opening the discussion of whether some health effects using supplements could be achieved following a balanced diet. In fact, a former editor of The Lancet wrote a letter entitled “Chateau Resveratrol,” where he emphasized that the potential health benefits of wine, if any, came from ethanol and not from the small amounts of resveratrol (CAS 501–36–0) present in wine (5). In this regard, mean concentrations of resveratrol in wine are around 3 mg/L. Although the rate of absorption of resveratrol is relatively good, its bioavailability after first past metabolism is <1%. Larger doses of resveratrol in supplements, typically containing between 250 and 500 mg, try to compensate for the poor bioavailability of resveratrol when it is not present in its natural hydroalcoholic matrix (wine) (6). In the case of olive oil, containing simple phenols like hydroxytyrosol, concentrations of the unaltered compound reaching the systemic circulation are about 0.1 to 1% of the dose ingested (7), with peak concentrations in the low nM range. On the other hand, concentrations of hydroxytyrosol metabolites are about 50 to 100 times higher depending on the dose and the matrix used for its administration (8).
Higher doses are also founded in the pharmacological principle that the higher the unaltered fraction of the phytochemical in the body, the larger the biological/pharmacological effect. Specifically for resveratrol, adverse effects are presumed to be less relevant compared with a synthetic molecule due to the natural origin of the molecule. However, several studies show that high amounts of apparently beneficial compounds used as dietary supplements may have deleterious health effects (e.g., vitamin C, β-carotene, or vitamin E) (9).
Most studies in humans show that circulating concentrations of plant phytochemicals following dietary consumption are negligible due to poor bioavailability, substantively lower than those tested in vitro and in vivo in animal models. This poor bioavailability is not only explained by poor absorption but also by the fact that phytochemicals undergo multiple physicochemical and enzymatic modifications starting in the oral cavity (10) and further due to the actions of gastric juices, the enzymatic activity of the gut microbiota, and an extensive first pass metabolism in intestinal epithelial cells and in the liver. In a recent report where the fate of extra-virgin olive oil (EVOO) phytochemicals during gastrointestinal digestion was investigated, the study highlighted the relevant changes on the EVOO phenolic profile triggered by gastrointestinal digestion. The authors suggest the need to account for actual bioaccessibility values rather than just considering raw concentrations (11). Also, a recent review stressed the relevance of biotransformations regulated by the gut microbiota (12). In this review, we postulate that some of the microbiota bioaccessible metabolites, or those generated in first pass metabolism of the absorbed fraction, are biologically active. This may be due to the chemical moieties previously incorporated in Phase II metabolic reactions (by sulfation, glucuronide conjugation, or methylation) being directly or indirectly freed, giving rise to significant intracellular concentrations of the unaltered ingested compound. Thus, biological activities of phytochemicals could be enhanced by metabolic processes rather than dependent upon concentrations of the unaltered phytochemical in the body.
The contribution of drug-metabolizing enzymes in the disposition and bioactivity of phytochemicals has seldom been explored. We recently provided evidence that the simple phenol tyrosol (CAS 501–94–0) is bioactivated in humans to hydroxytyrosol (CAS 10,597–60–1), a potent dietary antioxidant present in olive oil and wine, by Phase I metabolic enzymes (13). This metabolic activation results in biological effects, for example in endothelial function (14). The resulting bioactivation of tyrosol is modulated by sex and genetic variations in the activities of the isoenzymes of cytochrome P450 (CYP, i.e., CYP2A6 and CYP2D6) (14). In the same context, we have described that the Val158Met polymorphism in catechol-O-methyltransferase (COMT) affects the efficiency of the methylation of hydroxytyrosol to homovanillyl alcohol (CAS 2380–78–1), in so far as the concentrations of homovanillyl alcohol were associated with a lower risk of cardiovascular disease (CVD) and total mortality in elderly individuals (15). Additionally, we have presented evidence that other Phase II metabolites of tyrosol and hydroxytyrosol could be involved in biological activities elicited by these aforementioned simple phenols (16, 17).
The purpose of this review was to compile and evaluate the contribution of the gut microbiota, drug-metabolizing enzymes, and transport proteins to the biological activities elicited by dietary phytochemicals. Additionally, this review explores the sources of interindividual variability in each of the steps that phytochemicals undergo before eliciting their biological effects, with special emphasis on genetic and sex differences as sources of variability.
Current Status of Knowledge
Metabolic reactions regulated by enzymatic activities of the gut microbiota
Metabolic biotransformations by the gut microbiota
The human gut microbiota is a crucial component of dietary metabolism, as it provides many essential biochemical pathways that humans do not possess endogenously (18, 19). The metabolic capacity of the microbiota, distinct and complementary to that of the liver and gut mucosa, has proven to be greater than that of the host (18, 19) to the extent that it is now considered as a separate virtual organ (20, 21). In a similar way to the well-known effects that stomach pH or gastric enzymes have on the stability and bioavailability of phytochemicals, the microbiota also has significant effects on the digestion of foods, and in the profound biotransformation that nutrients and nonnutrient compounds undergo after ingestion.
The first site of polyphenol absorption is generally the small intestine [although some phytochemicals can be absorbed in the stomach (22)], where, being present in food matrices commonly conjugated to organic acids and sugars, the polyphenols need to be hydrolyzed prior to absorption. Some polyphenols may be hydrolyzed by the action of lactase-phlorizin hydrolase (LPH) or cytosolic β-glucosidase (CBG). LPH, located in the brush border of the epithelial cells, deglycosylates polyphenols, allowing them to enter the enterocyte. CBG is found inside the enterocyte and metabolizes the more polar glycosides carried inside the cells by specific transporters (23, 24). However, glycosides that are bound to sugars other than glucose (e.g., rhamnose) are not substrates for these hydrolytic enzymes (25). Moreover, the total uptake of dietary polyphenols in the small intestine, in the form of aglycones and monomeric and dimeric structures, is estimated to be ∼10% (26, 27). Hence, a major proportion of ingested polyphenols are transported to the large intestine, where an efficient hydrolysis of conjugated forms occurs, mediated by the enzymatic activity of the colonic microbiota. The fate of the small fraction of the compounds absorbed in the small intestine is strongly influenced by the presence of the enzymes that catalyze Phase I and II reactions, by those that hydrolyze the newly formed metabolites, and by the transporter proteins that can promote their entry into the blood circulation or their transport back into the intestinal lumen. Many of these aspects will be dealt with later in the following sections.
Gut microbiota and polyphenol bioavailability
The colonic microbiota exerts a fundamental role in determining the bioavailability of ingested phenolic compounds via different enzymatic activities. The transformations performed by the gut microbiota on polyphenol structures depend on their chemical structure, amount, type and position of specific functional groups, stereoisomerism, and polymerization degree.
Several studies including in vitro cultures using fecal inoculum and intervention studies in animals and humans have demonstrated that the gut microbiota can deconjugate the majority of dietary polyphenols (28, 29). The most abundant species of microorganism in the human gut belong to the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria phyla. All possess O-glycosidase activity (30). They also possess C-glycosidase activity but hydrolysis of C-glycosides is less efficient than that of O-glycosides, and only some species of bacteria of the Firmicutes phylum can perform this hydrolysis (31). Colonic microbiota also express α-rhamnosidases (32), in addition to β-glucosidases (33, 34), glucuronidases, and esterases (31). Microbial organisms can hydrolyze a wide range of conjugated compounds, resulting in the free forms of the polyphenols and facilitating their absorption. Once inside the enterocyte, the phytochemicals can be locally metabolized into Phase I/II metabolites. Conjugated and unconjugated phenolic compounds enter the enterohepatic circulation and reach the liver, where they undergo further biotransformations before being distributed to target tissues (23). Part of the conjugated compounds are excreted by the liver through enterohepatic recirculation, as bile components into the intestine, where microbial enzymes can regenerate deconjugated compounds that are available for reabsorption (33).
Polyphenol metabolism by the gut microbiota
Apart from assisting the intestinal absorption and bioavailability of phenolic compounds, the microbiota may further metabolize deconjugated products, giving rise to a pool of unique microbial catabolites, known as postbiotics, in addition to nonspecific degradation products. The evidence suggests that postbiotics can exert beneficial health effects. The relevance of the phenolic-derived postbiotics has been focused on as a key step to better understand the link between polyphenol consumption and the biological effects observed (31). Once more, microbial transformations differ depending on phenolic structure, polymerization degree, and spatial configuration.
In the case of flavonoids (flavonols, flavones, flavanones, and anthocyanidins), C-ring or A-ring (flavanols) cleavage is the first and most common reaction that occurs in the colon, giving rise to several products depending on the breaking position in the ring (31, 33, 35). C-ring cleavage is catalyzed by dioxygenases and some have been characterized, such as quercetin 2,3-dioxygenase, producing 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate (36–39). Another example is the degradation of the A-ring, as in the case of catechins, mediated by a complex reverse Claisen-driven reaction (40). After ring fission, bacterial flavonoid degradation follows a general pathway via demethylation and dehydroxylation. Many nonspecific metabolites are formed, such as various derivatives of phenylacetic, phenyl propionic, and benzoic acids (31, 33, 41).
Conversely, the formation of certain postbiotics by the gut microbiota is substrate specific. The most studied polyphenols are those derived from isoflavones, ellagitannins, or lignans (Table 1). The first example is the catabolism of the isoflavone daidzein (CAS 486–66–8), which undergoes sequential hydrogenation reactions to form dihydrodaidzein (CAS 17,238–05–0; 4′,7-dihydroxyisoflavone), tetrahydrodaidzein (4′,7-dihydroxyisoflavan-4-ol), and the unique biologically active metabolite (S)-equol (CAS 531–95–3, 4′-methoxy-7-isoflavanol). An alternative metabolic route produces O-desmethylangolensin (CAS 21,255–69–6) (35, 50, 51). Only 30–50% of people contain this kind of equol-producing bacteria and are able to convert daidzein to (S)-equol (42).
TABLE 1.
Examples of substrate-specific gut microbiota reactions and their interindividual variability
| Parent compound | Postbiotic | Described metabotype | Key strain described | Food sources1 | Reference |
|---|---|---|---|---|---|
| Daidzein | (S)-equol | Yes, only 30–50% of individuals | Coriobacteriaceae | Soy and soy products | (31, 42) |
| Ellagic acid | Urolithin A and urolithin B | Yes, Phenotype 0: nonproducers, Phenotype A: producers of urolithin A, Phenotype B: producers of urolithin A, isourolithin A, and urolithin B | Gordonibacter but other species may also be involved | Berries, chestnuts, and walnuts | (31, 43, 44) |
| Lignans | Enterolactone | No | Bacteroides, Clostridium, Eubacterium, and Eggerthella lenta | Flaxseed, sesame and in minor concentration: fruits, vegetables, grains, and oilseeds | (31, 45) |
| Xanthohumol and isoanthohumol | 8-prenylnaringenin | Likely, observed in ex vivo studies but not sufficiently demonstrated | Eubacterium | Beer and hop-derived products | (46, 47) |
| Naringin, hesperidin, and neohesperidin | Aglycones naringenin, hesperetin | Likely, observed high and low excreters but not sufficiently demonstrated | Bacteriodes and Eubacterium | Fruit and citrus juices | (48) |
Information regarding the food sources of the phenolic compounds was obtained from the database phenol-explorer.eu (49).
Similarly, ellagitannins undergo lactone cleavage to produce ellagic acid, which is further catabolized by the gut microbiota to form urolithins, dibenzopyran-6-one derivatives with different hydroxyl substitutions (43, 44). The metabolic pathway involves dehydration, driven by decarboxylation and reduction reactions, hydrolysis, and keto-enol tautomerism (35, 43, 52).
Another substrate-specific pathway is the catabolism of lignans. They are metabolized to the bioactive products enterodiol (CAS 80,226–00–2) and enterolactone (CAS 78,473–71–9) through a complex metabolic pathway involving several intermediary metabolites and a consortium of diverse bacterial species able to catalyze these biotransformations (45). To illustrate, pinoresinol (CAS 487–36–5) is subjected to 4 distinct chemical reactions, 2 sequential benzyl ether reductions, guaiacol demethylation, catechol dehydroxylation, and diol lactonization yielding the final products (45, 53).
Indeed, there is a 2-way relation between microbiota and polyphenols. Beyond the aforementioned role of the gut microbiota catalyzing the metabolism of polyphenols, the polyphenols and their metabolites themselves can also modulate the microbiota composition exerting a “prebiotic activity.” They can promote the growth of several microorganisms by being a source of nutrients, in the case of conjugated compounds, or by inhibiting the growth of others acting as antimicrobials (54–57). In general, polyphenols have been shown to influence the relative abundance of different gut-inhabiting bacteria, reducing the number of potential pathogens and enhancing the growth of beneficial species (58).
Individual variability in the gut microbiota metabolism and its biological relevance
An individual's microbiota composition is the result of complex interactions between several extrinsic factors such as diet, treatment with medications, physical activity, chronic stress, alcohol consumption, and smoking, and intrinsic factors such as host genetic background, age, race/ethnicity, and sex, among others (30, 55). The intestinal microbiota is a dynamic ecosystem that changes throughout life. Following the initial colonization of gut bacteria at birth, the species diversity increases equally in both sexes until adolescence, when a significant difference between males and females becomes evident (59, 60). In humans aged over 70 y, the microbiota composition returns to being homogeneous between the sexes and depends mostly on changes in intestinal physiological function (61, 62). Thus, sexual maturation and sex hormones are considered to be major determinants of sex differences in the gut microbiota (59, 63). In general, females have a higher ratio of bacterial cells to human cells (64) and a higher intestinal microbial diversity than males (65, 66). It is also worth considering that estrogens impact on microbiota and conversely, the gut microbiota may itself regulate circulating estrogens, as bacterial β-glucuronidases deconjugate a variety of endogenous molecules including sex hormones (67, 68).
At the species level, the human gut microbiota has been divided into 3 enterotypes, classified in European individuals by the variation in the levels of genera Bacteroides, Prevotella, and Ruminococcus (69), with some differences in Asian populations (70, 71).
As a result of this complex interplay of factors modulating the gut microbiota, some microbial metabolites are not present in individuals to the same extent, suggesting that the transformation of the parent polyphenols into their metabolites depends mostly on individual variation in the colonic microbiota (72, 73). In relation to several specific metabolites, “producer” and “nonproducer” individuals have been identified, depending on the presence of particular species or strains possessing the enzymatic machinery to catalyze different reactions within the phenolic compound. This concept has been defined by the term metabotype, describing a phenotype characterized by the generation of specific metabolites derived from the gut microbiota and the specific metabolism of the polyphenol precursors (see Table 1). In this context, different studies have emphasized the need to cluster individuals into metabotypes in an attempt to explain, at least in part, the interindividual variability in the health effects observed following dietary interventions rich in phenolic compounds (31).
The heterogeneity in microbiota composition and metabolic activity may explain why some individuals metabolize one of the most active isoflavones, daidzein, to S-equol (72). The reduced metabolite S-equol is produced via a series of consecutive reduction reactions due to the metabolic activities of bacterial species belonging mainly to the Coriobacteriaceae family (72). Individuals unable to produce S-equol do not possess the above-mentioned microbial species, thus the catabolism of daidzein ends with O-desmethylangolensin or other metabolites (74). S-equol is more stable and easily absorbed than its precursor daidzein (75). It also shows stronger estrogenic activity than any other isoflavone or isoflavone-derived metabolite (76, 77) and significant antioxidant activity, through the enhancement of antioxidant cellular defenses (72). S-equol has been shown to exert beneficial effects in the protection against estrogen-dependent and aging-associated processes, such as menopause, osteoporosis, CVD, and cancer (31, 72). However, only individuals that possess S-equol-producing microbes may fully benefit from daidzein consumption.
A large interindividual variation has also been highlighted in the metabolism of ellagic acid into urolithins. Individuals can be classified into 3 metabotypes according to the type and amount of urolithin produced following the intake of ellagitannin and/or ellagic acid: nonproducers (metabotype 0), producers of urolithin A (CAS 1143–70–00; 3,8-dihydroxy-urolithin) only (metabotype A), and producers of urolithin A, isourolithin A (CAS 174,023–48–4; 3,9-dihydroxy-urolithin), and urolithin B (CAS 1139–83–9; 3-hydroxy-urolithin) (metabotype B) (73). Some Gordonibacter species have been identified as responsible for this conversion, but other bacterial species may also catalyze these transformations (78). Urolithins have exhibited important biological activities and are considered to be the actual bioactive molecule at a systemic level (79). Urolithins are better absorbed in the intestine and reach target tissues at a higher concentration than ellagic acid itself (44). For instance, subjects with a metabotype A seem to have a lower risk of developing chronic illnesses, as urolithin A is the most active form among urolithins (73, 80).
The gut microbiota composition seems to be the most critical factor governing interindividual variability in the production of enterodiol and enterolactone from dietary lignans (81). Several bacteria involved in the different steps of this transformation have been identified, which include Bacteroides, Clostridium, Eubacterium, and Eggerthella lenta (81), and their activity has been found to be strain specific (82). Insufficient concentrations or the complete lack of some of these bacterial species may greatly influence an individual's lignan metabolism (81). Enterolactone possesses significant anticancerogenic activity, attributed to its antiproliferative, proapoptotic, anti-inflammatory, antiangiogenic, and antimetastatic activities. A high concentration of circulating enterolactone has been inversely correlated with the incidence of different cancers (83).
Not all unique microbial catabolites have been characterized nor linked to a biological effect. In fact, a clear association between the production of specific phenolic-derived microbial catabolite and health effects has been reported for only a few phenolics. There is a great need to enrich the characterization and to better understand the biological significance of the complex pool of metabolites formed by the microbiota. Individual variation in the composition of the gut microbiota can affect polyphenol metabolism capacity and thus explain partially, the interindividual variability in health effects observed after the consumption of dietary polyphenols.
Phase I reactions: cytochrome P450 regulated
Polyphenol biotransformation by cytochrome P450
Regarding the metabolic disposition of polyphenols, Phase II conjugation reactions are quantitatively the most common because their chemical structure, which includes a number of hydroxyl and phenol groupings, favors this type of reaction. Even so, the participation of Phase I metabolism in the clearance of polyphenols has to be considered. Phytochemicals act both as inhibitors of CYP-catalyzed reactions as well as inductors via their interactions with receptors of xenobiotics (e.g., AhR or PXR) (84, 85). These interactions may directly affect the development of various diseases such as cancer, CVDs, and diabetes and are relevant for the interpretation of drug–phytochemical interactions and drug effects (85). The contribution of phytochemicals to the induction and inhibition of CYPs is not specifically covered in this section but there are good reviews covering this topic.
The most important group of Phase I reactions are mediated by CYP enzymes and include oxidation, reduction, and hydrolysis reactions. Phase I metabolism is primarily located in the liver, but can also occur in the enterocytes, kidneys, and lungs. cyps are a superfamily of hemoproteins responsible for the oxidative metabolism of a wide range of xenobiotics (also known as xenometabolites) and are involved in the metabolism of many endogenous compounds including steroids, vitamins, and hormones (85, 92). A total of 57 CYP isoforms have been described in humans, nonetheless, a subset of isoforms belonging to the 1, 2, and 3 CYP families are responsible for the majority of the CYP-mediated reactions (93, 94).
Initially, Phase I reactions were considered exclusively a detoxification pathway to eliminate xenobiotics, producing metabolites with decreased or null biological activity. This is the case for phytochemicals such as caffeine (CAS 58–08–2) and capsaicin (CAS 404–86–4) (95, 96). Nonetheless, the literature confirms the involvement of CYPs in the bioactivation of certain dietary phytochemicals whose metabolism through the cyp family yields more biologically active metabolites or at least as active as the parent compound (87). Table 2 summarizes examples of well-known phenolic compounds that are bioactivated by means of cyp isoforms. Indeed, the biotransformation catalyzed by cyp within the flavonoids group of compounds, which all share similar structures, can trigger the conversion of 1 type of flavonoid into another, resulting in changes in their biological activities (97). For instance, flavanones can be metabolized into flavones, hydroxyflavanones, and isoflavones by CYPs, with all of the corresponding metabolites showing relevant biological activities (98). In the case of the polyphenol safrole (CAS 94–59–7), the bioactivation by means of CYP enzymes yields 1′-hydroxysafrole (CAS 5208–87–7), a metabolite that can be further transformed into procarcinogenic metabolites (91).
TABLE 2.
Phytochemical bioactivation by cytochrome P450 isoforms
| Parent compound | Active metabolite | Reaction | Isoforms involved | Food sources1 | Reference |
|---|---|---|---|---|---|
| Tyrosol | Hydroxytyrosol | 3’-hydroxylation | CYP2A6 CYP2D6 | Olive oil, wine, and beer | (13) |
| Trans-resveratrol | Piceatannol | 3’-hydroxylation | CYP1A1 CYP1A2 CYP1B1 | Grapes, wine | (86) |
| Kaempferol | Quercetin | 3’-hydroxylation | CYP1A2 | Tea, fruits, vegetables, and beans | (87, 88) |
| Tamarixetin | Quercetin | 4’-demethylation | CYP1A2CYP3A4 CYP2C9 | Fruits, vegetables, and nuts | (87) |
| Apigenin | Luteolin | 3’-hydroxylation | CYP1A2 CYP3A4 | Fruits, plant-derived beverages, and vegetables | (87, 88) |
| Naringenin | Eriodictyol | 3’-hydroxylation | CYP1A2 CYP3A4 | Herbs and fruits (grapefruit, sour orange, tomato, cocoa…) | (87) |
| Hesperetin/hesperidin | Eriodictyol | 4’-demethylation | CYP1A2 | Herbs and fruits (orange, lemon, tangerine, and peppermint) | (87) |
| Genistein | Orobol | 3’-hydroxylation | CYP1A2 | Soy products | (89, 90) |
| Safrole | 1’-hydroxysafrole | 1’-hydroxylation | CYP1A2 CYP2A6 CYP2C9 CYP2C19 CYP2D6 CYP2E1 | Spices (nutmeg, mace, cinnamon, anise, black pepper, and sweet basil) | (91) |
Information regarding the food sources of the phenolic compounds was obtained from the database phenol-explorer.eu (49).
The study of phytochemical metabolism is complex and not completely understood, and for this reason information regarding the impact of Phase I transformations of many compounds is still scarce, even in compounds frequently studied for their potential biological effects. It would seem that this is the case for the well-known and extensively researched phenolic compound curcumin (CAS 458–37–7), in which CYPs have been described to be the most likely enzymes mediating its metabolism to hexahydrocurcuminol (CAS 36,062–07–4) (99).
Individual variability in CYP metabolism of polyphenols and its biological relevance
The CYP family of enzymes shows high genetic variability in the population. This is commonly caused by single nucleotide polymorphisms (SNPs) and copy-number variations (CNVs) including duplications and deletions. The functional enzymatic status of the variants can range from nonfunctional to increased function, having an impact on the rate of the reactions metabolized by the CYP enzyme. The discipline of pharmacogenetics has made a great effort to standardize the terminology and functional status of the resulting alleles and phenotypes (94). Additionally, consortiums have released evidence-based guidelines in order to translate CYP genetic results into clinical practice, in many cases adapting drug dosage to an individual's genotype (100).
The polymorphic trait of CYPs together with their role in the metabolism of certain phenolic compounds leads to the speculation that these individual differences could render significant differences in the benefits obtained from the consumption of these compounds (87). The impact of polymorphisms in CYP1A2 has been studied in the context of caffeine and coffee intake. A meta-analysis concluded that rapid caffeine metabolizers (having an increased CYP1A2 activity caused by the rs762551 polymorphism) show greater coffee intake. This relation was more marked in young males and Caucasians, but not observable in females, elderly, or Asian populations (101). Polymorphisms in CYP1A2 also have an impact on the effects of caffeine on blood pressure, with a greater increase shown in slow caffeine metabolizers (rs762551 polymorphism) (102). Another example of the impact of polymorphic CYPs has been studied in the context of the bioactivation of tyrosol into hydroxytyrosol by CYP2A6 and CYP2D6. The rate of hydroxytyrosol formation was associated with the genetic profile of these CYPs: the carriers of nonfunctional and decreased function alleles were less capable of bioactivating tyrosol to hydroxytyrosol (14, 103). Consistent with this, the polymorphisms in these CYPs also had an impact on the magnitude of the cardioprotective effects rendered by a tyrosol-rich intervention (14). Regarding the polyphenol safrole, poor metabolizer phenotypes could reduce the relative risk of the detrimental effects derived from its consumption (91).
In the case of lignans, CYPs are not directly involved in their degradation or bioactivation. The isoform CYP1B1 is involved in triggering the modulatory effect that lignans exhibit on estrogen metabolism. CYP1B1 is involved in both estrogen biosynthesis and metabolic clearance. Metabolism favors the formation of compounds with weaker estrogenic activity. High exposures to estrogenic compounds are associated with osteoporosis and hormone-sensitive cancers such as breast cancer. On the contrary, high lignan exposures exhibit a protective effect. Genetic variation in CYP1B1 modulated the effect of a lignan-rich intervention on the estrogen metabolic profile (104, 105). The presence of alleles associated with a greater CYP1B1 activity triggered a significant increased formation of metabolites with weaker estrogenic activity (104).
The cyp family contributes to sex differences observed in the pharmacokinetics of a wide range of compounds. It is likely that most of these differences are influenced by sex hormone production and hormonal changes due to oral contraceptive consumption, pregnancy, and menopause (106, 107). Certain CYP isoforms show a sex dimorphism in their mRNA expression, resulting in distinct protein concentrations. Overall, this results in a sexual dimorphism in the metabolic rate of CYP-associated reactions. CYP3A4 expression is higher in women, yielding a higher clearance in the reactions mediated by this enzyme (93, 106–109). On the contrary, men exhibit higher expression of CYP1A2, CYP2E1 (93, 106–109), and higher CYP2D6 activity for certain substrates (109). In the case of CYP2A6, a functional difference has been observed between sexes, however, it is attributed to an inducible effect of estrogens on CYP2A6 transcription, resulting in a higher activity of the enzyme in premenopausal women compared with men (110).
The sexual dimorphism in CYP enzymes has an impact on the metabolism of some of the aforementioned phytochemicals. In the case of caffeine, sex differences and oral contraceptive use explained part of the interindividual variation observed in caffeine metabolism, independent of CYP1A2 genotype. Within women, oral contraceptive users were associated with decreased caffeine metabolism (111). In the case of tyrosol to hydroxytyrosol conversion, lower hydroxytyrosol formation has been observed in women following an equal dose of wine (103).
Finally, it is important to mention that certain phytochemicals can alter the activity of specific CYPs, thus adding a further degree of complexity. These alterations can be the result of induction/inhibition of CYP activity triggered by the phytochemical or direct competition for the same substrate-binding site (85). Therefore, the simultaneous consumption of therapeutic drugs and certain phytochemicals can alter the pharmacokinetics of drugs and hence, increase the risk of unwanted side effects. As a matter of example of the relevance of these interactions, well-known and serious consequences have been described with the coadministration of therapeutic drugs with St John's wort or grapefruit due to their induction and inhibition of CYP3A4, respectively. For further information refer to other relevant reviews (85, 112).
Overall, Phase I drug-metabolizing enzymes play an important role on the biological activities fostered by phytochemicals. Extensive CYP genetic variation, together with sex differences, could be important modifiers of the health effects attributed to their exposure and explain part of the variability observed in phytochemical-rich interventions.
Phase II metabolic reactions
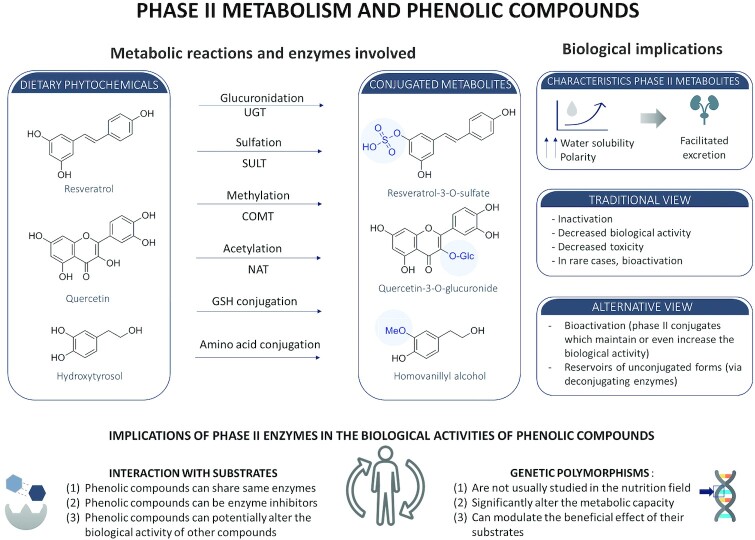
Phase II enzymes metabolize dietary phenolic compounds giving rise to conjugated metabolites. The 3 main Phase II reactions involved in the metabolism of dietary phenols are sulfation, glucuronidation, and methylation, which are catalyzed by sulfotransferases (SULTs), uridine 5′-diphosphate-glucuronosyltransferases (UGTs), and COMT, respectively. Phenolic compounds can also undergo alternative Phase II reactions such as acetylation (catalyzed by N-acetyltransferase), glutathione conjugation (catalyzed by glutathione-S-transferase), conjugation with amino acids, or bis-conjugations (Figure 1), but the quantitative contribution of the latter reactions is generally lower. The metabolic pathway of each compound depends on their affinity towards each Phase II metabolic enzyme, as well as on the quantity of expression and location of the enzyme. For instance, hydroxycinnamic acids are essentially sulfated (mainly by SULT1A1) and, to a lesser extent, glucuronidated (by UGT1A9) (113), whereas flavonoids are both sulfated and glucuronidated, but the rates of glucuronidation are faster than those of sulfation (114). The simple phenol hydroxytyrosol can be both sulfated and glucuronidated, glucuronides being more predominant at lower doses, and sulfates being the major metabolites found following high doses (115). Also, the polarity of the matrix (i.e., hydroalcoholic compared with fatty) influences the rate of Phase II metabolite formation. In the case of wine consumption, hydroxytyrosol sulfates are predominantly formed and in the case of olive oil, glucuro-conjugates are the most relevant metabolites (116). A crucial question regarding the potential biological activity of Phase II metabolites is to establish whether they act directly as conjugates or if they first undergo hydrolysis in the intra- and/or extracellular environment by deconjugating enzymes (15, 117). Table 3 outlines a list of Phase II reactions leading to metabolites with a described biological activity. It is worth mentioning that the conjugation patterns for Phase II reactions can be significantly different between human and animal models, and even between different rodent models (e.g., rats compared with mice). In an elegant example, Ottaviani et al. measured the metabolites of epicatechin following the oral administration of [2–14C](−)-epicatechin in humans, rats, and mice. The authors found that the metabolic profile was very different between the 3 species, evidencing the relevance of interspecies metabolic differences (118).
FIGURE 1.
Schematic representation of major Phase II metabolic reactions and their biological consequences. COMT, catechol-O-methyltransferase; GSH, glutathione; NAT, N-acetyltransferase; SULT, sulfotransferases; UGT, uridine glucuronyl transferases.
TABLE 3.
Selected examples of Phase II metabolic reactions of phytochemicals leading to bioactive metabolites
| Parent compound | Active metabolite(s) | Reaction | Food sources1 | Reference |
|---|---|---|---|---|
| Hydroxytyrosol and tyrosol | Hydroxytyrosol 3-O-sulfate; hydroxytyrosol 3-O-glucuronide; tyrosol 4-O-sulfate; tyrosol 4-O-glucuronide; homovanillyl alcohol | Sulfation, glucuronidation, and methylation | Olive oil, olives, wine, beers | (15, 122, 123) |
| Resveratrol | Resveratrol-3-O-glucuronide; resveratrol-3-O-sulfate; resveratrol-4′-O-sulfate; dihydro-resveratrol-3-O-glucuronide | Glucuronidation and sulfation | Grapes, wine | (124) |
| Quercetin | Quercetin 3-O-sulfate; quercetin 3-O-glucuronide; isorhamnetin-3-glucuronide | Sulfation and glucuronidation | Cocoa, berries, apples, onions | (125–128) |
| Epicatechin | Epicatechin sulfate; epicatechin glucuronide | Sulfation and glucuronidation | Tea, cocoa, wine, fruits, beans | (125) |
| (+)-Catechin | Catechin sulfate; catechin glucuronide | Sulfation and glucuronidation | Tea, cocoa, wine, fruits, beans | (126) |
| Daidzein | Daidzein 4′-sulfate | Sulfation | Soy and soy products | (129) |
Information regarding the food sources of the phenolic compounds was obtained from the database phenol-explorer.eu (49)
Sulfation
SULTs are cytosolic enzymes widely expressed in the liver, but also present in other tissues like the small intestine, brain, adrenal glands, kidneys, lungs, skin, breast, and blood (platelets) (117, 119). They catalyze the transfer of the sulfonate group (SO3–) from the cosubstrate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to an acceptor molecule (hydroxyl, amino, or sulfhydryl groups) generating a sulfate conjugate. Sulfation is an important pathway for the in vivo metabolism of exogenous (e.g., drugs, dietary compounds, and food additives) and endogenous compounds (e.g., steroid and thyroid hormones, bile salts, and monoamine neurotransmitters) (117, 120). Generally, sulfation decreases the biological activity of these molecules but in some cases, it can result in bioactivation and increase biological activity. This has been studied for drugs (e.g., the antidepressant minoxidil) and xenobiotics [e.g., mutagenicity of aromatic hydroxylamines and benzylic alcohols (121)] and also in dietary antioxidants (hydroxytyrosol sulfate) (122, 123).
SULTs display a wide interindividual variability, and this is partially explained by genetic variants. In humans there are 4 different SULT families (SULT1, SULT2, SULT4, and SULT6) and 14 different subtypes of human cytosolic SULTs (119). Additionally, SULT subfamilies can have genetic variants (SNPs and CVNs) that lead to different allozymes with different sulfation capacities. A well-known example is SULT1A1, whose genetic variants (caused mainly by SNPs) lead to 3 different alleles (*1, *2, and *3), which differ in frequency across different ethnicities, and affect SULT activity, the metabolism of phytochemicals, and the efficacy of drugs (130). The differential catalytic activity of the SULT1A1 allozymes has been studied in vitro with dietary flavonoids [e.g., chrysin (CAS 480–40–0), genistein (CAS 446–72–0), quercetin (CAS 117–39–5)] and the catalytic activity depends on the allozyme (Vmax *1 > *2 > *3) (131). Additional experiments with the dietary phenols apigenin (CAS 520–36–5), epicatechin (CAS 13,392–26–2), and resveratrol confirmed that allele-specific differences in SULT are common (132). Nevertheless, despite our current knowledge of SULT genetic variations and their potential impact on health, SULT polymorphisms are not commonly studied in nutritional interventions (133).
Besides genetic variation, SULT activity can be inhibited by the exposure of certain drugs, dietary phenols, and food additives (117, 119). Indeed, according to in vitro studies, many common dietary phenols are potent SULT inhibitors: quercetin, green tea catechins, and the food additives vanillin (CAS 121–33–5) and tartrazine (CAS 12,225–21–7) (117). Additional in vitro studies with SULT recombinant proteins have shown that grapefruit juice, orange juice, and teas (green, black, oolong) and their phenolic compounds (quercetin and epigallocatechin gallate) are inhibitors of SULT1A1 and/or SULT1A3. In light of these observations, the authors hypothesized that SULT inhibition could increase β2-agonist bioavailability, as they are known substrates of SULT1A3 (133). Orange juice and red wine contain phytochemicals known to inhibit SULT. This inhibition has been hypothesized to interfere with normal catecholamine deactivation, leading to deleterious health effects caused by high catecholamine concentrations (134, 135).
Taken together, current evidence from in vitro and preclinical studies supports the fact that dietary phenols can modify the biological activity of both endogenous and exogenous compounds by competing as substrates and/or inhibiting SULT (119). Interestingly, the recent FDA guidelines (January 2020) for industry to study clinical drug interaction have included the recommendation to conduct in vitro studies to find out if investigational drugs are metabolized by SULTs (136). Unfortunately, there are a lack of clinical trials evaluating the biological and pharmacological consequences of the inhibition of SULT induced by dietary phytochemicals, and further research is required to better understand the health consequences derived from diet-induced SULT inhibition.
Glucuronidation
UGTs are microsomal enzymes primarily expressed in the liver, but also present in the intestine, kidneys, heart, thymus, spleen, olfactory epithelium, brain, adrenal glands, and lungs (137). UGTs catalyze the transfer of a uronic acid (i.e., glucuronic acid) from uridine diphosphoglucuronic acid to exogenous (e.g., carcinogens, dietary compounds, and drugs) and endogenous compounds (e.g., bilirubin or hormones) (137). This reaction, known as glucuronidation, takes place in the endoplasmic reticulum (ER) and is one of the most quantitatively important Phase II reactions. As glucuronidation results in an increased solubility, it is generally considered a detoxification pathway and a major barrier that limits oral bioavailability (138, 139). Nevertheless, it is noteworthy that there are examples of compounds retaining activity after glucuronidation (e.g., daidzein or genistein) and even examples of bioactivation where the glucuronide is more potent than the unconjugated compound (e.g., morphine, codeine, or ezetimibe) (139).
The glucuronidation capacity varies significantly (from 3-fold to >100-fold, depending on the compound) between humans, and this can be explained by genetic and environmental factors (138). In a similar way to SULTs, UGTs are also genetically variable, changing regulation and expression. UGTs are divided into 4 subfamilies based on their amino acid sequence identity: UGT1, UGT2, UGT3, and UGT8. Many genetic polymorphic variants have been reported for UGT1A and UGT2B genes. As an example, the relevance of UGT1A6 and UGT1A1 genetic polymorphisms on cis- and trans-resveratrol glucuronidation was studied in 51 genotyped human liver microsomes. Interestingly, ≤5-fold variability was observed in trans-resveratrol (CAS 501–36–0) glucuronidation and cis-resveratrol (CAS 61,434–67–1) glucuronidation depending on UGT1A6 genotype (140). Also, as occurs with SULTs, epidemiological studies have found associations among UGT polymorphisms and the exposure of cancer-related dietary chemicals, but these relations are not fully understood (140). However, a few studies have shown the clinical significance of these polymorphisms (139). Although feasible, major challenges for this are: 1) that most members of the UGT family are genetically polymorphic, 2) that the relation between genotype and phenotype is still an area of research, and 3) that UGTs have distinct but sometimes overlapping substrate specificity towards dietary phytochemicals.
Besides genetic variation, several in vitro studies have shown that UGTs can be inhibited by dietary compounds. For instance, UGT2B17 (the key enzyme involved in testosterone glucuronidation) is modulated by green tea, white tea (141, 142), and red wine constituents (143). UGTs can also be inhibited by dietary anthocyanins like cyanidin (CAS 13,306–05–3) or delphinidin (CAS 8012–95–1) (144). Two questions remain to be answered in the field of glucuronidation of dietary antioxidants. Firstly, to what extent do glucuronides retain the biological activity of the parent compound in vivo? Secondly, what is the in vivo relevance of β-glucuronidase (the enzyme that carries out deglucuronidation)? This is further discussed below.
Methylation
Methyltransferases have received special attention due to their role in reducing disease risk (112). COMT is one of the most relevant methyltransferases expressed in almost all mammalian tissues. In humans, the highest COMT expression levels are found in the liver, followed by the kidneys, stomach, and intestine (145). This enzyme catalyzes O-methylation by transferring a methyl-group from S-adenosyl-Lmethionine (SAM) to one of the hydroxyl moieties of a catechol-containing substrate (146). We now know that it catalyzes the O-methylation of a great variety of endogenous (e.g., catecholamines, estrogens, melanin intermediates) and exogenous compounds (e.g., drugs and phytochemicals) (146). In a similar way to SULTs and UGTs, COMT is considered a detoxifying enzyme that reduces the formation of potentially mutagenic metabolites, protecting DNA from oxidative stress (146). COMT plays an important role in deactivating biologically active catechols and a decreased COMT activity has been related to the risk of CVDs, neurodegenerative diseases, and estrogen-induced hormonal cancers (145).
There are important interindividual differences in COMT activity. Sex is known to play a role, as males exhibit higher COMT expression in the liver compared with females (146–148). Genetics also contributes to these differences. Several polymorphisms have been reported in the COMT gene but the most studied and clinically relevant is the COMT rs4680 polymorphism, resulting in a Val158Met substitution (149). The substitution significantly reduces COMT activity, and determines the high-, intermediate-, and low-activity phenotypes (146). Since COMT deactivates catecholamines, the relevance of this polymorphism has been widely studied in brain-related disorders like mood disorders, schizophrenia, and substance dependence (149, 150).
Besides genetic variation, dietary flavonoids are both substrates and potent inhibitors of COMT (151). Indeed, COMT catalyzes the O-methylation of various phytochemicals such as tea flavanols [catechin (CAS 7295–85–4), epicatechin, epigallocatechin (CAS 970–74–1), epigallocatechin gallate (CAS 989–51–5)], flavonols [quercetin (CAS 117–39–5) and fisetin (CAS 528–48–3)], flavones [luteolin (CAS 491–70–3)], and phenolic compounds (hydroxytyrosol). Interestingly, flavonoids are good substrates for COMT and they have higher metabolic rates than most endogenous substrates (146).
Despite the general concept that COMT inactivates and decreases the biological activity of compounds, some in vitro and in vivo studies indicate that the corresponding O-methylated analogs maintain or even increase the biological activity of the parent compounds. For example, studies in human HepG2 cells found that the antiproliferative activity of the flavonols, fisetin and quercetin, was decreased in the presence of the COMT inhibitor entacapone, and that the O-methylated metabolites had higher antiproliferative effects (152). Also, a clinical trial on the beneficial effects of following the Mediterranean diet [PREDIMED (PREvención con DIeta MEDiterránea) study] investigated the relation between hydroxytyrosol and its O-methylated metabolite, homovanillyl alcohol, on CVDs and total mortality. Interestingly, urinary concentrations of homovanillyl alcohol were associated with a significantly lower total mortality and CVD risk (15). Taken together, COMT-catalyzed O-methylation of dietary phenolic catechols results in the formation of active metabolites. Evaluating the in vivo bioactivity of the methylated metabolites of phytochemicals and the clinical impact of COMT polymorphisms are 2 interesting research areas that can provide us with key aspects to better understand the health benefits of dietary phenols.
Deconjugating enzymes influence Phase-II metabolite activity
Deconjugating enzymes (e.g., sulfatases and glucuronidases) convert conjugated metabolites into unconjugated forms. They have, thus, a direct (although rarely explored in clinical trials) impact on the biological activity of phytochemicals and their metabolites. Deconjugation is mediated by glucuronidases (mainly by isoforms of the enzyme β-glucuronidase) and by sulfatase enzymes, steroid sulfatase (STS) and, above all, arylsulfatases (ARS). Sulfatases and β-glucuronidase are ubiquitously expressed, but the latter is usually found at higher concentrations in the small intestine, in the liver, and in tissues containing inflammatory cells such as lymphocytes, Kupffer cells (liver), and/or macrophages (153–155).
Once in the bloodstream, metabolites may first undergo extracellular deconjugation by enzymes in the endothelial cell wall and intracellularly in RBCs, which may play a pivotal role in the distribution and bioaccessibility of circulating phenols and their metabolites (156–158). Deconjugation by β-glucuronidase can then occur intracellularly in several tissues (e.g., liver and small intestine) because of the location of this enzyme in the lysosomes and in the microsomal fraction (155, 159, 160). It can also occur extracellularly, since β-glucuronidase was also found in the wall of intestinal cells (161). Furthermore, it may also take place via extracellular β-glucuronidase released by neutrophils and macrophages stressed with proinflammatory agents, and by bone marrow (161, 162). Although ARS-A and B are water-soluble enzymes, STS has a hydrophobic domain and is an integral membrane protein of the ER (163).
One of the best examples of the relevance of Phase II metabolites and the role of deconjugating enzymes in the biological effects elicited by phytochemicals is known as the “flavonoid paradox.” Quercetin, one of the most widely studied flavonoids, is extensively metabolized into methylated, glucuronidated, and sulfated conjugates. Glucuronides are then slowly hydrolyzed at the vascular level, yielding the parent aglycone which accumulates in tissues. Thus, quercetin conjugation is reversible and, at least regarding its vasodilator and antihypertensive effects, the conjugation-deconjugation cycle appears to be an absolute requirement. As such, glucuronides deliver free quercetin and its methylated form to tissues (164, 165, 124).
Several studies have shown that the activation of deconjugating enzymes is related to cell homeostasis, pathological conditions, and to the different tissues exposed to metabolites. A pioneering work of Shimoi et al. showed that luteolin monoglucuronide is converted to its free aglycone during inflammation using human neutrophils stimulated with ionomycin/cytochalasin B and rats treated with LPS (166). In addition, a higher β-glucuronidase activity was also found in the livers of hepatocarcinogenic rats with respect to the livers of healthy rats, corroborating the hypothesis that β-glucuronidases as well as sulfatases are specifically activated in inflammatory tissues as well as in a low pH environment, required for enzyme activation, and typical of developing cancers (153, 163, 167).
Concerning deconjugating enzymes, Gratz et al. (168) found a substantial interindividual variability of β-glucuronidase in plasma and verified the association of β-glucuronidase activity with age, sex, and BMI. Differences in the expression of different isoforms of sulfatase enzymes such as aryl-sulfatases and STSs were also observed comparing breast tumoral cells with normal cells (169). In tumor samples, an increased ARS-A expression was found compared with control tissue, whereas no differences were observed regarding the expression levels of ARS-B. Furthermore, STS mRNA expression was significantly higher in control cells compared with tumor tissue samples.
Moreover, Gimenez Bastida et al. showed that resveratrol derivatives do not undergo deconjugation in breast cancer cells, but they enter the cells via ATP-binding cassette (ABC) transporters and are biologically active on their own (124). In the literature, however, it is generally assumed that deconjugation of phenolic metabolites may promote their entry into cells and may be required before they can exert biological effects. Moreover, although deconjugated before entering the cells, metabolites can be reconjugated inside the cell, acting together with their free forms (17, 123).
Taken together, these findings suggest that polymorphisms of deconjugating enzymes likely promote interindividual differences in polyphenol metabolite efficacy in vivo and are therefore of relevance in the context of metabolite availability for target tissues.
Transporter proteins influence metabolite bioavailability and biological activity
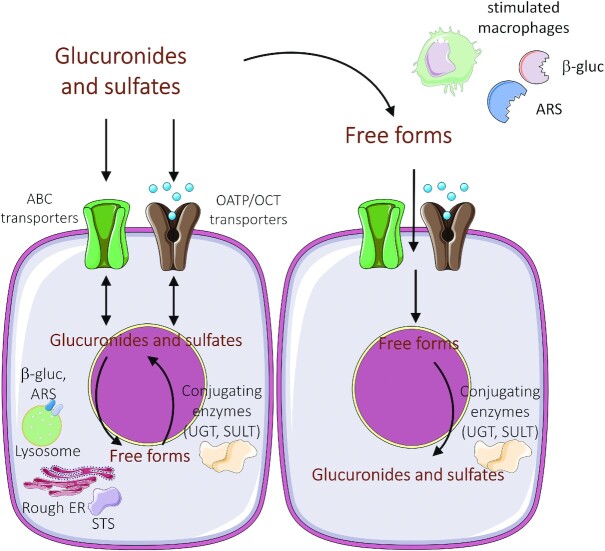
As stated earlier, a major issue which is currently debated is how and to what extent the metabolites of dietary phenolic compounds are bioaccessible to tissues and directly or indirectly involved in the biological effects attributed to the ingested free forms in vivo. A crucial step is that Phase I/II metabolites and their free forms must leave enterocytes or hepatocytes to be distributed to target tissues. Once the metabolites are formed, their bioaccessibility is primarily related to the transporters that regulate their concentration inside and outside of the target cells (170) (see Figure 2).
FIGURE 2.
The intra- and extracellular concentration of glucuronide and sulfate metabolites is regulated by transport proteins and by deconjugating enzymes. Expression, distribution, and activation of metabolizing enzymes and transporters are influenced by pathophysiological status (e.g., activation of macrophages) and by protein polymorphisms. ABC, ATP-binding cassette; ARS, arylsulfatases; β-gluc, β-glucuronidase; ER, endoplasmic reticulum; OATP, organic-anion-transporting polypeptides; OCT, organic cation transporter; SULT, sulfotransferase; STS, steroid sulfatase; UGT, UDP-glucuronosyltransferase.
The efflux of flavonoids and their metabolites is usually regulated by ABC transporters, which are ubiquitous integral membrane proteins that transport various ligands across the membrane (171). Members of ABC transporters such as P-glycoprotein 1 (PGP), multidrug resistance protein 2 (MRP2), and breast cancer resistance protein (BCRP) are widely expressed in all tissues and are known to be involved in the efflux of simple phenols as well as their glucuronides and sulfate conjugates. These Phase II metabolites are, in fact, too hydrophilic to diffuse across the cell membrane compared with their parent compounds, and can only leave cells via active transportation (172, 173).
Some polyphenols, like quercetin, competitively inhibit PGP, MRP1, and BCRP, affecting their activity (174), but it is unknown whether conjugated metabolites can also inhibit these transporters. In any case, the efflux of sulfate and glucuronide metabolites seems to be conjugate specific. Indeed, it has been shown that sulfate conjugates preferentially efflux back to the apical side of Caco-2/TC7 cell monolayers, whereas glucuronides were mostly transported to the basolateral side (175). In particular, it has been seen that the efflux of resveratrol sulfate is regulated by MRP2, whereas MRP3 (which is located in the basolateral membrane of enterocytes) and MRP4 have been found to selectively transport resveratrol glucuronide (164, 176). There is some evidence that sulfate and glucuronide metabolites, applied to apical and basolateral sides of the cells, are not able to permeate in either direction, suggesting conjugates cannot recross the cell monolayer once released to the exterior (175). All these findings may imply that, in the gut (especially the small intestine), sulfate conjugates are more likely to remain in the gut lumen relative to glucuronides, where they may act locally, be excreted in the feces, or be further metabolized by the gut microbiota.
Polymorphisms in ABC transporters have been identified. Genetic variations in its protein-coding gene, ABCB1, and their interethnic frequency differences, are expected to have an impact on the exposure of polyphenols in human populations (177). For instance, polymorphisms associated with reduced expression and function of MRP3 and MRP4 transporters are thought to induce intracellular accumulation of metabolites and decrease ATP-dependent bile acid sulfates/glucuronides export, whereas a reduced expression of PGP may lead to higher concentrations of metabolites in the blood circulation (166).
In humans, there are also other classes of influx/efflux transporters that modulate the cellular uptake of phytochemicals and drugs by moving substrates against a gradient. These uptake transporters include organic anion transporting proteins (OATPs), organic cation transporters (OCTs), concentrative nucleoside transporters (CNT), dipeptide transporters (PEPT), and monocarboxylate transporters (MCT) (178). It is not fully understood whether these transporters can mediate the uptake of conjugate metabolites as well as free forms, the former being more polar and not able to cross the cell membrane via passive diffusion.
The evaluation of individual variations in tissue distribution and differences in function caused by the genetic variation of transporters involved in metabolite efflux is of relevance to further study the health benefits of polyphenols. For instance, the uptake of resveratrol and its sulfates into targeted tissues is closely related to the expression of specific OATP isoforms (165). Therefore, individuals with low or no detectable expression of OATP1B1, OATP1B3, and OATP2B1 may show decreased response rates or even no response to resveratrol interventions. One relevant in vitro study showed that resveratrol-3-O-4-β-O-disulfate was transported, with lowered affinity, by OATP1B1 and OATP1B3 isoforms, whereas resveratrol-3-O-sulfate (CAS 858,127–11–4) was exclusively transported by OATP1B3 (165). Interestingly, no uptake of resveratrol-3-O-glucuronide (CAS 387,372–17–0) or resveratrol-4′β-O-glucuronide (CAS 387,372–20–5) was observed.
Conclusions
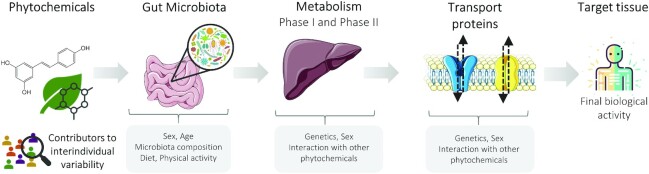
In conclusion, the present review highlights the relevance of the microbiota, Phase I/II reactions, transport proteins, and deconjugating enzymes on the final activity elicited by dietary phytochemicals, contributing in some cases, to their bioactivation (Figure 3). Therefore, their role must be further studied to correctly understand and predict the effects observed following their intake. Clinical research on the biological activities of dietary phytochemicals has shown high variability in results and highlighted important discrepancies between in vitro and preclinical studies. The microbiota, drug-metabolizing enzymes, and cell transporters are critical aspects that are prone to wide interindividual variability and could thus be underlying contributors to the heterogenicity of the results obtained in clinical studies. Age, genetics, sex, physiopathological conditions, and the interplay with other dietary phytochemicals are important sources of interindividual variability that must be considered when studying the health effects of these compounds.
FIGURE 3.
Schematic representation of the interplay between phytochemicals and microbiota, drug-metabolizing enzymes, and transport proteins before reaching the target tissue, all being potential sources of inter-individual variability.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—RD: conceived the idea of the manuscript; RD, AB, JRM, GS, and MD: wrote the manuscript sections; AB, JRM, GS, and MD: drafted the tables and figures; RFT, MF, and RD: revised the manuscript and provided critical review; and all authors: read and approved the final manuscript.
Notes
Supported by the Departament d'Economia i Coneixement de la Generalitat de Catalunya (Spain) 2017 SGR 138, Instituto Carlos III (ISCIII), CIBEROBN and FIS PI17/00223 , Sara Borrell contract (JRM, CD20/00001), Canada Research Chair in Pharmacogenomics, Centre for Addiction and Mental Health, and the CAMH Foundation. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author disclosures: RFT has consulted for Quinn Emanuel and Ethismos Research Inc on unrelated topics. All the other authors report no conflicts of interest.
AB, JR-M, and GS share first coauthorship.
Abbreviations used: ABC, ATP-binding cassette; ARS, arylsulfatases; β-gluc, β-glucuronidase; BCRP, breast cancer resistance protein; CBG, cytosolic β-glucosidase; COMT, catechol-O-methyltransferase; CVD, cardiovascular disease; CYP, cytochrome P450; ER, endoplasmic reticulum; EVOO, extra-virgin olive oil; LPH, lactase-phlorizin hydrolase; MRP, multidrug resistance protein; OATP, organic anion transporting protein; PGP, P-glycoprotein 1; SNP, single nucleotide polymorphism; STS, steroid sulfatase; SULT, sulfotransferase; UGT, uridine 5′-diphosphate-glucuronosyltransferase.
Contributor Information
Anna Boronat, Integrative Pharmacology and Systems Neurosciences Research Group, Hospital del Mar Medical Research Institute, Barcelona, Spain.
Jose Rodriguez-Morató, Integrative Pharmacology and Systems Neurosciences Research Group, Hospital del Mar Medical Research Institute, Barcelona, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Centre (CIBEROBN), Madrid, Spain; Department of Experimental and Health Sciences (UPF-CEXS), Universitat Pompeu Fabra, Barcelona, Spain.
Gabriele Serreli, Department of Biomedical Science, Pathology Section, Experimental Pathology Unit, University of Cagliari, Montserrato, Italy.
Montserrat Fitó, Physiopathology of Obesity and Nutrition Networking Biomedical Research Centre (CIBEROBN), Madrid, Spain; Cardiovascular Risk and Nutrition Research Group, Hospital del Mar Medical Research Institute, Barcelona, Spain.
Rachel F Tyndale, Campbell Family Mental Health Research Institute (CAMH), Toronto, Canada; Department of Pharmacology, Toxicology, and Psychiatry, University of Toronto, Toronto, Canada.
Monica Deiana, Department of Biomedical Science, Pathology Section, Experimental Pathology Unit, University of Cagliari, Montserrato, Italy.
Rafael de la Torre, Integrative Pharmacology and Systems Neurosciences Research Group, Hospital del Mar Medical Research Institute, Barcelona, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Centre (CIBEROBN), Madrid, Spain; Department of Experimental and Health Sciences (UPF-CEXS), Universitat Pompeu Fabra, Barcelona, Spain.
References
- 1. Aune D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: a review of the evidence. Adv Nutr. 2019;10:S404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajaram S, Jones J, Lee GJ. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv Nutr. 2019;10:422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forman HJ, Davies KJA, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiskirchen S, Weiskirchen R. Resveratrol: how much wine do you have to drink to stay healthy?. Adv Nutr. 2016;7:706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharp D. Château resveratrol. Lancet. 2003;361:1404. [DOI] [PubMed] [Google Scholar]
- 6. Ortuño J, Covas MI, Farre M, Pujadas M, Fito M, Khymenets O, Andres-Lacueva C, Roset P, Joglar J, Lamuela-Raventòs RMet al. Matrix effects on the bioavailability of resveratrol in humans. Food Chem. 2010;120:1123–30. [Google Scholar]
- 7. Pastor A, Rodríguez-Morató J, Olesti E, Pujadas M, Pérez-Mañá C, Khymenets O, Fitò M, Covas MI, Solà R, Motilva M-Jet al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J Chromatogr A. 2016;1437:183–90. [DOI] [PubMed] [Google Scholar]
- 8. de Bock M, Thorstensen EB, Derraik JGB, Henderson HV, Hofman PL, Cutfield WS. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol Nutr Food Res. 2013;57:2079–85. [DOI] [PubMed] [Google Scholar]
- 9. Bast A, Haenen GRMM. Ten misconceptions about antioxidants. Trends Pharmacol Sci. 2013;34:430–6. [DOI] [PubMed] [Google Scholar]
- 10. Ginsburg I, Kohen R, Shalish M, Varon D, Shai E, Koren E. The oxidant-scavenging abilities in the oral cavity may be regulated by a collaboration among antioxidants in saliva, microorganisms, blood cells and polyphenols: a chemiluminescence-based study. PLoS One. 2013;8:e63062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rocchetti G, Senizza B, Giuberti G, Montesano D, Trevisan M, Lucini L. Metabolomic study to evaluate the transformations of extra-virgin olive oil's antioxidant phytochemicals during in vitro gastrointestinal digestion. Antioxidants. 2020;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dey P. Gut microbiota in phytopharmacology: a comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. 2019;147:104367. [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez-Morató J, Robledo P, Tanner J-A, Boronat A, Pérez-Mañá C, Oliver Chen C-Y, Tyndale RF, De la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017;217:716–25. [DOI] [PubMed] [Google Scholar]
- 14. Boronat A, Mateus J, Soldevila-Domenech N, Guerra M, Rodríguez-Morató J, Varon C, Muñoz D, Barbosa F, Morales JC, Gaedigk Aet al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic Biol Med. 2019;143:471–81. [DOI] [PubMed] [Google Scholar]
- 15. De La Torre R, Corella D, Castañer O, Martínez-González MA, Salas-Salvador J, Vila J, Estruch R, Sorli JV, Aròs F, Fiol Met al. Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: virgin olive oil, wine, and catechol-methylation. Am J Clin Nutr. 2017;105:1297–304. [DOI] [PubMed] [Google Scholar]
- 16. Serreli G, Deiana M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019;10:6999–7021. [DOI] [PubMed] [Google Scholar]
- 17. Serreli G, Deiana M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants. 2018;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Ligget CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada Tet al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans JM, Morris LS, Marchesi JR. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–47. [DOI] [PubMed] [Google Scholar]
- 22. Talavéra S, Felgines C, Texier O, Besson C, Lamaison J-L, Rémésy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr. 2003;133:4178–82. [DOI] [PubMed] [Google Scholar]
- 23. Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130:2765–71. [DOI] [PubMed] [Google Scholar]
- 25. Arts ICW, Sesink ALA, Faassen-Peters M, Hollman PCH. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br J Nutr. 2004;91:841–7. [DOI] [PubMed] [Google Scholar]
- 26. Vissers MN, Zock PL, Katan MB. Bioavailability and antioxidant effects of olive oil phenols in humans: a review. Eur J Clin Nutr. 2004;58:955–65. [DOI] [PubMed] [Google Scholar]
- 27. Donovan JL, Crespy V, Manach C, Morand C, Besson C, Scalbert A, Rémésy C. Catechin is metabolized by both the small intestine and liver of rats. J Nutr. 2001;131:1753–7. [DOI] [PubMed] [Google Scholar]
- 28. Aura A-M, O'Leary KA, Williamson G, Ojala M, Bailey M, Puupponen-Pimiä R, Nuutila AM, Oksman-Caldentey K-M, Poutanen K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J Agric Food Chem. 2002;50:1725–30. [DOI] [PubMed] [Google Scholar]
- 29. Vollmer M, Esders S, Farquharson FM, Neugart S, Duncan SH, Schreiner M, Louis P, Maul R, Rohn S. Mutual interaction of phenolic compounds and microbiota: metabolism of complex phenolic apigenin-C- and kaempferol-O-derivatives by human fecal samples. J Agric Food Chem. 2018;66:485–97. [DOI] [PubMed] [Google Scholar]
- 30. Moszak M, Szulińska M, Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders — a review. Nutrients. 2020;12:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cortés-Martín A, Selma MV, Tomás-Barberán FA, González-Sarrías A, Espín JC. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol Nutr Food Res. 2020;64:e1900952. [DOI] [PubMed] [Google Scholar]
- 32. Bang SH, Hyun YJ, Shim J, Hong SW, Kim DH. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from Bifidobacterium dentium. J Microbiol Biotechnol. 2015;25:18–25. [DOI] [PubMed] [Google Scholar]
- 33. Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–501. [DOI] [PubMed] [Google Scholar]
- 34. Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7:216–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stevens JF, Maier CS. The chemistry of gut microbial metabolism of polyphenols. Phytochem Rev. 2016;15:425–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowater L, Fairhurst SA, Just VJ, Bornemann S. Bacillus subtilis YxaG is a novel Fe-containing quercetin 2,3-dioxygenase. FEBS Lett. 2004;557:45–8. [DOI] [PubMed] [Google Scholar]
- 37. Fetzner S. Ring-cleaving dioxygenases with a cupin fold. Appl Environ Microbiol. 2012;78:2505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirooka K, Fujita Y. Excess production of Bacillus subtilis quercetin 2,3-dioxygenase affects cell viability in the presence of quercetin. Biosci Biotechnol Biochem. 2010;74:1030–8. [DOI] [PubMed] [Google Scholar]
- 39. Schaab MR, Barney BM, Francisco WA. Kinetic and spectroscopic studies on the quercetin 2,3-dioxygenase from Bacillus subtilis. Biochemistry. 2006;45:1009–16. [DOI] [PubMed] [Google Scholar]
- 40. Appeldoorn MM, Vincken JP, Aura AM, Hollman PCH, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–92. [DOI] [PubMed] [Google Scholar]
- 41. Marín L, Miguélez EM, Villar CJ, Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int. 2015;2015:905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodríguez-Morató J, Xicota L, Fitó M, Farré M, Dierssen M, De La Torre R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules. 2015;20:4655–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomás-Barberán FA, González-Sarrías A, García-Villalba R, Núñez-Sánchez MA, Selma MV, García-Conesa MT, Espin JC. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res. 2017;61:e00901. [DOI] [PubMed] [Google Scholar]
- 44. Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–85. [DOI] [PubMed] [Google Scholar]
- 45. Bess EN, Bisanz JE, Yarza F, Bustion A, Rich BE, Li X, Kitamura S, Waligurski E, Ang QY, Albaet DLet al. Genetic basis for the cooperative bioactivation of plant lignans by Eggerthella lenta and other human gut bacteria. Nat Microbiol. 2020;5:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, Dhooge W, De Keukeleire D, Rabot S, Verstraete W, Van de Wiele T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr. 2006;136:1862–7. [DOI] [PubMed] [Google Scholar]
- 47. Paraiso IL, Plagmann LS, Yang L, Zielke R, Gombart AF, Maier CS, Sikora AE, Blakemore PR, Stevens JF. Reductive metabolism of xanthohumol and 8-prenylnaringenin by the intestinal bacterium Eubacterium ramulus. Mol Nutr Food Res. 2019;63:1800923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vallejo F, Larrosa M, Escudero E, Zafrilla MP, Cerdá B, Boza J, García-Conesa MT, Espín JC, Tomás-Barberán FA. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J Agric Food Chem. 2010;58:6516–24. [DOI] [PubMed] [Google Scholar]
- 49. Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart Det al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heinonen SM, Hoikkala A, Wähälä K, Adlercreutz H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol. 2003;87:285–99. [DOI] [PubMed] [Google Scholar]
- 51. Lee PG, Lee UJ, Song H, Choi KY, Kim BG. Recent advances in the microbial hydroxylation and reduction of soy isoflavones. FEMS Microbiol Lett. 2018;365:fny195. [DOI] [PubMed] [Google Scholar]
- 52. García-Villalba R, Selma M V, Espín JC, Tomás-Barberán FA. Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J Agric Food Chem. 2019;67:11099–107. [DOI] [PubMed] [Google Scholar]
- 53. Quartieri A, García-Villalba R, Amaretti A, Raimondi S, Leonardi A, Rossi M, Tomàs-Barberàn F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol Nutr Food Res. 2016;60:1590–601. [DOI] [PubMed] [Google Scholar]
- 54. Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wiciński M, Gębalski J, Mazurek E, Podhorecka M, Śniegocki M, Szychta P, Szychta P, Sawicka E, Malinowski B. The influence of polyphenol compounds on human gastrointestinal tract microbiota. Nutrients. 2020;12:20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wan MLY, Co VA, El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit Rev Food Sci Nutr. 2021;61(4):690–711. [DOI] [PubMed] [Google Scholar]
- 58. Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015;2015:850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. [DOI] [PubMed] [Google Scholar]
- 60. Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salles N. Basic mechanisms of the aging gastrointestinal tract. Dig Dis. 2007;25:112–17. [DOI] [PubMed] [Google Scholar]
- 62. Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19:26–30. [DOI] [PubMed] [Google Scholar]
- 63. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–40. [DOI] [PubMed] [Google Scholar]
- 65. Edogawa S, Peters SA, Jenkins GD, Gurunathan SV, Sundt WJ, Johnson S, Lennon RJ, Dyer RB, Camilleri M, Kashyap PCet al. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J. 2018;32:6615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Landa BB, Navas-Cortés JA, Tena-Sempere Met al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. [DOI] [PubMed] [Google Scholar]
- 68. Pollet RM, D'Agostino EH, Walton WG, Xu Y, Little MS, Biernat KA, Pellock SJ, Patterson LM, Creekmore BC, Isenberg HNet al. An atlas of β-glucuronidases in the human intestinal microbiome. Structure. 2017;25:967–977.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Tap J, Bruls T, Batto J-M, Bertalan Met al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, Hattori M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nakayama J, Watanabe K, Jiang J, Matsuda K, Chao S-H, Haryono P, La-ongkham O, Sarwoko M-A, Sujaya I.N, Zhao L.et al. Diversity in gut bacterial community of school-age children in Asia. Sci Rep. 2015;5:8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the Daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11:2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma MV, Espín JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem. 2014;62:6535–8. [DOI] [PubMed] [Google Scholar]
- 74. Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora — implications for health. Mol Nutr Food Res. 2007;51:765–81. [DOI] [PubMed] [Google Scholar]
- 75. Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NPet al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–19. [DOI] [PubMed] [Google Scholar]
- 76. Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TDet al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 77. Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev. 2011;69:432–48. [DOI] [PubMed] [Google Scholar]
- 78. Selma MV, Beltrán D, García-Villalba R, Espín JC, Tomás-Barberán FA. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–84. [DOI] [PubMed] [Google Scholar]
- 79. Alfei S, Turrini F, Catena S, Zunin P, Grilli M, Pittaluga AM, Boggia R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur J Med Chem. 2019;183:111724. [DOI] [PubMed] [Google Scholar]
- 80. Kang I, Kim Y, Tomás-Barberán FA, Espín JC, Chung S. Urolithin A, C, and D, but not iso-urolithin A and urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res. 2016;60:1129–38. [DOI] [PubMed] [Google Scholar]
- 81. Hålldin E, Eriksen AK, Brunius C, da Silva AB, Bronze M, Hanhineva K, Aura A-M, Landberg R. Factors explaining interpersonal variation in plasma enterolactone concentrations in humans. Mol Nutr Food Res. 2019;63:e1801159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clavel T, Henderson G, Engst W, Doré J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol Ecol. 2006;55:471–8. [DOI] [PubMed] [Google Scholar]
- 83. Mali AV, Padhye SB, Anant S, Hegde MV, Kadam SS. Anticancer and antimetastatic potential of enterolactone: clinical, preclinical and mechanistic perspectives. Eur J Pharmacol. 2019;852:107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shimada T, Tanaka K, Takenaka S, Murayama N, Martin MV, Foroozesh MK, Yamazaki H, Guengerich FP, Komori M. Structure−function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem Res Toxicol. 2010;23:1921–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Korobkova EA. Effect of natural polyphenols on CYP metabolism: implications for diseases. Chem Res Toxicol. 2015;28:1359–90. [DOI] [PubMed] [Google Scholar]
- 86. Piver B, Fer M, Vitrac X, Merillon J-M, Dreano Y, Berthou F, Lucas D. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol. 2004;68:773–82. [DOI] [PubMed] [Google Scholar]
- 87. Breinholt V, Offord E, Brouwer C, Nielsen S, Brøsen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem Toxicol. 2002;40:609–16. [DOI] [PubMed] [Google Scholar]
- 88. Wilsher NE, Arroo RR, Matsoukas M, Tsatsakis AM, Spandidos DA, Androutsopoulos VP. Cytochrome P450 CYP1 metabolism of hydroxylated flavones and flavonols: selective bioactivation of luteolin in breast cancer cells. Food Chem Toxicol. 2017;110:383–94. [DOI] [PubMed] [Google Scholar]
- 89. Breinholt VM, Rasmussen SE, Brosen K, Friedberg TH. In vitro metabolism of genistein and tangeretin by human and murine cytochrome P450s. Pharmacol Toxicol. 2003;93:14–22. [DOI] [PubMed] [Google Scholar]
- 90. Roberts-Kirchhoff ES, Crowley JR, Hollenberg PF, Kim H. Metabolism of genistein by rat and human cytochrome P450s. Chem Res Toxicol. 1999;12:610–6. [DOI] [PubMed] [Google Scholar]
- 91. Uno T, Obe Y, Ogura C, Goto T, Yamamoto K, Nakamura M, Kanamaru K, Yamagata H, Imaishi H. Metabolism of 7-ethoxycoumarin, safrole, flavanone and hydroxyflavanone by cytochrome P450 2A6 variants. Biopharm Drug Dispos. 2013;34:87–97. [DOI] [PubMed] [Google Scholar]
- 92. Sousa MC, Braga RC, Cintra BAS, de Oliveira V, Andrade CH. In silico metabolism studies of dietary flavonoids by CYP1A2 and CYP2C9. Food Res Int. 2013;50:102–10. [Google Scholar]
- 93. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. [DOI] [PubMed] [Google Scholar]
- 94. Tornio A, Backman JT. Cytochrome P450 in pharmacogenetics: An update. Adv Pharmacol. 2018;83:3–32. [DOI] [PubMed] [Google Scholar]
- 95. Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. 2018;70:384–411. [DOI] [PubMed] [Google Scholar]
- 96. Reilly CA, Ehlhardt WJ, Jackson DA, Kulanthaivel P, Mutlib AE, Espina RJ, Moody DE, Crouch DJ, Yost GS. Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem Res Toxicol. 2003;16:336–49. [DOI] [PubMed] [Google Scholar]
- 97. Nikolic D, van Breemen RB. New metabolic pathways for flavanones catalyzed by rat liver microsomes. Drug Metab Dispos. 2004;32:387–97. [DOI] [PubMed] [Google Scholar]
- 98. Kagawa H, Takahashi T, Ohta S, Harigaya Y. Oxidation and rearrangements of flavanones by mammalian cytochrome P450. Xenobiotica. 2004;34:797–810. [DOI] [PubMed] [Google Scholar]
- 99. Ireson CR, Jones DJL, Orr S, Coughtrie MWH, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11(1):105–11. [PubMed] [Google Scholar]
- 100. Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TEet al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Denden S, Bouden B, Haj Khelil A, Ben Chibani J, Hamdaoui MH. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: evidence from a meta-analysis. Genet Mol Res. 2016;15:7487. [DOI] [PubMed] [Google Scholar]
- 102. Yoshihara T, Zaitsu M, Shiraishi F, Arima H, Takahashi-Yanaga F, Arioka M, Kajioka S, Sasaguri T. Influence of genetic polymorphisms and habitual caffeine intake on the changes in blood pressure, pulse rate, and calculation speed after caffeine intake: a prospective, double blind, randomized trial in healthy volunteers. J Pharmacol Sci. 2019;139:209–14. [DOI] [PubMed] [Google Scholar]
- 103. Soldevila-Domenech N, Boronat A, Mateus J, Diaz-Pellicer P, Matilla I, Pérez-Otero M, Aldea-Perona A, De la Torre R. Generation of the antioxidant hydroxytyrosol from tyrosol present in beer and red wine in a randomized clinical trial. Nutrients. 2019;11:2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McCann SE, Wactawski-Wende J, Kufel K, Olson J, Ovando B, Kadlubar SN, Davis W, Carter L, Muti P, Shields PGet al. Changes in 2-hydroxyestrone and 16 -hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev. 2007;16:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chang H, Yao S, Tritchler D, Hullar MA, Lampe JW, Thompson LU, McCann SE. Genetic variation in steroid and xenobiotic metabolizing pathways and enterolactone excretion before and after flaxseed intervention in African American and European American women. Cancer Epidemiol Biomarkers Prev. 2019;28:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. [DOI] [PubMed] [Google Scholar]
- 107. Spoletini I, Vitale C, Malorni W, Rosano GMC. Sex differences in drug effects: interaction with sex hormones in adult life. Handb Exp Pharmacol. 2013:91–105. [DOI] [PubMed] [Google Scholar]
- 108. Anderson GD. Chapter 1 gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. [DOI] [PubMed] [Google Scholar]
- 109. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tanner JA, Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J Pers Med. 2017;7:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tian D-D, Natesan S, White JR, Paine MF. Effects of common CYP1A2 genotypes and other key factors on intraindividual variation in the caffeine metabolic ratio: an exploratory analysis. Clin Transl Sci. 2019;12:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hodges RE, Minich DM. Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab. 2015;2015:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wong CC, Meinl W, Glatt HR, Barron D, Stalmach A, Steiling H, Crozier A, Williamson G. In vitro and in vivo conjugation of dietary hydroxycinnamic acids by UDP-glucuronosyltransferases and sulfotransferases in humans. J Nutr Biochem. 2010;21:1060–8. [DOI] [PubMed] [Google Scholar]
- 114. Tang L, Zhou J, Yang CH, Xia BJ, Hu M, Liu ZQ. Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences. J Agric Food Chem. 2012;60:3223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kotronoulas A, Pizarro N, Serra A, Robledo P, Joglar J, Rubió L, Hernaéz A, Tormos C, Motilva M-J, Fitó Met al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol Res. 2013;77:47–56. [DOI] [PubMed] [Google Scholar]
- 116. Boronat A, Martínez-Huélamo M, Cobos A, de la Torre R. Wine and olive oil phenolic compounds interaction in humans. Diseases. 2018;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bamforth KJ, Jones AL, Roberts RC, Coughtrie MWH. Common food additives are potent inhibitors of human liver 17α-ethinyloestradiol and dopamine sulphotransferases. Biochem Pharmacol. 1993;46:1713–20. [DOI] [PubMed] [Google Scholar]
- 118. Ottaviani JI, Borges G, Momma TY, Spencer JPE, Keen CL, Crozier A, Schroeter H. The metabolome of 2–14C.(-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep. 2016;6:29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Marto N, Morello J, Monteiro EC, Pereira SA. Implications of sulfotransferase activity in interindividual variability in drug response: clinical perspective on current knowledge. Drug Metab Rev. 2017;49:357–71. [DOI] [PubMed] [Google Scholar]
- 120. Klaassen CD, Boles JW. The importance of 3'-phosphoadenosine 5'-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997;11:404–18. [DOI] [PubMed] [Google Scholar]
- 121. Jones AL, Hagen M, Coughtrie MWH, Roberts RC, Glatt H. Human platelet phenolsulfotransferases: CDNA cloning, stable expression in V79 cells and identification of a novel allelic variant of the phenol-sulfating form. Biochem Biophys Res Commun. 1995;208:855–62. [DOI] [PubMed] [Google Scholar]
- 122. Serreli G, Melis MP, Corona G, Deiana M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: insight into the mechanism of action. Food Chem Toxicol. 2019;125:520–7. [DOI] [PubMed] [Google Scholar]
- 123. Atzeri A, Lucas R, Incani A, Peñalver P, Zafra-Gómez A, Melis MP, Pizzala R, Morales JC, Deiana M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016;7:337–46. [DOI] [PubMed] [Google Scholar]
- 124. Giménez-Bastida JA, Ávila-Gálvez MÁ, Espín JC, González-Sarrías A. Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol Nutr Food Res. 2019;63:e1900629. [DOI] [PubMed] [Google Scholar]
- 125. Terao J. Dietary flavonoids as antioxidants in vivo: conjugated metabolites of (-)-epicatechin and quercetin participate in antioxidative defense in blood plasma. J Med Investig. 1999;46:159–68. [PubMed] [Google Scholar]
- 126. Koga T, Meydani M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr. 2001;73:941–8. [DOI] [PubMed] [Google Scholar]
- 127. Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, Needs PW, Kroon PA, Hughes DA. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–6. [DOI] [PubMed] [Google Scholar]
- 128. Suri S, Liu X, Rayment S, Hughes D, Kroon P, Needs P, Taylor MA, Tribolo S, Wilson VG. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br J Pharmacol. 2010;159:566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Montalesi E, Cipolletti M, Cracco P, Fiocchetti M, Marino M. Divergent effects of daidzein and its metabolites on estrogen-induced survival of breast cancer cells. Cancers (Basel). 2020;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Daniels J, Kadlubar S. Pharmacogenetics of SULT1A1. Pharmacogenomics. 2014;15:1823–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. 2006;69:2084–92. [DOI] [PubMed] [Google Scholar]
- 132. Ung D, Nagar S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab Dispos. 2007;35:740–6. [DOI] [PubMed] [Google Scholar]
- 133. Nishimuta H, Ohtani H, Tsujimoto M, Ogura K, Hiratsuka A, Sawada Y. Inhibitory effects of various beverages on human recombinant sulfotransferase isoforms SULT1A1 and SULT1A3. Biopharm Drug Dispos. 2007;28:491–500. [DOI] [PubMed] [Google Scholar]
- 134. Eagle K. Hypothesis Holiday sudden cardiac death: food and alcohol inhibition of SULT1A enzymes as a precipitant. J Appl Toxicol. 2012;32:751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Eagle K. Toxicological effects of red wine, orange juice, and other dietary SULT1A inhibitors via excess catecholamines. Food Chem Toxicol. 2012;50:2243–9. [DOI] [PubMed] [Google Scholar]
- 136. US Food and Drug Administration . In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme and Transporter Mediated Drug Interactions. Silver Spring, MD: US Food and Drug Administration; 2020. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. [Google Scholar]
- 137. Stingl JC, Bartels H, Viviani R, Lehmann ML, Brockmöller J. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. Pharmacol Ther. 2014;141:92–116. [DOI] [PubMed] [Google Scholar]
- 138. Mehboob H, Iqbal T, Jamil A, Khaliq T. Genetic polymorphism of UDP-glucuronosyltransferase (UGT2B15) and glucuronidation of paracetamol in healthy population. Pak J Pharm Sci. 2016;29:1037–41. [PubMed] [Google Scholar]
- 139. Wu B, Kulkarni K, Basu S, Zhang S, Hu M. First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. J Pharm Sci. 2011;100:3655–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Iwuchukwu OF, Ajetunmobi J, Ung D, Nagar S. Characterizing the effects of common UDP glucuronosyltransferase (UGT) 1A6 and UGT1A1 polymorphisms on cis- and trans-resveratrol glucuronidation. Drug Metab Dispos. 2009;37:1726–32. [DOI] [PubMed] [Google Scholar]
- 141. Jenkinson C, Petroczi A, Barker J, Naughton DP. Dietary green and white teas suppress UDP-glucuronosyltransferase UGT2B17 mediated testosterone glucuronidation. Steroids. 2012;77:691–5. [DOI] [PubMed] [Google Scholar]
- 142. Coll S, Matabosch X, Garrostas L, Monfort N, Perez-Maña C, Pizarro N, Mateus JA, Ezzel M, De la Torre R, Ventura R. The effect of tea consumption on the steroid profile. Drug Test Anal. 2018;10:1438–47. [DOI] [PubMed] [Google Scholar]
- 143. Jenkinson C, Petroczi A, Naughton DP. Red wine and component flavonoids inhibit UGT2B17 in vitro. Nutr J. 2012;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Szotáková B, Bártíková H, Hlaváčová J, Boušová I, Skálová L. Inhibitory effect of anthocyanidins on hepatic glutathione S-transferase, UDP-glucuronosyltransferase and carbonyl reductase activities in rat and human. Xenobiotica. 2013;43:679–85. [DOI] [PubMed] [Google Scholar]
- 145. Zhu B. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2005;3:321–49. [DOI] [PubMed] [Google Scholar]
- 146. Sak K. The Val158Met polymorphism in COMT gene and cancer risk: role of endogenous and exogenous catechols. Drug Metab Rev. 2017;49:56–83. [DOI] [PubMed] [Google Scholar]
- 147. Boudíková B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–9. [DOI] [PubMed] [Google Scholar]
- 148. Soeiro-De-Souza MG, Bio DS, David DP, Missio G, Lima B, Fernandes F, Machado-Vieira R, Moreno RA. Gender effects of the COMT Val158Met genotype on verbal fluency in healthy adults. Mol Med Rep. 2013;8:837–44. [DOI] [PubMed] [Google Scholar]
- 149. Kumar P, Rai V. Catechol-O-methyltransferase gene Val158Met polymorphism and obsessive compulsive disorder susceptibility: a meta-analysis. Metab Brain Dis. 2020;35:241–51. [DOI] [PubMed] [Google Scholar]
- 150. Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, Peeters F, Derom C, Vlietinck R, Mengelers Ret al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33:3030–6. [DOI] [PubMed] [Google Scholar]
- 151. van Duursen MBM, Sanderson JT, de Jong PC, Kraaij M, van den Berg M. Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. Toxicol Sci. 2004;81:316–24. [DOI] [PubMed] [Google Scholar]
- 152. Poór M, Zrínyi Z, Kőszegi T. Structure related effects of flavonoid aglycones on cell cycle progression of HepG2 cells: metabolic activation of fisetin and quercetin by catechol-O-methyltransferase (COMT). Biomed Pharmacother. 2016;83:998–1005. [DOI] [PubMed] [Google Scholar]
- 153. Ishisaka A, Kawabata K, Miki S, Shiba Y, Minekawa S, Nishikawa T, Mukai R, Terao J, Kawai Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS One. 2013;8:e80843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–72. [DOI] [PubMed] [Google Scholar]
- 155. Galindo P, Rodriguez-Gómez I, González-Manzano S, Dueñas M, Jiménez R, Menéndez C, Vargas F, Tamargo J, Santos-Buelga C, Pérez-Vizcaíno Fet al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS One. 2012;7:e32673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Perez-Vizcaino F, Duarte J, Santos-Buelga C. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric. 2012;92:1822–5. [DOI] [PubMed] [Google Scholar]
- 157. Menendez C, Dueñas M, Galindo P, González-Manzano S, Jimenez R, Moreno L, Zarzuelo MJ, Rodríguez-Gómez I, Duarte J, Santos-Buelga Cet al. Vascular deconjugation of quercetin glucuronide: the flavonoid paradox revealed?. Mol Nutr Food Res. 2011;55:1780–90. [DOI] [PubMed] [Google Scholar]
- 158. Fernández-Castillejo S, Macià A, Motilva M-J, Catalán Ú, Solà R. Endothelial cells deconjugate resveratrol metabolites to free resveratrol: a possible role in tissue factor modulation. Mol Nutr Food Res. 2019;63:1800715. [DOI] [PubMed] [Google Scholar]
- 159. Lu Q-Y, Zhang L, Eibl G, Go VLW. Overestimation of flavonoid aglycones as a result of the ex vivo deconjugation of glucuronides by the tissue β-glucuronidase. J Pharm Biomed Anal. 2014;88:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. O'Leary KA, Day AJ, Needs PW, Sly WS, O'Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett. 2001;503:103–6. [DOI] [PubMed] [Google Scholar]
- 161. Ha SK, Lee JA, Cho EJ, Choi I. Effects of catechol O-methyl transferase inhibition on anti-inflammatory activity of luteolin metabolites. J Food Sci. 2017;82:545–52. [DOI] [PubMed] [Google Scholar]
- 162. Kunihiro AG, Luis PB, Brickey JA, Frye JB, Chow H-HS, Schneider C, Funk JL. Beta-glucuronidase catalyzes deconjugation and activation of curcumin-glucuronide in bone. J Nat Prod. 2019;82:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ghosh D. Human sulfatases: a structural perspective to catalysis. Cell Mol Life Sci. 2007;64:2013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wang S, Li F, Quan E, Dong D, Wu B. Efflux transport characterization of resveratrol glucuronides in UDP-glucuronosyltransferase 1A1 transfected HeLa cells: application of a cellular pharmacokinetic model to decipher the contribution of multidrug resistance-associated protein 4. Drug Metab Dispos. 2016;44:485–8. [DOI] [PubMed] [Google Scholar]
- 165. Riha J, Brenner S, Böhmdorfer M, Giessrigl B, Pignitter M, Schueller K, Thalhammer T, Stieger B, Somoza V, Szekeres Tet al. Resveratrol and its major sulfated conjugates are substrates of organic anion transporting polypeptides (OATPs): impact on growth of ZR-75-1 breast cancer cells. Mol Nutr Food Res. 2014;58:1830–42. [DOI] [PubMed] [Google Scholar]
- 166. Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos. 2001;29:1521–4. [PubMed] [Google Scholar]
- 167. Oi N, Hashimoto T, Kanazawa K. A possible mechanism that flavonoids exert anticarcinogenesis with activation of β-glucuronidase in cancerous tissues. ACS Symp Ser. 2008;993:102–7. [Google Scholar]
- 168. Gratz M, Kunert-Keil C, John U, Cascorbi I, Kroemer HK. Identification and functional analysis of genetic variants of the human β-glucuronidase in a German population sample. Pharmacogenet Genomics. 2005;15:875–81. [DOI] [PubMed] [Google Scholar]
- 169. Miksits M, Wlcek K, Svoboda M, Thalhammer T, Ellinger I, Stefanzl G, Falany CN, Szekeres T, Jaeger W. Expression of sulfotransferases and sulfatases in human breast cancer: impact on resveratrol metabolism. Cancer Lett. 2010;289:237–45. [DOI] [PubMed] [Google Scholar]
- 170. Williamson G, Kay CD, CrozierA. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr Rev Food Sci Food Saf. 2018;17:1054–112. [DOI] [PubMed] [Google Scholar]
- 171. Jiang W, Hu M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012;2:7948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Williamson G, Aeberli I, Miguet L, Zhang Z, Sanchez M-B, Crespy V, Needs P, Kroon PA, Glavinas H, Krajcsi Pet al. Interaction of positional isomers of quercetin glucuronides with the transporter ABCC2 (cMOAT, MRP2). Drug Metab Dispos. 2007;35:1262–8. [DOI] [PubMed] [Google Scholar]
- 173. Brand W, van der Wel PAI, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IMCM. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–802. [DOI] [PubMed] [Google Scholar]
- 174. Ofer M, Wolffram S, Koggel A, Spahn-Langguth H, Langguth P. Modulation of drug transport by selected flavonoids: involvement of P-gp and OCT?. Eur J Pharm Sci. 2005;25:263–71. [DOI] [PubMed] [Google Scholar]
- 175. Barrington R, Williamson G, Bennett RN, Davis BD, Brodbelt JS, Kroon PA. Absorption, conjugation and efflux of the flavonoids, kaempferol and galangin, using the intestinal CaCo-2/TC7 cell model. J Funct Foods. 2009;1:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Delmas D, Lin HY. Role of membrane dynamics processes and exogenous molecules in cellular resveratrol uptake: consequences in bioavailability and activities. Mol Nutr Food Res. 2011;55:1142–53. [DOI] [PubMed] [Google Scholar]
- 177. Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sissung TM, Goey AKL, Ley AM, Strope JD, Figg WD. Pharmacogenetics of membrane transporters: a review of current approaches. Methods Mol Biol. 2014;1175:91–120. [DOI] [PMC free article] [PubMed] [Google Scholar]