Abstract
Background
Despite the therapeutic efficacy of immune checkpoint inhibitors (ICIs) in a subset of patients, consistent and easily obtainable predictors of efficacy remain elusive.
Methods
This study was conducted on 644 advanced non-small cell lung cancer (NSCLC) patients treated with ICI monotherapy between April 2013 and September 2020 at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital. Patient smoking history, clinicopathological characteristics, tumor mutation burden (TMB) by clinical targeted next-generation sequencing, and programmed death ligand-1 (PD-L1) tumor proportion score (TPS) by immunohistochemistry were prospectively collected. The association of smoking history with clinical outcomes of ICI monotherapy in metastatic NSCLC patients was evaluated after adjusting for other potential predictors. All statistical tests were 2-sided.
Results
Of 644 advanced NSCLC patients, 105 (16.3%) were never smokers, 375 (58.2%) were former smokers (median pack-years = 28), and 164 (25.4%) were current smokers (median pack-years = 40). Multivariable logistic and Cox proportional hazards regression analyses suggested that doubling of smoking pack-years is statistically significantly associated with improved clinical outcomes of patients treated with ICI monotherapy (objective response rate odds ratio = 1.21, 95% confidence interval [CI] = 1.09 to 1.36, P < .001; progression-free survival hazard ratio = 0.92, 95% CI = 0.88 to 0.95, P < .001; overall survival hazard ratio = 0.94, 95% CI = 0.90 to 0.99, P = .01). Predictive models incorporating pack-years and PD-L1 TPS yielded additional information and achieved similar model performance compared with using TMB and PD-L1 TPS.
Conclusions
Increased smoking exposure had a statistically significant association with improved clinical outcomes in metastatic NSCLC treated with ICI monotherapy independent of PD-L1 TPS. Pack-years may serve as a consistent and readily obtainable surrogate of ICI efficacy when TMB is not available to inform prompt clinical decisions and allow more patients to benefit from ICIs.
The treatment paradigm for metastatic non-small cell lung cancer (NSCLC) has been changed dramatically with the introduction of immune checkpoint inhibitors (ICIs) as systemic therapy. Despite the remarkable therapeutic benefit from ICIs, only a minority of patients have a durable response from ICI monotherapy, and consistent clinicopathological and genomic predictors of therapeutic efficacy remain elusive (1,2).
The use of programmed death ligand-1 (PD-L1) tumor proportion score (TPS) as a predictive biomarker for ICIs has been extensively studied and is the only FDA-approved biomarker for ICI patient selection in NSCLC. Despite the promising clinical benefit for NSCLC patients with PD-L1 TPS of 50% or greater with ICI monotherapy, the treatment recommendations for PD-L1 negative and those with PD-L1 TPS less than 50% are still unclear (3). In addition, tumor mutation burden (TMB) has also emerged as a potential predictive biomarker for ICIs (4). Higher TMB is associated with improved clinical responses to ICIs, and this possibly could be explained by higher probability of tumor neoantigen production and thus, illicit immunogenicity and induce remarkable response (1,5-7). However, lack of access to adequate tumor specimens for next-generation sequencing, prolonged turnaround time, high expense for TMB assessment, and variations across platforms and pipelines, including assay types, panel size, and types of mutations, included into TMB limit its standardization and widespread use (2,8). Moreover, the high failure rates for PD-L1 TPS and challenges in obtaining TMB values also plague the biomarker field for ICIs (5). Therefore, it is imperative to discover consistent and readily obtainable predictors to help clinicians make prompt treatment decisions, especially for metastatic patients with high symptom burden (4,9).
Tobacco smoking, as the leading cause of lung cancer, has been consistently reported to be associated with higher TMB and increased response to immunotherapy (10‐14). The clinical trials that have reported on smoking typically designated patients into never, former, or current smokers. Diverging results of the qualitative smoking effect on objective response rate (ORR) and progression-free survival (PFS) have been observed (3,6,7,15‐17). The qualitative smoking status typically collected in this manner has been unable to sufficiently capture quantitative smoking exposure and thus limits its potential being a predictor for ICI efficacy. Although imperfect, detailed smoking history may be a readily obtainable and consistent surrogate for TMB given its challenges in generating this correlative value. As such, we hypothesize that quantitative smoking pack-years provides information that is predictive of the efficacy of ICI monotherapy in metastatic NSCLC.
In this study, we aimed to assess the predictive impact of thoroughly collected smoking exposure on ORR, PFS, and overall survival (OS) of advanced NSCLC patients treated with ICI monotherapy to validate the potential clinical utility of cigarette exposure in addition to PD-L1 TPS in patients with metastatic NSCLC where TMB is a challenge to generate.
Methods
Clinical Samples and Study Population
Patients with advanced NSCLC who had consented to a correlative research study (DF/HCC protocol #02–180), received ICI monotherapy, and whose tumors underwent successful clinical targeted OncoPanel sequencing from April 2013 to September 2020 at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital were identified (18). Clinicopathological characteristics, including age at treatment initiation, gender, detailed smoking history, histology, Eastern Cooperative Oncology Group Performance Status (ECOG PS) and lines of treatment, were prospectively collected. The smoking status and smoking pack-years were consistently obtained from patients and recorded in the Thoracic Oncology Basic Assessment of Cancer and Clinical Outcomes database that has been previously described (19). Smoking status included never smokers (<100 cigarettes in a lifetime), former smokers (quit >12 months before diagnosis), and current smokers (quit <12 months before diagnosis or currently still smoking). Smoking pack-years, defined as packs per day (1 pack = 20 cigarettes) × years of smoking, was directly extracted from the Thoracic Oncology Basic Assessment of Cancer and Clinical Outcomes database.
TMB Assessment and PD-L1 Testing
Sample collection and DNA extraction were performed as previously described (20). TMB, defined as the number of somatic, nonsynonymous, base substitution, and small InDels mutations per Mb , was calculated from the Dana-Farber Cancer Institute OncoPanel next-generation sequencing platforms as previously described (18,20). OncoPanel was conducted only on tumor-derived samples, and potential polymorphisms were systematically filtered on the basis of the allele frequency at the population level of greater than 0.1% in the Exome Sequencing Project database (RRID: SCR_012761) and on an in-house panel of control samples (21). Several antibodies, including 22C3 (DAKO), SP263 (Ventana), and E1L3N (Danvers), were used to identify the PD-L1 TPS. PD-L1 TPS was reported as a percentage of tumor cells with positive membranous staining.
Clinical Outcomes
The ORR and PFS were determined using Response Evaluation Criteria In Solid Tumors version 1.1. ORR was defined as the proportion of patients with complete response or partial response. PFS was measured from ICI initiation to the date of disease progression or death, whichever occurred first. OS was defined as from the start of ICI to last contact or death, whichever occurred first.
Statistical Analysis
The Wilcoxon rank sum test and Fisher exact test were used to test for associations between continuous and categorical variables between groups. Time to event distributions were estimated using Kaplan–Meier methodology, and log-rank tests were used for testing the crude differences in event-time distributions between groups (22,23). The association between PFS and OS and independent variables, such as smoking exposure, TMB, and PD-L1 TPS, were estimated as a hazard ratio (HR) using Cox proportional hazards regression model and assessed using the score test (24). The proportional hazards assumption was assessed graphically and with Schoenfeld residuals. No indication of assumption violation was observed. Multivariable analyses on ORR, PFS, and OS consistently adjusted for age at treatment initiation, gender, histology, ECOG PS, PD-L1 TPS, and lines of treatment (24,25). Predictive logistic regression on ORR and Cox proportional hazards regression on PFS and OS were constructed in the training set (80%). Model performance was evaluated in the test set (20%) using 10-fold cross-validation based on the area under the curve (AUC) and 95% confidence interval of AUC was calculated based on 500 bootstrapping. The performance of the predictive models was assessed and validated by R software, version 3.6.1, R package survivalROC, pROC (26,27). Mediation analysis was conducted by R package mediation (28).
Base 2 log transformation was used for TMB and pack-years to meet the linearity assumption and to facilitate easy interpretation. Inverse probability weighting (IPW) was used to account for the potential selection bias resulting from missingness in PD-L1 TPS. All P values were 2-sided, and confidence intervals were at the 95% level, with a statistical significance level of .05.
Results
Study Population
A total of 644 metastatic NSCLC patients who were treated with ICI monotherapy were identified: 105 (16.3%) never smokers, 375 (58.2%) former smokers (median pack-years = 28), and 164 (25.4%) current smokers (median pack-years = 40). Never, former, and current smokers were well balanced in gender, histology, and ECOG PS (Table 1). PD-L1 TPS of at least 50% were common in former and current smokers compared with never smokers (P = .07). Most never smokers (82.9%) received ICI monotherapy in second-line settings and beyond, while former (37.1%) and current smokers (36.0%) were more likely to receive it as first-line treatment (P < .001). Among those who received it as first-line therapy, 145 (67.1%) had PD-L1 TPS of at least 50%. A total of 453 (70.3%) patients had known PD-L1 TPS, and TMB was available for all patients.
Table 1.
Baseline clinicopathological characteristics (N = 644)
| Characteristics | Never smoker (n = 105) | Former smoker (n = 375) | Current smoker (n = 164) | P a |
|---|---|---|---|---|
| Age, median (range), y | 63 (25-87) | 69 (35-92) | 63 (38-88) | <.001 |
| Gender, No. (%) | .80 | |||
| Female | 60 (57.1) | 207 (55.2) | 88 (53.0) | |
| Male | 45 (42.9) | 168 (44.8) | 77 (47.0) | |
| Histology, No. (%) | .27 | |||
| Nonsquamous cell carcinoma | 97 (92.4) | 332 (88.5) | 141 (86.0) | |
| Squamous cell carcinoma | 8 (7.6) | 43 (11.5) | 23 (14.0) | |
| ECOG PSb, No. (%) | .48 | |||
| 0-1 | 82 (78.1) | 298 (79.5) | 123 (75.0) | |
| ≥2 | 22 (21.0) | 74 (19.7) | 40 (24.4) | |
| Unknown | 1 (1.0) | 3 (0.8) | 1 (0.6) | |
| PD-L1 TPSc, No. (%) | .07 | |||
| Negative | 20 (19.0) | 48 (12.8) | 12 (7.3) | |
| 1%-49% | 23 (21.9) | 86 (22.9) | 41 (25.0) | |
| ≥50% | 30 (28.6) | 137 (36.5) | 56 (34.1) | |
| Unknown | 32 (30.5) | 104 (27.7) | 55 (33.5) | |
| Lines of therapyd, No. (%) | <.001 | |||
| 1 | 18 (17.1) | 139 (37.1) | 59 (36.0) | |
| ≥2 | 87 (82.9) | 236 (62.9) | 105 (64.0) | |
| Median pack-yearse (IQR) | 0 (0) | 28 (25) | 40 (26.3) | <.001 |
| Median TMB (IQR), (mut/Mb) | 7.6 (6.1) | 9.9 (6.8) | 12.2 (7.7) | <.001 |
P values were based on a Wald test and were 2-sided. ECOG PS = Eastern Cooperative Oncology Group Performance Status; IQR = interquartile range; NA = not applicable; PD-L1 TPS = programmed death ligand-1 tumor proportion score; TMB = tumor mutation burden.
ECOG PS not available for 5 patients.
PD-L1 TPS not available for 191 patients.
1L present first-line treatment setting; 2 L+ present second-line treatment setting and beyond.
Smoking pack-years not available for 1 patient.
Molecular Characteristics
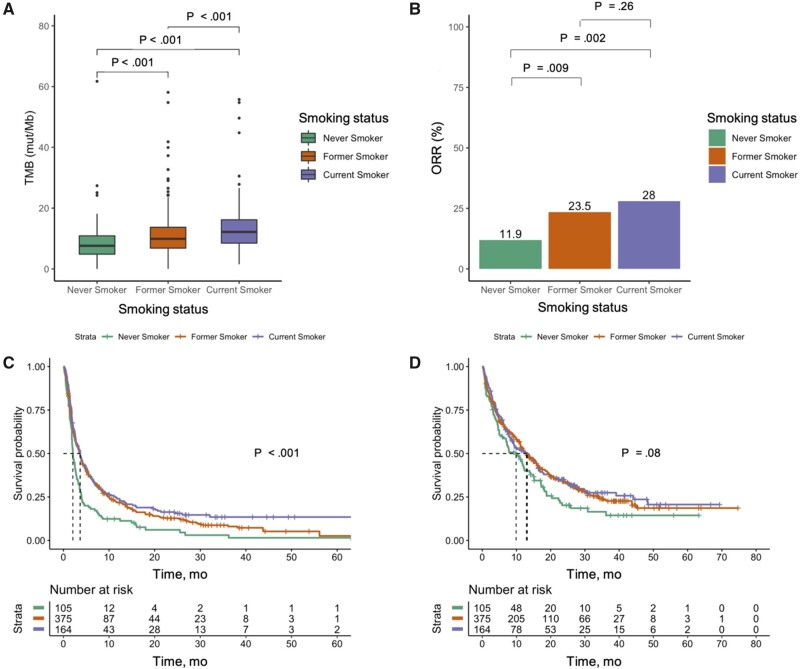
We compared the baseline molecular characteristics by smoking status. As expected, never smokers were more likely to harbor EGFR (37.1%), ALK (1.9%), HER2 (5.7%), and MET (5.7%) mutations compared with former (5.9%, 0.5%,1.6%, and 2.9%, respectively) and current smokers (2.4%, 0.6%, 0.6%, and 0.6%, respectively) (Supplementary Table 1, available online). KRAS mutations were more common in former (41.1%) and current smokers (39.0%) compared with never smokers (11.4%) (P < .001 for both comparisons). We also compared the TMB distribution across different smoking subgroups. Median TMB among current smokers (12.2 mut/Mb) was the highest, followed by former smokers (9.9 mut/Mb) and never smokers (7.6 mut/Mb) (P < .001 for both comparisons) (Figure 1, A).
Figure 1.
Clinical outcomes by smoking status. A) Distribution of tumor mutation burden (TMB) among never, former, and current smokers were presented. Clinical outcomes of (B) objective response rate (ORR), (C) progression-free survival according to Response Evaluation Criteria In Solid Tumors version 1.1, and (D) overall survival among never, former, and current smokers are presented.
Association Between Smoking History and Clinical Outcomes
Best objective responses were observed in 11.4%, 23.5%, and 28.0% of never, former, and current smokers, respectively (former vs never smokers, P = .009; current vs never smokers, P = .002; former vs current smokers, P = .26, respectively) (Figure 1, B; Supplementary Table 2, available online). In multivariable analysis, after controlling for PD-L1 TPS and other clinicopathological characteristics, smoking status was statistically significantly associated with increased ORR (former vs never smokers, OR = 2.07, 95% CI = 1.08 to 4.25, P = .04; current vs never smokers, OR = 3.04, 95% CI = 1.52 to 6.47, P = .003; current vs former smokers, OR = 1.34, 95% CI = 0.85 to 2.09, P = .20, respectively). Never smokers had a statistically significantly shorter PFS than former (2.07 months vs 3.65 months, HR = 0.74, 95% CI = 0.59 to 0.93, P = .01) and current smokers (2.07 months vs 3.68 months, HR = 0.60, 95% CI = 0.46 to 0.79, P < .001). Never smokers had a numerically shorter OS than former (9.9 months vs 12.9 months, HR = 0.85, 95% CI = 0.66 to 1.11, P = .23) and current smokers (9.9 months vs 13.2 months, HR = 0.78, 95% CI = 0.58 to 1.04, P = .10) (Figure 1, C and D; Table 2). Similar results were observed in sensitivity analysis based on complete dataset (n = 451) and using the IPW method to account for potential selection bias because of PD-L1 TPS missingness (Supplementary Table 3, available online).
Table 2.
Association between smoking status and ORR, PFS, and OS (N = 644)a
| Parameters | ORR |
PFS |
OS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||||
| OR (95% CI)b | P | OR (95% CI)c | P | HRd (95% CI) | P | HRe (95% CI) | P | HRd (95% CI) | P | HRe (95% CI) | P | |
| Smoker | ||||||||||||
| Former vs never smoker | 2.38 (1.29 to 4.75) | .009 | 2.07 (1.08 to 4.25) | .04 | 0.66 (0.53 to 0.83) | <.001 | 0.74 (0.59 to 0.93) | .01 | 0.77 (0.60 to 0.99) | .04 | 0.85 (0.66 to 1.11) | .23 |
| Current vs never smoker | 3.02 (1.56 to 6.27) | .002 | 3.04 (1.52 to 6.47) | .003 | 0.59 (0.45 to 0.76) | <.001 | 0.60 (0.46 to 0.79) | <.001 | 0.74 (0.56 to 0.99) | .04 | 0.78 (0.58 to 1.04) | .10 |
| Age | NA | NA | 1.01 (0.99 to 1.03) | .22 | NA | NA | 0.99 (0.98 to 1.00) | .07 | NA | NA | 1.00 (0.99 to 1.01) | .77 |
| Male vs female | NA | NA | 0.79 (0.53 to 1.18) | .25 | NA | NA | 1.14 (0.96 to 1.34) | .13 | NA | NA | 1.19 (0.99 to 1.44) | .07 |
| Squamous vs nonsquamous | NA | NA | 1.34 (0.72 to 2.39) | .34 | NA | NA | 1.04 (0.81 to 1.35) | .75 | NA | NA | 1.15 (0.87 to 1.52) | .34 |
| ECOG PS | ||||||||||||
| ≥2 vs 0-1 | NA | NA | 0.30 (0.16 to 0.54) | <.001 | NA | NA | 2.04 (1.67 to 2.49) | <.001 | NA | NA | 2.98 (2.39 to 3.71) | <.001 |
| Unknown vs 0-1 | 0.64 (0.03 to 4.77) | .70 | 0.75 (0.31,1.83) | .53 | NA | NA | 0.42 (0.10 to 1.69) | .22 | ||||
| PD-L1 TPS | ||||||||||||
| ≥50% vs negative | NA | NA | 4.00 (1.84 to 9.70) | <.001 | NA | NA | 0.55 (0.41 to 0.73) | <.001 | NA | NA | 0.63 (0.46 to 0.88) | .006 |
| 1%-49% vs negative | NA | NA | 1.44 (0.63 to 3.64) | .41 | NA | NA | 0.90 (0.68 to 1.18) | .44 | NA | NA | 0.99 (0.73 to 1.35) | .95 |
| Unknown vs negative | NA | NA | 2.15 (0.98 to 5.26) | .07 | NA | NA | 0.82 (0.62 to 1.07) | .15 | NA | NA | 0.97 (0.72 to 1.30) | .82 |
| 2L+ vs 1 L treatment | NA | NA | 0.90 (0.57 to 1.44) | .66 | NA | NA | 1.05 (0.86 to 1.29) | .62 | NA | NA | 1.31 (1.03 to 1.66) | .03 |
All P values were 2-sided. CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Status; HR = hazard ratio; NA = not applicable; OR = odds ratio; ORR = objective response rate; OS = overall survival; PFS = progression-free survival.
Unadjusted OR of objective response.
OR of objective response adjusted for age, gender, histology, ECOG PS, PD-L1 TPS, and lines of treatment.
Unadjusted HR.
HR adjusted for age, gender, histology, ECOG PS, PD-L1 TPS, and lines of treatment.
We next investigated whether pack-years is associated with clinical outcomes and found that doubling smoking pack-years was statistically significantly associated with improved clinical outcomes in multivariable analyses after adjusting for PD-L1 TPS and other clinicopathological characteristics (ORR OR = 1.21, 95% CI = 1.09 to 1.36, P < .001; PFS HR = 0.92, 95% CI = 0.88 to 0.95, P < .001; OS HR = 0.94, 95% CI = 0.90 to 0.99, P = .01) (Table 3). Similar results were observed in sensitivity analyses (Supplementary Table 4, available online). In stratified analysis by oncogenic driver mutation status, consistent improved trends in clinical response and outcomes with increased smoking exposure were observed in both groups. Numerically larger effects were consistently observed in patients with oncogenic driver mutations compared with wild-type patients (ORR OR = 1.16, 95% CI = 1.01 to 1.36, P = .04; PFS HR = 0.96, 95% CI = 0.90 to 1.00, P = .06; OS HR = 0.95, 95% CI 0.89 to 1.01, P = .11) (Supplementary Table 5, available online). We explored further the association between pack-years and clinical outcomes in metastatic NSCLC with PD-L1 TPS of at least 50% or less than 50% as a subgroup analysis in the patients with PD-L1 TPS available. A statistically significant effect on clinical outcomes was observed only in patients with PD-L1 TPS of at least 50% (ORR OR = 1.18, 95% CI = 1.01 to 1.41, P = .05; PFS HR = 0.88, 95% CI = 0.81 to 0.95, P = .002; OS HR = 0.87, 95% CI = 0.79 to 0.96, P = .007). A numerically larger effect on clinical outcomes was consistently observed in patients with PD-L1 TPS of at least 50% compared with those with PD-L1 TPS of less than 50% (Supplementary Table 6, available online). We further explored different PD-L1 TPS cutoffs of 55%, 60%, and 70%, and consistent results were observed. Of note, pack-years was statistically significantly associated with PFS regardless of PD-L1 TPS levels (Supplementary Table 7, available online). Collectively, pack-years was confirmed to be associated with clinical responses to ICI monotherapy in metastatic NSCLC.
Table 3.
Association between smoking pack-years and ORR, PFS, and OS (n = 643)a
| Parameters | ORR |
PFS |
OS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||||
| ORb (95% CI) | P | ORc (95% CI) | P | HRd (95% CI) | P | HRe (95% CI) | P | HRd (95% CI) | P | HRe (95% CI) | P | |
| Doubling pack-years | 1.20 (1.09 to 1.34) | <.001 | 1.21 (1.09 to 1.36) | <.001 | 0.91 (0.88 to 0.95) | <.001 | 0.92 (0.88 to 0.95) | <.001 | 0.95 (0.91 to 0.99) | .02 | 0.94 (0.90 to 0.99) | .01 |
| Age | NA | NA | 1.01 (0.99 to 1.03) | .51 | NA | NA | 1.00 (0.99 to 1.00) | .23 | NA | NA | 1.00 (0.99 to 1.01) | .93 |
| Male vs female | NA | NA | 0.74 (0.49 to 1.10) | .13 | NA | NA | 1.17 (0.99 to 1.39) | .06 | NA | NA | 1.22 (1.01 to 1.47) | .04 |
| Squamous vs nonsquamous | NA | NA | 1.29 (0.70 to 2.31) | .41 | NA | NA | 1.05 (0.81 to 1.36) | .71 | NA | NA | 1.16 (0.88 to 1.54) | .30 |
| ECOG PS | ||||||||||||
| ≥2 vs 0-1 | NA | NA | 0.30 (0.16 to 0.53) | <.001 | NA | NA | 2.03 (1.66 to 2.48) | <.001 | NA | NA | 2.99 (2.40 to 3.73) | <.001 |
| Unknown vs 0-1 | NA | NA | 0.62 (0.03 to 4.61) | .68 | NA | NA | 0.77 (0.32 to 1.86) | .56 | NA | NA | 0.42 (0.10 to 1.68) | .22 |
| PD-L1 TPS | ||||||||||||
| ≥50% vs negative | NA | NA | 4.14 (1.91 to 10.06) | <.001 | NA | NA | 0.54 (0.41 to 0.72) | <.001 | NA | NA | 0.63 (0.45 to 0.87) | .006 |
| 1%-49% vs negative | NA | NA | 1.48 (0.64 to 3.74) | .38 | NA | NA | 0.88 (0.67 to 1.17) | .39 | NA | NA | 0.97 (0.71 to 1.32) | .85 |
| Unknown vs negative | NA | NA | 2.25 (1.02 to 5.50) | .06 | NA | NA | 0.79 (0.61 to 1.05) | .11 | NA | NA | 0.96 (0.71 to 1.28) | .77 |
| 2L+ vs 1 L treatment | NA | NA | 0.90 (0.57 to 1.43) | .65 | NA | NA | 1.06 (0.86 to 1.30) | .61 | NA | NA | 1.30 (1.02 to 1.65) | .04 |
All P values were 2-sided. CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Status; HR = hazard ratio; 1 L = first-line treatment setting; 2 L+ = second-line treatment setting and beyond; NA = not applicable; OR = odds ratio; ORR = objective response rate; OS = overall survival; PD-L1 TPS = programmed cell death ligand-1 tumor proportion score; PFS = progression-free survival.
Unadjusted OR of objective response.
OR of objective response adjusted for age, gender, histology, ECOG PS, PD-L1 TPS, and lines of treatment.
Unadjusted HR.
HR adjusted for age, gender, histology, ECOG PS, PD-L1 TPS, and lines of treatment.
Validation of Clinical Utility
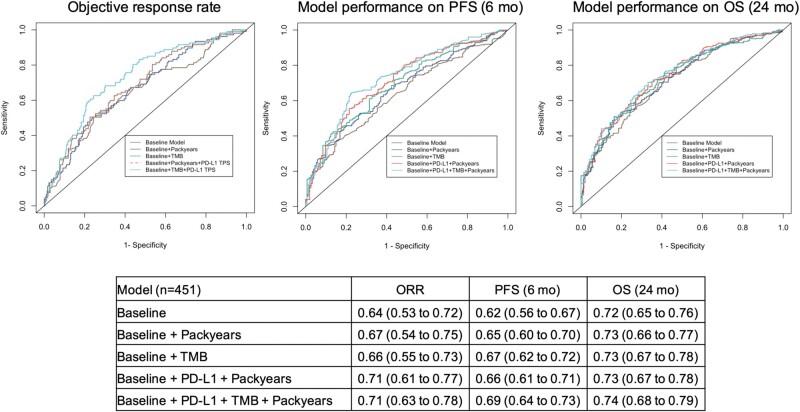
Approximately 30% of the patients in our cohort did not have PD-L1 TPS values. Therefore, predictive models were constructed and validated in the complete dataset (n = 451) to maximize accuracy. Baseline model was constructed on age at treatment initiation, gender, ECOG PS, histology, and lines of treatment, and they were consistently incorporated in the models with the addition of PD-L1 TPS, pack-years, and TMB. Incorporating smoking pack-years improved the model performance (ORR AUC = 0.67, 95% CI = 0.54 to 0.75; PFS AUC = 0.65, 95% CI = 0.60 to 0.70; OS AUC = 0.73, 95% CI = 0.66 to 0.77) compared with the baseline model (ORR AUC = 0.64, 95% CI = 0.53 to 0.72; PFS AUC = 0.62, 95% CI = 0.56 to 0.67; OS AUC = 0.72, 95% CI = 0.65 to 0.76), indicating the additional predictive value of pack-years on clinical outcomes in addition to PD-L1 TPS. Incorporating pack-years along with PD-L1 TPS (ORR AUC = 0.71, 95% CI = 0.61 to 0.77; PFS AUC 0.66 = 95% CI = 0.61 to 0.71; OS AUC = 0.73, 95% CI = 0.67 to 0.78) achieved similar model performance compared with the one using PD-L1 TPS and TMB (ORR AUC = 0.71, 95% CI = 0.63 to 0.77; PFS AUC = 0.68, 95% CI = 0.64 to 0.72; OS AUC = 0.74, 95% CI = 0.68 to 0.78), providing clinical evidence of using smoking pack-years as a surrogate for TMB (Figure 2). Sensitivity analysis using IPW showed consistent results (Supplementary Table 8, available online). Mediation analysis suggested that the effect of smoking information on clinical outcomes of ICI monotherapy was largely mediated by the increased TMB (Supplementary Table 9, available online).
Figure 2.
Model performance on objective response rate (ORR), progression-free survival (PFS) at 6 months, and overall survival (OS) at 24 months. Predictive logistic and Cox proportional hazards regression models were constructed and validated in the patients with complete information (n = 451) using 10-fold cross-validation. Receiver operating characteristics (ROC) area under the curve (AUC) illustrates the model performance on ORR, and time-dependent AUC on PFS at 6 months and on OS at 24 months using different predictors. The baseline model incorporated age at treatment initiation, gender, histology, Eastern Cooperative Oncology Group Performance Status, and lines of treatment, and they were consistently incorporated in all of the models. The table below the graphs displays the AUCs with 95% confidence intervals shown within parentheses. These 95% confidence intervals were calculated from 500 bootstrapping. PD-L1 TPS = programmed cell death ligand-1 tumor proportion score; TMB = tumor mutation burden.
Discussion
Our study comprehensively assesses the association between a quantitative assessment of smoking exposure and clinical outcomes of advanced NSCLC patients treated with ICI monotherapy. The study shows that after accounting for the PD-L1 TPS effect and other clinicopathological characteristics, a detailed smoking history was statistically significantly associated with better response for NSCLC patients treated with ICI monotherapy and informs patient outcomes, particularly when TMB assessment is not available.
A high TMB is associated with improved outcomes to immunotherapy, but a minority of patients in the large clinical trials have that data available at the time of patient entry or time of analyses. There is still a lack of prospective randomized data using of TMB as biomarkers for prospective patient stratification (2,8,17,29–34). In contrast, several studies have shown a correlation between smoking status and response to immunotherapy, and nearly all patients have that information available. Gainor et al (16) reported that heavy smokers have a numerically better ORR compared with light or never smokers in a retrospective study of 58 NSCLC patients treated with PD-(L)1 inhibitors. Consistently, in the KEYNOTE-001 trial, heavy smokers had prolonged PFS and OS compared with never smokers, a potential surrogate for higher TMB of tumors arising in patients with a smoking history (7). In the CheckMate 568 study, 98 patients (82%) of 120 were evaluable for TMB by whole exome sequencing at the time of protocol amendment, and a TMB greater than 10 mut/Mb was associated with an improved response to low-dose ipilimumab and nivolumab as first-line treatment of advanced NSCLC (2,35). Our study prospectively captured very detailed smoking information, which has not been included in many of the clinical trial reports of patients treated with immunotherapy. The information on cigarette smoking allowed us to assess the association between smoking history and clinical outcomes of ICI monotherapy, and our analysis included information on the contribution of PD-L1 TPS and TMB to the likelihood of response and patient outcomes. Our analyses showed both smoking history and PD-L1 TPS are complementary correlates of improved clinical outcomes. As expected, increased smoking exposure in our NSCLC patients treated with ICI monotherapy showed an association with increased TMB and improved clinical outcomes. This finding assists with clinical decisions on ICI treatment allocation given smoking information is available for more patients in real practice.
Our analysis suggests that smoking pack-years may provide additional predictive information above and beyond the routinely assessed PD-L1 TPS. Specifically, we observed trends towards better response and prolonged PFS and OS in patients with PD-L1 TPS of at least 50% compared with the PD-L1 TPS of less than 50% subgroup when smoking history was added , and this was consistently observed in OS and PFS when using different PD-L1 TPS cut points (Supplementary Table 7, available online). Although these differences by PD-L1 TPS cut points were not statistically significant, likely owing to the sample size in never smokers with PD-L1 TPS above the cut points, these findings suggest that there may be interactions between smoking-related molecular characteristics and immunological features. Additional subgroup analysis with detailed smoking history in larger prospective trials is necessary to confirm these findings.
In addition to the qualitative smoking effect that has been reported, a statistically significant dose-dependent association between log2 (pack-years) and log2 (TMB) was observed in our cohort (Supplementary Figure 1, available online). Consistent with our previous research findings in a larger cohort of advanced NSCLC (14), former and current smokers had higher TMB compared with never smokers controlling for age, gender, and histology. Although TMB is emerging as a predictor for ICI efficacy, it is a challenge to assess because of the need for tumor tissue, high expense, and the prolonged turnaround time and thus may not be available for early clinical decision making. The linear dose-dependent association between smoking pack-years and TMB as well as the extensive predictive modeling provide a clinically relevant implication that smoking pack-years may serve as a more easily obtainable surrogate for TMB. The improvement in predictive models, in particular for ORR, with addition of pack-years compared with the baseline model emphasizes the clinical relevance of collecting and using detailed smoking information when PD-L1 TPS and TMB are not available in real practice (Figure 2). Results from model comparison also suggest that low PD-L1 TPS could be complemented with an additional profile containing detailed smoking information and TMB.
Our study has several limitations. First, this is a retrospective study, and our observations should be validated in a larger prospective cohort study. Second, certain molecularly defined NSCLC, for example, EGFR-mutant, ALK-rearranged NSCLC were underrepresented (Supplementary Table 1, available online). These patients are more likely to receive targeted therapy, are typically excluded from most ICI treatment trials, and avoid or are offered immunotherapy in later treatment course (36). Nonetheless, this is the largest cohort of advanced NSCLC treated with ICI monotherapy and with detailed smoking history prospectively collected to date, and the distribution of other genetic alterations reflects real-world data. Third, a majority of the patients in our study received PD-(L)1 inhibitors, and further studies are necessary to elucidate the potential predictive effect of these variables on other types of immunotherapies. Lastly, the predictive model performance could be compromised by the absence of PD-L1 TPS values in 30% of our cohort, especially for the models using predictors other than PD-L1 TPS. Yet, this reflects the real situation of PD-L1 TPS and emphasizes the clinical significance in the discovery of consistent and obtainable predictors that are available in more patients. Model construction and external validation based on a larger cohort will be necessary before implementation in clinical practice.
In conclusion, this study provides important implications that smoking history may serve as an independent surrogate for TMB in ICI efficacy in advanced NSCLC. Improved clinical response and outcomes to ICI monotherapy with increased tobacco exposure are statistically significantly mediated by TMB, giving rise to a more robust immune response. A detailed smoking history should be collected in future clinical practice to make prompt clinical decisions and to enhance the proportion of patients who may benefit from ICIs.
Funding
This work was supported by the National Cancer Institute (5U01CA209414 to XW, BEJ, DCC, X. Lin); Sola Fund for Lung Cancer Research, LUNGSTRONG, the National Cancer Institute (P30-CA006516), Dana-Farber/Harvard Cancer Center Grant, Elaine and Gerald Schuster Fund for Lung Cancer Research to BEJ, and the National Institutes of Health (R35-CA197449 and U01HG009088 to X. Lin).
Notes
Roles of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: Authors have no disclosures.
Author contributions: Conceptualization: X.W, X.Lin, B.E.J, and D.C.C. Methodology: X.W, B.R, X.Lin, B.E.J, and D.C.C. Data curation: X.W, B.R, T.N, M.R, M.M.A, X.Lin, B.E.J, and D.C.C. Writing, review and editing: X.W, B.R, M.M.A, X.Lin, B.E.J, and D.C.C. Funding acquisition: X.Lin, B.E.J, D.C.C. Supervision: X.Lin, B.E.J, D.C.C.
Data Availability
The data underlying this article cannot be shared publicly because of the privacy of individuals who participated in the study. The data underlying this article will be shared on reasonable request to the corresponding authors.
Supplementary Material
References
- 1. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sholl LM, Hirsch FR, Hwang D, et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: a perspective from the international association for the study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15(9):1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 4. Gainor JF, Rizvi H, Jimenez Aguilar E, et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression >/=50. Ann Oncol. 2020;31(3):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Addeo A, Banna GL, Weiss GJ.. Tumor mutation burden-from hopes to doubts. JAMA Oncol. 2019;5(7):934–935. [DOI] [PubMed] [Google Scholar]
- 6. Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 7. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buttner R, Longshore JW, López-Ríos, et al. Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open. 2019;4(1):e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee Y, Clark EW, Milan MS, et al. Turnaround time of plasma next-generation sequencing in thoracic oncology patients: a quality improvement analysis. J Clin Oncol Precis Oncol. 2020;4:PO.20.00121. doi: 10.1200/PO.20.00121. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vokes NI, Liu D, Ricciuti B, et al. Harmonization of tumor mutational burden quantification and association with response to immune checkpoint blockade in non-small-cell lung cancer. J Clin Oncol Precis Oncol. 2019;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexandrov LB, Ju YS, Haase K, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354(6312):618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berland L, Heeke S, Humbert O, et al. Current views on tumor mutational burden in patients with non-small cell lung cancer treated by immune checkpoint inhibitors. J Thorac Dis. 2019;11(suppl 1):S71–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagahashi M, Sato S, Yuza K, et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J Surg Res. 2018;230:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Ricciuti B, Nguyen T, et al. Association between smoking history and tumor mutation burden in advanced non-small cell lung cancer. Cancer Res. 2021;81(9):2566–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norum J, Nieder C.. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): a review of the literature. ESMO Open. 2018;3(6):e000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricciuti B, Kravets S, Dahlberg SE, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin JJ, Cardarella S, Lydon CA, et al. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11(4):556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141(6):751–758. [DOI] [PubMed] [Google Scholar]
- 21.Garrido-Castro AC, Spurr LF, Hughes ME, et al. Genomic characterization of de novo metastatic breast cancer. Clin Cancer Res. 2020;27(4):1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bland JM, Altman DG.. The logrank test. BMJ. 2004;328(7447):1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc. 1988;83(402):414–425. [Google Scholar]
- 24. Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Methodol). 1972;34(2):187–220. [Google Scholar]
- 25. Hosmer Jr DW, Lemeshow S, Sturdivant RX. . Applied Logistic Regression Vol. 398. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 26. Heagerty PJ, Lumley T, Pepe MS.. SurvivalROC: time-dependent ROC curve estimation from censored survival data. Biometrics. 2000;56(2):337–344. [DOI] [PubMed] [Google Scholar]
- 27. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 2011;12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tingley D, Yamamoto T, Hirose KKeele L, Imai K. Mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59(5):1–38. [Google Scholar]
- 29. Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22): 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5): 843–852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7): 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–1448. [DOI] [PubMed] [Google Scholar]
- 34. Kowanetz M, Zou W, Shames D, et al. tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol. 2017;12(1):S321–S322. [Google Scholar]
- 35. Ready N, Awad MM, Otterson GA, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garassino MC, Cho B-C, Kim J-H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly because of the privacy of individuals who participated in the study. The data underlying this article will be shared on reasonable request to the corresponding authors.