ABSTRACT
High-glycemic index (high-GI) foods (so-called fast carbs) have been hypothesized to promote fat storage and increase risk of obesity. To clarify whether dietary GI impacts body weight, we searched PubMed and the Cochrane Database of Systematic Reviews for observational studies reporting associations between BMI and dietary GI, and for meta-analyses of randomized controlled trials (RCTs) comparing low-GI and high-GI diets for weight loss. Data on 43 cohorts from 34 publications, totaling 1,940,968 adults, revealed no consistent differences in BMI when comparing the highest with the lowest dietary GI groups. In the 27 cohort studies that reported results of statistical comparisons, 70% showed that BMI was either not different between the highest and lowest dietary GI groups (12 of 27 cohorts) or that BMI was lower in the highest dietary GI group (7 of 27 cohorts). Results of 30 meta-analyses of RCTs from 8 publications demonstrated that low-GI diets were generally no better than high-GI diets for reducing body weight or body fat. One notable exception is that low-GI diets with a dietary GI at least 20 units lower than the comparison diet resulted in greater weight loss in adults with normal glucose tolerance but not in adults with impaired glucose tolerance. While carbohydrate quality, including GI, impacts many health outcomes, GI as a measure of carbohydrate quality appears to be relatively unimportant as a determinant of BMI or diet-induced weight loss. Based on results from observational cohort studies and meta-analyses of RCTs, we conclude that there is scant scientific evidence that low-GI diets are superior to high-GI diets for weight loss and obesity prevention.
Keywords: glycemic index, carbohydrate quality, diet, obesity, body mass index, weight loss
Statement of Significance: Controversy exists regarding the impact of glycemic index on body weight, and high-glycemic foods (so-called fast carbs) have been hypothesized to promote fat storage and increase risk of obesity. Relying on a substantial body of evidence from epidemiological studies and meta-analyses of randomized controlled trials, the present work demonstrates that dietary glycemic index is unimportant as a determinant of BMI and diet-induced weight loss.
Introduction
The glycemic index (GI) was introduced in 1981 as a means to classify foods according to their effects on postprandial blood glucose (1). Since then, >10,000 scientific articles have been published on GI (PubMed search May 2021), and several popular books have extolled the purported health benefits of low-GI diets (2–4), including better weight control and reduced obesity risk (5, 6). High-GI foods are frequently referred to as “fast carbs.” A May 2021 Google search for “fast carbs” produced >47,000 results, many of which featured websites that portrayed fast carbs as unhealthier and more fattening than low-GI “slow carbs.” Despite popular perception of the superiority of low-GI diets for weight loss and obesity prevention, published research on the topic has produced conflicting interpretations of results (5–14).
Several highly cited reviews have been published on the health implications of a high-GI diet (5, 6, 10, 11, 14), and a 2015 scientific consensus statement concluded diets low in GI were “probably” relevant to the prevention of obesity (8). In contrast, the 2010 US Dietary Guidelines Advisory Committee concluded that there was strong and consistent evidence showing that dietary GI was not associated with body weight (7). The conclusion that GI is not strongly or consistently associated with body weight is also supported from the results of 3 previous narrative reviews (9, 12, 13). The 2015 and 2020 US Dietary Guidelines Advisory Committees made no recommendations for using GI in dietary guidelines (15, 16).
More recently, a 2020 review on the importance of carbohydrate quality over quantity indicated that systematic reviews and meta-analyses of >50 randomized controlled trials (RCTs) have shown that low-GI dietary patterns lead to weight loss (17). However, some RCTs used control diets for which GI was not reported. Moreover, dietary fiber, which impacts body weight (18), was not always measured or reported as the dietary GI changed. Thus, inadequate reporting and residual confounding make attributing changes in body weight entirely to a low-GI diet problematic.
Central to the hypothesized link between high-GI diets and excess body weight is the carbohydrate-insulin model of obesity (19). This model proposes that high-GI foods are particularly fattening because they elevate postprandial insulin secretion, which has direct effects on accelerating storage of fat. However, the validity of the carbohydrate-insulin model of obesity has been questioned (17, 20–22). Although postprandial increases in insulin are greater after high-GI meals (23), most intervention studies show that low-GI diets are no better than high-GI diets for reducing fasting insulin concentrations (24–27).
To clarify whether dietary GI is important for weight control and obesity prevention, we searched PubMed and the Cochrane Database of Systematic Reviews for meta-analyses of observational studies that compared BMI of individuals across dietary GI strata and RCTs that compared low-GI and high-GI diets for weight loss. For observational studies, the search strategy included “glycemic index” OR “glycaemic index” AND “body mass index.” For the RCTs, the search strategy included “glycemic index” OR “glycaemic index” AND “weight” OR “body fat” OR “obesity.” Search results were filtered for “meta-analysis” and were limited to adult populations. No restrictions were placed on date of publication. Of the 56 results for the RCT search, 8 publications were identified that presented 1 or more meta-analyses that compared low-GI and high-GI diets for changes in body weight and/or body fat indices. However, we found no meta-analyses of observational studies, so we removed the “meta-analysis” filter. This produced 892 results, of which 35 publications provided required data on GI and BMI. Reference lists and electronic citation records of all identified meta-analyses and observational studies were also reviewed for additional publications not found in the initial searches.
BMI in Relation to Dietary GI: Observational Cohort Studies
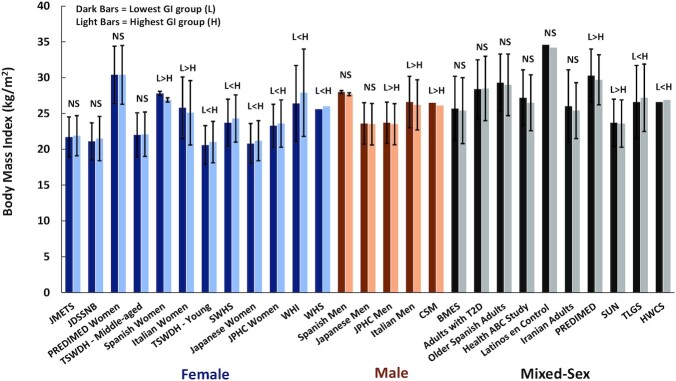
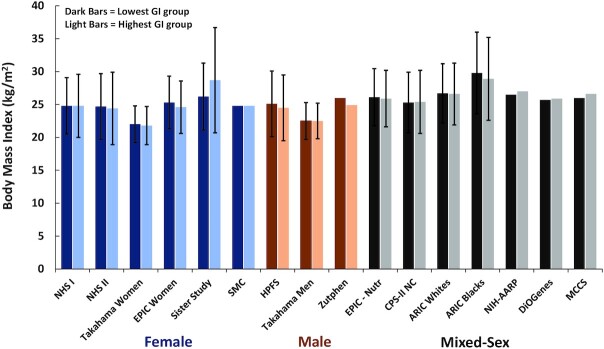
A total of 35 observational studies, including data from 43 cohorts, provided information on baseline BMI across tertiles, quartiles, or quintiles of dietary GI (28–62). These included 18 cohorts of women, 8 cohorts of men, and 17 cohorts of combined women and men, with a total of 1,940,968 adults. In 27 cohorts, statistical analyses were performed to determine whether BMI differed significantly in the highest compared with lowest GI groups (Figure 1). In 16 cohorts, no statistical analyses were reported to assess whether BMI differed between the highest and lowest GI groups (Figure 2).
FIGURE 1.
BMI of the lowest and highest categories of dietary GI in cohort studies in which statistical analysis of BMI differences across dietary GI categories was performed. Values are means ± SDs, except in the cohorts of Spanish women and men (±95% CI). Three cohort studies did not report SDs or CIs. NS = P > 0.05. L>H = BMI in the lowest GI group was significantly greater than in the highest GI group. L<H = BMI in the lowest GI group was significantly lower than in the highest GI group. PREDIMED, n = 3583 (30); PREDIMED women, n = 4010 (60); JMETS, n = 1354 (50); TSWDH, middle-aged, n = 3826; young, n = 3963 (48); SWHS, n = 64,328 (43); JDSSNB, n = 1050 (51); Japanese women, n = 3931 (62); JPHC, women, n = 38,941; men, n = 34,560 (28); WHI, n = 93,676 (33); WHS, n = 18,137 (38); Japanese men, n = 1995 (55); CSM, n = 4617 (61); BMES, n = 3654 (36); HWCS, n = 5830 (31); Spanish women, n = 4001, and men, n = 3669 (39); Italian women, n = 4242, and men, n = 3482) (53); adults with T2D, n = 640 (47); older Spanish adults, n = 343 (45); Health ABC Study, n = 1898 (54); Latinos en Control = Latinos with T2D, n = 238 (57); Iranian adults, n = 265 (49); SUN, n = 9267 (46); TLGS, n = 2457 (59). BMES, Blue Mountain Eye Study; CSM, Cohort of Swedish Men; GI, glycemic index; HWCS, Health Worker Cohort Study; JDSSNB, Japan Dietetic Students’ Study for Nutrition and Biomarkers; JMETS, Japanese Multi-centered Environmental Toxicants Study; JPHC, Japanese Public Health Center Study; Latinos en Control, Latinos with T2D; PREDIMED, PREvencion con Dieta MEDiterranea study; SUN, Seguimiento Universidad de Navarra; SWHS, Shanghai Women's Health Study; TLGS, Tehran Lipid and Glucose Study; TSWDH, Three-Generation Study of Women on Diets and Health; T2D, type 2 diabetes; WHI, Women's Health Initiative Observational Study; WHS, Women's Health Study.
FIGURE 2.
BMI of the lowest and highest categories of dietary GI in cohort studies in which no statistical analysis of BMI differences across dietary GI categories was reported. Values are means ± SDs. Five cohort studies did not report SDs. NHS I, n = 74,248 (29); NHS II, n = 90,411 (29); Takahama women (n = 14,445) and men (n = 11,856) (52); Sister Study, n = 866 (44); EPIC women, n = 334,849 (40); SMC, n = 61,433 (37); HPFS, n = 40,498 (29); Zutphen, n = 394 (56); EPIC-Nutr, n = 338,325 (41); CPS-II NC, n = 30,996 (34); ARIC (Whites, n = 11,478; Blacks, n = 4211) (42); NIH-AARP, n = 482,362 (35); DiOGenes, n = 89,432 (32); MCCS, n = 36,787 (58). ARIC, Atherosclerosis Risk in Communities Study; CPS-II NC, Cancer Prevention Study II Nutrition Cohort; DiOGenes, Diet, Obesity, and Genes cohort study; EPIC/EPIC-Nutr, European Prospective Investigation into Cancer and Nutrition; GI, glycemic index; HPFS, Health Professionals Follow-Up Study; MCCS, Melbourne Collaborative Cohort Study; NHS, Nurses’ Health Study; NIH-AARP, NIH-AARP Diet and Health Study; SMC, Swedish Mammography Cohort; Zutphen, Zutphen Elderly Study.
In the 27 cohorts for which statistical analyses were performed to compare BMI between GI groups, 12 showed no difference in BMI between the highest and lowest GI groups and 7 indicated that BMI was lower in the highest GI groups. Thus, in 70% of the 27 cohorts, dietary GI had either no association with BMI or high GI was associated with lower BMI. In only 8 of the 27 cohorts was BMI significantly lower in the group ingesting the lowest dietary GI.
BMI was not the primary outcome in any of these epidemiological studies. Thus, even when statistical differences were reported, the results must be viewed guardedly as BMI can be affected by many variables, such as total energy intake, fiber intake, and physical activity. Few, if any, adjustments were made for these potential confounders. In studies that did report statistical comparisons across GI groups regarding these potential confounders, no consistent pattern was evident. In studies that found BMI to be significantly lower in the highest GI groups, total energy intake in the highest GI group was significantly higher than in the lowest GI group in 3 cohorts (39, 46, 53) and lower in 1 cohort (28). Five cohorts that reported lower BMI in the highest GI group had significantly lower intakes of dietary fiber (28, 30, 39, 46, 53), whereas only 1 cohort with lower BMI in the highest GI group had significantly higher dietary fiber intake (61). Similar interactions were observed between physical activity and GI. For example, in 3 cohorts that showed lower BMI in the highest dietary GI groups, physical activity levels were also lower (39, 46, 61). In contrast, in only 1 study that showed lower BMI in the highest dietary GI group was physical activity level significantly higher (53).
Collectively, these results suggest that the significantly lower BMIs observed in the highest dietary GI group were not attributable to lower energy intake, lower fiber intake, or higher levels of physical activity. In contrast, in 4 of the 7 studies that reported higher BMIs in the groups with the highest dietary GI, the results could be due in part to significantly higher total energy intake (31), lower intake of fiber (28, 31, 33, 38), and less physical activity (28, 33).
In the 16 cohorts for which no statistical analysis was performed, the mean BMI for the lowest and highest GI groups was virtually the same. This was observed across male, female, and mixed-sex cohorts (Figure 2). The reported BMI in the highest GI group was lower than that in the lowest dietary GI group in 8 of 16 cohorts and was the same in 3 of the cohorts. These observations are consistent with results of studies that reported statistical comparisons. Additional data reported in these studies suggest that these observations were not materially influenced by differences in total energy intake, fiber intake, or physical activity. For example, in the Nurses’ Health Study (NHS) I and NHS II, BMI was either exactly the same in the highest and lowest quintiles of dietary GI (NHS I) or 0.3 kg/m2 lower in the highest quintile of dietary GI (NHS II), yet total energy intake was essentially the same across quintiles and physical activity levels were inversely related to GI (29). Among men in the Health Professionals Follow-Up Study, BMI was 0.6 kg/m2 lower in the highest compared with the lowest quintiles of GI despite similar total energy intake and lower levels of physical activity (29). Similarly, for men and women in the European Prospective Investigation into Cancer and Nutrition cohort, BMI was 0.2 kg/m2 lower in the highest compared with the lowest quintile of dietary GI, despite similar total energy and fiber intake and lower levels of physical activity (41).
In the DiOGenes (Diet, Obesity, and Genes) study, there was a trivial difference in BMI (in kg/m2) between the lowest (25.7) and highest (25.9) quintiles, yet the highest GI quintile consumed nearly 400 kcal/d more than the lowest GI quintile (32). Thus, the slightly higher BMI in the highest GI quintile could be simply due to increased energy intake.
Only 1 of the cohort studies reported data on body fat, assessed by DXA (31). For 5830 adults in the Health Worker Cohort Study, BMI was significantly higher in the highest dietary GI quartile (26.9) compared with the lowest dietary GI quartile (26.6), but percentage body fat did not differ across quartiles (30.9% in quartile 1 vs. 31.4% in quartile 4) (31).
Several epidemiological studies have reported that the prevalence of overweight and obesity differs by very little across dietary GI categories. In the DiOGenes cohort, the percentage of individuals with obesity was essentially the same in quintile 5 (12%) and quintile 1 (13%) of dietary GI, although the percentage of overweight participants was slightly higher in quintile 5 compared with quintile 1 (43% vs. 38%) (32). In the Black Women's Health Study, the percentage of women with a BMI >30 (range = 28.2% to 29.5%) was similar across quintiles of dietary GI (63). In the NIH-AARP Diet and Health Study, the percentage of men with a BMI >25 was similar for quintile 1 (68.8%) and quintile 5 (69.1%) of dietary GI, but the percentage of women with a BMI >25 was higher in quintile 5 (59.9%) than in quintile 1 (48.8%) (64). The higher prevalence of women with a BMI >25 in quintile 5 compared with quintile 1 of dietary GI could be attributed in part to higher daily energy intake (1651 vs. 1499 kcal/d), lower fiber intake (13.8 vs. 22.1 g/d), and a lower percentage who regularly engaged in physical activity (11.0% vs. 23.6%) (64). Also, in the Framingham Offspring Cohort, waist circumference was not different across quintiles of dietary GI (65). Among older adults in the National Diet and Nutritional Survey, dietary GI was not correlated with BMI, body weight, or waist-to-hip ratio (66). In sum, data from these cohort studies do not support the hypothesis that high dietary GI is associated with a higher BMI or greater prevalence of obesity.
Results from RCTs
Eight publications presented a total of 30 meta-analyses of RCTs comparing low-GI and high-GI diets (24–27, 67–70). The RCTs in these meta-analyses were behavioral interventions in which participants received dietary advice. In some instances, participants were supplied with key foods that were aligned with the intervention GI. These studies examined the effects of dietary GI on several anthropometric outcomes, including body weight, BMI, body fat, and waist circumference. Results of these meta-analyses are presented in Table 1, which includes information on the populations examined and the number of studies in each meta-analysis.
TABLE 1.
Effects of low-GI diets compared with high-GI diets on body weight, body fat, fat-free mass, and waist circumference: results from meta-analyses of RCTs1
| Meta-analysis | Subject characteristics | Number of RCTs included | Difference between low-GI and high-GI diets (kg or SMD) (95% CI) |
|---|---|---|---|
| Body weight (kg) | |||
| Kelly et al., 2004 (24) | At risk for CVD | 13 | 0.14 (−0.68, 0.95) kg |
| Thomas et al., 2007 (26) | Overweight, obese | 4 | −1.09 (−1.99, −0.18) kg |
| Ajala et al., 2013 (67) | T2D | 3 | 1.39 (−1.58, 4.36) kg |
| Schwingshackl et al., 2013 (69) | Overweight, obese, T2D | 14 | −0.62 (−1.28, 0.03) kg |
| Schwingshackl et al., 2013 (69) | Obese, T2D | 9 | −1.26 (−2.17, −0.34) kg |
| Schwingshackl et al., 2013 (69) | Overweight, T2D | 6 | 0.04 (−0.90, 0.98) kg |
| Clar et al., 2017 (68) | At risk for CVD | 20 | −0.16 (−0.54, 0.21) kg |
| Reynolds et al., 2019 (25) | Overweight, obese, IGT, T2D | 8 | −0.29 (−0.62, 0.03) kg |
| Zafar et al., 2019 (70) | NGT, IGT, T2D | 51 | −0.06 (−0.15, 0.03) SMD |
| Zafar et al., 2019 (70) | NGT, IGT, T2D; GI difference ≥20 units | 28 | −0.14 (−0.25, −0.03) SMD |
| Zafar et al., 2019 (70) | NGT; GI difference ≥20 units | 13 | −0.26 (−0.43, −0.09) SMD |
| Zafar et al., 2019 (70) | IGT; GI difference ≥20 units | 8 | −0.07 (−0.28, 0.14) SMD |
| Zafar et al., 2019 (70) | T2D; GI difference ≥20 units | 7 | −0.02 (−0.22, 0.18) SMD |
| Zafar et al., 2019 (27) | IGT, T1D, T2D | 22 | 0.00 (−1.92, 1.92) kg |
| Zafar et al., 2019 (27) | IGT, T1D, T2D; GI difference ≥20 units | 12 | −0.64 (−3.33, 2.05) kg |
| Body fat (kg) | |||
| Thomas et al., 2007 (26) | Overweight, obese | 4 | −1.13 (−1.89, −0.38) kg |
| Schwingshackl et al., 2013 (69) | Overweight, obese | 5 | −0.56 (−1.24, 0.12) kg |
| Reynolds et al., 2019 (25) | Overweight, obese, IGT, T2D | 5 | −0.27 (−0.79, 0.26) kg |
| Zafar et al., 2019 (70) | NGT, IGT, T2D | 24 | −0.09 (−0.19, 0.02) SMD |
| Zafar et al., 2019 (70) | NGT | 17 | −0.10 (−0.21, 0.01) SMD |
| Zafar et al., 2019 (70) | IGT | 5 | −0.06 (−0.38, 0.26) SMD |
| Zafar et al., 2019 (70) | NGT, IGT, T2D; GI difference ≥20 units | 12 | −0.15 (−0.35, 0.04) SMD |
| Zafar et al., 2019 (70) | NGT; GI difference ≥20 units | 7 | −0.28 (−0.52, −0.04) SMD |
| Zafar et al., 2019 (70) | IGT; GI difference ≥20 units | 4 | 0.17 (−0.24, 0.58) SMD |
| Body fat percentage | |||
| Zafar et al., 2019 (70) | NGT, IGT, T2D | 21 | 0.00 (−0.14, 0.13) SMD |
| Zafar et al., 2019 (70) | NGT | 17 | −0.10 (−0.21, 0.01) SMD |
| Zafar et al., 2019 (70) | IGT | 5 | −0.06 (−0.38, 0.26) SMD |
| Fat-free mass (kg) | |||
| Thomas et al., 2007 (26) | Overweight, obese | 2 | −0.13 (−0.03, 0.56) kg |
| Schwingshackl et al., 2013 (69) | Overweight, obese | 3 | −1.04 (−1.73, −0.35) kg |
| Waist circumference (cm) | |||
| Schwingshackl et al., 2013 (69) | Overweight, obese, T2D | 10 | 0.06 (−0.83, 0.96) kg |
CVD, cardiovascular disease; GI, glycemic index; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; RCT, randomized controlled trial; SMD, standardized mean difference; T1D, type 1 diabetes; T2D, type 2 diabetes.
Body weight
Eight publications, including a total of 15 meta-analyses, reported on body weight as an outcome measure. With only 2 exceptions (26, 70), low-GI diets provided no benefit over high-GI diets for weight loss. One small meta-analysis with only 4 RCTs reported that a low-GI diet produced significantly greater weight loss (∼1 kg) compared with a high-GI diet (26). In a meta-analysis of 28 RCTs by Zafar et al. (70) that included adults with normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and type 2 diabetes (T2D), significantly greater weight loss was observed for a low-GI diet compared with a high-GI diet (standardized mean difference = 0.14, corresponding to ∼1.8 kg), but only if the low-GI diet was ≥20 units lower than the high-GI diet. However, when analyzed by glucose tolerance, the greater weight loss with the low-GI diet was observed only in NGT, with no differences between diets in IGT and T2D. In the combined meta-analysis that included all 51 RCTs (28 RCTs with ≥20 units lower GI plus 23 RCTs with a GI difference <20 units), low-GI diets did not produce greater weight loss than high-GI diets (Table 1). In another meta-analysis by Zafar et al. (27), no significant differences in weight loss were observed among subjects with IGT, type 1 diabetes, or T2D, regardless of whether the GI of the low-GI diet was ≥20 units lower than the high-GI diets. Collectively, these meta-analyses suggest that weight loss with low-GI diets may be more effective than high-GI diets only if subjects have NGT and the GI difference between the diets is ≥20 units.
Fat mass and body fat percentage
Change in fat mass was reported in 4 of the 8 meta-analyses (25, 26, 69, 70), and 1 study also reported results for body fat mass and % body fat (70). In a meta-analysis of 4 RCTs with overweight and obese subjects, low-GI diets resulted in 1.13-kg greater body fat loss (26). Among individuals with NGT (70), consumption of a low-GI diet that differed by ≥20 units from the comparison high-GI diet resulted in more fat loss than the high-GI diet (Table 1). In contrast, among individuals with IGT, and in the combined group of individuals with IGT, NGT, and T2D, a low-GI diet that was ≥20 units lower than the high-GI diet did not result in greater fat loss (70). When the GI difference between diets was unstipulated, the percentage of fat loss did not differ between high- and low-GI diets (25, 69, 70). In the only meta-analysis that reported on changes in body fat percentage, no differences were observed between low-GI and high-GI diets among individuals with NGT, IGT, or T2D (70).
Fat-free mass
Two relatively small meta-analyses, that included only 2 or 3 RCTs, reported data on changes in fat-free mass in overweight and obese subjects. One reported no difference between diets (26), whereas one reported that low-GI diets resulted in significantly greater loss (1.04 kg) of fat-free mass (69).
Waist circumference
Only 1 meta-analysis has been published comparing the effects of low-GI and high-GI diets on waist circumference (69). This meta-analysis of 10 RCTs demonstrated that low-GI and high-GI diets did not differ with regard to changes in waist circumference.
Expected Compared with Observed Results: Problems with Interpreting Research Findings
High-GI meals consistently result in greater glucose and insulin secretion (23) and lower postprandial fat oxidation than low-GI meals (71). These findings are consistent with the carbohydrate-insulin model of obesity and the hypothesis that low-GI diets are associated with greater weight loss and reduced risk of obesity. However, data from observational studies and meta-analyses of RCTs do not substantiate the superiority of low-GI diets for weight loss or obesity prevention. Interestingly, body weight and fat loss were observed following low-GI diets in individuals with NGT but not in those with IGT (70), where insulin responses to a glycemic challenge are almost always greater. If the carbohydrate-insulin model of obesity were to hold true, it would be expected that individuals with the greatest insulin responses to meals would show the greatest reductions in body weight in response to a low-GI diet. It must be noted, however, that most intervention studies showed that low-GI diets are no better than high-GI diets for reducing fasting insulin (24–27). Even in controlled-feeding studies, fasting insulin concentration is not affected by the GI of the diet (72).
Several physiological and methodological limitations of GI research may help explain our null findings. High-GI meals rarely predict short-term energy intake (73). Also, GI values found in tables are measured under rigidly controlled laboratory conditions where foods are eaten singly by generally healthy young subjects and typically involve small sample sizes. This may not represent real-world eating situations because most foods are rarely ingested singly and in prescribed amounts. The glycemic response to a meal with carbohydrate-containing foods can change depending upon the macronutrient composition and dietary fiber content of the meal, preparation of the food, and the time of day that the food is consumed (74–78). Using the published or measured GI value of individual foods to determine the GI of a meal has been reported to overestimate the directly measured GI of a meal by 12 to 19 GI units (75). For perspective, in the observational studies presented in Figures 1 and 2, the difference in median GI between the highest and lowest dietary GI groups rarely exceeded 10 GI units.
Accurate assignment of GI values to foods from a diet record or FFQ is difficult (79). Factors that strongly affect a food's GI, such as variety and cooking/processing methods, are rarely specified in sufficient detail to ensure that the actual GI of the food eaten accurately reflects the assigned GI. For instance, the University of Sydney's GI database lists 27 values for brown rice ranging from 48 to 87 and 66 GI values for white rice ranging from 17 to 94 (80). Moreover, significant interindividual variability and intraindividual reproducibility in repeat GI testing under controlled conditions further complicate interpretation of GI data (81, 82). In a cohort of nearly 800 adults, the glycemic response to white bread varied by >5-fold when comparing the bottom 10% and top 10% of the individual postprandial glycemic responses (82). Finally, glycemic responses across individuals are highly heterogeneous and subject to variation due to age, genetics, physical activity, insulin sensitivity, and BMI (82).
Interpretation of results from observational studies is also limited by the inherent inaccuracies of self-reported diet and physical activity data. Energy intake data from self-report invariably underestimates energy intake determined from doubly-labeled water (DLW) (83, 84), and self-reported physical activity data may not accurately correspond to objectively measured physical activity from accelerometry or physical activity energy expenditure from DLW (85).
Conclusions
Data from observational cohort studies show no consistent association between BMI and dietary GI, and results of meta-analyses of RCTs provide little support for the notion that low-GI diets are superior for weight loss. In the 27 cohort studies that performed statistical comparisons of BMI by dietary GI, the majority (70%) reported either no differences in BMI between extremes of dietary GI or a significantly lower BMI in the highest dietary GI group. We acknowledge that our results may not include all published observational studies that show data on BMI and dietary GI. However, we doubt that additional cohorts missed in our search would change the interpretation regarding BMI–GI relations of the 43 cohorts presented herein. Moreover, previous reviews of the association between BMI and dietary GI (9, 12, 13) included <10 cohorts.
Similarly, results of RCTs generally do not support a case for greater weight loss with low-GI diets. The 1 notable exception is that low-GI diets with a dietary GI at least 20 units lower than the comparison diet resulted in greater weight loss, but this was only observed in individuals with NGT. Because low-GI diets may result in greater loss of fat-free mass (69), this may explain why none of the meta-analyses showed a benefit of low-GI diets for reducing body fat percentage. Also, it must be noted that the RCTs included in the meta-analyses were behavioral interventions in which participants received dietary advice. Although controlled-feeding trials are necessary to determine efficacy of dietary GI for weight loss, the RCTs used in the meta-analyses in Table 1 are more relevant to the effectiveness of dietary GI in real-world conditions.
In view of all the contrary evidence from observational cohort studies and meta-analyses of RCTs, it is surprising that the hypothesis that low-GI (“slow-carb”) diets are superior for weight loss and obesity prevention persists. Carbohydrate quality, including GI, clearly impacts many health outcomes (17, 18, 25), and several meta-analyses have reported higher risks of T2D, cardiovascular disease, and stroke associated with high-GI diets (25, 86–89). However, GI as a measure of carbohydrate quality appears to be unimportant as a determinant of BMI or diet-induced weight loss. We contend that GI is an imprecise measure of the glycemic response of a food when applied to foods in a meal, and that the GI assigned to foods from an FFQ may differ significantly from actual GI. Nutrient density, dietary fiber and whole-grain content of carbohydrates, and percentage of added sugar, are more important qualities (17, 18, 78). Further, a focus on staple carbohydrate foods and the positive nutrients they contribute to diet quality as compared with the detractor nutrients associated with indulgent foods is important in characterizing the quality of carbohydrates. As for body weight and obesity, there is scant scientific evidence that low-GI diets are superior to high-GI diets for weight loss and obesity prevention.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—GAG: wrote the initial draft of the manuscript with contributions from JMJ and SSA; and all authors: reviewed and commented on subsequent drafts of the manuscript and read and approved the final manuscript.
Notes
This work was supported in part by the Grain Foods Foundation.
Author disclosures: GAG, JMJ, and SSA are members of the Scientific Advisory Board of the Grain Foods Foundation; GAG is a member of the Scientific Advisory Boards of the Wheat Foods Council and Ardent Mills, LLC; JMJ and SSA are members of the Scientific Advisory Council to the Quality Carbohydrate Coalition.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: DiOGenes, Diet, Obesity, and Genes cohort study; DLW, doubly-labeled water; GI, glycemic index; IGT, impaired glucose tolerance; NGI, normal glucose tolerance; NHS, Nurses’ Health Study; RCT, randomized controlled trial; T2D, type 2 diabetes.
Contributor Information
Glenn A Gaesser, College of Health Solutions, Arizona State University, Phoenix, AZ, USA.
Julie Miller Jones, Department of Family, Consumer, and Nutritional Science, St. Catherine University, Minneapolis, MN, USA.
Siddhartha S Angadi, Department of Kinesiology, University of Virginia, Charlottesville, VA, USA.
References
- 1. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6. [DOI] [PubMed] [Google Scholar]
- 2. Brand Miller J. The glucose revolution: the authoritative guide to the glycemic index—the groundbreaking medical discovery. New York: Marlowe and Co; 1999. [Google Scholar]
- 3. Kessler DA. Fast carbs, slow carbs : the simple truth about food, weight, and disease. 1st ed. New York: HarperWave; 2020. [Google Scholar]
- 4. Ludwig D. Always hungry? Conquer cravings, retrain your fat cells, and lose weight permanently. 1st ed. New York: Grand Central Life & Style; 2016. [Google Scholar]
- 5. Brand-Miller JC, Holt SH, Pawlak DB, McMillan J.. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281S–5S. [DOI] [PubMed] [Google Scholar]
- 6. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 7. Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. [Internet]. 2010. Available from: https://www.dietaryguidelines.gov/sites/default/files/2019-05/2010DGACReport-camera-ready-Jan11-11.pdf. [Google Scholar]
- 8. Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Björck I, Brand-Miller JC, Brighenti F, Buyken AEet al. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. [DOI] [PubMed] [Google Scholar]
- 9. Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc. 2007;107(10):1768–80. [DOI] [PubMed] [Google Scholar]
- 10. Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266S–73S. [DOI] [PubMed] [Google Scholar]
- 11. O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249–55. [DOI] [PubMed] [Google Scholar]
- 12. Vega-Lopez S, Mayol-Kreiser S N. Use of the glycemic index for weight loss and glycemic control: a review of recent evidence. Curr Diab Rep. 2009;9(5):379–88. [DOI] [PubMed] [Google Scholar]
- 13. Vega-Lopez S, Venn BJ, Slavin JL. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients. 2018;10(10):1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76(1):274S–80S. [DOI] [PubMed] [Google Scholar]
- 15. Dietary Guidelines Advisory Committee. Scientific report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. [Internet]. Washington (DC): US Department of Agriculture, Agricultural Research Service; 2015. Available from: https://health.gov/dietaryguidelines/2015-scientific-report/. [Google Scholar]
- 16. Dietary Guidelines Advisory Committee. Scientific report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. [Internet]. Washington (DC): US Department of Agriculture, Agricultural Research Service; 2020. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf. [Google Scholar]
- 17. Sievenpiper JL. Low-carbohydrate diets and cardiometabolic health: the importance of carbohydrate quality over quantity. Nutr Rev. 2020;78(Suppl 1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Augustin LSA, Aas AM, Astrup A, Atkinson FS, Baer-Sinnott S, Barclay AW, Brand-Miller JC, Brighenti F, Bullo M, Buyken AEet al. Dietary fibre consensus from the International Carbohydrate Quality Consortium (ICQC). Nutrients. 2020;12(9):2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out.”. JAMA Intern Med. 2018;178(8):1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall KD, Guo J, Courville AB, Boring J, Brychta R, Chen KY, Darcey V, Forde CG, Gharib AM, Gallagher Iet al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med. 2021;27(2):344–53. [DOI] [PubMed] [Google Scholar]
- 21. Hall KD, Guyenet SJ, Leibel RL. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern Med. 2018;178(8):1103–5. [DOI] [PubMed] [Google Scholar]
- 22. Speakman JR, Hall KD. Carbohydrates, insulin, and obesity. Science. 2021;372(6542):577–8. [DOI] [PubMed] [Google Scholar]
- 23. Toh DWK, Koh ES, Kim JE. Lowering breakfast glycemic index and glycemic load attenuates postprandial glycemic response: a systematically searched meta-analysis of randomized controlled trials. Nutrition. 2020;71:110634. [DOI] [PubMed] [Google Scholar]
- 24. Kelly S, Frost G, Whittaker V, Summerbell C. Low glycaemic index diets for coronary heart disease. Cochrane Database Syst Rev. 2004(4):CD004467. [DOI] [PubMed] [Google Scholar]
- 25. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–45. [DOI] [PubMed] [Google Scholar]
- 26. Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007(3):CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zafar MI, Mills KE, Zheng J, Regmi A, Hu SQ, Gou L, Gou L, Chen LL. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2019;110(4):891–902. [DOI] [PubMed] [Google Scholar]
- 28. Abe SK, Inoue M, Sawada N, Ishihara J, Iwasaki M, Yamaji T, Shimazu T, Sasazuki S, Tsugane S. Glycemic index and glycemic load and risk of colorectal cancer: a population-based cohort study (JPHC Study). Cancer Causes Control. 2016;27(4):583–93. [DOI] [PubMed] [Google Scholar]
- 29. Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castro-Quezada I, Sanchez-Villegas A, Estruch R, Salas-Salvado J, Corella D, Schroder H, Alvarez-Pérez J, Ruiz-López MD, Artacho R, Ros Eet al. A high dietary glycemic index increases total mortality in a Mediterranean population at high cardiovascular risk. PLoS One. 2014;9(9):e107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denova-Gutierrez E, Huitron-Bravo G, Talavera JO, Castanon S, Gallegos-Carrillo K, Flores Y, Salmeron J. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J Nutr Metab. 2010;2010:170680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Du H, van der A DL, van Bakel MME, Slimani N, Forouhi NG, Wareham NJ, Halkjaer J, Tjønneland A, Jakobsen MU, Overvad Ket al. Dietary glycaemic index, glycaemic load and subsequent changes of weight and waist circumference in European men and women. Int J Obes. 2009;33(11):1280–8. [DOI] [PubMed] [Google Scholar]
- 33. Gangwisch JE, Hale L, Garcia L, Malaspina D, Opler MG, Payne ME, Rossom RC, Lane D. High glycemic index diet as a risk factor for depression: analyses from the Women's Health Initiative. Am J Clin Nutr. 2015;102(2):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartman TJ, McCullough ML, Hodge JM, Gaudet MM, Wang Y, Gapstur SM. Dietary energy density, glycemic load, glycemic index, and risk for endometrial cancer in the CPS-II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2018;27(1):113–15. [DOI] [PubMed] [Google Scholar]
- 35. Jiao L, Flood A, Subar AF, Hollenbeck AR, Schatzkin A, Stolzenberg-Solomon R. Glycemic index, carbohydrates, glycemic load, and the risk of pancreatic cancer in a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88(4):1104–10. [DOI] [PubMed] [Google Scholar]
- 37. Larsson SC, Giovannucci E, Wolk A. Dietary carbohydrate, glycemic index, and glycemic load in relation to risk of colorectal cancer in women. Am J Epidemiol. 2007;165(3):256–61. [DOI] [PubMed] [Google Scholar]
- 38. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, Manson JE, Liu S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57(3):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendez MA, Covas MI, Marrugat J, Vila J, Schroder H. Glycemic load, glycemic index, and body mass index in Spanish adults. Am J Clin Nutr. 2009;89(1):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romieu I, Ferrari P, Rinaldi S, Slimani N, Jenab M, Olsen A, Tjonneland A, Overvad K, Boutron-Ruault MC, Lajous Met al. Dietary glycemic index and glycemic load and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2012;96(2):345–55. [DOI] [PubMed] [Google Scholar]
- 41. Sieri S, Agnoli C, Grioni S, Weiderpass E, Mattiello A, Sluijs I, Sanchez MJ, Jakobsen MU, Sweeting M, van der Schouw YTet al. Glycemic index, glycemic load, and risk of coronary heart disease: a pan-European cohort study. Am J Clin Nutr. 2020;112(3):631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stevens J, Ahn K, Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care. 2002;25(10):1715–21. [DOI] [PubMed] [Google Scholar]
- 43. Yu D, Zhang X, Shu XO, Cai H, Li H, Ding D, Hong Z, Xiang YB, Gao YT, Zheng Wet al. Dietary glycemic index, glycemic load, and refined carbohydrates are associated with risk of stroke: a prospective cohort study in urban Chinese women. Am J Clin Nutr. 2016;104(5):1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson C, Milne GL, Park YM, Sandler DP, Nichols HB. Dietary glycemic index and glycemic load are positively associated with oxidative stress among premenopausal women. J Nutr. 2018;148(1):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castro-Quezada I, Artacho R, Molina-Montes E, Serrano FA, Ruiz-Lopez MD. Dietary glycaemic index and glycaemic load in a rural elderly population (60–74 years of age) and their relationship with cardiovascular risk factors. Eur J Nutr. 2015;54(4):523–34. [DOI] [PubMed] [Google Scholar]
- 46. de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Zazpe I, Vazquez-Ruiz Z, Benito-Corchon S, Bes-Rastrollo M. Glycemic load, glycemic index, bread and incidence of overweight/obesity in a Mediterranean cohort: the SUN project. BMC Public Health. 2014;14:1, 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farvid MS, Homayouni F, Shokoohi M, Fallah A, Farvid MS. Glycemic index, glycemic load and their association with glycemic control among patients with type 2 diabetes. Eur J Clin Nutr. 2014;68(4):459–63. [DOI] [PubMed] [Google Scholar]
- 48. Minobe N, Murakami K, Kobayashi S, Suga H, Sasaki S; Three-Generation Study of Women on Diets and Health Study. Higher dietary glycemic index, but not glycemic load, is associated with a lower prevalence of depressive symptoms in a cross-sectional study of young and middle-aged Japanese women. Eur J Nutr. 2018;57(6):2261–73. [DOI] [PubMed] [Google Scholar]
- 49. Moshtaq MA, Rahimi MH, Mollahosseini M, Khorrami-Nezhad L, Maghbooli Z, Mirzaei K, Pooyan S, Setayesh L. Association between dietary glycemic index and liver enzymes level among apparently healthy adults. Diabetes Metab Syndr. 2019;13(2):1597–602. [DOI] [PubMed] [Google Scholar]
- 50. Murakami K, Sasaki S, Takahashi Y, Okubo H, Hosoi Y, Horiguchi H, Oguma E, Kayama F. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr. 2006;83(5):1161–9. [DOI] [PubMed] [Google Scholar]
- 51. Murakami K, Sasaki S, Uenishi K; Japan Dietetic Students' Study for Nutrition and Biomarkers Group . Dietary glycemic index, but not glycemic load, is positively associated with serum homocysteine concentration in free-living young Japanese women. Nutr Res. 2014;34(1):25–30. [DOI] [PubMed] [Google Scholar]
- 52. Oba S, Nagata C, Nakamura K, Fujii K, Kawachi T, Takatsuka N, Shimizu H. Dietary glycemic index, glycemic load, and intake of carbohydrate and rice in relation to risk of mortality from stroke and its subtypes in Japanese men and women. Metabolism. 2010;59(11):1574–82. [DOI] [PubMed] [Google Scholar]
- 53. Rossi M, Bosetti C, Talamini R, Lagiou P, Negri E, Franceschi S, La Vecchia C. Glycemic index and glycemic load in relation to body mass index and waist to hip ratio. Eur J Nutr. 2010;49(8):459–64. [DOI] [PubMed] [Google Scholar]
- 54. Sahyoun NR, Anderson AL, Tylavsky FA, Lee JS, Sellmeyer DE, Harris TB; Health, Aging, and Body Composition Study . Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr. 2008;87(1):126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Suwazono Yet al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism. 2012;61(1):47–55. [DOI] [PubMed] [Google Scholar]
- 56. van Dam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr. 2000;54(9):726–31. [DOI] [PubMed] [Google Scholar]
- 57. Wang ML, Gellar L, Nathanson BH, Pbert L, Ma Y, Ockene I, Rosal MC. Decrease in glycemic index associated with improved glycemic control among Latinos with type 2 diabetes. J Acad Nutr Diet. 2015;115(6):898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27(11):2701–6. [DOI] [PubMed] [Google Scholar]
- 59. Hosseinpour-Niazi S, Sohrab G, Asghari G, Mirmiran P, Moslehi N, Azizi F. Dietary glycemic index, glycemic load, and cardiovascular disease risk factors: Tehran Lipid and Glucose Study. Arch Iran Med. 2013;16(7):401–7. [PubMed] [Google Scholar]
- 60. Castro-Quezada I, Sanchez-Villegas A, Martinez-Gonzalez MA, Salas-Salvado J, Corella D, Estruch R, Schröder, H, Álvarez-Pérez J, Ruiz-López MD, Artacho Ret al. Glycemic index, glycemic load and invasive breast cancer incidence in postmenopausal women: the PREDIMED study. Eur J Cancer Prev. 2016;25(6):524–32. [DOI] [PubMed] [Google Scholar]
- 61. Levitan EB, Mittleman MA, Wolk A. Dietary glycemic index, dietary glycemic load and mortality among men with established cardiovascular disease. Eur J Clin Nutr. 2009;63(4):552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murakami K, Sasaki S, Okubo H, Takahashi Y, Hosoi Y, Itabashi M. Dietary fiber intake, dietary glycemic index and load, and body mass index: a cross-sectional study of 3931 Japanese women aged 18–20 years. Eur J Clin Nutr. 2007;61(8):986–95. [DOI] [PubMed] [Google Scholar]
- 63. Krishnan S, Rosenberg L, Singer M, Hu FB, Djousse L, Cupples LA, Palmer JR. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med. 2007;167(21):2304–9. [DOI] [PubMed] [Google Scholar]
- 64. George SM, Mayne ST, Leitzmann MF, Park Y, Schatzkin A, Flood A, Hollenbeck A, Subar AF. Dietary glycemic index, glycemic load, and risk of cancer: a prospective cohort study. Am J Epidemiol. 2009;169(4):462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28(2):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Milton JE, Briche B, Brown IJ, Hickson M, Robertson CE, Frost GS. Relationship of glycaemic index with cardiovascular risk factors: analysis of the National Diet and Nutrition Survey for people aged 65 and older. Public Health Nutr. 2007;10(11):1321–35. [DOI] [PubMed] [Google Scholar]
- 67. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–16. [DOI] [PubMed] [Google Scholar]
- 68. Clar C, Al-Khudairy L, Loveman E, Kelly SA, Hartley L, Flowers N, Germanò R, Frost G, Rees K. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;7:CD004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. [DOI] [PubMed] [Google Scholar]
- 70. Zafar MI, Mills KE, Zheng J, Peng MM, Ye X, Chen LL. Low glycaemic index diets as an intervention for obesity: a systematic review and meta-analysis. Obes Rev. 2019;20(2):290–315. [DOI] [PubMed] [Google Scholar]
- 71. Scazzina F, Del Rio D, Benini L, Melegari C, Pellegrini N, Marcazzan E, Brighenti F. The effect of breakfasts varying in glycemic index and glycemic load on dietary induced thermogenesis and respiratory quotient. Nutr Metab Cardiovasc Dis. 2011;21(2):121–5. [DOI] [PubMed] [Google Scholar]
- 72. Kristo AS, Matthan NR, Lichtenstein AH. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: review of randomized controlled-feeding trials. Nutrients. 2013;5(4):1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun FH, Li C, Zhang YJ, Wong SH, Wang L. Effect of glycemic index of breakfast on energy intake at subsequent meal among healthy people: a meta-analysis. Nutrients. 2016;8(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meng H, Matthan NR, Ausman LM, Lichtenstein AH. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am J Clin Nutr. 2017;105(4):842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dodd H, Williams S, Brown R, Venn B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am J Clin Nutr. 2011;94(4):992–6. [DOI] [PubMed] [Google Scholar]
- 76. Henry CJK, Lightowler HJ, Newens KJ, Pata N. The influence of adding fats of varying saturation on the glycaemic response of white bread. Int J Food Sci Nutr. 2008;59(1):61–9. [DOI] [PubMed] [Google Scholar]
- 77. Marangoni F, Poli A. The glycemic index of bread and biscuits is markedly reduced by the addition of a proprietary fiber mixture to the ingredients. Nutr Metab Cardiovasc Dis. 2008;18(9):602–5. [DOI] [PubMed] [Google Scholar]
- 78. Schulz R, Slavin J. Perspective: defining carbohydrate quality for human health and environmental sustainability. Adv Nutr. 2021;12(4):1108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van Bakel MME, Slimani N, Feskens EJM, Du H, Beulens JW, van der Schouw YT, Brighenti F, Halkjaer J, Cust AE, Ferrari Pet al. Methodological challenges in the application of the glycemic index in epidemiological studies using data from the European Prospective Investigation into Cancer and Nutrition. J Nutr. 2009;139(3):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. The University of Sydney. The International Glycemic Index (GI) database [March 2, 2021]. [Internet]. Available from: http://www.glycemicindex.com/. [Google Scholar]
- 81. Vega-Lopez S, Ausman LM, Griffith JL, Lichtenstein AH. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care. 2007;30(6):1412–17. [DOI] [PubMed] [Google Scholar]
- 82. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan Met al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 83. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB; Energy Balance Measurement Working Group . Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39(7):1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schoeller DA, Bandini LG, Dietz WH. Inaccuracies in self-reported intake identified by comparison with the doubly labelled water method. Can J Physiol Pharmacol. 1990;68(7):941–9. [DOI] [PubMed] [Google Scholar]
- 85. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hardy DS, Garvin JT, Xu H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe and Asia: a dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2020;30(6):853–71. [DOI] [PubMed] [Google Scholar]
- 87. Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr. 2011;106(11):1649–54. [DOI] [PubMed] [Google Scholar]
- 88. Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJA, Augustin LSA, Sievenpiper JL, Barclay AW, Liu S, Wolever TMSet al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11(6):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma XY, Liu JP, Song ZY. Glycemic load, glycemic index and risk of cardiovascular diseases: meta-analyses of prospective studies. Atherosclerosis. 2012;223(2):491–6. [DOI] [PubMed] [Google Scholar]