Abstract
A 15-year-old male patient with progressive dyspnoea and exercise-related wheezing was analysed with spirometry, ECG and a cardiopulmonary exercise test with blood gas analysis. Earlier analysis by a paediatrician concluded no abnormalities. However, the previously performed spirometry test may have clarified the diagnosis in an earlier stage.
Severe hypoventilation was seen during the exercise test with hypercapnia and hypoxaemia while hearing a stridor during exercise. Eventually, a circular subglottic stenosis was seen on a CT scan of the chest. No malignancy or granulomatosis with polyangiitis was seen in biopsy and pathologic examination. There was no history of trauma, intubation or infection. Therefore, the diagnosis idiopathic subglottic stenosis was established. Bronchoscopic balloon dilation followed several times, leading to full recovery.
Keywords: respiratory medicine, sports and exercise medicine
Background
Spirometry is frequently used to analyse the cause of dyspnoea. The test gives a good reflection of a patient’s lung function, when performed correctly. However, incorrect technique can mimic an abnormal flow–volume curve. This case showed that a spirometry test should not simply be discarded as ‘incorrect technique’. The results of spirometry were consistently abnormal, which could have led to suspicion of pathology and diagnose in an earlier stage.
We will report a rare case of an idiopathic subglottic trachea stenosis. The cardiopulmonary exercise test (CPET) produced a clear reflection of the pathophysiological consequences of an idiopathic subglottic stenosis during exercise.
Case presentation
A 15-year-old male patient without a medical history was presented at the sports medicine department with complaints of progressive exercise-related dyspnoea. They were first noticed 7 months ago, where he found himself panting and wheezing during football. At the moment of presentation, he could barely exercise due to his complaints. He also experienced symptoms during cycling and stair climbing. There were no palpitations, no chest pain, dizziness or (near-)syncope during exercise. He frequently caught a cold. There was no history of smoking, no allergies and no familial predisposition to asthma or atopy. At the onset of complaints, he visited the general practitioner who prescribed salbutamol, which had no effect. The patient was referred to the paediatrician who concluded a dysfunctional breathing pattern during spirometry examination, without any signs of asthma. Thereafter the patient was referred to a paediatric physiotherapist for an exercise test (Bruce test) and breathing technique training. Exercise tolerance was very low (percentile 5–10) for his age and gender. Despite performing exercise in breathing technique, the complaints got worse over time and the patient was referred to a sports physician.
Investigations
Physical examination showed no abnormalities in heart, lungs, the musculoskeletal system and ear, nose and throat. Biometrics, an ECG, spirometry test and CPET including blood gas analysis were performed to analyse the cardiopulmonary and metabolic response and exercise tolerance according to Wasserman’s references.1
Biometrics
Length: 161 cm, weight: 57.0 kg, body mass index: 22.0 kg/m2.
Blood pressure: 111/57 mm Hg
Resting heart rate: 65 beats per minute.
Resting ECG
The resting ECG showed a sinus rhythm of 90 bpm, normal heart axis, normal conduction times and a normal repolarisation.
Spirometry
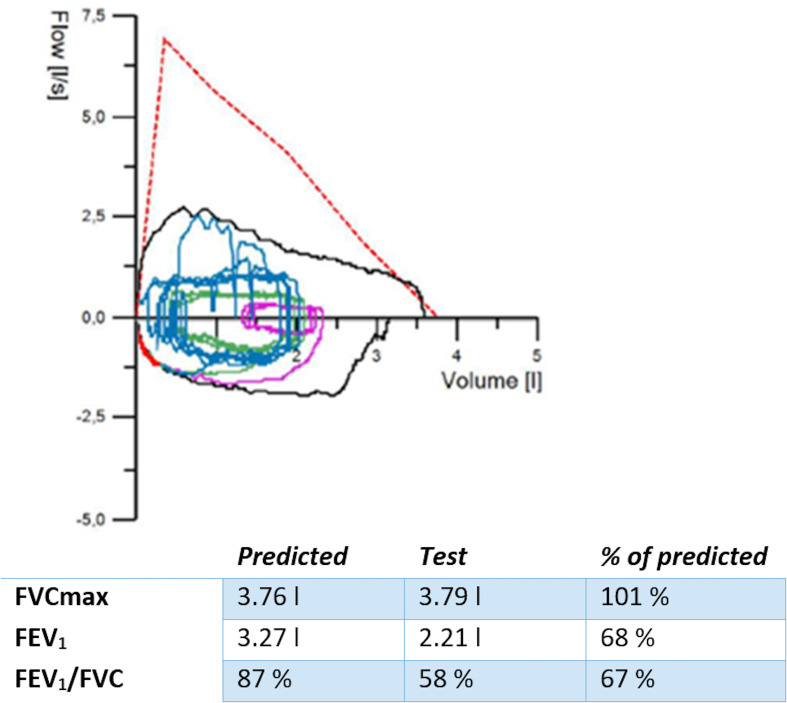
Spirometry (figure 1) showed low amplitude during inhalation and exhalation. The inspiratory and expiratory curves are both flattened. This might be caused by bad technique, poor effort or due to a fixed airway obstruction. There were no signs of restrictive lung disease as the forced vital capacity was normal.
Figure 1.
Spirometry. Y axis: flow (L/s). The negative values, lower part of the curve, represent inspiration. The positive values, upper part of the curve, represent expiration. X axis: ventilation volume in litres. The ratio of FEV1/FVC should be >70%. The flow curve is flattened during inspiration. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
The CPET
The CPET cycling test consisted of several phases. First, a 3-min resting phase was performed to measure resting metabolism parameters. Next, the exercise phase was performed. This phase consisted of a 3- min unloaded ‘warm-up’ period. This was followed by a RAMP protocol of 12 Watt per minute until maximum performance was reached. The exercise phase contributed to 14:34 min, followed by a recovery phase of 5 min. After analysis, the test could be considered a maximum performance test based on hypercapnia, a decrease in the partial arterial pressure of O2 (PaO2) and saturation during exercise. The measured oxygen consumption (VO2) peak was 32.9 mL/min/kg. This was 66% of the predicted value, corresponding with a very poor exercise tolerance.1 There was an inspiratory stridor during exercise.
Cardiovascular plots and electrocardiogram
Exercise electrocardiogram
The ECG during exercise showed no signs of myocardial ischaemia, arrhythmias or premature atrial or ventricular contractions. The maximum heart rate was relatively low at 173 bpm (32 bpm lower than the estimated heart rate of 220 − age).
Cardiovascular plots
All cardiovascular plots were normal, with a relatively low maximum heart rate.
Ventilatory plots
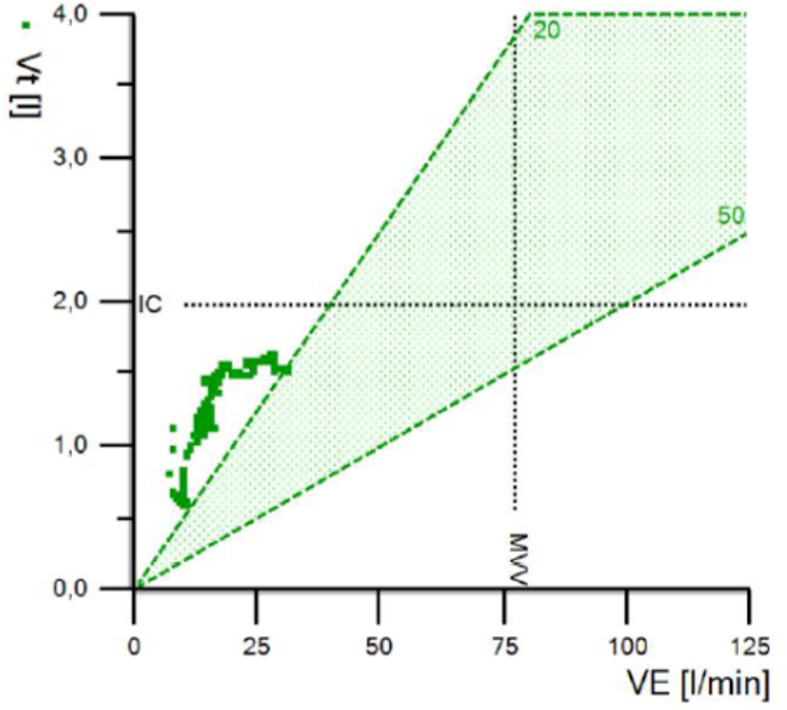
The ventilation rose to 31 L/min during exercise. The breathing reserve was very high (65%). This was calculated as the maximal voluntary ventilation minus the measured minute ventilation (figure 2). A high breathing reserve might be due to submaximal effort or hypoventilation.
Figure 2.
VE (L/min) on the Y axis in relation to the Vt (L) on the X axis. MVV (=40×FEV1). Nb: note that current software calculates MVV by 35×FEV1. The figure shows that tidal volume increases as the exercise phase progresses. however, ventilation only reaches 31 L/min. MVV, maximum voluntary ventilation; VE, minute ventilation; Vt, tidal volume.
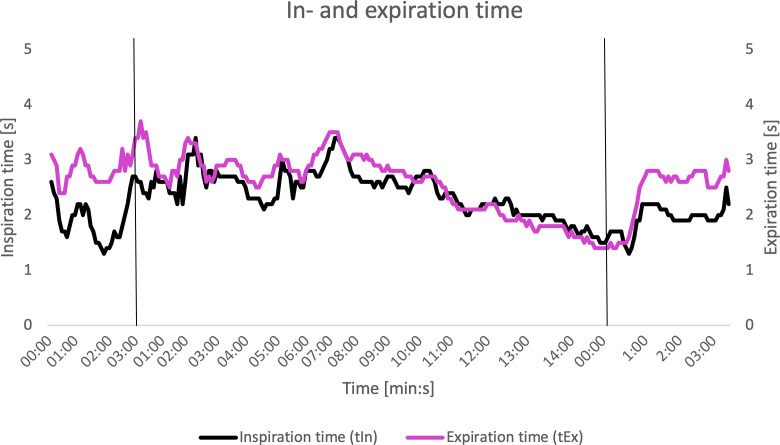
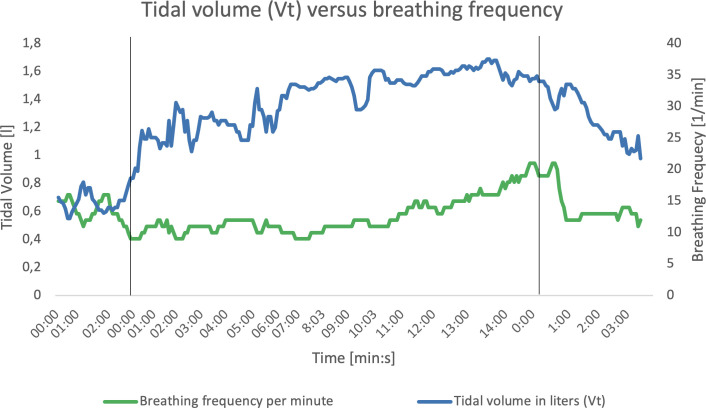
Inspiration and expiration time showed remarkable results (figure 3). During the resting phase, the inspiration and expiration time should have a relation of 1:2 or less. During maximum exertion the ratio should approach a ratio of 1:1. In this case, the inspiration time was already equal to the expiration time in the beginning of the exercise phase and so on. The delay in inspiration time suggests that flow during inspiration is affected. Also, the breathing frequency at maximal exertion was only around 20 breaths per minute (figure 4), where normally the breathing frequency may rise up to 55 per minute.2
Figure 3.
Relation of inspiration time and expiration time during test. The vertical black lines represent the beginning of the next phase. First: from rest to exercise phase. Second: from exercise phase to recovery phase. X axis: time progression (min:s). Y axis: inspiration and expiration (s).
Figure 4.
Relation of breathing frequency and tidal volume during test. The vertical black lines represent the beginning of the next phase. First: from rest to exercise phase. Second: from exercise phase to recovery phase. X axis: time progression in minutes. Y axis: tidal volume on the left side in litres, breathing frequency per minute on the right side.
Conclusion: very low ventilation with an inadequate breathing frequency and a paradoxical relation between inspiration time and expiration time during (sub)maximal exercise.
Gas exchange plots
There were no signs of significant dynamic hyperinflation during this test.
Blood gas analysis
Arterial blood for blood gas analysis was drawn during the resting phase and in the recovery phase, directly after maximum exertion (table 1). The Alveolar–arterial gradient (A–a gradient) is the measured difference in oxygen concentration between the alveoli and arterial blood. This measurement reflects the function and integrity of the alveolar–capillary membrane. In this case, a normal A–a gradient was seen during resting phase and recovery phase, meaning that there is no alveolar–capillary membrane dysfunction.
Table 1.
Blood gas analysis
| Time (s) |
pH | PaCO2 (kPa) |
PaO2 (kPa) |
Sat (%) |
BE (meq/L) |
Lactate (mmol/L) | A–a gradient | |
| Resting phase | 0:29 | 7.38 | 5.55 | 12.5 | 97 | −0.3 | 0.9 | 1.0 |
| Recovery phase | 0:27 | 7.20 | 8.11 | 10.3 | 92 | −5.5 | 4.1 | 0.5 |
A–a gradient, difference between alveolar concentration of oxygen and the arterial concentration of oxygen; BE, base excess; PaCO2, partial arterial pressure of CO2; PaO2, partial arterial pressure of O2; Sat, arterial saturation.
In the resting phase, the blood gas analysis was normal. In the recovery phase, there was a desaturation to 92% (arterial oxygen saturation, SaO2) with a fall in PaO2, a respiratory acidosis of 7.2 (pH) with severe hypercapnia of 8.11 kPa (partial arterial pressure of CO2, PaCO2) and a base excess of −5.5 meq/L, with a lactic acid production of 4.1 mmol/L at this point. The respiratory acidosis with a relatively low lactate production indicated hypoventilation.
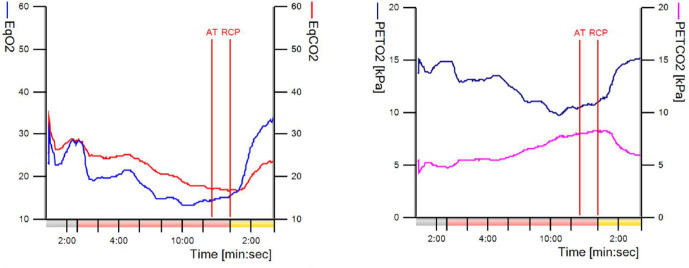
The anaerobic threshold (AT) was at 62% of the predicted VO2max and considered normal. The respiratory compensation point was not reached during the exercise phase as the EqCO2, the respiratory exchange ratio and the ventilation/ventilated CO2 slope showed no rise during exercise. Also, the PETCO2 keeps increasing during exercise up until the end of the test. There was no decrease in the exercise phase (figure 5). A decrease in PETCO2 was seen in the recovery phase. The O2 and CO2 equivalents (VE/VO2 and VE/VCO2) are very low at the AT, which indicate a very efficient breathing technique. The VE/VCO2 slope was 13.5 which is low. There are no signs of dead space ventilation.
Figure 5.
Ventilation versus blood gas analysis. The point where, aerobic energy production is supplemented by anaerobic mechanisms and lactate + H+ starts to increase. Buffering of H+ with lactate is possible due to the bicarbonate buffering system (Co2+ H2O ↔ H2CO3 ↔ HCO3− + H+), which leads to an increase of CO2. The rise in CO2 causes an increase in ventilation. CO2 then diffuses from circulation to alveoli and is exhaled. The point where respiratory compensation for metabolic acidosis becomes inadequate. From this point on H+ ions which accumulate from anaerobic mechanisms cannot be buffered entirely, which leads to acidosis. This causes a strong ventilatory stimulus, which can be seen as an increase in VE/VCO2 slope, a decline in the PETCO2 curve and an increase of EqCO2 curve. AT, anaerobic threshold; EqCO2, equivalent of CO2=VE/VCO2; EqO2, equivalent of O2=VE/VO2; PETCO2, partial and tidal pressure of CO2; PETO2, partial end tidal pressure of O2; RCP, respiratory compensation point; VCO2, carbon dioxide production; VE, minute ventilation.
Conclusion: ventilation-limited exercise test based on hypercapnia and hypoxaemia (at a normal A–a gradient) with a low VO2 peak value indicating alveolar hypoventilation. This suggests a mechanical obstruction in the upper airway. There is no suspicion of cardiac pathology
Differential diagnosis
Most likely the diagnosis was a fixed upper air way obstruction based on the flattened inspiratory and expiratory flow loops during spirometry (figure 1).3 This can primarily be a dysfunction of the trachea or subglottic region due to mechanical obstruction, which can have a (post-)traumatic, inflammatory, malignant or autoimmune origin. There was no history of (recent) intubation, mechanical ventilation or blunt trauma before onset of the complaints. Family history was negative for auto-immune diseases.
Treatment
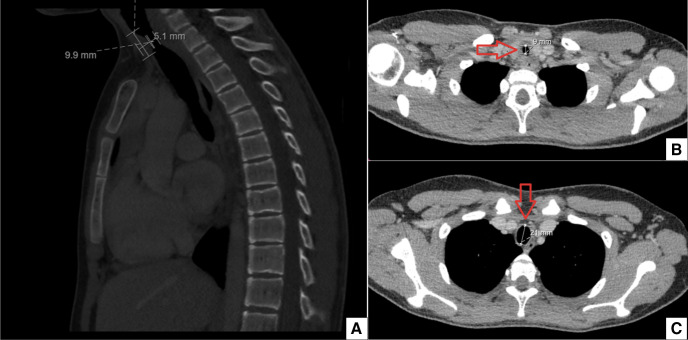
The patient was referred to a pulmonologist. An additional CT scan with intravenous contrast showed a circular tracheal stenosis of unknown origin. No surrounding tumour or lymphadenopathy was seen (figure 6). A bronchoscopy was performed which showed a significant stenosis, mass or web 2–3 cm distal to the vocal cords (figure 7). Further examination and treatment was performed in an Academic Centre, including a team of an otorhinolaryngologist and a paediatric rheumatologist. Physical examination of the head/neck region, skin and joints showed no abnormalities. A biopsy was taken during bronchoscopy with subsequently subglottic dilatation to 12 mm diameter. Pathological examination showed a sclerotic stroma with non-specific mildly active chronic inflammatory tissue with reactive changes. No malignancy was seen. Blood samples showed an ANA positive and ANCA negative result. Another ANCA test was performed with an added urine analysis to further exclude autoimmune aetiology. Both were negative. There were insufficient clincal arguments to diagnose granulomatosis with polyangiitis (GPA) or other autoimmune diseases at this point. The subglottic dilatation was repeated after 2 months. The patient’s symptoms improved significantly. Sports participation was possible again. The patient was advised to report future symptoms for further follow-up.
Figure 6.
(A) CT thorax saggittal plane showing the narrowed trachea. (B) Transversal plane showing the narrowed trachea of 9 mm. (C) Transversal plane showing normal diameter of the trachea of 21 mm.
Figure 7.
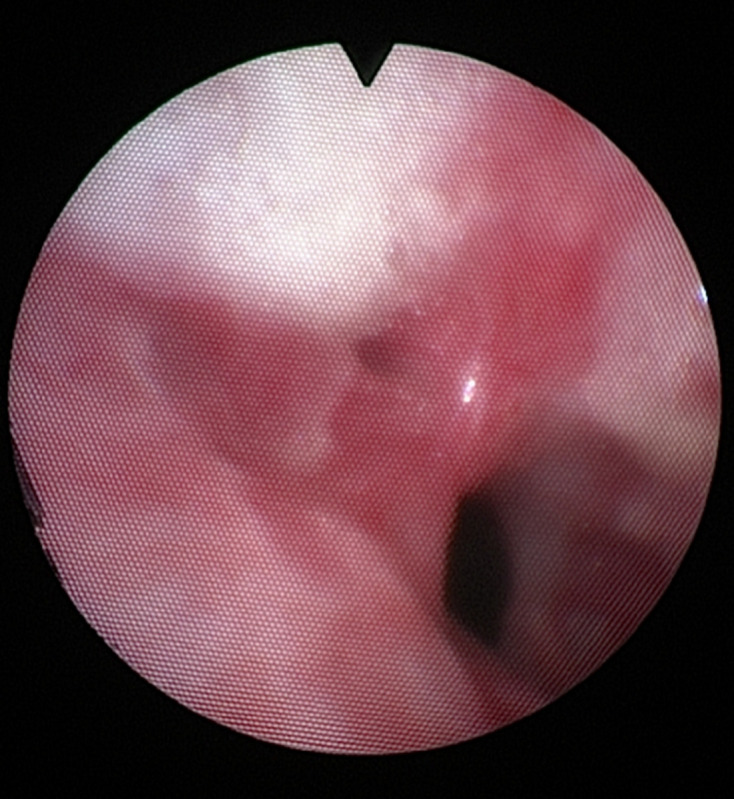
Subglottic stenosis, mass or web 2–3 cm under the vocal cords.
Outcome and follow-up
Despite extensive examination, the cause of the subglottic stenosis is still unknown. It was classified as idiopathic subglottic stenosis. Another dilatation followed after 1-year follow-up. Nowadays, the patient can exercise freely without any complaints. Further follow-up is only indicated when complaints recur.
Discussion
We found no cases in literature which measured the exercise-related consequences of a chronic subglottic obstruction in adolescents. No guidelines were found concerning idiopathic subglottic stenosis.
Idiopathic subglottic stenosis mostly affects adult female Caucasian patients and presents itself at middle-age. In these cases, there seems to be an association with gastro-oesophageal reflux disorder.4–6 Therefore, this case of a 15-year-old male patient seems very rare. However, an autoimmune origin of the subglottic stenosis can never be fully excluded. It is possible that future complaints occur. In retrospect, these may be matching to an autoimmune or systemic disease such as GPA, sarcoidosis and relapsing polychondritis.
Stenosis caused by GPA is reported at 10%–23%. The median age of patients with the diagnosis of subglottic stenosis and GPA is 26 years old. Symptoms were more frequent in patients before 20 years of age. Half of these patients had no active disease at presentation.6 Subglottic stenosis caused by sarcoidosis is only described in a few cases. Sarcoidosis mostly affects lobar, segmental and subsegmental airways and seems less likely.6
The future may reveal the true origin of the subglottic stenosis in this case.
Nevertheless, the consequences of the subglottic stenosis during exercise are severe. Usually during exercise, ventilation increases to stabilise PaCO2. However, due to a mechanical obstruction, increase in ventilation is simply not possible. Therefore, adequate wash-out of CO2 could not be effectuated, causing a rise in PaCO2 which triggers the breathing stimulus. One can imagine that this causes subjective dyspnoea in the patient. In retrospect, the diagnosis could have been made earlier based on the abnormal spirometry. The low amplitude during inspiration and expiration are typical findings in fixed upper airway obstruction.
Patient’s perspective.
The treatment delay was a bit annoying, especially because my complaints took a while and got worse over time. I wanted to exercise more than I could at that point. After the first dilatation, my complaints vanished entirely. For me, this was the most important. I’m glad that my case is published in a medical journal. In this way, students and doctors can learn from my personal story.
Learning points.
Listen: The progressive complaints and the inspiratory stridor narrow the differential diagnosis.
Spirometry test: do not assume ‘bad technique’ too soon during spirometry. Consistent flattening of the inspiration and expiration curve could indicate fixed upper airway obstruction.
Exercise test: low exercise tolerance can be caused by a ventilation-limited exercise test due to an upper airway obstruction.
Idiopathic subglottic stenosis can present itself as a primary ventilatory limited test without signs of a deficient alveolar–capillary membrane.
Footnotes
Contributors: RV and HG conceived of the presented case. HG was the primary treating physician. RV wrote the full text case report including figures. RV was responsible for communication between caretakers and the patient including his family. RV helped in retrieval of informed consent, reconstructing raw data of the cardiopulmonary exercise testing to figures, retrieval of follow-up information in the academic setting, retrieval of patients perspective and data collection and interpretation. SvB, TB and HG helped supervise the project, made critical review of the full text report including figures. All authors discussed the results and contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Cooper DM, Weiler-Ravell D, Whipp BJ, et al. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol Respir Environ Exerc Physiol 1984;56:628–34. 10.1152/jappl.1984.56.3.628 [DOI] [PubMed] [Google Scholar]
- 2.Wasserman K. Principles of exercise testing and interpretation. 5th edn. Philadelphia: Wolters Kluwer, 2012: 571. [Google Scholar]
- 3.Cotes JE, Maynard RL, Pearce SJ. Lung function. 7th ed. Willey-Blackwell, 2020: 808p. [Google Scholar]
- 4.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope 2016;126:1390–6. 10.1002/lary.25708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarnæs MT, Sandvik L, Brøndbo K. Idiopathic subglottic stenosis: an epidemiological single-center study. Eur Arch Otorhinolaryngol 2017;274:2225–8. 10.1007/s00405-017-4512-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravena C, Almeida FA, Mukhopadhyay S, et al. Idiopathic subglottic stenosis: a review. J Thorac Dis 2020;12:1100–11. 10.21037/jtd.2019.11.43 [DOI] [PMC free article] [PubMed] [Google Scholar]