ABSTRACT
There is growing evidence that supplementation with carnosine, or its rate-limiting precursor β-alanine, can ameliorate aspects of metabolic dysregulation that occur in diabetes and its related conditions. The purpose of this systematic review and meta-analysis was to evaluate the effect of carnosine or β-alanine supplementation on markers of glycemic control and insulin resistance in humans and animals. We performed a systematic search of 6 electronic databases up to 31 December 2020. Primary outcomes were changes in 1) fasting glucose, 2) glycated hemoglobin (HbA1c), and 3) 2-h glucose following a glucose-tolerance test. A set of additional outcomes included fasting insulin and homeostatic model assessment of β-cell function (HOMA-β) and insulin resistance (HOMA-IR). We assessed risk of bias using the Cochrane risk of bias (RoB) 2.0 (human studies) and the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) RoB (animal studies) tools; and used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess certainty. We used Bayesian hierarchical random-effects models, with informative priors for human data and noninformative priors for animal data. Inferences were made on posterior samples generated by Hamiltonian Markov Chain Monte Carlo using 90% credible intervals (90% CrI) and calculated probabilities. Twenty studies (n = 4 human, n = 16 rodent) were included, providing data for 2 primary outcomes (fasting glucose and HbA1c) and 3 additional outcomes (fasting insulin, HOMA-β, and HOMA-IR). The model provides evidence that supplementation decreases fasting glucose [humans: mean difference (MD)0.5 = –0.95 mmol · L–1 (90% CrI: –2.1, 0.08); rodent: MD0.5 = –2.26 mmol · L–1 (90% CrI: –4.03, –0.44)], HbA1c [humans: MD0.5 = –0.91% (90% CrI: –1.46, –0.39); rodents: MD0.5 = –1.05% (90% CrI: –1.64, –0.52)], HOMA-IR [humans: standardized mean difference (SMD)0.5 = –0.41 (90% CrI: –0.82, –0.07); rodents: SMD0.5 = –0.63 (90% CrI: –1.98, 0.65)], and fasting insulin [humans: SMD0.5 = –0.41 (90% CrI: –0.77, –0.07)]. GRADE assessment showed our certainty in the effect estimate of each outcome to be moderate (human outcomes) or very low (rodent outcomes). Supplementation with carnosine or β-alanine may reduce fasting glucose, HbA1c, and HOMA-IR in humans and rodents, and fasting insulin in humans; both compounds show potential as therapeutics to improve glycemic control and insulin resistance. This review was registered at PROSPERO as CRD42020191588.
Keywords: endocrinology, histidine, metabolic health, metabolism, nutrition, obesity
Statement of Significance: This study includes all available human and animal data to provide the most comprehensive assessment to date of the effects of carnosine and β-alanine supplementation on glycemic control and insulin resistance.
Introduction
Diabetes is a major public health problem; worldwide estimates show that 463 million people were living with diabetes in 2019—equivalent to 9.3% of the global population (1). Type 2 diabetes accounts for >90% of these cases, with the remaining made up of type 1 diabetes, gestational diabetes, and rarer types of diabetes (e.g., maturity-onset diabetes of the young). A hallmark of type 2 diabetes is poor glycemic control and insulin resistance (2), which present earlier in life as impaired fasting glucose or impaired glucose tolerance (also known as prediabetes). This represents a high-risk state that requires intervention, as a 45-y-old with prediabetes has a 74% lifetime risk of progression to type 2 diabetes (3). While lifestyle modifications are central to risk reduction, they can be challenging to implement, and long-term adherence limits their effectiveness (4). It is therefore essential to develop low-cost, novel therapies to improve glycemic control and help prevent or delay disease progression.
The multifunctional dipeptide carnosine is an emerging therapeutic that has the potential to contribute to the treatment or management of various chronic diseases (5). Carnosine is a member of the histidine-containing dipeptide (HCD) family and exists naturally in high concentrations in skeletal muscle, with smaller amounts in other excitable tissues (6–9). Dietary sources include meat, poultry, fish, and prawns (10); but the most efficient way to increase tissue stores is by supplementing with carnosine or its rate-limiting precursor β-alanine (11). Work from our research group shows that treatment with carnosine recovers glucolipotoxic inhibition of insulin-stimulated glucose uptake in skeletal muscle cells and decreases highly toxic lipid peroxidation products in pancreatic β-cells, leading to an increase in insulin secretion (12). Further evidence supports the role of carnosine in nonenzymatic detoxification of reactive aldehydes (13, 14), an effect that β-alanine supplementation potentiates in humans (15, 16). This has important clinical implications, as reactive aldehydes have been implicated in the etiology of diabetes (17). Collectively, this suggests that carnosine may be able to ameliorate aspects of the metabolic dysregulation that occurs in diabetes and its related conditions.
There is growing evidence from rodent studies that carnosine supplementation can prevent or delay the development of type 2 diabetes (18, 19). Initial human trials also show promise (20, 21), but there is currently no consensus on whether carnosine or β-alanine can be used to treat or manage diabetes. A recent meta-analysis of human studies sought to address this knowledge gap and concluded that supplementation with HCDs improved waist circumference, fasting glucose, and glycated hemoglobin (HbA1c) (22). The review, however, had several methodological shortcomings [for a commentary, see (23)], which included combining effects from studies using multi-ingredient supplements with those supplementing carnosine or β-alanine alone. They also included studies supplementing histidine in isolation, which is not a member of the HCD family, and is not rate limiting for carnosine synthesis in humans—at least under standard dietary conditions (24–27). This approach cannot explain whether the beneficial effects are due to carnosine, β-alanine, HCDs, or another supplement ingredient. Another meta-analysis on the topic also had methodological issues (28), which included an incomplete and inconsistent risk of bias assessment and the inclusion of the same study sample as 2 separate studies. It is also important to consider outcomes from animal studies, which can provide mechanistic insight and inform future human trials. Therefore, the purpose of this systematic review and meta-analysis was to evaluate the effect of carnosine or β-alanine supplementation on markers of glycemic control and insulin resistance in humans and animals.
Methods
The methods for this study were published in full as a protocol paper (29) and preregistered on PROSPERO (CRD42020191588). Our reporting follows the updated 2020 Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines (30).
Eligibility criteria
Table 1 outlines the eligibility criteria. The primary outcomes were changes in fasting glucose (includes plasma, serum, and blood glucose values), HbA1c, and 2-h glucose following a glucose-tolerance test (GTT). These outcomes represent the 3 clinical markers used in the diagnosis of type 1 diabetes, type 2 diabetes, prediabetes, and gestational diabetes (31, 32). A set of additional outcomes included changes in other markers of glycemic control and insulin resistance (Table 1). There were no restrictions on the timing or duration of supplementation, or on the study setting. We included English and non–English-language sources with the latter translated into English using freely available online tools (i.e., Google Translate).
TABLE 1.
Overview of PICOS eligibility criteria1
| Criteria | |
|---|---|
| Participants | Humans with type 1 diabetes, type 2 diabetes, prediabetes, gestational diabetes, impaired fasting glucose, or impaired glucose tolerance [according to WHO guidelines (31, 32)], or with overweight/obesity (BMI ≥ 25 kg/m2) where the relevant outcomes were collected and reported |
| Animal studies using a diabetes-related disease model (see human criteria), or overweight/obese animals where the relevant outcomes were reported | |
| No restrictions were applied on age or comorbidities, or on the methods used to induce disease in animal studies | |
| Intervention | Supplementation with carnosine or β-alanine. We excluded studies that used a multi-ingredient supplement intervention |
| Human studies included oral administration only, whereas animal studies also included administration by other means (e.g., intraperitoneal or intravenous injection) | |
| Comparator | Comparisons for human studies were between placebo and the experimental intervention |
| Comparisons for animal studies were between placebo or control (no intervention) and the experimental intervention | |
| We excluded studies without a control or placebo group | |
| Outcomes | Outcomes relating to glycemic control and insulin resistance: fasting glucose, HbA1c, 2-h glucose following a GTT, fasting insulin, C-peptide, homeostatic model assessment (HOMA) parameters (e.g., HOMA-IR, HOMA-β, HOMA-S) |
| Study designs | Studies were limited to nonrandomized and RCTs, including cluster RCTs. We excluded cohort studies, cross-sectional studies, case series, case reports, commentary, and review articles |
GGT, glucose-tolerance test; HbA1c, glycated hemoglobin; HOMA-β, homeostatic model assessment of β-cell function; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-S, homeostatic model assessment of insulin sensitivity; PICOS, Participant, Intervention, Comparator, Outcomes, Study designs; RCT, randomized controlled trial.
Information sources
We searched 6 electronic databases for potentially eligible studies—PubMed, Scopus, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and ProQuest—from the earliest record in each database up to 31 December 2020. This was supplemented by searching for trial protocols, reference lists and citation tracking of included studies, and relevant reviews. The authors also searched their personal files to identify any additional relevant material.
Search strategy and selection process
Search strategies were developed using key text words and medical subject headings (MeSH) related to the population, intervention, and outcomes. An academic librarian, not otherwise associated with the project, reviewed all searches using the Peer Review of Electronic Search Strategies (PRESS) checklist (33). The full search strategy for each database and the completed PRESS report are available in the Supplemental Methods. Two reviewers independently completed the initial searches (JJM and KJE-S), data extraction (JJM and ED), and assessment of risk of bias (JJM and ED); and 3 reviewers independently completed full-text screening (JJM, LS, and GGA). Disagreements for searches, data extraction, and risk of bias were referred to a third reviewer (CS) who provided a recommendation.
Titles and abstracts of articles from the initial searches were imported into a systematic review management platform (Covidence; Veritas Health Innovation Ltd.); duplicates were removed and remaining articles screened for potential eligibility. We obtained full texts for all articles that appeared to meet the inclusion criteria or where there was any uncertainty; multiple reports of the same study were handled by including the article that provided the most relevant outcome data. Reviewers used the reference manager functions to highlight eligibility criteria and add comments on each article to cross-reference decisions in the event of a disagreement. We contacted study authors to resolve issues regarding eligibility—for example, to clarify methods or obtain necessary data (maximum of 3 e-mail attempts). Reviewers were not blinded to journal titles or the study authors.
Data-collection process and items
We extracted data using a standardized spreadsheet based upon the Cochrane data collection form for intervention reviews (34). Data items included the following: 1) study characteristics (location, setting, study design, size, duration, funding sources, and study aim), 2) human participant characteristics (age, height, sex, body mass, BMI, body fat %, type and duration of condition, activity and exercise levels, and dietary information), 3) animal characteristics (age, body mass, source, species, strain, sex, genetic modification status, type and duration of condition, method used to induce disease, and housing conditions), 4) intervention characteristics (name, type of control used, dosage, frequency, duration, route of administration), 5) outcome characteristics (type of measure; sample sizes; baseline, interim, and postintervention measures of central tendency and dispersion; adherence to the intervention; dropouts; number and nature of side effects; and assessment of blinding to the intervention), and 6) information relevant to risk of bias and certainty assessment. We converted glucose values to millimoles per liter using a standard equation [mmol · L–1 = mg · dL–1 × 0.0555; (35)]. We converted all supplement doses to relative cumulative intake [mg · kg body weight (bw)–1]. Some animal studies reported the treatment dose as grams per liter dissolved in drinking water, which we multiplied by reported or normative drinking volumes, before converting to an estimate of relative cumulate intake. Where necessary, we extracted measures of central tendency and dispersion from figures using WebPlotDigitizer version 3.10 (https://apps.automeris.io/wpd/) or contacted study authors for additional data (maximum of 3 e-mail attempts). We converted SE to SD using a standard equation (SD = SE × √n).
Risk of bias assessment
Study risk of bias
We assessed risk of bias in human studies using the Cochrane risk of bias 2.0 tool (RoB 2) per protocol for parallel-group randomized trials (36), and in animal studies using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool (37). Reviewers assessed each study item as either “high risk,” “low risk,” “some concerns” (RoB 2), or “unclear risk” (SYRCLE) of bias. For human studies, we performed a summary judgment for overall risk of bias based upon RoB 2 recommendations.
Reporting bias
For human studies, we screened clinical trial registers to compare outcomes reported in the protocol with each published report. For animal studies, or where there was no preregistration or protocol, we compared the outcomes reported in the methods with the results section of each study. Small study bias, including publication bias, was explored by visually inspecting funnel plots and, where substantive asymmetry was present, conducting a multilevel extension of Egger's regression test (38).
Effect measures
We extracted and analyzed all outcomes as continuous measures. Mean difference (MD) effect sizes (not standardized) were calculated for the primary outcomes: modeling outcomes on the same absolute scale as the original measurement provides more clinically interpretable results. We also identified minimal important difference thresholds (fasting glucose: 1 mmol · L–1 reduction; HbA1c: 0.5% reduction) (39) and calculated the probability that the pooled effect size met or exceeded threshold values (see Data synthesis section). For additional outcomes, MD effect size estimates were standardized (SMD) using reported SDs to account for differences in measurement scales. We used standard threshold values of 0.2, 0.5, and 0.8 to describe effect size estimates as small, medium, and large (40), with values between 0 and 0.2 described as trivial. Data collected from human studies included both baseline and postintervention values and effect sizes were calculated with both sets of information (41), whereas effect sizes were calculated from postintervention values only in animal studies (42).
Data synthesis
Meta-analyses were conducted within a Bayesian framework, providing a flexible modeling approach to account for uncertainty in model parameters and underlying structures within the data. Bayesian models enable intuitive interpretation of results through reporting subjective probabilities rather than null hypothesis tests or frequentist confidence intervals (43). We planned to conduct 3-level Bayesian hierarchical models with noninformative priors for the between-study variance parameters and adhered to this for analyses of animal data. Due to limitations in the number of human studies and effect sizes, a deviation from the original protocol (29) was required. Instead, standard (2-level) Bayesian random-effects models were conducted, and an informative log-t distribution used as a prior for the between-study heterogeneity variance using a predictive distribution provided for biological markers in pharmacologic versus placebo or control studies (44). Informative priors were used in the human data to estimate the within-study variances and account for unknown correlations between baseline and postintervention values. This was achieved by assuming a uniform prior for each within-study variance based upon a correlation ranging from 0.5 to 0.9. Inferences from all analyses were performed on posterior samples generated by Hamiltonian Markov chain Monte Carlo simulations, and through use of the median value (0.5-quantile) and 90% credible intervals (90% CrI) and calculated probabilities.
Sensitivity and subgroup analyses were performed to examine the robustness of the main model results. Preplanned analyses included removing studies at high risk of bias, as well as meta-regressions to explore the effect of type of supplementation (carnosine or β-alanine), duration of supplementation, and the disease type. Following data extraction, additional sensitivity analyses were performed for clear outliers and for studies where the outcomes of interest were not elevated at baseline (human studies) or in the control group (animal studies). Due to heterogeneity across animal studies, meta-regressions for the dose-response of relative cumulative intake were also performed. Analyses were performed using R2OpenBUGS (45) and the R wrapper package brms interfaced with Stan to perform sampling (46).
Certainty assessment
The certainty of each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (47), across 5 domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Outcomes from human trials began with a high-quality rating, based upon their randomized controlled trial (RCT) design (as indicated by the eligibility criteria). This initial rating was subsequently maintained, or downgraded, based upon performance in each of the 5 domains, resulting in an overall rating of high, moderate, low, or very low for each outcome (47). Inconsistency was graded on visual inspection of effect size estimates, whether credible intervals overlapped, and between-study variability [τ (tau)]; these factors were considered within the context of the outcome values at baseline and the cumulative supplement dose, which could plausibly explain inconsistency (48). Human studies were not downgraded for indirectness, as our eligibility criteria narrowly selected for the population, intervention, and outcomes of interest; further, our primary outcomes are indirect by nature and used in clinical decision making. Animal studies were automatically graded down 1 level for indirectness, unless conducted in nonhuman primates (49). Imprecision was graded based on the pooled sample size and the width of the credible intervals: the interval crossed the null and simultaneously included large clinical benefit or harm (serious), or the interval included both a large clinical benefit and harm (very serious) (48, 49). Publication bias was graded as either detected or undetected (see “Outcome reporting bias”).
Results
Study selection and characteristics
Figure 1 depicts the search and selection process. Twenty studies were included in the data synthesis—7 studies in mice (n = 132 mice), 9 studies in rats (n = 159 rats), and 4 human studies (n = 172 participants)—providing data for 2 primary outcomes (fasting glucose and HbA1c) and 3 additional outcomes [fasting insulin, HOMA-IR, and homeostatic model assessment for steady-state β-cell function (HOMA-β)]. As all included animal studies were conducted in mice or rats, the term rodent(s) is used herein instead of the nonspecific term animal(s). Tables 2 and 3 summarize the characteristics of included human and rodent studies. Human populations included adults with type 2 diabetes (21, 50), children with type 1 diabetes (51), and nondiabetic adults with overweight or obesity (a subgroup exhibited impaired glucose tolerance) (20). Rodent disease models included 1) genetic modifications to develop obesity, hyperglycemia, and insulin resistance (18, 19, 52, 53); 2) dietary interventions to develop obesity, hyperglycemia or hyperinsulinemia, and insulin resistance (54–56); and 3) single or multiple streptozotocin injection(s) to induce pancreatic β-cell death, leading to hyperglycemia (57–65).
FIGURE 1.
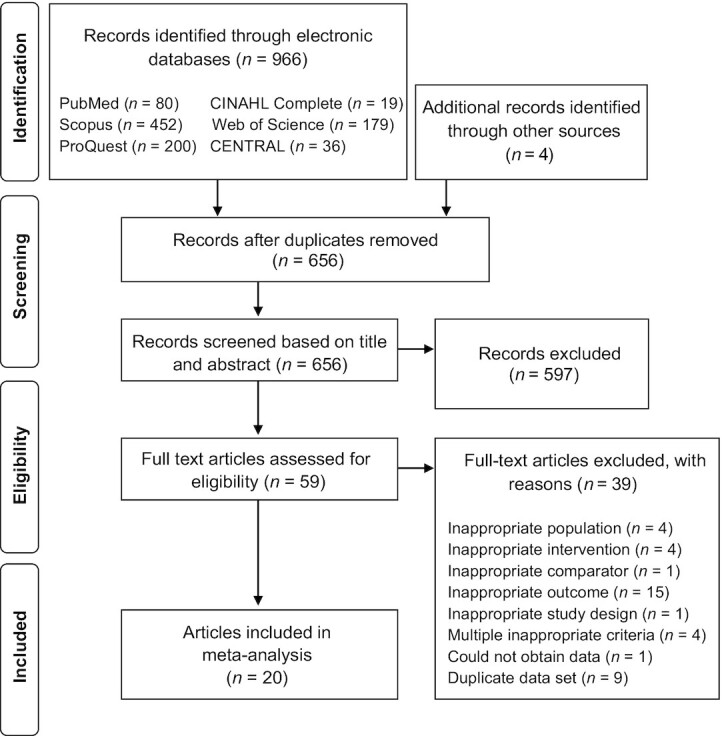
PRISMA flow diagram depicting the search and selection process. PRISMA, Preferred Reporting Items for Systematic review and Meta-Analysis.
TABLE 2.
Characteristics and outcomes for included human studies1
| Study (reference), country | Design and setting | Population | Participant characteristics (F/M, n/n) | Intervention | Extracted outcomes |
|---|---|---|---|---|---|
| de Courten et al. (20), Slovakia | RCT, research institute | n = 30 Nondiabetic, sedentary individuals with overweight or obesity | Int: n = 15 (3/12) | Int: carnosineCon: placebo (sucrose) 2 g · d−1 for 12 wk (2 × 1-g doses) | FG (mmol · L−1) |
| Age: 42 ± 7 y | FI (mU · L−1) | ||||
| BMI (kg/m2): 31.1 ± 4.6 | HbA1c (%) | ||||
| Con: n = 11 (4/7) | HOMA-β (%) | ||||
| Age: 43 ± 10 y | HOMA-IR | ||||
| BMI: 31.6 ± 3.7 | |||||
| Elbarbary et al. (51), Egypt | RCT, hospital: pediatric diabetes clinic | n = 90 Patients with type 1 diabetes (≥5 y duration), active diabetic nephropathy | Int: n = 45 (25/20) Age: 12.4 ± 3.4 yCon: n = 45 (22/23) Age: 13.3 ± 2.8 y | Int: carnosineCon: placebo1 g · d−1 for 12 wk (2 × 500-mg doses) | FG (mg · dL−1)HbA1c (%) |
| Houjeghani et al. (21), Iran | RCT, hospital | n = 54 Patients with type 2 diabetes, not receiving exogenous insulin | Int: n = 22 [13/10 (sic)] | Int: carnosine | FG (mg/dL) |
| Age: 43 ± 7.6 y | Con: placebo (cellulose) | FI (μIU · mL−1) | |||
| BMI: 29.1 ± 5.3 | 1 g · d−1 for 12 wk (2 × 500-mg doses) | HbA1c (%) | |||
| Con: n = 22 [9/12 (sic)] | HOMA-β (%) | ||||
| Age: 40.4 ± 5.1 y | HOMA-IR | ||||
| BMI: 28.3 ± 4.6 | |||||
| Nealon et al. (50), Australia | RCT, university | n = 12 Patients with type 2 diabetes, not receiving exogenous insulin | Int: n = 7 (2/5) | Int: β-alanine | FG (mmol · L−1) |
| Age: 62 ± 4.6 y | Con: placebo (maltodextrin) | FI (mU · L−1) | |||
| BMI: 30.9 ± 2.5 | 4 g · d−1 for 4 wk (3 × 1334-mg doses) | HOMA-β (%) | |||
| Con: n = 5 (1/4) | HOMA-IR | ||||
| Age: 66 ± 6.4 y | |||||
| BMI: 35.2 ± 8.5 |
Con, control group; FG, fasting glucose; FI, fasting insulin; HbA1c, glycated hemoglobin, HOMA-β, homeostatic model assessment for steady-state β-cell function; HOMA-IR, homeostatic model assessment for insulin resistance; Int, intervention group; RCT, randomized controlled trial.
TABLE 3.
Characteristics and outcomes for included animal studies1
| Study (reference), country | Population | Disease model and method | Intervention | Included outcomes |
|---|---|---|---|---|
| Albrecht et al. (18), Germany | Male BTBR ob/ob mice | Genetic modification to develop obesity, hyperglycemia, and insulin resistanceTreatment started at 6 wk old | Duration: 18 wk | FG (mg · dL−1) |
| 6 wk old | Int: carnosine, oral, dissolved in drinking water: | FI (ng/mL) | ||
| n = 15 per group | 1) 45 mg · kg bw−1 . d−1 | HbA1c (%) | ||
| Con: no intervention | ||||
| Aldini et al. (52), Italy | Male Zucker obese fa/fa rats | Genetic modification to develop obesity, hyperglycemia, and insulin resistanceTreatment started at 6 wk old | Duration: 24 wk | FG (mmol · L−1) |
| 5 wk old | Int: l-carnosine, oral, dissolved in drinking water: | FI (pmol · L−1) | ||
| n = 6 per group | 1) 30 mg · kg bw−1 . d−1 | HOMA-IR | ||
| Con: no intervention | ||||
| Al-Sawalha et al. (54), Jordan | Male Wistar rats | Dietary intervention to develop metabolic syndromeHFHC diet (sucrose, margarine, etc.) and 20% sucrose added to drinking waterTreatment started alongside diet | Duration: 16 wk | FG (mg · dL−1) |
| Young adults | Int: carnosine, injection: | FI (pg/mL) | ||
| n = 10 per group | 1) 250 mg · kg bw−1 × 5 per week | |||
| Con: vehicle only, injection | ||||
| Aydin et al. (57), Turkey | Male Wistar rats | Single STZ injection: 40 mg · kg bw−1 | Duration: 4 wk | FG (mg · dL−1) |
| 3–4 mo oldn = 8 per group | HF diet for 12 wk: 4 wk prior to and 8 wk following STZ injection | Int: carnosine, injection: 1) 250 mg · kg bw−1 × 5 per weekCon: vehicle only, injection | HbA1c (%) | |
| Glucose >200 mg · dL−1 (11.1 mmol ·L−1) considered diabeticTreatment started 4 wk after STZ injection | ||||
| Barca et al. (58), Italy | Male C57BL/6JB6 mice | Single STZ injection: 200 mg · kg bw−1 | Duration: 2 wk | FG (mg · dL−1) |
| 12 wk oldn = 5 per group | Glucose ≥250 mg · dL−1 (13.9 mmol ·L−1) considered diabetic | Int: carnosine, oral, dissolved in drinking water: 1) 1 g · L−1 (0.1% conc.) | ||
| Treatment started after disease induction | Con: no intervention | |||
| Giriş et al. (55), Turkey | Male Sprague-Dawley ratsAge NR | Dietary intervention to develop hyperglycemia and insulin resistance | Duration: 8 wkInt: carnosine, oral, dissolved in drinking water: | FG (mg · dL−1) FI (μU/mL) |
| n = 8 per group | High-fructose diet (60% fructose); isocaloric to the control group diet | 1) 120—150 mg · kg bw−1 . d−1Con: no intervention | HbA1c (%)HOMA-IR | |
| Treatment started alongside diet | ||||
| Hue et al. (53), Korea | Male C57BL/6J db/db mice7 wk old | Genetic modification to develop obesity, hyperglycemia, and insulin resistance | Duration: 8 wkInt: carnosine, oral administration: | FI (ng/mL)HbA1c (%) |
| n = 10 per group | Glucose >350 mg · dL−1 (19.4 mmol ·L−1) considered diabetic | 1) 6 mg · kg bw−1 . d−1 | ||
| 2) 30 mg · kg bw−1 . d−1 | ||||
| Treatment began after disease induction | 3) 150 mg · kg bw−1 . d−1 | |||
| Con: saline | ||||
| Hue et al. (59), Korea | Male ICR (CD-1) mice | Single STZ injection: 120 mg · kg bw−1 | Duration: 12 wk | HbA1c (%) |
| 5 wk old | Glucose >300 mg · dL−1 (16.7 mmol ·L−1) considered diabetic | Int: carnosine, oral administration: | ||
| n = 10 per group | 1) 6 mg · kg bw−1 . d−1 | |||
| Treatment started after disease induction | 2) 30 mg · kg bw−1 . d−1 | |||
| 3) 150 mg · kg bw−1 . d−1 | ||||
| Con: saline | ||||
| Liu et al. (60), China | Male C57BL/6J mice68 wk old | Multiple STZ injections: 50 mg · kg bw−1 for 5 consecutive days | Duration: 16 wk Int: carnosine, oral, dissolved in drinking water: | FG (mmol · L−1) |
| n = 6 per group | Glucose >300 mg · dL−1 (16.7 mmol ·L−1) considered diabetic | 1) 1000 mg · kg bw−1 . d−1Con: no intervention | ||
| Treatment started after disease induction | ||||
| Peters et al. (61), Germany | Male Sprague-Dawley rats | Single/double STZ injection: 50 mg · kg bw−1 | Duration: 24 wk | HbA1c (%) |
| Age NR n = 13 per group | Glucose >400 mg · dL−1 (22.2 mmol ·L−1) considered diabetic | Int: carnosine, oral, dissolved in drinking water: 1) 1000 mg · kg bw−1 . d−1 | ||
| Unilateral nephrectomy performed 4 wk after STZ injection; treatment started postsurgery | Con: no intervention | |||
| Pfister et al. (62), Germany | Male Wistar rats | Single STZ injection: 45 mg · kg bw−1 | Duration: 26 wk | HbA1c (%) |
| Age NR n = 7–8 per group | Glucose >250 mg · dL−1 (13.9 mmol ·L−1) considered diabetic | Int: carnosine, oral, dissolved in drinking water: 1) 1000 mg · kg bw−1 . d−1 | ||
| Treatment started 1 wk after disease induction | Con: no intervention | |||
| Riedl et al. (63), Germany | Male Wistar rats | Single STZ injection: 45 mg · kg bw−1 | Duration: 12 wk | HbA1c (%) |
| Age NR n = 7–9 per group | Glucose >250 mg · dL−1 (13.9 mmol ·L−1) considered diabetic | Int: carnosine, oral, dissolved in drinking water 1) 1000 mg · kg bw−1 . d−1 | ||
| Treatment started 1 wk after disease induction | Con: no intervention | |||
| Sauerhöfer et al. (19), Germany | Male and female | Genetic modification to develop obesity, hyperglycemia, and insulin resistance | Duration: 18 wk | FG (mg · dL−1) |
| C57BL/6J Leprdb db/db mice | Int: carnosine, oral, dissolved in drinking water: | FI (no units) | ||
| 4 wk old | Treatment started at 4 wk old | 1) 0.9 g · L−1 (4 mmol · L−1) | HbA1c (%) | |
| n = 8–16 per group | Con: no intervention | |||
| Soliman et al. (64), Egypt | Male albino rats | Single STZ injection: 40 mg · kg bw−1 | Duration: 4 wk | FG (mg · dL−1) |
| Age NR | Glucose >200 mg · dL−1 (11.1 mmol ·L−1) considered diabetic | Int: carnosine, injection, administered daily: | ||
| n = 10 per group | 1) 100 mg · kg bw−1 . d−1 | |||
| Treatment started after disease induction | 2) 200 mg · kg bw−1 . d−1 | |||
| Con: vehicle only, injection | ||||
| Stegen et al. (56), Belgium | Male Sprague-Dawley rats | Dietary intervention to develop obesity, hyperinsulinemia, and insulin resistance | Duration: 8 wk | FG (mmol · L−1) |
| 3 wk old | Int: carnosine, oral, dissolved in drinking water: | FI (pmol · L−1) | ||
| n = 9 per group | Hypercaloric HF diet (60% fat) | 1) 1697 mg · kg bw−1 . d−1 | HOMA-IR | |
| Treatment started alongside diet | Int: β-alanine, oral, dissolved in drinking water: | |||
| 2) 933 mg · kg bw−1 . d−1 | ||||
| Con: no intervention | ||||
| Yan et al. (65), Taiwan | Male BALB/cA mice3 wk old | Multiple STZ injections: 40 mg · kg bw−1 for five consecutive days | Duration: 6 wkInt: carnosine, oral, dissolved in drinking water: | FG (mmol · L−1)FI (nmol · L−1) |
| n = 8 per group | Glucose >200 mg · dL−1 (11.1 mmol ·L−1) considered diabetic | 1) 5 g · L−1 (0.5% conc.) Con: no intervention | ||
| Treatment started after disease induction |
bw, body weight; Con, control group; conc., concentration; FG, fasting glucose; FI, fasting insulin; HbA1c, glycated hemoglobin; HF, high-fat; HFHC, high-fat, high-carbohydrate; Int, intervention group; IR, insulin resistance; NR, not reported; STZ, streptozotocin.
Data from human studies included between-group pre- to postintervention changes, whereas rodent studies included between-group postintervention changes only (e.g., no baseline data were available), longitudinal repeated measures, and multiple treatment doses. Only single studies reported 2-h glucose following a GTT (20), homeostatic model assessment of insulin sensitivity (HOMA-S) (50), and C-peptide (18), so these outcomes were not included in the meta-analysis. We excluded 10 rodent studies that did not record glucose or insulin in the fasted state, or where the measurement type was unclear (66–75); some studies also recorded HbA1c, but this outcome was retained as it does not need to be recorded in the fasted state (62, 63). Two included studies were translated from Korean into English (53, 59). The supplementary information contains individual study data used in the analyses (Supplemental Human and Animal Data); and a list of all excluded full-text studies, including reasons for exclusion (Supplemental Methods).
Risk of bias assessment
Study risk of bias
There were no differences in the risk of bias for individual outcomes within studies, so we allocated a single rating at the study level. Of the 4 human studies, 1 study showed some concerns with risk of bias (51), the remaining 3 studies showed high risk of bias (20, 21, 50) (Supplemental Table 1). This was due to a lack of information on adherence rates or no adjustment for nonadherence in analyses, which led to a high risk of bias for domain 2: bias due to deviations from the intended outcomes. No studies had a prespecified analyses plan, which led to some concerns for domain 5: bias in selection of the reported results. The risk of bias profile, however, was different across studies and 50% of items showed low risk of bias. Nearly all rodent studies had the same risk of bias profile: 60% unclear, 38% low, and 2% high risk of bias (Supplemental Table 2). The majority of criteria were scored as unclear due to reporting issues, which is consistent with risk of bias assessments in previous animal studies (37).
Outcome reporting bias
Three human studies reported prospective trial registration [(51), NCT02928250; (21), IRCT2016011211689N2; (50), ACTRN12613000273785], with a further human study not reporting trial registration in the manuscript [(20), NCT02011100]. All outcomes included in this review were explicitly preregistered in 2 studies (21, 50), whereas the remaining studies did not preregister HbA1c (51), fasting glucose (20, 51), fasting insulin, or HOMA-β outcomes (20). All studies showed internal consistency when comparing the methods with the reported results.
Small study and publication bias
Due to the number of available studies, we only assessed small study bias for fasting glucose and HbA1c in rodent studies. Visual inspection of funnel plots revealed no substantive asymmetries, such that we did not conduct a quantitative assessment (Supplemental Figures 1 and 2).
Results of individual studies
Two human studies did not show any side effects from carnosine supplementation (20, 51), whereas the remaining studies did not report information on side effects (21, 50). Cumulative supplement intake in human studies ranged from 84 g to 168 g (carnosine) and 112 g (β-alanine), which translated to estimated relative cumulative intakes of 1.1 g · kg–1 to 1.7 g · kg–1 [carnosine (20, 21)] and 1.2 g · kg–1 [β-alanine (50)]. It was not possible to estimate the relative cumulative intake for Elbarbary et al. (51) as body weight was reported as a standard score. Relative cumulative intakes in rodent studies ranged from 315 mg · kg–1 to 182 g · kg–1 (carnosine) and 52.2 g · kg–1 (β-alanine). There were too few studies for each outcome to reliably explore the dose-response across human studies. Due to substantial variability, values from rodent studies were log-transformed prior to meta-regressions to assess the dose-response.
Primary outcome: fasting glucose
Human studies
The meta-analysis model (4 effect sizes from 4 studies) provided evidence for a decrease in fasting glucose with supplementation [MD0.5 = –0.95 mmol · L–1 (90% CrI: –2.12 to 0.08); τ0.5 = 0.97 mmol · L–1 (90% CrI: 0.48 to 2.3)] (Figure 2). A sensitivity analysis, removing data from studies where participants did not have elevated fasting glucose at baseline (20), was performed, which provided stronger evidence in favor of supplementation [MD0.5 = –1.5 mmol · L–1 (90% CrI: –2.49 to –0.54); τ0.5 = 0.54 mmol · L–1 (90% CrI: 0.05 to 1.99)]. The probability that the pooled effect size was less than or equal to the minimal important difference threshold (≥1 mmol · L–1 reduction) was estimated as P = 0.464 when including all studies and P = 0.841 when restricted to studies with elevated fasting glucose at baseline (where P closer to 1 indicates greater certainty based on posterior inferences).
FIGURE 2.
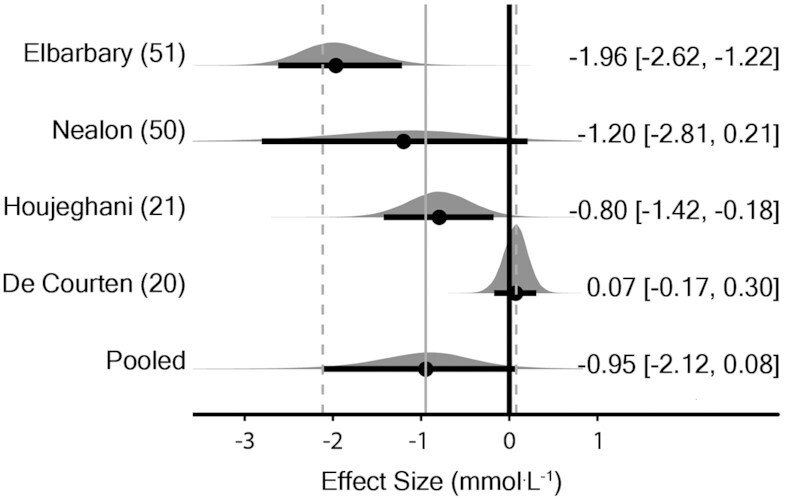
Bayesian forest plot of meta-analysis for fasting glucose in human studies. Each interval represents posterior “shrunken” estimates based on the random-effects model fitting and borrowing information across studies to reduce uncertainty. Circles represent the median value along with 90% credible intervals. Negative values show a reduction in fasting glucose in the intervention group compared with the control group. This analysis included 172 human participants (89 intervention/83 placebo).
Rodent studies
Data from 1 study containing 2 large effect sizes [–20.6 and –20.5 (64)] were deemed outliers and excluded due to the effect sizes being several-fold higher than all other studies reporting this outcome. The meta-analysis model (45 effect sizes from 10 studies) provided evidence to support a decrease in fasting glucose with supplementation [MD0.5 = –2.26 mmol · L–1 (90% CrI: –4.03 to –0.44); τ0.5 = 2.7 mmol · L–1 (90% CrI: 1.6 to 4.7); intraclass correlation coefficient (ICC)0.5 = 0.33 (90% CrI: 0.16 to 0.53)] (Figure 3). A sensitivity analysis was performed removing data from studies where the method to induce disease did not elevate fasting glucose (56) and this provided support in favor of supplementation [MD0.5 = –2.58 mmol · L–1 (90% CrI: –4.50 to –0.61); τ0.5 = 2.81 mmol · L–1 (90% CrI: 1.6 to 5.0); ICC0.5 = 0.33 (90% CrI: 0.16 to 0.54)]. The dose-response analysis with meta-regression of effect size on the log-transformed cumulative dose showed greater decreases in fasting glucose with higher doses [β0.5 = –1.7 mmol · L–1 (90% CrI: –2.7 to –0.68); τ0.5 = 4.1 mmol · L–1 (90% CrI: 2.5 to 6.8)] (Supplemental Figure 3).
FIGURE 3.
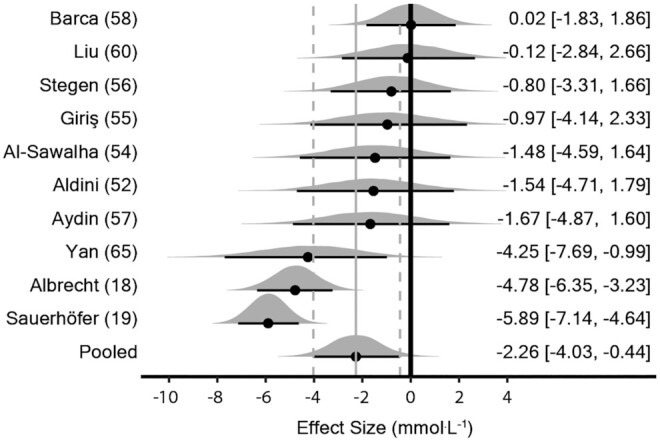
Bayesian forest plot of meta-analysis for fasting glucose in rodent studies. Each interval represents posterior “shrunken” estimates based on the random-effects model fitting and borrowing information across studies to reduce uncertainty. Circles represent the median value along with 90% credible intervals. Negative values show a reduction in fasting glucose in the intervention group compared with the control group. This analysis included 229 rodents (111 intervention/118 control).
Primary outcome: HbA1c
Human studies
The meta-analysis model (2 effect sizes from 2 studies) provided evidence for a decrease in HbA1c with supplementation [MD0.5 = –0.91% (90% CrI: –1.46 to –0.39); τ0.5 = 0.17% (90% CrI: 0.01 to 1.08)] (Figure 4). The probability that the pooled effect size was less than or equal to the minimal important difference threshold (≥0.5% reduction) was estimated as P = 0.921 (where P closer to 1 indicates greater certainty based on posterior inferences).
FIGURE 4.
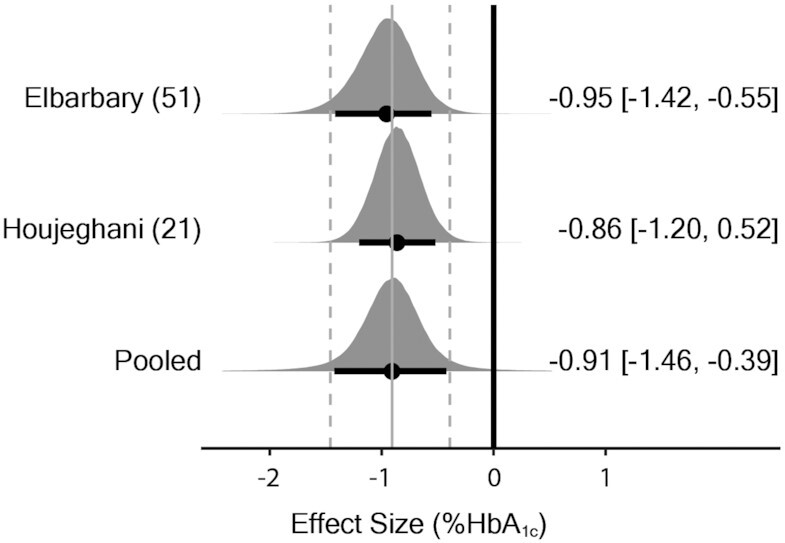
Bayesian forest plot of meta-analysis for HbA1c in human studies. Each interval represents posterior “shrunken” estimates based on the random-effects model fitting and borrowing information across studies to reduce uncertainty. Circles represent the median value along with 90% credible intervals. Negative values show a reduction in HbA1c in the intervention group compared with the control group. This analysis included 134 human participants (67 intervention/67 placebo). Both studies supplemented with carnosine. HbA1c (HbA1c), glycated hemoglobin.
Rodent studies
The meta-analysis model (16 effect sizes from 9 studies) provided evidence for a decrease in HbA1c with supplementation [random effects model: MD0.5 = –1.05% (90% CrI: –1.64 to –0.52); τ0.5 = 0.58% (90% CrI: 0.07 to 1.44); ICC0.5 = 0.11 (90% CrI: 0.00 to 0.53)] (Figure 5). Initially, no evidence of a dose response was obtained with meta-regression of effect size on the log-transformed cumulative dose [β0.5 = –0.03% (90% CrI: –0.32 to 0.27); τ0.5 = 0.62% (90% CrI: 0.08 to 1.52)] (Supplemental Figure 4). However, 1 effect size [cumulative dose: 182 g · kg · bw–1; effect size: 1.10 (62)] exhibited substantive leverage and removal of the point within a sensitivity analysis showed a possible dose-response effect in favor of greater decreases in HbA1c with higher doses [β0.5 = –0.18% (90% CrI: –0.50 to 0.13); τ0.5 = 0.57% (90% CrI: 0.08 to 1.39)] (Supplemental Figure 4).
FIGURE 5.
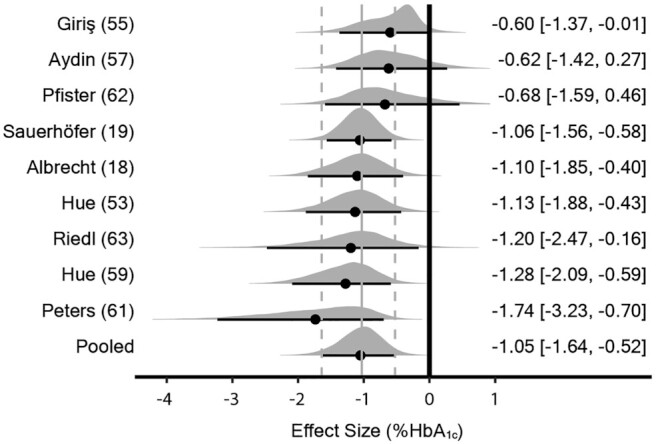
Bayesian forest plot of meta-analysis for HbA1c in rodent studies. Each interval represents posterior “shrunken” estimates based on the random-effects model fitting and borrowing information across studies to reduce uncertainty. Circles represent the median value along with 90% credible intervals. Negative values show a reduction in HbA1c in the intervention group compared with the control group. This analysis included 260 rodents (127 intervention/133 control). All studies supplemented with carnosine. HbA1c (HbA1c), glycated hemoglobin.
Additional outcomes: fasting insulin, HOMA-β, and HOMA-IR
Human studies
The meta-analysis models provided evidence of a small to medium effect for a decrease in HOMA-IR [SMD0.5 = –0.41 (90% CrI: –0.82 to –0.07); τ0.5 = 0.11 (90% CrI: 0.01 to 0.61); P(≤ –0.2) = 0.853; P(≤ –0.5) = 0.343; P(≤ –0.8) = 0.057] (Supplemental Figure 5); a small to medium effect in favor of decreased fasting insulin [SMD0.5 = –0.41 (90% CrI: –0.77 to –0.07); τ0.5 = 0.10 (90% CrI: 0.01 to 0.52); P(≤ –0.2) = 0.857; P(≤ –0.5) = 0.324; P(≤ –0.8) = 0.041] (Supplemental Figure 6); and a small effect in favor of decreased HOMA-β [SMD0.5 = –0.22 (90% CrI: –0.57 to 0.15); τ0.5 = 0.10 (90% CrI: 0.01 to 0.54); P(≤ –0.2) = 0.532; P(≤ –0.5) = 0.085; P(≤ –0.8) = 0.009] (Supplemental Figure 7) with supplementation.
Rodent studies
The meta-analysis models provided some evidence of a medium effect for decreased HOMA-IR [SMD0.5 = –0.63 (90% CrI: –1.98 to 0.65); τ0.5 = 0.72 (90% CrI: 0.06 to 2.84); ICC0.5 = 0.21 (90% CrI: 0.00 to 0.85); P(≤–0.2) = 0.745; P(≤–0.5) = 0.563; P(≤–0.8) = 0.364] (Supplemental Figure 8) and a small effect in favor of decreased fasting insulin [SMD0.5 = –0.31 (90% CrI: –1.33 to 0.57); τ0.5 = 1.03 (90% CrI: 0.16 to 2.72); ICC0.5 = 0.09 (90% CrI: 0.00 to 0.78); P(≤ –0.2) = 0.570; P(≤ –0.5) = 0.334; P(≤ –0.8) = 0.177] (Supplemental Figure 9) with supplementation.
Sensitivity and subgroup analyses
Sensitivity analyses were performed to explore the robustness of the main analyses against outliers (rodent studies: fasting glucose and dose-response for HbA1c) and where the outcomes were not elevated at baseline (rodent studies: fasting glucose) or in the control group (rodent studies: fasting glucose). Data for these are presented in the previous sections. A sensitivity analysis for risk of bias was not performed due to no human studies being at low risk of bias and all rodent studies having similar risk of bias profiles. Only 1 rodent study supplemented with β-alanine, so meta-regressions for the effect of supplementation type were not performed. Instead, a sensitivity analysis was performed to show that the main results were robust to the removal of the β-alanine data from Stegen et al. (56): fasting glucose [MD0.5 = –2.36 mmol · L–1 (–4.16 to –0.49); τ0.5 = 2.73 mmol · L–1 (1.61 to 4.68); ICC = 0.34 (0.17 to 0.54)], HOMA-IR [SMD0.5 = –0.64 (90% CrI: –1.82 to 0.47); τ0.5 = 0.75 (90% CrI: 0.08 to 2.77); P(≤ –0.2) = 0.793; P(≤ –0.5) = 0.608; P(≤ –0.8) = 0.383], and fasting insulin [SMD0.5 = –0.33 (90% CrI: –1.50 to 0.57); τ0.5 = 1.07 (90% CrI: 0.15 to 2.80); ICC0.5 = 0.15 (90% CrI: 0.00 to 0.82); P(≤ –0.2) = 0.597; P(≤ –0.5) = 0.375; P(≤ –0.8) = 0.213]. Because there were no substantive differences, data from both supplementation groups in Stegen et al. (56) were combined into a single pooled effect size for the study.
Certainty of evidence
There was moderate certainty in the effect estimates for human study outcomes. All outcomes were downgraded 1 level due to concerns with imprecision and risk of bias. It was decided not to rate down an additional level for the other outcomes, as there were no concerns with publication bias or inconsistency. There was very low certainty in the effect estimates for rodent study outcomes. All outcomes were downgraded 1 level, due to the prespecified criteria for indirectness and an additional level due to serious concerns with risk of bias and serious or very serious concerns with imprecision. The summary of findings table depicts the full GRADE assessment, with footnotes explaining each judgment (Supplemental Table 3).
Discussion
Summary of main findings
We included all available human and animal data to provide the most comprehensive assessment to date of the effects of carnosine and β-alanine supplementation on glycemic control and insulin resistance. Our main findings show that supplementation improves glycemic control across a range of disease types in humans (type 1 diabetic children, type 2 diabetic adults) and rodents (genetic models of obesity and diabetes, diet-induced metabolic syndrome, and pharmacological models of type 1 diabetes). As would be expected, there was no improvement in fasting glucose in normoglycemic populations. Of clinical relevance is the high probability that supplementation improves impaired fasting glucose (P = 0.841) and HbA1c (P = 0.921) beyond the minimal important difference thresholds (≥1 mmol · L–1 and ≥0.5% reduction). Data from animal studies support these findings, which strengthens our confidence in the effect. We also show evidence of a possible dose-response effect in animals, in favor of higher cumulative intakes causing greater reductions in fasting glucose and, possibly, HbA1c. The positive effects are driven primarily by carnosine supplementation, as only 1 human and 1 animal study supplemented with β-alanine (50, 56). There were insufficient data to assess the effect of supplementation on our other prespecified primary outcome: 2-h glucose following a GTT. Additional results show evidence of a small to medium effect in favor of supplementation reducing fasting insulin and HOMA-IR in humans. While there was some evidence in favor of supplementation reducing HOMA-β in humans, and fasting insulin and HOMA-IR in animals, it was not possible to rule out a neutral or negative effect for these outcomes.
Proposed mechanisms
The inconsistent effects for fasting insulin across animal studies could be due to the relationship between hyperinsulinemia and insulin resistance, where an improvement in one may, paradoxically, lead to a decline in the other [for a commentary, see (76)]. Two long-term studies showed that supplementation attenuated the development of hyperglycemia in genetically modified mice (18, 19). Both studies reported an increase in fasting insulin, and 1 reported a 2-fold increase in C-peptide—a specific marker of insulin secretion (18). This suggests that carnosine might enhance insulin secretion from pancreatic β-cells, which may compensate for peripheral insulin resistance, leading to an improvement in glycemic control. Further, as supplementation began prior to disease development, it is possible that carnosine can play a role in preventing or delaying disease progression. One human study supports this: a subgroup of participants with impaired glucose tolerance displayed normal 2-h glucose and reduced 2-h insulin following supplementation (20). This suggests that carnosine might also improve postprandial glucose disposal, potentially by reducing peripheral insulin resistance. Consistent with these hypotheses, work from our research group showed that treatment with carnosine reverses glucolipotoxic inhibition of insulin secretion in isolated mouse islets and INS-1 pancreatic β-cells, as well as insulin-stimulated glucose uptake in C2C12 skeletal muscle cells (12). Together, this suggests that carnosine might exert beneficial effects in multiple tissues.
Improvements in glycemic control can protect organs and tissues from complications associated with diabetes. In type 1 diabetic children, Elbarbary et al. (51) showed a large decrease in plasma ɑ1-microglobulin (–44%) and the urinary albumin to creatinine ratio (UACR; –58%), which suggests that carnosine might have a protective effect on kidney function and may lower the risk of diabetic nephropathy. In support, several rodent studies showed reductions in the UACR (18, 52, 60, 61), as well as reductions in blood urea nitrogen and serum creatinine (60). Interestingly, these improvements occurred in 2 studies without a change in fasting glucose (52, 60). The studies showed an 11-fold and 4-fold increase in kidney carnosine concentrations following supplementation, supporting prior research that the human kidney has an intrinsic system for metabolizing carnosine (8). It is possible that some of the beneficial effects of carnosine occur directly in the kidney and may be independent of its actions on glycemic control—with positive outcomes in both type 1 and type 2 diabetes.
The most plausible mechanism by which carnosine provides a therapeutic effect is through its ability to form stable adducts with reactive carbonyl species, such as acrolein, 4-hydroxynoneal, and methylglyoxal (13, 77). These toxic products increase with diabetes severity and cause deleterious modifications to proteins, lipids, and DNA—inducing inflammation and insulin resistance, and impairing insulin secretion (12, 78–80). By scavenging these products, carnosine reduces their reactivity, allowing them to be safely metabolized or excreted from the body (14, 81, 82), which limits downstream formation of advanced glycation and advanced lipid-oxidation end products (AGEs and ALEs). Indeed, human and rodent studies showed supplementation protected against oxidative stress, lipid peroxidation, AGEs, and ALEs (18, 21, 51, 52, 55–57, 60, 62, 63, 65). It is also possible that carnosine works indirectly by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling cascade, which enhances endogenous antioxidant and anti-carbonylation defense systems [for a detailed review, see (83)].
There is a debate over the location of these actions. Some studies suggest that carnosine can act on reactive species and inflammatory markers in plasma, meaning that raising plasma carnosine would be key to any potential therapeutic effects (56). Although this might be true for rodents, where low carnosinase activity means that carnosine readily circulates in plasma and fasted values can increase 25-fold with supplementation (56), it seems unlikely to be as important in humans given that carnosinase is highly active in enterocytes and plasma, rapidly hydrolyzing carnosine (84, 85). As such, plasma carnosine concentrations remain below the limit of detection (11, 86). In a subgroup of individuals with low plasma carnosinase activity, a single dose of carnosine (60 mg · kg–1; 4.2 g for a 70-kg individual) increased plasma carnosine concentrations to a peak of 73.3 μM, before returning to baseline within 1–2 h (87).
Carnosine might instead act in human tissues that synthesize it in situ—those expressing carnosine synthase and transporters for β-alanine and histidine—and which play an important role in the pathogenesis of insulin resistance and diabetes. Recent genetic studies support the tissue–carnosine hypothesis: overexpression of cardio-specific ATP-grasp domain-containing protein 1 (ATPGD1; carnosine synthase) increased carnosine and anserine concentrations in the myocardium of mice, which reduced protein-aldehyde adducts and gave protection against ischemia reperfusion injury (88), whereas knockout of glutamic acid decarboxylase–like 1 (GADL1) reduced carnosine concentrations in the olfactory bulb and skeletal muscle, leading to increased markers of oxidative stress (89). Based on this evidence, increasing tissue carnosine stores should be the primary goal of supplementation. The included human studies, however, used a dose or duration that would cause only a modest increase in tissue carnosine stores (90, 91). de Courten et al. (20) was the only human study to quantify the change in tissue carnosine, reporting a 33% increase in skeletal muscle stores after supplementing with 2 g · d–1 carnosine for 12 wk. In contrast, nonclinical studies often supplement with 3.2 g · d–1 to 6.4 g · d–1 of β-alanine, whereby large cumulative intakes (24 wk; 1075.2 g total) can lead to a 2-fold increase in skeletal muscle carnosine content (92). It is worth noting that Nealon et al. (50) used a high β-alanine dose and showed improvements in glycemic control and insulin resistance in a 4-wk period. The comparably low relative cumulative intakes in human studies, coupled with the evidence of a dose-response in rodent studies, suggest that there could be room for further improvement in human outcomes. Future studies should consider the type, dose, and duration of supplementation.
While our study provides evidence in favor of supplementation, we recommend caution as the results require replication in large-sample, high-quality studies. The GRADE assessment showed our certainty in the effect estimates from human studies is moderate, which suggests the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different (48). Further, the certainty in all outcomes from rodent studies was graded as very low, due to inconsistency, imprecision, and indirectness. Despite this, results from animal and human studies agree, which collectively adds weight to the main findings and shows that changes in rodent outcomes for glycemic control and insulin resistance may translate to humans. Given the inherent limitations in preclinical studies, however, caution is needed when interpreting the rodent outcomes for the purposes of choosing treatment options for humans.
Limitations in the research
Our study highlights several limitations in the existing evidence base, which future studies can improve upon. No human studies were at a low risk of bias. To address this, researchers should provide clear information on randomization and allocation concealment, publish a prespecified statistical analysis plan within the trial registration, and rigorously assess and report adherence and blinding to the intervention. All human studies were of short duration (≤3 mo) with modest cumulative intakes; it is possible that longer-duration studies with higher cumulative intakes could lead to better clinical outcomes, consistent with the dose-response results in rodents. This is particularly important since diabetic complications develop over many years and may not be captured in short-duration studies. Several rodent studies did not contain the relevant information to assess the risk of bias; researchers should aim to satisfy the SYRCLE criteria and follow animal study reporting guidelines [e.g., the ARRIVE guidelines (93)]. Preclinical researchers should also design studies that translate to human clinical outcomes; several rodent studies were excluded from the current analysis because they did not record glucose or insulin in the fasted state. While certain disease models cannot be fasted for prolonged periods due to ethical concerns, it is often possible to conduct a short-term fast during the light phase in rodents that is equivalent to an overnight fast in humans (94). Our study focused upon surrogate outcomes involved in diabetes diagnosis and clinical decision making. These are important, although future RCTs should collect data on key patient-centered outcomes, such as disease progression rates, diabetic symptoms, hospital admissions, and diabetic complications.
Conclusions
Our study provides evidence that supplementation with carnosine or β-alanine may reduce fasting glucose, HbA1c, and HOMA-IR in humans and rodents, and fasting insulin in humans. There is a need, however, for longer-term studies (>3 mo), in large samples, using dynamic methods to assess glycemic control and insulin resistance (e.g., GTTs and glucose clamp techniques). To improve the certainty in future findings, researchers should also explore dose-response effects in humans, whether treatment effects are the same for carnosine and β-alanine, and address shortcomings in study designs and reporting. Despite these caveats, our promising results indicate that carnosine and β-alanine supplementation remain viable therapeutics to improve glycemic control and insulin resistance in diabetes and its related conditions. The results of the current study provide a foundation upon which ongoing studies can be based, with the goal of defining whether this strategy is suitable for widespread population-level implementation.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Victoria Boskett, the Research Support Librarian who helped us complete the Peer Review of Electronic Search Strategies checklist. The authors’ responsibilities were as follows—JJM and CS: developed the initial idea for the study; JJM and KJE-S: performed the initial searches; JJM, LS, and GGA: performed the full-text screening; JJM and ED: performed the data extraction, risk of bias, and certainty assessment; PAS: performed the data analysis; JJM and PAS: interpreted the data analysis; JJM: drafted the first version of the manuscript; all authors: provided critical input and revised the manuscript, developed the pre-published protocol and study design, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study. ED is supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant numbers: 2019/05616-6 and 2019/26899-6). GGA has been supported by FAPESP (grant numbers: 2014/11948-8 and 2019/25032-9) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). MDT has received a British Council award to support a studentship focused on research into carnosine (grant number: 209524711).
Author disclosures: CS is the recipient of funding to support a PhD program of work from the Natural Alternatives International (NAI) and has received β-alanine supplements free of charge from NAI for use in experimental investigations; NAI have also supported open-access page charges for some manuscripts. GGA and ED have been co-investigators on projects partially supported by NAI. NAI have also supported open-access page charges for some manuscripts where GGA is the corresponding author. The other authors report no conflicts of interest.
Supplemental Figures 1–9, Supplemental Tables 1–3, Supplemental Methods, Supplemental Human Data, and Supplemental Animal Data are available from the link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AGE, advanced glycation end product; ALE, advanced lipid-oxidation end product; bw, body weight; CrI, credible interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; GTT, glucose-tolerance test; HbA1c, glycated hemoglobin; HCD, histidine-containing dipeptide; HOMA-β, homeostatic model assessment of β-cell function; HOMA-S, homeostatic model assessment of insulin sensitivity; ICC, intraclass correlation coefficient; MD, mean difference; PRESS, Peer Review Of Electronic Search Strategies; RCT, randomized controlled trial; SMD, standardized mean difference; SYRCLE, Systematic Review Center for Laboratory Animal Experimentation; UACR, urinary albumin to creatinine ratio.
Contributor Information
Joseph J Matthews, Sport, Health, and Performance Enhancement (SHAPE) Research Centre, Musculoskeletal Physiology Research Group, School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom; Research Centre for Life and Sport Sciences (CLaSS), School of Health and Life Sciences, Department of Sport and Exercise, Birmingham City University, Birmingham, United Kingdom.
Eimear Dolan, Applied Physiology and Nutrition Research Group, School of Physical Education and Sport, University of Sao Paulo, Sao Paulo, Brazil.
Paul A Swinton, School of Health Sciences, Robert Gordon University, Aberdeen, United Kingdom.
Lívia Santos, Sport, Health, and Performance Enhancement (SHAPE) Research Centre, Musculoskeletal Physiology Research Group, School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom.
Guilherme G Artioli, Applied Physiology and Nutrition Research Group, School of Physical Education and Sport, University of Sao Paulo, Sao Paulo, Brazil; Rheumatology Division, Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil.
Mark D Turner, Centre for Diabetes, Chronic Diseases, and Ageing, School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom.
Kirsty J Elliott-Sale, Sport, Health, and Performance Enhancement (SHAPE) Research Centre, Musculoskeletal Physiology Research Group, School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom.
Craig Sale, Sport, Health, and Performance Enhancement (SHAPE) Research Centre, Musculoskeletal Physiology Research Group, School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom.
Data Availability
Statistical code is available upon request. Data and other materials associated with the project are available in the Supplemental Methods, Supplemental Data, Supplemental Human Data, and Supplemental Animal Data files.
References
- 1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova Ket al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. 9th ed. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. [DOI] [PubMed] [Google Scholar]
- 3. Ligthart S, van Herpt TTW, Leening MJG, Kavousi M, Hofman A, Stricker BHC, van Hoek M, Sijbrands EJG, Franco OH, Dehghan Aet al. . Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):44–51. [DOI] [PubMed] [Google Scholar]
- 4. Lindahl B, Nilssön TK, Borch-Johnsen K, Røder ME, Söderberg S, Widman L, Johnson O, Hallmans G, Jansson JH. A randomized lifestyle intervention with 5-year follow-up in subjects with impaired glucose tolerance: pronounced short-term impact but long-term adherence problems. Scand J Public Health. 2009;37(4):434–42. [DOI] [PubMed] [Google Scholar]
- 5. Artioli GG, Sale C, Jones RL. Carnosine in health and disease. EJSS. 2019;19(1):30–9. [DOI] [PubMed] [Google Scholar]
- 6. Crush KG. Carnosine and related substances in animal tissues. Comp Biochem Physiol. 1970;34(1):3–30. [DOI] [PubMed] [Google Scholar]
- 7. Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci. 1988;85(9):3175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters V, Klessens CQ, Baelde HJ, Singler B, Veraar KA, Zutinic A, Drozak J, Zschocke J, Schmitt CP, de Heer E. Intrinsic carnosine metabolism in the human kidney. Amino Acids. 2015;47(12):2541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews JJ, Artioli GG, Turner MD, Sale C. The physiological roles of carnosine and β-alanine in exercising human skeletal muscle. Med Sci Sports Exercise. 2019;51(10):2098–108. [DOI] [PubMed] [Google Scholar]
- 10. Jones G, Smith M, Harris R. Imidazole dipeptide content of dietary sources commonly consumed within the British diet. Proc Nutr Soc. 2011;70(OCE6). [Google Scholar]
- 11. Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006;30(3):279–89. [DOI] [PubMed] [Google Scholar]
- 12. Cripps MJ, Hanna K, Lavilla C, Sayers SR, Caton PW, Sims C, De Girolamo L, Sale C, Turner MD. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake. Sci Rep. 2017;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aldini G, Carini M, Beretta G, Bradamante S, Facino RM. Carnosine is a quencher of 4-hydroxy-nonenal: through what mechanism of reaction?. Biochem Biophys Res Commun. 2002;298(5):699–706. [DOI] [PubMed] [Google Scholar]
- 14. Baba SP, Hoetker JD, Merchant M, Klein JB, Cai J, Barski OA, Conklin DJ, Bhatnagar A. Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. J Biol Chem. 2013;288(39):28163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carvalho VH, Oliveira AH, de Oliveira LF, da Silva RP, Di Mascio P, Gualano B, Artioli GG, Medeiros MH. Exercise and β-alanine supplementation on carnosine-acrolein adduct in skeletal muscle. Redox Biol. 2018;18:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoetker D, Chung W, Zhang D, Zhao J, Schmidtke VK, Riggs DW, Derave W, Bhatnagar A, Bishop D, Baba SP. Exercise alters and β-alanine combined with exercise augments histidyl dipeptide levels and scavenges lipid peroxidation products in human skeletal muscle. J Appl Physiol. 2018;125(6):1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes—second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47(sup1):39–48. [DOI] [PubMed] [Google Scholar]
- 18. Albrecht T, Schilperoort M, Zhang S, Braun JD, Qiu J, Rodriguez A, Pastene DO, Krämer BK, Köppel H, Baelde Het al. . Carnosine Attenuates the development of both type 2 diabetes and diabetic nephropathy in BTBR ob /ob mice. Sci Rep. 2017;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sauerhöfer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N, Floege J, Kriz W, Van der Woude F, Moeller MJ. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes. 2007;56(10):2425–32. [DOI] [PubMed] [Google Scholar]
- 20. De Courten B, Jakubova M, De Courten MP, Kukurova IJ, Vallova S, Krumpolec P, Valkovic L, Kurdiova T, Garzon D, Barbaresi Set al. . Effects of carnosine supplementation on glucose metabolism: pilot clinical trial. Obesity. 2016;24(5):1027–34. [DOI] [PubMed] [Google Scholar]
- 21. Houjeghani S, Kheirouri S, Faraji E, Jafarabadi MA. L-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor–α levels in patients with type 2 diabetes: a double-blind placebo-controlled randomized clinical trial. Nutr Res. 2018;49:96–106. [DOI] [PubMed] [Google Scholar]
- 22. Menon K, Marquina C, Liew D, Mousa A, de Courten B. Histidine-containing dipeptides reduce central obesity and improve glycaemic outcomes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2020;21(3):e12975. [DOI] [PubMed] [Google Scholar]
- 23. Matthews JJ, Sale C. Comments upon “Histidine-containing dipeptides reduce central obesity and improve glycaemic outcomes: a systematic review and meta-analysis of randomized controlled trials.” Obes Rev. 2020;21(7):e13036. [DOI] [PubMed] [Google Scholar]
- 24. Blancquaert L, Everaert I, Missinne M, Baguet A, Stegen S, Volkaert A, Petrovic M, Vervaet C, Achten E, De Maeyer Met al. . Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med Sci Sports Exerc. 2017;49(3):602–9. [DOI] [PubMed] [Google Scholar]
- 25. Church DD, Hoffman JR, Varanoske AN, Wang R, Baker KM, La Monica MB, Beyer KS, Dodd SJ, Oliveira LP, Harris RCet al. . Comparison of two β-alanine dosing protocols on muscle carnosine elevations. J Am Coll Nutr. 2017;36(8):608–16. [DOI] [PubMed] [Google Scholar]
- 26. Varanoske AN, Hoffman JR, Church DD, Wang R, Baker KM, Dodd SJ, Coker NA, Oliveira LP, Dawson VL, Fukuda DHet al. . Influence of skeletal muscle carnosine content on fatigue during repeated resistance exercise in recreationally active women. Nutrients. 2017;9(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varanoske AN, Hoffman JR, Church DD, Coker NA, Baker KM, Dodd SJ, Harris RC, Oliveira LP, Dawson VL, Wang Ret al. . Comparison of sustained-release and rapid-release β-alanine formulations on changes in skeletal muscle carnosine and histidine content and isometric performance following a muscle-damaging protocol. Amino Acids. 2019;51(1):49–60. [DOI] [PubMed] [Google Scholar]
- 28. Peng W, Mao P, Liu L, Chen K, Zhong Y, Xia W, Guo Q, Tan SC, Rahmani J, Varkaneh HKet al. . Effect of carnosine supplementation on lipid profile, fasting blood glucose, HbA1C and insulin resistance: a systematic review and meta-analysis of long-term randomized controlled trials. Complement Ther Med. 2020;48:102241. [DOI] [PubMed] [Google Scholar]
- 29. Matthews JJ, Dolan E, Swinton PA, Santos L, Artioli GG, Turner MD, Elliott-Sale KJ, Sale C. The effect of carnosine or β-alanine supplementation on markers of glycaemic control and insulin resistance in human and animal studies: a protocol for a systematic review and meta-analysis. Syst Rev. 2020;9(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. . PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;29:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. [Internet]. Geneva (Switzerland): World Health Organization; 2006; [cited 2019 May 25]. Available from: https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf. [Google Scholar]
- 32. World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. [Internet]. Geneva (Switzerland): World Health Organization; 2011; [cited 2019 May 25]. Available from: https://www.who.int/diabetes/publications/report-hba1c_2011.pdf. [PubMed] [Google Scholar]
- 33. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 34. The Cochrane Collaboration . Data collection form (for RCTs) [Internet]. Cochrane Training. [cited 2020 Oct 4]. Available from: https://training.cochrane.org/data-collection-form-rcts. [Google Scholar]
- 35. Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J; ALLHAT Collaborative Research Group . Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2006;166(20):2191–201. [DOI] [PubMed] [Google Scholar]
- 36. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SMet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 37. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Method. 2014;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernández-Castilla B, Declercq L, Jamshidi L, Beretvas SN, Onghena P, Van den Noortgate W. Detecting selection bias in meta-analyses with multiple outcomes: a simulation study. J Exp Educ. 2021;89(1):125–44. [DOI] [PubMed] [Google Scholar]
- 39. National Institute for Health and Care Excellence (NICE) . Type 2 diabetes in adults: clinical guideline update (NG28). [Internet]. National Institute for Health and Care Excellence (NICE); 2015 Dec; [cited 2021 Feb 16]. Available from: https://www.nice.org.uk/guidance/ng28/evidence/full-guideline-pdf-78671532569. [Google Scholar]
- 40. Cohen J. The effect size index: d. Statistical power analysis for the behavioral sciences. 2nd edition. Abingdon-on-Thames: Routledge; 1988. p. 284–8. [Google Scholar]
- 41. Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11(2):364–86. [Google Scholar]
- 42. Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105. [DOI] [PubMed] [Google Scholar]
- 43. Kruschke JK, Liddell TM. The Bayesian new statistics: hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull Rev. 2018;25(1):178–206. [DOI] [PubMed] [Google Scholar]
- 44. Rhodes KM, Turner RM, Higgins JPT. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol. 2015;68(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sturtz S, Ligges U, Gelman A. R2OpenBUGS: a package for running OpenBUGS from R. [Internet]. 2010. Available from: http://cran.ms.unimelb.edu.au/web/packages/R2OpenBUGS/vignettes/R2OpenBUGS.pdf. [Google Scholar]
- 46. Bürkner PC. brms: An R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80(1):1–28. [Google Scholar]
- 47. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer Het al. . GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 48. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris Set al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 49. Wei D, Tang K, Wang Q, Estill J, Yao L, Wang X, Chen Y, Yang K. The use of GRADE approach in systematic reviews of animal studies. J Evidence-Based Med. 2016;9(2):98–104. [DOI] [PubMed] [Google Scholar]
- 50. Nealon RS, Sukala WR, Coutts RA, Zhou S. The effect of 28 days of beta-alanine supplementation on exercise capacity and insulin sensitivity in individuals with type 2 diabetes mellitus: a randomised, double-blind and placebo-controlled pilot trial. J Nutr Sci Res. 2016;1(3):1–7. [Google Scholar]
- 51. Elbarbary NS, Ismail EAR, El-Naggar AR, Hamouda MH, El-Hamamsy M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: a randomized placebo-controlled trial. Pediatr Diabetes. 2018;19(3):470–7. [DOI] [PubMed] [Google Scholar]
- 52. Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, Yeum KJ, Negrisoli G, Carini M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15(6):1339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hue JJ, Kim JS, Kim JH, Nam SY, Yun YW, Jeong JH, Lee BJ. Antiglycemic effect of carnosine in diabetic mice. J Food Hyg Saf. 2009;24(4):391–7. [Google Scholar]
- 54. Al-Sawalha NA, Alshogran OY, Awawdeh MS, Almomani BA. The effects of l-carnosine on development of metabolic syndrome in rats. Life Sci. 2019;237:116905. [DOI] [PubMed] [Google Scholar]
- 55. Giriş M, Doğru-Abbasoğlu S, Kumral A, Olgaç V, Koçak-Toker N, Uysal M. Effect of carnosine alone or combined with α-tocopherol on hepatic steatosis and oxidative stress in fructose-induced insulin-resistant rats. J Physiol Biochem. 2014;70(2):385–95. [DOI] [PubMed] [Google Scholar]
- 56. Stegen S, Stegen B, Aldini G, Altomare A, Cannizzaro L, Orioli M, Gerlo S, Deldicque L, Ramaekers M, Hespel Pet al. . Plasma carnosine, but not muscle carnosine, attenuates high-fat diet-induced metabolic stress. Appl Physiol Nutr Metab. 2015;40(9):868–76. [DOI] [PubMed] [Google Scholar]
- 57. Aydın AF, Bingul I, Küçükgergin C, Doğan-Ekici I, Doğru Abbasoğlu S, Uysal M. Carnosine decreased oxidation and glycation products in serum and liver of high-fat diet and low-dose streptozotocin-induced diabetic rats. Int J Exp Pathol. 2017;98(5):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barca A, Gatti F, Spagnolo D, Ippati S, Vetrugno C, Verri T. Responsiveness of carnosine homeostasis genes in the pancreas and brain of streptozotocin-treated mice exposed to dietary carnosine. Int J Mol Sci. 2018;19(6):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hue JJ, Kim JS, Kim JH, Nam SY, Yun YW, Jeong JH, Lee BJ. Anti-glycemic effect of L-carnosine in streptozotocin-induced diabetic mice. Korean J Veterinary Res. 2010;50(2):105–11. [Google Scholar]
- 60. Liu XQ, Jiang L, Lei L, Nie ZY, Zhu W, Wang S, Zeng HX, Zhang SQ, Zhang Q, Yard Bet al. . Carnosine alleviates diabetic nephropathy by targeting GNMT, a key enzyme mediating renal inflammation and fibrosis. Clin Sci. 2020;134(23):3175–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peters V, Riedl E, Braunagel M, Höger S, Hauske S, Pfister F, Zschocke J, Lanthaler B, Benck U, Hammes HPet al. . Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul Pept. 2014;194-195:36–40. [DOI] [PubMed] [Google Scholar]
- 62. Pfister F, Riedl E, Wang Q, vom Hagen F, Deinzer M, Harmsen MC, Molema G, Yard B, Feng Y, Hammes HP. Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell Physiol Biochem. 2011;28(1):125–36. [DOI] [PubMed] [Google Scholar]
- 63. Riedl E, Pfister F, Braunagel M, Brinkkötter P, Sternik P, Deinzer M, Bakker SJ, Henning RH, van den Born J, Krämer BKet al. . Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem. 2011;28(2):279–88. [DOI] [PubMed] [Google Scholar]
- 64. Soliman KM, Mohamed AM, Metwally NS. Attenuation of some metabolic deteriorations induced by diabetes mellitus using carnosine. J Appl Sci. 2007;7:2252. [Google Scholar]
- 65. Yan S-L, Wang Z-H, Mong M-C, Yang Y-C, Yin M-C. Combination of carnosine and asiatic acid provided greater anti-inflammatory protection for HUVE cells and diabetic mice than individual treatments of carnosine or asiatic acid alone. Food Chem Toxicol. 2019;126:192–8. [DOI] [PubMed] [Google Scholar]
- 66. Ahshin-Majd S, Zamani S, Kiamari T, Kiasalari Z, Baluchnejadmojarad T, Roghani M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: possible involved mechanisms. Peptides. 2016;86:102–11. [DOI] [PubMed] [Google Scholar]
- 67. Forsberg EA, Botusan IR, Wang J, Peters V, Ansurudeen I, Brismar K, Catrina SB. Carnosine decreases IGFBP1 production in db/db mice through suppression of HIF-1. J Endocrinol. 2015;225(3):159–67. [DOI] [PubMed] [Google Scholar]
- 68. Lee Y-T, Hsu C-C, Lin M-H, Liu K-S, Yin M-C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol. 2005;513(1-2):145–50. [DOI] [PubMed] [Google Scholar]
- 69. Marzook EA, Marzook FA, Abd El Moneim AE. Radioprotective and anti-diabetic effects of Costus speciosus and carnosine. Trop J Pharmaceutical Res. 2020;19(1):121–7. [Google Scholar]
- 70. Mong M-C, Chao C-Y, Yin M-C. Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol. 2011;653(1-3):82–8. [DOI] [PubMed] [Google Scholar]
- 71. Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids. 2012;42(6):2411–16. [DOI] [PubMed] [Google Scholar]
- 72. Vahdatpour T, Nokhodchi A, Zakeri-Milani P, Mesgari-Abbasi M, Ahmadi-Asl N, Valizadeh H. Leucine-glycine and carnosine dipeptides prevent diabetes induced by multiple low-doses of streptozotocin in an experimental model of adult mice. J Diabetes Investig. 2019;10(5):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang Y, Wang Y, Kong Y, Zhang X, Zhang H, Gang Y, Bai L. Carnosine prevents type 2 diabetes-induced osteoarthritis through the ROS/NF-κB pathway. Front Pharmacol. 2018;9:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yapislar H, Aydogan S. Effect of carnosine on erythrocyte deformability in diabetic rats. Arch Physiol Biochem. 2012;118(5):265–72. [DOI] [PubMed] [Google Scholar]
- 75. Yay A, Akkuş D, Yapıslar H, Balcıoglu E, Sonmez MF, Ozdamar S. Antioxidant effect of carnosine treatment on renal oxidative stress in streptozotocin-induced diabetic rats. Biotech Histochem. 2014;89(8):552–7. [DOI] [PubMed] [Google Scholar]
- 76. Abdul-Ghani M, DeFronzo RA. Insulin resistance and hyperinsulinemia: the egg and the chicken. J Clin Endocrinol Metab. 2021;106(4):e1897–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hipkiss AR, Chana H. Carnosine protects proteins against methylglyoxal-mediated modifications. Biochem Biophys Res Commun. 1998;248(1):28–32. [DOI] [PubMed] [Google Scholar]
- 78. Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44(10):625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pillon NJ, Croze ML, Vella RE, Soulère L, Lagarde M, Soulage CO. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 2012;153(5):2099–111. [DOI] [PubMed] [Google Scholar]
- 80. Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. 2020;100(1):407–61. [DOI] [PubMed] [Google Scholar]
- 81. Regazzoni L, De Courten B, Garzon D, Altomare A, Marinello C, Jakubova M, Vallova S, Krumpolec P, Carini M, Ukropec Jet al. . A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci Rep. 2016;6(1):27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Szwergold BS. Carnosine and anserine act as effective transglycating agents in decomposition of aldose-derived Schiff bases. Biochem Biophys Res Commun. 2005;336(1):36–41. [DOI] [PubMed] [Google Scholar]
- 83. Aldini G, de Courten B, Regazzoni L, Gilardoni E, Ferrario G, Baron G, Altomare A, D'Amato A, Vistoli G, Carini M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: direct and indirect mechanisms. Free Radic Res. 2020;1–10. [DOI] [PubMed] [Google Scholar]
- 84. Asatoor AM, Bandoh JK, Lant AF, Milne MD, Navab F. Intestinal absorption of carnosine and its constituent amino acids in man. Gut. 1970;11(3):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gardner ML, Illingworth KM, Kelleher JE, Wood DI. Intestinal absorption of the intact peptide carnosine in man, and comparison with intestinal permeability to lactulose. J Physiol. 1991;439(1):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yeum KJ, Orioli M, Regazzoni L, Carini M, Rasmussen H, Russell RM, Aldini G. Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids. 2010;38(3):847–58. [DOI] [PubMed] [Google Scholar]
- 87. Everaert I, Taes Y, De Heer E, Baelde H, Zutinic A, Yard B, Sauerhöfer S, Vanhee L, Delanghe J, Aldini Get al. . Low plasma carnosinase activity promotes carnosinemia after carnosine ingestion in humans. AJP: Renal Physiology. 2012;302(12):F1537–44. [DOI] [PubMed] [Google Scholar]
- 88. Zhao J, Conklin DJ, Guo Y, Zhang X, Obal D, Guo L, Jagatheesan G, Katragadda K, He L, Yin X, Prodhan MA. Cardiospecific overexpression of ATPGD1 (carnosine synthase) increases histidine dipeptide levels and prevents myocardial ischemia reperfusion injury. J Am Heart Assoc. 2020;9(12):e015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mahootchi E, Homaei SC, Kleppe R, Winge I, Hegvik TA, Megias-Perez R, Totland C, Mogavero F, Baumann A, Glennon JCet al. . GADL1 is a multifunctional decarboxylase with tissue-specific roles in β-alanine and carnosine production. Sci Adv. 2020;6(29):eabb3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rezende NS, Swinton P, De Oliveira LF, Da Silva RP, da Eira Silva V, Nemezio K, Yamaguchi G, Artioli GG, Gualano B, Saunders Bet al. . The muscle carnosine response to beta-alanine supplementation: a systematic review with Bayesian individual and aggregate data E-max model and meta-analysis. Front Physiol. 2020;11:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Spelnikov D, Harris RC. A kinetic model of carnosine synthesis in human skeletal muscle. Amino Acids. 2019;51(1):115–21. [DOI] [PubMed] [Google Scholar]
- 92. Saunders B, de Salles Painelli V, De Oliveira LF, da Eira Silva V, Da Silva RP, Riani L, Franchi M, de Souza Gonçalves L, Harris RC, Roschel Het al. . Twenty-four weeks of β-alanine supplementation on carnosine content, related genes, and exercise. Med Sci Sports Exerc. 2017;49(5):896–906. [DOI] [PubMed] [Google Scholar]
- 93. du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl Uet al. . The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol. 2020;598(18):3793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim. 2013;47(4):225–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Statistical code is available upon request. Data and other materials associated with the project are available in the Supplemental Methods, Supplemental Data, Supplemental Human Data, and Supplemental Animal Data files.


