ABSTRACT
Although levodopa remains the most effective drug for symptomatic management of Parkinson's Disease (PD), treatment during advanced disease stages may raise unpredictable motor fluctuations and other complications. Counteracting these complications with other pharmacological therapies may prompt a vicious circle of side effects, and here, nutritional therapy may have great potential. Knowledge about the role of diet in PD is emerging and multiple studies have investigated nutritional support specifically with respect to levodopa therapy. With this systematic review, we aim to give a comprehensive overview of dietary approaches to optimize levodopa treatment in PD. A systematic search was performed using the databases of PubMed and Scopus between January 1985 and September 2020. Nutritional interventions with the rationale to optimize levodopa therapy in human PD patients were eligible for this study and their quality was assessed with the Cochrane risk-of-bias tool. In total, we included 22 papers that addressed the effects of dietary proteins (n = 10), vitamins (n = 7), fiber (n = 2), soybeans (n = 1), caffeine (n = 1), and ketogenic diets (n = 1) on levodopa therapy. Interventions with protein redistribution diets (PRDs), dietary fiber, vitamin C, and caffeine improved levodopa absorption, thereby enhancing clinical response and reducing motor fluctuations. Furthermore, supplementation of vitamin B-12, vitamin B-6, and folic acid successfully reduced high homocysteine concentrations that emerged from levodopa metabolism and promoted many metabolic and clinical complications, such as neuropathology and osteoporosis. In conclusion, dietary interventions have the potential to optimize levodopa efficacy and control side effects. Nutrition that improves levodopa absorption, including PRDs, fiber, vitamin C, and caffeine, is specifically recommended when fluctuating clinical responses appear. Supplements of vitamin B-12, vitamin B-6, and folic acid are advised along with levodopa initiation to attenuate hyperhomocysteinemia, and importantly, their potential to treat consequent metabolic and clinical complications warrants future research.
Keywords: Parkinson's Disease, levodopa, diet, nutritional therapy, vitamin B
Statement of significance: In contrast to adjuvant drug therapy to optimize levodopa treatment, nutritional therapy is a highly acceptable, nonpharmacologic approach to this matter with virtually no side effects. Of all dietary interventions, simple supplementation of vitamin B-12, vitamin B-6, and folic acid is highly promising given its ability to reduce drastic metabolic side effects of levodopa.
Introduction
Parkinson's Disease (PD) is a progressive neurodegenerative disease, primarily characterized by dopamine deficiencies and subsequent movement disabilities, including bradykinesia, balance problems, tremor, and rigidity (1). PD is estimated to affect 1% of the population >65 y, making it the second most common neurodegenerative disease (2, 3). Symptomatic treatment of the motor symptoms in PD is typically achieved with levodopa (3,4-dihydroxy-l-phenylalanine) therapy, which remains the first choice and most effective pharmacological agent (4, 5). This dopamine precursor is absorbed from the intestine by active transporters for large neutral amino acids (LNAAs). In contrast to peripheral dopamine, levodopa can reach the brain by using similar LNAA transporters in the blood–brain barrier (BBB). In the brain, levodopa is converted to dopamine (6, 7). Levodopa is typically co-administered with a DOPA decarboxylase inhibitor such as carbidopa (8, 9). This adjuvant prevents the peripheral breakdown of levodopa, thereby increasing the bioavailability and absorbance rate across the BBB.
The improvement of motor performance achieved by levodopa during early stages of PD is typically experienced as a “honeymoon” phase (10). Notably, as the disease progresses, the therapeutic window for levodopa narrows and detrimental complications may arise (Figure 1) (11, 12). This is consequent to, among others, desensitization of dopamine receptors and dopaminergic cell death and leads to inconsistent clinical responses to levodopa. These responses are typically characterized by unpredictable fluctuations in motor performance and extended periods of wearing-off phenomena (i.e., off-phase), involving rigidity and immobility, and tremor, before the next dose. Based on a cumulative literature review, 40% of patients with PD were estimated to experience complications within 5 y of levodopa treatment (13). Other studies even reported shorter incidence latencies of 2 y (14). In addition to fluctuations in therapeutic response, unfavorable side effects may become more pronounced with persistent levodopa therapy during advanced disease stages. Patients often develop levodopa-induced dyskinesia, a neurological disorder characterized by involuntary choreatic and dystonic movements of extremities (Figure 1) (15). Furthermore, side effects can arise as a result of, predominantly, catechol O-methyltransferase (COMT)-mediated levodopa metabolism (16). Elevated concentrations of homocysteine are reported in levodopa-treated patients (17–19), promoting serious cases of peripheral neuropathy (19–23) and increasing risks of cardiovascular disease (24, 25), cognitive decline (26, 27), and osteoporosis (28). To optimize levodopa efficacy and counteract complications, adjuvant drugs are proposed. For instance, entacapone prevents methylation of levodopa, MAO-B (monoamine oxidase-B) inhibitor limits breakdown of dopamine and levodopa in the brain, and amantadine may alleviate dyskinesias (29–32). Despite effectiveness of these therapies, targeting drug-induced side effects with other pharmacologic agents may lead to a vicious cycle of complications and side effects.
FIGURE 1.
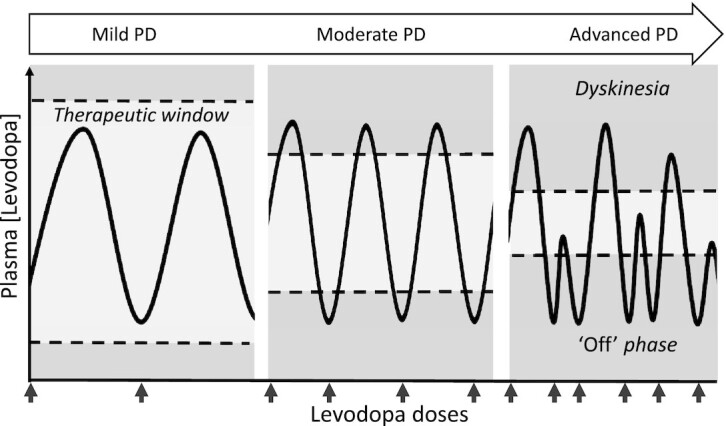
Therapeutic window of levodopa therapy in patients with PD. The therapeutic window of levodopa narrows as PD progresses. Excessive levodopa concentrations may lead to levodopa-induced dyskinesias and insufficient levodopa concentrations may lead to wearing-off phenomena. Responses to levodopa are of shorter duration and become unpredictable and inconsistent. PD, Parkinson's Disease.
Recently, the relation between nutrition and medicinal treatment has gained much attention since specific dietary patterns can increase drug efficacy (33, 34). Not surprisingly, nutritional interventions may play an important role in the optimization of levodopa therapy. To illustrate, absorption of levodopa is influenced by constipation problems inherent to PD and nutrition plays an important role in optimizing drug absorbance. Likewise, the fact that levodopa may cause deficiencies in vitamin B-12, vitamin B-6, and folic acid (35, 36), vitamins crucially involved in the alleviation of detrimental hyperhomocysteine concentrations, stresses the potential of nutritional interventions in preventing levodopa-related side effects. This dietary approach may be concurrent to, or even outweigh, pharmacologic approaches in terms of safety, side effects, invasiveness, and adherence to interventions. Although multiple studies investigated nutritional optimization of levodopa treatment in terms of both pharmacokinetics and pharmacodynamics, a comprehensive overview of the current evidence is lacking.
Here, we conduct a systematic review focusing on nutritional intervention trials that pursue the optimization of the therapeutic effects of levodopa while minimizing adverse side effects. Our vision is to propose a nonpharmacologic optimization strategy to improve pharmacologic management of PD.
Methods
Literature search strategy
The protocol of this systematic review was prospectively registered on PROSPERO (CRD42020211775) and written in accordance with Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (37). A systematic search was performed in the literature databases of PubMed and Scopus to identify relevant articles from January 1985 to September 2020. In addition, reference lists of selected papers were screened for articles that were missed during the primary search. To operationalize the search, 3 search components were coupled with the AND operator (Text Box 1). The first component covers the term Parkinson's Disease, the second component covers levodopa terms, and the third component comprises terms for diets and nutrition. Terms were searched for as Medical Subject Headings (MeSH), title or abstract or keyword search terms (Tiab) or both (All Fields). In PubMed, 3 overarching MeSH terms partly covering the field of nutrition and dietary therapy were added.
Text Box 1.
Combination of search components
#1 Parkinson [All Fields]
#2 levodopa* [Tiab] OR L-dopa [All Fields]
#3 diet* [Tiab] OR food OR micronutri* [Tiab] OR macronutri* [Tiab] OR nutrient [Tiab] OR nutrition [Tiab] OR vitamin* [Tiab] OR Diet, Food and Nutrition [Mesh] OR Nutritional Therapy [Mesh] OR Food-drug Interactions [Mesh]
Complete search: #1 AND #2 AND #3
Study selection process
Studies that met the inclusion criteria included the following: 1) English written clinical intervention trials, including randomized controlled trials, (randomized) crossover interventions and pre/post interventions; 2) levodopa-treated human PD patients; 3) a clear link between levodopa therapy and the rationale for dietary interventions; and 4) levodopa-related outcome measures. After removal of duplicates, a screening protocol was applied to systematically assess eligibility of the articles. A first selection was carried out based on titles and, where needed, short abstract screening. Complete abstracts of the remaining records were carefully screened for inclusion criteria, ultimately followed by thorough eligibility assessment of the full texts. The study selection process was performed by the first author (JTBK) in close collaboration with co-authors (OvdR and IACA) to reach consensus.
Risk-of-bias assessment
Assessment of the methodological quality of the included studies was carried out by evaluating their risk of bias (RoB). RoB is inversely related to quality, such that a low and high RoB indicate high and low overall study quality, respectively. Despite the inclusion of different intervention designs, all studies were assessed with the same revised Cochrane RoB tool to achieve consistency (38). Initially designed for randomized controlled trials, RoB evaluation with the Cochrane tool generates a strict judgment. The tool builds on evaluating the RoB for 5 individual domains, based on multiple questions per domain and a question–answer decision tree to infer the domain RoB. Domains include the following: 1) randomization process, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of outcome data, and 5) selection of reported results. The overarching RoB per study was considered either high (if ≥1 domains have high RoB), moderate (if ≥1 domains have moderate RoB), or low (if all domains have low RoB).
Data extraction and data synthesis
For each included study, information about study type, patient characteristics, levodopa medication, nutritional intervention, results, and RoB was systematically extracted (Table 1). To determine the role of dietary interventions in optimizing levodopa treatment, we strictly focused on outcome measures that are clearly related to levodopa treatment. The quality of studies is considered when weighing evidence and inferring conclusions. The latter is primarily based on studies with high or moderate quality.
TABLE 1.
Data extraction per field
| Data fields | Extracted data |
|---|---|
| Study information | Authors, year, and study design |
| Intervention | Investigated dietary source and intervention setup |
| Patient characteristics | Sample size, age, disease duration, and Hoehn and Yahr stages |
| Levodopa characteristics | Type of levodopa treatment and daily dose |
| Outcome measures | Operationalization of levodopa-related outcome measures |
| Results | Statistical significance, quantified differences, and effect sizes |
| Risk of bias | Judgment made by Cochrane risk-of-bias tool |
Results
Literature search
A systematic literature search was performed (Figure 2). The searches in PubMed and Scopus and the extensive search through reference lists identified 713 records. Based on abstract and title screening 681 studies were excluded, leaving 32 potentially relevant studies for full-text eligibility assessment. Adhering to the inclusion and exclusion criteria, 22 papers were included. Reasons for exclusion were no dietary intervention (n = 1), lack of levodopa-related outcome measure (n = 2), observational studies (n = 4), and overlapping samples between studies (n = 3). These 22 papers consisted of crossover interventions (n = 7), pre/post interventions (n = 6), randomized controlled trials (n = 4), randomized crossover designs (n = 4), and a prospective intervention (n = 1). All studies aimed to either maximize therapeutic effects of levodopa or minimize side effects. Types of dietary sources included dietary proteins (n = 10), dietary fiber (n = 2), vitamins (n = 7), caffeine (n = 1), soybeans (n = 1), and ketogenic diets (n = 1).
FIGURE 2.
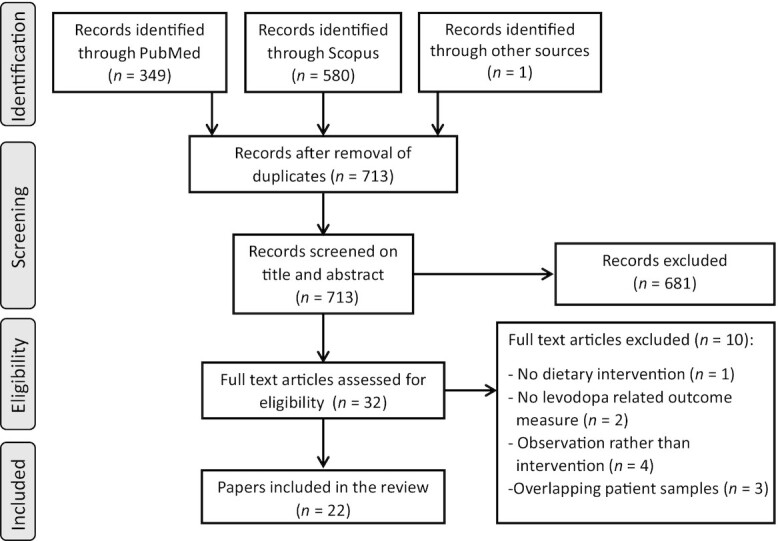
Flow diagram of systematic literature search.
Quality assessment and RoB
An overview of the RoB judgment per study is provided in Table 2. With the strict Cochrane RoB tool, nonrandomized studies automatically score high risks for domain 1 (randomization). To control for this deficit, domain 1 was not included in the overall adjusted RoB judgment for nonrandomized trials and this adjusted RoB was used for the remainder of the review. Three studies were rated as high risk due to significant carryover effects (39), lack of control groups (40), and insufficient clarity of reported results (41). A total of 13 and 6 studies were rated as low and moderate RoB, respectively. The main reasons for moderate RoB judgments were minor protocol adjustments during intervention (42), specific selection of reported results (43, 44), and incomplete intervention details (45–47). Given the inverse relation between RoB and overall study quality, articles with high RoB were not taken into consideration for the conclusion.
TABLE 2.
Risk-of-bias assessment by Cochrane RoB tool1
| Overall risk | |||||||
|---|---|---|---|---|---|---|---|
| First author, year (reference) | 1. Randomization | 2. Intervention | 3. Missing data | 4. Measurement data | 5. Selection results | Cochrane | Adjusted |
| Protein | |||||||
| Juncos, 1987 (48) | High2 | Low | Low | Low | Low | High | Low |
| Pincus, 1987 (49) | High2 | Low | Low | Low | Low | High | Low |
| Frankel, 1989 (42) | High2 | Moderate | Low | Low | Low | High | Moderate |
| Berry, 1991 (43) | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Bracco, 1991 (50) | High2 | Low | Low | Low | Low | High | Low |
| Karstaedt, 1992 (46) | High2 | Moderate | Low | Low | Low | High | Moderate |
| Karstaedt, 1993 (51) | High2 | Low | Low | Low | Low | High | Low |
| Simon, 2004 (39) | High | High | Low | Low | Low | High | High |
| Barichella, 2006 (52) | Low | Low | Low | Low | Low | Low | Low |
| Cucca, 2015 (44) | Low | Low | Moderate | Low | Low | Moderate | Moderate |
| Vitamins | |||||||
| Lamberti, 2005 (53) | High2 | Low | Low | Low | Low | High | Low |
| Postuma, 2006 (47) | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Lee, 2010 (28) | Low | Low | Low | Low | Low | Low | Low |
| Müller, 2013 (54) | High2 | Low | Low | Low | Low | High | Low |
| Rispoli, 2017 (40) | High2 | High | Low | Low | Low | High | High |
| Habibi, 2018 (41) | Low | Low | High | Moderate | High | High | High |
| Nagayama, 2004 (55) | High2 | Low | Low | Low | Low | High | Low |
| Fiber | |||||||
| Astarloa, 1992 (56) | High2 | Low | Low | Low | Low | High | Low |
| Fernandez, 2014 (57) | Low | Low | Low | Low | Low | Low | Low |
| Caffeine | |||||||
| Deleu, 2006 (58) | Low | Low | Low | Low | Low | Low | Low |
| Soybeans | |||||||
| Nagashima, 2016 (59) | High2 | Low | Low | Low | Low | High | Low |
| Ketogenic diet | |||||||
| Elbarbry, 2019 (45) | High2 | Low | Low | Low | Moderate | High | Moderate |
1The RoB, as measured with the Cochrane tool, is assessed for 5 individual domains: 1) randomization process, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of outcome data, and 5) selection of reported results. The RoB is defined as low, moderate, or high and is inversely related to the overall quality. Following the official Cochrane algorithm, the overall RoB is rated low (all individual domains have low RoB), moderate (moderate RoB at least in 1 domain), or high RoB (at least 1 domain high RoB or multiple essential domains moderate RoB). Nonrandomized trials automatically score a high RoB (indicated with a superscript “2”) for the randomization process with the Cochrane tool. Therefore, a high RoB in domain 1 is not taken into account for the overall adjusted RoB judgment of non-randomized studies. RoB, risk of bias.
2Nonrandomized trial.
PD motor symptoms
Most studies focused on levodopa-related motor improvements. With motor symptoms, motor disability, or motor performance, we explicitly refer to motor disabilities inherent to PD that are consequent to dopamine deficiencies. These symptoms include bradykinesia, imbalance, tremor, and rigidity and are represented by the off-phase in Figure 1 where levodopa is either absent or subthreshold. Periods with adequate levodopa concentrations, as demonstrated by clinical improvement in motor function, are marked as “on-phase” or “on periods.” Intuitively, the term “motor fluctuations” refers to the unpredictable switch between on- and off-phases. PD motor symptoms can be measured by subjective scales, simple motor tests, and test batteries including the New York University Rating Scale (NYURS), the New York University Disability Scale (NYUDS), and subscales of the Unified Parkinson's Disease Rating Scale (UPDRS). When levodopa concentrations reach above the upper threshold of its therapeutic window, dyskinesias are induced (Figure 1). These involuntary movements are often measured by the Abnormal Involuntary Movement Scale (AIMS). Finally, the Columbia rating scale measures a continuum ranging from PD motor symptoms (insufficient levodopa) to dyskinesia (excess of levodopa).
Dietary proteins
Multiple studies in the 1980s and 1990s evaluated the effect of dietary proteins on the effectiveness of levodopa, focusing both on pharmacokinetic profile and clinical responses. An overview of these studies is provided in Table 3. The influence of dietary proteins on levodopa was assessed by the administration of different amounts of protein. First, researchers examined the effect of high-protein diets (42, 48, 51).
TABLE 3.
Overview of studies investigating the effect of dietary proteins on levodopa treatment in patients with Parkinson's Disease1
| First author, year (reference); study design | Patient and levodopa characteristics | Dietary intervention | Outcome measures | Results | Risk of bias |
|---|---|---|---|---|---|
| Juncos, 1987 (48); crossover intervention | n = 6, motor fluctuationsMean ± SE age = 59 ± 2.5 yMean ± SE disease duration = 14 ± 2 yH&Y = III to VLevodopa/carbidopa useDose = usual daily dose | Single high-protein load (0.4 g protein/kg body weight, 33% above RDA for 1 meal) versus diet adhering to RDAFour-hour measurement after protein meal | -Plasma LNAA concentrations-Motor symptoms and dyskinesia severity (both measured with 4-point modified Colombia rating scale) | -Increased LNAA concentrations after high-protein load-High-protein load worsened motor response with mean increase of 1.7 points.-High-protein load reduced dyskinesia symptoms with mean reduction of 2.3 points-No effect of RDA meals on motor responses | Low |
| Pincus, 1987 (49); crossover intervention | n = 7, motor fluctuations Mean age (range) = 56 (46–63) y Mean disease duration (range) = 16 (8–22) y H&Y = — Levodopa/carbidopa use Dose (range) = 1243 (400–2800) mg/d | PRD (7 g protein until supper) versus high-protein diet (160 g protein until supper), both combined with normal supper meal (25 g protein); intervention diets on consecutive days | -Plasma LNAA concentrations -Plasma levodopa concentrations-Motor disability: measured by NYUDS -Dyskinesia severity (AIMS) | -Higher LNAA concentrations during high-protein diet versus PRD (1439 vs. 467 μmol/L; change, 71%; P = 0.005) -Higher levodopa concentrations during high-protein diet versus PRD (1.15 vs. 0.80 μmol/L; change, 30%; P = 0.025) -Lower motor disability (better motor performance) on PRD versus high-protein diet (12 vs. 31 points; change, 61%; P = 0.01)-Higher dyskinesia severity on PRD versus high-protein diet (16 vs. 2 points; change, 800%; P = 0.005) | Low |
| Frankel, 1989 (42); pre/post intervention | n = 4, levodopa-related motor fluctuations Mean age (range) = 65 (59–73) y Mean disease duration (range) = 10 (8–15) y H&Y = — Duodenal levodopa infusion Dose = 50 mg bolus, then continue infusion of 50 mg/h | Levodopa infusion for 7.5 h; single oral high-protein drink (60 mg protein) after 4-h infusion | -Plasma concentrations LNAA -Plasma concentrations levodopa -Motor performance: finger-tapping speed (taps/30 s) and walking speed (time/12 m) | -Increased LNAA concentrations after protein drink versus baseline (1913 vs. 1106 μmol/L; change, 72%) -Relatively unaltered levodopa concentrations after protein drink versus baseline (7.5 vs. 6.5 μmol/L; change, 15%) -Slower tapping speed (39 vs. 55 taps; change, 29%) and walking speed (14 vs. 10 s; change, 40%) after protein drink versus baseline | Moderate |
| Berry, 1991 (43); randomized crossover intervention | n = 9 Mean ± SE age = 60.6 ± 1.9 y Mean ± SE disease duration = 12.4 ± 1.4 y H&Y = 2.3 ± 0.2 Levodopa/carbidopa use Dose = 1000 mg/d | Together with levodopa dose, breakfast of “high-protein/low-carb” or “high-carb/low-protein” or balanced carb/protein (5/1) Interventions on 3 consecutive days, overnight wash-out | -Plasma concentrations LNAA -Plasma concentrations levodopa -Subjective motor assessment -Purdue pegboard test -Writing speed test | -Compared with baseline, LNAA concentrations increased after high protein (change, 24%), decreased after high carbohydrate (change, 18%) and was unaltered after balanced breakfast (change, 3%) -Increase in levodopa concentrations (% change from baseline), respectively, 1 and 2 h after high-protein (151%, 29%), high-carb (73%, 82%), and balanced breakfast (83%, 20%) -5 of 9 patients worse subjective motor assessment after high-protein, 3 of 9 worse dyskinesias after high-carb, 1 of 9 worse dyskinesia after balanced breakfast -Pegboard motor performance: steady increase 2 h after balanced breakfast, peak after 1 h, and decline after 2 h in high-protein and high-carb breakfast -No differences in writing time | Moderate |
| Bracco, 1991 (50); pre/post intervention | n = 16, motor fluctuations Mean age (range) = 65 (53–75) y Mean disease duration (range) = 9 (3–14) y H&Y = II-IV Levodopa/carbidopa use with different adjuvants Dose (range) = 625 (375–1000) mg/d | PRD (0.8 g protein/kg body weight, no protein before supper); evaluation at day 7 and 10, follow-up 1–12 mo | -Motor disability: measured by NYURS | -Lower motor disability (better motor performance) while on PRD (23 points) compared with baseline (34 points; P < 0.01) -5 of 16 patients: motor performance improved >20% -11 of 16 patients: motor performance improved up to 12% -3 of 16 patients developed dyskinesias | Low |
| Karstaedt, 1992 (46); crossover intervention | n = 43, with motor fluctuations Mean ± SE age = 69.3 ± 1.5 y Mean ± SE disease duration = 13.7 ± 1.0 y H&Y = — Levodopa/carbidopa use Dose = 839 mg/d | PRD (7 g protein before supper) versus normal hospital diet (protein content unknown) for 2–3 wk | -Motor disability: measured by NYUDS -Average “on” time (% of day) -Walking time 12 m back and forth | -Mean worst disability score lower during PRD (18.7 points) versus normal diet (31.4 points; change, 40.4%; P < 0.01) -On-time during PRD (76%) significantly higher compared with normal diet (17%; P < 0.01) -Faster walking time on PRD (6.3 s) compared with normal diet (16.0 s; P < 0.05) | Moderate |
| Karstaedt, 1993 (51); double-blind, placebo- controlled crossover | n = 18, motor fluctuations on PRD Mean ± SE age = 65.4 ± 2.0 y Mean ± SE disease duration = 11.9 ± 1.7 y H&Y = — Levodopa/carbidopa use Dose = usual daily dose | Single dose of aspartame (600 mg or 1200 mg) or placebo, 2 d intervention, wash-out overnight | -Motor disability: measured by NYUDS -Dyskinesia severity (AIMS) -Plasma levodopa concentrations -Walking speed | -No significant differences between aspartame (600 mg or 1200 mg) or placebo | Moderate |
| Simon, 2004 (39); crossover intervention | n = 20, advanced PD with motor fluctuations Mean ± SE age = 60 ± 10 y Mean disease duration = 10 y H&Y = II-IIII Levodopa use with different adjuvants Dose (± SE) = 213.7 ± 93.7 mg/d | Low-protein breakfast in the morning (7.6 g protein) vs. normal-protein lunch in the afternoon (38.7 g protein) | -Levodopa pharmacokinetics (Cmax, Tmax, AUC) | -Significant increase in AUC during normal-protein lunch (4736 μg/L ⋅ h) versus low-protein breakfast (3187 μg/L ⋅ h; P < 0.001) -After baseline corrections, no significant difference between morning and noon for Cmax, Tmax, and AUC -Carryover effect from morning levodopa concentrations | High |
| Barichella, 2006 (52); randomized single-blind crossover | n = 21, motor fluctuations Mean ± SE age = 60.6 ± 7.6 y Mean ± SE disease duration = 11.5 ± 4.3 y H&Y = II-III Levodopa use with different adjuvants Dose (± SE) = 567.5 ± 226.4 mg/d | PRD (15% protein intake in before supper) using LPP or balanced diet (60% protein intake before supper); both diets contain 0.8 g protein/kg body weight. 2 × 2 mo intervention, 2 mo wash-out | -On/off-periods (minutes/24 h, self-report) -Subjective improvement in postprandial motor blocks (GCI) | -Compared with balanced diet, PRD reduced total off-periods (271 vs. 164 min; P < 0.0001) and postprandial off-periods (79 vs. 49 minutes; P < 0.0001) -Compared with balanced diet, PRD versus prolonged total on-period (738 vs. 852 min; P < 0.0001) and postprandial on-periods (220 vs. 250 min; P < 0.0001) -Subjective improvement in 50% of patients | Low |
| Cucca, 2015 (44); randomized, double-blind, placebo-controlled trial | n = 22, fluctuating response to PRD Mean ± SE age both groups2 = 74 ± 3 y Mean ± SE disease duration both groups2 = 5.8 ± 1.5 y H&Y = — Levodopa use Dose = — | AA supplementation (2 × 8 g minimum of 1 h before levodopa) or placebo for 6 mo | -Oxidative stress, measured by ratio of GSH:GSSG -On/off motor periods | -Compared with baseline, AA supplementation decreased GSSG (5.2 vs. 2.4 μmol/L; P = 0.04) -No significant change in GSG:GSSG from baseline or placebo -No alterations on/off periods | Moderate |
—, results not available; AA, amino acid; AIMS, Abnormal Involuntary Movement Scale; Cmax, maximum concentration; GCI, global clinical impression; GSH, reduced form of glutathione; GSSG, oxidized form of glutathione; H&Y, Hoehn and Yarh stage; LNAA, large neutral amino acid; LPP, low-protein products; NYUDS, New York University Disability Scale; NYURS, New York University Rating Scale; PRD, protein redistribution diet; Tmax, time to reach maximum concentration; UPDRS, Unified Parkinson's Disease Rating Scale.
Age (intervention) = 74 ± 1 y, Disease duration (intervention) = 5.6 ± 1.5 y, Age (placebo) = 74 ± 4 y, Disease duration (placebo) = 6.0 ± 1.4 y.
The pre/post intervention study by Frankel et al. (42) assessed the impact of a single high-protein drink (60 mg protein) after 4 h of stable intraduodenal levodopa infusion in 4 patients with PD (mean age, 65 y) with motor fluctuations. Comparing the high-protein situations with baseline, an increase in plasma concentrations of LNAAs (1913 vs. 1106 μmol/L; change, 72%) was observed. Although plasma levodopa concentrations were relatively unaffected (7.5 vs. 6.5 μmol/L; change, 15%), both finger-tapping speed (39 vs. 55 taps/30 s; change, 29%) and walking speed (14 vs. 10 s/12 m; change, 40%) worsened in response to LNAA plasma increase.
Another study by Juncos et al. (48) included 6 patients (mean age, 59 y) with motor fluctuations. In a crossover intervention, patients consumed a single high-protein load (0.4 g protein/kg body weight) and a normal-protein diet adhering to the RDA. An increase in LNAA concentrations was observed after the high-protein load (data not reported). The normal diet left clinical responsiveness to levodopa unaffected, whereas the high-protein load diminished levodopa response as demonstrated by an increase in motor symptoms (1.7-point increase on the 4-point Colombia rating scale). Simultaneously, patients were less dyskinetic after the protein load (2.3-point decrease on the 4-point Colombia rating scale).
Karstaedt and Pincus (51) further investigated the influence of LNAAs on levodopa response. In 18 patients (mean age, 65.4 y) with motor fluctuations, they evaluated the effect of 1 single dose of aspartame (600 mg or 1200 mg). This placebo-controlled trial reported no impact of aspartame on either levodopa concentrations, dyskinesia, or motor symptoms.
Second, Berry and colleagues (43) evaluated the optimal protein-carbohydrate balance in 9 patients (mean age, 60.6 y). In a crossover trial, patients consumed breakfasts containing high-protein, low-carbohydrate (HPLC); high-carbohydrate, low-protein (HCLP); or balanced carbohydrate-protein (BCP) contents, and motor performance, LNAA plasma concentrations, and levodopa plasma concentrations were assessed. Compared with baseline, LNAA concentrations were increased for HPLC (+24%), decreased for HCLP (−18%), and unaltered for BCP (+3%). Levodopa baseline differences ∼1 and 2 h after breakfast plus levodopa dose increased for all breakfasts (HPLC: 151%, 29%; HCLP: 73%, 82%; BCP: 83%, 20%). Although 5 of 9 patients reported worse subjective motor assessment after HPLC, 3 of 9 reported worse dyskinesias after HCLP and 1 of 9 experienced worse dyskinesia after BCP. Although writing time was not affected by breakfast type, pegboard performance steadily improved after BCP but rapidly peaked and declined with the HPLC and HCLP breakfasts.
Third, several researchers evaluated the consequences of low-protein diets (39) and protein redistribution diets (PRDs), comparing the latter either with normal diets (46, 52, 50) or with high-protein diets (49). Simon et al. (39) performed a crossover intervention to further examine the role of LNAAs on the pharmacokinetic profile of levodopa. In 20 patients (mean age, 60 y) with advanced PD and motor fluctuations, a high-protein lunch (38.7 g protein) was compared with a low-protein breakfast (7.6 g protein). The amount of levodopa reaching the plasma, as measured by the AUC, was significantly higher during lunch than breakfast (4736 vs. 3187 μg/L ⋅ h; P < 0.001). However, this concerns a carryover effect since corrections for baseline levodopa concentrations prior to meal consumption diminished this finding. The maximal levodopa plasma concentration (Cmax) and the time to reach this maximal concentration (Tmax) did not differ.
In a pre/post intervention, 16 patients (mean age, 65 y) with motor fluctuations followed a PRD (50). During this diet, protein intake is limited during the day and compensated during supper (daily 0.8 g protein/kg body weight). PD motor disability, as measured by the NYURS, decreased on average from 34 points at baseline to 24 points during the PRD (change, 32%; P < 0.01). This corresponds to a baseline motor improvement of 20% in 5 of 16 patients and up to 12% in 11 of 16 patients.
In another crossover design, a PRD (7 g protein during the day, 25 g protein during supper) and a high-protein diet (160 g protein during the day, 25 g protein during supper) were administered on 2 consecutive days (49). In 7 patients (mean age, 56 y) with motor fluctuations, plasma concentrations of LNAAs significantly increased during the high-protein diet versus the PRD (1439 vs. 467 μmol/L; change, 71%; P = 0.005). Despite higher levodopa plasma concentrations during the high-protein diet versus the PRD (1.15 vs. 0.80 μmol/L; change, 30%; P = 0.025), PD motor disability, as measured by the NYUDS, was lowest during the PRD versus the high-protein diet (12 vs. 31 points; mean change, 61%; P = 0.01). This infers motor improvement during PRD as compared with the high-protein diet. In contrast, the PRD resulted in more severe dyskinesia symptoms compared with the high-protein diets (16 vs. 2 points; mean change, 800%; P = 0.005).
Karstaedt and Pincus (46) focused specifically on levodopa's pharmacodynamic profile in response to PRD. In their crossover intervention, 43 patients (mean age, 69.3 y) with motor fluctuations followed a PRD (7 g protein before supper, unlimited during supper) or a normal diet. Duration of diets as well as protein content of normal diets were not reported. Compared with the regular diet, the PRD enhanced the clinical efficacy of levodopa as measured by percentage of “on” time during the day (17% vs. 76%; P < 0.01). Similarly, walking speed (seconds/24 m) improved during PRD as compared with a regular diet (6.3 vs 16.0 s; P < 0.05) and the worst disability score (measured by the NYUDS) was reduced from 31.4 points (regular diet) to 18.7 points (PRD) (P < 0.01).
In a randomized crossover intervention (52), a PRD (15% of daily protein intake before supper) was compared with a normal diet (60% of daily protein intake before supper). Both diets adhered to the RDA of 0.8 g protein/kg body weight and the PRD diets strictly prescribed special low-protein products, initially formulated for renal deficiencies. In 21 patients (mean age, 60.6 y) with motor fluctuations, a 2-mo PRD was compared with 2 mo following a normal diet and results included significantly reduced total off-periods (164 vs. 271 min; P < 0.0001) and reduced postprandial off-periods (49 vs. 79 min; P < 0.0001). Likewise, the total on-period was prolonged during PRD versus normal diet (852 vs. 738 min; P < 0.0001), even as the postprandial on-period (250 vs. 220 min; P < 0.0001). In addition, 50% of the patients reported subjective improvement in postprandial motor blocks measured by the Clinical Global Impression Scale.
Rather than limiting dietary proteins, Cucca et al. (44) supplemented a mixture of multiple amino acids [predominantly (iso)leucine, l-valine, l-lysine, and l-threonine] to 22 patients (mean age, 74 y) for 6 mo with the aim to reduce oxidative stress. In a randomized placebo-controlled trial, patients ingested 8 g of amino acids twice daily, a minimum of 1 h prior to levodopa dose. Compared with baseline, amino acid supplementation significantly decreased oxidized glutathione (GSSG; 5.2 vs 2.4 μmol/L; P = 0.04). However, supplementation did not reduce levels of oxidative stress as measured by the ratio of GSSG to reduced glutathione (GSH). Interestingly, on/off-motor periods were not influenced by amino acid supplementation ≥1 h before levodopa administration.
Vitamin supplementation
Interventions with vitamin supplements to improve levodopa treatment involve B vitamins and vitamins C and D (Table 4). Five trials that examined the effects of several B vitamins (vitamin B-12, vitamin B-6, and folic acid), in levodopa-medicated PD patients mainly focused on the consequences of levodopa metabolism, resulting in hyperhomocysteine concentrations (40, 47, 53, 60, 54).
TABLE 4.
Overview of studies investigating the effect of vitamins on levodopa treatment in patients with Parkinson's Disease1
| First author, year (reference); study design | Patient and levodopa characteristics | Dietary intervention | Outcome measures | Results | Risk of bias |
|---|---|---|---|---|---|
| Nagayama, 2004 (55); crossover intervention | n = 67 Mean ±SE age = 77.8 ± 6.0 y Mean ±SE disease duration = 4.1 ± 3.0 y H&Y (±SE) = 3.1 ± 2.1 Use of levodopa Dose = — | Single tablet containing levodopa/ carbidopa/vitamin C (100:10:200 mg) or tablet of levodopa/carbidopa (100:10) without vitamin C; 180 min intervention, 1 wk wash-out | -Levodopa pharmacokinetics (Cmax, Tmax, and AUC) | -No pharmacokinetic differences between with vs. without vitamin C -Negative correlations between baseline levodopa response and levodopa increase after vitamin C (Cmax, P < 0.0001; Tmax, P < 0.001; AUC, P < 0.0001) -Within low baseline levodopa responders (AUC <2500 ng/mL · h): significant improvement with vitamin C vs. without vitamin C for Cmax (1470 vs. 960 ng/mL, P = 0.0017), Tmax (42 vs. 68 min, P = 0.012), and AUC (2080 vs. 1540 ng/mL ⋅ h, P = 0.002) -No changes within high-baseline AUC group | Low |
| Lamberti, 2005 (53); pre/post intervention | n = 20 Mean ± SE age = 65.1 ± 8.5 y Mean ± SE disease duration = 9.4 ± 4.2 y H&Y = II-III Levodopa use Dose (±SE) = 640 ± 240 mg/d n = 35 healthy controls Mean ± SE age = 64.1 ± 11 y | Oral supplementation of vitamin B-12 (500 μg/d) and folic acid (5 mg/d) for 5 wk | -Plasma homocysteine concentrations | -Homocysteine concentrations correlate with levodopa administration within all subjects (r2 = 0.33, P = 0.0003) and with levodopa dose within patients (r2 = 0.23, P = 0.03) -Within patients, B vitamin supplementation reduces homocysteine concentrations (10.5 μmol/L) compared with baseline (17.9 μmol/L, P < 0.0001) -Significant baseline difference between controls and patients (10.6 μmol/L, 17.9 μmol/L; P < 0.0001) diminished after B vitamin supplementation (10.6 vs. 10.5 μmol/L, NS) | Low |
| Postuma, 2006 (47); randomized, double-blind, placebo-controlled trial | n = 35, including levodopa-starters (n = 18) Mean ± SE age = 67.4 ± 7.2 y Mean ± SE disease duration = — H&Y = — All patients: dose (± SE) = 548 ± 264 mg/d Levodopa-starters: dose (median) = 450 mg/d | Supplementation with B vitamins (1 mg/d folic acid and 500 μg/d vitamin B-12) or entacapone (200 mg/d) together with levodopa dose for 6 wk Levodopa-starters: Start levodopa treatment 6 wk prior to intervention | -Serum homocysteine and 3-OMD concentrations | -Levodopa-starters: increased homocysteine concentrations after 6 wk levodopa treatment (10.0 μmol/L) compared with baseline (8.7 μmol/L, P = 0.029) -Baseline change in homocysteine concentrations significantly higher during B vitamin supplementation (from 12.1 to 9.46 μmol/L; change, −2.64) compared with placebo (from 12.2 to 11.2 μmol/L; change, −1.03; P = 0.047) -Entacapone did not differ from placebo -Missing data on: significance of baseline change within B vitamin group; effect of B vitamins within levodopa-starters; 3-OMD concentrations | Moderate |
| Lee, 2010 (60); randomized, open-label, controlled | n = 42, low BMD Mean ±SE age all groups2 = 66.9 ± 5.4 y Mean disease duration all groups2(range) = 46.7 mo (1–156 mo) Mean H&Y all groups2 (±SE) = 1.7 ± 0.5 Levodopa use Dose all groups2 (median/range) = 300 mg/d (0–900 mg/d) | B vitamin supplementation (5 mg/d folic acid, 1500 μg/d vitamin B-12) or antioxidant therapy (1200 mg/d α-lipoic acid) or control for 12 mo | -Serum homocysteine concentrations -BMD (g/cm2) at multiple skeletal sites: lumbar spine, femur neck, total femur, trochanter, femur shaft, Ward's triangle | -Compared with baseline, B vitamins reduced homocysteine concentrations (13.7 vs. 8.8 μmol/L; change; −35.2%; P < 0.001); baseline change within control group was +9.1% (13.0 μmol/L vs. 14.2 μmol/L, NS); significant difference between baseline change of B vitamin group (−35.2%) and control group (9.1%; change, −44.3%; P < 0.001; 95% CI = −70.6, −18.0) -Compared with baseline, BMD in controls decreases at most sites (range, −5.0 to 0.6%), BMD after B vitamins increases at most sites (range, −1.3 to 3.3%); BMD change between control and vitamin group was significant3 at lumbar spine, total femur, trochanter, and femur shaft -No change in homocysteine concentrations or BMD in antioxidant group versus baseline or control | Low |
| Müller, 2013 (54); pre/post intervention | n = 8 Mean ± SE age = 71.38 ± 5.09 y Mean ± SE disease duration = 19.25 ± 4.77 y H&Y (±SE) = 3.75 ± 0.71 Duodenal levodopa/carbidopa gel infusion Dose = — | Monthly intramuscular injection with vitamin B-12 (1000 μg) and vitamin B-6 (200 mg) and oral folic acid (5 mg/d) for 12 mo | -Plasma concentrations of levodopa, homocysteine, and 3-OMD | -Compared with baseline, no changes in levodopa and homocysteine concentrations -Compared with baseline (∼2000 ng/mL), significant increase in 3-OMD concentrations (∼16,000 ng/mL, P < 0.01) | Low |
| Rispoli, 2017 (40); prospective study | n = 30 consecutive patients Mean ± SE age = 67.43 ± 6.54 y Mean ± SE disease duration = 15.76 ± 6.27 y H&Y (±SE) = 2.90 ± 0.63 LCIG infusion Dose = — | For 10 d/mo, oral supplementation of vitamin B-12 (5 μg), folic acid (400 μg), vitamin B-6 (3.0 mg), and riboflavin (2.4 mg) Mean of 42.4 mo follow-up (range, 24–72) Integrative start of LCIG and vitamin supplementation → lack of control group | -Blood tests, including homocysteine plasma concentrations -Neurophysiological assessment of PNP: SAPs, and cMAP | -9 of 30 (30%) patients had pre-existing PNP prior to LCIG/B vitamin treatment -21 of 30 (70%) patients without pre-existing PNP; during integrative LCIG/B vitamin treatment, 4 of 21 (19%) developed PNP and 17 of 21 (81%) did not develop PNP -Compared with baseline concentrations, no significant change in homocysteine after LCIG/B vitamin treatment in any PNP group | High |
| Habibi, 2018 (41); randomized, double-blind, placebo-controlled trial | n = 120, with levodopa-induced dyskinesia Mean ± SE age both groups4 = 46.9 ± 12.3 y Mean disease duration4 = 7.5 y H&Y: = — Levodopa use Dose = — | Oral vitamin D3 1000 IU/d (0.025 mg/d) or placebo for 3 mo | -Duration (hours/day) of dyskinesia -Severity of dyskinesia (UPDRS IV) -Parkinson motor disability (UPDRS) | -No significant differences in duration and severity of dyskinesia, and Parkinson motor disability | High |
BMD, bone mineral density; cMAP, compound muscle action potential; Cmax, maximum concentration; H&Y, Hoehn and Yarh stage; LCIG, levodopa/carbidopa intestinal gel; NYUDS, New York University Disability Scale; NYURS, New York University Rating Scale; PNP, peripheral neuropathy; PRD, protein redistribution diet; SAP, sensory nerve action potential; Tmax, time to reach maximum concentration; UPDRS, Unified Parkinson's Disease Rating Scale; 3-OMD, 3-O-methyldopa.
Age (vitamin group) = 66.1 ± 6.5 y, Disease duration (vitamin group) = 56 mo (12–156 mo), H&Y (vitamin group) = 1.6 ± 0.5, Dose [vitamin group, median (range)] 300 mg/d (0–700), Age (antioxidant group) = 67.4 ± 4.9 y, Disease duration (antioxidant group) = 48 mo (10–132 mo), H&Y (antioxidant group) = 1.7 ± 0.5, Dose [antioxidant group, median (range)] 300 mg/d (0–900), Age (control) = 67.4 ± 4.9 y, Disease duration (control) = 36 mo (1–156 mo), H&Y (control) = 1.8 ± 0.5, Dose [control, median (range)] 300 mg/d (0–900).
Lumbar spine: P = 0.008, 95% CI = 1.3–7.7; Total femur: P = 0.004, 95% CI = 0.9–4.7; Trochanter: P = 0.033, 95% CI = 0.3–7.5; Femur shaft, P = 0.002, 95% CI = 1.1–4.6.
Age (intervention) = 44.02 ± 13.2 y, Disease duration (intervention) = 7.2 y, Age (placebo) = 49.9 ± 11.4 y, Disease duration (placebo) = 7.8 y.
Investigating homocysteine-lowering therapies, a pre/post intervention performed by Lamberti et al. (53) prescribed oral supplements of vitamin B-12 (500 μg/d) and folic acid (5 mg/d) to 20 patients (mean age, 65.1 y) for 5 wk. A healthy subject sample (n = 20; mean age, 64.1 y) was included as a control group. Considering all participants, levodopa administration (binary yes or no) correlated positively with homocysteine plasma concentrations (r2 = 0.33, P = 0.0003). Within patients, levodopa dose correlated positively with homocysteine plasma concentrations (r2 = 0.23, P = 0.03). Supplementation with B vitamins significantly reduced homocysteine concentrations compared with baseline (10.5 vs. 17.9 μmol/L; P < 0.0001). Moreover, initial significant differences between controls and patients (10.6 vs. 17.9 μmol/L; P < 0.0001) were eliminated after vitamin B-12 plus folic acid supplementation (10.6 vs. 10.5 μmol/L; NS).
A similar, but randomized, placebo-controlled trial (47) involved 35 patients (mean age, 67.4 y). Among them, 18 levodopa-naive patients started levodopa treatment 6 wk prior to the study and homocysteine plasma concentrations increased significantly during these weeks (8.7 vs. 10.0 μmol/L; P = 0.029). A 6-wk homocysteine-lowering intervention followed in which patients were either administered entacapone (200 mg/d), B vitamin supplements (1 mg/d folic acid and 500 μg/d vitamin B-12), or placebo. A comparison of baseline changes in homocysteine serum concentrations between the placebo group (12.2 vs. 11.2 μmol/L; change, −1.03 μmol/L) and the B vitamin group (12.1 to 9.46 μmol/L; change, −2.64 μmol) revealed significantly greater baseline changes in the B vitamin group (P = 0.047). Within the vitamin B-12 plus folic acid group, the statistical inference of this baseline change as well as the homocysteine-lowering effects within the levodopa starters were not reported. No significant effects were observed in the entacapone group.
A well-established link exists between levodopa-induced hyperhomocysteinemia and consequent osteoporosis (28). This randomized controlled study included 42 patients (mean age, 66.9 y) with low bone mineral density (BMD). Three experimental groups were supplemented either with folic acid (5 mg/d) plus vitamin B-12 (1500 μg/d), antioxidants (1200 mg α-lipoic acid/d), or nothing (control group). Per group, the effects on serum homocysteine concentrations and BMD were examined. Compared with baseline, 12 mo of vitamin B-12 plus folic acid significantly reduced serum homocysteine concentrations (13.7 vs. 8.8 μmol/L; change, −35.2%; P < 0.001). Within the control group, homocysteine concentrations changed by +9.1% from baseline (13.0 μmol/L) to 12 mo later (14.2 μmol/L). The difference in baseline changes between the B vitamin group (+9.1%) and the control group (−35.2%) was significant (difference = 44.3%, P < 0.001; 95% CI = −70.6, −18.0) . During this year, BMD decreased at most skeletal sites in the control group (range, −5.0% to 0.6%), whereas it increased at most skeletal sites in the B vitamin-supplemented patients (range, −1.3% to 3.3%). BMD differences between the control group and the group that received vitamin B-12 plus folic acid were significant at most sites (see Table 4). In contrast, antioxidant therapy had no significant effect on homocysteine concentrations or on BMD.
In addition to oral vitamin therapy, Müller et al. (54) included 8 patients (mean age, 71.4 y) on duodenal levodopa/carbidopa gel infusion and administered monthly intramuscular injections of vitamin B-6 (200 mg) and vitamin B-12 (1000 μg) combined with daily oral 5 mg folic acid for 12 mo. Compared with baseline, no significant changes were observed in levodopa plasma concentrations or homocysteine concentrations. Concentrations of levodopa metabolite 3-O-methyldopa (3-OMD) significantly increased after vitamin B-12 and vitamin B-6 supplementation (∼16,000 ng/mL) compared with baseline (∼2000 ng/mL; P < 0.01).
In a prospective study by Rispoli and colleagues (40), 30 consecutive patients (mean age, 67.43 y) started integrative levodopa/carbidopa intestinal gel infusion and B vitamin supplements for 10 d/mo consisting of vitamin B-12 (5 μg), folic acid (400 μg), vitamin B-6 (3.0 mg), and riboflavin (2.4 mg). No control group was included. During an average follow-up of 3.5 y, researchers monitored, among others, homocysteine concentrations and consequent peripheral neuropathy (PNP). Pre-existing PNP was established in 9 patients, whereas 21 patients had no history of PNP. Among the latter patient group, 4 developed PNP during integrative treatment and no significant elevations were observed in homocysteine concentrations.
Second, with regard to other vitamins, 1 study investigated the effects of vitamin C on levodopa pharmacokinetics (55). In a crossover intervention, 67 patients (mean age, 77.8 y) consumed a single tablet of levodopa/carbidopa/vitamin C (100/10/200 mg) and 1 levodopa/carbidopa tablet (100/10 mg) 1 wk later. Overall, Cmax, Tmax, and AUC revealed no pharmacokinetic enhancing effect of vitamin C. Nonetheless, low baseline levodopa responders demonstrated a higher benefit of vitamin C as indicated by a negative correlation between baseline levodopa concentrations and increases after vitamin C for all pharmacokinetic parameters (Cmax, P < 0.0001; Tmax, P < 0.001; AUC, P < 0.0001). Within low baseline levodopa responders (AUC <2500 ng/mL ⋅ h) comparing with vitamin C versus without vitamin C showed a significantly higher Cmax (1470 vs. 960 ng/mL; P = 0.0017), faster Tmax (42 vs. 68 min; P = 0.012), and greater AUC (2080 vs. 1540 ng/mL ⋅ h; P = 0.002).
Finally, in a randomized placebo-controlled trial, 120 patients (mean age, 46.9 y) with levodopa-induced dyskinesias took oral vitamin D supplements (1000 IU = 0.024 mg/d) or placebo for 3 mo (41). Comparing vitamin supplements with placebo, simple Student's t tests initially revealed significant treatment effects for severity of dyskinesia (UPDRS IV; 1.8 vs. 2.2; P = 0.024), duration of dyskinesia (1.2 vs. 2.2 h/d; P = 0.008), and PD motor disability (UPDRS; 18.0 vs. 19.2; P = 0.035). However, when adjusting for covariates of age, sex, duration of dyskinesia, and duration of PD, the effects of treatment were diminished. This implies that initially significant results were due to confounding differences between groups rather than vitamin D treatment.
Other dietary interventions
Other dietary interventions involved dietary fiber, caffeine, soybeans, and ketogenic diets (Table 5). The potency of dietary fiber in optimizing levodopa therapy has been twice investigated in PD patients. Astarloa et al. (56) included 19 patients (mean age, 67.3 y) with constipation problems. As compared with baseline values, consuming a diet rich in insoluble fiber (28 g fiber/d) for 3 mo significantly diminished constipation (P < 0.001), increased plasma levodopa concentrations (953.8 vs. 1266.6 ng/mL; P = 0.002), and reduced the levodopa metabolite 3-OMD (5786.8 vs. 5303.1 ng/mL; P = 0.034). Compared with baseline, the high-fiber diet reduced global motor disability as measured by the UPDRS (18 vs. 11 points; P < 0.001) and approached statistical significance for the assessment of upper extremities coordination (P < 0.059) and gait (P < 0.050).
TABLE 5.
Overview of studies investigating the effect of other dietary interventions on levodopa treatment in patients with Parkinson's Disease1
| First author, year (reference); study design | Patient and levodopa characteristics | Dietary intervention | Outcome measures | Results | Risk of bias |
|---|---|---|---|---|---|
| Fiber | |||||
| Astarloa, 1992 (56); pre/post intervention | n = 19, constipation Mean ± SE age = 67.3 ± 1.70 y H&Y = II-III Mean ± SE disease duration = 5.10 ± 0.94 y Mean ± SE disease duration = 5.10 ± 0.94 y Levodopa use with different adjuvants Dose = usual daily dose | DRIF (28 g/d fiber) for 2 mo | -Plasma concentrations of levodopa and 3-OMD -Parkinson (motor) disability (UPDRS, upper extremities coordination, gait) -Constipation (bowel movements and feces consistency) | -Compared with baseline, DRIF increased levodopa concentrations (953.8 vs. 1266.6 ng/mL, P = 0.002) and reduced 3-OMD concentrations (5786.8 vs. 5303.1 ng/mL, P = 0.034) -Compared with baseline, DRIF reduced motor disability (UPDRS; 18 vs. 11 points, P < 0.001) -DRIF caused borderline significant improvement in upper extremities coordination (P < 0.059) and gait (P < 0.050) -DRIF reduces constipation (P < 0.001) | Low |
| Fernandez, 2014 (57); randomized double-blind crossover intervention | n = 18 Mean ± SE age = 69.8 ± 4.2 y Mean ± SE disease duration = 1.3 ± 0.5 y H&Y = — Levodopa/carbidopa use Dose = usual daily dose | Plantago ovata husk (3.5 mg), in 200 mL water 3×/d or placebo before levodopa; 2 × 14-d intervention, 7 d wash-out | -Levodopa pharmacokinetics (Cmax, Tmax, and AUC) -Number of peaks in levodopa concentration, following single dose | -No significant differences in Cmax, Tmax, AUC -Plantago ovata husk treatment reduced number of concentration peaks: at baseline/placebo/fiber, the number of patients with multiple peaks was 8/9/2 | Low |
| Caffeine | |||||
| Deleu, 2006 (58); randomized double-blind crossover | n = 12 Mean ± SE age = 61 ± 9 y Mean ± SE disease duration = 6.3 ± 3.1 y H&Y = I-III Levodopa/carbidopa use Dose (±SE) = 604 ± 127 mg/d | Caffeine (200 mg) or placebo 15 min before single tablet of levodopa/carbidopa (250/25 mg); 4 d in total, 2-d intervention, 48-h wash-out | -Levodopa pharmacokinetics (Cmax, Tmax, and AUC) -Motor performance: walking speed (onset + magnitude), finger-tapping speed (onset + magnitude)-4-point dyskinesia scale | -Shorter Tmax with caffeine versus placebo (60 vs. 90 min, P = 0.003), no differences in Cmax and AUC -Comparing caffeine versus placebo, shorter walking onset (30 vs. 60 min, P = 0.03), higher walking magnitude (average increase of 44%, P < 0.0001), shorter tapping onset (30 vs. 90 min, P = 0.009), no difference in tapping magnitude -No difference in dyskinesia scores | Low |
| Soybeans | |||||
| Nagashima, 2016 (59); crossover intervention | n = 7, dyskinesia and wearing-off phenomena Mean ± SE age = 67.1 ± 7.9 y Mean ± SE disease duration = 10.7 ± 3.5 y H&Y (±SE) = 3.0 ± 0.6 Levodopa/carbidopa use with different adjuvants Dose = 378.6 mg/d | Tablet of levodopa/carbidopa in combination with 11 g ground soybeans versus tablet levodopa/carbidopa without soybeans; 3-h intervention, 1-wk wash-out | -Plasma concentrations of levodopa and 3-OMD -On-periods (minutes/3 h, self-reported) -Dyskinesia severity (mAIMS) | -Comparing levodopa AUC and 3-OMD AUC without versus with soybeans, no differences; comparing EMM (adjusted for covariates) with soybeans versus without soybeans, decrease in 3-OMD (317.0 vs. 374.6 ng/mL, P = 0.03) -Longer on-periods with soy consumption (80% increase, P = 0.028) -EMM of dyskinesia severity lower with soy (1.9) versus without soy (3.9, P < 0.001) | Low |
| Ketogenic diet | |||||
| Elbarbry, 2019 (45); pre/post intervention | n = 4 Age = — Disease duration = — H&Y = II-III Levodopa/carbidopa use Dose = — | Ketogenic diet (80% fat, 15% protein, 5% carbohydrates) for 12 wk | Levodopa pharmacokinetics (Cmax, Tmax, and AUC) | Compared with baseline, no changes in Cmax, Tmax, and AUC | Moderate |
Cmax, maximum concentration; DRIF, diet rich in insoluble fiber; EMM, estimated marginal means; H&Y, Hoehn and Yarh stage; (m)AIMS, (modified) Abnormal Involuntary Movement Scale; Tmax, time to reach maximum concentration; UPDRS, Unified Parkinson's Disease Rating Scale; 3-OMD, 3-O-methyldopa.
More recently, in a randomized crossover intervention including 18 patients with PD (mean age, 69.8 y) the water-soluble fiber Plantago ovata husk (3.5 mg, 3×/d) or placebo was prescribed immediately before levodopa administration (57). Fiber supplementation had no significant effect on pharmacokinetic parameters of levodopa (Cmax, Tmax, and AUC) but affected the number of plasma levodopa concentration peaks following a single levodopa dose. Although 8 and 9 patients demonstrated multiple levodopa peaks at baseline and with placebo, respectively, only 2 patients had >1 peak after fiber treatment.
In a randomized crossover study, the influence of caffeine on both the pharmacokinetics and pharmacodynamics of levodopa was assessed in 12 patients (mean age, 61 y). Subjects received caffeine (200 mg) or placebo 15 min prior to 1 single levodopa/carbidopa tablet (250/25 mg) with a 48-h wash-out time (58). Although caffeine did not influence Cmax and AUC, Tmax was significantly shorter compared with placebo (60 vs. 90 min; P = 0.003). Both walking onset and walking magnitude (difference between baseline and maximum effect) improved as compared with placebo (onset: 30 vs. 60 min; P = 0.03; magnitude: average increase, 44%; P < 0.0001). With regard to finger-tapping performance, patients had a faster onset time (30 vs. 90 min; P = 0.009) but magnitude was not affected by caffeine, nor did caffeine affect dyskinesia severity.
Another crossover intervention examined the influence of soybean consumption (11 g) along with a usual levodopa/carbidopa dose in 7 patients (mean age, 67.1 y) with dyskinesia and wearing-off symptoms (59). AUCs for levodopa and 3-OMD were initially not significant. Adjusting for covariates by applying the estimated marginal means (EMM; ng/mL) revealed a significant decrease in 3-OMD plasma after soybean ingestion compared with without soybean ingestion (317.0 vs. 374.6 ng/mL; P = 0.03). This was accompanied by a self-rated longer on-period (270 vs. 150 min; P = 0.028) and relief in dyskinesia symptoms (AIMS) with soybeans versus without soybeans (EMM of 1.9 vs. 3.9; P < 0.001).
Last, Elbarbry and colleagues (45) reported that a ketogenic diet (80% fat, 15% protein, 5% carbohydrates) did not influence pharmacokinetics and pharmacokinetics of levodopa. Four patients (mean age, unreported) followed the diet for 12 wk but, compared with baseline, the diet had no effect on Cmax, Tmax, or AUC.
Discussion
Dietary protein
In this systematic review we investigated the potential of nutritional interventions in optimizing levodopa efficacy or minimizing levodopa's side effects. Multiple studies assessed the influence of dietary protein on levodopa therapy by prescribing diets with different amounts of protein. High-protein interventions led to elevated plasma concentrations of LNAAs, accompanied by reduced effectiveness of levodopa, as demonstrated by a decline in motor performance (42, 48, 49). On the contrary, elimination of daytime dietary protein, by PRDs, improved and prolonged motor function in patients with fluctuating levodopa responses (46, 52, 50, 49). Based on these studies, we conclude that high amounts of protein interfere with levodopa therapy and PRDs prevent this interference, thereby improving levodopa bioavailability and motor function. The findings are in line with 2 earlier reviews by Cereda et al. (61) and Wang et al. (62) who covered this topic. In contrast to these reviews, we included clinical intervention trials evaluating all protein diets (high, PRD, and low) rather than only PRD trials (61) or a combination of clinical and preclinical, intervention, and observation studies (62). Despite these disparities, the findings substantiate the same, aforementioned conclusion.
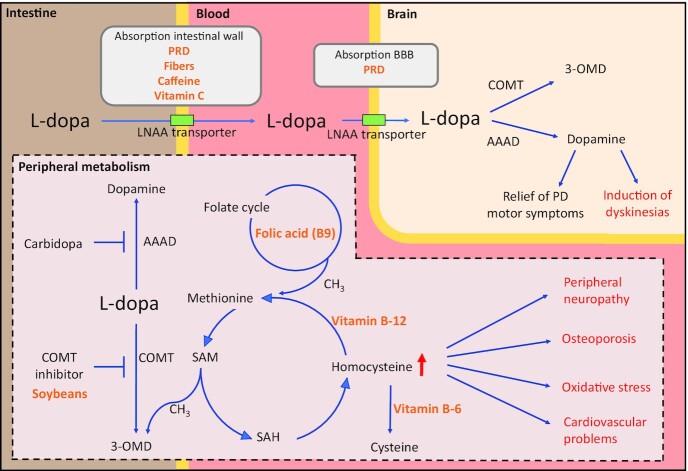
Competitive absorption between LNAAs and levodopa, which is also classified as an LNAA, underlies protein interference in levodopa-treated patients (Figure 3). Located on the intestinal wall, an active transport system with limited capacity is shared between levodopa and other LNAAs and brain influx is executed by similar carrier systems across the BBB (42). When protein is excessively consumed, competitive transport across the BBB forms a more prominent cause for motor fluctuations compared with competition at the intestine. The latter can be concluded from observations by Frankel et al. (42) and Pincus and Barry (49), where high LNAA plasma concentrations, first, do not obstruct levodopa from reaching the systemic blood circulation, but second, do diminish the anti-Parkinsonian response of levodopa, presumably by obstructing absorbance into the brain. Similarly, low LNAA concentrations minimally affect levodopa blood concentrations while they significantly improve clinical motor function, indicating facilitated transport across the BBB (49). Interestingly, although the effect is less pronounced, there is still competition between LNAAs and levodopa at the intestine. Therefore, a simultaneous plasma increase in levodopa and LNAAs following high-protein diets remains unexpected (49). As hypothesized, elevated levodopa plasma concentrations after high-protein diets may reflect a “closed levodopa gate” located at the BBB. The final observation that substantiates prominent transport competition at the BBB concerns the finding by Frankel et al. (42) and Nutt et al. (63) that circumventing gastrointestinal absorption using intravenous levodopa infusions does not prevent protein-related motor fluctuations.
FIGURE 3.
Levodopa pathway and nutritional interventions in patients with PD. Nutritional interventions (in orange) may interact at multiple sites within the levodopa metabolism pathway to increase the efficacy or temper the side effects of levodopa therapy. Levodopa is absorbed from the intestine to the systemic circulation and nutritional interventions that improve this absorption across the intestinal wall include PRDs, dietary fiber, caffeine, and vitamin C. Similarly, transportation across the BBB from the bloodstream to the brain is facilitated by PRDs. In the periphery, levodopa is partly metabolized by COMT-mediated methylation. Soybeans may potentially act as COMT inhibitors by preventing this metabolism. Furthermore, this methylation reaction requires CH3 donation by SAM, which is subsequently converted into SAH. Eventually, this leads to elevated homocysteine concentrations, a risk factor for peripheral neuropathy, osteoporosis, oxidative stress, and cardiovascular diseases. Alleviation of homocysteine concentrations occurs either through trans-sulfuration to cysteine or through remethylation to methionine. This first reaction requires vitamin B-6. The second reaction requires vitamin B-12 and donation of CH3 by the folate cycle, which, in turn, requires folic acid (vitamin B-9). B vitamin supplements, including vitamin B-12, vitamin B-6, and folic acid, can effectively attenuate hyperhomocysteinemia and have the potency to treat or prevent related complications. AAAD, aromatic l-amino acid decarboxylase; BBB, blood–brain barrier; CH3, methyl group; COMT, catechol O-methyltransferase; L-dopa, levodopa; LNAA, large neutral amino acid; PD, Parkinson's Disease; PRD, protein redistribution diet; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; 3-OMD, 3-O-methyldopa.
Adhering to daytime protein restrictions to improve levodopa brain availability constitutes 2 limitations. First, the development of levodopa-induced dyskinesias forms a major drawback for patients with PD (Figure 1). High-protein diets relieve dyskinesia symptoms (48, 49), whereas PRDs rather induce or worsen dyskinesias (50, 49). In the latter 2 PRD studies, enhanced levodopa efficacy permitted a reduction in levodopa dose in some patients to achieve similar motor improvements while avoiding dyskinesias. Despite 1 successful preclinical trial that prevented levodopa-induced dyskinesias in monkeys by administering DHA (64), a clinically approved nutritional approach is lacking. The second complication of limited protein consumption concerns the risk for weight loss and malnutrition, as indicated by Barichella et al. (52). Therefore, PRDs are generally preferred over low-protein diets to ensure sufficient protein intake. A limitation that applies to many protein-levodopa studies performed between 1980 and 2000 is the limited number of participants. Nevertheless, similar results that substantiate the inverse relation between dietary protein and levodopa efficacy are reported in a well-powered (n = 600) recent observational study by Barichella and colleagues (65). Finally, it is important to consider levodopa-optimizing interventions in light of PD disease stage. Clinical fluctuations to levodopa typically occur in patients with moderate and advanced PD and, therefore, this group may experience an above average benefit from PRDs. These patients require higher levodopa doses, achieved by PRDs, to relieve motor symptoms. Simultaneously, the risk for dyskinesia rises because the levodopa threshold for dyskinesias is relatively low (Figure 1).
In contrast to protein restriction, Cucca and colleagues (44) examined supplementation of amino acids. This method has been reported to reduce oxidative stress in PD (66), a phenomenon related to levodopa therapy and subsequent increase in homocysteine concentrations (see "Vitamins" section and Figure 3). Although oxidative stress was not attenuated, this study demonstrated that amino acid supplementation >1 h before levodopa intake did not influence motor performance. Thereby, Cucca et al. (44) substantiated the importance of timing of protein intake and levodopa administration during the day to achieve optimal levodopa effects.
Together, the results included in this systematic review provide substantial evidence for the inverse relation between dietary protein and motor performance in patients with fluctuating clinical responses. Protein restriction during the day, compensated for during dinner, provides a successful dietary approach to target these motor fluctuations. While on a PRD, the hazard of levodopa-induced dyskinesias is noteworthy and, in some patients, dose reductions are permitted and required to prevent their manifestation.
Vitamins
In this section, we switch from motor complications to metabolic side effects of levodopa. Supplementation with B vitamins, including vitamin B-12, vitamin B-6, and folic acid, was investigated in 5 independent trials and the combined results reveal a promising approach to reduce levodopa-induced hyperhomocysteinemia (40, 47, 53, 60, 54). The incidence of hyperhomocysteinemia, a metabolic condition marked by elevated concentrations of homocysteine, is extensively related to levodopa therapy (17, 19, 67, 68). The metabolic pathway that underlies this relation is schematically illustrated in Figure 3. Peripheral levodopa metabolism through COMT-mediated methylation produces 3-OMD (7, 69). To establish this reaction, S-adenosylmethionine donates a methyl group (CH3) and is then converted to S-adenosylhomocysteine, eventually resulting in elevated concentrations of homocysteine. Several B vitamins play an essential role in the attenuation of homocysteine accumulation (Figure 3). Alleviation of hyperhomocysteinemia occurs either by remethylation to methionine, requiring cofactor vitamin B-12 and methyl donation by one-carbon folate (folic acid) cycle, or by trans-sulfuration to cysteine, requiring vitamin B-6 (7, 69). Not surprisingly, deficiencies in these B vitamins, which often arise from levodopa medication, can further mediate levodopa-induced hyperhomocysteinemia (35, 36, 70).
Hyperhomocysteinemia is a well-established risk factor for multiple health complications. Levodopa-induced hyperhomocysteinemia has been reported to promote PNP (19, 21–23), osteoporosis (28, 71, 72), cognitive decline (26, 27), cardiovascular problems (24, 25), and accumulation of reactive oxygen species (Figure 3) (73). Hypothetically, vitamin B-12, vitamin B-6, and folic acid may play a role in the prevention of the consequences of hyperhomocysteinemia as these vitamins show promising homocysteine-lowering abilities. While of interest, this speculation is scarcely investigated. To illustrate, PNP is the most evident consequence of levodopa-induced hyperhomocysteinemia, estimated to occur in 55% of all levodopa-treated patients (23). Although this phenomenon is referred to as “the elephant in the room” (74), and supplementation of vitamin B-12, vitamin B-6, and folic acid has been repeatedly suggested (19, 35, 75, 76), it was only applied by Rispoli et al. (40). Their prospective study design and integrative vitamin supplementation (vitamin B-12, vitamin B-6, folic acid, and riboflavin) plus levodopa therapy obstructs drawing conclusions on the efficacy of these B vitamins in the prevention of PNP. Nevertheless, homocysteine concentrations did not increase despite initiation of levodopa treatment. This tentatively suggested a protective role of integrated levodopa therapy and B vitamin supplementation, including vitamin B-12, vitamin B-6, and folic acid. Concerning the risk for osteoporosis, Lee et al. (60) succeeded in normalization of low BMD in levodopa-treated PD patients by reducing homocysteine concentrations with vitamin B-12 and folic acid supplements. The fact that patients additionally took vitamin D right from the start suggests that the combination of vitamin B-12, folic acid, and vitamin D was more effective than vitamin D alone, presumably through a reduction in homocysteine concentrations.
Finally, 2 observations are noteworthy. The first involves a comparison between dietary B vitamin supplements, namely vitamin B-12 and folic acid, and pharmacologic entacapone administration. Specifically, COMT inhibitors, such as entacapone, prevent methylation of levodopa and thereby hypothetically alleviate hyperhomocysteinemia (Figure 3). Interestingly, Postuma et al. (47) reported larger homocysteine-lowering effects of vitamin B-12 and folic acid in comparison to entacapone, which gained interest for dietary interventions above pharmacologic treatment. It is noteworthy that homocysteine-lowering results of entacapone are controversial (77–79). Moreover, the effect of entacapone has been attributed to B vitamin status: the lower the folic acid concentrations, the higher the homocysteine-lowering effect of entacapone (80). Second, in contrast to other studies, Müller and colleagues (54) reported no homocysteine-lowering effect of vitamin B-12, vitamin B-6, and folic acid. However, a drastic increase in 3-OMD concentrations suggests accelerated COMT-mediated levodopa metabolism (Figure 3). This may occur when methyl group donors, including vitamins, are available in excess. Notably, this study differs from the other studies since both aforementioned B vitamins and levodopa were injected intravenously rather than orally consumed (47, 53, 60). Comparisons between studies are obstructed by these differences and current conclusions on homocysteine-lowering effects of vitamin B-12, vitamin B-6, and folic acid are restricted to oral treatment strategies.
Taken together, the herein reported studies yielded promising results and provide a potential dietary approach to reduce or even prevent levodopa-induced hyperhomocysteinemia using B vitamins including vitamin B-12, vitamin B-6, and folic acid. Despite ample observational studies on the relation between the status of these B vitamins, levodopa, and hyperhomocysteinemia-related complications, intervention studies are urgently needed to determine whether the aforementioned B vitamins can counteract these complications, including PNP, osteoporosis, and cardiovascular problems.
In addition to the B vitamins, vitamin C may beneficially affect the pharmacokinetics of levodopa in patients who initially demonstrate a low response to the drug (Figure 3) (55). Interestingly, vitamin C is often added as stabilizer for solutions of levodopa plus carbidopa (81, 82). This liquid levodopa formula is sometimes preferred over the solid compound, because it offers a more continuous delivery of the drugs. In addition to the stabilizing ability of vitamin C, more research is needed to draw conclusions regarding its beneficial effect on levodopa pharmacokinetics.
Other dietary interventions
Other investigated dietary sources include dietary fiber, caffeine, and soybeans. With regard to dietary fiber, 2 studies reported benefits for the maximization (56) and stabilization (57) of levodopa absorption (Figure 3). In the study by Astarloa et al. (56), the improved pharmacokinetics and motor performance, combined with reduced 3-OMD plasma concentrations, indicated that enhanced efficacy is established through faster absorption and limited premature metabolism. The consumption of Plantago ovata husk, applied in the study by Fernandez and colleagues (57), did not directly improve levodopa pharmacokinetics. However, it did promote the constancy and stability of gastrointestinal absorption of levodopa, as demonstrated by a reduced number of concentration peaks following a single levodopa dose. The latter study contrasts previous experiments in rabbits where Plantago ovata husk drastically increased the pharmacokinetic profile of levodopa (83, 84). Comparing the 2 clinical studies, it is noteworthy to mention that Astarloa et al. (56) included patients with constipation problems. Deficits in gastric emptying and constipation are common autonomic symptoms of PD (85), and partially underlie fluctuations in clinical response to levodopa due to inconsistent drug absorption (86, 87). Dietary fiber generally improves constipation problems and stool frequency in PD patients (88, 89). Intuitively, one might argue that the potency of dietary fiber to increase levodopa efficacy relies on its laxative properties and, thus, is more pronounced in constipated patients.
Similarly to dietary fiber, caffeine improves levodopa absorption time and motor performance (Figure 3) (58). These findings may rely on enhanced gastric emptying by caffeine, thereby accelerating drug absorption. However, the fact that caffeine increases energy expenditure challenges the attribution of improved motor performance to accelerated levodopa absorption rather than the direct effects of caffeine (90, 91). Furthermore, Deleu and colleagues (58) did not show any effect on levodopa-induced dyskinesias. This finding is in contrast to observations that coffee consumption can reduce the risk of levodopa-induced dyskinesias, presumably established through its antagonistic effect on the A2A receptors (92, 93).
Finally, results on soybeans from Nagashima et al. (59) are interesting but ambiguous since prolonged levodopa efficacy was observed in combination with a reduction in levodopa-induced dyskinesias. In addition, levodopa concentrations remained unaffected, whereas 3-OMD concentrations decreased, suggesting less levodopa metabolism (Figure 3). This combination of results led to the speculation that soybeans may contain several unexplored substances that influence levodopa pharmacodynamics and provide COMT-inhibition activity, as previously observed in cell cultures (94). Future studies are needed to examine the role of soybeans in levodopa-treated PD.
Conclusions
The efficacy of levodopa treatment in PD is not always optimal and is often accompanied by side effects. Specific dietary approaches may modulate the effect of levodopa by either optimizing its desired therapeutic effects or by reducing its side effects. Successful dietary methods to optimize the preferable effects typically promote maximal and continuous absorption of levodopa and include PRDs, fiber, caffeine, and vitamin C. Although the latter 3 rely predominantly on improved gastric emptying, PRD facilitates levodopa absorption by limiting protein interference. The control of levodopa-induced complications may primarily be established through managing its metabolic pathways. A complex interplay exists between levodopa; the status of vitamin B-12, vitamin B-6, and folic acid; elevated concentrations of homocysteine; and homocysteine-related consequences of PNP, osteoporosis, cognitive decline, and cardiovascular problems. In these pathways, therapy with B vitamins, including vitamin B-12, vitamin B-6, and folic acid, provides a highly promising homocysteine-attenuating approach, thereby targeting the backbone of many levodopa-induced metabolic complications. The downside of improved levodopa effectiveness concerns the increased risk of levodopa-induced dyskinesias and, so far, a clinically approved nutritional therapy to this complaint is lacking. These results clearly pave the way for future research directions. More investigation is needed to clarify the benefits of vitamin B-12, vitamin B-6, and folic acid supplements on complications that occur as a consequence of levodopa-induced hyperhomocysteinemia. Considering all the evidence, nutritional recommendations for patients with fluctuating clinical responses should maximize and stabilize levodopa absorption and include the adherence to a PRD and consumption of dietary fiber, caffeine, and vitamin C. Furthermore, vitamins B-12 and B-6 and folic acid could be supplemented to all patients with PD as an adjuvant to levodopa, and strict monitoring of metabolic pathway components is strongly recommended.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JTBK, OvdR, and IACA: designed the study; JTBK: conducted the literature search, screening, and data synthesis and wrote the initial draft; IACA and OvdR: were closely involved during the search and data synthesis, provided critical feedback to the initial draft, and contributed to the finalization of the manuscript; CV: provided critical feedback on the final draft and contributed significantly to sections devoted to Parkinson's Disease; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
IACA and OvdR contributed equally.
Abbreviations used: AIMS, Abnormal Involuntary Movement Scale; BBB, blood–brain barrier; BCP, balanced carbohydrate-protein; BMD, bone mineral density; Cmax, maximal plasma concentration; COMT, catechol O-methyltransferase; EMM, estimated marginal means; GSH, reduced glutathione; GSSG, oxidized glutathione; HCLP, high-carbohydrate/low-protein; HPLC, high-protein/low-carbohydrate; LNAA, large neutral amino acid; MeSH, Medical Subject Heading; NYUDS, New York University Disability Scale; NYURS, New York University Rating Scale; PD, Parkinson's disease; PNP, peripheral neuropathy; PRD, protein redistribution diet; RoB, risk of bias; Tiab, title or abstract or keyword search term; Tmax, time to reach maximal concentration; UPDRS, Unified Parkinson's Disease Rating Scale; 3-OMD, 3-O-methyldopa.
Contributor Information
Jikke T Boelens Keun, Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Anatomy and Neurosciences, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Ilse Ac Arnoldussen, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, The Netherlands; Department of Medical Imaging, Anatomy, Radboud University Medical Center, Nijmegen, The Netherlands; Donders Institute for Brain, Cognition, and Behaviour, Nijmegen, The Netherlands.
Chris Vriend, Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Anatomy and Neurosciences, Amsterdam Neuroscience, Amsterdam, The Netherlands; Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Psychiatry, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Ondine van de Rest, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, The Netherlands.
References
- 1. Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem. 2016;139:318–24. [DOI] [PubMed] [Google Scholar]
- 2. Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson's disease. J Neurol. 2008;255(S5):18–32. [DOI] [PubMed] [Google Scholar]
- 3. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–35. [DOI] [PubMed] [Google Scholar]
- 4. Salat D, Tolosa E. Levodopa in the treatment of Parkinson's disease: current status and new developments. J Parkinsons Dis. 2013;3:255–69. [DOI] [PubMed] [Google Scholar]
- 5. Smith Y, Wichmann T, Factor SA, Delong MR. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37(1):213–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camargo SMR, Vuille-Dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, Götze O, Verrey F. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson's disease. J Pharmacol Exp Ther. 2014;351(1):114–23. [DOI] [PubMed] [Google Scholar]
- 7. Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. 2010;257(Suppl 2):253–61. [DOI] [PubMed] [Google Scholar]
- 8. Nutt JG, Woodward WR, Anderson JL. The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: The mechanism of action in the treatment of parkinsonism. Ann Neurol. 1985;18(5):537–43. [DOI] [PubMed] [Google Scholar]
- 9. Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62(1):1–8. [DOI] [PubMed] [Google Scholar]
- 10. Levodopa/carbidopa and Parkinson's disease progression. Inpharma Wkly. 2004;NA;(1468):14. [Google Scholar]
- 11. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015;30(1):80–9. [DOI] [PubMed] [Google Scholar]
- 12. Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000;23(10 Suppl):S2–7. [DOI] [PubMed] [Google Scholar]
- 13. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–58. [DOI] [PubMed] [Google Scholar]
- 14. Holloway RG. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61(7):1044–53. [DOI] [PubMed] [Google Scholar]
- 15. Pandey S, Srivanitchapoom P. Levodopa-induced dyskinesia: clinical features, pathophysiology, and medical management. Ann Indian Acad Neurol. 2017;20(3):190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Espinoza S, Manago F, Leo D, Sotnikova TD, Gainetdinov RR. Role of catechol-O-methyltransferase (COMT)-dependent processes in Parkinson's disease and L-DOPA treatment. CNS Neurologic Disord Drug Targets. 2012;11(3):251–63. [DOI] [PubMed] [Google Scholar]
- 17. Hu XW, Qin SM, Li D, Hu LF, Liu CF. Elevated homocysteine levels in levodopa-treated idiopathic Parkinson's disease: a meta-analysis. Acta Neurol Scand. 2013;128(2):73–82. [DOI] [PubMed] [Google Scholar]
- 18. Müller T, Kuhn W. Homocysteine levels after acute levodopa intake in patients with Parkinson's disease. Mov Disord. 2009;24(9):1339–43. [DOI] [PubMed] [Google Scholar]
- 19. Rajabally YA, Martey J. Levodopa, vitamins, ageing and the neuropathy of Parkinson's disease. J Neurol. 2013;260(11):2844–8. [DOI] [PubMed] [Google Scholar]
- 20. Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behavior. 2017;7(5):e00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller T, Laar T van, Cornblath DR, Odin P, Klostermann F, Grandas FJ, Ebersbach G, Urban P, Valldeoriola F, Antonini A. Peripheral neuropathy in Parkinson's disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19(5):501–7. [DOI] [PubMed] [Google Scholar]
- 22. Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L. Levodopa-induced neuropathy: a systematic review. Mov Disord Clin Pract. 2019;6(2):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toth C, Breithaupt K, Ge S, Duan Y, Terris JM, Thiessen A, Wiebe S, Zochodne DW, Suchowersky O. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 2010 Jul;68(1):28–36. [DOI] [PubMed] [Google Scholar]
- 24. Günaydin ZY, Özer FF, Karagöz A, Bektaş O, Karataş MB, Vural A, Bayramoʇlu A, Çelik A, Yaman M. Evaluation of cardiovascular risk in patients with Parkinson disease under levodopa treatment. J Geriatr Cardiol. 2016;13(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogers JD, Sanchez-Saffon A, Frol AB, Diaz-Arrastia R. Elevated plasma homocysteine levels in patients treated with levodopa: association with vascular disease. Arch Neurol. 2003;60(1):59–64. [DOI] [PubMed] [Google Scholar]
- 26. Song IU, Kim JS, Park IS, Do K Y, Cho HJ, Chung SW, Lee KS. Clinical significance of homocysteine (Hcy) on dementia in Parkinson's disease (PD). Arch Gerontol Geriatr. 2013;57(3):288–91. [DOI] [PubMed] [Google Scholar]
- 27. Xie Y, Feng H, Peng S, Xiao J, Zhang J. Association of plasma homocysteine, vitamin B12 and folate levels with cognitive function in Parkinson's disease: a meta-analysis. Neurosci Lett. 2017;636:190–5. [DOI] [PubMed] [Google Scholar]
- 28. Lee SH, Kim MJ, Kim BJ, Kim SR, Chun S, Kim HK, Ryu JS, Kim GS, Lee MC, Chung SJet al. . Hyperhomocysteinemia due to levodopa treatment as a risk factor for osteoporosis in patients with Parkinson's disease. Calcif Tissue Int. 2010;86(2):132–41. [DOI] [PubMed] [Google Scholar]
- 29. Sharma VD, Lyons KE, Pahwa R. Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson's disease, Ther Clin Risk Manage. 2018;14:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stocchi F, Tagliati M, Olanow CW. Treatment of levodopa-induced motor complications. Mov Disord. 2008;23(Suppl 3):599–612. [DOI] [PubMed] [Google Scholar]
- 31. Schrag A. Entacapone in the treatment of Parkinson's disease. Lancet Neurol. 2005;4(6):366–70. [DOI] [PubMed] [Google Scholar]
- 32. DeMaagd G, Philip A. Parkinson's disease and its management: Part 4: treatment of motor complications. Pharm Ther. 2015;40(11):747–73. [PMC free article] [PubMed] [Google Scholar]
- 33. Bushra R, Aslam N, Khan AY. Food-drug interactions. Oman Med J. 2011;26(2):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yaheya M, Ismail M. Drug-food interactions and role of pharmacist. Asian J Pharm Clin Res. 2009;2(4):1–10. [Google Scholar]
- 35. Miller JW, Selhub J, Nadeau MR, Thomas CA, Feldman RG, Wolf PA. Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology. 2003;60(7):1125–9. [DOI] [PubMed] [Google Scholar]
- 36. Rojo-Sebastián A, González-Robles C, García de Yébenes J. Vitamin B6 deficiency in patients with Parkinson disease treated with levodopa/carbidopa. Clin Neuropharmacol. 2020;43(5):151–7. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Estarli M, Barrera ESAet al. . Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Rev Esp Nutr Humana y Diet. 2016;20(2):148–60. [Google Scholar]
- 38. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simon N, Gantcheva R, Bruguerolle B, Viallet F. The effects of a normal protein diet on LevoDOPA plasma kinetics in advanced Parkinson's disease. Parkinsonism Relat Disord. 2004;10(3):137–42. [DOI] [PubMed] [Google Scholar]
- 40. Rispoli V, Simioni V, Capone JG, Golfrè Andreasi N, Preda F, Sette E, Tugnoli E, Sensi M. Peripheral neuropathy in 30 duodopa patients with vitamins B supplementation. Acta Neurol Scand. 2017;136(6):660–7. [DOI] [PubMed] [Google Scholar]
- 41. Habibi AH, Anamoradi A, Shahidi GA, Razmeh S, Alizadeh E, Kokhedan KM. Treatment of levodopa induced dyskinesia with vitamin D: a randomized, double-blind, placebo-controlled trial. Neurol Int. 2018;10(3):7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frankel JP, Kempster PA, Bovingdon M, Webster R, Lees AJ, Stern GM. The effects of oral protein on the absorption of intraduodenal levodopa and motor performance. J Neurol Neurosurg Psychiatry. 1989;52(9):1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berry EM, Growdon JH, Wurtman JJ, Caballero B, Wurtman RJ. A balanced carbohydrate: protein diet in the management of Parkinson's disease. Neurology. 1991;41(8):1295–7. [DOI] [PubMed] [Google Scholar]
- 44. Cucca A, Mazzucco S, Bursomanno A, Antonutti L, Di Girolamo FG, Pizzolato G, Koscica N, Gigli GL, Catalan M, Biolo G. Amino acid supplementation in l-dopa treated Parkinson's disease patients. Clin Nutr. 2015;34(6):1189–94. [DOI] [PubMed] [Google Scholar]
- 45. Elbarbry F, Nguyen V, Mirka A, Zwickey H, Rosenbaum R. A new validated HPLC method for the determination of levodopa: application to study the impact of ketogenic diet on the pharmacokinetics of levodopa in Parkinson's participants. Biomed Chromatogr. 2019;33(1):e4382. [DOI] [PubMed] [Google Scholar]
- 46. Karstaedt PJ, Pincus JH. Protein redistribution diet remains effective in patients with fluctuating parkinsonism. Arch Neurol. 1992;49(2):149–51. [DOI] [PubMed] [Google Scholar]
- 47. Postuma RB, Espay AJ, Zadikoff C, Suchowersky O, Martin WRW, Lafontaine AL, Ranawaya R, Camicioli R, Lang AE. Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled study. Neurology. 2006;66(12):1941–3. [DOI] [PubMed] [Google Scholar]
- 48. Juncos JL, Fabbrini G, Mouradian MM, Serrati C, Chase TN. Dietary influences on the antiparkinsonian response to levodopa. Arch Neurol. 1987;44(10):1003–5. [DOI] [PubMed] [Google Scholar]
- 49. Pincus JH, Barry KM. Plasma levels of amino acids correlate with motor fluctuations in parkinsonism. Arch Neurol. 1987;44(10):1006–9. [DOI] [PubMed] [Google Scholar]
- 50. Bracco F, Malesani R, Saladini M, Battistin L. Protein redistribution diet and antiparkinsonian response to levodopa. Eur Neurol. 1991;31(2):68–71. [DOI] [PubMed] [Google Scholar]
- 51. Karstaedt PJ, Pincus JH. Aspartame use in Parkinson's disease. Neurology. 1993;43(3, Part 1):611–3. [DOI] [PubMed] [Google Scholar]
- 52. Barichella M, Marczewska A, De Notaris R, Vairo A, Baldo C, Mauri A, Savardi C, Pezzoli G. Special low-protein foods ameliorate postprandial off in patients with advanced Parkinson's disease. Mov Disord. 2006;21(10):1682–7. [DOI] [PubMed] [Google Scholar]
- 53. Lamberti P, Zoccolella S, Armenise E, Lamberti S V, Fraddosio A, de Mari M, Iliceto G, Livrea P. Hyperhomocysteinemia in L-dopa treated Parkinson's disease patients: effect of cobalamin and folate administration. Eur J Neurol. 2005;12(5):365–8. [DOI] [PubMed] [Google Scholar]
- 54. Müller T, Jugel C, Muhlack S, Klostermann F. Methyl group-donating vitamins elevate 3-O-methyldopa in patients with Parkinson disease. Clin Neuropharmacol. 2013;36(2):52–4. [DOI] [PubMed] [Google Scholar]
- 55. Nagayama H, Hamamoto M, Ueda M, Nito C, Yamaguchi H, Katayama Y, The effect of ascorbic acid on the pharmacokinetics of levodopa in elderly patients with Parkinson Disease. Clin Neuropharmacol. 2004;27(6):270–3. [DOI] [PubMed] [Google Scholar]
- 56. Astarloa R, Mena MA, Sánchez V, de la Vega L, de Yébenes JG. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin Neuropharmacol. 1992;15(5):375–80. [DOI] [PubMed] [Google Scholar]
- 57. Fernandez-Martinez MN, Hernandez-Echevarria L, Sierra-Vega M, Jose Diez-Liebana M, Calle-Pardo A, Carriedo-Ule D, Sahagún-Prieto A, Anguera-Vila A, Garcia-Vieitez JJ. A randomised clinical trial to evaluate the effects of Plantago ovata husk in Parkinson patients: changes in levodopa pharmacokinetics and biochemical parameters. BMC Complement Altern Med. 2014;14(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deleu D, Jacob P, Chand P, Sarre S, Colwell A. Effects of caffeine on levodopa pharmacokinetics and pharmacodynamics in Parkinson disease. Neurology. 2006;67(5):897–9. [DOI] [PubMed] [Google Scholar]
- 59. Nagashima Y, Kondo T, Sakata M, Koh J, Ito H. Effects of soybean ingestion on pharmacokinetics of levodopa and motor symptoms of Parkinson's disease—in relation to the effects of Mucuna pruriens. J Neurol Sci. 2016;361:229–34. [DOI] [PubMed] [Google Scholar]
- 60. Lee SH, Kim MJ, Kim B-J, Kim SR, Chun S, Ryu JS, Kim GS, Lee MC, Koh JM, Chung SJ. Homocysteine-lowering therapy or antioxidant therapy for bone loss in Parkinson's disease. Mov Disord. 2010;25(3):332–40. [DOI] [PubMed] [Google Scholar]
- 61. Cereda E, Barichella M, Pedrolli C, Pezzoli G. Low-protein and protein-redistribution diets for Parkinson's disease patients with motor fluctuations: a systematic review. Mov Disord. 2010;25(13):2021–34. [DOI] [PubMed] [Google Scholar]
- 62. Wang L, Xiong N, Huang J, Guo S, Liu L, Han C, Zhang G, Jiang H, Ma K, Kia Yet al. . Protein-restricted diets for ameliorating motor fluctuations in Parkinson's disease. Front Aging Neurosci. 2017;9(206):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nutt JG, Carter JH, Lea ES, Woodward WR. Motor fluctuations during continuous levodopa infusions in patients with Parkinson's disease. Mov Disord. 1997;12(3):285–92. [DOI] [PubMed] [Google Scholar]
- 64. Samadi P, Grégoire L, Rouillard C, Bédard PJ, Di Paolo T, Lévesque D. Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Ann Neurol. 2006;59(2):282–8. [DOI] [PubMed] [Google Scholar]
- 65. Barichella M, Cereda E, Cassani E, Pinelli G, Iorio L, Ferri V, Privitera G, Pasqua M, Valentino A, Monajemi Fet al. . Dietary habits and neurological features of Parkinson's disease patients: implications for practice. Clin Nutr. 2017;36(4):1054–61. [DOI] [PubMed] [Google Scholar]
- 66. Tosukhowong P, Boonla C, Dissayabutra T, Kaewwilai L, Muensri S, Chotipanich C, Joutsa J, Rinne J, Bhidayasiri R. Biochemical and clinical effects of whey protein supplementation in Parkinson's disease: a pilot study. J Neurol Sci. 2016;367:162–70. [DOI] [PubMed] [Google Scholar]
- 67. Ceravolo R, Cossu G, Bandettini di Poggio M, Santoro L, Barone P, Zibetti M, Frosini D, Nicoletti V, Manganelli F, Lodice Ret al. . Neuropathy and levodopa in Parkinson's disease: evidence from a multicenter study. Mov Disord. 2013;28(10):1391–7. [DOI] [PubMed] [Google Scholar]
- 68. O'Suilleabhain PE, Bottiglieri T, Dewey RB Jr, Sharma S, Diaz-Arrastia R. Modest increase in plasma homocysteine follows levodopa initiation in Parkinson's disease. Mov Disord. 2004;19(12):1403–8. [DOI] [PubMed] [Google Scholar]
- 69. Müller T. Motor complications, levodopa metabolism and progression of Parkinson's disease. Expert Opin Drug Metab Toxicol. 2011;7(7):847–55. [DOI] [PubMed] [Google Scholar]
- 70. dos Santos EF, Busanello ENB, Miglioranza A, Zanatta A, Barchak AG, Vargas CR, Saute J, Rose C, Carrion MJ, Camargo Det al. . Evidence that folic acid deficiency is a major determinant of hyperhomocysteinemia in Parkinson's disease. Metab Brain Dis. 2009;24(2):257–69. [DOI] [PubMed] [Google Scholar]
- 71. Behera J, Bala J, Nuru M, Tyagi SC, Tyagi N. Homocysteine as a pathological biomarker for bone disease. J Cell Physiol. 2017;232(10):2704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Herrmann M, Widmann T, Herrmann W. Homocysteine—a newly recognised risk factor for osteoporosis. Clin Chem Lab Med. 2005;43(10):1111–7. [DOI] [PubMed] [Google Scholar]
- 73. Müller T, Trommer I, Muhlack S, Mueller BK. Levodopa increases oxidative stress and repulsive guidance molecule A levels: a pilot study in patients with Parkinson's disease. J Neural Transm. 2016;123(4):401–6. [DOI] [PubMed] [Google Scholar]
- 74. Paul R, Borah A. L-dopa-induced hyperhomocysteinemia in Parkinson's disease: elephant in the room. Biochim Biophys Acta. 2016;1860(9);1989–97. [DOI] [PubMed] [Google Scholar]
- 75. Santos-García D, De La Fuente-Fernández R, Valldeoriola F, Palasí A, Carrillo F, Grande M, Mir P, de Fabregues O, Casanova J. Polyneuropathy while on duodenal levodopa infusion in Parkinson's disease patients: we must be alert. J Neurol. 2012;259(8):1668–72. [DOI] [PubMed] [Google Scholar]
- 76. Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology. 2011;77(22):1947–50. [DOI] [PubMed] [Google Scholar]
- 77. Lamberti P, Zoccolella S, Iliceto G, Armenise E, Fraddosio A, De Mari M, Livrea P. Effects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson's disease patients. Mov Disord. 2005;20(1):69–72. [DOI] [PubMed] [Google Scholar]
- 78. Kocer B, Guven H, Comoglu SS. Homocysteine levels in Parkinson's disease: is entacapone effective?. Biomed Res Int. 2016;2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nevrly M, Kanovsky P, Vranova H, Langova K, Hlustik P. Effect of entacapone on plasma homocysteine levels in Parkinson's disease patients. Neurol Sci. 2010;31(5):565–9. [DOI] [PubMed] [Google Scholar]
- 80. Zesiewicz TA, Wecker L, Sullivan KL, Merlin LR, Hauser RA. The controversy concerning plasma homocysteine in Parkinson disease patients treated with levodopa alone or with entacapone: effects of vitamin status. Clin Neuropharmacol. 2006;29(3):106–11. [DOI] [PubMed] [Google Scholar]
- 81. Kurth MC, Tetrud JW, Irwin I, Lyness WH, Langston JW. Oral levodopa/carbidopa solution versus tablets in Parkinson's patients with severe fluctuations: a pilot study. Neurology. 1993;43(5):1036–9. [DOI] [PubMed] [Google Scholar]
- 82. Metman LV, Hoff J, Mouradian MM, Chase TN. Fluctuations in plasma levodopa and motor responses with liquid and tablet levodopa/carbidopa. Mov Disord. 1994;9(4):463–5. [DOI] [PubMed] [Google Scholar]
- 83. Garcia JJ, Fernandez N, Carriedo D, Diez MJ, Sahagun A, Gonzalez A, Calle A, Sierra M. Hydrosoluble fiber (Plantago ovata husk) and levodopa I: experimental study of the pharmacokinetic interaction. Eur Neuropsychopharmacol. 2005;15(5):497–503. [DOI] [PubMed] [Google Scholar]
- 84. Fernandez N, Carriedo D, Sierra M, Diez MJ, Sahagun A, Calle A, Conzalez A, Garcia JJ. Hydrosoluble fiber (Plantago ovata husk) and levodopa. II: Experimental study of the pharmacokinetic interaction in the presence of carbidopa. Eur Neuropsychopharmacol. 2005;15(5):505–9. [DOI] [PubMed] [Google Scholar]
- 85. Yu QJ, Yu SY, Zuo LJ, Lian TH, Hu Y, Wang RD, Piao YS, Guo P, Liu L, Jin Zet al. . Parkinson disease with constipation: clinical features and relevant factors. Sci Rep. 2018;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, Tateno F, Tsuyusaki Y, Takahashi O. Plasma levodopa peak delay and impaired gastric emptying in Parkinson's disease. J Neurol Sci. 2012;319(1-2):86–8. [DOI] [PubMed] [Google Scholar]
- 87. Djaldetti R, Baron J, Ziv I, Melamed E. Gastric emptying in Parkinson's disease: patients with and without response fluctuations. Neurology. 1996;46(4):1051–4. [DOI] [PubMed] [Google Scholar]
- 88. Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SAet al. . Probiotics and prebiotic fiber for constipation associated with Parkinson disease. Neurology. 2016;87(12):1274–80. [DOI] [PubMed] [Google Scholar]
- 89. Christodoulides S, Dimidi E, Fragkos KC, Farmer AD, Whelan K, Scott SM. Systematic review with meta-analysis: effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment Pharmacol Ther. 2016;44(2):103–16. [DOI] [PubMed] [Google Scholar]
- 90. Bracco D, Ferrarra JM, Arnaud MJ, Jequier E, Schutz Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol Endocrinol Metab. 1995;269(4):e671–8. [DOI] [PubMed] [Google Scholar]
- 91. Dulloo AG, Geissler CA, Collins A, Miller DS. Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr. 1989;49(1):44–50. [DOI] [PubMed] [Google Scholar]
- 92. Nicoletti A, Zappia M. Coffee consumption and risk of levodopa-induced dyskinesia in Parkinson's disease: the FRAGAMP study. Mov Disord. 2015;30:1854–6. [DOI] [PubMed] [Google Scholar]
- 93. Wills AMA, Eberly S, Tennis M, Lang AE, Messing S, Togasaki D, Tanner CM, Kamp C, Chen JF, Oakes Det al. . Caffeine consumption and risk of dyskinesia in CALM-PD. Mov Disord. 2013;28(3):380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lehmann L, Jiang L, Wagner J. Soy isoflavones decrease the catechol-O-methyltransferase-mediated inactivation of 4-hydroxyestradiol in cultured MCF-7 cells. Carcinogenesis. 2008;29(2):363–70. [DOI] [PubMed] [Google Scholar]