Abstract
Background
Recent studies suggest that associations of ceramides (Cer) and sphingomyelins (SM) with health outcomes differ according to the fatty acid acylated to the sphingoid backbone. The purpose of this study was to assess associations of Cer and SM species with mortality.
Methods
The study population included participants from the Cardiovascular Health Study (CHS), a community-based cohort of adults aged ≥65 years who were followed from 1992–2015 (n = 4612). Associations of plasma Cer and SM species carrying long-chain (i.e., 16:0) and very-long-chain (i.e., 20:0, 22:0, 24:0) saturated fatty acids with mortality were assessed using Cox proportional hazards models.
Results
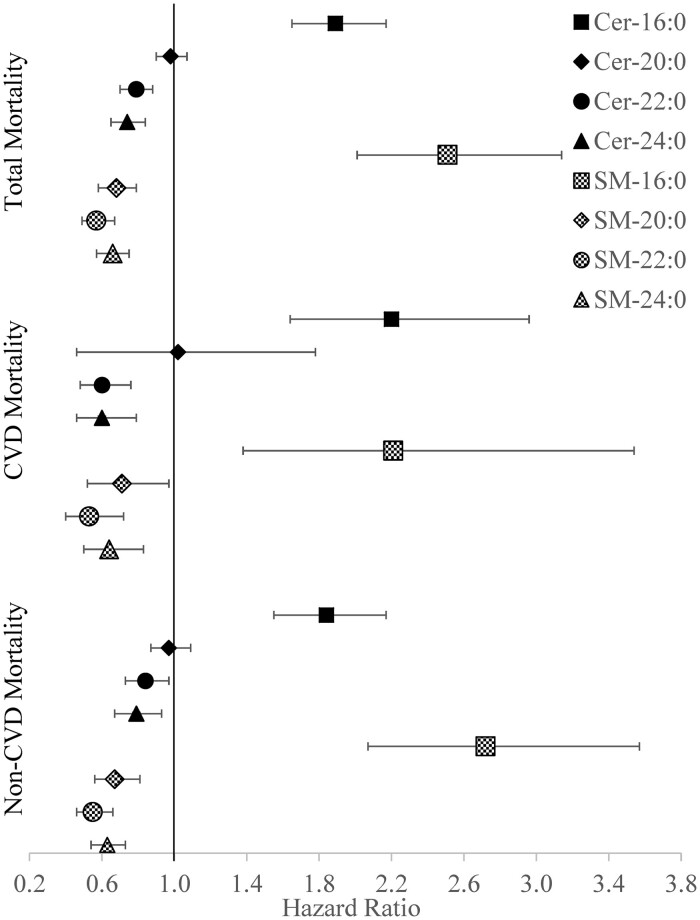
During a median follow-up of 10.2 years, 4099 deaths occurred. High concentrations of Cer and SM carrying fatty acid 16:0 were each associated with an increased risk of mortality. Conversely, high concentrations of several ceramide and sphingomyelin species carrying longer fatty acids were each associated with a decreased risk of mortality. The hazard ratios for total mortality per 2-fold difference in each Cer and SM species were: 1.89 (95% CI), 1.65–2.17 for Cer-16, 0.79 (95% CI, 0.70–0.88) for Cer-22, 0.74 (95% CI, 0.65–0.84) for Cer-24, 2.51 (95% CI, 2.01–3.14) for SM-16, 0.68 (95% CI, 0.58–0.79) for SM-20, 0.57 (95% CI, 0.49–0.67) for SM-22, and 0.66 (0.57–0.75) for SM-24. We found no association of Cer-20 with risk of death.
Conclusions
Associations of Cer and SM with the risk of death differ according to the length of their acylated saturated fatty acid. Future studies are needed to explore mechanisms underlying these relationships.
Introduction
There is considerable interest in the impact of circulating sphingolipid species on health outcomes. In particular, it is becoming increasingly apparent that ceramides and sphingomyelins with different saturated fatty acids have divergent biological activities, and that the associations of ceramides and sphingomyelins with health outcomes differ by the fatty acid acylated to the sphingoid backbone. In humans, high concentrations of circulating Cer-16 have been shown to be associated with development of cardiovascular diseases (CVD) and major adverse cardiac events (MACE), including mortality, among healthy individuals (1, 2), individuals at high risk for CVD (3), and individuals with underlying CVD (4–8). Whether circulating concentrations of ceramides carrying longer-chain fatty acids (i.e., Cer-20, Cer-22, and Cer-24) are associated with MACE is less clear; some studies report that high circulating concentrations of Cer-20, Cer-22, and Cer-24 are associated with an increased risk of MACE (3), while other studies report a decreased risk (2, 8), or no association (5, 6, 9). To our knowledge, no published studies have assessed whether sphingomyelins carrying long-chain (i.e., SM-16) or very-long-chain saturated (i.e., SM-20, SM-22, SM-24) fatty acids are associated with total or cause-specific mortality.
So far, most studies that have examined associations of ceramides with mortality have focused exclusively on populations with prevalent CVD (4–6, 8, 9) or at high risk of CVD (3), or they have only reported associations of sphingolipid species with composite endpoints (i.e., a combination of fatal and nonfatal cardiovascular-related outcomes) (1, 3–5, 9)—making it challenging to tease out the associations of circulating sphingolipids with risk of death in the general population. Further, few studies have assessed associations of sphingomyelin species with mortality. As sphingomyelins can generate ceramides through sphingomyelinases (10), better understanding as to whether ceramide and sphingomyelin species exhibit similar associations with risk of death may provide insights into biological mechanisms that may explain the impact of sphingolipids on health outcomes.
To our knowledge, no published community-based studies that have examined associations of ceramide species with risk of death have focused exclusively on an elderly population (1, 2). As biological function deteriorates with aging and senescence, it is unclear whether findings from younger populations are generalizable to the elderly, and more studies are needed to better understand this relationship.
The purpose of this paper was to examine the associations of 4 plasma ceramides and 4 sphingomyelin species concentrations with the risk of mortality among older adults who participated in the Cardiovascular Health Study (CHS), a large community-based prospective cohort study.
Materials and Methods
Design and Population
The CHS is a prospective cohort study of CVD and its risk factors among older adults (≥65 years) from 4 communities in the USA (Forsyth County, NC; Sacramento County, CA; Washington County, MD; Allegheny County, PA). Details on the design of the study are described in detail elsewhere (11). In brief, noninstitutionalized adults aged ≥65 years from each participating community were randomly selected and recruited to the study using Medicare beneficiary lists. In total, 5201 participants enrolled in the study in 1989–1990, and 687 participants (predominantly black) enrolled in 1992–1993. Written informed consent was obtained for all study participants.
Plasma ceramide and sphingomyelin species were measured for 4026 participants using plasma specimens from the 1994–1995 clinic visit and 586 participants (without available plasma specimens from the 1994–1995 clinic visit) using specimens from the 1992–1993 clinic visit. All 4612 participants with available sphingolipid data were included in this report.
Data Collection
Participants underwent study examinations once per year for the first 10 years of the study and then completed telephone surveys twice a year thereafter. Each study examination included a standardized interview to assess education, medical history, current medication use, smoking status, alcohol use, physical activity (usual walking habits, gait speed, and distance walked), activities of daily living (i.e., ability to perform routine self-care tasks, such as eating, bathing, toileting), and self-reported health status; a physical exam to assess weight, waist circumference, height, and blood pressure using standardized methods (11, 12); and a complete laboratory work-up (11, 12). Blood samples were collected after a 12-hour overnight fast and stored at −70 °C.
Sphingolipid Measurement
Ceramide and sphingomyelin species were measured using fasting EDTA-plasma samples collected in 1992–1993 or 1994–1995 and stored at −70 °C until extraction. This hypothesis-driven analysis focuses on 8 species of interest: 4 ceramide species (i.e., Cer-16, Cer-20, Cer-22, and a composite concentration of Cer-24 computed as the sum of the concentrations of 2 species of ceramides with 24:0 having the distinct “d181” and “d182” sphingoid backbones) and 4 sphingomyelin species (i.e., SM-16, SM-20, SM-22, SM-24). The detailed methodology and quality control procedures for the sphingolipid measurement have been reported previously (13, 14). Plasma lipids were extracted and sphingolipids quantified by liquid chromatography-tandem mass spectrometry at the University of Washington (Seattle, WA). Concentrations of ceramide and sphingomyelin species (expressed as µmol/L) were quantified as previously described using a single point calibrator added to each batch in 5 replicates. Coefficients of variation over 52 batches from an independent QC pool of EDTA plasma run in duplicate in each batch were <20% for the 8 sphingolipids of interest.
Mortality Assessment
Deaths were adjudicated by a centralized CHS events committee based on information from medical records, laboratory/diagnostic reports, death certificates, and/or interviews with next of kin. Details of CHS methods for surveillance and disease classification have been reported in detail previously (11, 15). We were most interested in the relationship of each sphingolipid with total mortality (primary analyses). In secondary analyses, we assessed the relationship of each sphingolipid with CVD mortality and non-CVD mortality. In exploratory analyses, we further subclassified CVD mortality as deaths from coronary heart disease (CHD), and non-CVD mortality as deaths from cancer, dementia, infections, or respiratory diseases; these subclassifications were mutually exclusive.
Statistical Analyses
Sphingolipid species concentrations were log (base 2) transformed due to skewness. Cox proportional hazards regression was used to examine the associations of levels of each circulating sphingolipid species with total and cause-specific mortality with entry at the time of the sphingolipid measurement and time-at-risk until death or the latest adjudicated date of follow-up (i.e., time scale is from time of sphingolipid measurement). Observed associations were quantified using hazard ratios (HRs) for death per 2-fold difference in sphingolipid species concentration. A Bonferroni correction was used to adjust for multiple comparisons; the significance threshold of 0.006 (0.05/8 sphingolipid species) was used. Schoenfeld residuals were reviewed to evaluate the proportional hazards assumption for each sphingolipid of interest (16).
Three levels of adjustment were used to examine associations of sphingolipids with risk of death. The first model (minimally adjusted model) included age, sex, race (black vs other), and enrollment site (Bowman Grey, Davis, Hopkins, Pittsburg). The second model (multivariate-adjusted model) additionally adjusted for education (no high school, high school/vocational school, college), smoking (yes/no), alcohol use (1 or more drink/week: yes/no), physical activity (linear), body mass index (BMI) (linear), low-density lipoprotein (LDL) cholesterol (linear), high-density lipoprotein (HDL) cholesterol (linear), triglycerides (linear, log-transformed), systolic blood pressure (linear), use of hypertension or lipid-lowering drugs (yes/no), self-reported health status (excellent/very good/good vs fair/poor), instrumental activities of daily living (2 or more vs 0/1), prevalent CVD (yes/no), and prevalent diabetes (yes/no). The third model (fully adjusted model) additionally adjusted for one of the other sphingolipid species: analyses of Cer-20, Cer-22, or Cer-24 included Cer-16; analyses of SM-20, SM-22, or SM-24 included SM-16; analyses of Cer-16 included Cer-22; analyses of SM-16 included SM-22. In sensitivity analyses, we further adjusted for C-reactive protein concentrations (linear) or prevalent heart failure (HF) (yes/no) to better understand if inflammation influenced observed associations. As it is possible that dietary intake of foods and nutrients known to impact circulating concentrations of long-chain or very-long-chain saturated fatty acids may also impact concentrations of specific sphingolipid species (17–23), we ran a sensitivity analysis that further adjusted for dietary intake of fruits and vegetables, total meat, saturated fat, carbohydrates, and total calories.
All covariates were assessed at the time of the sphingolipid measurement. As we found departure from the proportional hazards assumption for analyses of Cer-16, Cer-22, and all sphingomyelin species of interest with total mortality, we also conducted sensitivity analyses stratified by 5-year increment of survival time.
We examined potential interactions of each sphingolipid of interest with age, sex, race (black or other), BMI, and prevalent HF at the time of the sphingolipid measure since these factors may modify the associations of sphingolipids with risk of death. Likelihood ratio tests were used to evaluate the statistical significance of the multiplicative interaction term for each factor with each sphingolipid of interest modeled using covariates for the fully adjusted model (model 3) as described before. A Bonferroni correction was used to adjust for multiple interaction analyses; the significance threshold of 0.001 (0.05/40 based on 8 sphingolipids and 5 interactions) was used.
Missing values of education (n = 10), smoking (n = 50), alcohol consumption (n = 5), physical activity (n = 52), instrumental activities of daily living (n = 17), self-reported health status (n = 3), BMI (n = 8), LDL (n = 280), HDL (n = 203), triglycerides (n = 194), hypertension (n = 2) or lipid-lowering (n = 2) medication use, systolic blood pressure (n = 3), and C-reactive protein (n = 255) were multiply imputed with chained equations using information on age, sex, race, and prevalent diabetes. Twenty imputed datasets were generated and model fitting results were pooled using standard methods (24).
All statistical analyses were conducted using STATA v.16.0 (Stata Corp, College Station, TX).
Results
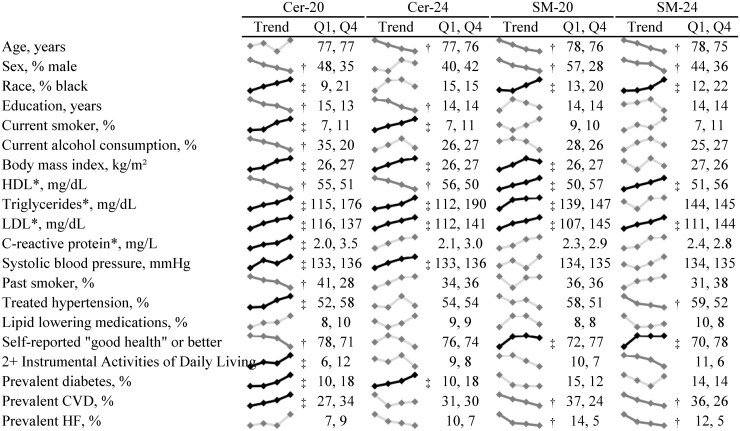
The distributions (mean, range) of circulating concentrations of sphingolipids species are shown in Table 1. Demographic and cardio-metabolic characteristics of study participants in general [mean (SD)] and according to quartile of sphingolipid species of interest are shown in Fig. 1 and Fig. 1 in the online Data Supplement. At baseline, the mean age of study participants was 77 ± 5 years, 59% were female, 84% were white, and 30% had prevalent CVD. Some baseline characteristics of study participants were consistent across quartiles of all sphingolipid species of interest, while others differed across individual sphingolipid species. For instance, participants with high concentrations of each species (i.e., Cer-16, Cer-20, Cer-22, Cer-24, SM-16, SM-20, SM-22, SM-24) had higher levels of systolic blood pressure and LDL cholesterol, and were more likely to report current smoking than participants with low circulating concentrations of each sphingolipid species. On the other hand, participants with higher circulating concentrations of ceramide species had lower HDL cholesterol, while those with higher circulating concentrations of sphingomyelin species had higher concentrations of HDL cholesterol. Participants with high concentrations of Cer-16 and Cer-20 were more likely to have prevalent CVD than participants with low concentrations of those ceramides, while participants with high concentrations of SM-20, SM-22, and SM-24 were less likely to have prevalent CVD than participants with low concentrations of those sphingomyelins. There were no observed associations of Cer-22, Cer-24, or SM-16 with prevalent CVD.
Table 1.
Concentrations (µM) of plasma sphingolipid species carrying different saturated fatty acids.
| Sphingolipid | Mean | Range |
|---|---|---|
| Cer-16 | 0.27 | 0.09–0.91 |
| Cer-20 | 0.08 | 0.01–0.25 |
| Cer-22 | 0.62 | 0.18–1.90 |
| Cer-24 | 4.48 | 1.55–9.98 |
| SM-16 | 125.2 | 48.9–227.1 |
| SM-20 | 17.7 | 6.19–36.4 |
| SM-22 | 26.6 | 9.60–63.2 |
| SM-24 | 14.3 | 4.59–33.2 |
Abbreviations: Cer-16, Cer-20, Cer-22, and Cer-24: ceramides with palmitic, arachidic, behenic, and lignoceric acid, respectively; SM-16, SM-20, SM-22-, and SM-24: sphingomyelins with palmitic, arachidic, behenic, and lignoceric acid, respectively.
Fig. 1.
Baseline characteristics of study participants according to quartiles of select ceramide and sphingomyelin species. The * denotes variables only measured at the exam in 1992–1993. To convert HDL and LDL cholesterol concentrations to mmol/L multiply by 0.0259. To convert triglycerides concentration to mmol/L, multiply by 0.0113. † indicates a significant inverse association and ‡ indicates a significant positive association based on a Bonferroni-corrected p-value of 0.05/20=0.0025 (based on 20 baseline characteristics of interest).
During a median follow-up time of 10.2 years (range 0–23 years), there were 4099 deaths. Higher concentrations of circulating Cer-16 and SM-16 were each associated with a higher risk of death after adjustment for age, sex, self-reported race, geographic area, education, smoking, alcohol use, BMI, activities of daily living, HDL and LDL cholesterol, triglycerides, systolic blood pressure, physical activity, use of hypertension or lipid-lowering drugs, self-reported health status, prevalent diabetes, CVD, and Cer-22 or SM-22, respectively (Table 2, Fig. 2). In the other direction, higher concentrations of Cer-22, Cer-24, SM-20, SM-22, and SM-24 were each associated with a lower risk of mortality (Table 2, Fig. 2). Cer-20 was not found to be associated with risk of death. Associations appeared generally similar when analyses were repeated using CVD or non-CVD mortality as outcomes of interest (Table 2, Fig. 2).
Table 2.
Hazard ratios (95% CI) for associations of circulating ceramides and sphingomyelins carrying saturated fatty acids with mortalitya.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Total mortality | |||
| Cer-16 | 1.68 (1.52, 1.85) | 1.61 (1.44, 1.80) | 1.89 (1.65, 2.17) |
| Cer-20 | 1.18 (1.10, 1.27) | 1.15 (1.06, 1.24) | 0.98 (0.90, 1.07) |
| Cer-22 | 1.08 (1.00, 1.17) | 1.05 (0.95, 1.15) | 0.79 (0.70, 0.88) |
| Cer-24 | 1.03 (0.94, 1.13) | 1.03 (0.92, 1.15) | 0.74 (0.65, 0.84) |
| SM-16 | 1.36 (1.17, 1.59) | 1.60 (1.34, 1.92) | 2.51 (2.01, 3.14) |
| SM-20 | 0.76 (0.68, 0.85) | 0.84 (0.73, 0.96) | 0.68 (0.58, 0.79) |
| SM-22 | 0.76 (0.68, 0.84) | 0.80 (0.71, 0.91) | 0.57 (0.49, 0.67) |
| SM-24 | 0.77 (0.70, 0.84) | 0.84 (0.75, 0.94) | 0.66 (0.57, 0.75) |
| CVD mortality | |||
| Cer-16 | 1.65 (1.31, 2.07) | 1.56 (1.22, 1.99) | 2.20 (1.64, 2.96) |
| Cer-20 | 1.26 (1.08, 1.46) | 1.17 (0.99, 1.38) | 1.02 (0.85, 1.23) |
| Cer-22 | 1.01 (0.85, 1.20) | 0.86 (0.71, 1.04) | 0.60 (0.48, 0.76) |
| Cer-24 | 1.00 (0.81, 1.23) | 0.86 (0.69, 1.08) | 0.60 (0.46, 0.79) |
| SM-16 | 1.21 (0.87, 1.69) | 1.33 (0.90, 1.98) | 2.21 (1.38, 3.54) |
| SM-20 | 0.82 (0.64, 1.04) | 0.82 (0.63, 1.08) | 0.71 (0.52, 0.97) |
| SM-22 | 0.77 (0.61, 0.96) | 0.71 (0.56, 0.91) | 0.53 (0.40, 0.72) |
| SM-24 | 0.78 (0.64, 0.96) | 0.78 (0.63, 0.97) | 0.64 (0.50, 0.83) |
| Non-CVD mortality | |||
| Cer-16 | 1.59 (1.39, 1.80) | 1.64 (1.42, 1.89) | 1.84 (1.55, 2.17) |
| Cer-20 | 1.11 (1.02, 1.21) | 1.14 (1.04, 1.26) | 0.97 (0.87, 1.09) |
| Cer-22 | 1.04 (0.95, 1.15) | 1.11 (0.98, 1.24) | 0.84 (0.73, 0.97) |
| Cer-24 | 0.99 (0.88, 1.12) | 1.08 (0.94, 1.24) | 0.79 (0.67, 0.93) |
| SM-16 | 1.40 (1.15, 1.70) | 1.68 (1.33, 2.11) | 2.72 (2.07, 3.57) |
| SM-20 | 0.74 (0.64, 0.86) | 0.84 (0.71, 0.99) | 0.67 (0.56, 0.81) |
| SM-22 | 0.72 (0.63, 0.83) | 0.79 (0.68, 0.92) | 0.55 (0.46, 0.66) |
| SM-24 | 0.76 (0.67, 0.85) | 0.82 (0.72, 0.94) | 0.63 (0.54, 0.73) |
Hazard ratios (95% CI) for mortality per 2-fold difference in sphingolipid species concentration.
Model 1 adjusted for age, sex, race, and enrollment site. Model 2 additionally adjusted for education, smoking, alcohol use, physical activity, BMI, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, use of hypertension medications, use of cholesterol-lowering medications, self-reported health status, activities of daily living, prevalent cardiovascular disease, and prevalent diabetes. Model 3 additionally adjusted for one of the other sphingolipid species: exposures Cer-20, Cer-22, and Cer-24 adjusted for Cer-16; exposure Cer-16 adjusted for Cer-22; exposures SM-20, SM-22, and SM-24 adjusted for SM-16; exposure SM-16 adjusted for SM-22. Abbreviations: Cer-16, Cer-20, Cer-22, Cer-24: ceramides with palmitic, arachidic, behenic, lignoceric acid respectively; CVD, cardiovascular diseases; SM-16, SM-20, SM-22-, SM-24: sphingomyelins with palmitic, arachidic, behenic, lignoceric acid, respectively.
Fig. 2.
Hazard ratios (95% CI) for associations of circulating ceramides and sphingomyelins carrying saturated fatty acids with mortality.
In exploratory analyses, we assessed the relationship of each sphingolipid of interest with subtypes of CVD and non-CVD mortality, including deaths related to CHD, cancer, dementia, infections, and respiratory diseases. In the CHS, CHD and cancer were the most common causes of death; CHD accounted for 23% of deaths and cancer accounted for 19% of deaths. Associations of the sphingolipids of interest with death from CHD and cancer were similar to the findings reported for total, CVD, and non-CVD morality, although associations with Cer-22 and Cer-24 did not reach statistical significance for deaths due to cancer (Supplemental Table 1). Likewise, the HRs for deaths from dementia, respiratory illness, and infections were similar to those for total, CVD, and non-CVD mortality for most sphingolipids, although several associations did not reach statistical significance, possibly due to limited power (Supplemental Table 2).
There were no statistically significant interactions between the sphingolipid species and age, sex, race, BMI, or HF at baseline when assessing risk of total mortality after correcting for multiple-testing (smallest P for interaction = 0.004). Sensitivity analyses that further adjusted for C-reactive protein concentrations, prevalent HF, or dietary intake of fruits and vegetables, total meat, saturated fat, carbohydrates, and total calories produced similar results (data not shown). Sensitivity analyses stratified by survival time indicated that the magnitude of associations of ceramides and sphingomyelins with mortality risk were strongest in the 5-year period closest to the sphingolipid measurement, and moved toward the null throughout the follow-up period—with results not achieving statistical significance in the strata of survival time 10 or more years post the sphingolipid measurement (data not shown).
Discussion
In this large, community-based cohort study among older adults, high concentrations of circulating ceramides and sphingomyelins carrying fatty acids 16:0 (i.e., Cer-16 and SM-16) were each associated with an increased risk of death. On the other hand, high concentrations of circulating ceramides and sphingomyelins carrying longer-chain fatty acids (i.e., Cer-22, Cer-24, SM-20, SM-22, SM-24) were each associated with a decreased risk of death. These findings suggest that relationships of circulating ceramides and sphingomyelins with risk of death differ by the length of the attached acylated saturated fatty acid.
Mechanisms that may explain observed associations are not fully understood. Ceramides and sphingomyelins are involved in multiple biological activities, including apoptosis (25, 26), inflammation (27), atherosclerosis (28–30), immune response (31), oxidative stress (27), mitochondrial dysfunction (32–36), and insulin resistance (37, 38), and the observed associations could be due to biological collectivity across multiple pathways. Results from studies in worm and mouse models suggest divergent associations of long-chain versus very-long-chain ceramides with apoptosis; Cer-16 induces apoptosis, while Cer-20 and Cer-22 protect against apoptosis (25, 26). Studies in multiple cell types (i.e., human fibroblasts, human leukemic cell lines) and organisms (i.e., yeast, worms, flies, mice) also suggest that sphingolipids are involved in regulating lifespan—although published studies have primarily focused on total sphingolipids rather than specific sphingolipid species (31). In yeast and worms, global inhibition of de novo sphingolipid synthesis by downregulating serine palmitoyltransferase, a key enzyme in sphingolipid metabolism, increased lifespan (7, 39). High concentrations of several sphingolipid species, particularly Cer-16, have been shown to stimulate chronic cellular senescence in adipocytes, hepatocytes, and myoblasts, which has unfavorable effects on cardio-metabolic and immune function, and longevity (31). High concentrations total plasma ceramides and sphingomyelin have also been shown to be associated with atherosclerosis in humans (28–30), with the likely mechanism being that over-accumulation of lipids in nonadipose compartments trigger ceramide synthesis and apoptosis (28). Additionally, high concentrations of Cer-16 have been shown to decrease T-cell proliferation and impair immune response and risk of mortality in rodents (31). High concentrations of total circulating ceramides have also been shown to trigger the synthesis of amyloid β-peptide in a human neuroglioma cell line—and amyloid β-peptide may increase risk of Alzheimer’s disease and subsequent mortality; inhibition of sphingomyelinases, a hydrolase enzyme that is involved in sphingolipid metabolism, has been shown to prevent Alzheimer’s disease (31). Although these studies point to relationships of circulating sphingolipid concentrations and longevity, more work is needed to better understand the influences of individual sphingolipid species on risk of death.
Our results show HRs that are strikingly similar in magnitude for ceramides and sphingomyelins carrying the same fatty acid. This suggests that the length of the acylated saturated fatty acid (i.e., 16:0 20:0, 22:0, and 24:0) attached to the ceramide or sphingomyelin may be driving observed associations. Previous work has shown that sphingomyelins can generate ceramides through sphingomyelinases, and the type of saturated fatty acid bound to sphingomyelin is maintained in the ceramide throughout this process (10). In CHS, similar associations of ceramides and sphingomyelins carrying the same fatty acids were also observed for incident HF and incident atrial fibrillation; high concentrations of Cer-16 and SM-16 were associated with increased risks of atrial fibrillation and HF (14, 40), while high concentrations of circulating Cer-20, Cer-22, Cer-24, SM-20, SM-22, and SM-24 were associated with decreased risk of atrial fibrillation, and Cer-22, SM-20, SM-22, and SM-24 were associated with decreased risk of HF (14, 40). This supports the hypothesis that specific sphingomyelin species affect risk of cardiovascular-related morbidity and mortality through biological processes in the generated ceramide (14, 40).
Most of the previous studies that assessed the relationships of sphingolipid species with mortality in humans focused primarily on populations with underlying CVD (4–6, 8, 9) and/or used composite endpoints that included not only death, but also major cardiovascular events (3–6, 9). These studies consistently report that Cer-16 is positively associated with cardiovascular-related morbidity and mortality in individuals with underlying cardiovascular diseases, while ceramides with longer-chain fatty acids show no association with these adverse outcomes in these populations. Three community-based studies have assessed the relationship of various sphingolipid species with risk of death (or a composite end point that included death) in healthy populations (1, 2, 40). In a large population-based study that assessed the relationships of Cer-16 and Cer-24 with fatal major adverse cardiovascular events, high circulating concentrations of Cer-16 (but Cer-24) were positively associated with fatal MACE, but only among participants who had experienced a previous cardiovascular event. Among participants who did not experience a cardiovascular event during follow-up, only the ratio of Cer-18/Cer-24 (but no individual sphingolipid species) was positively associated with fatal incident MACE (1). In contrast, a separate study reported that high circulating concentrations of Cer-16, Cer-22, and Cer-24 were each associated with an increased risk of a composite end point that included nonfatal acute myocardial infarction, nonfatal stroke, or cardiovascular-related death in a population of middle-aged or older adults at high risk of CVD (3). Differences in findings from these studies are difficult to explain but may reflect underlying differences in the study populations. Alternatively, the high correlation between ceramide species with lack of mutual adjustment for the different species in published studies may explain reported findings of similar associations across ceramide species.
To our knowledge, only one population-based study that combined data from 2 major cohort studies (i.e., Framingham Heart Study and the Study of Health in Pomerania) examined associations of specific ceramide species with mortality (CVD and non-CVD mortality) that was not part of a composite end point. Results indicated that ceramides containing 16:0 were associated with a 32% higher risk of total mortality (per 0.045 μg/mL increase in circulating concentrations), while ceramides containing 24:0 were associated with a 21% lower risk of total mortality (per 0.65 μg/mL increase in circulating concentrations); results were similar for the outcomes CVD morality and non-CVD mortality (2). These findings are consistent with results reported herein—and show different directions of associations of Cer-16 and Cer-24 with total mortality, CVD-related mortality, and non-CVD-related mortality. Our work extends these findings, indicating that circulating sphingomyelins are also associated with risk of mortality—with divergent associations of SM-16 versus SM-20, SM-22, and SM-24.
Many published studies designed to assess associations of ceramides with MACE or mortality focus largely on ceramide ratios (e.g., Cer-22/Cer-16, Cer-24/Cer-16) or ceramide risk scores as primary endpoints (2, 3, 5, 8, 9). However, modeling ratios of ceramides is tied to the underlying assumption that the magnitude of higher concentrations of the ceramides carrying the very-long-chain saturated fatty acid (e.g., Cer-22 or Cer-24) is equivalent to lower concentrations of Cer-16 on the ratio scale. Our work expands these findings—and suggest independent associations of specific ceramide and sphingomyelin species with risk of mortality; high circulating concentrations of Cer-16 and SM-16 are associated with an increased risk of mortality, while high circulating concentrations of Cer-22, Cer-24, SM-20, SM-22, and SM-24 are associated with a decreased risk of mortality.
Although there is a growing body of evidence that suggests associations of sphingolipid species with health outcomes, the utility of this information to inform clinical care is in its infancy (41). However, a growing body of literature suggests that sphingolipids may be modifiable through diet and drug therapies. In particular, intervention studies suggest that consuming a healthy diet high in fruits and vegetables, whole grains, low-fat dairy products, lean meats, and vegetable oil reduced plasma concentrations of Cer-22 and Cer-24:0 (42). Consuming a healthy diet may also negate the negative effects of ceramides on CVD risk (3). Other known therapies include drug therapy; a 14 day regimen of 40 mg of simvastatin reduced ceramides (i.e., Cer-16, Cer-18, Cer-20, and Cer-24) by approximately 25% in pre-/posttesting (43). Gastric bypass surgery among individuals with obesity has also been shown to lower concentrations of total circulating ceramides—and these effects were sustained for 6 months postoperatively (44). Although most of these studies are small (n < 50)—results suggest that ceramides may be modifiable by existing therapies. These studies also highlight the potential utility for measuring ceramides in the clinical setting in the future.
Although sphingolipids are not typically measured in the clinical setting, recent studies suggest that a risk score that includes ceramide and phosphatidylcholine (PC) species (i.e., ratio of Cer 24:1/Cer 24:0; ratio of Cer 18:0/PC 14:0/22:6; ratio of Cer 16:0/PC 16:0/22:5, and PC 16:0/16:0 s) predict risk of death in subjects with acute cardiac conditions (45, 46). Further, although ceramides and PCs are derived from distinct biosynthetic pathways (45, 47), combining ceramide and PC ratios in a risk score increased prediction of future cardiac events and death than using a risk score that comprised ceramide species alone (45). These findings demonstrate utility of assessing circulating ceramides (and PCs) in identifying subjects at high risk of recurrent coronary events and/or death in populations with underlying cardiac morbidity (45, 46).
This study has several strengths. CHS is a large cohort study of CVD and its risk factors in a population of older adults who reside in the USA. Assessing associations of circulating sphingolipids and risk of death in a large elderly population—a population with a high event rate—maximized study power. The sampling design, particularly the random-sampling of eligible adults from Medicare eligibility lists, and the standardized data collection methods utilized in the study reduced the likelihood of selection and recall biases. Finally, the availability of detailed data on a wide variety of demographic, behavioral, and health factors collected at the in-person examinations optimized the ability to control for potential confounders.
This study is not without limitations. Frozen samples collected in 1992–1993 or 1994–1995 were used to measure ceramide and sphingomyelin species, and ceramides and sphingomyelins carrying unsaturated acylated fatty acids were not measured as part of the study. Although is possible that long-term storage of samples may have impacted sphingolipid concentrations, any alterations in sphingolipid composition due to storage would be expected to be nondifferential (i.e., bias toward null) between those who die and those who do not during follow-up. Several demographic, behavioral, and clinical factors were assessed as potential confounders when examining the associations of the sphingolipids with mortality, but we cannot rule out residual confounding by unmeasured or imprecisely measured factors. Finally, as the CHS participants were on average 77 years of age at the time of sphingolipid measurement, it is unclear if the findings are generalizable to younger populations.
In conclusion, these findings suggest that plasma ceramides and sphingomyelins with different saturated fatty acids have divergent biological activities, and that the associations of ceramides and sphingomyelins with risk of death differ by acylated saturated fatty acid length in an older population. This work supports the need for future studies to better understand whether behavioral and drug therapies may affect specific species of plasma ceramides and sphingomyelins.
Data sharing: The data that support the findings of this study are part of the CHS. The CHS welcomes collaboration, and to receive access to the data, interested investigators must submit a manuscript proposal to be reviewed and approved by the CHS Presentations and Publications Committee. Details can be found at: https://chs-nhlbi.org.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations:
- Cer
ceramide
- SM
sphingomyelin
- CHS
Cardiovascular Health Study
- CVD
cardiovascular disease
- MACE
major adverse cardiac events
- CHD
coronary heart disease
- HR
hazard ratio
- BMI
body mass index
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- HF
heart failure
- PC
phosphadidylcholine
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content ; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
R.N. Lemaitre designed the research; A.N. Hoofnagle conducted the research; D.S. Siscovick, B.M. Psaty, and N. Sotoodehnia provided essential reagents or provided essential materials; P.N. Jensen, B. McKnight, and C.M. Sitlani analyzed data or performed statistical analysis; A.M. Fretts and R.N. Lemaitre wrote the paper; A.N. Hoofnagle, B. McKnight, C.M. Sitlani, D.S. Siscovick, B.M. Psaty, I.B. King, N. Sotoodehnia edited drafts of the paper; A.M. Fretts, P.N. Jensen, A.N. Hoofnagle, B. McKnight, C.M. Sitlani, D.S. Siscovick, B.M. Psaty, I.B. King, N. Sotoodehnia, and R.N. Lemaitre approved the manuscript. A.M. Fretts and R.N. Lemaitre are responsible for the overall content as guarantor, accepting full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
A.N. Hoofnagle, Clinical Chemistry, AACC.
Consultant or Advisory Role
B.M. Psaty, steering committee for the RURAL study, steering committee of the Yale Open Data Access Project funded by Johnson & Johnson.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
This research was supported by grants R01 HL128575 from the National Heart, Lung, and Blood Institute and R01 DK103657 and P30-DK035816 from theNational Institute of Diabetes and Digestive and Kidney Diseases. The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from National Institute of Aging.
Expert Testimony
None declared.
Patents
None declared.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments
The authors would like to thank the participants of the Cardiovascular Health Study; without their contributions, this work would have not been possible.
References
- 1. Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–30. [DOI] [PubMed] [Google Scholar]
- 2. Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, et al. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc 2018;7:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, et al. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial. Circulation 2017;135:2028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng JM, Suoniemi M, Kardys I, Vihervaara T, de Boer SP, Akkerhuis KM, et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 2015;243:560–6. [DOI] [PubMed] [Google Scholar]
- 5. Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, Oemrawsingh R, et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res 2018;59:1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P, et al. ; LIPID Study Investigators. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang X, Liu J, Dickson RC.. Down-regulating sphingolipid synthesis increases yeast lifespan. PLoS Genet 2012;8:e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meeusen J, Donato L, Bryant S, Baudhuin L, Berger P, Jaffe A.. Plasma ceramides: a novel predictor of major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol 2018;38:1933–9. [DOI] [PubMed] [Google Scholar]
- 10. Baranowski M, Górski J.. Heart sphingolipids in health and disease. Adv Exp Med Biol 2011;721:41–56. [DOI] [PubMed] [Google Scholar]
- 11. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 12. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP.. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–70. [PubMed] [Google Scholar]
- 13. Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen P, Fretts AM, et al. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes 2018;67:1663–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail 2019;12:e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
- 16. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- 17. Kris-Etherton PM, Mustad VA.. Chocolate feeding studies: a novel approach for evaluating the plasma lipid effects of stearic acid. Am J Clin Nutr 1994;60:1029S–36S. [DOI] [PubMed] [Google Scholar]
- 18. Tholstrup T. Influence of stearic acid on hemostatic risk factors in humans. Lipids 2005;40:1229–35. [DOI] [PubMed] [Google Scholar]
- 19. Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr 1999;70:1001–8. [DOI] [PubMed] [Google Scholar]
- 20. Garg ML, Blake RJ, Wills RB.. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 2003;133:1060–3. [DOI] [PubMed] [Google Scholar]
- 21. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J.. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 1996;97:2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, Artistizabal JC, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS ONE 2014;9:e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B.. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med 2000;225:191–9. [DOI] [PubMed] [Google Scholar]
- 24. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 25. Grosch S, Schiffmann S, Geisslinger G.. Chain length-specific properties of ceramides. Prog Lipid Res 2012;51:50–62. [DOI] [PubMed] [Google Scholar]
- 26. Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science 2008;322:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gulbins E, Li PL.. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol 2006;290:R11–26. [DOI] [PubMed] [Google Scholar]
- 28. Jiang XC, Goldberg IJ, Park TS.. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol 2011;721:19–39. [DOI] [PubMed] [Google Scholar]
- 29. Hornemann T, Worgall TS.. Sphingolipids and atherosclerosis. Atherosclerosis 2013;226:16–28. [DOI] [PubMed] [Google Scholar]
- 30. Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 2000;20:2614–8. [DOI] [PubMed] [Google Scholar]
- 31. Trayssac M, Hannun YA, Obeid LM.. Role of sphingolipids in senescence: implication in aging and age-related diseases. J Clin Invest 2018;128:2702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F.. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lang F, Ullrich S, Gulbins E.. Ceramide formation as a target in beta-cell survival and function. Expert Opin Ther Targets 2011;15:1061–71. [DOI] [PubMed] [Google Scholar]
- 34. Ishizuka N, Yagui K, Tokuyama Y, Yamada K, Suzuki Y, Miyazaki J, et al. Tumor necrosis factor alpha signaling pathway and apoptosis in pancreatic beta cells. Metabolism 1999;48:1485–92. [DOI] [PubMed] [Google Scholar]
- 35. Sjoholm A. Ceramide inhibits pancreatic beta-cell insulin production and mitogenesis and mimics the actions of interleukin-1 beta. FEBS Lett 1995;367:283–6. [DOI] [PubMed] [Google Scholar]
- 36. Shimabukuro M, Zhou YT, Levi M, Unger RH.. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 1998;95:2498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2006;45:42–72. [DOI] [PubMed] [Google Scholar]
- 38. Chavez JA, Summers SA.. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–94. [DOI] [PubMed] [Google Scholar]
- 39. Cutler RG, Thompson KW, Camandola S, Mack KT, Mattson MP.. Sphingolipid metabolism regulates development and lifespan in Caenorhabditis elegans. Mech Ageing Dev 2014;143-144:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen PN, Fretts AM, Hoofnagle AN, Sitlani CM, McKnight B, King IB, et al. Plasma ceramides and sphingomyelins in relation to atrial fibrillation risk: the Cardiovascular Health Study. J Am Heart Assoc 2020;9:e012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Summers SA. Could ceramides become the new cholesterol? Cell Metab 2018;27:276–80. [DOI] [PubMed] [Google Scholar]
- 42. Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, Seppanen-Laakso T, et al. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J Nutr 2015;146:662–72. [DOI] [PubMed] [Google Scholar]
- 43. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014;99:E45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang H, Kasumov T, Gatmaitan P, Heneghan HM, Kashyap SR, Schauer PR, et al. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity 2011;19:2235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2019;41:371–80. [DOI] [PubMed] [Google Scholar]
- 46. Gencer B, Morrow DA, Braunwald E, Goodrich EL, Hilvo M, Kauhanen D, et al. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. [Epub ahead of print] Eur J Prev Cardiol December 29, 2020. as doi: 10.1093/eurjpc/zwaa143. [DOI] [PubMed] [Google Scholar]
- 47. van Meer G, Voelker DR, Feigenson GW.. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008;9:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.