Abstract
Background
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is an emerging public health problem. This study explores the specifics of CRKP epidemiology in Colombia based on whole genome sequencing (WGS) of the National Reference Laboratory at Instituto Nacional de Salud (INS)’s 2013–2017 sample collection.
Methods
A total of 425 CRKP isolates from 21 departments were analyzed by HiSeq-X10®Illumina high-throughput sequencing. Bioinformatic analysis was performed, primarily using the pipelines developed collaboratively by the National Institute for Health Research Global Health Research Unit (GHRU) on Genomic Surveillance of Antimicrobial Resistance (AMR), and AGROSAVIA.
Results
Of the 425 CRKP isolates, 91.5% were carbapenemase-producing strains. The data support a recent expansion and the endemicity of CRKP in Colombia with the circulation of 7 high-risk clones, the most frequent being CG258 (48.39% of isolates). We identified genes encoding carbapenemases blaKPC-3, blaKPC-2, blaNDM-1, blaNDM-9, blaVIM-2, blaVIM-4, and blaVIM-24, and various mobile genetic elements (MGE). The virulence of CRKP isolates was low, but colibactin (clb3) was present in 25.2% of isolates, and a hypervirulent CRKP clone (CG380) was reported for the first time in Colombia. ST258, ST512, and ST4851 were characterized by low levels of diversity in the core genome (ANI > 99.9%).
Conclusions
The study outlines complex CRKP epidemiology in Colombia. CG258 expanded clonally and carries specific carbapenemases in specific MGEs, while the other high-risk clones (CG147, CG307, and CG152) present a more diverse complement of carbapenemases. The specifics of the Colombian situation stress the importance of WGS-based surveillance to monitor evolutionary trends of sequence types (STs), MGE, and resistance and virulence genes.
Keywords: Klebsiella pneumoniae, antimicrobial resistance, Carbapenemases, whole genome sequence (WGS)
Klebsiella pneumoniae is a pathogen that causes community and hospital-acquired infections worldwide; it is a threat to public health due to its high levels of antimicrobial resistance [1–3]. Carbapenem-resistant K. pneumoniae (CRKP) causes untreatable infections and high mortality [2].
The emergence and global spread of extremely resistant K. pneumoniae highlight the need for a greater understanding of resistance epidemiology, for which whole genome sequencing (WGS) methods provide a cost-effective alternative that complements routine methods [4]. Furthermore, the epidemic dynamics of CRKP differ across geographical regions, and it is important to understand local evolutionary and epidemiological variation to establish the most appropriate strategies of surveillance, infection control, and antibiotic stewardship [5, 6].
Colombia’s record of carbapenem use is one of the world’s highest [7]. According to the last Instituto Nacional de Salud (INS) nationwide report, the use of these antimicrobials exceeds more than 20 times the defined daily dose (DDD) of several countries in Europe and North America [7, 8].
In Colombia, K. pneumoniae is the most frequent pathogen found in intensive care units, with resistance to carbapenems reported in up to 15.6% of isolates [9–12]. The first reports of CRKP associated with KPC-2 were in 2005 and with KPC-3 in 2008 [13, 14]. Colombia is considered KPC endemic [15]. The CRKP clones associated with KPC-type carbapenemases commonly reported in Colombia are CG258, CG307, and CG14/15 [5, 16]. Other carbapenemases identified in CRKP are VIM and NDM-1 (reported since 2011 and 2012 respectively) [12, 17, 18]. The presence of different evolutionary mechanisms in the challenged healthcare system of Colombia, a medium-income country, may have created the conditions for a significant CRKP epidemic [5].
The present study aimed to extend the scope of previous CRKP reports in Colombia through WGS of CRKP isolates from the 21 most populated departments of Colombia, reported to the National Reference Laboratory (NRL), Instituto Nacional de Salud (INS) between 2013 and 2017. The objective was to increase our understanding of CRKP epidemiology in Colombia to support prevention and control strategies.
METHODS
Bacterial Isolates
The NRL manages Colombia’s antimicrobial resistance (AMR) surveillance program and adjusts the criteria for isolate submission according to the epidemiological behavior observed in the country and epidemiological alerts from the Pan American Health Organization (PAHO) (Supplementary Methods).
Between 2013 and 2017, the NRL received 811 clinical isolates of K. pneumoniae nonsusceptible to carbapenems with different sample size from the 21 departments of the country (see Figure 1). After sample selection (Supplementary Materials), 425 isolates remained that met the criteria: (1) originating from healthcare-associated infections (HAI); (2) year of isolation between 2013 and 2017; (3) phenotypic confirmation of K. pneumoniae bacterial identification by Vitek-2 (bioMérieux); (4) resistance to carbapenems determined by AST disk diffusion using CLSI guidelines for the corresponding year; and (5) genotypic confirmation of carbapenemases in K. pneumoniae.
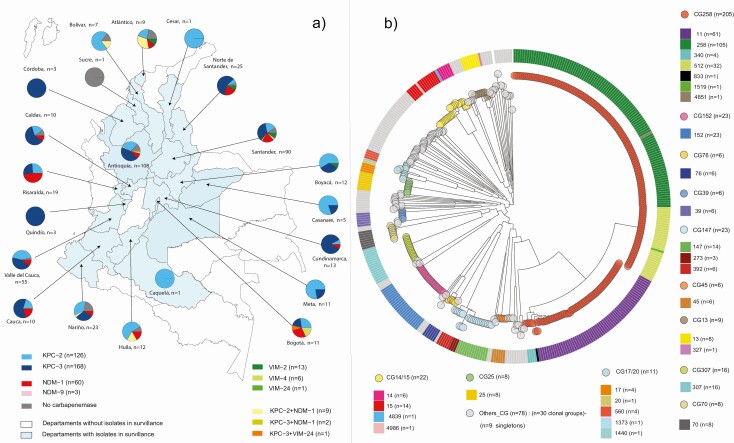
Figure 1.
Geographical distribution of mechanisms of resistance to carbapenems in Klebsiella pneumoniae and core genome tree in Colombia, 2013–2017. a) Map depicting the 21 departments that submitted isolates for surveillance. Isolates were classified according to the mechanism of resistance to carbapenem by color (see key). Pie chart shows the proportions of different mechanisms of resistance to carbapenems in each department. b) Circular representation of the transformed phylogenetic tree showing the genetic relationships among 421 Colombian K. pneumoniae CRKP isolates. Phylogenetic tree was built using 421/425 isolates; 4 were excluded from the analysis because they presented a high number of N in the multiple alignments. The 421 isolates were classified into 80 STs grouped in 42 CG and 9 singletons. Due to the number of different CGs, for the graph, CGs with ≥6 isolates were selected for easy viewing on the tree: CG258, CG13, CG14/15, CG17/20, CG25, CG39, CG45, CG70, CG76, CG147, CG152, and CG307. Remaining CG and singletons were classified as other-CG. Abbreviations: CG, clonal group; CRKP, carbapenem-resistant Klebsiella pneumoniae; ST. sequence type.
Whole Genome Sequencing
Genomic DNA was extracted from single-colony cultures using PureLink™ Genomic DNA Mini Kit (Invitrogen, USA). The DNA integrity was checked by electrophoresis in a 1% (w/v) agarose gel, the DNA purity was estimated using NanoDrop® spectrophotometer (Nanodrop Technologies Inc., USA), and the concentration was determined with a Qubit Fluorometer (Invitrogen, USA).
Subsequently, 1000 ng of DNA was shipped to the Wellcome Sanger Institute (Hinxton, UK) to sequence using Illumina HiSeq-X10 (San Diego, USA). DNA concentrations were confirmed using AccuBlue Broad Range assay (Biotium, Inc., Fremont, USA), followed by normalization and DNA library construction. DNA was sheared into 450–550 bp fragments using Covaris LC 220 ultrasonicator (Brighton, UK), followed by polymerase chain reaction (PCR)-based library preparation using Illumina adaptors and 384-indexed tags (NEBNext Ultra II FS DNA library kit). Afterward, size-selection, amplification, purification, and multiplexing were carried out, and libraries were pooled. Pool was quantified and normalized down to 4 nM using Biomek NXP workstation for (automated liquid handling; California, USA), Agilent Bioanalyzer 2100 (California, USA), and Roche Lightcycler 480) (Utah, USA), before denaturation and loading on the Illumina platform.
Quality Control and Bioinformatic Analysis
All genome sequence data were processed using versioned Nextflow workflows and associated Docker containers covering de novo assembly, mapping-based SNP phylogeny, MLST assignment, and AMR determinant detection. The workflow structure is summarized in protocols [19]. Major quality control parameters are available in Supplementary Table 1, including file size, number of contigs, genome size, N50. AMR determinants were detected [19], and virulence factor prediction was performed using ARIBA software (github.com/sanger-pathogens/ariba) in conjunction with the VFDB database (github.com/haruosuz/vfdb). MSLT prediction was assigned with ARIBA software using PubMLST databases downloaded on 20 December 2019. The isoforms of Tn4401 were determined with TETyper (github.com/aesheppard/TETyper) and annotated with Prokka v1.14.5. Additional AMR determinants, virulence factors, and typing (K-locus, wzi, and O-locus) were detected and species predicted using Kleborate and Kaptive as implemented on the PathogenWatch platform (https://pathogen.watch) [20, 21].
Single-nucleotide polymorphism (SNP) variants were called relative to a reference genome (K. pneumoniae strain K2044 [NCBI RefSeq NZ_CP026011.1]) and a maximum likelihood phylogeny inferred from pseudogenome alignment of the variants using IQ_TREE with GTR+G model and 1000 bootstrap replicates [19, 22]. High-quality views of trees and data were rendered using the ggtree R package (bioconductor.org/packages/ggtree). For clustering, clonal groups were defined using core genome SNP analysis using Prokka v1.14.5 gene annotation and a 70–90% ANI between isolates.
Raw sequence data were deposited at the European Nucleotide Archive under accession PRJEB29742 and visualized with Microreact (https://microreact.org/project/vcsgT8Ic4/66d1f105) [23].
RESULTS AND DISCUSSION
Geographic Spread of High-Risk Clones in Colombia
The 425 CRKP isolates analyzed in this study originated from 21 departments of Colombia, representing 90% of the total population (Figure 1A). These isolates belonged to 129 institutions (114 hospitals and 15 microbiology laboratories). The level of medical services was determined for 102 institutions, of which 68, 19, and 15 provided high, medium-high, and medium levels, respectively.
Of all isolates, 93.9% (n = 399) were multidrug-resistant (MDR), and 40.5% (n = 172) were resistant to at least one antibiotic from all classes tested (Supplementary Table 2).
A total of 80 sequence types (STs) were identified: 42 STs were reported for the first time in Colombia, including 10 novel STs: STs 4838–4841, 4851, 4852, 4984–4987 (Supplementary Table 2). The STs were classified into 42 clonal groups (CGs) and 9 singletons (Figure 1B). The most frequent CGs were: CG258, CG147, CG152, CG14/15, CG307, CG17/20, and CG13 (Figure 1B and Supplementary Table 3). These include known epidemic (“high-risk”) STs that are over-represented in the global public genome collections. Of the 7 most frequent CGs of this study, 6 had been previously reported in Colombia [5, 15, 16, 21, 24–29]. To the best of our knowledge, this is the first report of ST152 in the Americas.
CG258, the main CG identified in this study (48.9%, n = 208), presented the broadest geographic distribution (18 departments). CG17/20 (2.6%, n = 11) was reported in 7 departments. CG147 (5.4%, n = 23) and CG 14/15 (5.2%, n = 22) were present in 6 departments each, and CG152 (5.4%, n = 23) in 5 departments. Three geographically close departments, Antioquia, Santander, and Valle del Cauca (60%, n = 255) reported the simultaneous presence of the 7 main CGs.
All but 1 of the 7 main CGs showed increased and recent dissemination compared to previous reports in Colombia. Of CG258, ST11 was reported in 10 departments in this study, contrasting with previous reports, in which it was identified in only 2 departments [16, 30]. ST512, which was previously only detected in Medellin, is now reported in 4 departments [5, 16]. CG147 and CG307, which had been sporadically reported in Antioquia, were reported in 11 and 6 departments, respectively [16, 26, 29, 31]. CG152 is reported for the first time in Colombia in 5 departments (Supplementary Figure 1A). In this study, the prevalence of CG14/15 (5.2%) is lower compared to previous reports, of 30% between 2005 and 2007 and 8.3% between 2012 and 2014 [5]. This study represents the broadest geographical analysis of CRKP in Colombia and confirms their endemic status.
Most of the STs found in Colombia in this study have been reported globally. ST258, the dominant high-risk ST worldwide, was first reported in Colombia in 2006 following global clonal propagation [15, 21, 24, 25]. CG258 (ST258, ST11, ST512) has been identified as responsible for 68% of 112 K. pneumoniae outbreaks worldwide, reported between 2005 and 2016 [32]. The percentage of CRKP that belongs to CG258 in this study is lower than those reported in the United States (70%) by the Centers for Disease Control and Prevention (CDC), or in Israel (90%) [33–35]. ST11 is a prevalent clone in Asia, although its spread in Latin America had been only reported in Brazil [36–39]. ST512 is endemic in Italy, Israel, and Greece, ST147 in India, Italy, Tunisia, and Greece, and CG307 in Italy and the United States [16, 29, 31, 40–42]. The local samples behave as clones for ST258 and ST512 [35, 41], whereas ST11 shows local expansions and some diverged lineages of closely related samples against other clones reported globally [39]. Because differences in fitness, epidemicity, and host-related factors between CGs have been reported, molecular identification of specific high-risk clones in a region is of the utmost importance to implement appropriate infection control programs [32].
Detection and Distribution of Antimicrobial Resistance Mechanisms and Mobile Genetic Elements
Carbapenemase genes were identified in 389 CRKP genomes; 36 isolates were non-carbapenemase producers.
blaKPC
Among carbapenemase, blaKPC was identified in 294 isolates, and blaNDM and blaVIM in 63 and 20, respectively. Coproduction of blaKPC+NDM was found in 11 isolates and co-production of blaKPC+VIM in 1 isolate (Figure 1A). These findings confirm that, unlike in other regions of the world, blaKPC continues to dominate in Colombia, and blaOXA-48-like does not play a role in carbapenem resistance. They also indicate a recent expansion of blaNDM in Colombia.
We found 2 variants of blaKPC—blaKPC-3 (39%, n = 168) and blaKPC-2 (30%, n = 126)—across 64 different STs in multiple departments (Figure 1A). Additionally, a wide variety of mobile genetic elements with blaKPC were identified. The most common plasmid replicons in blaKPC-positive isolates were IncFIB(K) (86.7%), IncFII(K) (56.8%), ColRNAI (53.1%), IncR (39.1%), and IncFII (32.3%) (Table 1, Figure 2C). From the KPC-carrying isolates, 68% showed the presence of the Tn4401 transposon. In the isolates carrying blaKPC-2, the gene was located in 3 Tn4401 isoforms and in non-Tn4401 elements (NTEKPC) (n = 52) (Table1, Figure 2D). In contrast, blaKPC-3 was localized in Tn4401a in ST512 and ST1519 isolates and in Tn4401b in 82.5% of ST258. The clonal lineage ST258/512 accounts for 47% of all KPC genes reported in this study, whereas this percentage is >70% in the EuSCAPE sample collection [43]. The variant blaKPC-2 is associated with a greater variety of mobile genetic elements than in previous reports and is found in different CGs, including CG307, CG14/15, and CG147 (Table1), suggesting that its propagation is associated with successful Horizontal Gene Transfer (HGT) [5, 17]. In contrast, blaKPC-3 is correlated with the presence of 2 types of Tn4401 elements in the clonal lineage ST258/512, suggesting dissemination by clonal expansion. These observations confirm and extend the suggested compartmentalization of the genes encoding for the 2 KPC variants in Colombia by Rojas et al [5]. This horizontal transfer and promiscuity of KPC-2-carrying mobile genetic elements might have appeared in Colombia by selective pressure due to increased use of carbapenems [7].
Table 1.
Characteristics of Carbapenem-resistant K. pneumoniae Isolates
| Resistance mechanisms to carbapenems | Number of Isolates (% of Total) | Number of STs | Number of Departments | Carbapenemase Gene Variants | Kpc Element (Tn4401) | Plasmid Replicon Type |
|---|---|---|---|---|---|---|
| Carbapenemase blaKPC-like |
294 (69.1) | 64 STs (ST258, n = 106); (ST11, n = 34); (ST 512, n = 32); (other STs, n = 122) |
17 departments (Antioquia = 77) (Atlántico = 2) (Bogotá = 5) (Bolivar = 5) (Boyacá = 11) (Caldas = 8) (Caquetá = 1) (Casanare = 5) (Cauca = 8) (Córdoba = 3) (Cundinamarca = 12) (Huila = 7) (Meta = 9) (Nariño = 15) (N. Santander = 17) (Quindio = 3) (Risaralda = 10) (Santander = 50) (Valle del Cauca = 46) |
KPC-2 (n = 126) KPC-3 (n = 168) |
Tn4401_truncC (n = 7) Tn4401a (n = 48) Tn4401b (n = 145) No-Tn4401 (n = 94) |
IncFIB_K = 255 IncFII_K = 167 ColRNAI = 156 IncR = 115 IncFII = 95 IncI2 = 70 IncN = 46 IncFIB_pQil = 35 IncX3 = 33 Others = 215 (25 replicons) |
| Carbapenemase blaNDM-like |
63 (14.8) | 8 STs (ST11, n = 17); (ST152, n = 21); (others STs, n = 25) |
12 departments (Antioquia = 10) (Atlantico = 1) (Bogota = 3) (Caldas = 1) (Cauca = 2) (Cundinamarca = 1) (Huila = 2) (Nariño = 2) (N.Santander = 5) (Risaralda = 9) (Santander = 21) (Valle del Cauca = 6) |
NDM-1 (n = 60) NDM-9 (n = 3) |
- | Inc._1 = 62 IncFIB_K = 51 IncFII_K = 35 ColRNAI = 22 IncR = 17 FIA_pBK30683 = 13 Others = 38 (12 replicons) |
| Carbapenemase blaVIM-like |
20 (4.7) | 8 STs (ST11, n = 4); (ST15, n = 4); (ST70, n = 4) (ST147, n = 5); (ST273, n = 1); (ST307, n = 1); (ST340, n = 2); (ST833, n = 2); |
7 departments (Antioquia = 4) (Atlántico = 1) (Bogotá = 2) (Boyacá = 1) (Cesar = 1) (N.Santander = 2) (Santander = 9) |
VIM-2 (n = 13); VIM-4 (n = 6); VIM-24 (n = 1) |
- | Inc._1 = 20 IncFIB_K = 15 IncR = 14 Col_pHAD28 = 7 Col440I = 6 IncFII_K = 6 FIA_pBK30683 = 4 ColRNAI = 3 Others = 15 (9 replicons) |
| Coproduction of carbapenemases blaKPC-like and blaNDM-like | 11 (2.6) | 8 STs (ST11, n = 2); (ST25, n = 2); (ST76, n = 1); (ST152, n = 1); (ST219, n = 1); (ST258, n = 1); (ST340, n = 1); (ST392, n = 2); |
3 departments (Antioquia = 3); (Santander = 2); (Huila = 1) |
KPC-2+NDM-1 (n = 9); KPC-3+NDM-1 (n = 2) |
Tn4401a (n = 1) Tn4401b (n = 2) No-Tn4401 (n = 8) |
IncFIB_K = 8 IncFII_K = 8 Inc._1 = 6 IncN = 5 IncR = 4 ColRNAI = 3 Col_pHAD28 = 2 FIA_pBK30683 = 1 IncFIB_pQil = 1 IncFII = 1 IncI2 = 1 IncM1 = 1 |
| Coproduction of carbapenemases blaKPC-like and blaVIM-like | 1 (0.23) | 1 ST (ST3660, n = 1) |
1 department (Bogota = 1) |
KPC2+VIM-4 (n = 1) | Tn4401b (n = 1) | Inc._1 = 1 IncFIB_K = 1 IncFII_K = 1 IncR = 1 |
| ESBL gene + porin defects, but no carbapenemase | 22 (5.2) | 18 STs (ST11 n = 1); (ST15 n = 1); (ST25 n = 1) (ST29 n = 1) (ST35 n = 1) (ST37 n = 1) (ST40 n = 1) (ST70 n = 2) (ST147 n = 1) (ST258 n = 1) (ST300 n = 1) (ST307 n = 1) (ST340 n = 1) (ST405 n = 1) (ST629 n = 1) (ST4841 n = 2) (ST4986 n = 1) (ST4987 n = 1) |
9 departments (Antioquia = 8) (Bolivar = 1) (Caldas = 1) (Huila = 1) (Nariño = 2) (N. Santander = 1) (Santander = 5) (Sucre = 1) (Valle del Cauca = 2) |
- | - | IncFIB_K = 15 IncFII_K = 15 IncR = 6 IncFIB_pKPHS1 = 4 IncFIB_pNDM_Mar = 4 IncHI1B_pNDM_MAR = 2 FIA_pBK30683 = 2 Col_pHAD28 = 2 Others = 17 (14 replicons) |
| ESBL gene, but no carbapenemase | 7 (1.64) | 6 STs (ST11 n = 1); (ST39 n = 1); (ST40 n = 1) (ST147 n = 2) (ST1373 n = 1) (ST1758 n = 1) |
3 departments (Santander = 3); (Antioquia = 2) (Atlantico = 2) |
- | IncFIB_K = 7 IncFII_K = 3 IncR = 3 Col_pHAD28 = 2 Col156 = 1 Col440I = 1 Col440II = 1 FIA_pBK30683 = 1 IncFIB_pKPHS1 = 1 IncN = 1 |
|
| Porin defects, but no carbapenemases | 6 (1.41) | 3 STs (ST1198 n = 1); (ST70 n = 3); (ST14 n = 2) |
2 departments (Antioquia = 3) (Nariño = 3); |
- | - | IncFIB_K = 6 Inc._1 = 3 IncFIB_pKPHS1 = 3 IncFII_K = 3 IncR = 1 IncX4_1 = 1 ColRNAI = 1 |
| No Carbapenemase, no Porin, no BLEE | 1 (0.23) | 1 STs (ST4984 n = 1); |
1 department (Antioquia = 1) |
- | - | IncFIB_K = 1 IncR = 1 |
Abbreviations: ESBL, extended spectrum β-lactamase; ST, sequence type.
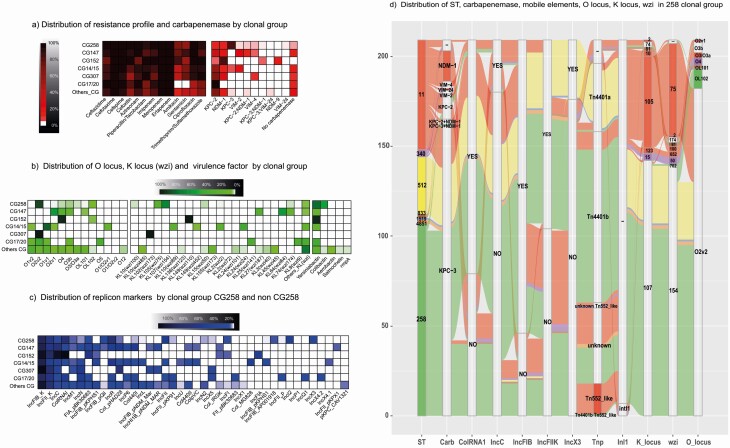
Figure 2.
Clonal group heat maps, virulence factors, resistance profile, carbapenemases, replicons, and alluvial of the CG258. Heatmap analyzes different clonal groups (each row of the heatmap represents a clonal group). a) Heatmap displaying the distribution of antimicrobials and the mechanism of resistance to carbapenems (y-axis) in the clonal group. b) Heatmap displaying the distribution of O locus, K locus (wzi), and virulence factors (y-axis) found in the clonal group. c) Heatmap displaying the distribution of the replicon type (y-axis) found in the clonal group. d) Alluvial diagram showing the “flow” of the presence of the most important transposable elements associated with carbapenemase and ST of CG258. Abbreviation: ST, sequence type.
blaNDM
Two variants of blaNDM were found: blaNDM-1 (n = 60) and blaNDM-9 (n = 3). This is the first report of blaNDM-9 in the Americas. Previous local blaNDM reports were found in 6 departments (n = 15) [12]. Additionally, the first description of blaNDM-1 genetic context was described in a Bogotá outbreak (n = 6) [18]. However, it is the first time that the dissemination of blaNDM-1 is reported in 12 departments in Colombia (Figure 1A). Also, this is the first description of blaNDM-9 prevalence and genetic context. The 2 variants were associated mainly with ST152 (33%) and ST11 (27%) (Supplementary Figure 1B). An IncC plasmid was carried by 98.4% of blaNDM isolates. Colocation of both determinants (IncC-blaNDM) in the same contig could be confirmed in 15.9% of isolates, using Artemis (Supplementary Figure 2). The IncC replicon is common in blaNDM isolates worldwide and has been previously reported in Colombia [28, 44]. Other isolates showed incomplete replicons due to short read sequencing limitations. The genetic context was identical in all isolates, including variants blaNDM-1 and blaNDM-9 (Supplementary Figure 2). A TnEc-like transposon from Tn3 family was identified in all genomic contexts with NDM genes (within 3093 bp) [28]. We conclude that the presence of NDM is associated with the expansion of the clones ST152 and ST11 and horizontal transfer of NDM-1 via the vehicle of an IncC plasmid.
blaVIM
Three blaVIM variants—blaVIM-2 (n = 13), blaVIM-4 (n = 6) and blaVIM-24 (n = 1) – were reported in 20 isolates from 7 departments, belonging to 8 STs, mainly ST147 (23.8%, n = 5), ST11 (19%, n = 4), and ST15 (19%, n = 4). The 3 blaVIM variants were found within 2 different class 1 integrons, known to be responsible for the mobilization of VIM genes (Supplementary Figure 2) [42, 45]. Our results contrast with those reported globally, where blaVIM-1 is the most common variant in VIM-carrying CRKP [42]. BlaVIM-24 was reported previously in an extremely resistant CRKP 2011 case report (n = 1) [17]. The observation that blaVIM-2 is the most common allele of VIM in P. aeruginosa in Colombia suggests that HGT may have been responsible for the intra-species transfer of the gene [45]. Further work will be carried out to investigate this hypothesis and the potential directionality of the transfer.
Co-productions
The 11 isolates with co-production of blaKPC+NDM came from 3 departments and were associated with 8 STs. In the isolates co-harboring blaKPC-3+NDM-1, it was observed that blaKPC-3 incorporated with Tn4401a exhibited a genetic environment, including [trpR (Tn3)-ISPsy42(Tn3)-ISKpn7(IS21)-blaKPC-3-ISKpn6(IS1182)-aacA4], and that the vehicle for blaNDM-1 was similar (TnEc-like). Coproducers of blaKPC-2+NDM-1 contained the same blaNDM-1 platform (TnEc-like), but the blaKPC-2 genetic environment revealed a diversity of genomic contexts. In the isolate co-producers of blaKPC-3+VIM-24, the blaVIM-24 gene was found associated with class-1 integron in IncC, and blaKPC-3 exhibited Tn4401b complemented with Tn2 transposases (Supplementary Figure 2). CRKPs with co-production of at least 2 genes have been previously reported worldwide, including blaKPC and blaVIM in Italy, blaKPC-2+NDM-1 in China and Brazil, and blaNDM-1+OXA-181 in Singapore [46]. The 3 coproductions reported in this study have been previously sporadically reported in Colombia [11, 12, 28], but this is the first report of the full replicon. Monitoring the dynamics of CRKPs with coproductions in the surveillance program is essential because of the potential risks they present, and because the way they emerge and whether they are only transient or have transmission capacity remain uncertain [43, 46].
CRKP Isolates not Harboring Carbapenemases
In 36 CRKP isolates not harboring a carbapenemase gene in their genomes, the resistance to carbapenems could be attributed to the presence of extended spectrum β-lactamase (ESBL) genes (86%, n = 31), especially blaCTX-M-15 (22/31); in 24 of the 31 isolates, these ESBL genes were in combination with porin truncations (Supplementary Table 2).
Porin truncations or mutations were found in 52% of all isolates. These truncations result in a nonfunctional pore that acts in concert to lower carbapenem concentrations, limiting antibiotic influx [47]. In 1 isolate, no resistance mechanisms were identified (Supplementary Table 3).
The enzymatic hydrolysis of carbapenems by carbapenemases was identified as the primary mechanism of carbapenem resistance, and the vast majority of carbapenemase producer isolates (91%, n = 385) were resistant simultaneously to all screening carbapenems (IPM, MEM, ETP) (Supplementary Figure 3). Possible explanations include the preferential use of carbapenems in hospitals for the treatment of serious infections caused by carbapenem resistance isolates, as well as the circulation of blaKPC-2 and blaKPC-3-harboring isolates promoting the progression of carbapenem resistance in Colombia, which is a KPC endemic country.
Additional AMR determinants to aminoglycosides (n = 390), fluoroquinolones (n = 378), phenicols (n = 317), ESBL and/or AmpC (n = 248), and tetracyclines (n = 219) were observed in the majority of isolates (Supplementary Table 2).
Our results outline the increasing complexity of the CRKP population, resistance mechanisms, and lineages in Colombia, which will require adaptations of the conventional infection control measures that were successful in areas where the objective was to curb the expansion of a single dominant clone.
Detection of Virulence Mechanisms and Serotypes
In general, we observed acquired virulence mechanisms in <30% of CRKP isolates in this study, which is in line with global trends [48]. However, we additionally observed an over-representation of colibactin genes among the isolates and the first observation in Colombia of a hypervirulent CRKP isolate.
The CG258 isolates exhibited different types of K-locus (KL), wzi, and O-locus (OL). The most common profile was KL107-wzi154:O2v2 (STs 258, 512, 1519, and 4851), present in 62.5% of isolates (Figures 2A, 2B, Supplementary Table 3) [21, 40, 49, 50]. The profile KL105-wzi75:O2v2, detected in 24% of isolates, is unusual for ST11 [21]. In China, where ST11 is endemic, the dominant KLs are KL47 and KL64, but the occurrence of capsule switching has been suggested in the case of this particular ST [37, 51]. In CG258, 76.9% of isolates were carriers of yersiniabactin (ybt), mainly of the ybt17 (105 isolates of ST258 and 2 of ST4851) and ybt10 (50 isolates of ST11 and 1 of ST258) lineages (Figures 2A, 2B, Supplementary Table 3). Details for other CGs are shown in Figure 2A and Supplementary Table 3.
It is remarkable to note that isolates of ybt17/ colibactin (clb3) lineages represented 25.2% of all isolates, while the spread of clb3 among CRKP is usually around 10% [52]. However, most isolates ybt17+clb3+ST258 have been reported in the literature as not being colibactin producers, due to a deletion in clbJ/clbK [50, 52].
Only 1 isolate (RB 877) from this study exhibited determinants associated with hypervirulence, such as ybt16, aerobactin (iuc2), salmochelin (iro2), and rmpA_3 (KpVP2). This ST380 isolate was recovered in Antioquia in 2013 and is a producer of KPC-2, with the profile KL2-wzi203:O1v1 (Table 1, Supplementary Table 2). CG380 has been classified as a hypervirulent clone [48]. This is the first time a hypervirulent CRKP is reported in Colombia.
The 2 cases of virulence elements reported in this study illustrate the risk of convergence of resistance and virulence, either through the acquisition of virulence plasmid(s) by CRKP or through the acquisition of resistance mobile genetic element(s) by virulent clone. The latter appears to be more difficult [52]. This emphasizes the need for genomic surveillance programs to include both resistance and virulence locus information.
Genetic Diversity Among CRKP Isolates
The phylogenetic analysis by SNP reflected the MLST distribution into STs as described above. Additionally, it highlights the genomic diversity within each ST. Of interest, we observed ST11, ST147, ST152, and ST307 with low nucleotide divergence (0.05–0.47%), suggesting clonality (Figure 1B). However, a deeper analysis by ST showed distinct clades within each ST (Supplementary Figure 4).
To our knowledge, this is the first report of blaNDM +VIM-carrying ST11 isolates in Colombia, although they have been reported worldwide [42, 44, 53]. There were several distinct clades of ST11, with different serotypes, resistance genes, and plasmid replicons (Supplementary Figure 4). Importantly, ST11 holds isolates with blaKPC, blaNDM, or blaVIM. From those, blaNDM was observed alongside IncC1 and Tn552-like, although blaKPC was associated to IncFIB and Tn4401, and VIM isolates with IntI1, all 3 with no clear phylogenetic relationship (see microreact). This opens the possibility of multiple introductions or the emergence of ST11 into Colombia. After comparing with globally reported ST11 samples, isolates relate mostly to Brazil and European samples [39]. This CRKP diversity in ST11 calls for further investigation in their introduction pathways.
The ST147 showed several clades with differences in carbapenemase genes, related to Inc-type elements, discarding the clonality of the group (Supplementary Figure 4). Similarly, ST307 showed different clades some with blaKPC, blaNDM, or blaVIM, alongside IncFIB mobile elements and ESBL genes. The apparent clonality may come from the acquisition of carbapenemase genes in multiple events, related to mobile elements. This is in accordance with ST147 found related to Inc-type plasmids, and ST307 to blaOXA48-like plasmid genes, both related to carbapenemase genes HGT [43].
ST152 was found as an emerging clone with a genomic profile characterized by blaNDM, several Inc-type mobile elements and hypervirulence provided by the presence of yersiniabactin, similar to that reported in Saudi Arabia and other countries as a hypervirulent ST of importance in urinary tract infections [54]. All previous analyses enhances the importance of WGS for AMR surveillance, as differential findings between STs and genes reveal nonclonality that may be translated into differential treatment of CRKP infections.
Among this study’s limitations were: having only voluntary surveillance isolates at the national level; and limited data of clinical and epidemiological information for a more exhaustive investigation. Nevertheless, the information obtained is essential for national surveillance and provides insights into pathogen genetics for planning effective surveillance.
CONCLUSION
This study allowed a detailed description of CRKP epidemiology in Colombia that should be taken into account when establishing efficient WGS-based surveillance programs in the country.
There are 7 predominant high-risk STs in Colombia. In addition to the previously described CG258 with KPC, we showed a complex, recent and significant expansion of multiple clones of interest and carbapenemases, urging reinforcement of the CRKP genomic surveillance process.
The dissemination of carbapenemase genes is based on a complex combination of mechanisms. KPC-3 occurs by the expansion of CG258, mainly ST258 and ST512 carrying an IncFII replicon plasmid with the insertion of transposon Tn4401-like. The spread of KPC-2 and VIM genes was associated with the horizontal transfer of different plasmid replicons and mobile elements. NDM-1 dissemination was associated with the expansion of clones CG152 and ST11 (CG258) and possible HGT related to IncC. The high proportion of HTG mechanisms in disseminating carbapenem resistance genes could have resulted from higher selective pressure due to overuse of antibiotics in a challenged healthcare system. This result shows the importance of including mobile genetic elements in the CRKP surveillance program in Colombia.
The hypervirulent strain report and the over-representation of the colibactin virulence factor urge close monitoring of the potential convergence of virulence and resistance of CRKP in Colombia.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
The authors acknowledge all public health laboratories from the National Reference Laboratory (NRL) for providing the isolates CRKP obtained under the previous National Surveillance by the Laboratory of Antimicrobial Resistance Surveillance Network.
Financial support. This work was supported by Official Development Assistance (ODA) funding from the National Institute for Health Research [grant number 16_136_111] and the Wellcome Trust grant number 206194. This research was commissioned by the National Institute for Health Research using Official Development Assistance (ODA) funding. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
MK reports support from Centre for Genomic Pathogen Surveillance (CGPS). M. A. reports support from Wellcome Connecting Science.
Potential conflicts of interest. M. K. reports grants or contracts, support for attending meetings/travel, and receipt of equipment, materials, drugs, medical writing, gifts or other services from National Institute for Health Research (NIHR). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplement sponsorship. The supplement is sponsored by the UK National Institute for Health Research Global Health Research Unit on Genomic Surveillance of AMR.
Acknowledgments. Members of the NIHR Global Health Research Unit on Genomic Surveillance of Antimicrobial Resistance: Khalil Abudahab, Harry Harste, Dawn Muddyman, Ben Taylor, Nicole Wheeler, and Sophia David of the Centre for Genomic Pathogen Surveillance, Big Data Institute, University of Oxford, Old Road Campus, Oxford, UK. and Wellcome Genome Campus, Hinxton, UK; Gabriel Beltran, Felipe Delgadillo and Erik C. D. Osma Castro of the Colombian Integrated Program for Antimicrobial Resistance Surveillance (COIPARS), CI Tibaitatá, Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA), Tibaitatá–Mosquera, Cundinamarca, Colombia; K. L. Ravikumar, Geetha Nagaraj, Varun Shamanna, Vandana Govindan, Akshata Prabhu, D. Sravani, M. R. Shincy, Steffimole Rose, and Ravishankar K.N of the Central Research Laboratory, Kempegowda Institute of Medical Sciences, Bengaluru, India; Iruka N Okeke, Anderson O. Oaikhena, Ayorinde O. Afolayan, Jolaade J Ajiboye, and Erkison Ewomazino Odih of the Department of Pharmaceutical Microbiology, Faculty of Pharmacy, University of Ibadan, Oyo State, Nigeria; Celia Carlos, Marietta L. Lagrada, Polle Krystle V. Macaranas, Agnettah M. Olorosa, June M. Gayeta, and Elmer M. Herrera of the Antimicrobial Resistance Surveillance Reference Laboratory, Research Institute for Tropical Medicine, Muntinlupa, the Philippines; Ali Molloy, alimolloy.com; John Stelling, The Brigham and Women’s Hospital; and Carolin Vegvari, Imperial College London.
Contributor Information
NIHR Global Health Research Unit on Genomic Surveillance of Antimicrobial Resistance:
Khalil Abudahab, Harry Harste, Dawn Muddyman, Ben Taylor, Nicole Wheeler, Sophia David, Gabriel Beltran, Felipe Delgadillo, Erik C D Osma, K L Ravikumar, Geetha Nagaraj, Varun Shamanna, Vandana Govindan, Akshata Prabhu, D Sravani, M R Shincy, Steffimole Rose, K N Ravishankar, Iruka N Okeke, Anderson O Oaikhena, Ayorinde O Afolayan, Jolaade J Ajiboye, Erkison Ewomazino Odih, Celia Carlos, Marietta L Lagrada, Polle Krystle V Macaranas, Agnettah M Olorosa, June M Gayeta, Elmer M Herrera, Ali Molloy, John Stelling, and Carolin Vegvari
References
- 1. Bengoechea JA, Sa Pessoa J. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev 2018; 43:123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caneiras C, Lito L, Melo-Cristino J, Duarte A. Community- and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: virulence and antibiotic resistance. Microorganisms 2019; 7. doi: 10.3390/microorganisms7050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. Available at: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. Accessed 18 August 2021.
- 4. Köser CU, Ellington MJ, Peacock SJ. Whole-genome sequencing to control antimicrobial resistance. Trends Genet 2014; 30:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rojas LJ, Weinstock GM, De La Cadena E, et al. An analysis of the epidemic of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: convergence of two evolutionary mechanisms creates the “perfect storm”. J Infect Dis 2017; 217:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argimón S, Masim MAL, Gayeta JM, et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat Commun 2020; 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez Rubio A. Consumo de antibioticos en el ámbito hospitalario. Colombia, 2017. Available at: https://www.ins.gov.co/buscador-eventos/Informesdeevento/CONSUMO DE ANTIBIÓTICOS 2017.pdf. Accessed 18 August 2021. [Google Scholar]
- 8. European Centre for Disease Prevention and Control. Antimicrobial consumption in the EU/ EEA Annual Epidemiological Report for 2019 Key facts. Stockholm, 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf. Accessed 18 August 2021. [Google Scholar]
- 9. INS. Resultados del Programa de Vigilancia por Laboratorio de Resistencia antimicrobiana en Infecciones Asociadas a la Atención en Salud (IAAS) 2015. 2016. Available at: https://www.ins.gov.co/BibliotecaDigital/informe-vigilancia-por-laboratorio-resistencia-antimicrobiana-y-whonet-iaas-2015.pdf. Accessed 18 August 2021.
- 10. INS. Resultados del Programa de Vigilancia por Laboratorio de Resistencia antimicrobiana en Infecciones Asociadas a la Atención en Salud (IAAS) 2016. 2017. Available at: http://www.ins.gov.co/buscador-eventos/Informacin de laboratorio/Informe Vigilancia por Laboratorio Resistencia Antimicrobiana y Whonet IAAS 2016.pdf. Accessed 18 August 2021.
- 11. INS. Resultados del Programa de Informe de Resultados de la Vigilancia por Laboratorio de Resistencia antimicrobiana en Infecciones Asociadas a la Atención en Salud (IAAS) 2017. 2018. Available at: http://www.ins.gov.co/buscador-eventos/Informacin de laboratorio/Informe Vigilancia por Laboratorio Resistencia Antimicrobiana y Whonet IAAS 2017.pdf. Accessed 18 August 2021.
- 12. Ovalle MV, Saavedra SY, González MN, Hidalgo AM, Duarte C, Beltrán M. Results of the national surveillance of antimicrobial resistance of Enterobacteriaceae and gram negative bacilli in health care-associated infections in Colombia, 2012-2014. Biomedica 2017; 37:473–85. [DOI] [PubMed] [Google Scholar]
- 13. Villegas MV, Lolans K, Correa A, et al. ; Colombian Nosocomial Resistance Study Group . First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother 2006; 50:2880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–6. [DOI] [PubMed] [Google Scholar]
- 15. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ocampo AM, Chen L, Cienfuegos AV, et al. A two-year surveillance in five Colombian tertiary care hospitals reveals high frequency of non-CG258 clones of carbapenem-resistant Klebsiella pneumoniae with distinct clinical characteristics. Antimicrob Agents Chemother 2016; 60:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montealegre MC, Correa A, Briceño DF, et al. ; Colombian Nosocomial Resistance Study Group . Novel VIM metallo-beta-lactamase variant, VIM-24, from a Klebsiella pneumoniae isolate from Colombia. Antimicrob Agents Chemother 2011; 55:2428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escobar Pérez JA, Olarte Escobar NM, Castro-Cardozo B, et al. Outbreak of NDM-1-producing Klebsiella pneumoniae in a neonatal unit in Colombia. Antimicrob Agents Chemother 2013; 57:1957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Underwood A. GHRU (Genomic Surveillance of Antimicrobial Resistance) retrospective 1 bioinformatics methods. protocols.io: 2020. Available at: https://gitlab.com/cgps/ghru/pipelines/dsl2/pipelines/amr_prediction. Accessed 7 January 2021.
- 20. Lam MMC, Wick RR, Wyres KL, Holt KE. Genomic surveillance framework and global population structure for Klebsiella pneumoniae. bioRxiv 2020:2020.12.14.422303. Available at: http://biorxiv.org/content/early/2020/12/14/2020.12.14.422303.abstract, https://www.biorxiv.org/content/biorxiv/early/2020/12/14/2020.12.14.422303.full.pdf. Accessed 18 August 2021.
- 21. Wyres KL, Wick RR, Gorrie C, et al. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom 2016; 2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 2015; 32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Argimón S, Abudahab K, Goater RJE, et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb Genom 2016; 2:e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 2014; 22:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 2015; 59:5873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cienfuegos-Gallet AV, Ocampo de Los Ríos AM, Sierra Viana P, et al. Risk factors and survival of patients infected with carbapenem-resistant Klebsiella pneumoniae in a KPC endemic setting: a case-control and cohort study. BMC Infect Dis 2019; 19:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Apolito D, Arena F, Conte V, et al. Phenotypical and molecular assessment of the virulence potential of KPC-3-producing Klebsiella pneumoniae ST392 clinical isolates. Microbiol Res 2020; 240:126551. [DOI] [PubMed] [Google Scholar]
- 28. Rojas LJ, Wright MS, De La Cadena E, et al. Initial assessment of the molecular epidemiology of blaNDM-1 in Colombia. Antimicrob Agents Chemother 2016; 60:4346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peirano G, Chen L, Kreiswirth BN, Pitout JDD. Emerging antimicrobial resistant high-risk clones among Klebsiella pneumoniae: ST307 and ST147. Antimicrob Agents Chemother 2020; 64:e01148–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia-Fulgueiras V, Zapata Y, Papa-Ezdra R, et al. First characterization of K. pneumoniae ST11 clinical isolates harboring blaKPC-3 in Latin America. Rev Argent Microbiol 2020; 52:211–6. [DOI] [PubMed] [Google Scholar]
- 31. Villa L, Feudi C, Fortini D, et al. Diversity, virulence, and antimicrobial resistance of the KPC-producing Klebsiella pneumoniae ST307 clone. Microb Genom 2017; 3. doi: 10.1099/mgen.0.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 2017; 41:252–75. [DOI] [PubMed] [Google Scholar]
- 33. Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 2009; 53:3365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwaber MJ, Lev B, Israeli A, et al. ; Israel Carbapenem-Resistant Enterobacteriaceae Working Group . Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011; 52:848–55. [DOI] [PubMed] [Google Scholar]
- 35. Pathogenwatch. Collection of genomes KPN ST258. Available at: https://pathogen.watch/collection/zshue8ur9z7a-globalcomparisonkpnst258. Accessed 18 August 2021.
- 36. Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother 2011; 66:307–12. [DOI] [PubMed] [Google Scholar]
- 37. Zhou K, Xiao T, David S, et al. Novel subclone of carbapenem-resistant Klebsiella pneumoniae sequence type 11 with enhanced virulence and transmissibility, China. Emerg Infect Dis 2020; 26:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pereira PS, de Araujo CF, Seki LM, Zahner V, Carvalho-Assef AP, Asensi MD. Update of the molecular epidemiology of KPC-2-producing Klebsiella pneumoniae in Brazil: spread of clonal complex 11 (ST11, ST437 and ST340). J Antimicrob Chemother 2013; 68:312–6. [DOI] [PubMed] [Google Scholar]
- 39. Pathogenwatch. Collection of genomes KPN ST11. Available at: https://pathogen.watch/collection/32fk0u6hb2bi-globalcomparisonkpnst11. Accessed 18 August 2021.
- 40. David S, Reuter S, Harris SR, et al. ; EuSCAPE Working Group; ESGEM Study Group . Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol 2019; 4:1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pathogenwatch. Collection of genomes KPN ST512. Available at: https://pathogen.watch/collection/9g0wo3trv31d-globalcomparisonkpnst512. Accessed 18 August 2021.
- 42. Matsumura Y, Peirano G, Devinney R, et al. Genomic epidemiology of global VIM-producing Enterobacteriaceae. J Antimicrob Chemother 2017; 72:2249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. David S, Cohen V, Reuter S, et al. ; European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group; ESCMID Study Group for Epidemiological Markers (ESGEM) . Integrated chromosomal and plasmid sequence analyses reveal diverse modes of carbapenemase gene spread among Klebsiella pneumoniae. Proc Natl Acad Sci U S A 2020; 117:25043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 2019; 32. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Correa A, Del Campo R, Perenguez M, et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob Agents Chemother 2015; 59:2421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao H, Liu Y, Wang R, Wang Q, Jin L, Wang H. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 2020; 51. doi: 10.1016/j.ebiom.2019.102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong JLC, Romano M, Kerry LE, et al. OmpK36-mediated carbapenem resistance attenuates ST258 Klebsiella pneumoniae in vivo. Nat Commun 2019; 10. doi: 10.1038/s41467-019-11756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wyres KL, Wick RR, Judd LM, et al. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLoS Genet 2019; 15:e1008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lam MMC, Wyres KL, Judd LM, et al. Tracking key virulence loci encoding aerobactin and salmochelin siderophore synthesis in Klebsiella pneumoniae. Genome Med 2018; 10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol 2020; 18:344–59. [DOI] [PubMed] [Google Scholar]
- 51. Zhao Q, Guo L, Wang LF, Zhao Q, Shen DX. Prevalence and characteristics of surgical site hypervirulent Klebsiella pneumoniae isolates. J Clin Lab Anal 2020; 34:e23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lam MMC, Wick RR, Wyres KL, et al. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom 2018; 4:e000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roach D, Waalkes A, Abanto J, et al. Whole genome sequencing of Peruvian Klebsiella pneumoniae identifies novel plasmid vectors bearing carbapenem resistance gene NDM-1. Open Forum Infect Dis 2020; 7:ofaa266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Al-Agamy MH, El-Mahdy TS, Radwan HH, Poirel L. Cooccurrence of NDM-1, ESBL, RmtC, AAC(6’)-Ib, and QnrB in clonally related Klebsiella pneumoniae isolates together with coexistence of CMY-4 and AAC(6′)-Ib in Enterobacter cloacae isolates from Saudi Arabia. Biomed Res Int 2019; 2019:6736897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.