ABSTRACT
Activation of brown adipose tissue (BAT) and promotion of white adipose tissue (WAT) browning is considered a potential tool to combat obesity and cardiometabolic disorders. The use of plant-based dietary components has become one of the most used strategies for activating BAT and promoting WAT browning in rodents. The main reason is because plant-based dietary components are usually recognized as safe when the dose is properly adjusted, and they can easily be administrated by being added to the diet or dissolved in water. The present systematic review aimed to study the effects of plant-based dietary components on activation of BAT and promotion of WAT browning in rodents. A systematic search of PubMed and Scopus (from 1978 to 2019) identified eligible studies. Studies assessing the effects of plant-based dietary components added to diet and/or water on uncoupling protein 1 (UCP1) expression in BAT and/or WAT were included. Studies that used dietary components of animal origin, did not specify the effects on UCP1, or were conducted in other species different from mice or rats were excluded. Of 3919 studies identified in the initial screening, 146 studies were finally included in the review. We found that tea extract catechins, resveratrol, capsaicin and capsinoids, cacao extract flavanols, and quercetin were the most studied components. Scientific evidence suggests that some of these dietary components activate BAT and promote WAT browning via activation of the AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) pathways. These findings reveal that there is strong scientific evidence supporting the use of plant-based dietary components to activate BAT and promote WAT browning in rodents and thus to potentially combat obesity and cardiometabolic disorders.
Keywords: brown fat, food ingredients, thermogenesis, UCP1, obesity, beigeing
Statement of Significance: This is the first systematic review that affords a critical analysis of the effects of plant-based dietary components on BAT activation and promotion of WAT browning in rodents. The findings of the present systematic review support the use of plant-based dietary components to activate BAT and promote WAT browning in rodents and thus to potentially combat obesity and cardiometabolic disorders.
Introduction
Obesity is a global epidemic that increases the risk of morbidity and reduces life span, being closely related to an increased risk of developing cardiometabolic disorders (1, 2). Brown adipose tissue (BAT) is considered a target tissue to combat obesity (3) and cardiovascular disease (4), as BAT activation increases energy expenditure, reduces adiposity, and effectively protects against diet-induced obesity in mice (5, 6). The thermogenic capacity of BAT is driven by uncoupling protein 1 (UCP1) activity, located in the inner mitochondrial membrane of brown adipocytes (7). Interestingly, UCP1 can also be expressed in beige adipocytes [brown-like adipocytes within white adipose tissue (WAT)], a process known as WAT browning (8). The resulting increase in energy expenditure due to BAT activation goes beyond heat generation, improving glucose and lipid metabolism (9–11). Furthermore, it seems that BAT also exerts an endocrine function through the so-called batokines (adipokines released by brown adipocytes), which could partially explain improvements in metabolism (12, 13). The existence of UCP1-independent thermogenic mechanisms is also known, yet their relevance in terms of energy expenditure remains poorly understood (14).
Cold exposure is the canonical stimulus for BAT activation (14), this being primarily mediated through β-3 adrenergic receptor (β3-AR) stimulation in rodents (15). There are, however, other ways to stimulate BAT activation and to promote WAT browning (both understood as an increase in UCP1 expression), such as the pharmacologic agonism of the β3-AR (16) and the glucagon-like peptide 1 (GLP1) receptor (17). Increasing evidence suggests that plant-based dietary components, which can easily be added to the diet or dissolved in water (18), can also boost BAT activation and promote WAT browning (19–21). Moreover, a significant fraction of these dietary components is Generally Recognized As Safe (GRAS) in the United States, which results in a large list of potential candidates. This might explain the massive increase in the number of publications on this topic.
Therefore, the main goal of the present systematic review was to study the effects of plant-based dietary components on BAT activation and promotion of WAT browning in rodents.
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (22).
Inclusion and exclusion criteria
The inclusion criteria were as follows—1) meeting the definition of plant-based dietary components: naturally occurring isolated dietary component, selected isolated fraction of plant extracts, or whole-plant extracts of vegetal origin, and thus excluding those of animal origin (i.e., conjugated linoleic, fish oil, or omega-3 fatty acids from animal origin); 2) administration: orally via mixture with diet or dissolved in water; 3) type of rodent: mice and rats; 4) UCP1 expression: measurements of UCP1 gene/protein expression in BAT and/or WAT; 5) original papers (not reviews); and 6) articles written in the English language.
The exclusion criteria were 1) plant-based dietary components categorized as toxic (i.e., alcohol or ephedrine), 2) inclusion of a control group with a different type of diet from the intervention group, and 3) housed animals at different temperatures from the intervention group.
Eligibility for inclusion and exclusion criteria was evaluated by reading 1) title and abstract (n = 3919) and 2) full text, but only when the information provided in the title and abstract did not allow to decide on the inclusion or exclusion of the study. We read the full text of ∼300 publications.
Data-collection process
The following data were extracted from each included study: 1) plant-based dietary component; 2) daily dose (single dose for acute studies); 3) species (sex); 4) age (at the beginning of the intervention, weeks); 5) duration of intervention with the plant-based dietary component (weeks); 6) housing temperature (°C); 7) light cycle (lights on:light off); 8) humidity (%); 9) food and water access (e.g., ad libitum or time-restricted); 10) type of diet; 11) sample size (intervention group); 12) activation of BAT and promotion of WAT browning, studies that measured UCP1 at gene (qRT-PCR) and/or protein (immunoblot and immunostaining assays) expression levels in 1 or both tissues; and 13) reference.
Search strategy
We used 3 different term resources from the National Library of Medicine for indexing articles for PubMed in our search—1) medical subject heading (MeSH) terms: used for ceiling the search to publications where that term is the major focus of the article; 2) text words (tw): this includes all words and numbers in the title, abstract, MeSH terms, MeSH subheadings, publication types, and other relevant sections; and 3) Supplementary concept: this includes chemical or organism-specific indexed terms. Search terms related to the main goal of the current systematic review were combined using the following strategy in PubMed: (“Adipose Tissue, Brown”[MeSH] OR “Adipocytes, Brown”[MeSH] OR “browning”[tw] OR “beigeing”[tw] OR ((“brown”[tw] OR “beige”[tw] OR “brite”[tw]) AND (“fat”[tw] OR “adipose”[tw] OR “adipocyte”[tw] OR “adipocytes”[tw] OR “thermogenesis”[tw]) OR (“Uncoupling Protein 1”[MeSH] OR “Uncoupling Protein 1”[tw] OR “UCP1”[tw] OR “Ucp1 protein, rat” [Supplementary concept]) OR (“Ucp1 protein, mouse” [Supplementary concept]) AND (“Food”[MeSH] OR “food”[tw] OR “foods”[tw] OR “condiment”[tw] OR “condiments”[tw] OR “spice”[tw] OR “spices”[tw] OR “dietary”[tw] OR “diet”[tw] OR “diets”[tw] OR “carbohydrate”[tw] OR “carbohydrates”[tw] OR “grain”[tw] OR “grains”[tw] OR “fiber”[tw] OR “fibers”[tw] OR “prebiotic”[tw] OR “prebiotics”[tw] OR “probiotic”[tw] OR “probiotics”[tw] OR “fruit”[tw] OR “fruits”[tw] OR “seed”[tw] OR “seeds”[tw] OR “nuts”[tw] OR “intake”[tw] OR “vegetable”[tw] OR “vegetables”[tw] OR “flavoring”[tw] OR “flavouring”[tw] OR “Flavonoids”[MeSH] OR “Flavonoids”[tw] OR “Flavonoid”[tw] OR “Anthocyanins”[tw] OR “Anthocyanin”[tw] OR “Catechins”[tw] OR “Catechin”[tw] OR “Flavanones”[tw] OR “Flavanone”[tw] OR “Flavones”[tw] OR “Flavone”[tw] OR “Flavonolignans”[tw] OR “Flavonolignan”[tw] OR “Isoflavones”[tw] OR “Isoflavone”[tw]) AND (“Animal Experimentation”[MeSH] OR “Murinae”[MeSH] OR “murinae”[tw] OR “rat”[tw] OR “rats”[tw] OR “mouse”[tw] OR “mice”[tw] OR “murine”[tw] OR “rodent”[tw] OR “rodents”[tw]) NOT (“humans”[MeSH] NOT “murinae”[mesh]). Publication date range was set from the identification of UCP1 as the inner mitochondria component driving the thermogenic process in BAT in 1978 (23) until 30 November 2019.
Results
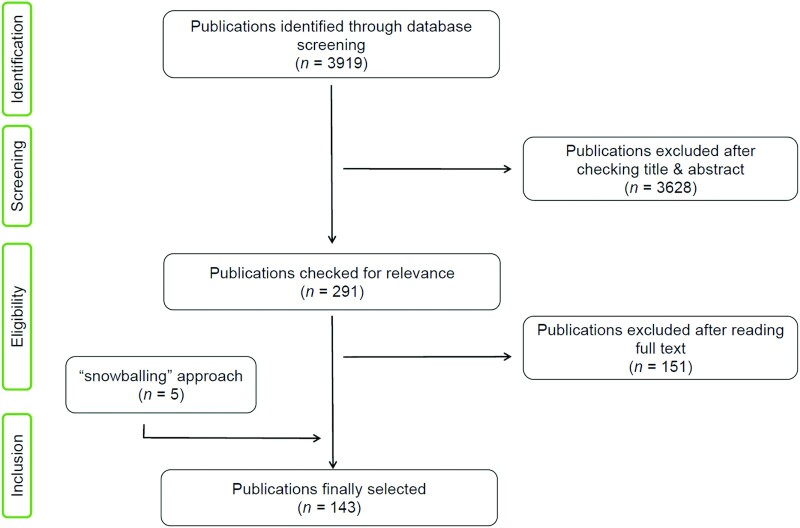
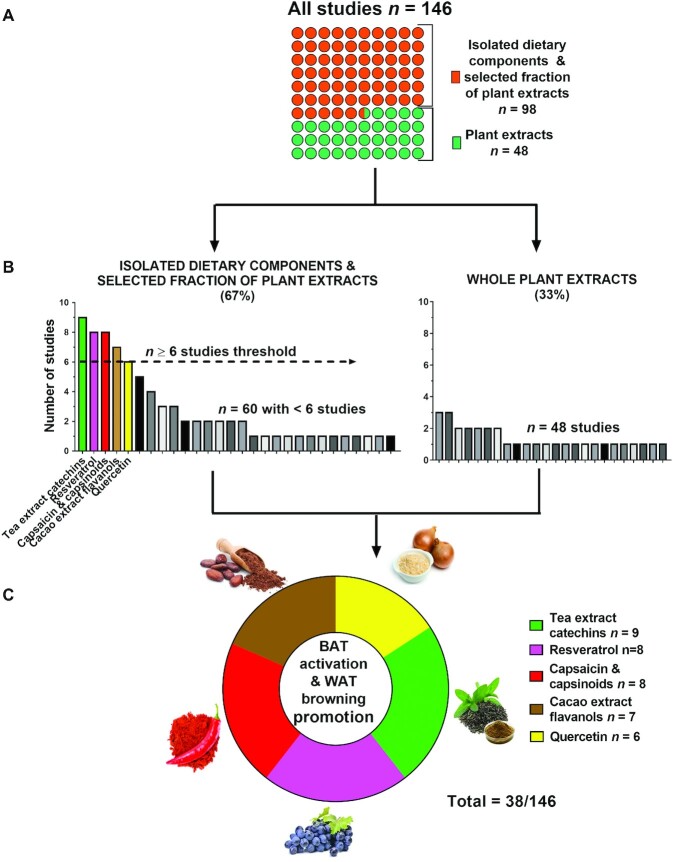
A total of 3919 publications were identified in the search (Figure 1). No additional studies meeting the inclusion criteria were identified after adapting the search terms to the Scopus database (data not shown). A total of 143 publications (including 146 different studies) were included after applying the inclusion and exclusion criteria. Supplemental Table 1 depicts all the plant-based dietary components included with references, sorted by the number of studies and name of the dietary component. Supplemental Table 2 shows the study set-ups and the main results of the 146 studies included (Supplemental References) in the systematic review. Notably, 98 of 146 studies (67%) used an isolated plant-based dietary component or a selected isolated fraction of the plant extracts, whereas the rest (n = 48, 33%) used the whole-plant extract. Because of the heterogeneity of the methods and information availability of the included studies, no quality-assessment scale system could be applied.
FIGURE 1.
Flowchart of the literature search and study selection process.
General results
For a better comprehension of the results and due to the elevated number of included studies, we have focused on those plant-based dietary components that were investigated in 6 or more studies. The most studied plant-based dietary components were as follows: 1) tea extract catechins (n = 9 studies) (24–32), 2) resveratrol (n = 8 studies) (33–40), 3) capsaicin and capsinoids (n = 8 studies) (41–48), 4) cacao extract flavanols (n = 7 studies) (49–55), and 5) quercetin (n = 6 studies) (56–61), which constitute a subset of n = 38 studies to be considered for the next analysis (see Figure 2 and Table 1). When we applied a less strict threshold (n ≥ 3), the studies using monosaccharides/sweeteners, curcumin, leucine, menthol, garlic, and Puerariae flowers were included (Supplemental Figure 1). Of the 86 different plant-based dietary components included in the 146 studies, 14% were studied twice and 73% were studied only once. Among the subset of the 38 selected studies, 96% of the studies that measured UCP1 expression reported a significant activation of BAT (24, 25, 29–35, 37–39, 41, 42, 45, 49–55, 57), whereas 84% of the studies that measured UCP1 expression reported a significant promotion of WAT browning (24, 26–28, 36, 40, 42–44, 46, 56–61). Some studies found that an upregulation in AMP-activated protein kinase (AMPK) signaling was involved in BAT activation (29, 31, 38, 39, 50, 52, 54, 55) and promotion of WAT browning (36, 56, 57, 59). Accordingly, other studies found that an upregulation of the sirtuin 1 (SIRT1) signaling was involved in BAT activation (30, 33–35, 45) and promotion of WAT browning (44).
FIGURE 2.
Process to select the most studied plant-based dietary components that activate BAT and/or promote WAT browning in rodents. (A) The number of isolated dietary components and selected fraction of plant extract studies versus plant extract studies after the initial screening. (B) Histogram depicting isolated dietary components and selected fractions of plant extract studies (n ≥ 6) (left) and plant studies (right). (C) Donut diagram depicting the most studied plant-based dietary components. BAT, brown adipose tissue; WAT, white adipose tissue.
TABLE 1.
Summary of the main findings on the effect of plant-based dietary components on BAT activation and WAT browning1
| Dietary component | Studies, n | Species (sex) | Type | Dose | BAT activation2 | WAT browning2 | Ref |
|---|---|---|---|---|---|---|---|
| Tea extract catechins | 9 | Sprague Dawley rats (male) | Tea catechins extract | 100 mg/(kg · d) | + | + | (24) |
| Sprague Dawley rats (male) | Tea catechins extract | 0.5/100 g diet | + | ? | (25) | ||
| C57BL/6J mice (male) | Decaffeinated green tea catechins | 7.7 g/(kg · d) | ? | + | (26) | ||
| Sprague Dawley rats (male) | Tea catechins extract | 77.5 mg/kg diet or 155 mg/kg diet | ? | + | (27) | ||
| C57BL/6J mice (male) | Green tea leaves extract | 0.5% diet | ? | + | (28) | ||
| IRC | Tea catechins extract | 10 mg/kg (single dose) | + | ? | (29) | ||
| C57BL/6J mice (male) | Epigallocatechin-3-gallate | 2 g/(L· d) | + | ? | (30) | ||
| C57BL/6J mice (male) | Epigallocatechin-3-gallate | 0.2% wt:wt | + | ? | (31) | ||
| C57BL/6J mice (male) | Epigallocatechin-3-gallate | 1% wt:wt | + | ? | (32) | ||
| Resveratrol | 8 | NR (male mice) | Resveratrol | 25 mg/d | + | ? | (33) |
| Wistar Rat (male) | Resveratrol | 30 mg/(kg · d) | + | ? | (34) | ||
| ? (male mice) | Resveratrol | 4 kg/(g · d) | + | ? | (35) | ||
| cd1 mice (female) | Resveratrol | 0.1% wt:wt | ? | + | (36) | ||
| OLETF rats (male) | Resveratrol | 10 mg/(kg · d) | + | ? | (38) | ||
| cd1 mice (female) | Resveratrol | 0.1% wt:wt | + | ? | (39) | ||
| C57BL/6 mice (male) | Resveratrol (R) or oxyresveratrol (OR) | 0.5% (R); 0.1% or 0.5% oxyresveratrol (OR) | ? | + | (40) | ||
| Wistar rat (male) | Resveratrol | 30 mg/(kg · d) | + | ? | (37) | ||
| Capsaicin and capsinoids | 8 | Std ddY mice (male | Capsiate | 10 mg/kg (single dose + 2 wk) | + | ∼ | (41) |
| Swiss albino mice (male) | Dihydrocapsiate | 2 or 10 mg/(kg · d) | ? | + | (46) | ||
| C57BL/6N mice (male) | Capsinoids | 0.3% wt:wt | ∼ | + | (47) | ||
| C57BL/6J mice (male) | Capsinoids | 0.3% wt:wt | ? | ∼ | (43) | ||
| C57BL/6J (male) | Capsinoids | 0.3% wt:wt | + | ∼ | (48) | ||
| Swiss albino mice (male) | Capsaicin | 2 mg/(kg · d) | + | + | (42) | ||
| B6.129×1 (male) | Capsaicin | 0.1% wt:wt | ? | + | (44) | ||
| B6.129×1 (male) | Capsaicin | 0.003, 0.01, 0.03% wt:wt | + | ? | (45) | ||
| Cacao extract flavanols | 7 | C57BL/J mice (male) | Flavan-3-ol fraction | 50 mg/(kg · d) | + | ? | (49) |
| Wistar rats (male) | Flavan-3-ol fraction | 0.2% diet | + | ? | (51) | ||
| C57BL/6J mice (male) | (−)-Epicatechin | 15 mg/(kg · d) | + | ? | (50) | ||
| IRC mice (male) | Flavan-3-ol fraction | 10 mg/kg (single dose) | + | ? | (52) | ||
| C57BL/6 mice (male) | Cacao liquor procyanidin extract | 0.5 or 2% wt:wt | + | ? | (55) | ||
| IRC mice (male) | Flavan-3-ol fraction | 10 mg/kg (single dose) | + | ? | (54) | ||
| IRC mice (male) | B-type procyanidins | 10 mg/kg (single dose) | + | ? | (53) | ||
| Quercetin | 6 | C57BL/6 mice (male) | Isoquercitrin or quercetin | 0.02%, 0.1% and 0.5% wt:wt | ? | + | (56) |
| C57BL/6 mice (male) | Pentamethylquercetin | 0.4% g/g | ? | + | (60) | ||
| C57BL/6J mice (male) | Onion peel extract | 0.5% | ? | + | (59) | ||
| C57BL/6J mice (male) | Quercetin | 0.01% wt:wt | ∼ | + | (58) | ||
| C57BL/6J mice (male) | Quercetin | 0.05% wt:wt | + | + | (57) | ||
| Sprague Dawley rats (male) | Quercetin | 0.36% and 0.72% | ? | + | (61) |
n ≥ 6 studies. BAT, brown adipose tissue; Ref, reference; UCP1, uncoupling protein 1; WAT, white adipose tissue.
BAT activation and WAT browning: (+) UCP1 expression significantly increased, (∼) UCP1 expression unchanged, (?) UCP1 expression was not studied.
Tea catechins, resveratrol, capsaicin, and capsinoids promote BAT activation and WAT browning
Tea extract catechins promoted BAT activation in 6 of 9 studies (24, 25, 29–32) and increased WAT browning in 4 studies (24, 26–28), whereas resveratrol promoted BAT activation in 6 of 8 studies (33–35, 37–39) and increased WAT browning in 2 studies (36, 40). Capsaicin and capsinoids promoted BAT activation in 4 of 8 studies (41, 42, 45, 48), while 1 study showed no BAT activation (43). Among these 8 studies, 5 reported that capsaicin and capsinoids promoted WAT browning (42–44, 46, 48), while 3 reported no significant effects on WAT browning (41, 43, 48).
Cacao extract flavanols activate BAT and quercetin promotes WAT browning
Cacao extract flavanols promoted BAT activation in all studies (n = 7) (49–55), while none of them evaluated the effect of cacao extract flavanols on WAT browning. Quercetin promoted BAT activation in 1 of 6 studies (57), while 1 study reported no BAT activation (58).
Discussion
In this systematic review, we investigated rodent studies evaluating the effects of plant-based dietary components on the activation of BAT and promotion of WAT browning. Tea extract catechins were the most studied plant-based dietary component, followed by resveratrol, capsaicin and capsinoids, cacao flavanols, and quercetin. Sixty-seven percent of the studies used isolated dietary components or a selected fraction of the plant extracts, whereas the remaining 33% used whole-plant extracts. AMPK and SIRT1 signaling pathways were upregulated and linked to the activation of BAT and promotion of WAT browning. Collectively, these findings support the use of plant-based dietary components to activate BAT and promote WAT browning in rodents and thus to potentially combat obesity and cardiometabolic disorders.
Tea from plants of Camellia sinensis is one of the world's most consumed beverages (62). The thermogenic response to tea extract catechins seems to be driven by the transient receptor potential (TRP) vanilloid subfamily member 1 (TRPV1) and TRP ankyrin 1 (TRPA1) channels expressed in the gut (63), and brown and white adipocyte membranes (64). Interestingly, the most abundant and bioactive tea catechin, epi-gallocatechin gallate (EGCG), and its autoxidation products can activate TRPV1 and TRPA1 in intestinal enteroendocrine cells at doses equivalent to those expected in the gut after tea catechin ingestion (65, 66). Therefore, it is likely that tea catechins could activate BAT via TRP channels located in the sensory neurons of the gut via a gut–sympathetic nervous system (SNS)–BAT axis (67). BAT activation by tea catechins will be ultimately driven via sympathetic activation of β-AR on brown adipocytes and by the inhibition of catechol-O-methyl transferase (COMT) by tea catechins, a catecholamine-degrading enzyme (68, 67). However, COMT activity is not inhibited by high doses of EGCG in humans, indicating a negligible role of COMT in the catechin effects in vivo (69, 70). This is explained by the much lower circulating concentrations of catechins after a single ingestion observed (∼0.1 μM at maximum) (71), compared with the half-maximal inhibitory concentration for the COMT activity (∼14 μM) (72). Notably, green tea leaf extracts are also rich in caffeine, a phosphodiesterase inhibitor (73). Since the phosphodiesterase enzyme degrades cAMP, its inhibition could enhance the protein kinase A (PKA) thermogenic pathway, as PKA is positively allosterically modulated by cAMP (74). Nonetheless, further studies are needed to confirm a link between tea extract catechins and the activation of the gut-SNS-BAT axis.
Capsaicinoids refers to a subgroup of secondary metabolites of the genus Capsicum plant, and are known for being pungent. The most important capsaicinoid is capsaicin, being responsible for the pungent effects of chili peppers through the activation of TRPV1 channels in the gut (75). Capsinoids, which include capsiate, dihydro-capsiate, and nor-dihydro-capsiate, activate TRPV1 and TRPA1 channels and are significantly less pungent than capsaicin (76). Both capsaicin and capsinoids activate BAT and promote WAT browning in rodents, probably via SNS adrenal catecholamine secretion (77). Congruently, the intragastric administration of capsinoids promotes BAT activation via TRPV1 agonism and sympathetic activation (77). However, BAT activation after capsinoid treatment was abolished either after vagal afferent denervation (78) or in UCP1-knockout mice (48). These results strengthen the idea that capsaicin and capsinoid effects rely on a gut-SNS-BAT axis.
Resveratrol is one of the most well-known polyphenols with antioxidant properties. It is mainly found in grape skin and seeds, but also in berries and nuts. Whereas resveratrol activates BAT (33–35, 37–39) and promotes WAT browning in mice and rats (36, 40), the gut-SNS-BAT connection has not yet been demonstrated. It seems that the thermogenic properties of resveratrol are directly mediated at the intracellular level, by an upregulation of the thermogenic pathways and related makers such as FNDC5 (type I membrane protein) and SIRT1 (79). Nevertheless, further investigation is warranted to confirm the mechanisms driving BAT activation after resveratrol ingestion.
Cacao beans are rich in flavonoids that constitute up to 10% of the dry weight of the bean (80). Cacao extract flavanols promote BAT activation, yet less is known about their effect on WAT browning. BAT activation by cacao extract flavanols is driven by an increase in catecholamine secretion and the consequent activation of the β3-ARs on brown adipocytes (52–54). Furthermore, it is important to consider that cacao extract flavanols can also be a source of theobromine and caffeine, substances that can boost the sympathetic response (81), thereby activating BAT. Further studies are needed to pinpoint the mechanism by which cacao extract flavanols activate BAT.
Quercetin is the most abundant flavonoid in onions, and it can also be found in other vegetables and fruits. Similar to cacao extract flavanols, quercetin drives its thermogenic activation through sympathetic stimulation (57). Quercetin upregulates β3-AR in WAT (57); therefore, the promotion of WAT browning by quercetin could be explained by a higher sensibilization of white adipocytes to catecholamines. However, the thermogenic mechanisms explaining the promotion of WAT browning by quercetin remain to be elucidated.
Last, we have analyzed in-depth those studies included after applying the sensitivity threshold of n ≥ 3 (i.e., monosaccharides/sweeteners, curcumin, leucine, menthol, garlic, and Puerariae flowers). However, the current scientific evidence is not strong enough to support these plant-based dietary components as BAT activators and promoters of WAT browning and further investigation is warranted to confirm their use.
We observed that 18 of 38 studies reported that either AMPK and/or SIRT1 pathways were upregulated in BAT and/or WAT in response to plant-based dietary components. AMPK is considered one of the major controllers of the cellular response to energetic stress and mitochondrial homeostasis (82) and plays a significant role in the metabolism of brown and beige adipocytes (83). Previous AMPK-null mouse studies have shown that AMPK is necessary for cold-induced and β-adrenergic BAT activation and WAT browning (84), whereas the specific pharmacological activation (A-769662 injection) of AMPK promotes WAT browning (85). SIRT1, which also has fuel-sensing properties similar to AMPK, is important for the activation of BAT and promotion of WAT browning (86). A whole-body SIRT1 heterozygous knockout (SIRT1+/−) mouse model study showed less BAT activation, higher adiposity, and insulin resistance (86), suggesting that SIRT1 activation is needed for normal BAT function.
BAT has an important endocrine role orchestrated by the release of batokines, with an impact on metabolism both at local and systemic levels (12, 13). Therefore, it is not surprising that many of the studies included in this systematic review also reported significant improvements in glucose and lipid metabolism along with BAT activation/recruitment and/or WAT browning. Collectively, these findings suggest that the potential clinical relevance of plant-based dietary components goes beyond thermogenic effects, as BAT activation and WAT browning might be driving the additional metabolic improvements potentially through BAT-mediated endocrine mechanisms. Future studies should address the connection between the secretory role of BAT and the metabolic improvements elicited by plant-based dietary components.
Limitations of the systematic review
Selected dietary components
It is important to highlight that, although we considered a reasonably wide spectrum of plant-based dietary components, the search strategy focused on a select group of compounds (see Methods section). Thus, certain groups of compounds, such as carotenoids, were not explicitly included.
Species included in the search
The present systematic review is focused on studies conducted exclusively in rodent models (mice and rats). Therefore, these findings cannot be transferable to other species.
In light of the aforementioned limitations, we contend that future systematic reviews on this topic should focus on specific and well-defined groups of plant-based dietary components. This would strengthen the scope of the studies by allowing them to use more specific search strategies and to include a wider spectrum of animal models (i.e., non-rodents).
Limitations and possible bias of the included studies
Heterogenous composition of plant-based dietary component extracts
There were differences in the composition of the plant-based dietary component on those studies using tea extract catechins (24–29) and cacao extract flavanols (50, 53, 55). Thus, a comparison of the results between these studies should be made carefully. Future studies should standardize the composition of plant-based dietary components to enable a critical comparison of the results across studies.
Dose
Dose variations in the plant-based dietary components used in the studies also hamper the comparison of results between studies.
Authorship
Of note, 5 of 7 studies that used cacao extract flavanols were conducted by the same laboratory (49, 51–54). The Ajinomoto Company was involved in 3 of 4 studies using capsinoids (43, 47, 48), whereas 2 of 3 capsaicin studies were conducted by members of the same laboratory (44, 45). Two of 8 resveratrol studies were conducted by a US-China collaboration (36, 39), while a Spanish group conducted 2 of 8 studies (34, 37). Therefore, these findings should be replicated by independent laboratories.
Analysis of thermogenic pathways
The assessment of AMPK and SIRT1 pathways was likely based on previous evidence and, therefore, untargeted approaches (RNA sequencing or proteomics) should be performed to demonstrate that both signaling pathways are the main pathways that activate BAT and promote WAT browning.
Assessment of BAT activity
The studies included lack of evaluation of actual BAT activity through either 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) scans (the current gold standard) (87), direct interscapular BAT temperature measurements, or infrared thermography assessments. Notably, it is important to consider that many of the studies measured UCP1 at the gene expression level, which cannot be considered a proxy of thermogenesis. While mRNA to protein ratio is thought to be constant (88), it could vary depending on specific tissues and genes. Future studies should include UCP1 protein assessments.
BAT activation and BAT recruitment
The studies evaluated the effects of plant-based dietary components over a period of time by measuring UCP1 mRNA or protein concentrations in BAT, which reflects BAT recruitment. Even though BAT recruitment is likely a consequence of repeated BAT activation, only a capsinoids study (42) and 2 cacao flavanol extract studies (53, 55) showed an acute activation of pre-existing BAT. Thus, future studies should investigate BAT activation (and not only recruitment) after chronic plant-based dietary component interventions.
Safety of plant-based dietary components
It has been shown that certain catechins at high concentrations may be responsible for the hepatotoxic effects of green tea extract (70), or β-carotene supplementation could increase the risk of lung cancer in smokers (89). Future studies investigating the safety of supplementation with plant-based dietary components are needed.
Translational research: future lines
Only 2 studies have evaluated the effect of oral tea extract catechins on human BAT activity (90, 91), showing that tea extract catechins increase cold-induced thermogenesis, resting metabolic rate, and BAT density in BAT-positive individuals. Sun et al. (92) reported a significant increase in BAT glucose uptake after capsinoid ingestion. In light of the present results, resveratrol, cacao extract flavonols, and quercetin could be potential activators of human BAT, although their BAT-activating properties have never been tested in humans. Furthermore, whether plant-based dietary components promote WAT browning in humans remains unexplored. Experimental procedures, robust study designs, and use of the gold-standard techniques for assessing BAT activity and WAT browning must be used in future studies.
Conclusions
To date, the most studied plant-based dietary components for activating BAT and promoting WAT browning in mice and rats are tea extract catechins, resveratrol, capsaicin and capsinoids, cacao extract flavanols, and quercetin. The findings of the present systematic review support the use of plant-based dietary components to activate BAT and promote WAT browning in rodents and thus potentially to combat obesity and cardiometabolic disorders. It seems that a part of these effects could be dependent on the upregulation of AMPK and SIRT1 signaling pathways, yet further studies are needed to confirm the mechanisms driving these findings. Studies in humans are warranted to understand the impact of plant-based dietary components on BAT metabolism and WAT browning.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. N van der Werf, Information Specialist/Lecturer Information Literacy from Walaeus Bibliotheek of the Leiden University Medical Center (LUMC; Leiden, The Netherlands), for her expertise and extremely helpful contribution to the PubMed literature search. The authors’ responsibilities were as follows—FJOP and JRR: conceived and designed the analysis; FJOP: collected the data and performed the analysis; FJOP, BMT, and ASC: contributed data and analysis tools; FJOP, BMT, and JRR wrote the manuscript; and all authors: critically reviewed and read and approved the final manuscript.
Notes
The study was supported by the Spanish Ministry of Economy and Competitiveness via Retos de la Sociedad (DEP2016-79512-R) and European Regional Development Funds (ERDF), the Spanish Ministry of Education (FPU 16/02828), the University of Granada Plan Propio de Investigación 2016–Excellence actions: Unit of Excellence on Exercise and Health (UCEES), and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF: ref. SOMM17/6107/UGR). BM-T is supported by an individual postdoctoral grant from the Fundación Alfonso Martin Escudero, 2020. This study is part of a PhD thesis conducted in the Biomedicine Doctoral Studies of the University of Granada, Spain.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1, Supplemental Tables 1–2 and Supplemental References of the studies included, are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AMPK, AMP-activated protein kinase; BAT, brown adipose tissue; COMT, catechol-O-methyl transferase; EGCG, epi-gallocatechin gallate; MeSH, medical subject heading; PKA, protein kinase A; SIRT1, sirtuin 1; SNS, sympathetic nervous system; TRP, transient receptor potential; TRPA1, TRP ankyrin 1; TRPV1, transient receptor potential vanilloid subfamily member 1; tw, text word(s); UCP1, uncoupling protein 1; WAT, white adipose tissue; β-3AR, β-3 adrenergic receptor.
Contributor Information
Francisco J Osuna-Prieto, PROFITH (PROmoting FITness and Health through Physical Activity) Research Group, Department of Physical Education and Sport, Faculty of Sport Sciences, University of Granada, Granada, Spain; Department of Analytical Chemistry, University of Granada, Granada, Spain; Research and Development of Functional Food Centre (CIDAF), Health Science Technological Park Avda. Del Conocimiento, Granada, Spain.
Borja Martinez-Tellez, PROFITH (PROmoting FITness and Health through Physical Activity) Research Group, Department of Physical Education and Sport, Faculty of Sport Sciences, University of Granada, Granada, Spain; Department of Medicine, Division of Endocrinology, and Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, The Netherlands.
Antonio Segura-Carretero, Department of Analytical Chemistry, University of Granada, Granada, Spain; Research and Development of Functional Food Centre (CIDAF), Health Science Technological Park Avda. Del Conocimiento, Granada, Spain.
Jonatan R Ruiz, PROFITH (PROmoting FITness and Health through Physical Activity) Research Group, Department of Physical Education and Sport, Faculty of Sport Sciences, University of Granada, Granada, Spain.
References
- 1. Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand?. Vasc Health Risk Manage. 2019;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obesity. 2010;17(2):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira R, McFarlane S. The role of brown adipose tissue in cardiovascular disease protection: current evidence and future directions. Int J Clin Res Trials. 2019;4(2):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–9. [DOI] [PubMed] [Google Scholar]
- 6. Bachman ES, Dhillon H, Zhang C-Y, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–5. [DOI] [PubMed] [Google Scholar]
- 7. Bargut TCL, Aguila MB, Mandarim-de-Lacerda CA. Brown adipose tissue: updates in cellular and molecular biology. Tissue Cell. 2016;48(5):452–60. [DOI] [PubMed] [Google Scholar]
- 8. Herz CT, Kiefer FW. Adipose tissue browning in mice and humans. J Endocrinol. 2019;241(3):R97–109. [PubMed] [Google Scholar]
- 9. Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng Y-Het al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch Cet al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–6. [DOI] [PubMed] [Google Scholar]
- 11. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22(4):546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villarroya J, Cereijo Rn, Gavald-Navarro A, Peyrou M, Giralt M, Villarroya F. New insights into the secretory functions of brown adipose tissue. J Endocrinol. 2019;243(2):R19–27. [DOI] [PubMed] [Google Scholar]
- 13. Villarroya F, Gavaldà-Navarro A, Peyrou M, Villarroya J, Giralt M. The lives and times of brown adipokines. Trends Endocrinol Metab. 2017;28(12):855–67. [DOI] [PubMed] [Google Scholar]
- 14. Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019;29(1):27–37. [DOI] [PubMed] [Google Scholar]
- 15. Shin JH, Lee SH, Kim YN, Kim IY, Kim YJ, Kyeong DS, Lim HJ, Cho SY, Choi J, Wi YJet al. AHNAK deficiency promotes browning and lipolysis in mice via increased responsiveness to beta-adrenergic signalling. Sci Rep. 2016;6(1):23426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hao L, Scott S, Abbasi M, Zu Y, Khan MSH, Yang Y, Wu D, Zhao L, Wang S. Beneficial metabolic effects of mirabegron in vitro and in high-fat diet-induced obese mice. J Pharmacol Exp Ther. 2019;369(3):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H, Liang H, Weng J. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia. 2016;59(5):1059–69. [DOI] [PubMed] [Google Scholar]
- 18. Saito M, Yoneshiro T, Matsushita M. Food ingredients as anti-obesity agents. Trends Endocrinol Metab. 2015;26(11):585–7. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Li X, Fang H, Guo F, Li F, Chen A, Huang S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr Metab. 2019;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonet ML, Mercader J, Palou A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie. 2017;134:99–117. [DOI] [PubMed] [Google Scholar]
- 21. Li H, Qi J, Li L. Phytochemicals as potential candidates to combat obesity via adipose non- shivering thermogenesis. Pharmacol Res. 2019;147:104393. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl. 1978;32:89–93. [DOI] [PubMed] [Google Scholar]
- 24. Yan J, Zhao Y, Zhao B. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Science China Life Sciences. 2013;56(9):804–10. [DOI] [PubMed] [Google Scholar]
- 25. Nomura S, Ichinose T, Jinde M, Kawashima Y, Tachiyashiki K, Imaizumi K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J Nutr Biochem. 2008;19(12):840–7. [DOI] [PubMed] [Google Scholar]
- 26. Sae-Tan S, Rogers CJ, Lambert JD. Decaffeinated green tea and voluntary exercise induce gene changes related to beige adipocyte formation in high fat-fed obese mice. J Funct Foods. 2015;14:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L-H, Chien Y-W, Liang C-T, Chan C-H, Fan M-H, Huang H-Y. Green tea extract induces genes related to browning of white adipose tissue and limits weight-gain in high energy diet-fed rat. Food Nutr Res. 2017;61:1347480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neyrinck AM, Bindels LB, Geurts L, Van Hul M, Cani PD, Delzenne NM. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;49:15–21. [DOI] [PubMed] [Google Scholar]
- 29. Kudo N, Arai Y, Suhara Y, Ishii T, Nakayama T, Osakabe N. A single oral administration of theaflavins increases energy expenditure and the expression of metabolic genes. PLoS One. 2015;10(9):e0137809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mi Y, Qi G, Fan R, Ji X, Liu Z, Liu X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochimica et Biophysica Acta (BBA) Molecular Basis of Disease. 2017;1863(6):1575–89. [DOI] [PubMed] [Google Scholar]
- 31. Lee M, Shin Y, Jung S, Kim Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr Res. 2017;61:1325307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou J, Mao L, Xu P, Wang Y. Effects of (-)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients. 2018;10:1325307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp Neurol. 2010;225(1):74–84. [DOI] [PubMed] [Google Scholar]
- 34. Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141(2):1530–5. [DOI] [PubMed] [Google Scholar]
- 35. Andrade JMO, Frade ACM, Guimaraes JB, Freitas KM, Lopes MTP, Guimaraes ALS, de Paula AMB, Coimbra CC, Santos SHS. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53(7):1503–10. [DOI] [PubMed] [Google Scholar]
- 36. Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes. 2015;39(6):967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trepiana J, Gómez-Zorita S, Fernández-quintela A, González M, Portillo MP. Effects of resveratrol and its analogue pterostilbene, on NOV/CCN3 adipokine in adipose tissue from rats fed a high-fat high-sucrose diet. J Physiol Biochem. 2019;75(3):275–83. [DOI] [PubMed] [Google Scholar]
- 38. Ku CR, Cho YH, Hong ZY, Lee H, Lee SJ, Hong SS, Lee EJ. The effects of high fat diet and resveratrol on mitochondrial activity of brown adipocytes. Endocrinol Metab. 2016;31(2):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Liang X, Yang Q, Fu X, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol enhances brown adipocyte formation and function by activating AMP-activated protein kinase (AMPK) α1 in mice fed high-fat diet. Mol Nutr Food Res. 2017;61(4):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pan M-H, Koh Y-C, Lee T-L, Wang B, Chen W-K, Nagabhushanam K, Ho C-T. Resveratrol and oxyresveratrol activate thermogenesis via different transcriptional coactivators in high-fat diet-induced obese mice. J Agric Food Chem. 2019;67(49):13605–16. [DOI] [PubMed] [Google Scholar]
- 41. Masuda Y, Haramizu S, Oki K, Ohnuki K, Watanabe T, Yazawa S, Kawada T, Hashizume SI, Fushiki T. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol. 2003;95(6):2408–15. [DOI] [PubMed] [Google Scholar]
- 42. Baboota RK, Murtaza N, Jagtap S, Singh DP, Karmase A, Kaur J, Bhutani KK, Boparai RK, Premkumar LS, Kondepudi KKet al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J Nutr Biochem. 2014;25(9):893–902. [DOI] [PubMed] [Google Scholar]
- 43. Ohyama K, Nogusa Y, Suzuki K, Shinoda K, Kajimura S, Bannai M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. Am J Physiol Endocrinol Metab. 2015;308(4):E315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173(15):2369–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, Thyagarajan B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes. 2017;41(5):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baboota RK, Khare P, Mangal P, Singh DP, Bhutani KK, Kondepudi KK, Kaur J, Bishnoi M. Dihydrocapsiate supplementation prevented high-fat diet-induced adiposity, hepatic steatosis, glucose intolerance, and gut morphological alterations in mice. Nutr Res. 2018;51:40–56. [DOI] [PubMed] [Google Scholar]
- 47. Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes. 2016;65(5):1410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okamatsu-Ogura Y, Tsubota A, Ohyama K, Nogusa Y, Saito M, Kimura K. Capsinoids suppress diet-induced obesity through uncoupling protein. 1–9. -dependent mechanism in mice. J Funct Foods. 2015;19: 1–9. [Google Scholar]
- 49. Watanabe N, Inagawa K, Shibata M, Osakabe N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis. 2014;13(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Varela CE, Rodriguez A, Romero-Valdovinos M, Mendoza-Lorenzo P, Mansour C, Ceballos G, Villarreal F, Ramirez-Sanchez I. Browning effects of (−)-epicatechin on adipocytes and white adipose tissue. Eur J Pharmacol. 2017;811:48–59. [DOI] [PubMed] [Google Scholar]
- 51. Osakabe N, Hoshi J, Kudo N, Shibata M. The flavan-3-ol fraction of cocoa powder suppressed changes associated with early-stage metabolic syndrome in high-fat diet-fed rats. Life Sci. 2014;114(1):51–6. [DOI] [PubMed] [Google Scholar]
- 52. Matsumura Y, Nakagawa Y, Mikome K, Yamamoto H, Osakabe N. Enhancement of energy expenditure following a single oral dose of flavan-3-ols associated with an increase in catecholamine secretion. PLoS One. 2014;9(11):e112180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakagawa Y, Ishimura K, Oya S, Kamino M, Fujii Y, Nanba F, Toda T, Ishii T, Adachi T, Suhara Yet al. Comparison of the sympathetic stimulatory abilities of B-type procyanidins based on induction of uncoupling protein-1 in brown adipose tissue (BAT) and increased plasma catecholamine (CA) in mice. PLoS One. 2018;13(7):e0201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamio N, Suzuki T, Watanabe Y, Suhara Y, Osakabe N. A single oral dose of flavan-3-ols enhances energy expenditure by sympathetic nerve stimulation in mice. Free Radic Biol Med. 2016;91:256–63. [DOI] [PubMed] [Google Scholar]
- 55. Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys. 2012;527(2):95–104. [DOI] [PubMed] [Google Scholar]
- 56. Jiang H, Yoshioka Y, Yuan S, Horiuchi Y, Yamashita Y, Croft KD, Ashida H. Enzymatically modified isoquercitrin promotes energy metabolism through activating AMPKα in male C57BL/6 mice. Food Funct. 2019;10(8):5188–202. [DOI] [PubMed] [Google Scholar]
- 57. Choi H, Kim C-S, Yu R. Quercetin upregulates uncoupling protein 1 in white /brown adipose tissues through sympathetic stimulation.J Obes Metab Syndr. 2018;27(2):102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuipers E, Dam A, Held N, Mol I, Houtkooper R, Rensen P, Boon M. Quercetin lowers plasma triglycerides accompanied by white adipose tissue browning in diet-induced obese mice. Int J Mol Sci. 2018;19(6):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem. 2017;42:62–71. [DOI] [PubMed] [Google Scholar]
- 60. Han Y, Wu J-Z, Shen J-Z, Chen L, He T, Jin M-W, Liu H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci Rep. 2017;7(1):1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moon J, Do H-J, Kim OY, Shin M-J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem Toxicol. 2013;58:347–54. [DOI] [PubMed] [Google Scholar]
- 62. Yang CS, Chen G, Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J Trad Complement Med. 2014;4(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu X, Yu M, Liu Y, Yu S. TRP channel functions in the gastrointestinal tract. Semin Immunopathol. 2016;38(3):385–96. [DOI] [PubMed] [Google Scholar]
- 64. Gao P, Yan Z, Zhu Z. The role of adipose TRP channels in the pathogenesis of obesity. J Cell Physiol. 2019;284(8):12483–97. [DOI] [PubMed] [Google Scholar]
- 65. Kurogi M, Kawai Y, Nagatomo K, Tateyama M, Kubo Y, Saitoh O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem Senses. 2015;40(1):27–46. [DOI] [PubMed] [Google Scholar]
- 66. Yoneshiro T, Saito M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr Opin Clin Nutr Metab Care. 2013;16(6):625–31. [DOI] [PubMed] [Google Scholar]
- 67. Dulloo AG. The search for compounds that stimulate thermogenesis in obesity management: from pharmaceuticals to functional food ingredients. Obes Rev. 2011;12(10):866–83. [DOI] [PubMed] [Google Scholar]
- 68. Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69(10):1523–31. [DOI] [PubMed] [Google Scholar]
- 69. Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K, Stangl V. The activity of catechol-O-methyltransferase (COMT) is not impaired by high doses of epigallocatechin-3-gallate (EGCG) in vivo. Eur J Pharmacol. 2014;740:645–51. [DOI] [PubMed] [Google Scholar]
- 70. Hu J, Webster D, Cao J, Shao A. The safety of green tea and green tea extract consumption in adults—results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–33. [DOI] [PubMed] [Google Scholar]
- 71. Takahashi M, Miyashita M, Suzuki K, Bae S, Kim H, Wakisaka T, Matsui Y, Takeshita M, Yasunaga K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br J Nutr. 2014;112(9):1542–50. [DOI] [PubMed] [Google Scholar]
- 72. Kadowaki M, Ootani E, Sugihara N, Furuno K. Inhibitory effects of catechin gallates on O-methyltranslation of protocatechuic acid in rat liver cytosolic preparations and cultured hepatocytes. Biol Pharm Bull. 2005;28(8):1509–13. [DOI] [PubMed] [Google Scholar]
- 73. Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(S1):S252–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res. 2016;30(5):732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bhave G, Zhu W, Wang H, Brasier D, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–31. [DOI] [PubMed] [Google Scholar]
- 76. Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, Yazawa S, Tominaga M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol. 2012;165(5):1476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol. 2011;110(3):789–98. [DOI] [PubMed] [Google Scholar]
- 78. Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, Higashi T, Tominaga M, Inoue K, Fushiki T. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem. 2009;73(12):2690–7. [DOI] [PubMed] [Google Scholar]
- 79. Andrade JMO, Barcala-Jorge AS, Batista-Jorge GC, Paraíso AF, Freitas KM, Lelis D de F, Guimarães ALS, de Paula AMB, Santos SHS. Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed Pharmacother. 2019;112:108634. [DOI] [PubMed] [Google Scholar]
- 80. Rusconi M, Conti A. Theobroma cacao L., the food of the gods: a scientific approach beyond myths and claims. Pharmacol Res. 2010;61(1):5–13. [DOI] [PubMed] [Google Scholar]
- 81. Martínez-Pinilla E, Oñatibia-Astibia A, Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front Pharmacol. 2015;6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Desjardins EM, Steinberg GR. Emerging role of AMPK in brown and beige adipose tissue (BAT): implications for obesity, insulin resistance, and type 2 diabetes. Curr Diabetes Rep. 2018;18(10):80. [DOI] [PubMed] [Google Scholar]
- 84. Mottillo EP, Desjardins EM, Crane JD, Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A, Sakamoto Ket al. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016;24(1):118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu L, Zhang L, Li B, Jiang H, Duan Y, Xie Z, Shuai L, Li J, Li J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. 2018;9:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu F, Zheng X, Lin B, Liang H, Cai M, Cao H, Ye J, Weng J. Diet-induced obesity and insulin resistance are associated with brown fat degeneration in SIRT1-deficient mice. Obesity. 2016;24(3):634–42. [DOI] [PubMed] [Google Scholar]
- 87. Frankl J, Sherwood A, Clegg DJ, Scherer PE, Öz OK. Imaging metabolically active fat: a literature review and mechanistic insights. Int J Mol Sci. 2019;20(21):5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Silva GM, Vogel C. Quantifying gene expression: the importance of being subtle. Mol Syst Biol. 2016;12(10):885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Middha P, Weinstein SJ, Männistö S, Albanes D, Mondul AM. β-Carotene supplementation and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: the role of tar and nicotine. Nicotine Tob Res. 2019;21(8):1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K, Katsuragi Y, Kameya T, Sugie H, Saito M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr. 2017;105(4):873–81. [DOI] [PubMed] [Google Scholar]
- 91. Nirengi S, Amagasa S, Homma T, Yoneshiro T, Matsumiya S, Kurosawa Y, Sakane N, Ebi K, Saito M, Hamaoka T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. SpringerPlus. 2016;5(1):1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun L, Camps SG, Goh HJ, Govindharajulu P, Schaefferkoetter JD, Townsend DW, Verma SK, Velan SS, Sun L, Sze SKet al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am J Clin Nutr. 2018;107(1):62–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.