ABSTRACT
Healthy maternal diets can lower the odds of developing pre-eclampsia, a direct and second leading cause of maternal death, globally. However, there is a research gap in low- and middle-income countries (LMIC), which bear a disproportionate burden of these deaths. The objectives of this systematic review were to: 1) evaluate the association between dietary patterns in pregnancy and hypertensive disorders, including pre-eclampsia for pregnant and postpartum women in LMIC, and 2) compile barriers and facilitators to an adequate maternal diet. A systematic search was performed on MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health, Web of Science, Cochrane Central Register of Controlled Trials, African Journals Online, the WHO Regional Databases, 2 trial registries, Google Scholar, and reference lists. Included in the analysis were primary research studies of dietary patterns during pregnancy, with pregnancy hypertension outcome(s), and conducted in LMIC. Included studies were assessed using ROBINS-I risk of bias. Thirteen studies were included, of which 5 studies were included in a meta-analysis (Review Manager 5). Lower odds of pre-eclampsia were associated with adequate (compared with no or low) consumption of vegetables (OR: 0.38; 95% CI: 0.18, 0.80; I2 = 85%; P = 0.01) and adequate (compared with no or low) consumption of fruit (OR: 0.42; 95% CI: 0.24, 0.71; I2 = 79%; P = 0.008). No firm conclusions could be drawn about the impact on pre-eclampsia odds of any of the following during pregnancy: high consumption of meat or grains; a “Western” diet; or alcohol consumption. More LMIC-based research is needed to explore whether the apparent beneficial effects of fruits and vegetables on pre-eclampsia incidence might be enhanced when maternal malnutrition is prevalent, and/or whether other sociodemographic factors might contribute.
Keywords: low- and middle-income countries (LMIC), resource-constrained, pregnancy hypertension, pre-eclampsia, maternal diets, systematic review, meta-analysis
In pregnant women in low- and middle-income countries, maternal diets high in vegetables can lower the odds of developing pre-eclampsia by 62%, and by 58% for diets high in fruits.
Introduction
Sustainable Development Goal 3.1 aims to reduce the global maternal mortality ratio (MMR) to 70 per 100,000 live births, but reaching this target will not be achieved without reducing the burden of maternal deaths in low- and middle-income countries (LMIC) (1–3). In 2017, low-income countries had an MMR of 462 per 100,000 compared with 11 per 100,000 in high-income countries (HICs) (1). More than half of all maternal deaths are associated with 3 leading direct causes: maternal hemorrhage (27%), hypertensive disorders (14%), and sepsis (11%) (4). Whereas there have been dramatic decreases in hemorrhage- and sepsis-related maternal deaths, reductions in pregnancy hypertension-related deaths have been much slower (3, 4). As a percentage of overall maternal deaths, the proportion of pregnancy hypertension-related deaths has remained relatively steady between 1990 and 2013 (4). Hypertensive disorders of pregnancy (HDPs) are associated with an estimated 46,000 maternal deaths, 416,000 stillbirths, and 1.5–2 million neonatal deaths annually (5). Pre-eclampsia, the most serious of the HDPs (6), is characterized by new-onset hypertension at ≥20 wk of gestation with proteinuria and/or signs of organ damage, most commonly the liver and kidneys (6–8). It is a pregnancy-specific inflammatory disorder related to abnormal vascular development of the placenta or pre-existing maternal risk factors for cardiovascular disease such as hypertension, renal disease, overweight, or diabetes (9).
Dietary intake during pregnancy has been proposed to influence the risk of pre-eclampsia, with micronutrients specifically implicated due to their antioxidant, anti-inflammatory, or vasoactive properties (6). However, the evidence remains inconclusive (6), suggesting a need to look at overall dietary patterns. A systematic review found that healthy diet interventions reduced the risk of pre-eclampsia by 33% (7), and a meta-analysis of 4 observational studies found that a healthy dietary pattern characterized by high intake of fruits, vegetables, whole-grain foods, fish, and poultry was associated with lower likelihood of pre-eclampsia (8). However, all 4 of the studies included in the meta-analysis were from HICs, with 3 from Norway and 1 from The Netherlands. There is a need to explore the impact of maternal diet on pre-eclampsia risk in resource-constrained settings where there are high burdens of both maternal deaths and maternal undernutrition. The primary objective of this review was to evaluate the association between dietary patterns in pregnancy and hypertension for pregnant and postpartum women in LMIC. The secondary objective was to understand risk factors associated with maternal dietary patterns, including identifying barriers and facilitators to obtaining an adequate maternal diet.
Methods
This review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (10). A review protocol was registered with PROSPERO (CRD42020165154) prior to conducting the review.
Searches were conducted on MEDLINE Ovid, Embase, the Cumulative Index to Nursing and Allied Health (CINAHL), Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), African Journals Online (AJOL), and the WHO Regional Databases. WHO Regional Databases included African Index Medicus, Latin American and Caribbean Health Sciences Literature, Index Medicus for the Eastern Mediterranean Region, Index Medicus for the South-East Asian Region, and Western Pacific Region Index Medicus. Additionally, searches were supplemented by reviewing clinicaltrials.gov, ICTRP (International Clinical Trials Registry Platform) Search Portal (WHO), and Google Scholar, and scanning reference lists. Searches were conducted from database inception to February 2020. Based on the PECOS (Participants, Exposures, Context, Outcomes, Study design) research framework (Supplemental Table 1), search terms broadly included dietary diversity, dietary pattern, diet quality, developing countries, resource-constrained, LMIC, maternal death, pregnancy hypertension, pregnancy-induced hypertension, pre-eclampsia, gestational hypertension, chronic hypertension, and eclampsia (Supplemental Table 2). We used dietary patterns and outcomes definitions as defined by study authors. Because the focus is on maternal dietary patterns as the exposure, searches primarily sought observational cohort studies but also searched for randomized controlled trials to identify interventions to improve maternal dietary diversity or patterns. After scoping searches, case-controlled studies were added to the full searches. Case-controlled studies are less expensive, quicker, and simpler to facilitate (11, 12), which can make them more feasible in resource-limited LMIC settings—a focus of this review. Studies without a comparison group or longitudinal follow-up, such as cross-sectional studies, were not included in the review to reduce potential risks of bias (11).
Titles and abstracts were independently screened by 2 reviewers (MWK, SO) and discrepancies were resolved by discussion with a third reviewer (KS). Full texts were independently assessed for their eligibility by the 2 reviewers (MWK, SO), with the third reviewer (KS) providing an independent assessment for discrepancies. Studies were screened for inclusion if they were studies with 1) primary data collection; 2) women of reproductive age during periconception, pregnancy, or postpartum; 3) conducted in LMIC; and 4) reported maternal dietary patterns in association with pregnancy hypertension outcomes (Table 1). Only English language articles were included due to capacities of the reviewer team. Search results were uploaded in Mendeley (Elsevier) to remove duplicates, then the reference list was uploaded to Excel (Microsoft) for study selection.
TABLE 1.
Eligibility criteria
| Inclusion | Exclusion | |
|---|---|---|
| Population | Studies with women of reproductive age (15–49 y) during prepregnancy, pregnancy, or postpartum (≤42 d after delivery) | Studies with women outside 15–49 y. Studies with men, children, or infants without separated outcomes for women |
| Exposure | Studies that report maternal dietary patterns such as food intake frequencies and diversity | Studies that reported on nutritional status without discussion of food groups or items |
| Context | Studies conducted in ≥1 low- and middle-income countries (as classified by the World Bank) | Excluded if study does not specify outcomes of interest specifically for low- and middle-income countries |
| Outcomes | Studies that report associations between maternal dietary patterns and pregnancy hypertension outcomes (chronic hypertension, gestational hypertension, pre-eclampsia, superimposed pre-eclampsia) | Studies that report on infant or child health outcomes only. Studies that do not report on pregnancy hypertension outcomes |
| Study design | Case-controlled, cohort studies, randomized controlled trials. Studies with primary data collection | Cross-sectional surveys, uncontrolled pre-post studies, and studies without primary data collection and sufficient methodological data, including reviews, commentaries and editorials, letters to the editor, study protocols, conference abstracts and proceedings |
| Language | Studies published in the English language | Excluded if not written in the English language |
| Other | No restrictions on the time of publication |
Details about study design, country, population characteristics, exclusion criteria, age of participants, dietary assessment, dietary patterns and food groups identified, outcome measured, comparative risk, relative effect, and risk factors associated with pre-eclampsia incidence and dietary patterns were extracted into a data extraction sheet on Excel (Microsoft) with independent review by 2 reviewers. Potential confounding factors and co-interventions were also extracted in preparation for quality assessment. To access study quality, we evaluated each included study with the ROBINS-I risk of bias tool, a conceptually rigorous tool for nonrandomized studies that uses the Cochrane-approved risk of bias approach and focuses on internal validity (13). No studies were excluded from the narrative synthesis on the basis of their quality assessment. However, following the guidance for using ROBINS-I, studies with high risk of bias (critical risk of bias) were excluded from meta-analyses (14). Evidence from studies of similar design, data collection methodology, sample, and outcomes reported was pooled using Review Manager (RevMan 5) and reported as ORs with 95% CIs. Evidence was pooled according to the Mantel–Haenzel random-effects model for analyses with substantial heterogeneity (I2 > 50%), and sensitivity analyses conducted to explore the impact of study quality on heterogeneity levels (15). Funnel plots to estimate publication bias were planned if there were >10 included studies in the meta-analysis.
Results
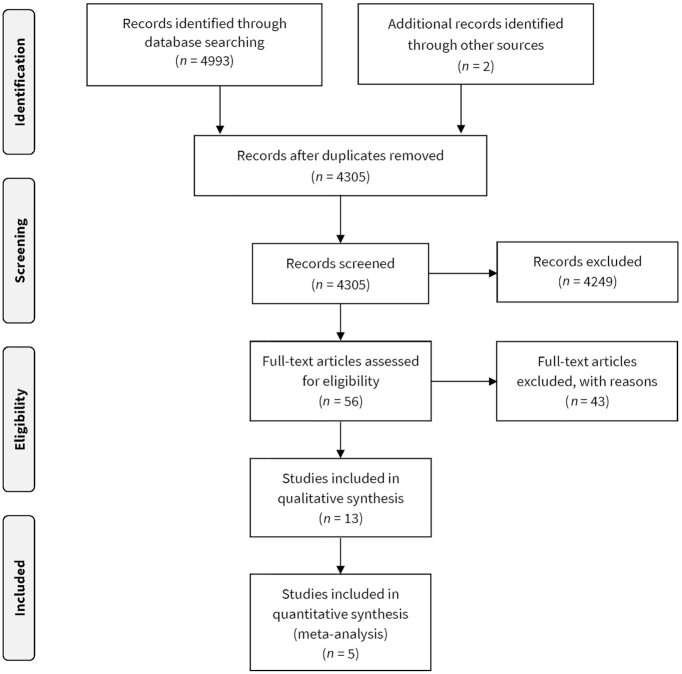
We identified a total of 4993 records from our database searches (2648 from MEDLINE, 1801 from Embase, 446 from Web of Science, 14 from CINAHL, 1 from AJOL, 32 from WHO Regional Databases, 43 from CENTRAL, 6 from clinicaltrials.gov, and 2 from the ICTRP Search Portal) (Figure 1). Two additional unique records were located through Google Scholar and reference list searching. After removal of duplicates and screening against the eligibility criteria, 13 studies were included in the review. Forty-three full-text articles were excluded. Reasons for exclusion included that the study did not investigate the population (16–19), exposure (20–24), or outcome of interest (19, 21, 25–51), was not conducted in an LMIC (52–54), was a conference abstract only (55), was a cross-sectional survey design (56, 57), or were duplicates (29, 58) (Supplemental Table 3).
FIGURE 1.
PRISMA flow diagram.
Characteristics of included studies
One article was published prior to 2000 (59), 1 between 2001 and 2010 (60), and 11 between 2011 and 2020 (61–71). Overall, there were 7 studies from sub-Saharan Africa and 6 studies from Asia and the Middle East. There were 5 studies from Ethiopia (62–64, 66, 68), which was the country with the most studies, followed by Iran with 4 studies (65, 67, 70, 71). There was a study each from mainland China (69), Turkey (61), Democratic Republic of Congo (60), and Zimbabwe (59). All studies were of a prospective design with dietary intake data collected, pregnancy monitoring, and recording of morbidities. The most common study design was prospective case-controlled, with 7 unmatched (59, 61, 62, 64, 68, 70, 71) and 3 matched studies (63, 65, 66). In addition, there were 2 prospective cohort studies (60, 67) and 1 secondary analysis of a randomized controlled trial (69). Nine studies reported pre-eclampsia as an outcome (59, 61–65, 68, 70, 71) whereas 3 studies reported both pre-eclampsia and gestational hypertension as study outcomes (60, 67, 69). One study reported HDPs, including pre-eclampsia, gestational hypertension, or superimposed pre-eclampsia (66). For more information on study characteristics, see Table 2 (see Supplemental Table 4 for dietary pattern classifications from each included study, Supplemental Table 5 for outcome definitions from each included study, and Supplemental Table 6 for reported study outcomes by study).
TABLE 2.
Characteristics of included studies1
| Reference | Study design | Country, n participants | Cases, n | Population characteristics | Age, y | Dietary assessment method, period | Dietary pattern | Outcome |
|---|---|---|---|---|---|---|---|---|
| Abbasi et al. 2019 (70) | Prospective unmatched case-controlled study | Iran, 510 | 170 cases, 340 controls | Pregnant women visiting 3 hospitals, 11 urban and 7 rural health care centers in Lorestan province, August 2015–2016 | Cases: 24; controls: 26 (median) | 198-item FFQ, 20–40 wk (cases: 35 ± 5 wk; controls: 26 ± 6 wk) | Healthy; Western; Iranian traditional | PE |
| Atkinson et al. 1998 (59) | Prospective unmatched case-controlled study | Zimbabwe, 374 | 180 cases, 194 controls | Pregnant women delivering at Harare Maternity Hospital or 1 of 9 clinics in suburbs of Harare city, June 1995 to April 1996 | Cases: 25.6 ± 6.4; controls: 24.8 ± 7.9 (mean + SD) | Questionnaire, during postpartum hospital stay (retrospective recall for the month prior to birth) | Meat; poultry; fruit; fish; vegetables; and dairy | PE |
| Endeshaw et al. 2015 (62) | Prospective unmatched case-controlled study | Ethiopia, 453 | 151 cases, 302 controls | Pregnant women attending antepartum or intrapartum care at any public health facility in Bahir Dar City, June to September 2014 | Range: 25 to 29: 33% of cases; 36% of controls | Questionnaire, during pregnancy (20 wk onwards) and delivery care for cases; during delivery care for controls | Fruit; vegetable; animal product; coffee; alcohol | PE |
| Grum et al. 2017 (64) | Prospective unmatched case-controlled study | Ethiopia, 291 | 97 cases, 194 controls | Pregnant women delivering at Gandhi and Sewditu Memorial hospitals in Addis Ababa, December 2015 to February 2016 | 25.42 ± 5.33 (mean + SD) | Questionnaire, during delivery care services | Fruit; alcohol; nutritional counseling during ANC | PE |
| Grum et al. 2018 (68) | Prospective unmatched case-controlled study | Ethiopia, 243 | 81 cases, 162 controls | Pregnant women delivering at Gandhi and Sewditu Memorial hospitals in Addis Ababa, December 2015 to February 2016 | 27.22 ± 5.03 (mean + SD) | Questionnaire, during delivery care services | Fruit; vegetable; coffee; nutritional counseling during ANC | PE |
| Gulsen and Guner 2012 (61) | Prospective unmatched case-controlled study | Turkey, 247 | 92 cases, 155 controls | Pregnant women from Konya and neighboring cities hospitalized with pre-eclampsia at the Selcuk University Meran Medical School clinic, October 2004 to May 2005 | Range: 20 to 34: 65% of cases; 88% of controls | Questionnaire, second to third trimesters | Milk; yogurt; yogurt drink; cheese; dairy desserts; egg; meat; chicken; meat product; fish; legumes; bread; vegetables; fruit | PE |
| Hajianfar et al. 2018 (67) | Prospective cohort study | Iran, 812 | NA | Healthy pregnant women in Isfahan referred to health centers during the first trimester of pregnancy in 2015–2016 | Range: 20–40 y | 117-item FFQ, 8–16 wk | Western; traditional; healthy | GH; PE |
| Kahsay et al. 2018 (66) | Prospective matched case-controlled study | Ethiopia, 330 | 110 cases, 220 controls | Pregnant women after 20 wk gestation attending antenatal care clinics at 7 public hospitals in Tigray region, June to November 2017 | Cases: 27.6 ± 5.6; controls: 26.7 ± 5.8 (mean + SD) | 18-question FFQ, during pregnancy (20 wk onwards) and delivery care | Fruits; vegetables | HDP (GH, PE, SIP) |
| Longo-Mbenza et al. 2008 (60) | Prospective cohort study | DR Congo, 238 | PE: 7 (2.9%); GH: 4 (1.7%) | Black pregnant women admitted to the rural Evangelical Hospital in Kimpese, January to March 2003 | 27 ± 6.4 (mean + SD) | Questionnaire, first trimester | Vegetables; meat | GH; PE |
| Mekie et al. 2020 (63) | Prospective matched case-controlled study | Ethiopia, 321 | 107 cases, 214 controls | Nulliparous women delivering at 3 high-volume hospitals in West Amhara, January to April 2018 | 22.92 ± 4.64 (mean + SD) | Questionnaire, during pregnancy | Fruits; vegetables; coffee; alcohol; nutritional counseling during ANC | PE |
| Mi et al. 2019 (69) | Randomized controlled trial (secondary analysis) | China, 987 | PE: 19 (1.9%) | Pregnant women enrolled at preconception and prenatal care in 3 rural counties in Shaanxi Province, December 2015 to February 2018 | 25.3 ± 4.2 (mean + SD) | 107-item FFQ, during pregnancy | Vegetable; meat; fruit; snack; wheat staple | GH; PE |
| Sheikhi et al. 2018 (65) | Prospective matched case-controlled study | Iran, 125 | 62 cases, 63 controls | Pregnant women attending Iman-Ali Hospital, June to September 2015. | Cases: 29.3 ± 6.7; controls: 25.8 ± 6.2 (mean + SD) | 169-item FFQ, 30–36 wk | Meat, beans, nuts and eggs; milk; grains; vegetables; fruit | PE |
| Zareei et al. 2019 (71) | Prospective unmatched case-controlled study | Iran, 182 | 82 cases, 100 controls | Pregnant women referred to the gynaecology ward of vali-e-Asr Hospital in 2016 | Cases: 28.96 ± 5.85; controls: 27.91 ± 4.93 (mean + SD) | 169-item FFQ, during pregnancy | Unhealthy; healthy | PE |
ANC: antenatal care; GH: gestational hypertension; HDP: hypertensive disorders of pregnancy; NA, not available; PE: pre-eclampsia; SIP: superimposed pre-eclampsia.
Quality assessment
Overall, 7 studies were classed as moderate risk of bias, 4 as serious risk of bias, and 2 as critical risk of bias (see Supplemental Figure 1 for ROBINS-I risk of bias quality assessment of each included study, and Supplemental Figure 2 for summary risk of bias). All but 2 studies (60, 61) adjusted for important baseline confounders. Over half of the studies controlled for maternal age (9/13, 69.2%) and a number of studies controlled for parity/gravidity (6/13, 46.2%), prepregnancy BMI (6/13, 46.2%), and family history of hypertension (4/13, 30.8%) (see Supplemental Table 7 for adjustment factors). The majority of studies also excluded women with a prepregnancy history of hypertension (7/13 excluded, 1/13 adjusted, 61.5% total). Based on a list of potential risk factors for pre-eclampsia described by Schoenaker and colleagues (72), a number of confounding variables were unaccounted for in all studies, including ethnicity, season of infant birth, maternal height, and smoking status. Some studies considered ethnicity in participant characteristics to understand the composition of study samples; however, no analyses adjusted for ethnicity. Few studies adjusted for socioeconomic status (SES), history of pregnancy hypertension, blood pressure in early pregnancy, gestational age, pregnancy interval, gestational weight gain, physical activity, total energy intake, and dietary supplement use (1–3 studies each). Some studies controlled for confounders beyond the list described by Schoenaker and colleagues (72), including rural/urban residence; educational level; occupation; cohabitation; family planning and whether the current pregnancy was planned; drug use; anemia; urinary tract infections; multiple pregnancy; antenatal care visits; differences in physical activities prior to, as opposed to during, the pregnancy; gestational diabetes; and history of miscarriage, stillbirth, and pregnancy termination. Due to the need for a clinical diagnosis for pre-eclampsia and other forms of pregnancy hypertension, 12 of 13 studies recruited participants at a health facility; only the randomized controlled trial also recruited in the community setting (69). Pregnancy hypertension was often diagnosed by clinicians external to the dietary study. All studies relied on self-report of dietary intake patterns through FFQs facilitated by trained interviewers, of which 6 studies used validated FFQs (65–67, 69–71).
Healthy dietary patterns
Four studies employed principal component analysis (PCA) to elicit dietary patterns from habitual diets of study participants. Study participants were scored on consumption frequency of foods characterizing dietary patterns and divided into quartiles (67, 69, 71) or tertiles (70). High adherence to a dietary pattern included those in the fourth quartile or third tertile, whereas first quartile or tertile were classified as low adherence.
Three Iranian studies described a healthy dietary pattern characterized by frequent consumption of vegetables, fruit, and dairy products, particularly low-fat dairy (67, 70, 71). These studies found that women who scored higher on the healthy dietary pattern had lower odds of developing pre-eclampsia. Case-controlled studies (2 studies) found that high adherence to a healthy dietary pattern significantly reduced the risk of pre-eclampsia by 82–87% [tertile 3 compared with tertile 1 adjusted OR: 0.13; 95% CI: 0.07, 0.22; P < 0.001 (70), quartile 4 compared with quartile 1 adjusted OR: 0.22; 95% CI: 0.09, 0.53; P = 0.001 (71)] compared with low adherence. A prospective cohort study found that high adherence to a healthy dietary pattern reduced the risk of pre-eclampsia by 51% (quartile 4 compared with quartile 1 adjusted OR: 0.49; 95% CI: 0.24, 1.00; P = 0.05) (67).
A secondary analysis of a randomized controlled trial in China identified 5 main food patterns: vegetable, meat, fruit, snack, and wheat staple (70). Women who scored high (quartile 4) in the vegetable dietary pattern characterized by frequent consumption of mushrooms, leafy and cruciferous vegetables, root vegetables, melon vegetables, and legumes had an 80% reduced risk of pre-eclampsia incidence (adjusted RR: 0.20; 95% CI: 0.04, 0.98; P = 0.041) compared with those who scored low (quartile 1) (69). A higher vegetable dietary pattern score was associated with lower risk of proteinuria (adjusted RR quartile 4 compared with quartile 1: 0.44; 95% CI: 0.24, 0.80; P-trend = 0.015). Vegetable dietary pattern was not associated with risk of gestational hypertension or proteinuria. Meat, fruit, snack, and wheat staple dietary patterns were not associated with pre-eclampsia risk.
In contrast, a case-controlled study in Iran found that high consumption of a Western dietary pattern characterized by red meat and/or processed meat, fried potatoes, pickles, sweets, pizza, and low intake of vegetables was associated with a 4-fold increase in the odds of developing pre-eclampsia (adjusted OR: 4.37; 95% CI: 3.04, 6.28; P < 0.001) (70). An Iranian cohort study also found that a Western dietary pattern was associated with double the odds of developing pre-eclampsia (adjusted OR: 2.08; 95% CI: 1.00, 4.36; P = 0.02) (67). Slightly different than in the case-controlled study, the cohort study defined a Western dietary pattern as high consumption of processed meats, fruits, fruit juice, citrus, nuts, fish, desserts and sweets, sugar, saturated fat, sweet fruit, potato, legumes, coffee, egg, pizza, high-fat dairy, whole grain, and soft drinks (67). A meta-analysis was not possible due to heterogeneity of study design and reporting formats.
Vegetable dietary pattern
Among 7 studies that examined frequency of vegetable consumption in association with risk of developing pregnancy hypertension, 1 Ethiopian study examining HDP (66) and 2 studies from Zimbabwe (59) and Iran (65) reporting pre-eclampsia outcomes did not find a significant association. Four studies found significant associations. Three case-controlled studies from Ethiopia reported that adequate vegetable consumption (≥1–3 servings/wk) was associated with a 5–58% reduced odds of developing pre-eclampsia [adjusted OR: 0.46; 95% CI: 0.24, 0.90; P = 0.023 (62), adjusted OR: 0.42; 95% CI: 0.22, 0.82; P < 0.05 (63), daily compared with no intake adjusted OR: 0.95; 95% CI: 0.01, 0.71; P < 0.05 (68)]. One of these studies found that the relation between adequate vegetable intake and pre-eclampsia incidence was only significantly protective after adjustment for daily but not 1 serving/wk (68). Additionally, a prospective cohort study from Democratic Republic of Congo found that 3 daily servings of vegetables was associated with a 12% reduced risk of pre-eclampsia incidence (RR: 0.88; 95% CI: 0.6, 0.98; P < 0.01) (60).
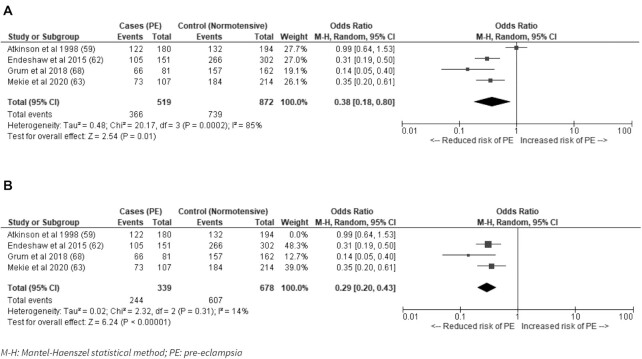
A meta-analysis of pooled evidence from the 4 case-controlled studies that measured pre-eclampsia and could be grouped as dichotomous indicators resulted in a pooled OR = 0.38 (95% CI: 0.18, 0.80; I2 = 85%; P = 0.01) for adequate consumption of vegetables compared with low or no consumption and risk of developing pre-eclampsia (Figure 2A). A sensitivity analysis excluding studies classed as serious risk of bias lowered the heterogeneity between studies without changing the direction of the effect (OR: 0.29; 95% CI: 0.20, 0.43; I2 = 14%; P < 0.00001) (Figure 2B). One study with a serious risk of bias that defined low consumption as <120 servings/mo was responsible for a majority of the heterogeneity (59). The 3 remaining studies evaluated consumption of vegetables at least once per week (62, 63, 68).
FIGURE 2.
(A) Adequate consumption of vegetables compared with low or no consumption for the risk of developing pre-eclampsia in low- and middle-income countries; (B) Sensitivity analysis excluding studies classed as serious risk of bias. M-H, Mantel–Haenzel random-effects model; PE, pre-eclampsia.
Fruit dietary pattern
Among 7 studies that examined frequency of fruit consumption in association with risk of developing pregnancy hypertension, 3 studies from Zimbabwe (59), Iran (65), and Ethiopia (64) reporting pre-eclampsia outcomes did not find a significant association. Three studies from Ethiopia reported that adequate fruit consumption (≥1–3 servings/wk) was associated with a 49–77% reduced odds of developing pre-eclampsia [adjusted OR: 0.51; 95% CI: 0.29, 0.91; P = 0.023 (62), adjusted OR: 0.45; 95% CI: 0.24,0.87; P < 0.05 (63), adjusted OR: 0.23; 95% CI: 0.06, 0.91; P < 0.05 (68)]. A study from Ethiopia found that regular fruit consumption ≥2–4 times/wk was associated with lower odds of developing HDP compared with women with low consumption (adjusted OR: 0.19; 95% CI: 0.09, 0.42; P < 0.001) (66).
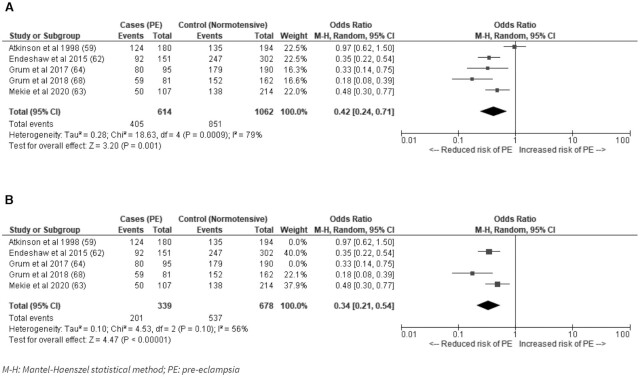
A meta-analysis of the 5 case-controlled studies that measured pre-eclampsia and could be grouped as dichotomous indicators found a pooled OR = 0.42 (95% CI: 0.24, 0.71; I2 = 79%; P = 0.001) (Figure 3A) for adequate consumption of fruits compared with low or no consumption for the risk of developing pre-eclampsia. A sensitivity analysis excluding studies with serious risk of bias lowered the heterogeneity between studies although the variability was still moderate (OR: 0.34; 95% CI: 0.21, 0.54; I2 = 56%; P < 0.00001) (Figure 3B). The 3 remaining studies also evaluated consumption of fruits at least once per week (62, 63, 68). The 2 studies with a serious risk of bias classified low consumption of fruit as ≤12 servings in the month before delivery (59) and no fruit intake during pregnancy (64).
FIGURE 3.
(A) Adequate consumption of fruits compared with low or no consumption for the risk of developing pre-eclampsia in low- and middle-income countries; (B) Sensitivity analysis excluding studies classed as serious risk of bias. M-H, Mantel–Haenzel random-effects model; PE, pre-eclampsia.
Other dietary patterns
Other food groups, including meat, poultry, fish, dairy and other animal products, and grains dietary patterns, were inconsistently defined among studies. A study from Zimbabwe suggested that higher consumption of meat might be associated with risk of pre-eclampsia (adjusted OR: 1.9; 95% CI: 1.5, 5.6 for women with 12–45 servings, and adjusted OR: 2.9; 95% CI: 0.8, 3.0 for women with 45–72 servings, compared with ≤11 servings in the month before delivery; P-trend = 0.142), whereas poultry, fish, and dairy were not (59). A study from Ethiopia found that overall animal product intake was not associated with pre-eclampsia (62), whereas a study from Iran found that meat, beans, nuts, eggs, and milk were not associated with pre-eclampsia (65). The same Iranian study found that higher consumption of grains was associated with a 2-fold increase in the risk of developing pre-eclampsia (adjusted OR: 2.00; 95% CI: 1.11, 3.61; P < 0.05) (65).
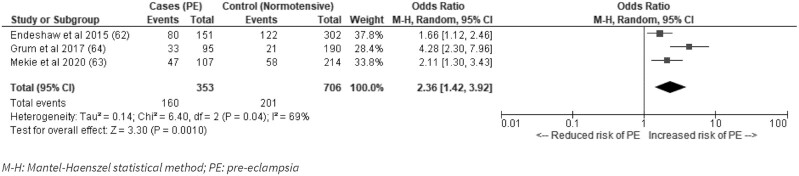
The association between coffee consumption and alcohol with risk of developing pre-eclampsia or HDPs was explored in 5 studies from Ethiopia (62–64, 66, 68). None of 4 studies looking at coffee consumption found a significant association (62, 63, 66, 68). Three Ethiopian studies found that alcohol use during pregnancy was associated with pre-eclampsia incidence (OR: 2.36; 95% CI: 1.42, 3.92; I2 = 69%; P = 0.001) (Figure 4), although 2 studies were nonsignificant after adjusting for confounders (62, 63).
FIGURE 4.
Alcohol use during pregnancy and risk of pre-eclampsia in low- and middle-income countries. M-H, Mantel–Haenzel random-effects model; PE, pre-eclampsia.
Factors associated with dietary patterns
Three studies from Ethiopia found that women who reported receiving dietary advice during antenatal care had a 48–83% reduced odds of developing pre-eclampsia [adjusted OR: 0.52; 95% CI: 0.29, 0.96; P < 0.05 (63), adjusted OR: 0.22; 95% CI: 0.10, 0.48; P < 0.001 (64), adjusted OR: 0.17; 95% CI: 0.05, 0.60, P < 0.05 (68)].
Other studies highlighted the influence of socioeconomic factors on dietary patterns (61, 62, 67, 71). A study from Turkey found that low education and income were associated with lower quality maternal diets and observed that a majority of the women with pre-eclampsia tended to meet their protein needs with low-quality, cheap vegetable protein products instead of animal products (61). A study from Iran found that women with high adherence to a healthy diet characterized by vegetables, fruits, low-fat dairy, poultry, and red meat were more likely to be employed, have graduated from university, and have husbands who were also employed (67). A study from Ethiopia reflected that due to poverty, “regular intake of green vegetables, fruit and animal products is impossible for most of the population, including pregnant women” (62).
Discussion
To the best of our knowledge, this is the first review examining maternal dietary patterns in association with pregnancy hypertension specifically in LMIC. Three studies identified that a healthy dietary pattern characterized by high consumption of fruits, vegetables, and low-fat dairy products was associated with a 51–82% risk reduction in incidence of pre-eclampsia (67, 70, 71). Maternal diets with no or low consumption of vegetables were associated with 2.6 times the odds of developing pre-eclampsia compared with women who consumed vegetables during their pregnancies. Similarly, no or low consumption of fruit was associated with 2.3 times the odds of developing pre-eclampsia compared with women who consumed fruits during their pregnancies. Limited data were found to suggest that high consumption of meat (59), grains (65), a “Western” style diet characterized by foods with high sugar content, fried and/or processed (67, 70) foods, or alcohol consumption during pregnancy (64) might be associated with higher pre-eclampsia incidence rates; however, mixed results and inconsistent definitions between studies preclude conclusions.
With 10 of the 13 included studies published within the past decade, there has been growing interest in maternal dietary patterns as a risk factor for pre-eclampsia in LMIC. However, the literature available is mostly limited to 2 countries, Ethiopia and Iran; this limits the broader relevance of the observed findings. Moderate-to-serious risk of bias in a majority of studies limits the quality of the evidence, as shown by substantially reduced heterogeneity in pooled outcomes when studies with a serious risk of bias were excluded. Additionally, although the review was limited to cohort and case-controlled studies and randomized controlled trials, all of which have lower risk of bias than cross-sectional studies, the evidence is drawn mostly from case-controlled studies with few large prospective cohort studies available. This weakness in the evidence base suggests a clear need for additional such studies to confirm the relation to pre-eclampsia.
Although the quality and completeness of current evidence is low, the association between healthy maternal dietary patterns with lower risk of pre-eclampsia incidence in LMIC is supported by the similar direction of evidence from HICs. A previous review including 2 case-controlled studies from the United States and 2 cohort studies from Norway suggested that maternal diets rich in fruits and vegetables had a beneficial effect on pre-eclampsia (72). A review with 4 publications based on cohort studies in Western Europe found a 22% lower odds of pre-eclampsia incidence in women who consumed a healthy dietary pattern characterized by high consumption of fruits, vegetables, whole-grain foods, fish, and poultry (9). Three of these studies were based on the Norwegian Mother and Child Cohort (MoBa), which found a 28% reduced odds of developing pre-eclampsia in women with high adherence to a dietary pattern characterized by vegetables, plant foods, and vegetable oils [tertile 3 compared with tertile 1 OR: 0.72; 95% CI: 0.62, 0.85; n = 23,423) (73)], particularly those who reported frequent consumption of organic vegetables (74). The New Nordic diet and the Mediterranean diet, characterized by the high intake of fruits and vegetables, whole grains, fish, legumes, and vegetable oil, were associated with lower rates of pregnancy hypertension in cohort studies from Norway (MoBa) (75), The Netherlands (Generation R) (76), Greece (54), and Australia (the Australian Longitudinal Study on Women's Health) (77). The Project Viva cohort from the United States also found that women with higher consumption of vegetables, fruits, fiber, and foods rich in folate, calcium, and iron, white compared with red meat, and polyunsaturated compared with saturated fatty acids/trans fats were 13% less likely to develop pre-eclampsia (OR: 0.87; 95% CI: 0.76, 1.00; n = 1777) (78).
As in studies conducted in HICs, this review finds that healthy diets characterized by adequate consumption of fruits and vegetables are associated with lower risk of pre-eclampsia incidence. However, although the odds of developing pre-eclampsia were lowered by 13–28% by healthy maternal dietary patterns between 5 cohort studies in Western Europe and the United States (73–76, 78), the potential risk reduction could be even larger in LMIC settings. Four studies from Iran and China reported that healthy maternal diets characterized by high intake of vegetables was associated with a 51–82% risk reduction in incidence of pre-eclampsia (67, 69–71). This could be related to high rates of maternal malnutrition in LMIC, and the advantages offered by a diversified diet, rich in micronutrients (79).
Maternal malnutrition, frequent in LMIC, is characterized by imbalanced macronutrients and inadequate micronutrient intakes, such as in iron, folate, calcium, and zinc, and has been documented in a review of 62 studies (79). A multicountry study from Pakistan, Democratic Republic of Congo, Guatemala, and India found that inadequate dietary diversity was found in ≥80% of poor, rural women during their first trimester, and >80% had inadequate intakes of folate, vitamin B-12, and choline (37). Researchers also found inadequate intakes of calcium, thiamine, riboflavin, and vitamin B-6 were common, but rates varied between countries (37). Additionally, a study in South Africa and Zimbabwe in women with a history of pre-eclampsia found high rates of overweight and obesity (74% from South Africa and 60% from Zimbabwe with BMI above normal) alongside high rates of undernutrition (46). Over 80% to 100% of women in the study had below recommended intakes of calcium, iron, folate, magnesium, zinc, and selenium, and a majority of women had lower protein intakes than the estimated average requirement (71% in South Africa, 98% in Zimbabwe) (46). Two Ethiopian studies included in the review also described heightened risk of pre-eclampsia incidence with increased midupper arm circumference while noting low overall consumption of fruits and/or vegetables (62, 63). Higher rates of maternal malnutrition including both obesity and overweight alongside nutritional deficiencies in LMIC highlight the importance of adequate diet for maternal health, and the potential impact that healthy maternal diets can have on reducing risk of pre-eclampsia and improving maternal outcomes in general. Although not reporting specifically on pregnancy hypertension, cohort studies from Ethiopia and Nepal reported that consumption of dark-green leafy vegetables in particular was associated with lower odds of adverse pregnancy outcomes (80) and maternal mortality (39).
Although the current review suggests the potential for healthy maternal diets, it is likely that SES plays an important role and it is unclear whether access to fruits and vegetables on a regular basis is directly related to a lower incidence of pre-eclampsia or indirectly because access to higher quality maternal diets is representative of higher SES. A number of the included articles suggest the potential importance of SES, but the discussion was limited (61, 62, 67, 71). Studies from rural Bangladesh examining determinants of improved household food security and dietary diversity identified household wealth and female literacy as important factors (41, 44). Despite having knowledge about their importance during pregnancy, dietary diversity and consumption of micronutrient-rich foods were low in pregnant women of lower SES (44). A Tanzanian cross-sectional survey found that higher dietary diversity was associated with HDP, but researchers highlighted that could have been driven by urban residence, greater prepregnancy BMI, and HIV-positive status (81). More research is needed to understand the influence of SES on the relation between maternal diets and adverse pregnancy outcomes, such as pre-eclampsia, in LMIC.
Strengths of our review include the use of multiple reviewers, a comprehensive search strategy employing multiple databases and gray literature, and use of quality assessments. A limitation of the current review is the exclusion of non-English-language articles, which might have excluded some studies. Publication bias was not evaluated due to the relatively small number of studies included in the meta-analyses and there is potential of an increased risk of making a type I error that results from testing multiple study outcomes in meta-analyses. Additionally, inconsistent definitions of dietary patterns limited the ability to pool evidence, particularly beyond frequency of fruit and vegetable consumption. Furthermore, although material dietary diversity was a focus outlined in the protocol, it remains a research gap in the literature. Studies tend to look at consumption of different food groups but not necessarily to see if the same woman is consuming multiple food groups.
Conclusions
Maternal diet is increasingly recognized as critical to the health of the mother as well as to the healthy development of her child. Pre-eclampsia is the second leading direct cause of maternal deaths worldwide and there is evidence that healthy dietary patterns characterized by regular consumption of fruits and vegetables can be related to lower incidence of pre-eclampsia. This association was previously demonstrated in research conducted in HICs. The review suggests that the association can also be found in women in LMIC, perhaps with an even stronger potential effect. However, the completeness and quality of evidence from LMIC is currently low, with few large prospective cohort studies investigating this topic. Further research with larger cohorts is needed to systematically evaluate the association, investigate deeper into dietary diversity and the consumption of different food groups, and explore the potential interaction with SES.
Supplementary Material
ACKNOWLEDGEMENTS
This manuscript is part of the PRECISE (PREgnancy Care Integrating translational Science, Everywhere) Network. We would like to express our gratitude to the PRECISE Team for their support. The PRECISE Conceptual Framework Working Group includes: King's College London (Peter von Dadelszen, Laura A Magee, Lucilla Poston, Hiten D Mistry, Marie-Laure Volvert, Cristina Escalona Lopez, Sophie Moore, Rachel Tribe, Andrew Shennan, Tatiana Salisbury, Lucy Chappell, Rachel Craik); Aga Khan University, Nairobi (Marleen Temmerman, Angela Koech Etyang, Sikolia Wanyonyi, Geoffrey Omuse, Patricia Okiro, Grace Mwashigadi); Centro de Investigação de Saúde de Manhiça (Esperança Sevene, Helena Boene, Corssino Tchavana, Euse-bio Macete, Carla Carillho, Lazaro Quimice, Sonia Maculuve); Donna Russell Consulting (Donna Russell); Imperial College London (Ben Baratt); London School of Hygiene and Tropical Medicine (Joy Lawn, Hannah Blencowe, Veronique Filippi, Matt Silver); Midlands State University (Prestige Tatenda Makanga, Liberty Makacha, Yolisa Dube, Newton Nyapwere, Reason Mlambo); MRC Unit The Gambia at LSHTM (Umberto D'Alessandro, Anna Roca, Melisa Martinez-Alvarez, Hawanatu Jah, Brahima Diallo, Abdul Karim Sesay, Fatima Touray, Abdoulie Sillah); University of Oxford (Alison Noble, Aris Papageorghiou); St George's, University of London (Judith Cartwright, Guy Whitley, Sanjeev Krishna, Rosemarie Townsend, Asma Khalil); University of British Colombia [Marianne Vidler, Joel Singer, Jing (Larry) Li, Jeffrey Bone, Mai-Lei (Maggie) Woo Kinshella, Kelly Pickerill, Ash Sandhu, Domena Tu, Rajavel Elango]; and University of Malawi (William Stones).
The authors’ responsibilities were as follows—MWK: conceptualized the review; MWK, SO, KS: conducted the review; MWK: conducted the formal analyses and wrote the first draft of the manuscript; MV, LAM, PvD, SEM, RE: reviewed all versions of the paper and contributed to the interpretation and the structure of the paper; and all authors: read and approved the final manuscript.
Notes
The PRECISE Network is funded by the UK Research and Innovation Grand Challenges Research Fund GROW Award scheme (grant number: MR/P027938/1). MWK is supported by the Vanier Canada Graduate Scholarship funded by the Government of Canada through the Canadian Institutes of Health Research (CIHR); and Canadian Institutes of Health Research (FRN 10321) to RE.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–7 and Supplemental Figures 1–2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AJOL, African Journals Online database; CENTRAL, Cochrane Central Register of Controlled Trials database; CINAHL, Cumulative Index to Nursing and Allied Health database; HDP, hypertensive disorder of pregnancy; HIC, high-income country; ICTRP, International Clinical Trials Registry Platform; LMIC, low- and middle-income countries; MMR, maternal mortality ratio; MoBa, Norwegian Mother and Child Cohort; PCA, principal component analysis; PRECISE, PREgnancy Care Integrating translational Science, Everywhere; SES, socioeconomic status.
Contributor Information
Mai-Lei Woo Kinshella, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada.
Shazmeen Omar, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada.
Kerri Scherbinsky, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada; Department of Pediatrics, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada.
Marianne Vidler, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada.
Laura A Magee, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada; Department of Women & Children's Health, King's College London, London, United Kingdom.
Peter von Dadelszen, Department of Obstetrics and Gynaecology, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada; Department of Women & Children's Health, King's College London, London, United Kingdom.
Sophie E Moore, Department of Women & Children's Health, King's College London, London, United Kingdom; MRC Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Rajavel Elango, Department of Pediatrics, BC Children's and Women's Hospital and University of British Columbia, Vancouver, British Columbia, Canada; School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada.
References
- 1. WHO, UNICEF, UNFPA, World Bank Group , The United Nations Population Division. Trends in maternal mortality 2000 to 2017. Geneva: World Health Organization; 2019. [Google Scholar]
- 2. WHO . Maternal mortality. [Internet]. 2019; [cited August 20, 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/maternal-mortality
- 3. Institute for Health Metrics and Evaluation Client Services . Making the world a healthier place for mothers: trends and opportunities for action in maternal health. Seattle (WA): IHME; 2019. [Google Scholar]
- 4. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014;2(6):e323–33. [DOI] [PubMed] [Google Scholar]
- 5. von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;36:83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Dadelszen P, Ayres de Campos D, Barivalala W. Classification of the hypertensive disorders of pregnancy. In: Magee L, von Dadelszen P, Stones W, Mathai M, editors. The FIGO textbook of pregnancy hypertension. London: The Global Library of Women's Medicine; 2016. p.33–61. [Google Scholar]
- 7. Achamrah N, Ditisheim A. Nutritional approach to preeclampsia prevention. Curr Opin Clin Nutr Metab Care. 2018;21(3):168–73. [DOI] [PubMed] [Google Scholar]
- 8. Allen R, Rogozinska E, Sivarajasingam P, Khan KS, Thangaratinam S. Effect of diet- and lifestyle-based metabolic risk-modifying interventions on preeclampsia: a meta-analysis. Acta Obstet Gynecol Scand. 2014;93(10):973–85. [DOI] [PubMed] [Google Scholar]
- 9. Kibret KT, Chojenta C, Gresham E, Tegegne TK, Loxton D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: a systematic review and meta-analysis. Public Health Nutr. 2019;22(3):506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne J, Higgins J, Elbers R, Reeves B, and the development group for ROBINS-I . Risk of bias in non-randomized studies of interventions (ROBINS-I): detailed guidance. [Internet]. Updated October 12, 2016[cited April 1, 2020]. Available from: http://www.riskofbias.info [Google Scholar]
- 15. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VA. Cochrane handbook for systematic reviews of interventions. 2nd ed.Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 16. Savy M, Martin-Prevel Y, Danel P, Traissac P, Dabire H, Delpeuch F. Are dietary diversity scores related to the socio-economic and anthropometric status of women living in an urban area in Burkina Faso?. Public Health Nutr. 2008;11(2):132–41. [DOI] [PubMed] [Google Scholar]
- 17. Safdar N, Bertone-Johnson E, Cordeiro L, Jafar T, Cohen N. Dietary patterns associated with hypertension among the low income urban population in Pakistan. FASEB J. 2013;27(1):622.11.23139156 [Google Scholar]
- 18. Safdar N, Bertone-Johnson E, Cordeiro L, Jafar T, Cohen N. Dietary patterns and their association with hypertension among Pakistani urban adults. Asia Pac J Clin Nutr. 2015;24(4):710–19. [DOI] [PubMed] [Google Scholar]
- 19. Desalegn BB, Lambert C, Riedel S, Negese T, Biesalski HK. Ethiopian Orthodox fasting and lactating mothers: longitudinal study on dietary pattern and nutritional status in rural Tigray. Int J Environ Res Public Health. 2018;15(8):1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martins EB, Nunez Urquiza RM. [Energy intake, maternal nutritional status and intrauterine growth retardation.] Cad Saude Publica. 2003;19(1):279–85. [DOI] [PubMed] [Google Scholar]
- 21. Tran N, Nguyen L, Berde Y, Low Y, Tey S. Maternal nutritional adequacy and gestational weight gain and their associations with birth outcomes among Vietnamese women. BMC Pregnancy Childbirth. 2019;19(1):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Babaei M. Nutritional status of pregnant women and urine calcium-to-creatinine ratio during 24th-28th weeks of pregnancy and their relationship with the incidence of hypertensive disorders during pregnancy. J Kermanshah Univ Med Sci. 2018;22(1):e69638. [Google Scholar]
- 23. Nucci L, Schmidt M, Duncan B, Fuchs S, Fleck E, Santos Britto M. Nutritional status of pregnant women: prevalence and associated pregnancy outcomes. Rev Saude Publica. 2001;35(6):502–7. [DOI] [PubMed] [Google Scholar]
- 24. Arredondo A, Torres C, Orozco E, Pacheco S, Huang F, Zambrano E, Bolaños-Jiménez F. Socio-economic indicators, dietary patterns, and physical activity as determinants of maternal obesity in middle-income countries: evidences from a cohort study in Mexico. Int J Health Plann Mgmt. 2019;34(1):e713–25. [DOI] [PubMed] [Google Scholar]
- 25. Harding KL, Matias SL, Mridha MK, Vosti SA, Hussain S, Dewey KG, Stewart CP. Eating down or simply eating less? The diet and health implications of these practices during pregnancy and postpartum in rural Bangladesh. Public Health Nutr. 2017;20(11):1928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lukmanji Z, Hertzmark E, Spiegelman D, Fawzi WW. Dietary patterns, nutrient intake, and sociodemographic characteristics in HIV-infected Tanzanian pregnant women. Ecol Food Nutr. 2013;52(1):34–62. [DOI] [PubMed] [Google Scholar]
- 27. Mridha M, Matias S, Arnold C. Factors associated with nutritional status and dietary practices of Bangladeshi adolescents in early pregnancy. Ann N Y Acad Sci. 2018;1416(1):66–76. [DOI] [PubMed] [Google Scholar]
- 28. Ravaoarisoa L, Rakotonirina J, Randriamanantsaina L, de Dieu Marie Rakotomanga J, Dramaix MW, Donnen P. Food consumption and undernutrition variations among mothers during the post-harvest and lean seasons in Amoron'i Mania Region, Madagascar. BMC Public Health. 2019;19(1):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang L, Shang L, Yang W, Li D, Qi C, Xin J, Wang S, Yang L, Zeng L, Chung MC. High starchy food intake may increase the risk of adverse pregnancy outcomes: a nested case-control study in the Shaanxi province of Northwestern China. BMC Pregnancy Childbirth. 2019;19(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen PH, Kim SS, Sanghvi T, Mahmud Z, Tran LM, Shabnam S, Aktar B, Haque R, Afsana K, Frongillo EAet al. Integrating nutrition interventions into an existing maternal, neonatal, and child health program increased maternal dietary diversity, micronutrient intake, and exclusive breastfeeding practices in Bangladesh: results of a cluster-randomized program eval. J Nutr. 2017;147(12):2326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu MS, He JR, Chen Q, Lu J, Wei X, Zhou Q, Chan F, Zhang L, Chen N, Qiu Let al. Maternal dietary patterns during pregnancy and preterm delivery: a large prospective cohort study in China. Nutr J. 2018;17(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Todd C, Chowdhury Z, Mahmud Z, Islam N, Shabnam S, Parvin M, Bernholc A, Martinez A, Aktar B, Afsana Ket al. Maternal nutrition intervention and maternal complications in 4 districts of Bangladesh: a nested cross-sectional study. PLoS Med. 2019;16(10):e1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agarwal DK, Agarwal A, Singh M, Satya K, Agarwal S, Agarwal KN. Pregnancy wastage in rural Varanasi: relationship with maternal nutrition and sociodemographic characteristics. Indian Pediatr. 1998;35(11):1071–9. [PubMed] [Google Scholar]
- 34. Na M, Mehra S, Christian P, Ali H, Shaikh S, Shamim AA, Labrique AB, Klemm RD, Wu LS, West KP Jr. Maternal dietary diversity decreases with household food insecurity in rural Bangladesh: a longitudinal analysis. J Nutr. 2016;146(10):2109–16. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed S, Hassen K, Wakayo T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: the role of nutritional factors. Nutr J. 2018;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zerfu TA, Mekuria A. Pregnant women have inadequate fiber intake while consuming fiber-rich diets in low-income rural setting: evidences from analysis of common “ready-to-eat” stable foods. Food Sci Nutr. 2019;7(10):3286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lander RL, Hambidge KM, Westcott JE, Tejeda G, Diba TS, Mastiholi SC, Khan US, Garcés A, Figueroa L, Tshefu Aet al. Pregnant women in four low-middle income countries have a high prevalence of inadequate dietary intakes that are improved by dietary diversity. Nutrients. 2019;11(7):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chomat AM, Solomons NW, Koski KG, Wren HM, Vossenaar M, Scott ME. Quantitative methodologies reveal a diversity of nutrition, infection/illness, and psychosocial stressors during pregnancy and lactation in rural Mam-Mayan mother-infant dyads from the Western Highlands of Guatemala. Food Nutr Bull. 2015;36(4):415–40. [DOI] [PubMed] [Google Scholar]
- 39. Christian P, Katz J, Wu L, Kimbrough-Pradhan E, Khatry SK, LeClerq SC, West KP Jr. Risk factors for pregnancy-related mortality: a prospective study in rural Nepal. Public Health. 2008;122(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wesolowska E, Jankowska A, Trafalska E, Kaluzny P, Grzesiak M, Dominowska J, Hanke W, Calamandrei G, Polańska K. Sociodemographic, lifestyle, environmental and pregnancy-related determinants of dietary patterns during pregnancy. Int J Environ Res Public Health. 2019;16(5):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris-Fry H, Azad K, Kuddus A, Shaha S, Nahar B, Hossen M, Younes L, Costello A, Fottrell E. Socio-economic determinants of household food security and women's dietary diversity in rural Bangladesh: a cross-sectional study. J Health Popul Nutr. 2015;33(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alaofe H, Douglas T, Burney J. The impact of solar market gardens on dietary diversity, women's nutritional status and components of women's empowerment in the Kalale district of northern Benin. Ann Nutr Metab. 2017;71(Suppl 2):1311–12. [Google Scholar]
- 43. Alaofe H, Burney J, Naylor R, Taren D. The impact of a Solar Market Garden programme on dietary diversity, women's nutritional status and micronutrient levels in Kalalé district of northern Benin. Public Health Nutr. 2019;22(14):2670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shamim AA, Mashreky SR, Ferdous T, Tegenfeldt K, Roy S, Rahman A, Rashid I, Haque R, Rahman Z, Hossen Ket al. Pregnant women diet quality and its sociodemographic determinants in Southwestern Bangladesh. Food Nutr Bull. 2016;37(1):14–26. [DOI] [PubMed] [Google Scholar]
- 45. Gomes CB, Malta MB, Papini SJ, Benício M, Corrente JE, Carvalhaes M. Adherence to dietary patterns during pregnancy and association with maternal characteristics in pregnant Brazilian women. Nutrition. 2019;62:85–92. [DOI] [PubMed] [Google Scholar]
- 46. Cormick G, Betrán AP, Harbron J, Purnat TD, Parker C, Hall D, Seuc AH, Roberts JM, Belizán JM, Hofmeyr GJ. Are women with history of pre-eclampsia starting a new pregnancy in good nutritional status in South Africa and Zimbabwe?. BMC Pregnancy Childbirth. 2018;18(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Workicho A, Belachew T, Ghosh S, Kershaw M, Lachat C, Kolsteren P. Burden and determinants of undernutrition among young pregnant women in Ethiopia. Matern Child Nutr. 2019;15(3):e12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zerfu TA, Umeta M, Baye K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am J Clin Nutr. 2016;103(6):1482–8. [DOI] [PubMed] [Google Scholar]
- 49. Teixeira JA, Castro TG, Grant CC, Wall CR, Castro AL, Francisco RPV, Vieira SE, Saldiva SR, Marchioni DM. Dietary patterns are influenced by socio-demographic conditions of women in childbearing age: a cohort study of pregnant women. BMC Public Health. 2018;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agbozo F, Abubakari A. Does gestational intake of adequate diets using the FAO women's dietary diversity indicator affect haemoglobin levels at delivery and newborn health outcomes? Preliminary findings from a prospective cohort study in Volta region, Ghana. Trop Med Int Heal. 2017;22(Suppl 1):331–2. [Google Scholar]
- 51. Agbozo F, Abubakari A, Der J, Jahn A. Maternal dietary intakes, red blood cell indices and risk for anemia in the first, second and third trimesters of pregnancy and at predelivery. Nutrients. 2020;12(3):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann Epidemiol. 2007;17(9):663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clausen T, Slott M, Solvoll K, Drevon CA, Vollset SE, Henriksen T. High intake of energy, sucrose, and polyunsaturated fatty acids is associated with increased risk of preeclampsia. Am J Obstet Gynecol. 2001;185(2):451–8. [DOI] [PubMed] [Google Scholar]
- 54. Parlapani E, Agakidis C, Karagiozoglou-Lampoudi T, Sarafidis K, Agakidou E, Athanasiadis A, Diamanti E. The Mediterranean diet adherence by pregnant women delivering prematurely: association with size at birth and complications of prematurity. J Matern Fetal Neonatal Med. 2019;32(7):1084–91. [DOI] [PubMed] [Google Scholar]
- 55. O'Sullivan A, McNamara A, Muhimbula H, Massawe G, Mtingele A, O'Connor D. Food security and nutrition status of mothers in nutritionally vulnerable regions of Tanzania. Ann Nutr Metab. 2015;67(Suppl 1):185. [Google Scholar]
- 56. Agrawal S, Fledderjohann J, Vellakkal S, Stuckler D. Adequately diversified dietary intake and iron and folic acid supplementation during pregnancy is associated with reduced occurrence of symptoms suggestive of pre-eclampsia or eclampsia in Indian women. PLoS One. 2015;10(3):e0119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Agrawal S. Frequency of consumption of specific food items and symptoms of preeclampsia and eclampsia in Indian women. Int J Med Public Health. 2014;4(4):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hajianfar H, Esmaillzadeh A, Feizi A, Shahshahan Z. The association between major dietary patterns and pregnancy-related complications. Arch Iran Med. 2018;21(10):443–51. [PubMed] [Google Scholar]
- 59. Atkinson JO, Mahomed K, Williams MA, Woelk GB, Mudzamiri S, Weiss NS. Dietary risk factors for pre-eclampsia among women attending Harare Maternity Hospital, Zimbabwe. Cent Afr J Med. 1998;44(4):86–92. [PubMed] [Google Scholar]
- 60. Longo-Mbenza B, Tshimanga KB, Buassa-bu-Tsumbu B, Kabangu MJR. Diets rich in vegetables and physical activity are associated with a decreased risk of pregnancy induced hypertension among rural women from Kimpese, DR Congo. Niger J Med. 2008;17(3):265–9. [PubMed] [Google Scholar]
- 61. Gulsen S, Guner A. Nutrition habits and blood test results of preeclamptic and healthy pregnant women. Res J Med Sci. 2012;6(4):175–80. [Google Scholar]
- 62. Endeshaw M, Abebe F, Bedimo M, Asart A. Diet and pre-eclampsia: a prospective multicentre case-control study in Ethiopia. Midwifery. 2015;31(6):617–24. [DOI] [PubMed] [Google Scholar]
- 63. Mekie M, Mekonnen W, Assegid M. Cohabitation duration, obstetric, behavioral and nutritional factors predict preeclampsia among nulliparous women in West Amhara Zones of Ethiopia: age matched case control study. PLoS One. 2020;15(1):e0228127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grum T, Seifu A, Abay M, Angesom T, Tsegay L. Determinants of pre-eclampsia/eclampsia among women attending delivery services in selected public hospitals of Addis Ababa, Ethiopia: a case control study. BMC Pregnancy Childbirth. 2017;17(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sheikhi M, Rezaei E, Hosseini SH, Shahrakipoor M, Sheikhi M, Soltani S. Nutritional status in preeclamptic women: a case-control study in south east of Iran. Nutr Food Sci Res. 2018;5(1):15–21. [Google Scholar]
- 66. Kahsay HB, Gashe FE, Ayele WM. Risk factors for hypertensive disorders of pregnancy among mothers in Tigray region, Ethiopia: matched case-control study. BMC Pregnancy Childbirth. 2018;18(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hajianfar H, Esmaillzadeh A, Feizi A, Shahshahan Z, Azadbakht L. Major maternal dietary patterns during early pregnancy and their association with neonatal anthropometric measurement. Biomed Res Int. 2018;2018:4692193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grum T, Hintsa S, Hagos G. Dietary factors associated with preeclampsia or eclampsia among women in delivery care services in Addis Ababa, Ethiopia: a case control study. BMC Res Notes. 2018;11:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mi B, Wen X, Li S, Liu D, Lei F, Liu R, Shen Y, Chen Y, Zeng L, Liu Xet al. Vegetable dietary pattern associated with low risk of preeclampsia possibly through reducing proteinuria. Pregnancy Hypertens. 2019;16:131–8. [DOI] [PubMed] [Google Scholar]
- 70. Abbasi R, Bakhshimoghaddam F, Alizadeh M. Major dietary patterns in relation to preeclampsia among Iranian pregnant women: a case-control study. J Matern Fetal Neonatal Med. [Internet]2019. doi:10.1080/14767058.2019.1686474. [DOI] [PubMed] [Google Scholar]
- 71. Zareei S, Homayounfar R, Naghizadeh MM, Ehrampoush E, Amiri Z, Rahimi M, Tahamtani L. Dietary pattern in patients with preeclampsia in Fasa, Iran. Shiraz E Med J. 2019 Nov 1;20(11):e86959. [Google Scholar]
- 72. Schoenaker D, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med. 2014;12(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brantsæter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, Magnus P, Meltzer HM. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139(6):1162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Torjusen H, Brantsæter AL, Haugen M, Alexander J, Bakketeig LS, Lieblein G, Stigum H, Næs T, Swartz J, Holmboe-Ottesen Get al. Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian mother and child cohort study. BMJ Open. 2014;4(9):e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hillesund ER, Øverby NC, Engel SM, Klungsøyr K, Harmon QE, Haugen M, Bere E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol. 2014;29(10):753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Timmermans S, Steegers-Theunissen RPM, Vujkovic M, Bakker R, Den Breeijen H, Raat Het al. Major dietary patterns and blood pressure patterns during pregnancy: the Generation R study. Am J Obstet Gynecol. 2011;205(4):337.e1–337.e12. [DOI] [PubMed] [Google Scholar]
- 77. Schoenaker D, Soedamah-Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women's Health. Am J Clin Nutr. 2015;102(1):94–101. [DOI] [PubMed] [Google Scholar]
- 78. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109(6):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16(8):1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zerfu TA, Pinto E, Baye K. Consumption of dairy, fruits and dark green leafy vegetables is associated with lower risk of adverse pregnancy outcomes (APO): a prospective cohort study in rural Ethiopia. Nutr Diabetes. 2018;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mwanri AW, Kinabo JL, Ramaiya K, Feskens EJM. High blood pressure and associated risk factors among women attending antenatal clinics in Tanzania. J Hypertens. 2015;33(5):940–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.