ABSTRACT
Normal placental development and proper angiogenesis are essential for fetal growth during pregnancy. Angiogenesis involves the regulatory action of many angiogenic factors and a series of signal transduction processes inside and outside the cell. The obstruction of placental angiogenesis causes fetal growth restriction and serious pregnancy complications, even leading to fetal loss and pregnancy cessation. In this review, the effects of placental angiogenesis on fetal development are described, and several signaling pathways related to placental angiogenesis and their key regulatory mediators are summarized. These factors, which include vascular endothelial growth factor (VEGF)-VEGF receptor, delta-like ligand 4 (DLL-4)-Notch, Wnt, and Hedgehog, may affect the placental angiogenesis process. Moreover, the degree of vascularization depends on cell proliferation, migration, and differentiation, which is affected by the synthesis and secretion of metabolites or intermediates and mutual coordination or inhibition in these pathways. Furthermore, we discuss recent advances regarding the role of functional nutrients (including amino acids and fatty acids) in regulating placental angiogenesis. Understanding the specific mechanism of placental angiogenesis and its influence on fetal development may facilitate the establishment of new therapeutic strategies for the treatment of preterm birth, pre-eclampsia, or intrauterine growth restriction, and provide a theoretical basis for formulating nutritional regulation strategies during pregnancy.
Keywords: angiogenesis, placenta, signaling pathways, amino acids, fatty acids
Introduction
During fetal development, the placenta undergoes high levels of both vasculogenesis and angiogenesis. The initiation, maturation, and maintenance of placental vasculature are critical to the success of pregnancy. During pregnancy, women are potentially threatened by obstetrical complications, such as preterm birth, pre-eclampsia, and intrauterine growth restriction (IUGR), which can be mainly attributed to the imbalance of human placental angiogenesis (1, 2). Accumulated evidence suggests that the occurrence of IUGR may be associated with decreased placental blood flow and/or impaired angiogenesis at the fetal-maternal interface (3, 4). Previous studies on IUGR placenta have shown that the development of terminal villi in IUGR placentas was significantly lower than that in normal term placentas based on the structure of the vascular tree (4, 5). Therefore, the normal regulation of placental angiogenesis is one of the irreplaceable key factors for fetal survival and development as well as successful pregnancy outcome.
The placenta, an important connecting organ between mother and fetus, is critical in providing a place for sufficient material exchange to meet the demands of fetal growth and development. Despite their classification into many types anatomically (such as epitheliochorial placenta and hemochorial placenta), mammalian placentas seem very similar in function, with no direct contact between fetal and maternal blood (6). For example, the porcine placenta belongs to an epitheliochorial type, with its surface being attached to maternal endometrium, and 6 layers of tissues to separate the fetus from the maternal blood (6). Angiogenesis is defined as the biological process of forming new blood vessels from pre-existing ones (7). Moreover, inasmuch as extensive angiogenesis occurs in the endometrium, fetal-maternal border, and placenta, and the material exchange between fetal and maternal blood is carried out through the capillary network in placental cotyledons, the importance of angiogenesis as the main biological event that takes place in the placenta for fetal development is self-evident (8). Collectively, during pregnancy, the degree of vascularization, blood flow, and the exchange capacity of material at the fetal-maternal interface are important factors affecting fetal development, especially angiogenesis, which plays a key role in this biological event.
Placental Angiogenesis Is an Irreplaceable Key Factor for Fetal Development
In our previous studies and others, normal angiogenesis and vascular development are shown as the basis for maintaining the blood flow in the uterus and placenta (umbilical cord) of humans and animal model organisms (8–10), ensuring the supply of nutrients and oxygen essential to the growth and development of the embryo (fetus).
Placental angiogenesis is generally found to be abnormal in the pathological study of human compromised pregnancies (11, 12). For example, IUGR fetuses with impaired respiratory gas exchange with the mother are at greater risk of chronic hypoxia, coupled with a reduction in the volume, surface, length, and density of the placental villi and capillaries (3, 12). Moreover, the occurrence of pre-eclampsia is characterized by the imbalance of angiogenic factors, which affects the feto-placental villus perfusion and oxygenation quality, resulting in impaired angiogenesis (11, 13). In the cotyledons of human placenta, the fetal-placental circulation is established through the formation of villi (the principal architecture of the placenta) as well as its internal blood vessels, where the material exchange between mother and fetus takes place. Angiogenesis is the driving force promoting the formation of villi in the early placenta and the continuous maturation of terminal villi during the second and third trimesters, meanwhile, the continuous remodeling of angiogenesis can contribute to the effective connection between the arterio-venous circuit and the parent intermediate villus (14). Suppressing placental angiogenesis can cause impoverished placental development and reduced blood flow to the placenta, leading to pregnancy syndrome and ultimately fetal growth failure.
In animal experiments, manipulating maternal nutrition or reducing blood flow to the placenta (by various approaches) can affect fetal growth and may lead to long-term complications after birth (13, 15). The study of sow placentas found that as pregnancy progresses, the generation of new blood vessels and the shortening of the hemotrophic diffusion distance can optimize blood nutrient transport and ensure the efficiency of maternal/fetal hemotrophic exchange during gestation (16, 17). Furthermore, even at a given placental size, fetal growth may vary because of differences in placental vascularization and efficiency (18). The lightest placenta within the same litter may have higher transport capacity and placental efficiency to compensate for the reduction in placental size (17, 19, 20). However, poor angiogenesis and growth in the placenta and endometrium can result in reduced placenta-fetal blood flow, inadequate maternal nutrition to the fetus, and eventually fetal growth retardation (18, 21). A previous study found that the placentas that supplied the lightest porcine fetuses suffered from impaired angiogenesis (22). In addition, in porcine placentas, the placentas that supplied small fetuses were shown to increase the width and remodeling of the bilayers, as well as the vascularity of the bilayer, thereby improving the efficiency of the placenta, trying to save the IUGR fetus, but ultimately failing (22). Similarly, when pregnant female rats were threatened by stressors such as nutrient restriction, the placenta tried to maintain fetal growth by maximizing the capacity for materno-fetal nutrient transfer and a compensatory increase in vascularity, despite a decrease in placental weight in the third trimester of pregnancy (23, 24). These results further indicate that adequate placental development and angiogenesis ensure placental blood flow and are essential for fetal development.
The placenta begins to develop rapidly after embryo implantation during early gestation, and the placental blood vessels continue to grow throughout pregnancy. Actually, in the second half of gestation, fetal weight increases exponentially, whereas uteroplacental growth slows or ceases in mammals (25). However, as pregnancy advances, placental transport capacity could continue to increase due to a steady increase in the extraction rate of substances from the uterus or umbilical blood (i.e., by increasing the arterial-venous concentration difference) during pregnancy (including the later stages of pregnancy), as well as a continuous increase in blood flow (21, 25, 26). Collectively, adequate placental blood circulation is essential for normal fetal development, indicating that the degree of placental angiogenesis and vasodilation may produce a significant impact on fetal growth and development, including the later stages of pregnancy.
Signaling Pathways and Key Regulators for Placental Angiogenesis
Unlike pathological angiogenesis, placental angiogenesis is a normal physiological process with steps similar to those of any other organ. Angiogenesis is a multistage process involving endothelial cells and the extracellular matrix and is regulated by the complex interaction of pro- and anti-angiogenic factors, which, if damaged, can lead to pregnancy complications and even the loss of pregnancy (7, 14, 27). The angiogenic growth stages include the proteolytic degradation of the extracellular matrix (basal and reticular lamina), migration and proliferation of endothelial cells to form a vessel sprout, tube formation, and recruitment of pericytes or smooth muscle cells to form a mature vessel (7). In general, the process of angiogenesis is jointly initiated and modulated by a variety of growth factors and their related signaling pathways, such as fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and angiopoietin (1, 28). In the normal placenta, various proangiogenic factors (VEGF, PIGF, angiopoietin, etc.) and antiangiogenic factors [such as soluble VEGF receptor-1 (sVEGFR-1)] have been found, and they all play a vital role in one or more signaling pathways. In the following sections, we focus on the signaling pathways involved in angiogenesis and their regulatory roles in placental development.
VEGF/VEGFR signaling pathway
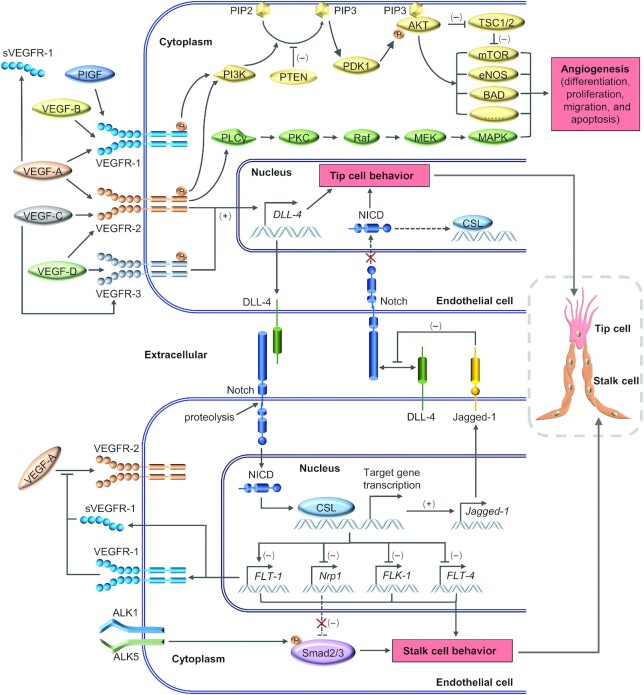
Many studies on angiogenesis have confirmed VEGF and its receptor as the key mediators of angiogenesis. Throughout angiogenesis, the VEGF/VEGFR signaling pathway is directly or indirectly involved in the regulatory process and related to other signaling pathways, playing an important role in angiogenesis (29). VEGF, a highly specific mitogen for endothelial cells (30), can cause vascular endothelial cell proliferation and induce angiogenesis in vivo, with the confirmed VEGF family members including VEGF-A, -B, -C, -D, -E, -F, and PIGF. Furthermore, there are 3 types of receptors for VEGFs: VEGF receptor-1 (VEGFR-1, also known as Flt-1), VEGFR-2 (KDR or Flk-1), and VEGFR-3 (Flt-4). In the VEGF/VEGFR signaling pathway (Figure 1), VEGF binds to the extracellular portion of the receptors, inducing their dimerization and activation of tyrosine kinase, leading to autophosphorylation of the receptor and the subsequent signal transduction (31, 32).
FIGURE 1.
Overview of the VEGF/VEGFR signaling pathway and DLL-4/Notch signaling pathway in endothelial cells. Vascular endothelial growth factor receptor (VEGFR) exists in 3 subtypes, including VEGFR-1 (encoded by FLT-1), -2 (encoded by FLK-1), and -3 (encoded by FLT-4), which can be activated by different vascular endothelial growth factor (VEGF) family ligands, and a soluble form of VEGFR-1 (sVEGFR-1) can be used as a potent antiangiogenic factor to sequester VEGF-A. Among all the ligands, the ligand VEGF-A is a major regulator of the activation of VEGFR-2 autophosphorylation. Downstream signaling is triggered by phosphorylated VEGFR, such as the phospholipase-Cγ (PLCγ) and PI3K-AKT pathways, determining cell proliferation and migration, tubule formation, and vascular permeability. In addition, VEGFR-2- and -3-mediated signals also induce the expression of delta-like ligand 4 (DLL-4) in endothelial cells to activate Notch signal transduction on adjacent endothelial cells. The activation of Notch signaling upregulates the expression of VEGFR-1 and sVEGFR-1 and downregulates the expression of VEGFR-2, VEGFR-3, and neuropilin-1 (Nrp1), repressing the function of VEGFR-2, inhibiting the tip cell phenotype and inducing the stalk cell phenotype. The downregulation of Nrp1 relieves its inhibition on Smad2/3 and can promote the differentiation of endothelial cells into stalk cells. Additionally, Notch signaling also affects the expression of Jagged-1 in the stalk cell, which can compete with DLL-4 for binding to the Notch receptor and inhibit Notch signaling in tip cells. AKT, V-Akt Murine Thymoma Viral Oncogene Homolog; ALK, activin receptor-like kinase; BAD, BCL2 associated agonist of cell death; CSL, CBFl/Suppressor of Hairless/Lag-1; MAPK, mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NICD, Notch intracellular domain; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphatidylinositol-3-kinase; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; Raf, RAF proto-oncogene serine/threonine-protein kinase; TSC1/2, tuberous sclerosis proteins 1/2. +, represents promotion; –, represents inhibition.
VEGF/VEGFR-2, a major angiogenic signal transduction pathway in mammals, mainly plays a role in embryonic vasculogenesis and angiogenesis (33, 34). The activation of the receptor, VEGFR-2, is influenced by the different variants of VEGF-A, a major regulator of angiogenesis, and VEGFR-2 is considered to be the main transducer of VEGF-A that affects the differentiation, proliferation, migration, and angiogenesis of endothelial cells (33, 35, 36). The activation of VEGFR-2 affects several downstream signaling pathways crucial to endothelial biology and participates in the regulation of mammalian placental angiogenesis by mediating multiple intermediates. These pathways, such as the phospholipase-Cγ (PLCγ)/protein kinase C/mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol-3-kinase (PI3K)-AKT pathway (Figure 1), play a critical role in regulating angiogenesis (29, 31, 35).
The PI3K/AKT cascade downstream of VEGFR-2 can control the biological process of placental angiogenesis by influencing the activation state of many effector molecules in endothelial cells of the placenta. In mammals, the kinases of the V-Akt murine thymoma viral oncogene homolog 1 (AKT) family consist of 3 isoforms, AKT1, AKT2, and AKT3. In the PI3K/AKT cascade (Figure 1), growth factors activate PI3K through transmembrane signal transduction to phosphorylate phosphatidylinositol-4,5-bisphosphate and thus produce phosphatidylinositol-3,4-bisphosphate/phosphatidylinositol-3,4,5-trisphosphate (37), leading to the translocation of AKT to the plasma membrane through binding the amino-terminal pleckstrin homology domain to AKT, as well as interaction with and activation of phosphoinositide-dependent kinase-1 (PDK1) (38–40). Subsequently, the activated PDK1 phosphorylates AKT to regulate many downstream effects of AKT (40). For example, phosphorylating the mammalian target of rapamycin (mTOR) can promote the proliferation and angiogenesis of endothelial cells (41); phosphorylating BCL2 associated agonist of cell death (BAD) can inhibit cell apoptosis (42); and activating endothelial NO synthase (eNOS) is important for new vessel formation and vasodilation (43). Studies have shown that AKT1 is widely expressed in mouse placenta, including all types of trophoblastic cells and vascular endothelial cells (43). AKT1 is a major component of signal transduction devices responsible for placental development and fetal growth. Placental vascularization was shown to be significantly reduced in AKT1-null mice, resulting in placental insufficiency, fetal growth impairment, and neonatal death (43). In addition, FGF2- and VEGF-activated PI3K/AKT1 pathways actively participate in the proliferation of placental endothelial cells and the expression of endothelial NO (44). In ovine fetoplacental artery endothelial cells, blocking the PI3K/AKT1 pathway with wortmannin, its specific inhibitor, can effectively inhibit all these angiogenic cellular processes (including cell proliferation, migration, and differentiation) (45). These results indicate that the signal cascade between VEGF/VEGFR and PI3K/AKT1 plays an important role in placental angiogenesis.
VEGFR-1, also a receptor of VEGF-A, can bind specifically to VEGF-B and PIGF. Aside from the major signal transduction role of VEGFR-2 in normal physiological angiogenesis, VEGFR-1 also regulates endothelial cells through crosstalk with VEGFR-2, dimerization, heterodimer-independent transphosphorylation, and regulation of receptor expression levels, whose synergy significantly enhances the signal (33, 46). Interestingly, VEGFR-1 has a higher affinity for VEGF-A than VEGFR-2 (∼10-fold), but a weaker tyrosine kinase activity (32, 47). In the past, this characteristic of VEGFR-1 with high affinity but weak tyrosine kinase activity was generally interpreted as an inhibitory receptor of VEGF-A and a negative regulator of physiological angiogenesis in early embryos (35, 48, 49). However, several studies have found that the activation of VEGFR-1 also plays an important role in regulating angiogenesis through special pathways. Cell experiments in vitro confirmed VEGFR-1 as a signaling receptor that activates eNOS and regulates NO-induced angiogenesis, which is dependent on the PI3K signaling pathway (50). In pathological models such as tumors and inflammatory bowel disease, PIGF, a specific ligand of VEGFR-1 (49), can directly stimulate the PI3K/AKT signaling pathway to affect cell migration and tube formation (51, 52). PIGF is highly expressed in placental trophoblastic cells throughout pregnancy and regulates endothelial cell function (53). Under normal physiological conditions, PIGF deficiency does not seem to affect embryonic angiogenesis, but in many pathological disorders, PIGF plays an important role in regulating angiogenesis, indicating the effect of PIGF on angiogenesis in normal pregnancy is very slight (54).
Apart from encoding the mRNA for a full-length receptor, the VEGFR-1 gene also encodes a short mRNA for sVEGFR-1 (55). sVEGFR-1, a natural inhibitor of VEGF-A, does not have tyrosine kinase activity, but only retains the ligand-binding domain, which can bind VEGF with high affinity and block its downstream biological signaling. It has been confirmed that the placenta can produce sVEGFR-1, which is abundantly expressed in both trophoblastic and endothelial cells of the placenta (56, 57), indicating its important role in avoiding excessive placental angiogenesis as a negative regulator of VEGF-A.
Delta-like ligand 4/Notch signaling pathway
Among the signaling pathways related to placental angiogenesis in mammals, aside from the VEGF signaling pathway, the Notch signaling pathway is also crucial for angiogenesis. The Notch signaling pathway, a highly conserved pathway in evolution, participates in cell fate control, as well as cell proliferation and apoptosis during development, thus affecting tissue differentiation and organ formation through signal transmission between adjacent cells (58–60). In mammals, there are 4 Notch receptors (Notch 1–4) and 5 Notch ligands: delta-like ligand 1 (DLL-1), DLL-3, DLL-4, Jagged-1, and Jagged-2, which can be detected in placental tissues and primary cell types involved in angiogenesis, such as endothelial cells and trophoblasts, and show different dynamic changes in different placental cells with the progression of pregnancy (61, 62). Among them, the Notch ligand, DLL-4, is essential to angiogenesis during embryonic and placental vascular development. Here, we mainly discuss the DLL-4/Notch signaling pathway (Figure 1).
In the process of angiogenesis induced by angiogenic factors such as VEGF, tip cells (the leading special endothelial cells of the sprouts) guide the direction of microvascular sprouting, followed by the proliferation of endothelial stalk cells and the formation of capillary lumen (63). This process is regulated by the DLL-4/Notch signaling pathway to ensure the balanced expression of these 2 types of cells and the normal development of blood vessels. Tip cells, which play a role in probing directional cues and guiding the nascent sprout, are characterized by high expression of VEGFR-2 and VEGFR-3, as well as a high level of DLL-4 expression and low Notch signaling activity (63–66). In contrast, stalk cells have a relatively high level of Notch signaling (66, 67). In tip cells, the VEGF stimulation of both VEGFR-2 and VEGFR-3 promotes the expression of DLL-4 (68), which binds to Notch receptors in adjacent stalk cells and induces Notch signaling, leading to 2-fold proteolysis of transmembrane Notch receptors and the migration of the Notch intracellular domain (NICD) into the nucleus (59, 60, 67). In the nucleus, NICD forms a complex with the transcription factor RBP-Jk (also known as CSL for CBF1/Su (H)/lag-1), and converts CSL molecules into transcriptional activators (59, 60, 69). This process initiates the transcription of the Notch target gene Jagged-1, resulting in a higher-level expression of Notch ligand Jagged-1 (65). Although few DLL-4 ligands can be expressed in the membrane of stem cells, high-level Jagged-1 competes with DLL-4 for binding to Notch receptors on tip cells, whereas Jagged-1 has weak signaling capacity and antagonizes DLL-4/Notch signaling (35, 65, 67), thus effectively suppressing the Notch signaling transduction from stalk cells to tip cells (Figure 1).
VEGF-VEGFR signaling is not only able to induce the DLL-4/Notch pathway but can also be affected by the feedback signal provided by DLL-4. DLL-4/Notch can regulate several components of the VEGF pathway, involving DLL-4 expressed in tip cells, which can upregulate the expression levels of VEGFR-1 and sVEGFR-1 in adjacent endothelial cells and reduce the expression levels of VEGFR-2, VEGFR-3, and neuropilin-1 in stalk cells, thus reducing the ability of endothelial cells to respond to VEGF and regulating tip cell formation and vessel branching (35, 64, 70–72). It has been reported that the local spatial secretion of sVEGFR-1 can precisely inactivate VEGF-A on either side of the sprout, but leave a corridor with intact VEGF-A in front of the sprout, guiding the emerging sprout to advance in the proper direction (73). In particular, unlike its function as a coreceptor for VEGFRs, neuropilin-1 does not function upstream, but as a downstream effector in tip/stem formation mediated by Notch signaling (74, 75). Furthermore, neuropilin-1 plays a key role in inhibiting the stalk cell phenotype and promoting the tip cell phenotype by limiting Smad2/3 activation downstream of ALK1 and ALK5 (75). Therefore, Notch signaling downregulates the expression of Neuropilin-1, leading to the differentiation of endothelial cells toward stalk cells. Overall, the VEGF signaling pathway and DLL-4/Notch signaling pathway form a feedback loop (Figure 1), where VEGF can promote the production of DLL-4, while DLL-4/Notch signaling is fed back to the VEGF pathway to limit the response of vascular endothelial cells to VEGF and counteract excessive angiogenesis, thereby playing a joint regulatory role in the proliferation and differentiation of vascular endothelial cells.
Wnt signaling pathway
The Wnt signaling pathway plays an important role in mediating angiogenesis by affecting the function of many known angiogenic factors. Compared with other signaling pathways, the Wnt signaling pathway not only induces the proliferation of cells, but also has the ability to shape the growing tissues, acting as the directional factor in this process (76).
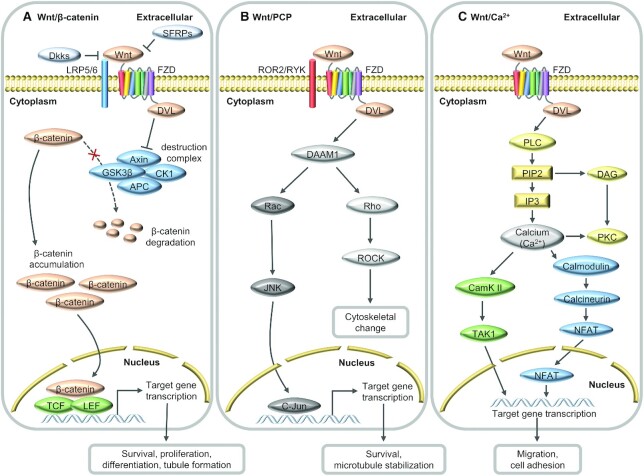
The Wnt signaling pathway can be subdivided into a canonical pathway (Wnt/β-catenin cascade) and a noncanonical pathway. The Wnt signaling pathway is usually composed of Wnt ligand proteins, receptors on the cell membrane, signal transduction parts in the cytoplasm, and transcriptional regulation parts in the nucleus. In the canonical Wnt signaling pathway (Figure 2A), the frizzled (FZD) and LDL receptor-related protein 5/6 (LRP5/6) coreceptors on the cell membrane combine with acylated Wnt to activate the dishevelled (DVL) protein, inhibiting the activity of the β-catenin destruction complex bound to cytoplasmic β-catenin and preventing the degradation of β-catenin by proteasomes to accumulate cytosolic β-catenin (77–82). Subsequently, β-catenin regulates the conversion of the T-cell factor/lymphoid-enhancing factor (TCF/LEF) nuclear complex into a transcriptional activator, inducing the transcription of target genes for biological events such as cell proliferation and differentiation, cell survival, and tubule formation (77, 78, 83). In recent years, researchers have paid more and more attention to the noncanonical Wnt signaling pathway, which mainly includes Wnt/planar cell polarity (Wnt/PCP) and Wnt/Ca2+ signaling pathways. In the Wnt/PCP signaling pathway (Figure 2B), Wnt ligands bind with FZD and receptor tyrosine kinase-like orphan receptor 2 (ROR2)/receptor-like tyrosine kinase (RYK) coreceptors to recruit DVL proteins to aggregate the disheveled-associated activator of morphogenesis 1 (DAAM1), thereby activating Rho or Rac1 proteins, and then regulating downstream transcription factors (77, 80, 81, 83). In the Wnt/Ca2+ signaling pathway (Figure 2C), Wnt ligands bind to FZD receptors to recruit DVL and activate heterotrimeric G protein, leading to the activation of phospholipase C, followed by increasing intracellular Ca2+ to activate calmodulin-dependent protein kinase II and regulate cell adhesion and migration (79, 84, 85).
FIGURE 2.
Overview of the Wnt signaling pathway. (A) Wnt/β-catenin signaling pathway. In the presence of Wnt ligands, the activity of the β-catenin destruction complex is inhibited, and β-catenin is accumulated in the cytoplasm, initiating transcription of target genes. The dotted arrow indicates that in the absence of Wnt ligands, β-catenin is phosphorylated and ubiquitinated by the destruction complex and subsequently degraded by the proteasome. In addition, extracellular factors such as dickkopfs (Dkks) and secreted Frizzled-related proteins (SFRPs) can inhibit the canonical Wnt signaling. (B) Wnt/planar cell polarity (PCP) signaling pathway. Aggregation of disheveled associated activator of morphogenesis 1 (DAAM1) activates Rho or Rac and subsequently regulates the transcription of target genes. (C) Wnt/Ca2+ signaling pathway. The activation of frizzled (FZD) stimulates the release of calcium (Ca2+), which in turn promotes the signaling of calmodulin-dependent protein kinase II (CamK II) and nuclear factor of activated T cell (NFAT) pathways, leading to changes in gene transcription to affect the biological processes such as cell migration and adhesion. APC, adenomatous polyposis coli; CK1, casein kinase1; DAG, diacylglycerol; DVL, dishevelled; GSK3β, glycogen synthase kinase-3β; IP3, inositol 1,4,5-trisphosphate; JNK, c-Jun N-terminal kinase; LEF, lymphoid-enhancing factor; LRP5/6, LDL receptor-related protein 5/6; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; ROCK, Rho-associated protein kinase; ROR2, receptor tyrosine kinase-like orphan receptor; RYK, receptor-like tyrosine kinase; TCF, T-cell factor.
Canonical Wnt signaling has been shown to be the key to the differentiation of invasive trophoblasts in the early placenta (86), and the expression of TCF-3/4 and β-catenin in these cells is markedly increased (87). In addition, a large number of studies have found that the normal transduction of Wnt/β-catenin signals is essential for mammalian placental development. In mouse experiments, the deletion of some components in the canonical Wnt pathway, such as the deletion of R-spondin3 (a soluble activator of the canonical Wnt pathway) or the deletion of both TCF-1 and LEF-1, will lead to the failure of interaction between chorion and allantois, resulting in severe defects in placental formation (88, 89). In bovine trophoblasts, β-catenin showed high levels of cellular accumulation and nuclear translocation, particularly in binucleate trophoblasts (90). Furthermore, extracellular factors such as dickkopfs (Dkks) and secreted Frizzled-related proteins (SFRPs) can inhibit canonical Wnt signaling by binding to Wnt ligands or the LRP family of Wnt coreceptors. Previous studies have shown that SFRP1-4 is expressed in the placenta throughout pregnancy, mainly in the villous syncytiotrophoblasts and the extravillous trophoblasts (91–93). In a coculture setting of trophoblasts and decidua cells, the close proximity between the 2 types of cells induces the enhanced production and action of Wnt antagonists in trophoblasts to regulate canonical Wnt signaling (94). In studies on pathological pregnancy, compared with normal pregnancy, patients with pre-eclampsia showed abnormal expression of Wnt-2, β-catenin, Dkk1, and SFRP4 in the placenta (93, 95). In the placenta of the rat IUGR model, the upregulation of SFRP4 reduced the entry of β-catenin into the nucleus, leading to suppression of the Wnt signal and negative influence on VEGF expression in the labyrinth zone of the placenta (96). Moreover, smoking-induced inhibition of Wnt signaling in the placenta of the IUGR fetus was accompanied by a significant increase in SFRP1 expression, and impaired proliferation of trophoblast cells (91). These results confirmed the important contribution of the canonical Wnt signaling pathway to placental development and function, whereas the placental Wnt signal in pathological pregnancy was abnormally regulated by antagonists such as SFRP1, SFRP4 and Dkk1, suggesting the molecular regulatory mechanism of Wnt signaling may be one of the most important factors affecting placental angiogenesis, but the specific mechanism needs to be further explored.
Aside from the aforementioned canonical Wnt signaling pathway, the role of the noncanonical Wnt signaling pathway in placental angiogenesis has also attracted more and more attention. Related studies have found that relevant Wnt ligands derived from endothelial cells capable of causing noncanonical Wnt signal transduction are regulatory factors for endothelial cell survival, proliferation, and subsequent vascular pruning during developmental and pathological angiogenesis, with an important role in vessel remodeling (97). Fourteen out of 19 Wnt proteins have been reported to be expressed in human placental tissues, including Wnt-1, -2, -2b, -3, -4, -5a, -5b, -6, -7a, -7b, -9b, -10a, -10b, and -11, with different expression patterns in various stages of placental development and angiogenesis (98), forming a highly complex signal transduction network with receptors and their upstream and downstream signal transduction components. However, there is no clear consensus on which ligand-receptor interactions activate which pathways (canonical or noncanonical, etc.).
Table 1 summarizes the reports on the expression of these Wnt ligands in placental tissues and their relation with placental angiogenesis. The high expression of Wnt-1, -7b, -10a, and -10b in first trimester cytotrophoblasts (98) may suggest that these ligands play specific roles in early placental function and differentiation, such as regulating cell proliferation and migration. In the human placenta, Wnt-2 is expressed at all stages of placental development (93, 98). The downregulation or deletion of Wnt-2 expression may impair placental function and normal angiogenesis, and is related to pregnancy complications (99–101). Additionally, Wnt-3a can stimulate the invasion and migration of trophoblasts and the secretion of matrix metalloproteinase-2, involving the canonical Wnt pathway (86, 87). Interestingly, Wnt-3a can also promote the movement of trophoblast cells by activating the β-catenin-independent PI3K-AKT pathway (86). Wnt-4, produced by cytotrophoblast cells as well as extravillous trophoblast cells, can promote endometrial stromal decidualization by activating β-catenin (102). In the human placenta, Wnt-5a, the Wnt ligand with the highest expression in both trophoblast and decidua cells (94), can be secreted by different decidual and placental cell types (103). Wnt-5a regulates downstream transcription factors by initiating the noncanonical signaling pathway or regulating the canonical signaling pathway to finely coordinate the self-localization of cells and the connecting mechanism between endothelial cells, thereby promoting cell adhesion and migration (103–105), indicating that Wnt-5a may be a key regulator of placental development and angiogenesis, but the detailed mechanism is still unclear. Moreover, Wnt-7a can activate canonical and noncanonical Wnt pathways, depending on the type of FZD receptor binding to it on the cell (106). Wnt-11 can bind to FZD and activate DVL independently of LRP-5 or -6, thereby activating the Wnt/PCP and Wnt/Ca2+ pathways, and inhibiting the canonical pathway (107, 108).
TABLE 1.
Summary of relevant reports on the role of the Wnt ligand in placental angiogenesis1
| Description | Reference |
|---|---|
| Wnt-1 | |
| Wnt-1 is mainly expressed in first trimester cytotrophoblasts, differentiated extravillous trophoblasts, and villous fibroblasts. | (98) |
| Wnt-2 | |
| Wnt-2 is expressed at all stages of placental development in human placenta. | (93, 98) |
| The birth of intrauterine growth restriction fetuses is associated with high DNA methylation levels of WNT-2 in human placenta,which is accompanied by epigenetic downregulation of placental Wnt-2 expression. | (99, 100) |
| Wnt-2 deletion resulted in edema and disruption of the labyrinthine zone in the placenta of mice, accompanied by anapparent decrease in the number of capillaries. | (101) |
| Wnt-3a | |
| Wnt-3a can stimulate the invasion and migration of trophoblasts and the secretion of matrix metalloproteinase-2, involving thecanonical Wnt pathway. Wnt-3a can also promote trophoblast cell movement by activating the β-catenin-independent PI3K-AKT pathway. | (86) |
| Wnt-3a can promote the invasion and migration of trophoblasts, which can be blocked by the addition of dickkopf-1. | (87) |
| Wnt-4 | |
| Wnt-4, produced by cytotrophoblast cells as well as extravillous trophoblast cells, promotes endometrial stromaldecidualization by activating β-catenin. | (102) |
| Wnt-4 can activate noncanonical Wnt signaling pathways independently of LRP-5/6. | (98) |
| Wnt-5a | |
| In human placenta, Wnt-5a can be secreted by different decidual and placental cell types. Wnt-5a secreted by villous and decidual stromal cells can promote the proliferation and survival of trophoblast cells byactivating the MAPK pathway. | (103) |
| Wnt-5a is the Wnt ligand with the highest expression in both trophoblast and decidua cells. | (94) |
| Wnt-5a can directly inhibit the canonical pathway in a similar Ca2+-independent manner or activate the Wnt/β-cateninsignaling pathway in the presence of FZD-4 and LRP-5. | (105) |
| Wnt-5b | |
| Wnt-5b can activate the noncanonical Wnt signaling pathway independently of LRP-5/6. | (98) |
| Wnt-7a | |
| Wnt-7a can activate canonical and noncanonical Wnt pathways, depending on the type of FZD receptor that binds to it on thecell.Secreted Frizzled-related protein 4 can directly bind to Wnt-7a, thus inhibiting the activation of Wnt/β-catenin signaling inboth an autocrine and paracrine manner. | (106) |
| Wnt-7b | |
| Wnt-7b is mainly expressed in first trimester cytotrophoblasts. | (98) |
| The homozygous mutation of Wnt-7b in mice resulted in placental abnormality and death at midgestational stages. | (109) |
| Wnt-10a | |
| Wnt-10a is mainly expressed in first trimester cytotrophoblasts but is absent in differentiated extravillous trophoblasts andvillous fibroblasts. | (98) |
| Wnt-10b | |
| Wnt-10b is mainly expressed in first trimester cytotrophoblasts but is absent in differentiated extravillous trophoblasts andvillous fibroblasts. | (98) |
| Wnt-11 | |
| Wnt-11 can bind to FZD and activate dishevelled independently of LRP-5 or -6, thereby activating Wnt/Planar cell polarity andthe Wnt/Ca2+ pathways and inhibiting the canonical pathway. | (107, 108) |
AKT, V-Akt murine thymoma viral oncogene homolog; FZD, frizzled; LRP, LDL receptor-related protein; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3-kinase.
In summary, studies involving the deletion or inhibition of various components of the Wnt signaling pathway have shown various defects in the placenta, especially those related to angiogenesis, indicating the dependence of placental vascular development on the Wnt signaling pathway.
Hedgehog signaling pathway
The Hedgehog (Hh) pathway is one of the important signaling mechanisms to regulate cell growth and differentiation during embryonic development. The Hh gene, encoding a secretory signaling protein, was first identified in Drosophila as a segment-polarity gene, and the Hh family of secreted proteins, Sonic Hedgehog (Shh), Indian Hedgehog, and Desert Hedgehog, have been found in mammals. Recent studies have demonstrated the existence of both canonical and noncanonical Hedgehog signaling pathways.
The components of the canonical Hedgehog signaling pathway include Hh ligands, receptor complexes composed of the 7-transmembrane protein smoothened (Smo) and the 12-transmembrane domain receptor Patched (Ptch), and downstream transcription factor glioma-associated oncogene homologues (GLI; including GLI-1, GLI-2, and GLI-3) (110–112). In the absence of Hh ligands, Ptch can inhibit the activity of Smo (112). GLI, the intracellular signal molecule of the Hh signal pathway, forms a large protein complex with the kinesin-related protein costal 2 (Cos2), the protein kinase Fused (Fu), and suppressor of Fused (SuFu), which binds to microtubules (110, 113). A proteolytic process regulates the activity of GLI-3 and GLI-2. In the absence of Hh stimulation, proteolytic events remove the transactivation domain of GLI-3 in the primary cilium (114, 115), leading to the transport of the GLI-3 repressor protein into the nucleus to perform transcriptional repressor functions. In the presence of Hh ligands, the binding of an Hh ligand to Ptch causes Ptch internalization, thereby abolishing the inhibitory effect of Ptch on Smo and causing activation of the Hh signaling pathway (110, 112, 116). Deregulated Smo can inhibit the downstream proteolytic processing of GLI-2 and GLI-3, resulting in the production of full-length activated GLI proteins from the complex and the transfer of full-length GLI into the nucleus, thereby promoting the expression of Hh target genes (115–117).
Three Hh ligands can all activate downstream signaling cascades of the Hedgehog canonical pathway in a similar manner (118, 119), with the Shh ligand, a mitogen, as a key component of the Hedgehog signaling pathway in regulating specific cell proliferation and differentiation. Studies have shown that Hh signaling plays an important role in the regulation of angiogenesis and embryo growth during embryonic development. However, Hh does not signal directly to endothelial cells via the canonical pathway to modulate their behavior but mediates them by other regulatory factors (119, 120). In fact, none of the Hh ligands can induce GLI target genes in endothelial cells (111). Studies have found that Shh ligands induce the synthesis of proangiogenic cytokines (such as VEGF, ANG-1, etc.) in tissue fibroblasts through classical pathways, thus promoting the formation of new blood vessels (121). Through overexpression of Shh in low Hh-expressing DLD-1 xenografts, canonical Hh signaling was found to enhance tumor angiogenesis by inducing the expression of VEGF-A in stromal perivascular cells (122). Instead of functioning as an on-off switch for VEGF expression, Hh maintains VEGF expression levels within the very tight boundaries required for normal endothelial development (120). Additionally, recent reports have shown that the expression of Smo and GLI-1 genes in canonical Hh signaling pathways is mediated by basic FGF (bFGF), which in turn promotes fibroblast migration (114).
Furthermore, the response of cells and tissues to Hh ligands also occurs through noncanonical pathways. Studies have found that direct regulatory effects in endothelial cells can be produced by the noncanonical pathways of Hh ligands (GLI-independent pathways), including 1) Hh signaling via activation of Ptch and Smo in an GLI-independent manner to stimulate tubulogenesis; 2) Hh signaling via activation of Ptch in an GLI- and Smo-independent way, mainly involved in cell apoptosis and proliferation (111, 114, 115). Endothelial cells derived from yolk sac-derived endothelial cells and primary embryonic fibroblasts in an in vitro wound-healing assay can directly respond to Shh stimulation by enhancing migration (118). Three Hh isoforms have been reported to stimulate the small GTPase RhoA independently of GLI in endothelial cells, which is implicated in tubulogenesis in response to VEGF by affecting the reorganization of the actin cytoskeleton and cell contraction of 3-dimensional collagen I (111, 123). The activity of the Rho/Rho-associated protein kinase (ROCK) pathway is necessary for tubulogenesis induced by Shh. Moreover, endothelial cell migration and tube formation can be directly enhanced by increasing osteopontin and matrix metalloproteinase-9 expression via ROCK modulation (123).
Collectively, the canonical Hh signaling pathway has no direct regulatory effect on endothelial cells, but indirectly regulates their activity by influencing other proangiogenic factors. In contrast, Hh signaling can act on endothelial cells through noncanonical pathways, thereby directly modulating the phenotype and angiogenesis of endothelial cells.
Regulatory Effects of Functional Nutrients on Placental Angiogenesis
Functional amino acids
Functional amino acids, the amino acids that participate in and regulate key metabolic pathways and are precursors of many low-molecular-weight substances with enormous physiological importance, play an important role in the healthy growth and production of organisms (124). The arginine family of amino acids (AFAA), which belongs to the functional amino acids group, plays an important role in promoting placental angiogenesis and growth. AFAA, including arginine, citrulline, glutamic, glutamine, proline, and ornithine, have important physiological, metabolic, nutritional, and immune functions, and can be interconvertible in most mammals via complex interorgan-metabolic pathways (125–127). They stimulate placental growth (including angiogenesis) and the transfer of nutrients from mother to fetus by regulating protein synthesis and the production of metabolites such as NO and polyamines (126–129). These metabolites play important roles in the growth and development of placentas and embryos/fetuses during gestation by regulating angiogenesis, utero-placental blood flow and nutrient transport, cell proliferation and migration, and tissue formation (21, 128, 130).
Arginine, a conditionally essential amino acid in humans and mammals, affects placental growth and development, promoting placental angiogenesis and growth, improving placental blood circulation, and increasing nutrient transport from mother to fetus during pregnancy, which ultimately affects the development and survival of embryos or fetuses (131–133). The complex metabolic pathways of arginine have been well characterized (127, 129), so this review will not describe them excessively. Some of the arginine not used for protein synthesis can be metabolized to ornithine in the body as the precursor for the synthesis of proline and polyamines (putrescine, spermidine, and spermine) (134, 135). Besides, arginine, the sole endogenous source of NO, can synthesize NO through the metabolic pathway of NOS (130, 136). Both humans and mammals are seriously threatened by IUGR during pregnancy. There is evidence that IUGR is associated with the reduced transport of amino acids and decreased synthesis of polyamines and NO in the placenta (130, 137, 138). A large number of studies have shown that arginine supplementation can significantly improve the synthesis of placental NO and polyamines to regulate a number of critical mediators of angiogenesis at the interface of endothelial cells (as well as placental cytotrophoblasts and syncytiotrophoblasts), including ornithine decarboxylase, eNOS, VEGFs (e.g., VEGF-A and PIGF), VEGFRs (e.g., VEGFR-1, VEGFR-2, and sVEGFR-1), and angiopoietin (e.g., angiopoietin-1 and angiopoietin-2) (133, 138–143). NO, a major vasodilator and angiogenic factor in vivo, is involved in the regulation of placental angiogenesis and blood flow (138). In the metabolic pathway of arginine NO synthase, arginine can mainly increase the activity of NOS and the production of NO in the placenta by promoting the synthesis and bioavailability of tetrahydrobiopterin (an essential cofactor for NO synthesis from arginine) (130, 136). With the increasing demand for biosynthesis of NO during pregnancy, the expression of eNOS in the placenta increases significantly, mainly located in syncytiotrophoblasts and vascular endothelial cells (144, 145). The increase of NO production during pregnancy contributes to the maintenance of low fetoplacental vascular resistance, and has a certain relation with the increase of uteroplacental blood flow (145). Additionally, NO synthesis and arginine transport in the placenta increases significantly during early pregnancy, with a second small peak present in late gestation, which corresponds to the continuous transfer of nutrients and oxygen and the rapid formation of placental blood vessels to support the rapid development of fetuses (146–148). The putrescine metabolic synthesis pathway in mammalian placentas involves arginase and arginine decarboxylase, which can metabolize arginine to ornithine and agmatine, respectively, and subsequently be converted into putrescine (129, 134). What is unusual is a lack of arginase activity in the pig placenta, and the synthesis of polyamines from arginine has not been detected (149), suggesting that the porcine placenta cannot use arginine to synthesize polyamines. Therefore, the lack of arginase in the porcine placenta maximizes the placental transfer of arginine from maternal and fetal blood to ensure the rapid growth and development of the fetus. In contrast, ovine placentas possess high activities of arginase and ornithine decarboxylase (the enzyme that converts ornithine to putrescine), and arginine can be actively converted into polyamines in ovine placental tissues (150).
Proline regulates the angiogenesis of placentas by synthesizing polyamines. As mentioned above, the special situation of porcine placenta makes it unable to synthesize polyamines directly from arginine, so the major amino acid for the synthesis of putrescine, spermidine, and spermine in porcine placenta is not arginine, but proline. Studies have shown that proline in the porcine placenta can be extensively catabolized by proline oxidase and ornithine aminotransferase to yield intermediate Δ1-pyrroline-5-carboxylate and then ornithine (149). Moreover, the concentrations of polyamines are positively correlated with proline oxidase activity and polyamine synthesis in the placenta (149). Previous reports have shown that mice fed a diet supplemented with 0.50% proline from day 0.5 to day 12.5 of gestation showed elevated mRNA levels of VEGF, VEGFR, NOS2, and NOS3 in the placenta (151), suggesting the dietary supplementation of proline may regulate placental angiogenesis by stimulating angiogenic factors.
Glutamine catabolism has recently been reported to be essential for the proliferation and migration of endothelial cells and vessel sprouting. Numerous studies have shown that glutamine is transported to the fetus in large quantities through the placenta, but few reports have shown the actions and mechanisms of glutamine in placental development and angiogenesis. The placental intake of glutamine and glutamate decreased in the IUGR fetus compared with normal fetus (152). Glutamine, the donor of most of the carbon in the tricarboxylic acid cycle and the nitrogen of some nonessential amino acids, maintains the growth of endothelial cells and is consumed heavily by the proliferating endothelial cells (153, 154). Glutamine can be hydrolyzed into ammonia and glutamate by glutaminase. Inhibiting the expression of glutaminase 1 in vitro or in vivo impaired endothelial cell sprouting, proliferation, and migration, and reduced the competitiveness of tip cells in vessel sprouting (153, 154). In addition, glutamine is used for de novo asparagine synthesis by asparagine synthetase in endothelial cells, and the inhibition of de novo asparagine synthesis or asparagine uptake will reduce endothelial cell proliferation and vessel sprouting (154). These results show the basic role of glutamine and its metabolic synthesis in angiogenesis, but the precise mechanism by which glutamine and its metabolites regulate endothelial cell motility and their specific role in the placenta remains to be studied.
The role of AFAA and their metabolites in placental angiogenesis is also related to the activation of mTOR. Functional amino acids also regulate DNA and protein synthesis in mammalian placentas, uteruses, and fetuses through the mTOR signaling pathway, affecting cell proliferation, migration, and differentiation, which are necessary for angiogenesis, growth of trophectoderm and placenta, and embryonic development. mTOR is a key nutrient sensor to support placental growth. Arginine stimulates the growth and protein synthesis of porcine conceptus trophectoderm cells in vitro, involving the activation of the mTOR cell signaling pathway (143). Phosphorylation of mTORC1 was enhanced in the placenta of mice supplemented with proline, whereas phosphorylation of GCN2, the negative regulator of mTORC1, was inhibited (151). In porcine trophectoderm cells, supplementation with putrescine can increase protein synthesis via the activation of the mTOR signaling pathway, thus promoting cell proliferation (128). Similarly, glutamine can also regulate the phosphorylation of the mTOR signaling pathway and promote protein synthesis in the placenta (154). At present, although how these amino acids stimulate mTOR signaling transduction has not been clarified, the role of functional amino acids in placental angiogenesis and growth cannot be ignored, and in-depth analysis of their complex regulatory mechanisms will provide new insights into dietary amino acid supplementation.
In addition to the above-mentioned AFAA, the effects of other functional amino acids such as tryptophan and tyrosine on placental angiogenesis cannot be ignored. Tryptophan, a precursor for physiologically active metabolites such as serotonin and melatonin, is also involved in fetal growth, placental development, and regulation of placental function. During the first trimester, the human placenta preferentially metabolizes tryptophan to serotonin, which is involved in fetal development and the synthesis of downstream metabolites such as melatonin (155). Local synthesis of melatonin and expression of its receptor have been confirmed in human placental tissues and villous trophoblasts (156). Studies on dietary melatonin supplementation in pregnant ewes have shown that melatonin can improve the umbilical artery blood flow of a normal or IUGR fetus to maintain fetal growth (157), and increase the sensitivity of the feto-placental artery to vasorelaxation in response to the endothelium-dependent vasodilator bradykinin (158). Bradykinin is actively secreted in the porcine uterus of early pregnancy and plays a potential role in porcine embryonic development and placental angiogenesis (159). Similar to VEGF, bradykinin can also regulate angiogenesis by stimulating NO, with multiple interactions between bradykinin-activated transmembrane signals and VEGF signals to converge in the eNOS-NO pathway (27).
Tyrosine, a precursor to the production of naturally occurring catecholamines, includes norepinephrine, epinephrine, and dopamine. The study of tumor angiogenesis has shown that norepinephrine and epinephrine stimulate tumor angiogenesis, but it is the opposite for dopamine (160). Meanwhile, both norepinephrine and epinephrine mostly inhibit the angiogenic process in cutaneous wounds, whereas dopamine produces positive or negative effects on angiogenesis depending on the type of dopamine receptor involved (161), which means that the effectiveness of catecholamines on angiogenesis is affected by the physiological status and the receptor type. Related reports have confirmed the secretion of catecholamines in ovine trophectoderm cells, and catecholamines can promote the synthesis of polyamines, as well as cell proliferation and migration (162). In a study of a mouse knockout model, deletion of the α2B-adrenoceptor gene was shown to cause defective angiogenesis in the placental labyrinth, which may be related to the failure to inhibit VEGFR-1/sVEGFR-1 expression by α2B-adrenoceptor signaling (163). These results suggest that catecholamines may play a crucial role in placental angiogenesis.
In summary, current studies have shown that the regulation of functional amino acids on placental angiogenesis is mainly achieved by promoting the synthesis of their metabolites (such as NO, polyamines, melatonin, and catecholamines) to regulate tissue growth, thus affecting placental angiogenesis and growth. In addition, AFAA can stimulate placental protein synthesis by activating mTOR cell signals, but how these amino acids activate signal transduction in this pathway is still unclear.
Fatty acids
A growing number of studies have found that fatty acids also play an important role in regulating angiogenesis in tumors and organs including the placenta. Essential fatty acids, including linoleic acid and α-linolenic acid, belong to the n–6 (ω-6) and n–3 (ω-3) families, respectively, and other fatty acids can be synthesized gradually from them. They play crucial biological roles in the body, including in the structure and function of cell membranes, modulating the expression of genes related to homeostasis in cells, and acting as precursors to some signaling molecules (164–166). Moreover, dietary essential fatty acids (including linoleic acid and α-linolenic acid) need to be converted into their long-chain metabolites in the body to exert their comprehensive biological actions. In general, n–3 long-chain PUFAs (LCPUFAs), including EPA and DHA, are generally supposed to have potent antiangiogenic effects in cancer cells and other cell systems, inhibiting the production of many important angiogenic factors, such as VEGF, platelet-derived growth factor, PGE2, and NO (167–169). Meanwhile, n–6 LCPUFAs, including arachidonic acid (AA or ARA), have proangiogenic effects, encouraging the transcription of angiogenic growth factors, increasing the migration and proliferation of endothelial cells, and promoting angiogenesis (169–171). Apart from an antiangiogenic role in cancer cells, in normal physiological angiogenesis, n–3 LCPUFAs are also shown to downregulate VEGFR-2 in endothelial cells, thus weakening the VEGF signal (172, 173). A large number of cell experiments in vitro have shown that EPA and DHA can significantly suppress the proliferation, migration, and tubule formation of endothelial cells (172–176), indicating the antiangiogenic effect of n–3 fatty acids. These fatty acids can influence angiogenesis via a variety of mechanisms. In addition to regulating the expression of the aforementioned angiogenic factors, they can also affect the expression of other regulatory factors, such as eicosanoids, cyclo-oxygenase, fatty acid-binding proteins (FABPs), and NO (167, 175, 177).
Recent studies have found that, contrary to the antiangiogenic effect in cancer cells and some other cellular systems, n–3 LCPUFAs can promote the secretion of VEGF by some placental cells, which is essential for fetal growth and early placental formation (178). Studies on the effects of n–3 LCPUFA intake by pregnant women in India on the placenta and fetus have shown a positive correlation between placental weight and placental DHA concentrations of preterm fetuses, but an inverse correlation of n–3 fatty acid deficiency with placental angiogenesis, vasculogenesis, and its size (179, 180). Compared with other fatty acids, DHA uptake is relatively abundant for placental trophoblast cells in the first and third trimester of pregnancy (181, 182). Johnsen et al. (183) observed that DHA can stimulate mRNA expression and protein secretion of VEGF in first trimester trophoblast cells (HTR8/SVneo cell line). Furthermore, DHA promotes tube formation by increasing capillary tube length and tube numbers of extravillous trophoblast cells (182), suggesting that DHA may affect placental formation by stimulating VEGF-mediated angiogenesis in placental trophoblastic cells in early pregnancy, but the specific mechanism needs to be further explored.
Different from the effect of DHA on VEGF expression, other fatty acids [oleic acid (OA), EPA, AA] can stimulate mRNA expression and protein secretion of angiopoietin-like 4 (ANGPTL4) in HTR8/SVneo cells without affecting the synthesis of VEGF, and their angiogenic effects (as measured by tube formation) were in the order of DHA > EPA > AA > OA (178, 181, 183). The angiogenic effects of AA, EPA, and OA were significantly reduced in ANGPTL4 knocked down cells (181), indicating the involvement of ANGPTL4 in fatty acid-induced angiogenesis. Compared with VEGF, ANGPTL4 has a weaker role in promoting angiogenesis, but the physiological significance of fatty acids in increasing ANGPTL4 expression is still unclear. Furthermore, some data have shown that EPA can promote fetal growth and placental angiogenesis through the SIRT1-independent inflammatory pathway and inhibit the hypoxia inducible factor-1α pathway (184), but the potential role of EPA in angiogenesis needs further investigation. Recent studies have also found that an increase in EPA concentration in the body is accompanied by a decrease in total cholesterol and VLDL cholesterol (185). Moreover, estrogen, a cholesterol derivative found in endothelial cells and smooth muscle cells of the uterine artery, whose receptor activation promotes eNOS activation and NO production, is one of the factors mediating estrogen-induced vasodilation in the pregnant uterus and placenta (186). These reports suggest a potential link between n–3 fatty acids and estrogen, but few studies have been performed on their action in placental blood vessel formation.
Based on the above, we further discuss the causes for the controversy over the role of n–3 LCPUFAs in angiogenesis. Firstly, the effect of n–3 LCPUFAs on angiogenesis may be dose-dependent and related to their mode of use. For example, treatment of mesenchymal stromal cells from placentas with lower concentrations (5 μM) of DHA and EPA (1:1) enhanced their tube formation ability in vitro and upregulated both bFGF and VEGF-A, but showed antiangiogenic effects at high concentrations (187). In addition, supplementation of modified fish oil and/or 5-methyltetrahydrofolate in the diet of pregnant women found that the combination of DHA and folic acid supplementation during pregnancy could stimulate the proliferation of placental trophoblasts, but not DHA or folic acid alone (188). Secondly, different lipid mediators produced by the metabolism of n–3 LCPUFAs vary in their effects on angiogenesis. LCPUFAs can be rapidly metabolized by cyclo-oxygenase, cytochrome P450, and lipoxygenase enzymes to generate a series of lipid mediators (171). In retinal pathological angiogenesis, epoxydocosapentaenoic acid (EDP), a metabolite produced by n–3 LCPUFA through cytochrome P450 epoxygenases (CYP2C8), can promote the production of VEGF-A and enhance the formation of neovascularization (189). On the contrary, EDPs play an inhibitory role in tumor angiogenesis and human umbilical vein endothelial cell migration, with VEGFR-2 as a potential cell target (190), which also suggests that the role of CYP2C8 is tissue specific. Furthermore, PGs produced by cyclo-oxygenase metabolism may also be a potential factor for LCPUFAs in placental angiogenesis. n–3 LCPUFAs are able to inhibit VEGF expression in colon cancer cells possibly through the mechanism of negative regulation of the cyclo-oxygenase-2/PGE2 pathway (167). The PG member PGI2, a critical endothelial vasodilator in the body, increases significantly during pregnancy, but previous studies have found that PGI2 has only a weak vasodilatation effect on human placental microvessels versus nonplacental vessels (191, 192). Similar to PGI2, the bioactive PGI3 metabolized by EPA through cyclo-oxygenase in vascular endothelial cells can also inhibit vasoconstriction and induce neovascularization (193), but the regulatory mechanism of PGI3 in the placenta is still poorly understood. Additionally, a series of metabolites (such as 4-hydroxy-DHA, 15-hydroxy-EPA, and 17-hydroxy-DHA) formed under the action of lipoxygenase can mediate the antiangiogenic effects of n–3 LCPUFAs (171, 194). Recently, placental vascular-associated cell types (such as placental vascular smooth muscle and extravillous trophoblasts) have been shown to express G protein-coupled receptor (GPR) 18 to regulate inflammation by binding to resolvin D2, a specialized pro-resolving lipid mediator derived from n–3 LCPUFAs (195). In view of the correlation between inflammatory stimulation and vascular function, the role of GPR18 and resolvin D2 in the placenta and their relation with pathological pregnancy need to be further studied. Finally, the discrepancies in the action of n–3 LCPUFAs can be attributed to the different characteristics of different organs or cells and their microenvironment under different physiological or pathological conditions. Studies on revascularization after cerebral ischemia show that n–3 LCPUFAs can stimulate astrocytes to release angiopoietin-2, thereby facilitating endothelial proliferation and barrier formation by potentiating VEGF-PLCγ1/Src signaling in endothelial cells (196). In the in vitro culture of mature adipocytes, it was found that EPA could upregulate VEGF-A production and release in adipocytes, especially under treatment with 250 μM EPA (197). These different results in cancer cells, endothelial cells, trophoblastic cells, astrocytes, adipocytes, and other cells may all be related to the different characteristics or states of these cells.
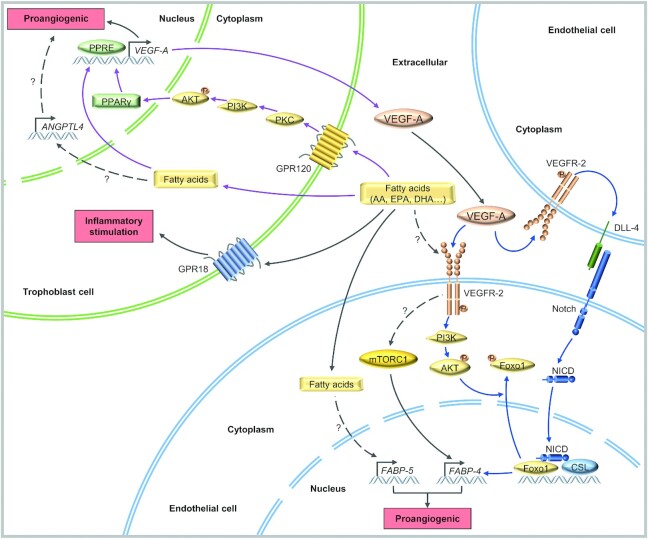
The uptake and metabolism of long-chain fatty acids are tightly regulated by several transport/binding proteins, including FABPs, fatty acid translocase, and fatty acid transport proteins. The cytosolic proteins, FABPs, have the function of binding, transporting, and facilitating the signaling of fatty acids and other lipophilic molecules. FABP-1, -3, -4, -5, and FABPpm have been confirmed to be expressed in trophoblasts and placental tissues (198, 199). In the past decade, FABP-4 has been proven as a novel target of the VEGF/VEGFR-2 pathway and a positive regulator of cell proliferation and angiogenesis in endothelial cells, mainly involved in most VEGF-mediated angiogenesis in endothelial cells (200, 201). Fatty acids, such as EPA and the cis-9, trans-11 isomer of conjugated linoleic acid, were found to promote tube formation in HTR8/SVneo cells, coupled with an increase in FABP-4 expression (181, 202), suggesting that FABP-4 may be an important mediator of fatty acids affecting angiogenesis. FABP-4, which is expressed in the labyrinthine area of mouse placenta and mainly in endothelial cells (203), can promote cell proliferation, migration, lipid accumulation, and morphogenesis, with a role in enhancing the angiogenic responses of endothelial cells (201, 204, 205), indicating that its activity is crucial for placental development and angiogenesis. The study of endothelial cells and placental trophoblast cells revealed that FABP-4 could induce their growth, proliferation, and tubular formation in vitro (205, 206), and a mechanism study unveiled that FABP-4 is induced by VEGF-A through the DLL-4/Notch-dependent pathway and may also be modulated by forkhead box protein O1 phosphorylation induced by AKT activation (207). Inhibition of the DLL-4/Notch signal leads to a decrease in FABP-4 response to VEGF-A, and FABP-4 transcription is induced by NICD release (207). Additionally, mTORC1 is also one of the downstream signaling pathways of FABP-4 expression induced by VEGF in endothelial cells, and PI3K/AKT and p38MAPK pathways are not involved in this process, which needs further elucidation (205). Furthermore, FABP-5 has recently been shown to play a role in promoting angiogenic responses of endothelial cells both in vitro and ex vivo, namely promoting endothelial cell proliferation and migration (204). Unlike FABP-4, FABP-5 expression was found to be regulated in a dose-dependent manner by an extracellular lipid mixture rather than VEGF-A or bFGF, and FABP-5 can enhance the death of apoptotic cells under certain conditions (such as serum starvation) (204). Similarly, in the spinal cord injury model, a diet rich in DHA was shown to increase FABP-5 mRNA expression (208), suggesting that fatty acids may be an important factor in inducing the proangiogenic effect of FABP-5. These results indicate that fatty acids may promote early placental angiogenesis by affecting the expression of FABPs, but the mechanism of their connection and the specific role of FABPs in placental angiogenesis need to be further studied.
Peroxisome proliferator-activated receptor (PPAR), a nuclear receptor protein functioning as a transcription factor, can be activated by several LCPUFAs (such as AA, DHA, and linoleic acid) and their metabolites, with an important role in cell differentiation, tissue development, metabolism, and other processes. All PPAR isotypes (PPARα, PPARβ/δ, and PPARγ) were found to be expressed in the placenta, mainly in trophoblasts at high levels, with PPARγ and PPARβ/δ playing key roles during implantation and placentation (209, 210). LCPUFAs (such as DHA and EPA) are known to regulate PPAR expression (211), and PPAR can indirectly participate in placental angiogenesis and regulate the secretion of angiogenic factors (212, 213). However, information is limited about whether PPAR mediates the effect of fatty acids or their derivatives on placental angiogenesis and the specific mechanism. In adipocytes, EPA has been reported to enhance the binding of PPARγ to the PPAR response element (PPRE) in the VEGF-A promoter region, leading to the increased expression of VEGF-A, and this pathway is subject to the synergistic effect of GPR120 (197). GPR120 is also a kind of LCPUFA receptor, whose activation can promote tumor angiogenesis and metastasis, mainly mediated by the PI3K/AKT/NF-κB signaling pathway (214, 215). Further studies can focus on whether LCPUFAs regulate placental angiogenesis through this pathway.
In summary, n–3 fatty acids and n–6 fatty acids in the placenta can jointly regulate angiogenesis, but controversy remains on the pro/antiangiogenic effect of n–3 fatty acids in the placenta. Additionally, the regulatory mechanism involved between fatty acids and placental angiogenesis can be speculated to be related to the mediating role of FABPs and PPARs (Figure 3), which needs to be confirmed by further studies.
FIGURE 3.
The proposed molecular mechanisms by which fatty acids regulate placental angiogenesis. Fatty acids, such as arachidonic acid (AA), EPA, and DHA, activate G-protein coupled receptor 120 (GPR120) on trophoblasts, thereby inducing activation of the PI3K/AKT signaling pathway. In the downstream cascade, the binding of peroxisome proliferator-activated receptor γ (PPARγ) to the PPAR-response element (PPRE) in the vascular endothelial growth factor-A (VEGF-A) promoter region is specifically enhanced (purple arrow). Note that fatty acids can directly activate PPARγ to enhance the transcription and release of VEGF-A. VEGF-A induces the expression of fatty acid-binding protein 4 (FABP-4) through delta-like ligand 4 (DLL-4)-Notch signal transduction between endothelial cells and is negatively regulated by forkhead box protein O1 (Foxo1) phosphorylation and cytoplasmic translocation induced by AKT activation (blue arrow). Additionally, fatty acids activate the GPR18 and mammalian target of rapamycin complex 1 (mTORC1) and regulate the expression of angiopoietin-like 4 (ANGPTL4) and FABP-5, which may also affect placental angiogenesis, although their exact molecular mechanisms have not been determined. AKT, V-Akt Murine Thymoma Viral Oncogene Homolog; CSL, CBFl/Suppressor of Hairless/Lag-1; NICD, Notch intracellular domain; PI3K, phosphatidylinositol-3-kinase; PKC, protein kinase C. Dashed arrows indicate hypothetical relations not studied directly.
Conclusion
A series of studies on the mechanism of various signaling pathways and the effects of functional nutrients on placental angiogenesis have laid an important theoretical basis for providing the nutritional environment essential for optimal placental development and angiogenesis. The pathways related to angiogenesis in the placenta include but are not limited to the VEGF, Notch, Wnt, and Hedgehog signaling pathways introduced in this article, and the effectiveness of research on the mechanism of a single signaling pathway is greatly restricted by the complexity of the pathways involved in the angiogenesis process in the placenta, as well as their interrelation and interaction mechanism. To date, although there have been many reports about the regulatory effect of angiogenic factors on the placental angiogenic phenotype, the specific mechanism of signal transduction induced by these angiogenic factors in vitro is difficult to fully prove in vivo since the placenta is an organ with complex tissue structure and highly active synthetic metabolism. Additionally, amino acids and fatty acids are not only used in metabolic synthesis, but also as special functional nutrients to regulate placental angiogenesis. The fact that nutrients are exchanged and transported between mother and fetus through the placenta often leads to the neglect of the functional regulation of nutrients on placental development and angiogenesis. There is no doubt that the anabolism of amino acids plays an important role in placental angiogenesis, but the activation mechanism of amino acids in the mTOR signaling pathway needs to be further explored. For fatty acids, n–3 and n–6 fatty acids have been shown to have effects on angiogenesis, but specific pathways and key regulatory mediators have not been clearly identified. Moreover, the controversy of fatty acids in placental tissues and cells is different from previous research results, involving potential determinants such as dose ratio, intermediates, and microenvironment, which can be used as strategic guides for future research. Finally, in view of the potential relation between maternal nutrition and placental angiogenesis, apart from ensuring the health of the mother during pregnancy, it is necessary to improve nutrition by adding some special nutrients to promote placental growth and fetal development. Taken together, more research is needed to gradually deepen our understanding of the effects of functional nutrients on the regulation mechanism of placental angiogenesis.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—all authors: wrote and revised the manuscript, and read and approved the final manuscript.
Notes
Supported by the Project of National Natural Science Foundation China (No. 31790411 and 31902165) and Natural Science Foundation of Guangdong Province (2019A1515011443).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AA, arachidonic acid; AFAA, the arginine family of amino acids; AKT, V-Akt murine thymoma viral oncogene homolog; ANGPTL4, angiopoietin-like 4; Dkk, dickkopf; DLL, delta-like ligand; DVL, dishevelled; EDP, epoxydocosapentaenoic acid; eNOS, endothelial NO synthase; FABP, fatty acid-binding protein; FGF, fibroblast growth factor; FZD, frizzled; GLI, glioma-associated oncogene homologues; GPR, G protein-coupled receptor; Hh, Hedgehog; IUGR, intrauterine growth restriction; LCPUFA, long-chain PUFA; LEF, lymphoid-enhancing factor; LRP, LDL receptor-related protein; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NICD, Notch intracellular domain; OA, oleic acid; PCP, planar cell polarity; PI3K, phosphatidylinositol-3-kinase; PIGF, placental growth factor; PLCγ, phospholipase-Cγ; PPAR, peroxisome proliferator-activated receptor; PPRE, PPAR-response element; Ptch, a 12-transmembrane domain receptor Patched; ROCK, Rho-associated protein kinase; SFRP, secreted Frizzled-related protein; Smo, 7-transmembrane protein smoothened; TCF, T-cell factor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Contributor Information
Zihao Huang, Guangdong Laboratory of Lingnan Modern Agriculture, Guangdong Provincial Key Laboratory of Animal Nutrition Control, and National Engineering Research Center for Breeding Swine Industry, College of Animal Science, South China Agricultural University, Guangzhou, China.
Shuangbo Huang, Guangdong Laboratory of Lingnan Modern Agriculture, Guangdong Provincial Key Laboratory of Animal Nutrition Control, and National Engineering Research Center for Breeding Swine Industry, College of Animal Science, South China Agricultural University, Guangzhou, China.
Tongxing Song, Huazhong Agricultural University, College of Animal Science and Technology, Wuhan, China.
Yulong Yin, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, Hunan, China.
Chengquan Tan, Guangdong Laboratory of Lingnan Modern Agriculture, Guangdong Provincial Key Laboratory of Animal Nutrition Control, and National Engineering Research Center for Breeding Swine Industry, College of Animal Science, South China Agricultural University, Guangzhou, China.
References
- 1. Umapathy A, Chamley LW, James JL. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis. 2020;23(2):105–17. [DOI] [PubMed] [Google Scholar]
- 2. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CP, Bajoria R, Aplin JD. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am J Obstet Gynecol. 2002;187(3):764–9. [DOI] [PubMed] [Google Scholar]
- 4. Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175(6):1534–42. [DOI] [PubMed] [Google Scholar]
- 5. Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995;172(2):518–25. [DOI] [PubMed] [Google Scholar]
- 6. Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol Diabetes. 1994;102(03):122–34. [DOI] [PubMed] [Google Scholar]
- 7. Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–4. [DOI] [PubMed] [Google Scholar]
- 8. Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–52. [DOI] [PubMed] [Google Scholar]
- 9. Hu C, Yang Y, Deng M, Yang L, Shu G, Jiang Q, Zhang S, Li X, Yin Y, Tan Cet al. . Placentae for low birth weight piglets are vulnerable to oxidative stress, mitochondrial dysfunction, and impaired angiogenesis. Oxidative Medicine and Cellular Longevity. 2020;2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu C, Yang Y, Li J, Wang H, Cheng C, Yang L, Li Q, Deng J, Liang Z, Yin Yet al. . Maternal diet-induced obesity compromises oxidative stress status and angiogenesis in the porcine placenta by upregulating Nox2 expression. Oxid Med Cell Longev. 2019;2019:2481592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(2–3):127–39. [DOI] [PubMed] [Google Scholar]
- 12. Mayhew TM, Wijesekara J, Baker PN, Ong SS. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta. 2004;25(10):829–33. [DOI] [PubMed] [Google Scholar]
- 13. Escudero C, Roberts JM, Myatt L, Feoktistov I. Impaired adenosine-mediated angiogenesis in preeclampsia: potential implications for fetal programming. Front Pharmacol. 2014;5:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138(6):895–902. [DOI] [PubMed] [Google Scholar]
- 15. Anderson CM, Lopez F, Zimmer A, Benoit JN. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod. 2006;74(3):538–44. [DOI] [PubMed] [Google Scholar]
- 16. Cristofolini A, Fiorimanti M, Campos M, Sanchis E, Diaz T, Moschetti E, Merkis C. Morphometric study of the porcine placental vascularization. Reprod Domest Anim. 2018;53(1):217–25. [DOI] [PubMed] [Google Scholar]
- 17. Burton GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33(Suppl):S23–7. [DOI] [PubMed] [Google Scholar]
- 18. Campos PH, Silva BA, Donzele JL, Oliveira RF, Knol EF. Effects of sow nutrition during gestation on within-litter birth weight variation: a review. Animal. 2012;6(5):797–806. [DOI] [PubMed] [Google Scholar]
- 19. McIntyre KR, Hayward CE, Sibley CP, Greenwood SL, Dilworth MR. Evidence of adaptation of maternofetal transport of glutamine relative to placental size in normal mice, and in those with fetal growth restriction. J Physiol. 2019;597(19):4975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krombeen SK, Shankar V, Noorai RE, Saski CA, Sharp JL, Wilson ME, Wilmoth TA. The identification of differentially expressed genes between extremes of placental efficiency in maternal line gilts on day 95 of gestation. BMC Genomics. 2019;20(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stenhouse C, Hogg CO, Ashworth CJ. Associations between fetal size, sex and placental angiogenesis in the pig. Biol Reprod. 2019;100(1):239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588(3):527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borowicz P, Reynolds LP. Placental programming: more may still be less. J Physiol. 2010;588(3):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73(6):1839–51. [DOI] [PubMed] [Google Scholar]
- 26. Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70(6):1806–13. [DOI] [PubMed] [Google Scholar]
- 27. Valdes G, Corthorn J. Review: the angiogenic and vasodilatory utero-placental network. Placenta. 2011;32(Suppl 2):S170–75. [DOI] [PubMed] [Google Scholar]
- 28. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–7. [DOI] [PubMed] [Google Scholar]
- 29. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–25. [DOI] [PubMed] [Google Scholar]
- 30. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13(1):18–32. [DOI] [PubMed] [Google Scholar]
- 31. Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39(5):469–78. [DOI] [PubMed] [Google Scholar]
- 32. Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–7. [DOI] [PubMed] [Google Scholar]
- 33. Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437(2):169–83. [DOI] [PubMed] [Google Scholar]
- 34. Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72(6):835–46. [DOI] [PubMed] [Google Scholar]
- 35. Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim M, Park HJ, Seol JW, Jang JY, Cho YS, Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya Met al. . VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med. 2013;5(9):1415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22(7):267–72. [DOI] [PubMed] [Google Scholar]
- 38. Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272(13):8474–81. [DOI] [PubMed] [Google Scholar]
- 39. Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272(50):31515–24. [DOI] [PubMed] [Google Scholar]
- 40. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7(4):261–9. [DOI] [PubMed] [Google Scholar]
- 41. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Frontiers in Molecular Neuroscience. 2011;4. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8(4):287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Z-Z, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–31. [DOI] [PubMed] [Google Scholar]
- 44. Zheng J, Wen Y, Song Y, Wang K, Chen DB, Magness RR. Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation. Biol Reprod. 2008;78(1):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol. 2012;227(6):2480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono Fet al. . Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9(7):936–43. [DOI] [PubMed] [Google Scholar]
- 47. Yamaguchi S, Iwata K, Shibuya M. Soluble Flt-1 (soluble VEGFR-1), a potent natural antiangiogenic molecule in mammals, is phylogenetically conserved in avians. Biochem Biophys Res Commun. 2002;291(3):554–9. [DOI] [PubMed] [Google Scholar]
- 48. Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70. [DOI] [PubMed] [Google Scholar]
- 49. Ceci C, Atzori MG, Lacal PM, Graziani G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: experimental evidence in different metastatic cancer models. Int J Mol Sci. 2020;21(4):1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay Det al. . Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99(7):715–22. [DOI] [PubMed] [Google Scholar]
- 51. Zhou Y, Tu C, Zhao Y, Liu H, Zhang S. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: potential implications of inflammation bowel disease. Biochem Biophys Res Commun. 2016;470(4):967–74. [DOI] [PubMed] [Google Scholar]
- 52. Akrami H, Mahmoodi F, Havasi S, Sharifi A. PlGF knockdown inhibited tumor survival and migration in gastric cancer cell via PI3K/Akt and p38MAPK pathways. Cell Biochem Funct. 2016;34(3):173–80. [DOI] [PubMed] [Google Scholar]
- 53. Khaliq A, Dunk C, Jiang J, Shams M, Li XF, Acevedo C, Weich H, Whittle M, Ahmed A. Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for “placental hyperoxia” in intrauterine growth restriction. Lab Invest. 1999;79(2):151–70. [PubMed] [Google Scholar]
- 54. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck Het al. . Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–83. [DOI] [PubMed] [Google Scholar]
- 55. Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci. 1993;90(22):10705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7(2):205–10. [DOI] [PubMed] [Google Scholar]
- 57. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E. Amniotic fluid-soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000;95(3):353–7. [DOI] [PubMed] [Google Scholar]
- 58. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6. [DOI] [PubMed] [Google Scholar]
- 59. Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181(3):393–409. [DOI] [PubMed] [Google Scholar]
- 60. Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–24. [DOI] [PubMed] [Google Scholar]
- 61. Haider S, Meinhardt G, Velicky P, Otti GR, Whitley G, Fiala C, Pollheimer J, Knofler M. Notch signaling plays a critical role in motility and differentiation of human first-trimester cytotrophoblasts. Endocrinology. 2014;155(1):263–74. [DOI] [PubMed] [Google Scholar]
- 62. Levin HI, Sullivan-Pyke CS, Papaioannou VE, Wapner RJ, Kitajewski JK, Shawber CJ, Douglas NC. Dynamic maternal and fetal Notch activity and expression in placentation. Placenta. 2017;55:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima Det al. . VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci. 2007;104(9):3225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–35. [DOI] [PubMed] [Google Scholar]
- 66. Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano Net al. . Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–80. [DOI] [PubMed] [Google Scholar]
- 67. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci. 2015;112(3):761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23(4):543–53. [DOI] [PubMed] [Google Scholar]
- 70. Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107(3):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen Ret al. . Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454(7204):656–60. [DOI] [PubMed] [Google Scholar]
- 72. Funahashi Y, Shawber CJ, Vorontchikhina M, Sharma A, Outtz HH, Kitajewski J. Notch regulates the angiogenic response via induction of VEGFR-1. J Angiogenes Res. 2010;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17(3):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–45. [DOI] [PubMed] [Google Scholar]
- 75. Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizan P, Geudens I, Collins RT, Franco CA, Abrahams CLet al. . Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun. 2015;6:7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99. [DOI] [PubMed] [Google Scholar]
- 77. Dejana E. The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107(8):943–52. [DOI] [PubMed] [Google Scholar]
- 78. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. [DOI] [PubMed] [Google Scholar]
- 79. van Amerongen R, Mikels A, Nusse R. Alternative Wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1(35):re9. [DOI] [PubMed] [Google Scholar]
- 80. Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–77. [DOI] [PubMed] [Google Scholar]
- 81. Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132(20):4421–36. [DOI] [PubMed] [Google Scholar]
- 82. Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(1):a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev. 2018;70(1):68–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11(1):63–9. [DOI] [PubMed] [Google Scholar]
- 85. Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417(6886):295–9. [DOI] [PubMed] [Google Scholar]
- 86. Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knofler M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151(1):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knofler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168(4):1134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301(1):218–26. [DOI] [PubMed] [Google Scholar]
- 89. Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a-/–like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev. 1999;13(6):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lu W, Tu Z, Wang S, Lu J, Wang Q, Wang W, Wang B, Wang H, Ni H, Guo Y. Spatiotemporal expression of Wnt signaling pathway components during bovine placental development. Theriogenology. 2013;80(8):893–902. [DOI] [PubMed] [Google Scholar]
- 91. Wang A, Zsengeller ZK, Hecht JL, Buccafusca R, Burke SD, Rajakumar A, Weingart E, Yu PB, Salahuddin S, Karumanchi SA. Excess placental secreted Frizzled-related protein 1 in maternal smokers impairs fetal growth. J Clin Invest. 2015;125(11):4021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang LJ, Lo HF, Lin CF, Ng PS, Wu YH, Lee YS, Cheong ML, Chen H. SFRP3 negatively regulates placental extravillous trophoblast cell migration mediated by the GCM1-WNT10B-FZD7 axis. FASEB J. 2019;33(1):314–26. [DOI] [PubMed] [Google Scholar]
- 93. Zhang Z, Zhang L, Zhang L, Jia L, Wang P, Gao Y. Association of Wnt2 and sFRP4 expression in the third trimester placenta in women with severe preeclampsia. Reprod Sci. 2013;20(8):981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nayeem SB, Dharmarajan A, Keelan JA. Paracrine communication modulates production of Wnt antagonists and COX1-mediated prostaglandins in a decidual-trophoblast co-culture model. Mol Cell Endocrinol. 2015;405:52–62. [DOI] [PubMed] [Google Scholar]
- 95. Zhang Z, Li H, Zhang L, Jia L, Wang P. Differential expression of beta-catenin and Dickkopf-1 in the third trimester placentas from normal and preeclamptic pregnancies: a comparative study. Reprod Biol Endocrinol. 2013;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hewitt DP, Mark PJ, Dharmarajan AM, Waddell BJ. Placental expression of secreted Frizzled related protein-4 in the rat and the impact of glucocorticoid-induced fetal and placental growth restriction. Biol Reprod. 2006;75(1):75–81. [DOI] [PubMed] [Google Scholar]
- 97. Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 2014;141(8):1757–66. [DOI] [PubMed] [Google Scholar]
- 98. Sonderegger S, Husslein H, Leisser C, Knofler M. Complex expression pattern of Wnt ligands and Frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007;28(Suppl A):S97–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xiao X, Zhao Y, Jin R, Chen J, Wang X, Baccarelli A, Zhang Y. Fetal growth restriction and methylation of growth-related genes in the placenta. Epigenomics. 2016;8(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ferreira JC, Choufani S, Grafodatskaya D, Butcher DT, Zhao C, Chitayat D, Shuman C, Kingdom J, Keating S, Weksberg R. WNT2 promoter methylation in human placenta is associated with low birthweight percentile in the neonate. Epigenetics. 2011;6(4):440–9. [DOI] [PubMed] [Google Scholar]
- 101. Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–53. [DOI] [PubMed] [Google Scholar]
- 102. Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154(1):446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Meinhardt G, Saleh L, Otti GR, Haider S, Velicky P, Fiala C, Pollheimer J, Knofler M. Wingless ligand 5a is a critical regulator of placental growth and survival. Sci Rep. 2016;6:28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Carvalho JR, Fortunato IC, Fonseca CG, Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF, Santos NC, Carvalho FA, Franco CA. Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. Elife. 2019;8:e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Carmon KS, Loose DS. Secreted Frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–28. [DOI] [PubMed] [Google Scholar]
- 107. Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319(9):1317–23. [DOI] [PubMed] [Google Scholar]
- 108. Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279(23):24659–65. [DOI] [PubMed] [Google Scholar]
- 109. Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237(2):324–32. [DOI] [PubMed] [Google Scholar]
- 110. Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304(5678):1755–9. [DOI] [PubMed] [Google Scholar]
- 111. Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9(3):570–9. [DOI] [PubMed] [Google Scholar]
- 112. Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–6. [DOI] [PubMed] [Google Scholar]
- 113. Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90(2):225–34. [DOI] [PubMed] [Google Scholar]
- 114. Salaritabar A, Berindan-Neagoe I, Darvish B, Hadjiakhoondi F, Manayi A, Devi KP, Barreca D, Orhan IE, Suntar I, Farooqi AAet al. . Targeting Hedgehog signaling pathway: paving the road for cancer therapy. Pharmacol Res. 2019;141:466–80. [DOI] [PubMed] [Google Scholar]
- 115. Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5(246):re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Choudhry Z, Rikani AA, Choudhry AM, Tariq S, Zakaria F, Asghar MW, Sarfraz MK, Haider K, Shafiq AA, Mobassarah NJ. Sonic Hedgehog signalling pathway: a complex network. Ann Neurosci. 2014;21(1):28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Carballo GB, Honorato JR, de Lopes GPF, Spohr T. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hochman E, Castiel A, Jacob-Hirsch J, Amariglio N, Izraeli S. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J Biol Chem. 2006;281(45):33860–70. [DOI] [PubMed] [Google Scholar]
- 119. Giarretta I, Gaetani E, Bigossi M, Tondi P, Asahara T, Pola R. The Hedgehog signaling pathway in ischemic tissues. Int J Mol Sci. 2019;20(21):5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moran CM, Myers CT, Lewis CM, Krieg PA. Hedgehog regulates angiogenesis of intersegmental vessels through the VEGF signaling pathway. Dev Dyn. 2012;241(6):1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara Tet al. . The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7(6):706–11. [DOI] [PubMed] [Google Scholar]
- 122. Chen W, Tang T, Eastham-Anderson J, Dunlap D, Alicke B, Nannini M, Gould S, Yauch R, Modrusan Z, DuPree KJet al. . Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci. 2011;108(23):9589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann Cet al. . Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49(3):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wu G. Functional amino acids in nutrition and health. Amino Acids. 2013;45(3):407–11. [DOI] [PubMed] [Google Scholar]
- 125. Wu G, Bazer FW, Davis TA, Jaeger LA, Johnson GA, Kim SW, Knabe DA, Meininger CJ, Spencer TE, Yin Y-L. Important roles for the arginine family of amino acids in swine nutrition and production. Livestock Science. 2007;112(1–2):8–22. [Google Scholar]
- 126. Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56(9):427–38. [DOI] [PubMed] [Google Scholar]
- 127. Morris SM Jr. Arginine: beyond protein. Am J Clin Nutr. 2006;83(2):508S–12S. [DOI] [PubMed] [Google Scholar]
- 128. Kong X, Wang X, Yin Y, Li X, Gao H, Bazer FW, Wu G. Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod. 2014;91(5):106. [DOI] [PubMed] [Google Scholar]
- 129. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci. 2010;88(suppl_13):E195–204. [DOI] [PubMed] [Google Scholar]
- 131. Weckman AM, McDonald CR, Baxter JB, Fawzi WW, Conroy AL, Kain KC. Perspective: L-arginine and L-citrulline supplementation in pregnancy: a potential strategy to improve birth outcomes in low-resource settings. Adv Nutr. 2019;10(5):765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Li X, Bazer FW, Johnson GA, Burghardt RC, Frank JW, Dai Z, Wang J, Wu Z, Shinzato I, Wu G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids. 2014;46(2):375–84. [DOI] [PubMed] [Google Scholar]
- 133. Herring CM. Dietary Arginine Supplementation Enhances Placental Water Transport and Angiogenesis in Gestating Gilts [dissertation]. College Station: Texas A&M University; 2018. [Google Scholar]
- 134. Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids. 2013;45(2):241–56. [DOI] [PubMed] [Google Scholar]
- 135. Wu Z, Hou Y, Hu S, Bazer FW, Meininger CJ, McNeal CJ, Wu G. Catabolism and safety of supplemental L-arginine in animals. Amino Acids. 2016;48(7):1541–52. [DOI] [PubMed] [Google Scholar]
- 136. Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35(1):21–7. [DOI] [PubMed] [Google Scholar]
- 137. Finch AM, Yang LG, Nwagwu MO, Page KR, McArdle HJ, Ashworth CJ. Placental transport of leucine in a porcine model of low birth weight. Reproduction. 2004;128(2):229–35. [DOI] [PubMed] [Google Scholar]
- 138. Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32(11):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–13. [DOI] [PubMed] [Google Scholar]
- 140. Cui X, Chen J, Zacharek A, Roberts C, Yang Y, Chopp M. Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. J Neurosci Res. 2009;87(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Arnal JF, Munzel T, Venema RC, James NL, Bai CL, Mitch WE, Harrison DG. Interactions between L-arginine and L-glutamine change endothelial NO production. An effect independent of NO synthase substrate availability. J Clin Invest. 1995;95(6):2565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McDonald CR, Cahill LS, Gamble JL, Elphinstone R, Gazdzinski LM, Zhong KJY, Philson AC, Madanitsa M, Kalilani-Phiri L, Mwapasa Vet al. . Malaria in pregnancy alters L-arginine bioavailability and placental vascular development. Sci Transl Med. 2018;10(431):eaan6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, Bazer FW, Wu G. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. 2012;23(9):1178–83. [DOI] [PubMed] [Google Scholar]
- 144. Rossmanith WG, Hoffmeister U, Wolfahrt S, Kleine B, McLean M, Jacobs RA, Grossman AB. Expression and functional analysis of endothelial nitric oxide synthase (eNOS) in human placenta. Mol Hum Reprod. 1999;5(5):487–94. [DOI] [PubMed] [Google Scholar]
- 145. Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272(2 Pt 2):R441–63. [DOI] [PubMed] [Google Scholar]
- 146. Wu G, Bazer FW, Johnson GA, Herring C, Seo H, Dai Z, Wang J, Wu Z, Wang X. Functional amino acids in the development of the pig placenta. Mol Reprod Dev. 2017;84(9):870–82. [DOI] [PubMed] [Google Scholar]
- 147. Kwon H, Wu G, Meininger CJ, Bazer FW, Spencer TE. Developmental changes in nitric oxide synthesis in the ovine placenta. Biol Reprod. 2004;70(3):679–86. [DOI] [PubMed] [Google Scholar]
- 148. Vonnahme KA, Ford SP. Placental vascular endothelial growth factor receptor system mRNA expression in pigs selected for placental efficiency. J Physiol. 2004;554(1):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wu G, Bazer FW, Hu J, Johnson GA, Spencer TE. Polyamine synthesis from proline in the developing porcine placenta. Biol Reprod. 2005;72(4):842–50. [DOI] [PubMed] [Google Scholar]
- 150. Kwon H, Wu G, Bazer FW, Spencer TE. Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod. 2003;69(5):1626–34. [DOI] [PubMed] [Google Scholar]
- 151. Liu N, Dai Z, Zhang Y, Chen J, Yang Y, Wu G, Tso P, Wu Z. Maternal L-proline supplementation enhances fetal survival, placental development, and nutrient transport in mice. Biol Reprod. 2019;100(4):1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McIntyre KR, Vincent KMM, Hayward CE, Li X, Sibley CP, Desforges M, Greenwood SL, Dilworth MR. Human placental uptake of glutamine and glutamate is reduced in fetal growth restriction. Sci Rep. 2020;10(1):16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim B, Li J, Jang C, Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017;36(16):2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, Visnagri A, Yuldasheva N, Goveia J, Cruys Bet al. . Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36(16):2334–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Karahoda R, Abad C, Horackova H, Kastner P, Zaugg J, Cerveny L, Kucera R, Albrecht C, Staud F. Dynamics of tryptophan metabolic pathways in human placenta and placental-derived cells: effect of gestation age and trophoblast differentiation. Front Cell Dev Biol. 2020;8:574034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lanoix D, Beghdadi H, Lafond J, Vaillancourt C. Human placental trophoblasts synthesize melatonin and express its receptors. J Pineal Res. 2008;45(1):50–60. [DOI] [PubMed] [Google Scholar]
- 157. Lemley CO, Meyer AM, Camacho LE, Neville TL, Newman DJ, Caton JS, Vonnahme KA. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2012;302(4):R454–67. [DOI] [PubMed] [Google Scholar]
- 158. Shukla P, Lemley CO, Dubey N, Meyer AM, O'Rourke ST, Vonnahme KA. Effect of maternal nutrient restriction and melatonin supplementation from mid to late gestation on vascular reactivity of maternal and fetal placental arteries. Placenta. 2014;35(7):461–6. [DOI] [PubMed] [Google Scholar]
- 159. Vonnahme KA, Fernando SC, Ross JW, Ashworth MD, DeSilva U, Malayer JR, Geisert RD. Porcine endometrial expression of kininogen, factor XII, and plasma kallikrein in cyclic and pregnant gilts. Biol Reprod. 2004;70(1):132–8. [DOI] [PubMed] [Google Scholar]
- 160. Sarkar C, Chakroborty D, Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. J Neuroimmune Pharmacol. 2013;8(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chakroborty D, Goswami S, Basu S, Sarkar C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. FASEB J. 2020;34(11):14093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Elmetwally MA, Lenis Y, Tang W, Wu G, Bazer FW. Effects of catecholamines on secretion of interferon tau and expression of genes for synthesis of polyamines and apoptosis by ovine trophectoderm. Biol Reprod. 2018;99(3):611–28. [DOI] [PubMed] [Google Scholar]
- 163. Muthig V, Gilsbach R, Haubold M, Philipp M, Ivacevic T, Gessler M, Hein L. Upregulation of soluble vascular endothelial growth factor receptor 1 contributes to angiogenesis defects in the placenta of alpha 2B-adrenoceptor deficient mice. Circ Res. 2007;101(7):682–91. [DOI] [PubMed] [Google Scholar]
- 164. Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30(1):39–103. [DOI] [PubMed] [Google Scholar]
- 165. Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259(2):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Doi O, Doi F, Schroeder F, Alberts AW, Vagelos PR. Manipulation of fatty acid composition of membrane phospholipid and its effects on cell growth in mouse LM cells. Biochim Biophys Acta. 1978;509(2):239–50. [DOI] [PubMed] [Google Scholar]
- 167. Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25(12):2303–10. [DOI] [PubMed] [Google Scholar]
- 168. Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D, Steward W, Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45(12):2077–86. [DOI] [PubMed] [Google Scholar]
- 169. Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111(7):3514–21. [DOI] [PubMed] [Google Scholar]
- 170. Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25(53):7019–28. [DOI] [PubMed] [Google Scholar]
- 171. Gong Y, Fu Z, Liegl R, Chen J, Hellstrom A, Smith LE. Omega-3 and omega-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr. 2017;106(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Murota SI, Onodera M, Morita I. Regulation of angiogenesis by controlling VEGF receptor. Ann N Y Acad Sci. 2000;902(1):208–13.; discussion 12–3. [DOI] [PubMed] [Google Scholar]
- 173. Yang SP, Morita I, Murota SI. Eicosapentaenoic acid attenuates vascular endothelial growth factor-induced proliferation via inhibiting Flk-1 receptor expression in bovine carotid artery endothelial cells. J Cell Physiol. 1998;176(2):342–9. [DOI] [PubMed] [Google Scholar]
- 174. Chao CY, Lii CK, Ye SY, Li CC, Lu CY, Lin AH, Liu KL, Chen HW. Docosahexaenoic acid inhibits vascular endothelial growth factor (VEGF)-induced cell migration via the GPR120/PP2A/ERK1/2/eNOS signaling pathway in human umbilical vein endothelial cells. J Agric Food Chem. 2014;62(18):4152–8. [DOI] [PubMed] [Google Scholar]
- 175. Matesanz N, Park G, McAllister H, Leahey W, Devine A, McVeigh GE, Gardiner TA, McDonald DM. Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2010;51(12):6815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kim HJ, Vosseler CA, Weber PC, Erl W. Docosahexaenoic acid induces apoptosis in proliferating human endothelial cells. J Cell Physiol. 2005;204(3):881–8. [DOI] [PubMed] [Google Scholar]
- 177. Zhong Y, Catheline D, Houeijeh A, Sharma D, Du L, Besengez C, Deruelle P, Legrand P, Storme L. Maternal omega-3 PUFA supplementation prevents hyperoxia-induced pulmonary hypertension in the offspring. Am J Physiol Lung Cell Mol Physiol. 2018;315(1):L116–L32. [DOI] [PubMed] [Google Scholar]
- 178. Basak S, Mallick R, Duttaroy AK. Maternal docosahexaenoic acid status during pregnancy and its impact on infant neurodevelopment. Nutrients. 2020;12(12):3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Basak S, Vilasagaram S, Duttaroy AK. Maternal dietary deficiency of n-3 fatty acids affects metabolic and epigenetic phenotypes of the developing fetus. Prostaglandins, Leukotrienes Essent Fatty Acids. 2020;158:102109. [DOI] [PubMed] [Google Scholar]
- 180. Dhobale MV, Wadhwani N, Mehendale SS, Pisal HR, Joshi SR. Reduced levels of placental long chain polyunsaturated fatty acids in preterm deliveries. Prostaglandins, Leukotrienes Essent Fatty Acids. 2011;85(3–4):149–53. [DOI] [PubMed] [Google Scholar]
- 181. Basak S, Duttaroy AK. Effects of fatty acids on angiogenic activity in the placental extravillious trophoblast cells. Prostaglandins, Leukotrienes Essent Fatty Acids. 2013;88(2):155–62. [DOI] [PubMed] [Google Scholar]
- 182. Basak S, Das MK, Duttaroy AK. Fatty acid-induced angiogenesis in first trimester placental trophoblast cells: possible roles of cellular fatty acid-binding proteins. Life Sci. 2013;93(21):755–62. [DOI] [PubMed] [Google Scholar]
- 183. Johnsen GM, Basak S, Weedon-Fekjaer MS, Staff AC, Duttaroy AK. Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta. 2011;32(9):626–32. [DOI] [PubMed] [Google Scholar]
- 184. Peng J, Zhou Y, Hong Z, Wu Y, Cai A, Xia M, Deng Z, Yang Y, Song T, Xiong Jet al. . Maternal eicosapentaenoic acid feeding promotes placental angiogenesis through a Sirtuin-1 independent inflammatory pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(2):147–57. [DOI] [PubMed] [Google Scholar]
- 185. Rao A, Briskey D, Nalley JO, Ganuza E. Omega-3 eicosapentaenoic acid (EPA) rich extract from the microalga nannochloropsis decreases cholesterol in healthy individuals: a double-blind, randomized, placebo-controlled, three-month supplementation study. Nutrients. 2020;12(6):1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bai J, Qi QR, Li Y, Day R, Makhoul J, Magness RR, Chen DB. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int J Mol Sci. 2020;21(12):4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mathew SA, Bhonde RR. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol Res. 2018;132:90–8. [DOI] [PubMed] [Google Scholar]
- 188. Klingler M, Blaschitz A, Campoy C, Cano A, Molloy AM, Scott JM, Dohr G, Demmelmair H, Koletzko B, Desoye G. The effect of docosahexaenoic acid and folic acid supplementation on placental apoptosis and proliferation. Br J Nutr. 2006;96(01):182–90. [DOI] [PubMed] [Google Scholar]
- 189. Shao Z, Fu ZJ, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui ZH, Pei Det al. . Cytochrome P450 2C8 omega 3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization-brief report. Arterioscler Thromb Vasc Biol. 2014;34(3):581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam Set al. . Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci. 2013;110(16):6530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Feng X, Zhang Y, Zhang Y, Yang X, Man D, Lu L, Xu T, Liu Y, Yang C, Li Het al. . Prostaglandin I2 mediates weak vasodilatation in human placental microvessels. Biol Reprod. 2020;103(6):1229–37. [DOI] [PubMed] [Google Scholar]
- 192. Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R245–58. [DOI] [PubMed] [Google Scholar]
- 193. Ohnishi H, Saito Y. Eicosapentaenoic acid (epa) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J Atheroscler Thromb. 2013;20(12):861–77. [DOI] [PubMed] [Google Scholar]
- 194. Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. Omega-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113–115:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ulu A, Sahoo PK, Yuil-Valdes AG, Mukherjee M, Van Ormer M, Muthuraj PG, Thompson M, Anderson Berry A, Hanson CK, Natarajan SKet al. . Omega-3 fatty acid-derived resolvin D2 regulates human placental vascular smooth muscle and extravillous trophoblast activities. Int J Mol Sci. 2019;20(18):4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wang JY, Shi YJ, Zhang LL, Zhang F, Hu XM, Zhang WT, Leak RK, Gao YQ, Chen L, Chen J. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hasan AU, Ohmori K, Konishi K, Igarashi J, Hashimoto T, Kamitori K, Yamaguchi F, Tsukamoto I, Uyama T, Ishihara Yet al. . Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPAR gamma mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2015;406(C):10–8. [DOI] [PubMed] [Google Scholar]
- 198. Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty acid-binding proteins in primary term human trophoblasts. Am J Obstet Gynecol. 2007;197(5):516.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Campbell FM, Dutta-Roy AK. Asymmetric distribution of the plasma membrane fatty acid-binding protein (FABPpm) in the human placenta. Biochem Soc Trans. 1996;24(2):249S. [DOI] [PubMed] [Google Scholar]
- 200. Ghelfi E, Yu CW, Elmasri H, Terwelp M, Lee CG, Bhandari V, Comhair SA, Erzurum SC, Hotamisligil GS, Elias JAet al. . Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am J Pathol. 2013;182(4):1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23(11):3865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Basak S, Duttaroy AK. cis-9,trans-11 conjugated linoleic acid stimulates expression of angiopoietin like-4 in the placental extravillous trophoblast cells. Biochim Biophys Acta. 2013;1831(4):834–43. [DOI] [PubMed] [Google Scholar]
- 203. Makkar A, Mishima T, Chang G, Scifres C, Sadovsky Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta. 2014;35(10):802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Yu CW, Liang X, Lipsky S, Karaaslan C, Kozakewich H, Hotamisligil GS, Bischoff J, Cataltepe S. Dual role of fatty acid-binding protein 5 on endothelial cell fate: a potential link between lipid metabolism and angiogenic responses. Angiogenesis. 2016;19(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil Get al. . Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15(3):457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Basak S, Sarkar A, Mathapati S, Duttaroy AK. Cellular growth and tube formation of HTR8/SVneo trophoblast: effects of exogenously added fatty acid-binding protein-4 and its inhibitor. Mol Cell Biochem. 2018;437(1–2):55–64. [DOI] [PubMed] [Google Scholar]
- 207. Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289(33):23168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Figueroa JD, Serrano-Illan M, Licero J, Cordero K, Miranda JD, De Leon M. Fatty acid binding protein 5 modulates docosahexaenoic acid-induced recovery in rats undergoing spinal cord injury. J Neurotrauma. 2016;33(15):1436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Fournier T, Tsatsaris V, Handschuh K, Evain-Brion D. PPARs and the placenta. Placenta. 2007;28(2–3):65–76. [DOI] [PubMed] [Google Scholar]
- 210. Tarrade A, Schoonjans K, Pavan L, Auwerx J, Rochette-Egly C, Evain-Brion D, Fournier T. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab. 2001;86(10):5017–24. [DOI] [PubMed] [Google Scholar]
- 211. Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19(3):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Meher A, Sundrani D, Joshi S. Maternal nutrition influences angiogenesis in the placenta through peroxisome proliferator activated receptors: a novel hypothesis. Mol Reprod Dev. 2015;82(10):726–34. [DOI] [PubMed] [Google Scholar]
- 213. Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, Desvergne B. PPARgamma in placental angiogenesis. Endocrinology. 2010;151(10):4969–81. [DOI] [PubMed] [Google Scholar]
- 214. Wu Q, Wang H, Zhao X, Shi Y, Jin M, Wan B, Xu H, Cheng Y, Ge H, Zhang Y. Identification of G-protein-coupled receptor 120 as a tumor-promoting receptor that induces angiogenesis and migration in human colorectal carcinoma. Oncogene. 2013;32(49):5541–50. [DOI] [PubMed] [Google Scholar]
- 215. Zhang M, Qiu S. Activation of GPR120 promotes the metastasis of breast cancer through the PI3K/Akt/NF-kappaB signaling pathway. Anticancer Drugs. 2019;30(3):260–70. [DOI] [PubMed] [Google Scholar]