Abstract
Timely diagnosis of leptospirosis is important to ensure a favorable clinical outcome. The definitive serologic assay, the microscopic agglutination test (MAT), requires paired sera and is not useful for guiding early clinical management. The only screening test approved for use in the United States, the indirect hemagglutination assay (IHA), has not undergone extensive field evaluation. To assess the performance of the leptospirosis IHA in Hawaii, serum from patients evaluated for leptospirosis between 1992 and 1997 were tested with the IHA at the Hawaii State Laboratories Division and with the MAT at the Centers for Disease Control and Prevention. Leptospirosis was considered confirmed by a fourfold rise in MAT titer and/or a positive culture. A total of 92 (41%) of 226 specimens from 114 persons with confirmed leptospirosis were found positive by IHA. Only 18 (15%) of 119 specimens obtained within 14 days of onset were IHA positive, compared to 74 (69%) of 107 specimens collected more than 14 days after onset (P <0.001). Repeat testing ultimately resulted in 78 (68%) of the confirmed cases having at least one specimen found positive by IHA. Thirteen different presumptive infecting serogroups were identified among 251 specimens with an MAT titer of ≥200 and obtained from persons with confirmed or probable leptospirosis. Fifty (68%) of 73 specimens with Icterohaemorrhagiae as the presumptive infecting serogroup were found positive by IHA, compared to 44 (47%) of 93 specimens with Australis as the presumptive infecting serogroup (P, 0.01). The IHA test was positive for 3 (1%) of 236 specimens from 154 persons without leptospirosis. The sensitivity of the leptospirosis IHA in Hawaii was substantially below figures reported previously, particularly early in the course of illness, limiting its usefulness for diagnosing acute infection. Since the presumptive infecting serogroup affected IHA results and the prevalence of serovars varies with geography, the performance of the IHA should be assessed locally. More sensitive leptospirosis screening tests are needed in Hawaii.
Leptospirosis is a major public health problem throughout the world, particularly in the tropics, where annual incidence rates have been estimated to be as high as 3,000 per 100,000 persons (6). Persons in temperate climates are also at risk of acquiring this serious and potentially fatal illness, as demonstrated by the recent outbreak among triathletes in Illinois (4).
Clinical recognition of leptospirosis is difficult because leptospires can affect many different organ systems, resulting in a wide variety of clinical presentations. Consequently, leptospirosis is often misdiagnosed as influenza, aseptic meningitis, encephalitis, dengue fever, hepatitis, or gastroenteritis. Timely diagnosis of leptospirosis is essential because prompt, specific treatment, as early in the illness as possible, is important in ensuring a favorable clinical outcome (1).
Laboratory confirmation of leptospirosis is challenging. The sensitivity of blood cultures is low (e.g., 45% in one study) (8), and culturing or isolation requires special media and up to 6 weeks of incubation. The definitive diagnostic serologic assay is the microscopic agglutination test (MAT), performed on human specimens in the United States by the Centers for Disease Control and Prevention (CDC). The MAT is a time-consuming, difficult test requiring technical expertise and the maintenance of multiple live serovars (5). Moreover, because a fourfold rise in titer between acute- and convalescent-phase samples is necessary for serologic confirmation, the MAT is not useful for guiding clinical management early in the course of the patient's illness.
Several screening tests for leptospirosis have been developed. The U.S. Food and Drug Administration has approved one of these, the indirect hemagglutination assay (IHA), for commercial use. Previously published reports have found the IHA to be highly sensitive (7, 10) and useful in the investigation of patients suspected of having acute leptospirosis (7).
Hawaii accounts for the majority of all leptospirosis cases acquired in the United States each year, with a reported annual incidence rate approximately 100 times that of the mainland United States (R. Burr, D. Sasaki, T. Aye, and H. Domen, Abst. Int. Conf. Emerg. Infect. Dis., 1998). Because of the high incidence, the Hawaii State Laboratories Division (SLD) routinely offers Leptospira testing services to the local medical community, including screening with the commercially available IHA and referral of specimens to the CDC for testing by the MAT. A preliminary review of the IHA and MAT results from patients evaluated in Hawaii in 1997 suggested that the IHA might not be as sensitive as described in other geographic settings. Here we present the findings of a comprehensive analysis of the sensitivity and specificity of the IHA in which we examined the effect of the presumptive infecting serogroup on test performance.
MATERIALS AND METHODS
Patient samples.
Physicians throughout Hawaii considering a diagnosis of leptospirosis were encouraged to submit acute- and convalescent-phase patient sera to SLD for evaluation. To ascertain the sensitivity of the IHA, all specimens from persons with leptospirosis and onset between 1 January 1992 and 31 December 1997 were examined. According to the leptospirosis case definition endorsed by the Council of State and Territorial Epidemiologists and CDC (2), cases of leptospirosis were classified as “confirmed” if there was isolation of Leptospira from a clinical specimen or a fourfold or greater increase in the Leptospira MAT titer between acute- and convalescent-phase serum specimens obtained ≥2 weeks apart and studied at the same laboratory. Cases of leptospirosis were classified as “probable” if there was a clinically compatible illness and a Leptospira MAT titer of ≥200 in one or more serum specimens without a fourfold rise in titer or a positive culture.
A total of 226 serum specimens were collected from 114 patients with confirmed leptospirosis; 95 cases (83%) were confirmed by a fourfold rise in MAT titer, 11 (10%) were confirmed by a positive culture, and 8 (7%) had both a fourfold rise in titer and a positive culture. An additional 71 cases of probable leptospirosis were identified, 38 (54%) of which had at least one MAT titer of ≥800. To ensure the validity of our findings, we restricted the analysis of the overall sensitivity of the IHA to specimens from confirmed cases. The potential association between presumptive infecting serogroup and IHA test performance was examined with all specimens (n = 251) from confirmed or probable cases with an MAT titer of ≥200.
For the analysis of IHA specificity, we also examined specimens from persons who had an illness initially suspected of being leptospirosis and who were later found to be negative by MAT testing. This analysis was limited to patients evaluated between 1 January 1996 and 31 December 1997 because detailed data on patients for whom leptospirosis was ruled out were not available for prior years. For the purpose of the specificity analysis, patients were considered to be free of leptospirosis if they had at least one serum specimen obtained ≥14 and ≤90 days after illness onset and MAT titers for all specimens were <200. A total of 154 cases were identified as true negative.
Culturing and serogrouping.
Blood culturing and serogrouping of isolates were performed as previously described (11), with one minor variation. Inoculated blood cultures were incubated for 6 weeks only and examined weekly, instead of weekly examinations for 5 weeks followed by monthly examinations for 4 months.
IHA.
A commercially available IHA purchased from MRL Diagnostics (Cypress, Calif.) was performed at SLD as previously described (10). Fifty microliters of a 1:50 dilution of each serum specimen was added to U-bottom microtiter plate wells in duplicate. Twenty-five microliters of either antigen-coated test cells or uncoated control cells was added to the wells and mixed. Plates were incubated at room temperature (20 to 25°C) for 1 h. Hemagglutination was read on a scale of 0 to ≥1+. Positive wells were defined as those having ≥1+ agglutination. A 1+ reaction was described as a granular or irregular ring covering more than 50% of the well bottom and looking distinctly different from the reaction found in wells to which uncoated control cells were added. Positive and negative control sera were tested each time the test was performed.
MAT.
The presence of antibodies to Leptospira antigens was determined by the MAT as described previously by Cole et al. (5). The antigen panel included the following 17 serogroups (serovars): Australis (australis and bratislava), Autumnalis (autumnalis), Ballum (ballum), Bataviae (bataviae), Canicola (canicola), Celledoni (celledoni), Cynopteri (cynopteri), Djasiman (djasiman), Grippotyphosa (grippotyphosa), Hebdomadis (borincana), Icterohaemorrhagiae (copenhageni, icterohaemorrhagiae, and mankarso), Javanica (javanica), Mini (georgia), Pomona (pomona), Pyrogenes (alexi and pyrogenes), Sejroe (wolffi), and Tarassovi (tarassovi). The antigens were 4- to 7-day-old live cells in PLM-5 broth (Intergen Co., Purchase, N.Y.) adjusted to the turbidity of a 0.5 McFarland standard. Serial twofold dilutions of serum in phosphate-buffered saline (50 μl/well), starting at 1:50, were mixed with an equal volume of antigen. After incubation at room temperature for 1.5 to 4 h, the reactions were read by dark-field microscopy. The reported titer was the reciprocal of the highest dilution that agglutinated at least 50% of the leptospires relative to the buffer control. Samples positive at a titer of 12,800 were reported as having an MAT titer of ≥12,800 and were not diluted further. As a positive control for each antigen, homologous rabbit antiserum was run in parallel with patient serum specimens.
Determination of presumptive infecting serogroup.
For any given specimen with an MAT titer of ≥200, the serogroup showing the highest titer was considered to be the presumptive infecting serogroup. When there was more than one serogroup with the same high titer, the presumptive infecting serogroup for that sample was designated indeterminate.
Data analysis.
Frequencies, comparisons of proportions (odds ratios [OR]), and chi-square tests for linear trends were calculated with Epi Info version 6.04; geometric mean titers were computed with Excel software; and 95% confidence intervals (CI) for proportions were calculated with the Fleiss quadratic equation.
RESULTS
IHA sensitivity.
The IHA was positive for 92 (41%; 95% CI, 34 to 47%) of the 226 specimens from confirmed cases and from 17 (49%; 95% CI, 32 to 66%) of 35 specimens from the 19 individuals whose infection was verified by culturing. Repeat testing ultimately resulted in 78 (68%; 95% CI, 59 to 77%) of the 114 confirmed cases having at least one specimen test positive by the IHA. The proportion of cases with one or more positive IHA results increased to 74% (95% CI, 49 to 90%) when the analysis was restricted to the 19 individuals with culture-proven infection.
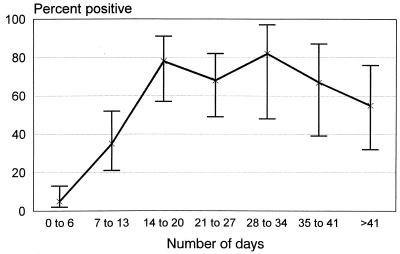
The proportion of specimens found positive by the IHA increased with the length of time from illness onset and reached a plateau of nearly 80% for specimens collected 14 to 20 days postonset (Fig. 1). The geometric mean titer for the 156 specimens from confirmed cases with an MAT titer of greater than zero followed a similar temporal pattern (Table 1). Specimens with higher MAT titers were significantly more likely to be found positive by the IHA (P, <0.0001) (Table 2).
FIG. 1.
Percent of leptospirosis IHA results positive over time from illness onset to specimen collection. A total of 226 specimens from 114 cases were examined. 95% CI for the percentages are indicated by brackets.
TABLE 1.
Geometric mean titers in the leptospirosis MAT over time from illness onset to specimen collection
| Days after onset when specimen was collected | Number of specimens
|
Geometric mean MAT titera | |
|---|---|---|---|
| Total | With an MAT titer of ≥100 (%) | ||
| 0–6 | 79 | 27 (34) | 233 |
| 7–13 | 40 | 26 (65) | 1,162 |
| 14–20 | 27 | 26 (96) | 2,801 |
| 21–27 | 34 | 33 (97) | 2,649 |
| 28–34 | 11 | 11 (100) | 3,630 |
| 35–41 | 15 | 14 (93) | 2,498 |
| 42 or more | 20 | 19 (95) | 1,152 |
| Total | 226 | 156 (69) | 1,407 |
Geometric mean titer calculations were limited to 156 specimens obtained from patients with confirmed leptospirosis and with an MAT titer of ≥100.
TABLE 2.
Percentage of leptospirosis IHA results positive relative to MAT titers of specimens
| MAT titer of specimen | No. of specimens
|
95% CIa | |
|---|---|---|---|
| Total | Positive by IHA (%) | ||
| Negative | 70 | 2 (3) | 0–11 |
| 100 | 15 | 0 (0) | 0–25 |
| 200 | 15 | 3 (20) | 5–49 |
| 400 | 24 | 10 (42) | 23–63 |
| 800 | 17 | 8 (47) | 24–71 |
| 1,600 | 15 | 9 (60) | 33–83 |
| 3,200 | 26 | 22 (85) | 64–95 |
| 6,400 | 17 | 15 (88) | 62–98 |
| ≥12,800 | 27 | 23 (85) | 65–95 |
| Total | 226 | 92 (41) | 34–47 |
95% CI for the percentage of specimens found positive by the IHA.
IHA specificity.
A total of 236 IHAs were performed on specimens obtained from the 154 patients without evidence of leptospirosis. The IHA was positive for three specimens (1%) from two separate individuals (1%). In this population, therefore, the specificity of the IHA was 99%.
Presumptive infecting serogroup.
Thirteen different presumptive infecting serogroups were identified among the 251 specimens with an MAT titer of ≥200 from confirmed and probable cases. The presumptive infecting serogroup was Australis for 93 (37%) of the specimens, Icterohaemorrhagiae for 73 (29%), indeterminate for 18 (7%), and 1 of the remaining 11 serogroups for 72 (27%), none of which individually accounted for more than 7% of the total.
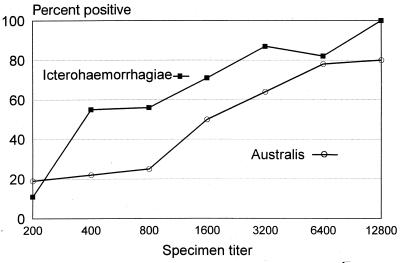
The proportion of IHA results that were positive was significantly associated with the presumptive infecting serogroup of the specimen tested. A total of 50 (68%) of 73 specimens with Icterohaemorrhagiae as the presumptive infecting serogroup were found positive by IHA, compared to 44 (47%) of 93 specimens with Australis as the presumptive infecting serogroup (OR, 2.4; 95% CI, 1.2 to 4.8; P, 0.01). This association remained significant (P, <0.05) when the analysis was restricted to convalescent-phase sera (i.e., those obtained 2 or more weeks after onset), and the trend persisted after stratification by MAT titer (Fig. 2). No significant differences in the proportion of positive IHA results were found for specimens with other presumptive infecting serogroups.
FIG. 2.
Percent of leptospirosis IHA results positive relative to MAT titer and presumptive infecting serogroup. Data are from 93 specimens with presumptive infecting serogroup Australis and 73 specimens with presumptive infecting serogroup Icterohaemorrhagiae.
DISCUSSION
In Hawaii, less than half of all specimens collected from patients with confirmed leptospirosis were found positive by the IHA; the IHA was particularly insensitive for samples collected within the first 2 weeks following illness onset. Testing of convalescent-phase sera ultimately resulted in about two-thirds of all patients with confirmed leptospirosis being correctly identified by the IHA.
Our results are in contrast with those reported from the earliest laboratory-based evaluation of the leptospirosis IHA conducted at the CDC in 1975 (10), and a recent field evaluation of the currently available IHA (7). In these settings, the sensitivities of the IHA were 92% (by sample tested) and 100% (by patient), respectively; the specificity of the IHA in Hawaii (99%) was similar to that reported in the two previous studies (94 to 95%).
There are several possible explanations for the difference in the sensitivities of the IHAs in Hawaii and the other field evaluation. First, each study used different criteria to define a case of leptospirosis. In the Hawaii study, a confirmed case was defined by a fourfold change in MAT titer or a positive culture; in the Barbados study, the case definition included patients with an initial MAT titer of ≥800 or an immunoglobulin (IgM) titer of ≥160 in an enzyme-linked immunosorbent assay (ELISA). Both an MAT titer of ≥800 and an ELISA IgM titer of ≥160 are markers of specimens with relatively high levels of antibody. Our data indicate that there is a positive association between antibody titers and the proportion of positive IHAs, a phenomenon also noted by Sulzer et al. (10). Evaluating the IHA with specimens which have already been determined to have high levels of antibody might therefore be expected to favorably bias estimates of the sensitively of the IHA. This is especially true early in the illness, because defining cases in this manner disproportionately identifies those with high levels of IgM and/or IgG antibodies in the initial specimens.
A second possible explanation for the relatively low sensitivity of the IHA in our study compared to that reported by Levett and Whittington (7) is differences in case ascertainment. Leptospirosis surveillance in Hawaii is community based; physicians suspecting leptospirosis submit serum samples from individuals treated as inpatients or outpatients. In Barbados, only specimens from persons hospitalized for suspected leptospirosis were included in the IHA evaluation. In general, the severity of illness for patients admitted to the hospital is greater than that for those who are treated as outpatients. Although we did not directly measure the effect of the severity of illness on the sensitivity of the IHA, we did reanalyze our data, examining only individuals who had confirmed leptospirosis and who were also hospitalized; we found that the sensitivity of the IHA increased to 73%. Variances in case ascertainment methods may therefore explain at least some of the difference in reported IHA sensitivities.
A third possible explanation for the low sensitivity of the IHA in our study is false-positive MAT results; i.e., the MAT mistakenly indicated leptospirosis while the IHA was appropriately negative. In our opinion, this hypothesis seems very unlikely, given that previous evaluations have found the MAT to be highly specific. Sehgal et al. reported that none of 140 patients with illnesses other than leptospirosis tested by the MAT had significant titers (9). Moreover, we found that the sensitivities of the IHA were similar among cases diagnosed by MAT and those confirmed by culturing; false-positive MAT results cannot explain the suboptimal performance of the IHA in culture-proven infections.
A fourth explanation is that the sensitivity of the currently available IHA is affected by the presumptive infecting serogroup. Rapid screening tests for leptospirosis, like the IHA, use single, broadly reacting antigens to detect the patient's immune response to the infecting leptospires. Because the prevalence of endemic leptospire serogroups varies from place to place, the sensitivity of the IHA should reflect the ability of the IHA antigen to react with the specific leptospire serovars frequently causing infection in a particular location. From 1992 through 1997, the most commonly identified serogroup among cases in Hawaii was Australis, followed by serogroup Icterohaemorrhagiae. Compared to specimens containing serogroup Icterohaemorrhagiae, we found that specimens in which the presumptive infecting serogroup was Australis had a significantly lower proportion of positive results when tested by the IHA. This result suggests that the current IHA might not detect serovars in the Australis serogroup as well as it does serovars in the Icterohaemorrhagiae serogroup. Although Levett and Whittington (7) did not provide data on the presumptive infecting serogroup for all specimens tested in their evaluation, information from other sources indicates that serogroup Australis is uncommon in Barbados (0 of 93 positive specimens in a survey from 1980 to 1982) (6). Therefore, the difference in IHA sensitivities reported in Hawaii and Barbados may be partially accounted for by the difference in the leptospire serogroups prevalent in the two locations.
Some investigators have recently questioned whether the infecting serogroup can be accurately determined via serology (P. N. Levett, meeting of the International Leptospirosis Society, Marysville, Australia, 22 to 25 August 1999). Limited data from Hawaii, however, indicate that there is a close correlation between the serovar isolated by culturing and the serogroup determined by the MAT at the CDC. For 18 (90%) of 20 isolates which were either serogrouped or serotyped between 1990 and 1998, the presumptive infecting serogroup correlated exactly with the servovar isolated. For the remaining two isolates, the serogroup was identified, but there were equally high MAT titers for at least one other serogroup.
Our findings are not directly comparable to those from the original evaluation of the leptospirosis IHA (10), because the CDC used serovar andamana to serve as the broad, cross-reacting antigen, while the currently licensed commercial test uses serovar patoc; both are saprophytic leptospires. Sulzer et al. (10) explained that their laboratory chose andamana because this antigen had a “somewhat broader cross-reaction pattern among the serogroups than our previous method in which patoc extract was used. …” Because only 2% of the 229 positive samples in that evaluation were specimens in which the presumptive infecting serogroup was Australis, it is not possible to speculate on whether the IHA with serovar andamana would have performed better than the current IHA in Hawaii.
This study is limited in that data from persons who were tested for leptospirosis and found to be negative were available for only a 2-year period, while the number of cases confirmed during those years was too small to calculate meaningful estimates of the positive and negative predictive values of the IHA in our setting.
Because timely diagnosis and treatment of leptospirosis are important in ensuring a desirable clinical outcome, a sensitive and specific rapid test would be of great benefit to patients and physicians in Hawaii. Conversely, a poorly performing test may actually have a negative impact on diagnostic follow-up and clinical management. Over the past several years, we observed that 78% of patients who had a negative result on an initial IHA did not have a convalescent-phase sample drawn for testing by the MAT, compared to 12% of those who had an initial IHA result which was positive (OR, 26; P, <0.0001; data not shown). We are concerned, therefore, that false-negative IHA screening results might be discouraging physicians from retaining leptospirosis in the differential diagnosis and that appropriate treatment may not be initiated. Moreover, the incidence of leptospirosis reported from Hawaii would probably increase substantially if a larger proportion of the patients initially evaluated for this illness went on to have convalescent-phase sera tested by the MAT, despite having had a negative IHA result for the acute-phase specimen. While physicians seem to readily recognize the need to verify a positive screening test result with a confirmatory test, confirming a negative IHA screening result by the MAT appears to be less intuitive.
Our study reaffirms the need to consider the setting in which a serologic test is performed when interpreting the results. This is particularly true when the test relies on antigenic cross-reactivity and there is a great degree of antigenic variability in the pathogen based on geographic location. Because serovars vary from one environment to another, it is important that laboratories validate the performance of available leptospirosis screening tests to determine which test shows the greatest sensitivity and specificity for the environment in which it will be used.
Better screening tests for diagnosing leptospirosis are urgently needed in Hawaii. At present, the IHA is the only screening test approved by the U.S. Food and Drug Administration for use in the United States, but many others, including IgM ELISAs, are available elsewhere in the world. Future work should attempt to identify leptospirosis screening tests which exhibit high sensitivity and specificity when used in a variety of diverse environments, including islands in the Pacific Ocean.
ACKNOWLEDGMENTS
We thank Jo Manea, Chester Wakida, Charles Middleton, Audrey Asahina, Mark Hanne, Glenn Kobayashi, and Henry Higa from the Hawaii State Department of Health for assistance with case investigations and laboratory support. We are also grateful to Robbin Weyant and Tatjana Popovic of the Centers for Disease Control and Prevention for editorial review of the manuscript.
REFERENCES
- 1.Benenson A S, editor. Control of communicable diseases manual. 16th ed. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46:49. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States. Morbid Mortal Weekly Rep. 1996;45:74. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998. Morbid Mortal Weekly Rep. 1998;47:673–676. [PubMed] [Google Scholar]
- 5.Cole J R, Sulzer C R, Pursell A R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everard C O R, Maude G H, Hayes R J. Leptospiral infection: a household serosurvey in urban and rural communities in Barbados and Trinidad. Ann Trop Med Parasitol. 1990;84:255–266. doi: 10.1080/00034983.1990.11812465. [DOI] [PubMed] [Google Scholar]
- 7.Levett P N, Whittington C U. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis. J Clin Microbiol. 1998;36:11–14. doi: 10.1128/jcm.36.1.11-14.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki D M, Pang L P, Minette H P, Wakida C K, Fujimoto W J, Manea S J, et al. Active surveillance and risk factors for leptospirosis in Hawaii. Am J Trop Med Hyg. 1993;48:35–43. doi: 10.4269/ajtmh.1993.48.35. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal S C, Vijayachari P, Sharma S, Sugunan A P. LEPTO Dipstick: a rapid and simple method for serodiagnosis of acute leptospirosis. Trans R Soc Trop Med Hyg. 1999;93:161–164. doi: 10.1016/s0035-9203(99)90293-6. [DOI] [PubMed] [Google Scholar]
- 10.Sulzer C R, Glosser J W, Rogers F, Jones W L, Frix M. Evaluation of an indirect hemagglutination test for the diagnosis of human leptospirosis. J Clin Microbiol. 1975;2:218–221. doi: 10.1128/jcm.2.3.218-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyant R S, Bragg S L, Kaufmann A F. Leptospira and Leptonema. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 739–745. [Google Scholar]