ABSTRACT
Advanced glycation end products (AGEs) are involved in the development of several age-related complications. The protective role of soluble receptors for AGEs (sRAGE) against deleterious effects of AGEs has been indicated in several studies. However, findings on the association of AGEs or sRAGE with mortality are equivocal. In this meta-analysis we aimed to present a quantitative estimation of the association between circulating AGEs or sRAGE and all-cause or cardiovascular disease (CVD) mortality. A comprehensive literature search was performed to determine relevant publications through the online databases including PubMed, Scopus, and Web of Science up to 29 November 2020. Prospective observational studies assessing the association between circulating AGEs or sRAGE and all-cause or CVD mortality were included. Seven studies with a total of 3718 participants and 733 mortality cases (345 CVD deaths) were included in the meta-analysis for assessing the association between circulating AGEs and mortality. Our results showed that higher circulating AGEs were associated with increased risk of all-cause (pooled effect measure: 1.05; 95% CI: 1.01, 1.09; P = 0.018, I2 = 77.7%) and CVD mortality (pooled effect measure: 1.08; 95% CI: 1.01, 1.14; P = 0.015, I2 = 80.2%), respectively. The association between sRAGE and mortality was assessed in 14 studies with a total of 16,335 participants and 2844 mortality cases (419 CVD deaths). Serum concentrations of sRAGE were not associated with the risk of all-cause mortality (pooled effect measure: 1.01; 95% CI: 1.00, 1.01; P = 0.205, I2 = 75.5%), whereas there was a significant link between sRAGE and the risk of CVD mortality (pooled effect measure: 1.02; 95% CI: 1.00, 1.04; P = 0.02, I2 = 78.9%). Our findings showed that a higher serum AGE concentration was associated with increased risk of all-cause and CVD mortality. In addition, higher circulating sRAGE was related to increased risk of CVD mortality. This review was registered at PROSPERO as CRD42021236559.
Keywords: advanced glycation end products, soluble receptor for advanced glycation end products, AGEs, sRAGE, mortality, cardiovascular diseases
Statement of Significance: To our knowledge, this is the first study that systematically assessed the association of circulating AGEs or sRAGE with mortality from all-cause and CVDs. Higher circulating concentrations of AGEs were associated with all-cause and CVD mortality. In addition, higher circulating sRAGE was related to increased risk of CVD mortality.
Introduction
Advanced glycation end products (AGEs), such as pentosidine, N-carboxymethyl-lysine (CML), and N-carboxyethyl-lysine (CEL) are considered as a complex and diverse group of bioactive compounds generated via nonenzymatic glycation of proteins, lipids, and nucleic acids (1). AGEs are involved in the development of microvascular and macrovascular diseases by inducing covalent bonds with proteins such as collagen, increasing the formation of extracellular matrix compounds, and enhancing oxidative stress via interaction with their receptors (2). The 2 major sources of AGEs in the human body are the endogenous AGEs that are produced under hyperglycemic and oxidative stress conditions and exogenous AGEs found in foods, mainly highly processed foods (3). A positive relation between dietary AGEs and the serum AGE concentration has been reported (4). The role of both circulating and diet-derived AGEs in the development of chronic diseases such as metabolic syndrome and cardiovascular diseases (CVDs) has been implicated (5, 6). Moreover, interaction of circulating AGEs with their multiligand receptors (RAGEs) leads to generation of reactive oxygen species (ROS) and induction of several inflammatory signaling cascades including activation of the NF-κB–ROS pathway (7, 8). RAGE is known as a transmembrane glycoprotein that belongs to the immunoglobulin superfamily. In addition to the cell surface form, there is also a soluble isoform of RAGE (sRAGE), circulating in blood and other body fluids (9). sRAGE functions as a decoy receptor, binding to circulating AGEs, thus inhibiting intracellular RAGE signaling cascades and related inflammatory responses (10). Although the precise function of sRAGE is not fully understood, evidence indicates a protective role for this molecule against several age-related diseases. Indeed, the plasma concentration of sRAGE has been reported to be reduced in conditions such as cardiometabolic diseases (11, 12). However, increased concentrations of sRAGE in diabetic and or end-stage renal disease (ESRD) patients have been reported (13, 14). Additionally, several studies suggested that high circulating concentrations of sRAGE are a consequence of increased RAGE activation and ongoing inflammation (9, 15). Despite suggested mechanisms and the observed relation with chronic diseases, evidence regarding AGEs or sRAGE as beneficial biomarkers for assessing the risk of mortality is equivocal (16). Findings from a previous meta-analysis showed that skin autofluorescence-indicated AGEs were positively associated with increased risk of mortality in high-risk subjects (17). However, to our knowledge, there is no meta-analysis assessing the relation of plasma AGEs and sRAGE with mortality, except for one that was conducted in patients suffering from acute respiratory distress syndrome (18). Therefore, this systematic review and meta-analysis aimed to evaluate the association of circulating AGEs and sRAGE with all-cause and cardiovascular mortality in the general population and/or patients with chronic conditions.
Methods
The present systematic review and meta-analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (19). The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42021236559.
Search strategy
A comprehensive literature search was performed to determine relevant publications through the online databases including PubMed, Scopus, and Web of Science up to 29 November 2020. Medical Subject Heading (MeSH) terms related to AGEs and sRAGE in combination with keywords related to mortality were searched (Supplemental Table 1). In addition, the first 4 pages of Google Scholar and the references lists of included articles were assessed to determine other, potentially relevant, articles. The literature search was not limited by language or date. Two reviewers independently evaluated the output of the literature search to identify eligible articles. Any disagreement was resolved by discussion and consultation with the first corresponding author (HA).
Inclusion criteria
Titles, abstracts, and full texts of the studies were screened by 2 independent authors and any disagreements were resolved through consultation with the first corresponding author author (HA).
We considered the following inclusion criteria for eligibility of the studies in this systematic review and meta-analysis: 1) all prospective observational studies that considered serum concentration of AGEs and/or sRAGE as the exposure and mortality from all-cause and/or CVD as the main outcome or as one of the outcomes; 2) studies conducted in general population or in patients with noncommunicable chronic disease; 3) studies that involved subjects aged >18 y old; and 4) publications in which ORs, RRs, or HRs with their 95% CIs were used to report the associations between serum concentration of AGEs or sRAGE and all-cause or CVD mortality.
Exclusion criteria
The letters, comments, reviews, meta-analyses, and ecological studies as well as studies performed in children or adolescents and in patients with cancer, sepsis, acute, or critical illness were excluded. We also excluded the studies that considered endogenous sRAGE as outcomes, a follow-up duration <1 y, studies reporting the effect measures for mortality in combination with morbidity, studies with insufficient data, and those that reported arbitrary units (AUs) for exposures instead of conventional units.
Data extraction
After reviewing the full text of identified studies, all required data were separately extracted by 2 investigators, based on a predefined screening form that was checked by a third researcher. Extracted information for each eligible article was as follows: the last name of the first author, publication year, study design, country, participant's characteristics, duration of follow-up, exposure, method used for outcome assessment, comparison categories, and relevant effect measures of comparison categories with the corresponding 95% CI. Studies that reported the data for subjects with or without diabetes mellitus (DM)/renal dysfunction separately were considered as distinct studies. In the studies in which several risk estimates were presented for mortality risk, we selected the fully adjusted effect measures. In the cases that published data were insufficient for meta-analysis, required information was requested by contacting the corresponding author of the original studies. Cross-checking of all data was performed to minimize potential errors, and any disagreement in extracted data was resolved through discussion.
Risk-of-bias assessment
The quality of studies was evaluated using the Newcastle-Ottawa Scale for observational studies (20). This checklist consists of 3 main sections assessing 1) selection, 2) comparability, and 3) outcomes. A maximum score of 9 for each study represents the highest quality. In the current analysis, quality scores >6 were considered high-quality and scores ≤6 were considered low-quality studies. Any disagreement between researchers was resolved by the principal investigator.
Statistical analysis
All extracted effect measures (HRs, ORs, and RRs) were considered as identical to RRs. Log RRs with their relevant SEs were calculated using RRs and their 95% CI. To take between-study heterogeneity into account, the overall effect measures were calculated using a random-effects model (21). Cochrane's Q test and I2 were used to assess between-study heterogeneity (22). Sensitivity analysis was performed to detect whether overall effect measures might be dependent on a specific study (23). Moreover, publication bias was checked using visual examination of funnel plots asymmetry and evaluated statistically by Egger's regression asymmetry test. A trim-and-fill analysis was done to explore the influence of possible unpublished articles on the obtained overall results (24, 25). To identify possible sources of heterogeneity, we performed a subgroup analysis. The subgroup analyses were based on type of AGEs, health status of study participants, participants’ age, duration of follow-up, effect measure type, and statistical adjustment for major confounding variables [BMI, age, sex, smoking, chronic disease morbidity, and C-reactive protein (CRP)]. The method developed by Greenland and Longnecker (26) and Orsini et al. (27) was applied to measure the linear dose–response relation across categories of circulating AGEs or sRAGE. In the current method, the distribution of mortality cases and the adjusted risk estimates (ORs, RRs, or HRs) for at least 3 categories of circulating AGEs or sRAGE were needed. The midpoint of exposure was considered in each separate category. If the amount of the exposure was reported as a range, we computed the midpoint in each distinct category using the mean of the minimum and maximum measures. For open-ended categories (lowest and highest categories), the lengths of their intervals were considered to be identical to those of the closest intervals. If an effect measure indicted the lowest versus the highest category of exposures, we calculated the highest compared with the lowest estimates by the Orsini et al. (27) approach (Supplemental Tables 2–4). STATA's metan procedure was used for statistical analyses (version 14.2; StataCorp). Values <0.05 were statistically considered significant.
Results
Literature search
Figure 1 presents the detailed processes of study selection. Overall, 9581 publications were obtained through the primary search. After removing of duplicated and irrelevant studies, 43 potentially eligible studies remained for further evaluation. After assessment of full texts, another 23 publications were also removed: 11 articles that were conducted in patients suffering from cancer and acute or critical illness, 2 that reported combined mortality and morbidity for heart failure, 1 with insufficient data (28), 1 with a duration length <1 y, 1 that had a prediction for mortality, and 7 that reported arbitrary units instead conventional units of exposers (29–35). Thus, meta-analysis was done on the remaining 20 studies.
FIGURE 1.
Flow chart of study selection process.
Study characteristics
Table 1 presents the characteristics of the included articles investigating associations between circulating AGEs and mortality. Exposures were CML in 5 studies (36–40), CEL in 2 studies (39, 40), and pentosidine in 2 studies (41, 42). A total of 3718 participants and 733 mortality cases (345 CVD deaths) were included in the current meta-analysis. The endpoints were all-cause mortality in 7 studies (36–39, 41, 42) and CVD mortality in 6 studies (36–38, 40–42). There was 1 study with missing data on mortality cases (42). The included studies varied based on mean age from 42.5 to ≥65 y and follow-up length from 2.6 to 12.3 y. Except for 1 study (38), all the studies included both men and women. Although 2 studies were based on the older community-dwelling population (37, 38), 5 studies targeted special groups with different conditions: 2 on patients with ESRD (36, 41), 3 others on type 1 DM (39), kidney transplant recipients (40), or chronic kidney disease (CKD) (42). Except for 2 studies (36, 41), 5 remaining studies adjusted for potential confounders. Most studies adjusted for age (37–40, 42), sex (37, 39, 40, 42), and BMI (37–40). Some studies also controlled for some conventional risk factors, including chronic disease morbidity (37, 40, 42), renal function indices (37, 38, 40), smoking (39, 40), total cholesterol (37, 39), and high-sensitivity CRP (hs-CRP) (40, 42). As shown in Table 2, of 14 studies investigating associations between sRAGE and mortality, 5 reported both all-cause and CVD mortality (16, 38, 43–45), 8 reported all-cause mortality (46–53), and 1 study (54) was based on CVD mortality. A total of 16,335 participants with 2844 mortality cases (419 CVD deaths) were included in the analysis. One study did not report mortality cases (44). The mean duration of follow-up ranged from 1.9 to 18 y. Three studies had a community-based design (16, 45, 47), whereas 5 studies targeted special groups with different conditions: 3 on DM (44, 46, 50), 3 on dialysis patients (43, 51, 52), 2 on older subjects (38, 53), and 3 on CKD (48), kidney transplant recipients (49), or chronic heart failure (54). The mean age of participants ranged from 18 to 75 y. Most studies adjusted for age (16, 38, 43–53), sex (43–53), and chronic disease morbidity (16, 43–45, 48–51, 53). Some studies also controlled for several major risk factors, including BMI (16, 38, 45, 47, 49, 50), renal function indices (16, 38, 49, 53, 54), CRP (44, 47–49, 51), and smoking (16, 45–47, 50). In total, the included studies originated from the Netherlands (n = 3), United States (n = 3), Sweden (n = 3), Germany (n = 2), Spain (n = 2), Italy (n = 2), and single studies from Japan, Finland, Korea, and mixed countries.
TABLE 1.
Main characteristics of studies examining the association between circulating AGEs and the risk of mortality1
| First author/year (reference) | Country | Study design | Follow-up duration, y | Population | Age (mean or range), y | Sex | Exposure | Outcome assessment | Effect measures (95% CI) | Study quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | ||||||||||
| Schwedler et al./2002 (36) | Germany | Prospective cross-sectional study | 2.6 | Hemodialysis patients | 62.7 | M/F | CML | Continuous contacts with the primary care physicians | RR 0.97 (0.95, 1) | 6 |
| Koyama et al./2007 (41) | Japan | Cohort | 9.2 | Patients with ESRD | 55.6 | M/F | Pentosidine | Medical records | HR 1.04 (0.95, 1.14) | 6 |
| Semba et al./2009 (37) | Italy | Cohort | 6 | Older community-dwelling adults | ≥65 | M/F | CML | Death certificates | HR 1.13 (1.04, 1.2) | 8 |
| Semba et al./2009 (38) | Italy | Cohort | 4.5 | Older community-dwelling women | ≥65 | F | CML | National Death Index | HR 1.05 (0.99, 1.11) | 7 |
| Nin et al./2011 (39) | Netherlands | Cohort | 12.3 | T1DM | 42.5 | M/F | CML | Death certificates | HR 1.05 (0.98, 1.15) | 8 |
| Nin et al./2011 (39) | Netherlands | Cohort | 12.3 | T1DM | 42.5 | M/F | CEL | Death certificates | HR 1.33 (0.98, 1.78) | 8 |
| Machowska et al./2016 (42) | Sweden | Prospective cross-sectional study | 5 | CKD | 61.6 | M/F | Pentosidine | NR | RR 1.01 (1, 1.02) | 6 |
| Sotomayor et al./2019 (40) | Netherlands | Cohort | 6.9 | Kidney transplant recipients | 51 | M/F | CML | NR | HR 1.19 (1, 1.43) | 7 |
| Sotomayor et al./2019 (40) | Netherlands | Cohort | 6.9 | Kidney transplant recipients | 51 | M/F | CEL | NR | HR 1.5 (1.12, 1.99) | 7 |
| CVD mortality | ||||||||||
| Schwedler et al./2002 (36) | Germany | Prospective cross-sectional study | 2.6 | Hemodialysis patients | 62.7 | M/F | CML | Continuous contacts with the primary care physicians | RR 0.99 (0.94, 1.02) | 6 |
| Koyama et al./2007 (41) | Japan | Cohort | 9.2 | Patients with ESRD | 55.6 | M/F | Pentosidine | Medical records | HR 1.03 (0.92, 1.17) | 6 |
| Semba et al./2009 (37) | Italy | Cohort | 6 | Older community-dwelling adults | ≥65 | M/F | CML | Death certificates | HR 1.16 (1.02, 1.3) | 8 |
| Semba et al./2009 (38) | Italy | Cohort | 4.5 | Older community-dwelling women | ≥65 | F | CML | National Death Index | HR 1.09 (1.01, 1.18) | 7 |
| Machowska et al./2016 (42) | Sweden | Prospective cross-sectional study | 5 | Patients with CKD | 61.6 | M/F | Pentosidine | NR | RR 1.01 (1, 1.02) | 8 |
| Sotomayor et al./2019 (40) | Netherlands | Cohort | 6.9 | Kidney transplant recipients | 51 | M/F | CML | NR | HR 1.46 (1.19, 1.82) | 7 |
| Sotomayor et al./2019 (40) | Netherlands | Cohort | 6.9 | Kidney transplant recipients | 51 | M/F | CEL | NR | HR 1.84 (1.25, 2.71) | 7 |
AGE, advanced glycation end product; CEL, N-carboxyethyl-lysine; CKD, chronic kidney disease; CML, N-carboxymethyl-lysine; CVD, cardiovascular disease; NR, not reported; T1DM, type 1 diabetes mellitus.
TABLE 2.
Main characteristics of studies examining the association between sRAGE and the risk of mortality1
| First author/year (reference) | Country | Study design | Follow-up duration, y | Population | Age (mean or range), y | Sex | Outcome assessment | Effect measures (95% CI) | Study quality score |
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||||
| Gros et al./2007 (49) | Netherlands | Cohort | 4.2 | Renal transplant recipients | 51 | M/F | Follow-up | HR 0.98 (0.97, 0.99) | 7 |
| Semba et al./2009 (38) | Italy | Cohort | 4.5 | Older community-dwelling women | ≥65 | F | National Death Index | HR 1.03 (0.99, 1.06) | 7 |
| Nakashima et al./2010 (43) | Sweden | Prospective case-control | 3.4 | Hemodialysis patients | 67 | M/F | NR | HR 1.00 (0.99, 1.01) | 6 |
| Nin et al./2010 (46) | Netherlands | Cohort | 12.3 | T1DM | ≥18 | M/F | Death certificates | HR 1.04 (1.01, 1.07) | 8 |
| Thomas et al./2011 (44) | Finland | Cohort | 9.1 | T1DM | 38 | M/F | National Death Registry | HR 1.03 (1.01, 1.05) | 7 |
| Selvin et al. 2013 (47) | USA | Cohort | 18 | Middle-aged adults | 56.6 | M/F | Hospital surveillance | HR 0.98 (0.96, 0.99) | 9 |
| Isoyama et al. 2014 (48) | Sweden | Cohort | 1.9 | CKD | 56 | M/F | NR | HR 1.01 (0.98, 1.04) | 6 |
| Thomas et al./2015 (50) | Mixed countries | Case-cohort | 5 | T2DM | 66.9 | M/F | NR | HR 1.00 (1.00, 1.01) | 6 |
| Jung et al./2017 (51) | Korea | Cohort | 3.6 | Hemodialysis patients | 58.1 | M/F | NR | HR 1.00 (0.99, 1.01) | 5 |
| Dozio et al./2018 (52) | Italy | Cohort | 2 | Dialysis patients | 63.7 | M/F | NR | OR 1.04 (0.99, 1.09) | 6 |
| Ho et al./2018 (45) | USA | Cohort | 14.3 | General population | 62 | M/F | Follow-up with annual health history | HR 0.99 (0.99, 1.00) | 9 |
| Butcher et al./2019 (53) | Spain | Cohort | 6 | Older adults | 75 | M/F | National Death Index | HR 1.03 (1.00, 1.05) | 7 |
| Ebert et al./2020 (16) | Germany | Cohort | 12 | Men without DM or renal dysfunction | 65 | M | Health department of the city | HR 1.00 (0.99, 1.01) | 9 |
| Ebert et al./2020 (16) | Germany | Cohort | 12 | Women without DM or renal dysfunction | 64 | F | Health department of the city | HR 1.01 (0.99, 1.02) | 9 |
| Ebert et al./2020 (16) | Germany | Cohort | 12 | Men with DM or renal dysfunction | 65 | M | Health department of the city | HR 0.99 (0.98, 1.01) | 9 |
| Ebert et al./2019(16) | Germany | Cohort | 12 | Women with DM or renal dysfunction | 64 | F | Health department of the city | HR 1.00 (0.99, 1.01) | 9 |
| CVD mortality | |||||||||
| Semba et al./2009 (38) | Italy | Cohort | 4.5 | Older community-dwelling women | ≥65 | F | National Death Index | HR 1.04 (0.99, 1.09) | 7 |
| Nakashima et al./2010 (43) | Sweden | Prospective case-control | 3.4 | Hemodialysis patients | 67 | M/F | NR | HR 1.00 (0.99, 1.02) | 6 |
| Raposeiras-Roubín et al./2011 (54) | Spain | Cohort | 1.3 | Outpatients with CHF | 72 | M/F | Follow-up | HR 1.26 (1.09, 1.45) | 6 |
| Thomas et al./2011 (44) | Finland | Cohort | 9.1 | T1DM | 38 | M/F | National Death Registry | HR 1.06 (1.03, 1.09) | 7 |
| Ho et al./2018 (45) | USA | Cohort | 14.3 | General population | 62 | M/F | Follow-up with annual health history | HR 1.02 (1.00, 1.03) | 9 |
| Ebert et al./2020 (16) | Germany | Cohort | 12 | General population | 65 | M | Health department of the city | HR 0.99 (0.98, 1.00) | 9 |
| Ebert et al./2020 (16) | Germany | Cohort | 12 | General population | 64 | F | Health department of the city | HR 1.01 (0.99, 1.02) | 9 |
CHF, chronic heart failure; CKD, chronic kidney disease; CVD, cardiovascular diseases; DM, diabetes mellitus; NR, not reported; sRAGE, soluble receptor for advanced glycation end products; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Meta-analysis
AGEs and all-cause mortality
Seven studies with 9 effect measures analyzed the association of circulating concentrations of AGEs with the risk of all-cause mortality. There was a significant positive association between serum AGE concentration and overall mortality, as a 100-μg/L increment in serum AGEs was associated with a 5% higher risk of all-cause mortality (pooled effect measure: 1.05; 95% CI: 1.01, 1.09; P = 0.018) and there was evidence of substantial heterogeneity (I2 = 77.7%, P < 0.01) (Figure 2). In the subgroup analysis (Table 3), CEL (effect measure: 1.42; 95% CI: 1.15, 1.74) and pentosidine (effect measure: 1.01; 95% CI: 1.00, 1.02) concentrations had stronger positive associations with the risk of all-cause mortality. As well, stronger positive associations between serum AGEs and mortality risk were observed in populations without DM and renal dysfunction (effect measure: 1.08; 95% CI: 1.03, 1.13), in subjects aged <60 y (effect measure: 1.08; 95% CI: 1.02, 1.14), among those studies with a follow-up duration >6 y (effect measure: 1.10; 95% CI: 1.05, 1.15) (Table 3), and those that reported an HR for their analysis and studies that adjusted for potential confounders including BMI, age, sex, and smoking (Supplemental Table 5). A sensitivity analysis indicated that the association of circulating AGEs and all-cause mortality largely depended on the study conducted by Semba et al. (37), so that, after elimination of this study from analysis, the pooled estimate was not significant (effect measure: 1.03; 95% CI: 0.99, 1.07) compared with that from the main analysis. While visual inspection of the funnel plot showed evidence of asymmetry, Egger's test (P = 0.052) did not confirm publication bias evidence among studies.
FIGURE 2.
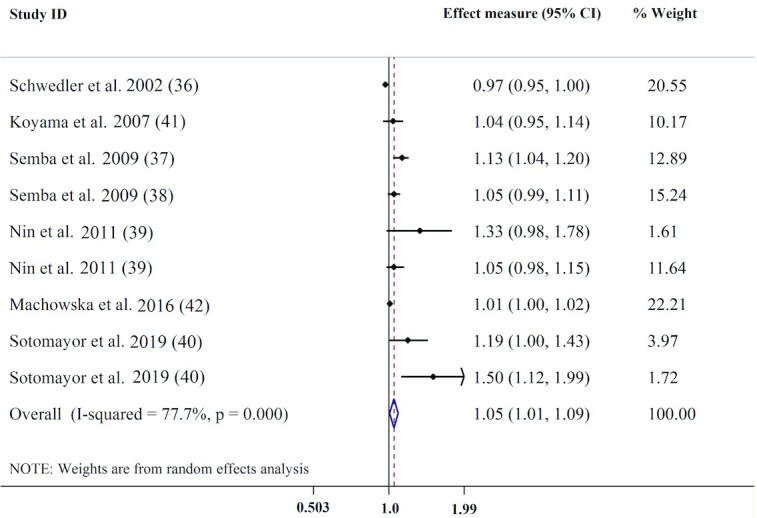
Forest plot for the association between circulating AGEs and all-cause mortality. AGE, advance glycation end product.
TABLE 3.
Subgroup analysis for the association of circulating AGEs and the risk of mortality1
| Variables | Number of effect measures | I2 | Effect measures (95% CI) | P value 2 |
|---|---|---|---|---|
| All-cause mortality | ||||
| Overall | 9 | 77.7 | 1.05 (1.01, 1.09) | 0.018 |
| AGE type | ||||
| CML | 5 | 83.6 | 1.00 (0.98, 1.02) | 0.005 |
| CEL | 2 | 0.00 | 1.42 (1.15, 1.74) | |
| Pentosidine | 2 | 0.00 | 1.01 (1.00, 1.02) | |
| Health status | ||||
| DM or renal dysfunction | 7 | 75.1 | 1.01 (1.00, 1.02) | 0.002 |
| Without DM or renal dysfunction | 2 | 59.5 | 1.08 (1.03, 1.13) | |
| Follow-up duration | ||||
| ≤6 y | 3 | 81.0 | 1.01 (1.00, 1.02) | <0.001 |
| >6 y | 6 | 50.3 | 1.10 (1.05, 1.15) | |
| Participants’ age | ||||
| <60 y | 5 | 56.3 | 1.08 (1.02, 1.14) | 0.013 |
| ≥60 y | 4 | 85.4 | 1.01 (1.00, 1.02) | |
| CVD mortality | ||||
| Overall | 7 | 80.2 | 1.08 (1.01, 1.14) | 0.015 |
| AGE type | ||||
| CML | 4 | 84.8 | 1.03 (1.00, 1.07) | 0.005 |
| CEL | 1 | — | 1.84 (1.25, 2.71) | |
| Pentosidine | 2 | 0.0 | 1.01 (1.00, 1.02) | |
| Health status | ||||
| DM or renal dysfunction | 5 | 81.7 | 1.01 (1.00, 1.02) | 0.005 |
| Without DM or renal dysfunction | 2 | 0.0 | 1.11 (1.04, 1.19) | |
| Follow-up duration | ||||
| ≤6 y | 4 | 36.3 | 1.01 (1.00, 1.02) | <0.001 |
| >6 y | 3 | 72.6 | 1.26 (1.14, 1.40) | |
| Participants’ age | ||||
| <60 y | 3 | 85.4 | 1.16 (1.05, 1.28) | 0.008 |
| ≥60 y | 4 | 68.7 | 1.01 (1.00, 1.02) | |
| Adjustment | ||||
| Adjusted | 5 | 86.2 | 1.01 (1.00, 1.02) | 0.346 |
| Not adjusted | 2 | 0.0 | 0.99 (0.96, 1.03) | |
AGE, advanced glycation end product; CEL, N-carboxyethyl-lysine; CML, N-carboxymethyl-lysine; CVD, cardiovascular disease; DM, diabetes mellitus.
P value for overall effect and subgroup differences.
AGEs and CVD mortality
Seven effect measures extracted from 6 studies were included for meta-analysis of the serum concentration of AGEs and CVD mortality. Higher serum AGE concentration was noticeably associated with higher pooled effect measures for CVD mortality, as a 100-μg/L increment in serum AGE was associated with 8% higher risk of CVD mortality (pooled effect measure: 1.08; 95% CI: 1.01, 1.14; P = 0.015). There was substantial evidence of heterogeneity across studies (I2 = 80.2%, P < 0.01) (Figure 3). Based on subgroup analysis, there was a stronger association between CEL (effect measure: 1.84; 95% CI: 1.25, 2.71) concentration and the risk of CVD mortality. The positive association of serum concentrations of AGEs with the risk of CVD mortality was also stronger in the population without DM and renal dysfunction (effect measure : 1.11; 95% CI: 1.04, 1.19), in subjects aged <60 y (effect measure: 1.16; 95% CI: 1.05, 1.28), among studies with a follow-up duration of >6 y (effect measure: 1.26; 95% CI: 1.14, 1.40) (Table 3), those that reported an HR for their analysis, and studies that adjusted for potential confounders including age and smoking (Supplemental Table 5). A sensitivity analysis revealed that 2 effect measures from studies conducted by Sotomayor et al. (40) and Semba et al. (37) had the largest effect on overall effect measure. After exclusion of the effect measure related to CVD mortality in older adults (37), the pooled estimate was not significant compared with that from the main analysis. Removing the effect measures related to CML (40) also modified the significance for CVD mortality. Visual examination of the funnel plot as well as the results from Egger's test (P = 0.03) showed possible evidence of publication bias across studies. Thus, the trim-and-fill method was used and showed that adding missed studies changed the overall effect measure (pooled effect measure: 1.02; 95% CI: 0.95, 1.09).
FIGURE 3.
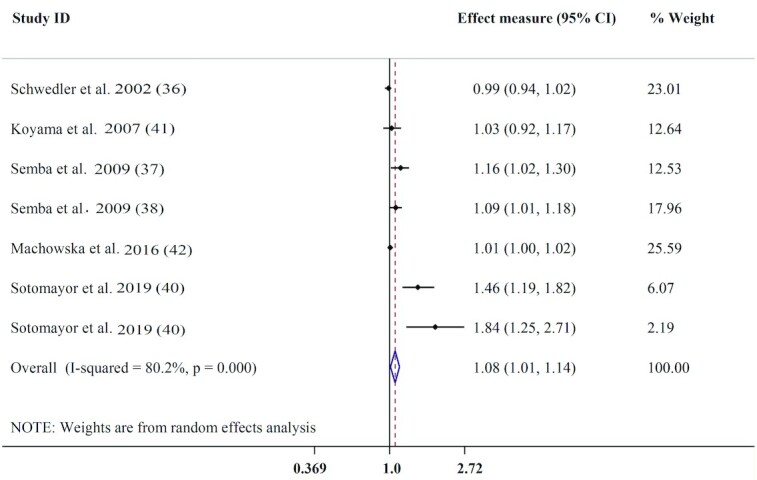
Forest plot for the association between circulating AGEs and CVD mortality. AGE, advance glycation end product; CVD, cardiovascular disease.
sRAGEs and all-cause mortality
Sixteen effect measures from 13 articles were included in the meta-analysis of association between circulating sRAGE concentration and all-cause mortality. Serum concentrations of sRAGE (per 100 ng/L) were not associated with the risk of all-cause mortality (pooled effect measure: 1.01; 95% CI: 1.00, 1.01; P = 0.205) (Figure 4). There was also substantial heterogeneity between studies (I2 = 75.5%, P < 0.001). Subgroup analysis showed stronger positive associations between serum sRAGE and all-cause mortality in subjects aged ≥60 y (effect measure: 1.00; 95% CI: 1.00, 1.01) (Table 4), and among studies that did not adjust for BMI, chronic disease morbidity, and CRP (Supplemental Table 6). A sensitivity analysis indicated that excluding each study had no significant effect on the pooled effect measure. While visual examination of the funnel plot and Egger's test (P = 0.043) showed evidence of publication bias, Begg's test did not confirm evidence of publication bias (P = 0.09). We ran the trim-and-fill method and observed that adding missed studies did not alter the overall effect measure (pooled effect measure: 1.00; 95% CI: 0.99, 1.01).
FIGURE 4.
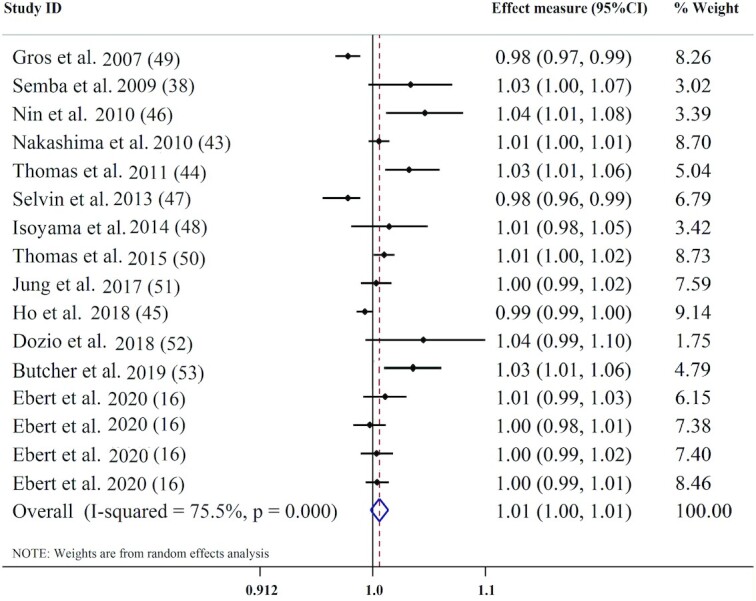
Forest plot for association between circulating sRAGE and all-cause mortality. sRAGE, soluble receptor for advance glycation end products.
TABLE 4.
Subgroup analysis for the association of sRAGE and the risk of mortality1
| Variables | Number of effect measures | I2 | Effect measures (95% CI) | P value 2 |
|---|---|---|---|---|
| All-cause mortality | ||||
| Overall | 16 | 75.5 | 1.01 (1.00, 1.01) | 0.205 |
| Health status | ||||
| DM or renal dysfunction | 10 | 76.7 | 1.00 (1.00, 1.01) | 0.209 |
| Without DM or renal dysfunction | 6 | 74.5 | 1.00 (0.99, 1.00) | |
| Follow-up duration | ||||
| ≤6 y | 8 | 77.7 | 1.00 (1.00,1.01) | 0.240 |
| >6 y | 8 | 74.2 | 1.00 (0.99, 1.00) | |
| Participants’ age | ||||
| <60 y | 6 | 84.8 | 0.99 (0.99, 1.00) | 0.016 |
| ≥60 y | 10 | 59.1 | 1.00 (1.00, 1.01) | |
| CVD mortality | ||||
| Overall | 7 | 78.9 | 1.02 (1.00, 1.04) | 0.020 |
| Health status | ||||
| DM or renal dysfunction | 2 | 89.7 | 1.02 (1.01, 1.03) | 0.190 |
| Without DM or renal dysfunction | 5 | 76.5 | 1.01 (1.00, 1.02) | |
| Follow-up duration | ||||
| ≤6 y | 3 | 81.8 | 1.01 (1.00, 1.03) | 0.734 |
| >6 y | 4 | 82.7 | 1.01 (1.00, 1.02) | |
CVD, cardiovascular disease; DM, diabetes mellitus; sRAGE, soluble receptor for advanced glycation end products.
P value for overall effect and subgroup differences
sRAGEs and CVD mortality
Seven effect measures from 6 publications were analyzed for the association between sRAGE concentration and CVD mortality. The results revealed that, per a 100-ng/L increment in serum sRAGE, the risk of CVD mortality significantly increased by 2% (pooled effect measure: 1.02; 95% CI: 1.00, 1.04; P = 0.020), with evidence of substantial heterogeneity (I2 = 78.9%, P < 0.001) (Figure 5). Subgrouping based on health status and follow-up duration did not modify the positive association between circulating sRAGE concentrations and the risk of CVD mortality and heterogeneity (Table 4). However, stronger positive associations between serum sRAGE and CVD mortality were seen among studies that did not adjust for age, smoking, and chronic disease morbidity (Supplemental Table 6). Additionally, subgroup analysis based on participants’ age was not possible due to the small number of related studies. A sensitivity analysis indicated that the pooled effect measure shifted to the nonsignificant level after excluding the study conducted by Thomas et al. (44) (effect measure: 1.01; 95% CI: 0.99, 1.02). Visual inspection of the funnel plot and Egger's test (P = 0.016) showed evidence of publication bias. Therefore, we used the trim-and-fill method and found that adding missed studies altered the overall estimate for CVD mortality (pooled effect measure: 0.99; 95% CI: 0.99, 1.03).
FIGURE 5.
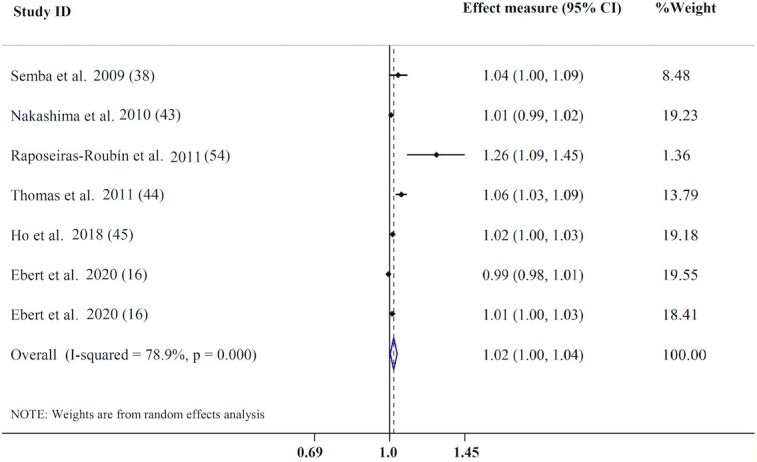
Forest plot for association between circulating sRAGE and CVD mortality. CVD, cardiovascular disease; sRAGE, soluble receptor for advance glycation end products.
Quality assessment
Supplemental Table 7 presents the quality assessments for the included studies. Quality scores of cohort studies with 9-point scales were 7 in 5 studies, 6 in 5 studies, 9 in 3 studies, 8 in 3 studies, and 5 in 1 study. Appraisal of included cross-sectional (with 10-point scales) and case-control (with 9-point scales) studies also led to average quality scores of 6–8 and 6, respectively.
Discussion
This systematic review and meta-analysis revealed a significant positive relation between circulating AGEs and death from all causes and CVDs. The positive association between serum AGE concentration and mortality from all causes and CVDs was stronger in studies conducted in populations without diabetes or renal dysfunction; those conducted in subjects <60 y; studies that controlled for BMI, age, sex, and smoking (for all-cause mortality); those that adjusted for age and smoking (for CVD mortality); and those with follow-up >6 y. Furthermore, subgroup analysis based on type of AGEs revealed a higher association of CEL and pentosidine with all-cause mortality. Moreover, CEL concentrations were more strongly related to the risk of CVD mortality. With regard to sRAGE, our findings showed no clear relation between circulating sRAGE and all-cause mortality. However, circulating concentration of sRAGE was positively associated with increased risk of CVD mortality. The positive association between serum concentration of sRAGE and CVD mortality was stronger among studies that did not adjust for age, smoking, and chronic disease morbidity. To the best of our knowledge, this is the first study that systematically reviewed the existing literature on the association of circulating AGEs or sRAGE with mortality from all causes and CVDs. In this study, we showed that each 100-μg/L increment in serum AGEs was associated with 5% and 8% higher risk of all-cause and CVD mortality, respectively. In line with the present finding, a previous meta-analysis showed that skin autofluorescence-indicated AGE concentrations were significantly correlated with increased risk of all-cause mortality in high-risk participants (17). The pivotal role of AGEs in the pathogenesis of DM, CVD, and renal disease has been implicated (55). The 2 major sources of circulating AGEs are endogenous-form and diet-derived AGEs. Approximately 10% of ingested diet-derived AGEs are absorbed, of which approximately two-thirds is accumulated in tissues and one-third excreted (56, 57). Restriction of dietary AGEs was associated with lower risk of metabolic syndrome and CVD (5, 6). In animals, a low-AGE diet increased life span in a magnitude that was comparable to the energy restriction (58). In 2 population-based studies, Kilhovd et al. (30, 34) showed that increased serum AGE concentrations in women with and without type 2 diabetes were associated with increased risk of total and CVD mortality, suggesting that the role of AGEs in mortality may not be related to diabetes. Conversely, in hemodialysis patients, high concentrations of serum fluorescent AGEs and CML were not linked to increased mortality and were even associated with better survival (36). Among circulating AGEs, CML (a potential ligand for RAGE), CEL (a marker of intracellular glycation), and pentosidine (a cross-link AGE) were the most assessed AGEs in the primary studies. Given their molecular properties, it may be thought that each AGE could induce detrimental effects via different pathways. In this regard, our subgroup analysis revealed that CEL and pentosidine were more associated with all-cause mortality compared with CML. CEL was also more associated with CVD mortality compared with the other 2 AGEs. However, Nin et al. (39) in a study in type 1 diabetic patients demonstrated that none of these 3 AGEs were independently associated with mortality. The authors also suggested that the destructive impacts of CEL, CML, and pentosidine on CVD risk largely overlap. Nevertheless, the results of our analysis should be interpreted with caution due to the high heterogeneity between studies on CML and the small number of studies conducted on CEL and pentosidine. Furthermore, of the 2 studies investigating the link of pentosidine with all-cause mortality, only 1 study (42) controlled for the confounding variables. Interestingly, the results of subgroup analysis for health status showed that the association of circulating AGEs with both CVD and all-cause mortality was more noticeable in patients without diabetes or renal dysfunction. The pivotal role of chronic hyperglycemia in the pathogenesis of atherosclerosis and CVD outcomes in subjects with DM or CKD is undeniable (59, 60). However, detection of AGEs in the atheroma of nondiabetic subjects with concomitant coronary heart disease has indicted the critical role of AGEs and oxidative stress in the development of atherosclerosis (61). In CKD patients, the contribution of circulating AGEs to the development of CVD and mortality is debatable and other conventional risk factors (e.g., CRP and diabetes) in ESRD patients seem to exert a more crucial role for cardiovascular events than elevated serum AGE concentration (62, 63). However, the number of studies in the subgroup of “without DM or renal dysfunction” was very small (n = 2 studies) to discern a definitive conclusion. A subgroup analysis by age showed that a higher concentration of circulating AGEs was more associated with the risk of all-cause and CVD mortality in subjects <60 y old. Although this finding is in contrast to the previous studies indicating the role of AGEs in pathogenesis of many age-related complications, understanding the association between age-related diseases and circulating AGEs has been difficult, due to the following reasons: 1) the existing variety among AGEs; 2) the slow formation of AGEs, which may take decades to identify in humans; and 3) lack of appropriate techniques to quantify a target AGE (64). In the interpretation of our results, the role of other contributing factors such as dietary intake in the association with mortality should not be ignored. Although we use the most fully adjusted effect measures, our findings might still be influenced by other confounding factors, as none of the included studies controlled for dietary factors. Of the 3 studies conducted in subjects aged <60 y, one did not control for confounding variables (41). Additionally, the small number of studies included in the subgroup of individuals aged >60 y and the high heterogeneity between studies make our findings difficult to interpret. Nevertheless, the role of AGEs in the progression of chronic age-related complications and related mortality in younger patients cannot be ruled out (65–67).
In the field of age-related complications, more attention has been directed toward sRAGE, a circulating receptor that mediates cytoprotective effects against deleterious events caused by the interaction of circulating AGEs with RAGE (68). Nevertheless, findings on sRAGE concentrations in cardiometabolic diseases and mortality are equivocal (69, 70). In this study, each 100-ng/L increment in circulating sRAGE was associated with a 2% higher risk of CVD mortality, while no association between sRAGE and all-cause mortality was observed. This finding is in contrast with previous reports indicating the protective role of sRAGE against CVD and related mortality (35, 47). In a cohort study by Larsen et al. (35), increased serum sRAGE concentrations were linked to reduced risk for incident of major cardiovascular events over 16.5 y and morality during 21 y of follow-up in middle-aged subjects without previously confirmed CVD. Steady-state circulating sRAGE may represent favorable effects in the general population, but its association with mortality appears to be complicated and affected partially by the pathophysiological background. Despite its protective role through blocking intracellular signaling, circulating concentrations of sRAGE may not be necessarily sufficient to counteract proinflammatory binding components of RAGE, including AGEs (71). On the other hand, the increased sRAGE concentrations may be the result of the compensatory increase in the AGE–RAGE axis activity by stress and subsequently increased RAGE shedding into the circulation (9). Therefore, the discrepancy between our findings with previous studies might be related to the different pathophysiological condition of patients in each study. In 2 population-based cohorts, plasma sRAGE was an independent predictor for mortality among only frail older subjects, even after adjusting for confounding variables. Indeed, sRAGE could be a useful prognostic biomarker for risk evaluation and stratification (sRAGE threshold: 1800 pg/mL) of frail older individuals (53). In additoin, in community-dwelling women, elevated circuiting sRAGE concentrations were linked to increased risk of mortality. This was the same for our subgroup analysis that revealed a more significant relation between sRAGE and all-cause mortality in subjects aged >60 y. Recent evidence indicates that aging is not merely an aspect of being old but also, besides natural aging, acute and serious stressors can contribute to the rapid formation of AGEs. Aging is particularly accompanied with reduced anti-AGE defense; therefore, as previously mentioned, aging can contribute to the increased AGE–RAGE axis activity, RAGE shedding, and increased concentrations of circulating sRAGE. On the other hand, increased AGE–RAGE axis activity contributes to further generation of ROS, which, in turn, causes further AGE formation, inflammation, and finally tissue dysfunction (72). Nevertheless, whether increased concentrations of sRAGE are a biological response to bind circulating AGEs and thus preventing AGE–RAGE interaction is not fully understood. An inverse association between plasma sRAGE and renal function has been reported in previous studies. Therefore, the high concentrations of sRAGE might be related to the declined renal function during aging (48, 73). As a biomarker of morbidity risk, it seems that sRAGE can act differently in subjects with longstanding chronic disease. However, we failed to detect any association between circulating sRAGE and CVD mortality based on subjects’ pathophysiological condition. More studies focusing on the role of sRAGE in healthy populations and those with compromised conditions are needed to discern a definitive conclusion.
The present review is accompanied by several strengths. As mentioned previously, this is the first systematic review and meta-analysis, to our knowledge, that assessed circulating concentrations of AGEs or sRAGE in association with all-cause and CVD mortality. Considering the relation between circulating AGEs and sRAGE, this study could present a more detailed picture of the relation of serum AGEs or sRAGE concentrations with mortality. In addition, we considered the most common types of circulating AGEs, including CEL, CML, and pentosidine, to evaluate the association of each separate AGE with mortality.
Conversely, the present study faced some limitations that could compromise our findings. We performed a meta-analysis of observational studies; thus, our results cannot be interpreted as a causal association. In general, higher circulating AGEs are associated with higher endogenous formation and/or higher dietary intakes of AGEs. Therefore, short-term generation of AGEs due to dietary intake may not be directly linked to chronic diseases. Although most studies adjusted for confounding variables, none controlled for dietary intakes of AGEs or other nutrients present in the diet. As the reduced AGE intake was associated with lower circulating AGE concentration, further long-term studies are needed to investigate the effect of the restriction of diet-derived AGEs on circulating concentrations of AGEs or sRAGE and their related mortality effect. As most of the included studies controlled for different sets of covariates that may lead to different interpretations, caution should be taken when interpreting our results. The cause of death in the description of all-cause mortality could differ among included studies; thus, our findings on the associations of serum AGE and sRAGE concentrations with the risk of mortality could be affected potentially by misclassification bias. As there was evidence of noticeable publication bias for the relation of circulating AGEs and sRAGE with CVD mortality, our findings for CVD mortality could be modified by the results of unpublished studies.
In conclusion, in this study we found that each 100-μg/L increment in the serum AGE concentration was associated with 5% and 8% higher risks of all-cause and CVD mortality, respectively, which is consistent with its deleterious effects on cardiometabolic and other age-related diseases. Moreover, each 100-ng/L increment in circulating sRAGE was associated with a 2% higher risk of CVD mortality. The application of these findings in the general population should be done prudently due to high heterogeneity between studies included in this meta-analysis. Although our findings can be used to assess the risk of cardiovascular and all-cause mortality, more well-designed prospective observational studies considering other confounding variables (dietary intakes) are required to assess the association of circulating AGEs and sRAGE with mortality and also to establish an optimal circulation of circulating AGEs and particularly sRAGE in different pathophysiological contexts.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by the Student Research Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (no. 3011193). The authors’ responsibilities were as follows—ES-Z and FHS: contributed to the study conception, study design, data collection, interpretation, and drafting the manuscript; MM and NBR: contributed to data extraction; HA: participated in the study conception, revising the paper critically, and approving the version of the manuscript being submitted; FHS: conducted statistical analysis; and all authors: read and approved final manuscript.
Notes
This study was funded by the Student Research Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran. (no. 3011193).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AGE, advanced glycation end product; CEL, N-carboxyethyl-lysine; CKD, chronic kidney disease; CML, N-carboxymethyl-lysine; CRP, C-reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; ESRD, end-stage renal disease; RAGE, receptor(s) for advanced glycation end products; ROS, reactive oxygen species; sRAGE, soluble receptor(s) for advanced glycation end products.
Contributor Information
Elham Sharifi-Zahabi, Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Fatemeh Hajizadeh Sharafabad, Department of Clinical Nutrition, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Hadi Abdollahzad, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Mahsa Malekahmadi, Research Institute for Gastroenterology and Liver, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Nadya Bahari Rad, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- 1. Semba RD, Cotch MF, Gudnason V, Eiríksdottir G, Harris TB, Sun K, Klein R, Jonasson F, Ferrucci L, Schaumberg DA. Serum carboxymethyllysine, an advanced glycation end product, and age-related macular degeneration: the Age, Gene/Environment Susceptibility–Reykjavik Study. JAMA Ophthalmol. 2014;132(4):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma C, Kaur A, Thind S, Singh B, Raina S. Advanced glycation end-products (AGEs): an emerging concern for processed food industries. J Food Sci Technol. 2015;52(12):7561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagata C, Wada K, Yamakawa M, Nakashima Y, Koda S, Uji T, Oba S. Dietary intake of Nε-carboxymethyl-lysine, a major advanced glycation end product, is not associated with increased risk of mortality in Japanese adults in the Takayama Study. J Nutr. 2020;150(10):2799–805. [DOI] [PubMed] [Google Scholar]
- 4. Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, Yee K, Tansman L, Chen X, Mani V. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity?. J Clin Endocrinol Metab. 2015;100(5):1957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sohouli MH, Fatahi S, Sharifi-Zahabi E, HO S, Tripathi N, Lari A, Pourrajab B, Kord-Varkaneh H, Găman M-A, Shidfar F. The impact of low advanced glycation end products diet on metabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;12:766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohouli MH, Sharifi-Zahabi E, Lari A, Fatahi S, Shidfar F. The impact of low advanced glycation end products diet on obesity and related hormones: a systematic review and meta-analysis. Sci Rep. 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prasad K, Mishra M. AGE–RAGE stress, stressors, and antistressors in health and disease. Int J Angiology. 2018;27(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ott C, Jacobs K, Haucke E, Santos AN, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008;22(10):3716–27. [DOI] [PubMed] [Google Scholar]
- 10. Detzen L, Cheng B, Chen C-Y, Papapanou PN, Lalla E. Soluble forms of the receptor for advanced glycation endproducts (RAGE) in periodontitis. Sci Rep. 2019;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25(5):1032–7. [DOI] [PubMed] [Google Scholar]
- 12. Dozio E, Briganti S, Delnevo A, Vianello E, Ermetici F, Secchi F, Sardanelli F, Morricone L, Malavazos AE, Romanelli MMC. Relationship between soluble receptor for advanced glycation end products (sRAGE), body composition and fat distribution in healthy women. Eur J Nutr. 2017;56(8):2557–64. [DOI] [PubMed] [Google Scholar]
- 13. Challier M, Jacqueminet S, Benabdesselam O, Grimaldi A, Beaudeux J-L. Increased serum concentrations of soluble receptor for advanced glycation endproducts in patients with type 1 diabetes. Clin Chem. 2005;51(9):1749–50. [DOI] [PubMed] [Google Scholar]
- 14. Kalousová M, Hodková M, Kazderová M, Fialová J, Tesař V, Dusilová-Sulková S, Zima T. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47(3):406–11. [DOI] [PubMed] [Google Scholar]
- 15. Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, Charlton-Menys V, Bao W, DeMicco DA, Preston GM. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60(9):2379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebert H, Lacruz ME, Kluttig A, Simm A, Greiser KH, Tiller D, Kartschmit N, Mikolajczyk R. Association between advanced glycation end products, their soluble receptor, and mortality in the general population: results from the CARLA study. Exp Gerontol. 2020;131:110815. [DOI] [PubMed] [Google Scholar]
- 17. Cavero-Redondo I, Soriano-Cano A, Álvarez-Bueno C, Cunha PG, Martínez-Hortelano JA, Garrido-Miguel M, Berlanga-Macías C, Martínez-Vizcaíno V. Skin autofluorescence–indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jabaudon M, Blondonnet R, Pereira B, Cartin-Ceba R, Lichtenstern C, Mauri T, Determann RM, Drabek T, Hubmayr RD, Gajic O. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med. 2018;44(9):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cro S, Morris TP, Kenward MG, Carpenter JR. Reference-based sensitivity analysis via multiple imputation for longitudinal trials with protocol deviation. Stata J. 2016;16(2):443–63. [PMC free article] [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 27. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 28. Paradela-Dobarro B, Agra RM, Álvarez L, Varela-Román A, García-Acuña JM, González-Juanatey JR, Álvarez E, García-Seara FJ. The different roles for the advanced glycation end products axis in heart failure and acute coronary syndrome settings. Nutr Metab Cardiovasc Dis. 2019;29(10):1050–60. [DOI] [PubMed] [Google Scholar]
- 29. Hanssen NM, Beulens JW, Van Dieren S, Scheijen JL, Spijkerman AM, van der Schouw YT, Stehouwer CD, Schalkwijk CG. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes. 2015;64(1):257–65. [DOI] [PubMed] [Google Scholar]
- 30. Kilhovd B, Juutilainen A, Lehto S, Rönnemaa T, Torjesen P, Hanssen K, Laakso M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007;50(7):1409–17. [DOI] [PubMed] [Google Scholar]
- 31. Roberts MA, Thomas MC, Fernando D, Macmillan N, Power DA, Ierino FL. Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrology Dialysis Transplantation. 2006;21(6):1611–7. [DOI] [PubMed] [Google Scholar]
- 32. Suliman ME, Heimbürger O, Bárány P, Anderstam B, Pecoits-Filho R, Ayala ER, Qureshi AR, Fehrman-Ekholm I, Lindholm B, Stenvinkel P. Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J Am Soc Nephrol. 2003;14(6):1614–22. [DOI] [PubMed] [Google Scholar]
- 33. Wagner Z, Molnár M, Molnár GA, Tamaskó M, Laczy B, Wagner L, Csiky B, Heidland A, Nagy J, Wittmann I. Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis. 2006;47(2):294–300. [DOI] [PubMed] [Google Scholar]
- 34. Kilhovd BK, Juutilainen A, Lehto S, R̈önnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol. 2005;25(4):815–20. [DOI] [PubMed] [Google Scholar]
- 35. Grauen Larsen H, Marinkovic G, Nilsson PM, Nilsson J, Engström G, Melander O, Orho-Melander M, Schiopu A. High plasma sRAGE (soluble receptor for advanced glycation end products) is associated with slower carotid intima-media thickness progression and lower risk for first-time coronary events and mortality. Arterioscler Thromb Vasc Biol. 2019;39(5):925–33. [DOI] [PubMed] [Google Scholar]
- 36. Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002;62(1):301–10. [DOI] [PubMed] [Google Scholar]
- 37. Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57(10):1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, Walston J, Guralnik JM, Fried LP. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res. 2009;21(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving H-H, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011;34(2):442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sotomayor CG, Gomes-Neto AW, van Londen M, Gans RO, Nolte IM, Berger SP, Navis GJ, Rodrigo R, Leuvenink HG, Schalkwijk CG. Circulating advanced glycation endproducts and long-term risk of cardiovascular mortality in kidney transplant recipients. Clin J Am Soc Nephrol. 2019;14(10):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koyama H, Shoji T, Fukumoto S, Shinohara K, Shoji T, Emoto M, Mori K, Tahara H, Ishimura E, Kakiya R. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27(1):147–53. [DOI] [PubMed] [Google Scholar]
- 42. Machowska A, Sun J, Qureshi AR, Isoyama N, Leurs P, Anderstam B, Heimburger O, Barany P, Stenvinkel P, Lindholm B. Plasma pentosidine and its association with mortality in patients with chronic kidney disease. PLoS One. 2016;11(10):e0163826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Bárány P, Heimbürger O, Stenvinkel P, Lindholm B. Effect of circulating soluble receptor for advanced glycation end products (sRAGE) and the proinflammatory RAGE ligand (EN-RAGE, S100A12) on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas M, Söderlund J, Lehto M, Mäkinen V-P, Moran J, Cooper M, Forsblom C, Groop P-H. Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia. 2011;54(10):2669–77. [DOI] [PubMed] [Google Scholar]
- 45. Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, Hwang SJ, Massaro JM, Larson MG, Levy D. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc. 2018;7(14):e008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving H-H, Tarnow L, Rossing P, Stehouwer CD. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes. 2010;59(8):2027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selvin E, Halushka MK, Rawlings AM, Hoogeveen RC, Ballantyne CM, Coresh J, Astor BC. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes. 2013;62(6):2116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Isoyama N, Leurs P, Qureshi AR, Bruchfeld A, Anderstam B, Heimburger O, Bárány P, Stenvinkel P, Lindholm B. Plasma S100A12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease stage 5 patients. Nephrol Dialysis Transplant. 2015;30(1):84–91. [DOI] [PubMed] [Google Scholar]
- 49. Gross S, van Ree RM, Oterdoom LH, de Vries AP, van Son WJ, de Jong PE, Navis GJ, Zuurman MW, Bierhaus A, Gans RO. Low levels of sRAGE are associated with increased risk for mortality in renal transplant recipients. Transplantation. 2007;84(5):659–63. [DOI] [PubMed] [Google Scholar]
- 50. Thomas MC, Woodward M, Neal B, Li Q, Pickering R, Marre M, Williams B, Perkovic V, Cooper ME, Zoungas S. Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care. 2015;38(10):1891–7. [DOI] [PubMed] [Google Scholar]
- 51. Jung ES, Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY. Associations between soluble receptor for advanced glycation end products (sRAGE) and S100A12 (EN-RAGE) with mortality in long-term hemodialysis patients. J Korean Med Sci. 2017;32(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dozio E, Ambrogi F, de Cal M, Vianello E, Ronco C, Corsi Romanelli MM. Role of the soluble receptor for advanced glycation end products (sRAGE) as a prognostic factor for mortality in hemodialysis and peritoneal dialysis patients. Mediators Inflamm. 2018;2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Butcher L, Carnicero JA, Gomez Cabrero D, Dartigues J-F, Pérès K, Garcia-Garcia FJ, Rodriguez-Mañas L, Erusalimsky JD, FRAILOMIC Consortium . Increased levels of soluble receptor for advanced glycation end-products (RAGE) are associated with a higher risk of mortality in frail older adults. Age Ageing. 2019;48(5):696–702. [DOI] [PubMed] [Google Scholar]
- 54. Raposeiras-Roubín S, BK R-J, Grigorian-Shamagian L, Moure-González M, Seoane-Blanco A, Varela-Román A, Almenar-Bonet L, Álvarez E, González-Juanatey JR. Relation of soluble receptor for advanced glycation end products to predict mortality in patients with chronic heart failure independently of Seattle Heart Failure Score. Am J Cardiol. 2011;107(6):938–44. [DOI] [PubMed] [Google Scholar]
- 55. Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype?. J Gerontol A Biol Sci Med Sci. 2010;65A(9):963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koschinsky T, He C-J, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci. 1997;94(12):6474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vlassara H, Palace M. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87–101. [DOI] [PubMed] [Google Scholar]
- 58. Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, Vlassara H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007;170(6):1893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link?. Kidney Int. 2001;59(2):407–14. [DOI] [PubMed] [Google Scholar]
- 60. Chen S-c, Tseng C-H. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabetic Stud. 2013;10(2-3):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care. 2001;24(9):1620–3. [DOI] [PubMed] [Google Scholar]
- 62. Stein G, Busch M, Müller A, Wendt T, Franke C, Niwa T, Franke S. Are advanced glycation end products cardiovascular risk factors in patients with CRF?. Am J Kidney Dis. 2003;41(3):S52–56. [DOI] [PubMed] [Google Scholar]
- 63. Busch M, Franke S, Müller A, Wolf M, Gerth J, Ott U, Niwa T, Stein G. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int. 2004;66(1):338–47. [DOI] [PubMed] [Google Scholar]
- 64. Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28(3):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hwang J, Shin CH, Yang S. Clinical implications of Nε-(carboxymethyl) lysine, advanced glycation end product, in children and adolescents with type 1 diabetes. Diabetes Obesity Metab. 2005;7(3):263–7. [DOI] [PubMed] [Google Scholar]
- 66. Berg TJ, Clausen JT, Torjesen PA, Dahl-Jørgensen K, Bangstad H-J, Hanssen KF. The advanced glycation end product Nepsilon-(carboxymethyl) lysine is increased in serum from children and adolescents with type 1 diabetes. Diabetes Care. 1998;21(11):1997–2002. [DOI] [PubMed] [Google Scholar]
- 67. Heier M, Margeirsdottir HD, Torjesen PA, Seljeflot I, Stensæth KH, Gaarder M, Brunborg C, Hanssen KF, Dahl-Jørgensen K. The advanced glycation end product methylglyoxal-derived hydroimidazolone-1 and early signs of atherosclerosis in childhood diabetes. Diabetes Vasc Dis Res. 2015;12(2):139–45. [DOI] [PubMed] [Google Scholar]
- 68. Prasad K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases?. Mol Cell Biochem. 2019;451(1-2):139–44. [DOI] [PubMed] [Google Scholar]
- 69. Egaña-Gorroño L, López-Díez R, Yepuri G, Ramirez LS, Reverdatto S, Gugger PF, Shekhtman A, Ramasamy R, Schmidt AM. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in diabetes and cardiovascular disease: insights from human subjects and animal models. Front Cardiovasc Med. 2020;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujisawa K, Katakami N, Kaneto H, Naka T, Takahara M, Sakamoto F, Irie Y, Miyashita K, Kubo F, Yasuda T. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis. 2013;227(2):425–8. [DOI] [PubMed] [Google Scholar]
- 71. Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ. RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ramasamy R, Vannucci SJ, Yan SSD, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. [DOI] [PubMed] [Google Scholar]
- 73. O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.