Abstract
In humans, excessive gestational weight gain during pregnancy is associated with an increased risk for executive function deficits in the offspring. Our previous work has confirmed this finding in mice, as offspring from dams fed a 60% high fat (HF) diet during breeding, gestation, and lactation demonstrate impulsive-like behavior in the 5 choice serial reaction time task (5CSRTT). Because the prefrontal cortex (PFC), which plays a key role in executive function, undergoes substantial postnatal adolescent pruning and microglia are actively involved in synaptic refinement, we hypothesized that microglia may play a role in mediating changes in brain development after maternal HF diet, with a specific focus on microglial activity during adolescence. Therefore, we treated male and female offspring from HF or control diet (CD) dams with PLX3397-formulated diet (PLX) to ablate microglia during postnatal days 23–45. After PLX removal and microglial repopulation, adult mice underwent testing to evaluate executive function. Adolescent PLX treatment did increase the control male dropout rate in learning the basic FR1 task, but otherwise had a minimal effect on behavior in control offspring. In males, HF offspring learned faster and performed better on a simple operant task (fixed ratio 1) without an effect of PLX. However, in HF offspring this increase in FR1 responding was associated with more impulsive errors in the 5CSRTT while PLX eliminated this association and decreased impulsive errors specifically in HF offspring. This suggests that adolescent PLX treatment improves executive function and particularly impulsive behavior in adult male HF offspring, without an overall effect of perinatal diet. In females, maternal HF diet impaired reversal learning but PLX had no effect on performance. We then measured gene expression in adult male PFC, nucleus accumbens (NAC), and amygdala (AMG), examining targets related to synaptic function, reward, and inflammation. Maternal HF diet increased PFC synaptophysin and AMG psd95 expression. PFC synaptophysin expression was correlated with more impulsive errors in the 5CSRTT in the HF offspring only and PLX treatment eliminated this correlation. These data suggest that adolescent microglia may play a critical role in mediating executive function after perinatal high fat diet in males.
1. INTRODUCTION
Perinatal high fat diet has been shown to adversely affect offspring executive function and cognition, including increased impulsive behavior (Grissom et al., 2015; McKee et al., 2017; Tozuka et al., 2010). These behaviors can contribute to poor social/emotional regulation, and impulsive choice of highly palatable obesogenic foods (S. D. Bilbo and Tsang, 2010; Grissom et al., 2015; Peleg-Raibstein et al., 2016, 2012; Sasaki et al., 2013; Smith and Reyes, 2017; Vucetic et al., 2010), which may in part contribute to the increased risk for metabolic and psychiatric disorders associated with perinatal high fat diet (Hale et al., 2015; Ralevski and Horvath, 2015; Rivera et al., 2015; Sullivan et al., 2015, 2011). Currently, the neurobiological mechanisms underlying these behavioral deficits are unknown.
Our lab previously found that maternal HF diet in mice caused deficits in adult offspring operant behavior (Grissom et al., 2015; McKee et al., 2017). Male and female HF offspring had delayed learning and decreased motivation as measured by fixed and progressive ratio testing (Grissom et al., 2015). Using the 5 choice serial reaction time task (5CSRTT), male and female HF offspring were found to have delayed learning and increased errors in the 5CSRTT (Grissom et al., 2015). Additionally, in a related task that assessed attentional threshold (5CSRTT-titration), female HF offspring were unable to reach the performance levels of controls (McKee et al., 2017), while male HF offspring had increased premature errors, indicating an increase in impulsivity (Grissom et al., 2015). The executive function deficits in HF offspring are associated with epigenetic, reward-related, and chemokine gene expression changes in the prefrontal cortex (PFC) (Grissom et al., 2015; McKee et al., 2017). In response to maternal HF diet, there are also significant gene expression changes in the nucleus accumbens (NAC) (Carlin et al., 2013; Vucetic et al., 2010) and amygdala (AMG) (Glendining et al., 2018; Sasaki et al., 2013), which are highly interconnected with the PFC and important for other behavioral phenotypes in HF offspring (S. Bilbo and Tsang, 2010; Carlin et al., 2013; Glendining et al., 2018; Sasaki et al., 2013).
Maternal HF diet also increases pro-inflammatory cytokine and chemokine gene expression in the offspring brain (S. D. Bilbo and Tsang, 2010; Grissom et al., 2016; Kang et al., 2014; McKee et al., 2017). Microglia, the resident immune cells of the brain, may contribute to offspring behavioral deficits through increases in neuroinflammatory signals (Smith and Reyes, 2017). In utero and throughout the early postnatal period, microglia sculpt synapses to establish appropriate brain connectivity for proper behavioral function in adulthood (Frost and Schafer, 2016; Kopec et al., 2017a; Paolicelli et al., 2011; Schafer et al., 2012; Zhan et al., 2014). Maternal HF diet causes dendritic spine instability and decreases the number of cortical dendritic spines in early adolescent male offspring, an effect that persists into adulthood (Hatanaka et al., 2016). Adolescent and adult HF offspring also have decreased dendritic complexity in the PFC and AMG, respectively (Janthakhin et al., 2017; Rincel et al., 2017). We suggest that microglial-mediated pruning could contribute to these HF diet-induced reductions in dendritic complexity. For PFC development, dendritic spines undergo significant overproduction and subsequent pruning from P30–45 as the PFC establishes connectivity with the AMG (Arruda-Carvalho et al., 2017; Gourley et al., 2012; Pattwell et al., 2016), and microglia eliminate dopamine receptors in the male NAC from P30–38 (Kopec et al., 2017a). Consequently, we sought to test that the adolescent period may be a critical period for the effects of microglia on executive function, along with examining this for the first time in females.
Therefore, we propose that maternal HF diet may affect microglial activity, leading to altered adolescent synaptic development and executive function deficits in adulthood. To test this idea, we ablated microglia during adolescence in HF versus control diet offspring using PLX3397 (PLX), a tyrosine kinase inhibitor for the colony-stimulating factor 1 receptor. We hypothesized that adolescent PLX treatment would improve operant performance and executive function in adult male and female HF offspring. With this study, our primary goal was to assess behavioral function, which can provide a rationale for future detailed molecular and histochemical investigations. PLX treatment occurred during early/mid adolescence, timed to when the PFC undergoes synaptic maturation, refines connections with the AMG, and microglia prune NAC dopamine receptors (Arruda-Carvalho et al., 2017; Gourley et al., 2012; Kopec et al., 2017a; Pattwell et al., 2016). Microglia then repopulated by the time behavioral testing began in adulthood. To identify potential molecular changes underlying behavioral changes, gene expression of microglial, synaptic, epigenetic, and reward-related genes was measured in adult male PFC, NAC, and AMG.
2. METHODS
2.1. Animal subjects
Subjects were F1 hybrid male and female mice bred in-house from male DBA/2J and sexually naïve female C57BL/6J mice. This hybrid strategy increases genetic diversity to enhance generalizability of the findings. Female C57BL/6J mice arrived at 7 weeks of age and acclimated to the facility for 1 week prior to breeding. The temperature and humidity-controlled vivarium had a 12-hour light cycle (6am on, 6pm off). Dams were singly housed and offspring were group housed (n=3–4/cage) in standard cages with corncob bedding and water available ad libitum. Outside of maternal and adolescent dietary manipulations, mice received house chow (Teklad LM-485 diet, 7912). Food was available ad libitum until behavioral testing (see below). Experimental procedures were all conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.2. Maternal/perinatal diets - high fat (HF) vs. control diet (CD)
See Figure 1 for the experimental timeline. Male DBA/2J and female C57BL/6J mice were harem bred for 10 days prior to singly housing the dams. From breeding initiation through pregnancy and lactation, dams were fed either control 5755 (CD; n = 16) or 60% high fat 58G9 (HF; n = 16) purified diets (Test Diet, Richmond IN USA) (McKee et al., 2017). When necessary, small litters were combined with as few modifications as possible to allow for 8–10 pups per dam to normalize access to nutrition across litters. Pups were weaned at postnatal day (P)21, with a maximum of 1–2 mice per litter per sex used as experimental mice, and kept on the respective CD or HF diets from the dams until P23 when they received their assigned adolescent dietary treatment. Pups were born over a period of 4 days and ages denote that of the youngest litters. Due to practical constraints, all experimental events were performed on the same date, when mice would have a spread in age of up to 4 days.
Figure 1:
Experimental timeline for breeding, perinatal diet, adolescent treatment, and adulthood behavioral testing. Behavioral testing was initiated at 11 weeks (females) or 12 weeks (males) and continued until 21 weeks of age. (GD = gestational day; P = postnatal day; HFD = high fat diet; FR = fixed ratio 1; PR = progressive ratio; 5CSRTT = 5 choice serial reaction time task)
2.3. Adolescent diets - PLX3397 treated (PLX) vs. untreated diet (NT)
Upon weaning (P21), mice were housed 3–4/cage and the CD and HF diet offspring were assigned to not treated (NT) and PLX3397-treated groups (PLX) to create 4 total groups per sex (CD NT, n = 16 males, n = 16 females; HF NT, n = 15 males, n = 15 females; CD PLX, n = 16 males, n = 16 females; HF PLX, n = 15 males, n = 15 females). The colony stimulating factor-1 receptor tyrosine kinase inhibitor, PLX3397 (Selleck Chemicals, Houston TX USA) was formulated into AIN-76A rodent diet (Research Diets Inc., New Brunswick NJ USA) at a dose of 290mg PLX3397/kg diet, with macro and micronutrients matching the CD. In mice, this dose of 290mg/kg eliminates 50% of microglia in 3 days, 70–90% in 7 days, and >99% in 21 days (Renee et al., 2015). Mice received their respective adolescent treatment diets beginning on P23. Untreated (NT) mice received a formulated control diet to match the PLX diet, without the addition of the PLX3397 drug. The mice stayed on these diets until P45, after which all mice were switched to house chow for the remainder of the study (Teklad LM-485 diet, 7912). This treatment period encompasses 1 week of PLX exposure prior to and continued through the surge and subsequent normalization in PFC dendritic spines and PFC-AMG connectivity from P30–45 (Arruda-Carvalho et al., 2017; Gourley et al., 2012; Pattwell et al., 2016), along with the microglial-mediated elimination of dopamine receptors in the male NAC from P30–38 (Kopec et al., 2017a).
2.4. Operant testing
At 10 weeks of age, male and female offspring were moved into separate behavioral testing rooms with a reversed light cycle (9am off, 9pm on) for behavioral training and testing during the dark phase (10am – 5pm). Mice were group housed 3–4 mice/ cage and food restricted for 1 week to achieve a body weight 85–90% of their baseline body weight prior to behavioral apparatus exposure, with this weight maintained throughout testing. Due to the large number of mice required for the study and limited number of behavioral apparatuses, males were tested in FR1, PR, and 5CSRTT using Bussey-Saksida Mouse Touch Screen Chambers and females were tested in FR1, PR, and reversal learning using ROBucket operant chambers since the ROBuckets cannot execute the 5CSRTT (Devarakonda et al., 2016; Lloyd et al., 2018). Mice engaged in operant training/testing once daily for 5 days per week.
2.5. Female operant procedures in ROBuckets
Inside of the ROBucket chamber used for the females, there was a face plate with 3 holes, equipped with photo interrupter sensors (Devarakonda et al., 2016; Lloyd et al., 2018). The center hole was the magazine that dispenses sucrose reward, while the lateral holes were designated as either active or inactive. When the mouse nosepoked at the active hole, the sensor created a click sound to indicate that reward was dispensed in the center magazine. Entries into the inactive hole had no programmed outcomes. The ROBucket programs did not have an inter trial interval, but there was 1 sec of inactivity after each active poke. Sessions lasted for 45 min and chambers were wiped clean with 20% ethanol after each mouse. Because estrous cycle previously had no effect on operant performance in the context of maternal HF diet, we used freely cycling females without monitoring estrous phase (Grissom et al., 2015). Female mice received 24 h home cage access to 20% sucrose with 0.2% grape Kool Aid to habituate to the palatable reward used for operant testing that began 3 days later at 11 weeks of age.
2.5.1. Female fixed ratio 1 (FR1) and progressive ratio (PR)
For 3 days, females habituated to the ROBucket chambers with the power off and no reinforcer present. After habituation, female mice performed fixed ratio 1 (FR1) for 15 days, where sucrose dispensed after each active poke. Criteria for successfully learning FR1 were: more than 20 active pokes in 45 min and a discrimination ratio of 3:1 (active : inactive) (based on (Devarakonda et al., 2016)). Three mice failed to reach this criteria by day 15 and were removed from all analyses (n = 1 each of CD NT, HF NT, HF PLX). After 15 days of FR1, mice went through two consecutive days of progressive ratio (PR) testing. After each trial, the number of active pokes required for a sucrose delivery increased following the formula r = 5e0.2n – 5, rounded to the nearest whole number (Lloyd et al., 2018; Richardson and Roberts, 1996). Mice timed out if they made no response for 5 min or were capped at 75 min. Session duration, active pokes, inactive pokes, and sucrose deliveries were recorded.
2.5.2. Female reversal
After PR, mice took 5 days off and then performed 3 FR1 sessions to maintain consistent responding. The following day, the active and inactive holes were switched. Mice then performed FR1 for 4 consecutive days with the reversed holes to learn that the previously inactive hole had become the active hole. Day 1 of reversal is when the mice first encounter this change in hole valence. Then day 2 was our primary performance measure of interest, during which the mice had to remember that the holes had been reversed.
2.6. Male operant procedures in Bussey-Saksida Mouse Touch Screen Chambers
Male mice received 24 h home cage access to chocolate Yoohoo to habituate to the palatable reward used in the operant procedure that began 3 days later at 12 weeks of age. This protocol used Bussey-Saksida Mouse Touch Screen Chambers connected to computers with Abet II and Whisker software (Lafayette Instrument, Lafayette IN USA). See Table S1 for a list of the pre-programmed schedules used. The chambers contained 5 touch screens in the front and a magazine in the back. The chocolate Yoohoo feeder pulse reward was 280 ms for all trials for the entire experiment. All sessions were 30 min, except for progressive ratio, which had a limit of 1 h.
2.6.1. Male FR1 and PR
After 3 days of habituation to the operant chamber and magazine reward, mice underwent 15 days of FR1, but a data collection error on day 10 removed this day from analysis for a total of 14 days. Each FR1 trial consisted of the center touch screen illuminating (stimulus) and staying lit until the mouse touched (response). Immediately after the response, the magazine light turned on and reward dispensed followed by a 1 s ITI. The remaining 4 lateral pads were inactive/unlit for FR1 but used to record blank touches. Mice remained on FR1 until the cohort reached the criteria of at least 70 trials in the 30 min session for at least 2 consecutive days. Mice that failed to reach criteria (n = 3; CD PLX) were eliminated from analysis. After FR1, mice performed two consecutive days of progressive ratio (PR) testing. The trials were the same format as FR1, except the number of responses required to turn off the stimulus and obtain a reward increased progressively after each set of 3 successful trials. Mice were required to perform 3 trials each of 1 response, 2 responses, 4, 7, 11, 16, 22, 29, 37, etc. until they quit responding for 10 min or until 1 h had passed, whichever came first.
2.6.2. Male 5 choice serial reaction time task (5CSRTT)
After PR, mice progressed to the 5 choice serial reaction time task (5CSRTT), where any 1 of the 5 touchscreen areas became active options and the ITI increased to 5 s. For a correct trial, the mouse had to make a correct touch response during stimulus presentation. An omission was failure to make a touch response and an incorrect trial was a touch response to an unlit screen during stimulus presentation. A premature response was any touch made during the ITI, before stimulus presentation. Incorrect, omitted, and premature responses received a 5 s time out, during which the house light illuminated. For correct trials, a 1 sec tone played while the magazine light illuminated and reward dispensed. We analyzed the percent of omissions, incorrect, and accuracy since they are contingent on stimulus presentation. Then we analyzed the total number of premature responses, as previously reported (Fletcher et al., 2007; Funk et al., 2019), since they are non-contingent to stimulus presentation and can occur at any point during the ITI.
The mice started learning the 5CSRTT with a 16 s stimulus presentation. After achieving more than 20 total trials and over 50% correct trials for 2 consecutive testing days, they progressed to shorter stimuli at their own pace. These included sessions with stimuli presentations of 16 sec, 8 sec, and 4 sec. After passing criteria for the 4 s stimulus, mice underwent testing in a more difficult version of the 5CSRTT, termed titration. For titration, the stimuli adjusted within a single session based on the mouse’s individual performance (Martin et al., 2015; McKee et al., 2017). The levels of stimuli were 10, 8, 6, 4, 2, 1.5, 1, 0.9, 0.8, 0.7 s, etc. until the lowest achievable stimulus. After each correct trial, the stimulus duration decreased one level. After an omission or incorrect trial, the stimulus duration increased. The stimulus stayed the same after premature responses. This resulted in approximately 50% correct trials during a titration session. No tone played during titration. In addition to the three mice that did not pass FR1, three more mice did not reach titration during the 23 days of 5CSRTT testing and were also excluded from the analysis (n = 1 CD PLX, n = 1 CD NT, 1 HF PLX).
2.7. Male gene expression
Three days following the final day of titration in the 5CSRTT, male mice (21 weeks) were euthanized at a time of day when they normally would have started behavioral testing (but without testing). Male brains were collected into RNAlater®, stored at 4°C overnight, and at −20°C until dissection. Using a brain block, 1–2 mm sections were dissected to obtain the medial prefrontal cortex (PFC; 1 mm sections; AP +1.2 – 2.2, DV −2.2 – 0.0, ML ± 0 – 0.6), nucleus accumbens (NAC, 1 mm sections; AP +1.2 – 2.2, DV −2.2 – 3.6, ML ± 0 – 2.4), and amygdala (AMG; 2 mm sections; AP −0.5 – 2.5, DV −4 – 5.4, ML ± 1.6 – 3.8) (Paxinos & Franklin Mouse Brain Atlas, 2nd edition). RNA was extracted using TRIzol® reagent (ThermoFisher Scientific, Waltham MA USA) and RNeasy columns (Qiagen, Valencia CA USA). Male brain tissue samples were removed from RNAlater® and homogenized in 500 μl TRIzol® reagent with a TissueLyser system (Qiagen). Samples were incubated with 100 μl chloroform for 20 min and centrifuged 15 min at 12000g, all at 4°C. The supernatant was mixed with 100% ethanol and transferred to RNeasy columns, where it was washed with buffers RWT and RPE (Qiagen). After elution, RNA yield and purity was assessed via Epoch microplate spectrophotometer (BioTek, Winooski VT USA). A High Capacity Reverse Transcriptase kit (Applied Biosystems, Foster City, CA, USA) was used for cDNA synthesis.
TaqMan assays with TaqMan Preamp Master Mix (ThermoFisher Scientific) were used to preamplify 32 target genes in cDNA samples, following the manufacturer protocol (Fluidigm, South San Francisco, CA, USA) and as previously published (McKee et al., 2017). For high throughput qPCR, samples were measured via 96.96 Dynamic Array Integrated Fluidic Circuit with Biomark HD machine (Fluidigm). See Table S2 for a full list of targets and assay IDs. From the panel of 5 housekeeping genes (actb, gapdh, hprt1, ppia, ywhaz), CT values were input into NormFinder with Log transformation to determine the most stable housekeeping gene pair for each brain region. Target gene expression was normalized to the geomean of the most stable housekeeping gene pair and expressed as fold change using the ddCT method, using the CD NT group as the reference group.
2.8. Immunohistochemistry (IHC)
Using animals from the same pregnancy cohort used in the behavior study, behaviorally naïve male mice were transcardially perfused with 4% paraformaldehyde for verification of microglial elimination (P45) and repopulation (P66) after 3 weeks of PLX treatment (n = 3/group per time point). Perfused brains were collected and stored in 4% paraformaldehyde for 4 h, transferred to 30% sucrose overnight, sectioned on a microtome at 35 μm and stored in cryoprotectant. For immunohistochemistry, sections were washed 5 × 5 min in PBS before transfer to blocking solution (0.2% bovine serum albumin, 0.4% Triton X-100). After 2 h incubation in blocking solution, sections were incubated in primary rabbit antibody overnight. P45 samples were incubated with 1:1500 anti-Iba1 (Synaptic Systems, Goettingen Germany) and P66 samples were incubated with 1:2000 anti-Iba1 (Wako Chemicals USA, Inc.). Following primary antibody incubation, samples were washed with PBS and incubated 1:500 in goat anti-rabbit cyanine3 IgG for 1 h. Sections were washed, mounted to slides, and coverslipped with DABCO® mounting media. Representative images 40x were captured using Zeiss Axiovision 4.6 software with Apotome Deconvolution. For Iba1 quantification in the PFC, NAC, and AMG after repopulation, Iba1 positive cells (Wako) were counted as previously described, using 7μm Z-stack projection images (Smith et al., 2016). Values from the right and left hemispheres, along with consecutive projection images from the same Z-stack, were averaged together to give one value per animal (n = 3/group: male CD NT, male CD PLX, male HF NT, male HF PLX).
2.9. Statistics
All data are displayed as mean ± SEM. Since males and females were tested using different behavioral apparatuses, data were analyzed separately without sex as a factor. Single time point data were analyzed via 2-way ANOVAs with diet (CD vs. HF) and treatment (NT vs. PLX) as factors, using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Prism 7 was also used for Pearson correlations (behavior × behavior or gene × behavior) and Chi2 analysis on behavioral criterion outcomes, in the event that any more than 1 mouse per experimental group failed criterion. Multiple time point data were analyzed via General Linear Model with Repeated Measures (3-way RM ANOVA), with diet and treatment as between subjects factors and day/week as the within subjects factor, using IBM SPSS Statistics 24 (SPSS Inc.). The 3-way RM ANOVAs used the Greenhouse Geisser within-subjects correction. Bonferroni correction was employed for all post hoc tests, as warranted. The significance threshold was set at p = 0.05, except for the correlation data, the threshold was adjusted to p = 0.0125 to account for the 4 correlations run (1 per experimental group within a sex).
3. RESULTS
3.1. PLX3397 verification
After 3 weeks of PLX3397 treatment, a subset of behaviorally naïve male mice from the experimental cohort were used to verify that PLX eliminated microglia (n = 3/group). PLX eliminated nearly all microglia in both CD and HF offspring, with very few isolated microglia observed sporadically throughout the brain (Fig S1 A–D). Another subset of mice (n=3/group) verified that microglia had repopulated in the medial prefrontal cortex after 3 weeks recovery from the PLX treatment and prior to starting behavioral training and testing (Fig S1 E–H). The repopulated microglia in PLX treated animals (Fig S1 G–H) looked different than NT microglia (Fig S1 E–F), with thinner processes. Despite a small sample size (n = 3), we quantified male Iba1 positive cells as a preliminary analysis. In the PFC, there was a diet*treatment interaction [F(1, 8) = 8.2; p = 0.021], but no post hoc tests were significant (Fig S1 I). In the NAC, there was a main effect of PLX [F(1, 8) = 10.9; p = 0.011] to increase Iba1 cells (Fig S1 J). In the AMG, there were no significant differences in Iba1 cell counts. Examination of body weights showed that HF offspring weighed more at weaning (Fig S2 C, D, G, H), consistent with our previous data (Vucetic et al., 2010). PLX treatment decreased food intake and body weight acutely (graphs and statistical results in Fig S2, 3), however, these effects diminished over time and there were no differences in body weight between the groups by the time they started behavioral training and testing. See Fig S2–3 for a description of the statistical results for food intake and body weight. PLX3397 also caused a distinct graying of the fur, which was heterogenous in nature, seemed to affect males more than females, with coat color only partially restored during recovery from PLX.
3.2. FR1 and PR
Contrary to our prediction, HF diet improved FR1 learning rate and performance in male offspring without significant effects of PLX. The 14 days of FR1 revealed a between-subjects main effect of diet [F(1, 53) = 11.5; p = 0.001] and within-subjects day*diet interaction [F(13, 689) = 2.8; p = 0.019] for the number of correct trials performed per session. HF male offspring performed more trials than CD on days 2 and 4–13 of FR1 training (p < 0.05; Fig 2 A). As a measure of non-rewarded general responsivity, we analyzed incorrect or blank touches to the lateral 4 inactive touchscreens during FR1. General responsivity failed to explain the performance we observed in FR1, as this revealed a different within-subjects day x diet interaction [F(13, 689) = 2.4; p = 0.048]. HF offspring made fewer blank touches on days 10–13 (p < 0.05; Fig 2 B, E). In calculating percent accuracy ((correct trials / correct + blank)*100), we found a within-subjects day x diet interaction [F(13, 689) = 2.6; p = 0.031] and between-subjects main effect of diet [F(1, 53) = 13.1; p = 0.001]. The HF males were more accurate than CD males on days 2 and 4–13 (p < 0.05; Fig 2 C). For FR1 trials, blank touches, or accuracy there were no effects or interactions with PLX treatment. Immediately prior to learning FR1, there were no differences between any of the groups in the number of infrared beam breaks during the 3 habituation days, indicating that there were no differences in locomotion (data not shown). Finally, there was a significant main effect of perinatal diet [F(1, 55) = 7.3; p = 0.0093] for the number of days needed to reach criterion, whereby HF offspring reached criterion in fewer days (Fig 3 A). Because 3/16 CD PLX males failed to reach criterion in FR1 but all of the mice in the other groups successfully passed, we conducted a Chi2 analysis on dropout rates between groups. The pass vs. fail outcomes were significantly different, indicating that PLX increased the dropout rate in CD offspring [X2 (3, N = 62) = 9.06, p = 0.028] (Fig S4). In PR, there were no differences in motivation assessed by breakpoint (Fig 3 B).
Figure 2:
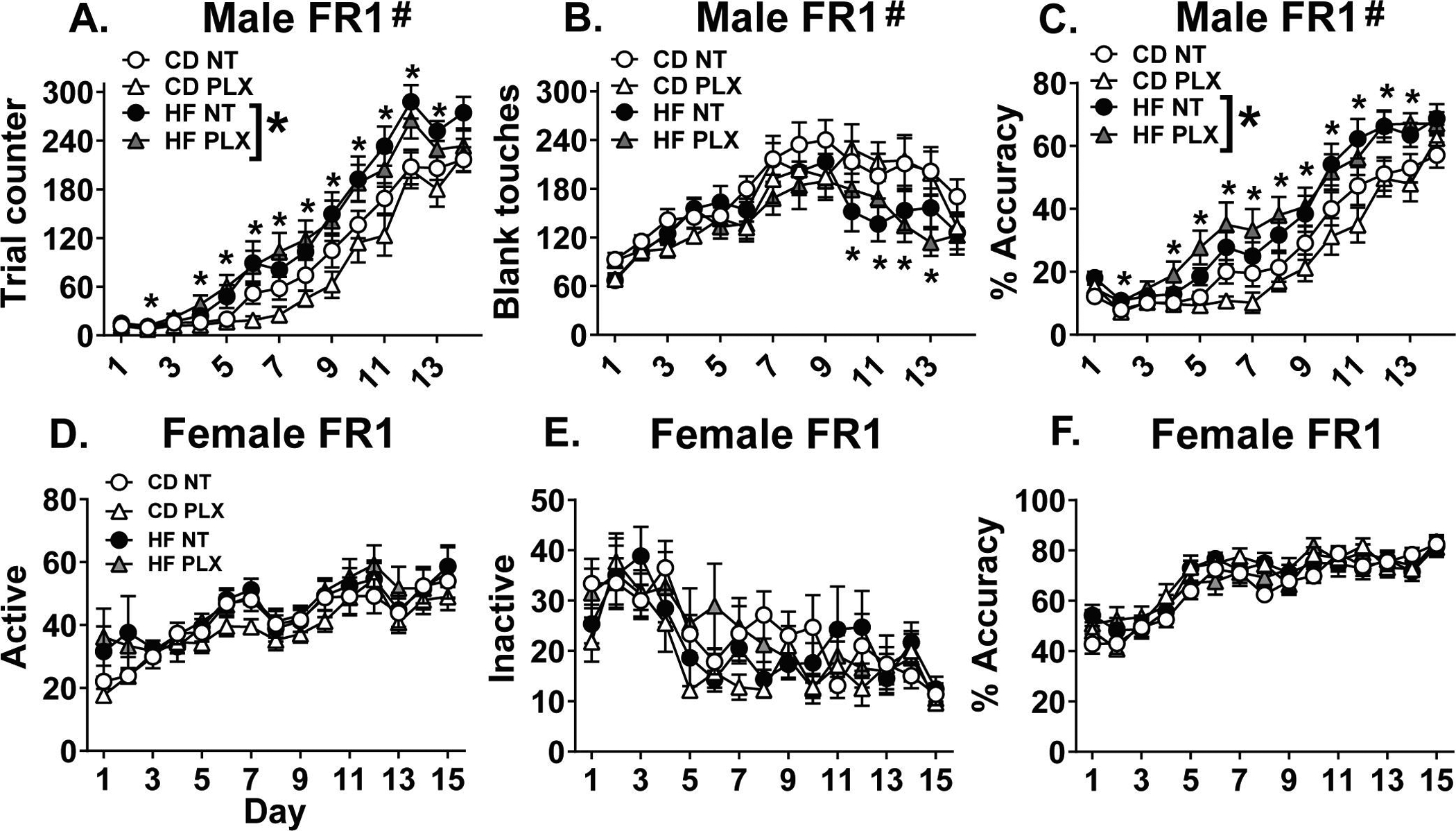
Across 14 days of fixed ratio 1 (FR1) in the males using Bussey touchscreen chambers, maternal HF diet (A) increased trials performed on days 2 and 4–13, (B) reduced blank or incorrect touches on days 10–13, and (C) increased accuracy on days 2 and 4–13. Across the 15 days of FR1 in the females using ROBuckets, there were no significant differences in (D) active pokes, (E) inactive pokes, or (F) accuracy. (n = 15–16/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (Graph title = # interaction between perinatal diet and day, HF * main effect of diet; *p < 0.05 HF vs. CD)
Figure 3:
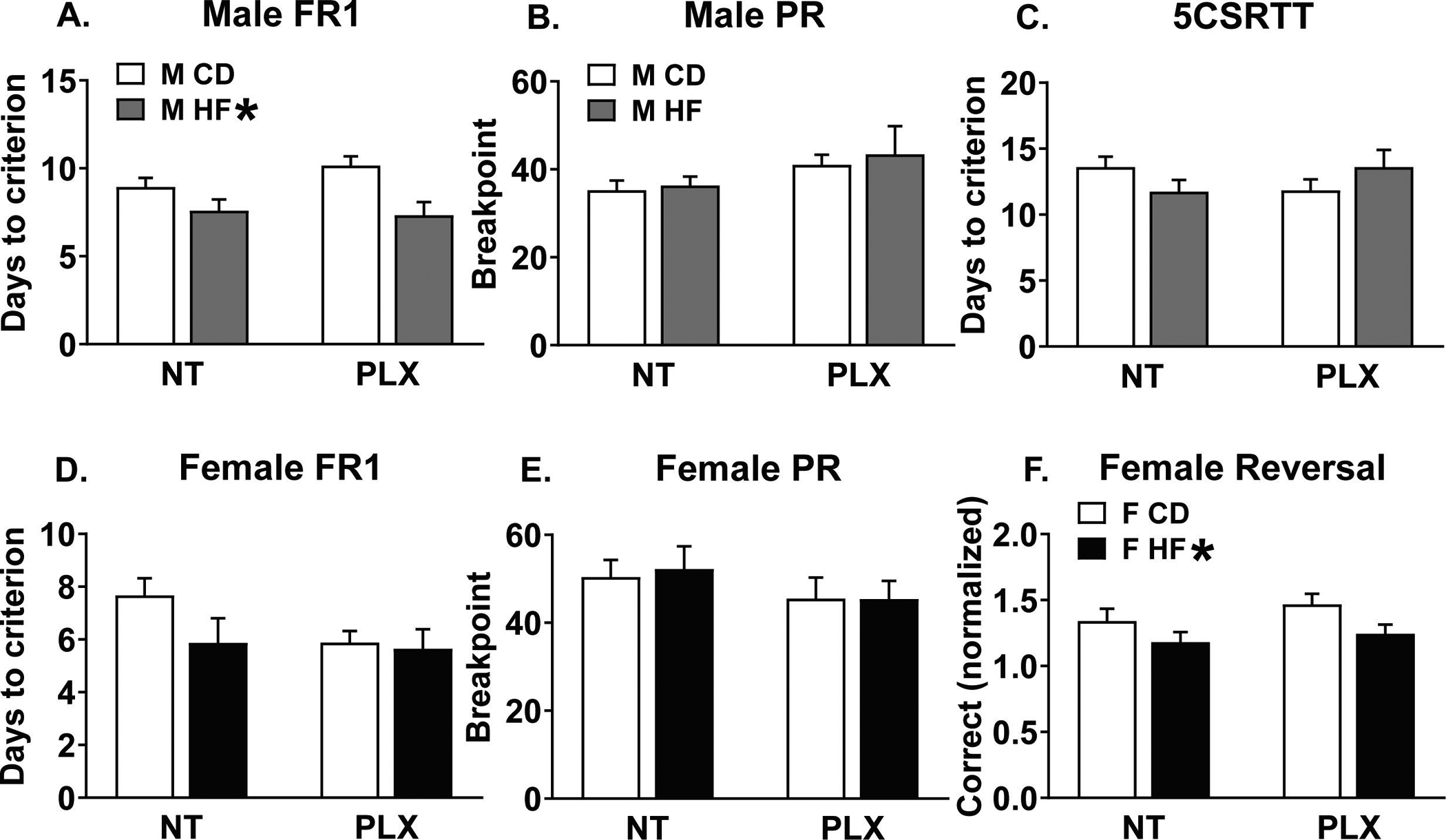
(A) In males, maternal HF diet decreased days needed to reach criterion for FR1. (B) No differences in male breakpoint using PR. (C) No differences in the days needed to reach criterion in the 5CSRTT. (D) No differences in female learning in FR1 (E) or breakpoint using PR. (F) Maternal HF diet decreased normalized correct responses in the recall of a reversal task. (n = 15–16/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (HF * = main effect of perinatal high fat diet)
In the females, using the ROBucket apparatus for 15 days of FR1, neither perinatal diet nor PLX treatment affected active pokes (Fig 2 D), inactive pokes (Fig 2 E), accuracy (Fig 2 F), or days to criterion (Fig 3 D). Similar to the males, there were no differences in breakpoint in PR (Fig 3 E).
3.3. Female reversal task
Due to the highly variable number of responses observed in the female ROBuckets, we normalized reversal correct pokes to the number of correct pokes achieved on the final day of FR1. In analyzing the second day of the reversal task, there was a main effect of HF diet decreasing correct responses [F(1, 55) = 5.6; p = 0.021], indicating that HF female offspring performed worse on reversal (Fig 3 F), but there was no effect of PLX treatment.
3.4. Male 5CSRTT
For the 5CSRTT, male mice progressed through a series of schedules with decreasing stimulus durations: 16 s, 8 s, and 4 s. All groups completed all three of the sessions to reach titration in a comparable number of days (Fig 3 C). There were also no differences between groups to reach criterion for any one of the three sessions. We then analyzed the first day that the mice reached criterion for each of the 16, 8, and 4 s sessions by 2-way ANOVAs. With the 16 s stimulus, there was a significant main effect of diet on the percent of omissions [F(1, 52) = 4.7; p = 0.034], with HF diet decreasing omissions (Fig 4 A). With the 8 s stimulus, there was a main effect of treatment on the percent of incorrect errors [F(1, 52) = 7.6; p = 0.008], with PLX decreasing incorrect trials (Fig 4 B). At the most challenging level (4 s stimulus), there was a main effect of treatment and significant diet x treatment interaction for the number of premature responses ([F(1, 52) = 4.7; p = 0.035]; [F(1, 52) = 4.1; p = 0.049]). PLX reduced premature responding specifically in the HF group and not the CD group (p < 0.05; Fig 4 C), although without an effect of HF diet in either the NT or PLX groups. Across the 16, 8, and 4 s stimuli, there were no other significant effects between groups for the different types of errors made. To examine whether performance in FR1 related to impulsive errors in the 5CSRTT, we correlated peak FR1 responses (day 12) with premature responses during the 4 s stimulus and found a significant positive correlation overall (Fig 4 D; p < 0.05). Post hoc correlations on individual groups revealed a positive correlation only in the HF NT group, which was not present in the HF PLX group, nor either of the control groups (Fig 4 D; p < 0.0125 adj).
Figure 4:
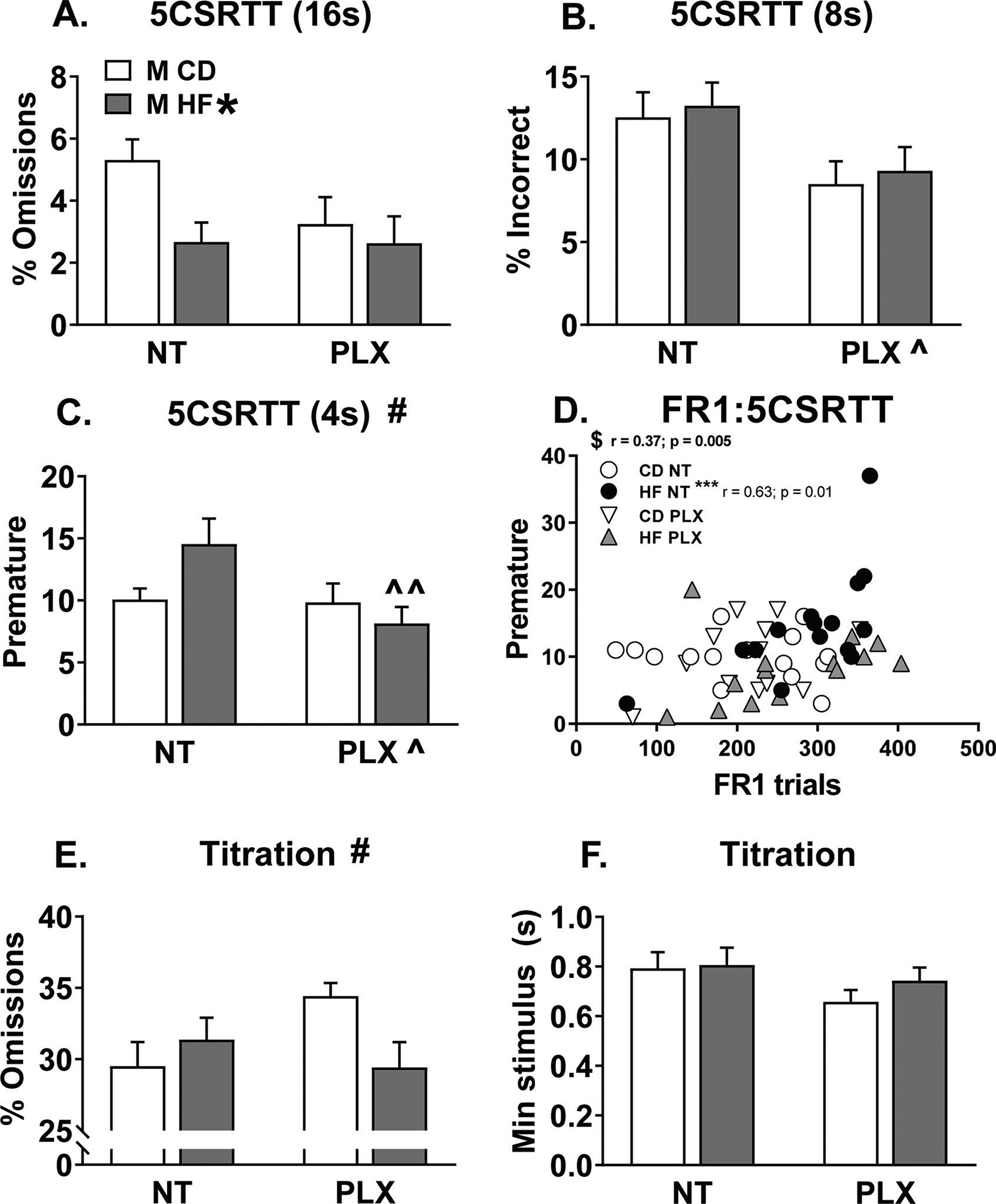
Male 5 choice serial reaction time task (5CSRTT) (A) With the 16 s stimulus, HF diet decreased the percent of omissions. (B) With the 8 s stimulus, PLX treatment decreased the percent of incorrect errors. (C) Then with the most challenging 4 s stimulus, PLX reduced premature responses specifically in the HF PLX vs. HF NT group. (D) Premature responses with the 4 s stimulus were significantly correlated with peak FR1 responses (day 12), specifically in the HF NT group. (E) With a titrated stimulus, HF diet and PLX treatment had an interaction effect on the percent of omissions but not post hocs reached significance. (F) There were no differences in the minimum achieved stimulus. (n = 15–16/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (Graph title # = significant interaction between perinatal diet and adolescent treatment; HF * = main effect of perinatal high fat diet; PLX ^ = main effect of adolescent treatment; ^^p < 0.05 vs. HF NT; $ p < 0.05 significant overall correlation; ****p < 0.0125 adj)
Upon reaching criterion for the 4 s stimulus, male mice underwent titration testing where the stimulus adjusted throughout the session based on performance (see methods). On the final day of titration prior to tissue collection, there was a significant diet x treatment interaction for the percent of omissions [F(1, 52) = 4.8; p = 0.033], but no post hoc group comparisons were significantly different (Fig 4 E). There were no differences in the minimum stimulus achieved (Fig 4 F) or in premature responding (not shown) across groups in titration. Together, adolescent PLX treatment seemed beneficial for performance in the 5CSRTT because it reduced premature and omitted responses in the HF group and reduced the percentage of incorrect responses overall.
3.5. Gene expression
Given the largely null behavioral data in the females, gene expression analyses were only completed for male animals. Using NormFinder analysis, the most stable housekeeping gene pairs were ywhaz & ppia (NAC, PFC) and ywhaz & gapdh (AMG). The geomean of these pairs was used to calculate fold change, respectively, for each brain region. The CD NT group served as the reference group for which fold change was normalized to 1. Of the 27 gene targets investigated, significant differences were detected for 12 targets in the AMG, 7 targets in the NAC, and 2 targets in the PFC. Interestingly, the amygdala appeared to be more sensitive to the combined effects of perinatal HFD and adolescent PLX treatment, as significant diet x treatment interaction effects were observed for 5 genes in the AMG, only 1 gene in the NAC, and none in the PFC. Eleven genes were unchanged in all of the brain regions analyzed (cnr1, cx3cl1, dnmt3a, drd2, gad1, gephryin, cr3, mecp2, oprd1, oprm1, and penk). See Table S3 for a complete list of statistics for the gene expression data. A consistent effect observed across regions was that PLX treatment in adolescence significantly increased c1qa expression in adult male brain (Fig 5 A–C). For synaptic-related genes, HF diet increased expression of synaptophysin (syp) in the PFC (Fig 5 D) and dlg4 (psd95) and slc17a7 (vglut1) in the AMG (Fig 5 E, F). Additionally, PLX decreased psd95 expression in AMG (Fig 5 E). For reward related genes, HF diet decreased oprk1 and TH expression in the AMG (Fig 6 A, B) and interacted with PLX to reduce TH, drd1a, and ppp1r1b (darpp-32) expression specifically in the HF PLX treated group (Fig 6 B, C, D). In the NAC, PLX decreased slc6a3 (DAT) and TH expression (Fig 6 E, F). Then some subtle regional differences were detected in microglial-related gene expression in the NAC versus AMG. In the NAC, HF diet increased cx3cr1 expression and the HF PLX group had increased csf1r expression compared to the CD PLX group, while in the AMG, PLX decreased cx3cr1 expression (Table S3).
Figure 5:
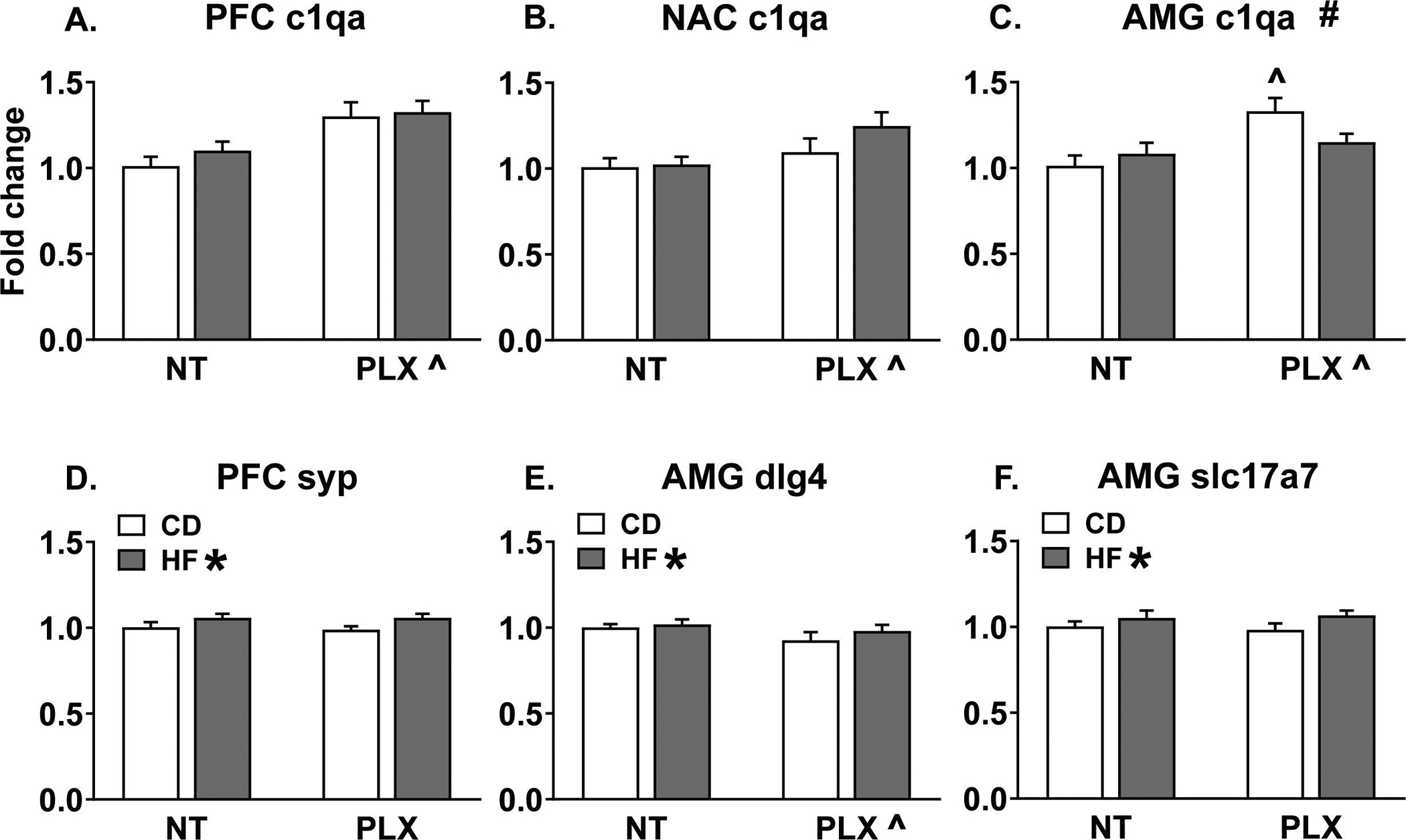
Adolescent PLX treatment increased c1qa expression measured in adult males in the (A) PFC, (B) NAC, and (C) AMG. In the AMG, this effect was specific to the CD group. (D) Maternal HF diet increased synaptophysin expression in the PFC. (E) Maternal HF diet increased AMG dlg4 (psd95) expression while PLX treatment reduced it. (F) Maternal HF diet increased slc17a7 (vglut1) expression in the AMG. (n = 7–8/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (Graph title # = significant interaction between perinatal diet and adolescent treatment; HF * = main effect of perinatal high fat diet; PLX ^ = main effect of adolescent treatment; ^p < 0.05 vs. CD NT)
Figure 6:
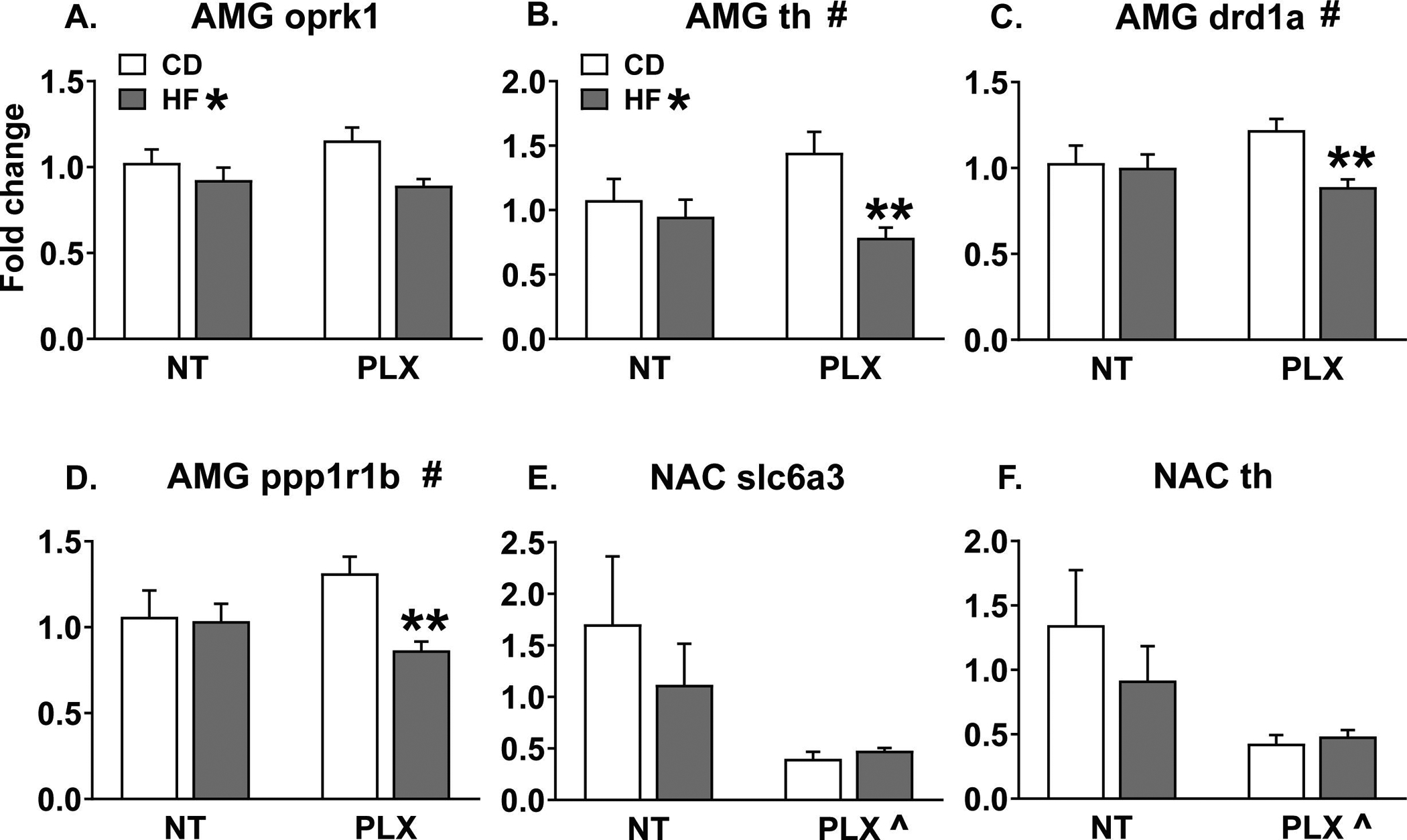
In the adult male AMG, maternal HF diet decreased (A) oprk1 and (B) TH expression and interacted with adolescent PLX treatment to reduce (B) TH, (C) drd1a, and (D) ppp1r1b (darpp32) expression specifically in the PLX group. In the NAC, PLX treatment decreased (E) slc6a3 (DAT) and (F) TH expression. (n = 7–8/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (Graph title # = significant interaction between perinatal diet and adolescent treatment; HF * = main effect of perinatal high fat diet; PLX ^ = main effect of adolescent treatment; **p < 0.05 vs. CD PLX)
3.6. Correlations between behavior and gene expression
Because our a priori hypothesis centered on how the PFC is affected by maternal HF diet and subsequent PLX treatment, we ran Pearson correlations between significant PFC gene expression fold changes (c1qa, syp) and behavioral outcomes on the final day of behavioral testing (titration). In the HF NT group only, PFC synaptophysin positively correlated with increased premature errors (r = 0.82; p < 0.0125 adj) (Fig 7). Importantly, PLX treatment abolished this correlation with impulsivity in the 5CSRTT. This suggests that adolescent PLX treatment may promote a beneficial association between synaptic changes and executive function, specifically impulse control, in HF offspring.
Figure 7:
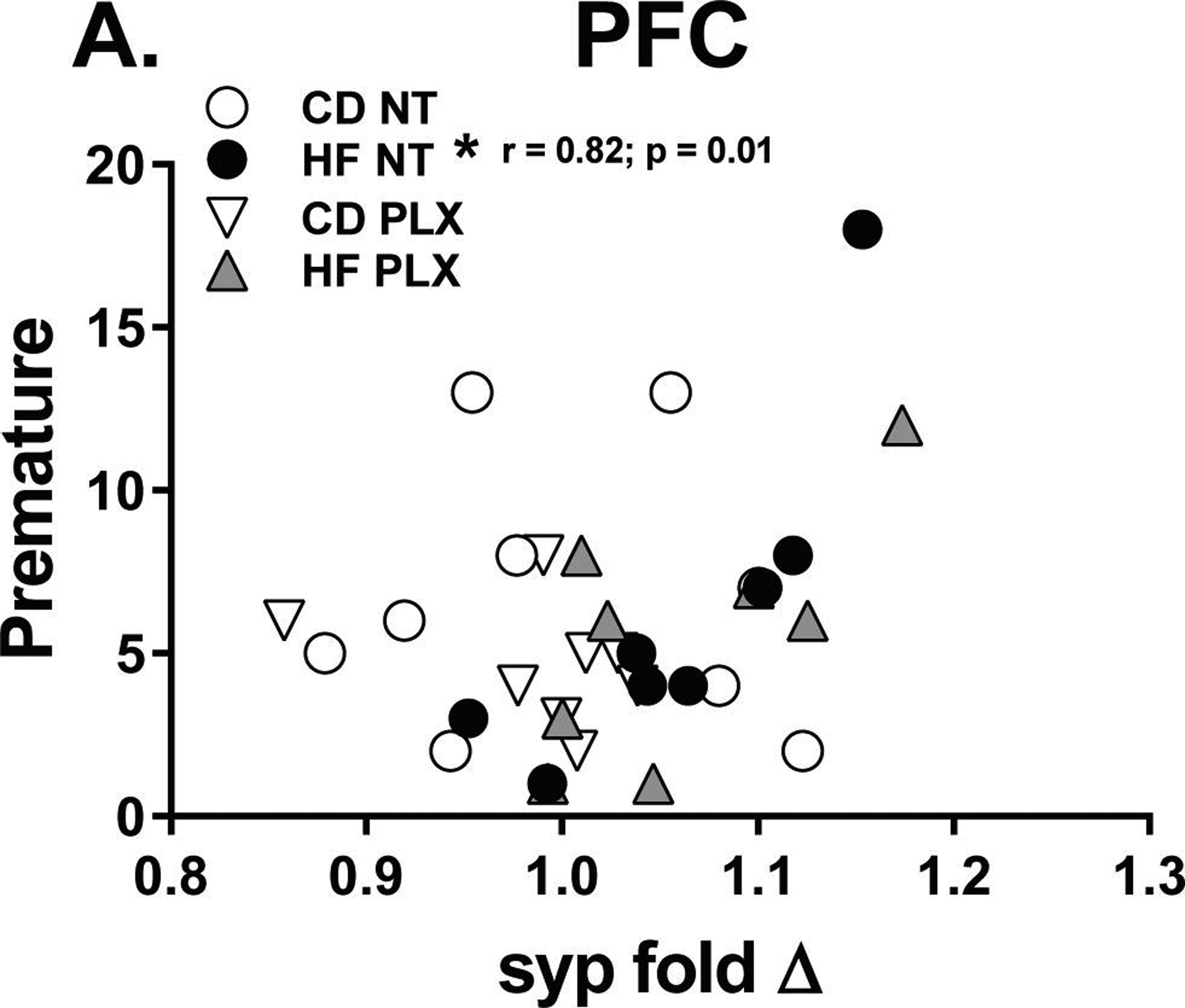
In the PFC, synaptophysin fold change positively correlated with increased (A) premature errors in the final day of testing, with the titration schedule of the 5CSRTT. This was only observed in the HF NT group. (n = 7–8/group) (HF = offspring of maternal high fat diet; CD = offspring of maternal control diet; PLX = adolescent PLX3397 treatment; NT = not treated during adolescence) (*p < 0.0125 adj)
4. DISCUSSION
Male offspring exposed to perinatal HF diet learned more quickly and were more accurate in a basic FR1 task without a significant effect of adolescent PLX3397 treatment. However, this increase in FR1 responding correlated with more premature errors in the 5CSRTT, specifically in HF mice while PLX treatment eliminated this association. Notably, PLX reduced premature responses in HF males in the most challenging 5CSRTT progression, although without a significant effect of HF diet. This demonstrates that ablating microglia during adolescence, a time when the prefrontal cortex undergoes surges in spine formation and subsequent pruning of dendritic spines (Arruda-Carvalho et al., 2017; Gourley et al., 2012; Pattwell et al., 2016), reduces impulsivity in adult HF male offspring. Together, this shows that microglia ablation improves executive function after perinatal HF diet in males and supports our hypothesis that microglia may be important for executive function after perinatal HF diet, although less so in CD offspring. Interestingly, adolescent microglial ablation may impair basic learning in a subset of control males without affecting executive function and had no effect on females exposed to either maternal diet.
In females, maternal HF diet decreased performance in the reversal task, while PLX had no effect. Maternal diet or PLX treatment failed to change any other behavioral measures in the females, although we did not test them in the 5CSRTT. Previous work on adolescent spine dynamics has been done primarily in males (Arruda-Carvalho et al., 2017; Pattwell et al., 2016) and evidence suggests that females have a different neurodevelopmental trajectory in adolescence where some of the key events occur earlier than in males, such as peaks in gray matter volume and dopamine receptor expression. (Kopec et al., 2017b; Sisk and Zehr, 2005). While maternal HF diet produced a deficit in reversal learning, it is possible that our current PLX treatment window favored male adolescent development. Future studies must address female dendritic spine dynamics, along with responsivity to PLX treatment. Alternatively, the circuits important for performance in reversal learning versus the 5CSRTT may be differentially affected by PLX treatment. While the 5CSRTT and reversal task can both assess behavioral impulsivity (Izquierdo A, 2013; Robbins, 2002), the predominant brain circuits recruited differ. While the medial PFC is most important for the 5CSRTT, reversal learning is highly dependent on the orbitofrontal cortex and our present spatial-based reversal task likely relies heavily on the hippocampus as well (Graf et al., 2018; Izquierdo et al., 2017; Izquierdo A, 2013; McTighe et al., 2009; Robbins, 2002). PLX treatment may have no effect on HF diet-induced changes in the hippocampus and/or orbitofrontal cortex but may reverse or alleviate some of the changes within the medial PFC, although this would have to be tested in both sexes. Finally, we clearly cannot rule out differences between the Bussey touchscreen chambers used for males and the ROBuckets used for females. Future studies on microglial ablation during neurodevelopmental periods can investigate treatment windows optimized for females in addition to males, along with comparing reversal learning, 5CSRTT performance, and gene expression in males and females in the same operant chambers.
Examination of male gene expression revealed that adolescent PLX treatment increased c1qa expression in PFC, NAC, and AMG. This change is evident after prolonged (14 week) recovery from PLX. Microglia are a dominant source of c1qa in the brain (Fonseca et al., 2017), so it is possible that this increase is an overshoot or compensation after recovery from adolescent microglial ablation. Future quantitative and cell-specific analyses after microglial repopulation will be important to inform whether this increase in c1qa represents an increase in expression/cell or an increase in cell number, and whether it reflects expression on microglia or neurons. Further, c1q is crucial for microglial-mediated synaptic remodeling and pruning (Perry and O’Connor, 2008; Stevens et al., 2007), so this suggests that adolescent PLX treatment may delay cortical refinement until well after microglial repopulation following PLX removal. Excessive complement activation is also associated with many disease states (Alexander et al., 2008), so this elevation in c1qa expression could indicate an underlying pathology. This sustained increase in c1qa expression after PLX recovery should be a topic of future investigation. Interestingly, other microglial-related genes, such as csf1r and cx3cr1 had expression patterns that varied in the NAC versus AMG. Our small sample size for IHC in the males suggest that maternal HF diet may differentially affect Iba1 cell repopulation in the PFC and that Iba1 cells are increased in the AMG after recovery. These regional differences may impact our findings because these changes could affect offspring behavior and are unexplored in females. Together, long-term microglial repopulation after PLX with and without maternal HF diet should be more thoroughly investigated, along with regional brain differences during recovery trajectory and in the context of behavioral testing in both males and females.
Maternal HF diet increased expression of synaptic genes, demonstrated by increased synaptophysin expression in the PFC and increased dlg4 (psd95) and slc17a7 (vglut1) expression in the AMG. PFC synaptophysin was positively correlated with increased premature errors in the HF untreated mice. Adolescent PLX treatment abolished this correlation. This suggests that maternal HF diet may induce synaptic remodeling in the PFC that could contribute to impulsivity while microglial ablation prevents this association.
Maternal HF diet decreased reward-related gene expression in the AMG of mice treated with PLX during adolescence. The behavioral improvements with PLX and HF diet seem to follow the same pattern as the decreases in AMG expression of dopamine D1 receptor, κ opioid receptor, tyrosine hydroxylase, and dopamine phosphoprotein (darpp-32/ppp1r1b). These modest but statistically significant changes require further investigation, but this clear pattern of reward system-related effects suggest a role for the AMG in regulating appetitive-based cognitive tasks after maternal HF diet as a focus for future investigation. Recent studies further support the importance of the AMG in maternal HF diet phenotypes because offspring display decreased AMG dendritic complexity, altered AMG gene expression, and increased anxiety-like behavior (S. D. Bilbo and Tsang, 2010; Glendining et al., 2018; Janthakhin et al., 2017; Peleg-Raibstein et al., 2012; Sasaki et al., 2013). In the NAC, PLX exposure decreased tyrosine hydroxylase (TH) and dopamine transporter (slc6a3/DAT) expression. Previous work with our maternal HF diet model demonstrated increased TH and DAT expression in the NAC of adult offspring, however these dams were maintained on the HF diet for 3 months prior to breeding (Vucetic et al., 2010). Therefore, an extended history of maternal HF diet exposure may be necessary to increase dopaminergic-related gene expression in offspring NAC.
Previous work from our group demonstrated that maternal HF diet delayed learning rate and responses in FR1, decreased motivation in PR, and decreased learning rate and increased errors in the 5CSRTT (Grissom et al., 2015). In our current study, we find that maternal HF diet increased FR1 performance in males but impaired reversal learning in females. However, in HF untreated males, this increase in FR1 responding correlated with impulsive errors in the 5CSRTT. Importantly, PLX eliminated this association and was specifically beneficial for HF offspring as the task became more challenging to assess executive function rather than general learning. Our male results agree with our previous data showing increased impulsive behavior in HF offspring (Grissom et al., 2015) and with prior reports in rats of maternal HF diet increasing responses in FR1(Naef et al., 2011). Further, our data suggest that perinatal HF-diet induced increased FR1 responding may drive or underlie impulsive behavior, while PLX blocks this association. The reversal deficit that we now report in females agrees with and adds to our previous work in the females that demonstrated cognitive and executive function deficits following maternal HF diet. Importantly, adolescent PLX treatment does not reverse this deficit in females and seems particularly beneficial for male HF offspring executive function and impulse control.
During adult operant training and testing, we observed little to no effect of adolescent PLX treatment. While there was no effect of PLX in females or in either sex learning the more complicated executive function tasks, PLX increased the male dropout rate at the initial FR1 stage in CD offspring, but not HF offspring (Fig S4). In adult mice, microglia are important for learning because microglial ablation via diphtheria toxin interferes with spine formation to impair learning (Parkhurst et al., 2013). It is possible that the 3 week recovery time from PLX was insufficient to support new learning in the FR1 task in a subset of CD males (n = 3/16). HF males may have recovered faster or the increased FR1 responding seen with HF diet may have prevented an increase in dropout rate. However, there were no other effects of PLX on learning or performance in CD offspring, suggesting that adolescent microglia do not play a central role in shaping executive function, or that our PLX3397 treatment was insufficient to disrupt these processes. An important consideration regarding the PLX treatment is the fact that repopulated microglia have the potential to be different from untreated microglia, and therefore, microglial differences at the time of testing may affect adult performance. Future studies designed to fully characterize repopulated microglia will be important for understanding the implications of developmental microglial ablation strategies.
5. CONCLUSION
Overall, microglial ablation with PLX3397 during the adolescent period improved operant-based executive function and impulse control in adult male HF offspring, despite minimal effects of HF diet itself. PLX treatment had surprisingly little effect on male and no effect on female executive function in CD offspring. Of the brain regions investigated, the amygdala was most sensitive to gene expression changes due to maternal HF diet or adolescent PLX treatment. The PFC had the fewest number of gene expression changes, but these changes correlated with behavioral performance. Maternal HF diet increased PFC synaptophysin that was associated with increased impulsive errors in the 5CSRTT, while adolescent PLX treatment abolished this association. However, the small but statistically significant gene expression fold changes warrant investigation at the protein level to determine biological significance. In females, maternal HF diet impaired reversal learning while PLX had no effect, indicating a possible sex-dependent effect of microglial ablation on adolescent neurodevelopment, or a difference in circuits important for performance in the 5CSRTT versus a reversal task. Together, this investigation reveals a role for microglia during the adolescent period for the development of executive function deficits after perinatal HF diet in male mice.
Supplementary Material
Highlights.
Adolescent microglial ablation reduces impulsivity in male HF offspring.
Adolescent microglial ablation has little impact on adult executive function in CD offspring.
Maternal HF diet impairs female reversal learning while microglial ablation has no effect.
Male gene expression in prefrontal cortex and amygdala may underlie behavioral changes.
ACKNOWLEDGEMENTS
We would like to acknowledge our funding to T.M. Reyes: MH106330. We would like to thank Hetty Rodriguez for processing our samples via Fluidigm BioMark (Molecular Profiling, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA USA). Finally, we would like to thank our undergraduate researchers who graciously dedicated their time to help with the operant behavioral testing and training: Helen Ashdown, Lindsey Miller, Alexis Ford, Megan Steele, and Emilia Drenkhan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ, 2008. The complement cascade: Yin-Yang in neuroinflammation - Neuro-protection and - degeneration. J. Neurochem 107, 1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu W-C, Cummings KA, Clem RL, 2017. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. J. Neurosci 37, 2976–2985. doi: 10.1523/JNEUROSCI.3097-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S, Tsang V, 2010. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115. doi: 10.1096/fj.09-144014 [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V, 2010. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115. doi: 10.1096/fj.09-144014 [DOI] [PubMed] [Google Scholar]
- Carlin JL, George R, Reyes TM, 2013. Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS One 8. doi: 10.1371/journal.pone.0063549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarakonda K, Nguyen KP, Kravitz AV, 2016. ROBucket: A low cost operant chamber based on the Arduino microcontroller. Behav. Res. Methods 48, 503–509. doi: 10.3758/s13428-015-0603-2 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA, 2007. Opposing effects of 5-HT2A and 5-HT2C receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl). 195, 223–234. doi: 10.1007/s00213-007-0891-z [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Chu SH, Hernandez MX, Fang MJ, Modarresi L, Selvan P, MacGregor GR, Tenner AJ, 2017. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J. Neuroinflammation 14, 1–15. doi: 10.1186/s12974-017-0814-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JL, Schafer DP, 2016. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 26, 587–597. doi: 10.1016/j.tcb.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Tamadon S, Coen K, Fletcher PJ, Lê AD, 2019. Kappa opioid receptors mediate yohimbine-induced increases in impulsivity in the 5-choice serial reaction time task. Behav. Brain Res 359, 258–265. doi: 10.1016/j.bbr.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendining KA, Fisher LC, Jasoni CL, 2018. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 96, 132–141. doi: 10.1016/j.psyneuen.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ, 2012. Arg Kinase Regulates Prefrontal Dendritic Spine Refinement and Cocaine-Induced Plasticity. J. Neurosci 32, 2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf R, Longo JL, Hughes ZA, 2018. The location discrimination reversal task in mice is sensitive to deficits in performance caused by aging, pharmacological and other challenges. J. Psychopharmacol 32, 1027–1036. doi: 10.1177/0269881118779383 [DOI] [PubMed] [Google Scholar]
- Grissom NM, George R, Reyes TM, 2016. Suboptimal nutrition in early life affects the inflammatory gene expression profile and behavioral responses to stressors. Brain. Behav. Immun doi: 10.1016/j.bbi.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Grissom NM, Herdt CT, Desilets J, Lidsky-Everson J, Reyes TM, 2015. Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology 40, 1353–1363. doi: 10.1038/npp.2014.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Spencer SJ, Conti B, Jasoni CL, Kent S, Radler ME, Reyes TM, Sominsky L, 2015. Diet, behavior and immunity across the lifespan. Neurosci. Biobehav. Rev 58, 46–62. doi: 10.1016/j.neubiorev.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Wada K, Kabuta T, 2016. Maternal high-fat diet leads to persistent synaptic instability in mouse offspring via oxidative stress during lactation. Neurochem. Int 97, 99–108. doi: 10.1016/j.neuint.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A, 2017. The neural basis of reversal learning: An updated perspective. Neuroscience 345, 12–26. doi: 10.1016/j.neuroscience.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, J. J, 2013. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl). 219, 607–620. doi: 10.1007/s00213-011-2579-7.Reversal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janthakhin Y, Rincel M, Costa AM, Darnaudéry M, Ferreira G, 2017. Maternal high-fat diet leads to hippocampal and amygdala dendritic remodeling in adult male offspring. Psychoneuroendocrinology 83, 49–57. doi: 10.1016/j.psyneuen.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, Fryer JD, 2014. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflammation 11, 156. doi: 10.1186/s12974-014-0156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec A, Smith CJ, Ayre NR, Sweat SC, Bilbo SD, 2017a. Microglial elimination of dopamine D1 receptors defines sex-specific changes in nucleus accumbens development and social play behavior during adolescence. bioRxiv 211029. doi: 10.1101/211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec A, Smith CJ, Ayre NR, Sweat SC, Bilbo SD, 2017b. Microglial elimination of dopamine D1 receptors defines sex-specific changes in nucleus accumbens development and social play behavior during adolescence. bioRxiv 02129, 211029. doi: 10.1101/211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KR, Yaghoubi SK, Makinson RA, McKee SE, Reyes TM, 2018. Housing and testing in mixed-sex rooms increases motivation and accuracy during operant testing in both male and female mice. Neurobiol. Learn. Mem 150, 20–24. doi: 10.1016/j.nlm.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Grigg A, Kim SA, Ririe DG, Eisenach JC, 2015. Assessment of attention threshold in rats by titration of visual cue duration during the five choice serial reaction time task. J. Neurosci. Methods 241, 37–43. doi: 10.1016/j.jneumeth.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SE, Grissom NM, Herdt CT, Reyes TM, 2017. Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet–fed dams. FASEB J. fj.201601172R. doi: 10.1096/fj.201601172R [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM, 2009. A new touchscreen test of pattern separation: Effect of hippocampal lesions. Neuroreport 20, 881–885. doi: 10.1097/WNR.0b013e32832c5eb2 [DOI] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD, 2011. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176, 225–236. doi: 10.1016/j.neuroscience.2010.12.037 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–8. doi: 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan WB, 2013. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609. doi: 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, Lee FS, 2016. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun 7, 11475. doi: 10.1038/ncomms11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Luca E, Wolfrum C, 2012. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res 233, 398–404. doi: 10.1016/j.bbr.2012.05.027 [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Sarker G, Litwan K, Krämer SD, Ametamey SM, Schibli R, Wolfrum C, 2016. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 6. doi: 10.1038/tp.2016.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, O’Connor V, 2008. C1q: The perfect complement for a synaptic feast? Nat. Rev. Neurosci 9, 807–811. doi: 10.1038/nrn2394 [DOI] [PubMed] [Google Scholar]
- Ralevski A, Horvath TL, 2015. Developmental programming of hypothalamic neuroendocrine systems. Front. Neuroendocrinol 39, 52–58. doi: 10.1016/j.yfrne.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Renee M, Elmore P, Najafi AR, Koike MA, Nazih N, Spangenberg EE, Rice RA, Kitazawa M, Nguyen H, West BL, Green KN, 2015. CSF1 receptor signaling is necessary for microglia viability, which unmasks a cell that rapidly repopulates the microglia- depleted adult brain. Neuron 82, 380–397. doi: 10.1016/j.neuron.2014.02.040.CSF1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS, 1996. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods doi: 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Rincel M, Lépinay AL, Janthakhin Y, Soudain G, Yvon S, da Silva S, Joffre C, Aubert A, Séré A, Layé S, Theodorou V, Ferreira G, Darnaudéry M, 2017. Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct. Funct doi: 10.1007/s00429-017-1526-8 [DOI] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, Sullivan EL, 2015. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci 9, 1–16. doi: 10.3389/fnins.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, 2002. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl). 163, 362–380. doi: 10.1007/s00213-002-1154-7 [DOI] [PubMed] [Google Scholar]
- Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO, 2013. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience 240, 1–12. doi: 10.1016/j.neuroscience.2013.02.044 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705. doi: 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL, 2005. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol 26, 163–174. doi: 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Smith BL, Reyes TM, 2017. Offspring neuroimmune consequences of maternal malnutrition: Potential mechanism for behavioral impairments that underlie metabolic and neurodevelopmental disorders. Front. Neuroendocrinol 47, 109–122. doi: 10.1016/j.yfrne.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BL, Schmeltzer SN, Packard BA, Sah R, Herman JP, 2016. Divergent effects of repeated restraint versus chronic variable stress on prefrontal cortical immune status after LPS injection. Brain. Behav. Immun 57, 263–270. doi: 10.1016/j.bbi.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AMM, Lambris JD, Smith SJ, John SWM, Barres BA, 2007. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 131, 1164–1178. doi: 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Riper KM, Lockard R, Valleau JC, 2015. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm. Behav 76, 153–161. doi: 10.1016/j.yhbeh.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL, 2011. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93, 1–8. doi: 10.1159/000322038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, Wada K, 2010. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int 57, 235–247. doi: 10.1016/j.neuint.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM, 2010. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151, 4756–4764. doi: 10.1210/en.2010-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT, 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci 17, 400–406. doi: 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


