ABSTRACT
The results from epidemiologic studies suggest that vitamin D receptor (VDR) gene polymorphisms are potentially associated with Alzheimer disease (AD) and mild cognitive impairment (MCI), but this association has yet to be confirmed. Here, we conducted a meta-analysis based on a larger sample size to clarify the contribution of VDR gene polymorphisms to MCI and AD susceptibility. The PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure databases were searched to obtain studies published before 30 October, 2020. The case group includes MCI and AD patients, and the matched controls were without any cognitive complaints. ORs and 95% CIs were used to assess the strength of the association. Ten case–control studies with 3573 participants and 4 loci of ApaI rs7975232, BsmI rs1544410, FokI rs10735810, and TaqI rs731236 were included in the meta-analysis. The global assessment indicated an association between the BsmI polymorphism and increased odds of MCI in the allelic model (b compared with B; OR: 1.77; 95% CI: 1.24, 2.54), the dominant model (bb + Bb compared with BB; OR: 2.04; 95% CI: 1.32, 3.16), and the heterozygote model (Bb compared with BB; OR: 1.97; 95% CI: 1.26, 3.09). In contrast, the ApaI polymorphism was protective against MCI in all models. The dominant model (tt + Tt compared with TT; OR: 1.44; 95% CI: 1.17, 1.79) and the homozygous model (tt compared with TT; OR: 1.43; 95% CI: 1.02, 2.00) revealed an association between the TaqI polymorphism of the VDR gene and increased odds of AD, particularly for Caucasian subjects. Egger's linear regression test found no publication bias. This meta-analysis indicated that VDR ApaI and BsmI, and TaqI gene polymorphisms may be important predictors of MCI and AD, respectively, with population discrepancies. More research is needed to further confirm these associations, especially considering gene–gene interactions, gene–environment interactions, and other confounding factors.
Keywords: Alzheimer disease, mild cognitive impairment, polymorphisms, single nucleotide polymorphisms, vitamin D receptor
Statement of Significance: Compared with previous studies on the same subject, this study included more data and found that the dominant and homozygous gene models of a TaqI gene polymorphism may increase the risk of Alzheimer disease. This study investigated the association between vitamin D receptor gene polymorphisms and mild cognitive impairment and for the first time indicated that the allele, dominant, and heterozygous gene models of BsmI polymorphisms are related to an increased risk of mild cognitive impairment.
Introduction
Alzheimer disease (AD) is characterized by cognitive decline and memory loss, with insidious onset and a long incubation period (1), before which mild cognitive impairment (MCI) constitutes a typical prodromal stage. With the aging of the population, incidence has gradually increased and has become a public health issue of general concern. Patients with MCI have biomarker evidence of Alzheimer brain changes (e.g., abnormal amounts of amyloid-β) and subtle problems with memory and thinking, but these deficits do not interfere with the individual's ability to perform daily activities (2). In particular, they have a higher risk of developing AD over time than older adults with normal cognition (3). The 2019 World Alzheimer's Disease Report shows that 50 million people worldwide suffer from dementia, and this number is expected to reach 152 million by 2050 (4). Unfortunately, the specific pathogenesis is still ambiguous despite many explorations.
It has been hypothesized that early identification of genetic risk factors may contribute to the prevention and treatment of cognitive decline. Recently, genome-wide association analysis has been used to screen out numerous candidate genes associated with the risk of MCI and AD, including β-amyloid precursor protein (5), presenilin 1/2, and apoE (6). The vitamin D receptor (VDR) gene has been extensively investigated in recent years. Vitamin D is a fat-soluble steroid hormone that exerts biological effects by combining with VDRs. Epidemiologic investigations have shown that low serum vitamin D concentrations increase the risk of cognitive decline, and that the elderly with vitamin D deficiency have a higher risk of AD (7). There is increasing evidence that vitamin D prevents the progression of AD by removing β-amyloid deposits, inhibiting hyperphosphorylation τ protein, alleviating inflammation, regulating Ca2+ homeostasis, and reducing antioxidative stress (8, 9). In addition, vitamin D deficiency increases the risk of cardiovascular disease (10), which is considered a risk factor for AD. Because serum 25-hydroxyvitamin D deficiency has been reported to be associated with an increased risk of cognitive decline, the hypothesis exists that allelic variation in the VDR gene might contribute to cognitive impairment.
Genetic variation indicates a link between genes and diseases. Among several types of genetic variation, single-nucleotide polymorphisms (SNPs) are prominent in ≥1% of the population. Polymorphisms in gene-regulatory regions may affect gene expression levels and protein functions (11). The biological function of active vitamin D benefits from binding to VDR, which is a ligand-dependent nuclear transcription factor expressed in multiple system organs and tissues throughout the body. The VDR gene is located on chromosome 12 (12q13-14) and consists of 9 exons and 8 introns, exceeding 100 kb (12–14) in length. Among the VDR SNPs, ApaI rs7975232, BsmI rs1544410, FokI rs10735810, and TaqI rs731236 are the most concerning (15). The BsmI and ApaI restriction sites are located in the 8th intron, the TaqI restriction sites are in the 9th exon, and the FokI restriction sites are situated in the 5'-end 16 of the gene (16).
Recent evidence supports the contention that VDR gene polymorphisms play a key role in AD and MCI by maintaining the biological function of vitamin D. However, those results are contradictory and uncertain owing to small sample sizes and limited statistical power. Although a previous meta-analysis established the association between VDR TaqI and ApaI polymorphisms and AD susceptibility (17), the results were limited to only 2 studies at the time. Notably, the role of VDR polymorphisms in MCI has yet to be investigated. Based on the aforementioned background, the present meta-analysis provides an overview of current knowledge to accurately assess the contribution of VDR gene polymorphisms to AD and MCI susceptibility.
Methods
Search strategy
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (18). Two independent reviewers searched the China National Knowledge Infrastructure, PubMed, Embase, and Cochrane Library databases from inception to 20 November, 2020. The following combination of keywords was used and adjusted according to the characteristics of the database: (vitamin D receptor or VDR or FokI or rs10735810 or BsmI or rs1544410 or ApaI or rs7975232 or TaqI or rs731236) and (polymorphism or variant or mutation) and (mild cognitive impairment or Alzheimer's disease). We limited the search languages to English and Chinese. The reference lists of the retrieved articles were also searched to identify other potentially relevant studies.
Inclusion and exclusion criteria
All studies were strictly screened following the inclusion/exclusion criteria to minimize heterogeneity: 1) case–control studies were included; 2) the relation between VDR gene polymorphisms and the risk of MCI or AD was reported; 3) the VDR gene polymorphisms and genotype distribution data were reported; and 4) ORs and corresponding 95% CIs were used to assess the frequency of genotypes between cases and controls. Studies that met the following conditions were not included: 1) commentary articles, editorials, conference abstracts, case reports, and duplicate studies; 2) studies that did not report the genotype and frequency of the 2 comparative populations; and 3) studies that did not report complete data.
Data extraction and quality assessment
Standardized data collection tables were used to extract the following information: first author, publication year, country, ethnicity, genotyping method, control source, numbers of cases and controls, population characteristics, and primary observed results. “A” and “a” were used to indicate the wild-type allele and mutant alleles of ApaI loci, respectively (A > a); “B” and “b” were used to indicate the wild-type allele and mutant alleles of BsmI loci, respectively (B > b). The same applies to the FokI and TaqI loci. The quality of each study was assessed through the Newcastle-Ottawa Scale (NOS) (19), which uses a “star” classification system, ranging from 0 (worst) to 9 (best). Studies with a score ≥7 are considered high-quality studies, whereas studies with a score ≤6 are considered of moderate quality or low quality. Two researchers independently completed the data extraction and quality assessment. Any disagreements were resolved through discussion or consultation with a third researcher.
Statistical analysis
All statistical analyses were performed using Stata 15.1 software (StataCorp). Pooled ORs and 95% CIs were used to assess the strength of the relations between VDR gene polymorphisms and the risk of MCI and AD. The chi-square test was used to evaluate the genotype frequency of the control group in relation to the Hardy–Weinberg equilibrium (P < 0.05 was considered significant imbalance). Five genetic models were used for analysis: the allelic, dominant, recessive, heterozygous, and homozygous models. For the ApaI variant, the 5 models are represented by a compared with A, aa + Aa compared with AA, aa compared with Aa + AA, Aa compared with AA, and aa compared with AA. For the BsmI variant, the 5 models are represented by b compared with B, bb + Bb compared with BB, bb compared with Bb + BB, Bb compared with BB, and bb compared with BB. For the FokI variant, the 5 models are represented by f compared with F, ff + Ff compared with FF, ff compared with Ff + FF, Ff compared with FF, and ff compared with FF. For the TaqI variant, the 5 models are represented by t compared with T, tt + Tt compared with TT, tt compared with Tt + TT, Tt compared with TT, and tt compared with TT. Heterogeneity between the included studies was assessed through the I2 statistic and P values (20); I2 ≤ 50% was considered to indicate low heterogeneity (the fixed-effects model was used), whereas I2 > 50% was considered to indicate substantial heterogeneity (the random-effects model was used). A sensitivity analysis evaluated the stability of the results by removing each study in turn. Further subgroup analysis was carried out by stratification by ethnicity (Caucasian and Asian). Publication bias was evaluated using a visual funnel plot and Egger's linear regression test. A 2-sided P < 0.05 was considered significant.
Results
Literature search
Eighty-six potentially relevant studies were retrieved from 4 databases, 7 records were identified through other sources, 31 of which were excluded owing to duplication. The remaining 62 records were filtered based on titles and abstracts, of which 33 were excluded because they were irrelevant to the subject. We reviewed the full texts of the remaining 29 studies and determined 10 case–control studies that met the inclusion criteria (21–30). Two of them reported MCI (29, 30), 7 reported AD (22–28), and 1 reported both MCI and AD (21). Figure 1 summarizes the detailed search process.
FIGURE 1.
Flowchart of the literature retrieval and selection process.
Study characteristics and quality assessment
Ten studies published from 2003 to 2019 were included in the meta-analysis, involving 3573 participants. Four studies were conducted in Asia (22, 24, 29, 30), 2 in the Americas (21, 28), and 4 in Europe (23, 25–27). All participants were >60 y old. Most of the control participants were healthy subjects or without cognitive impairment. The association of the VDR gene ApaI polymorphism was examined in 8 case–control studies (21–26, 29, 30), the association of the BsmI polymorphism was examined in 7 studies (21–23, 25, 27, 29, 30), the association of the FokI polymorphism was examined in 6 studies (21–23, 25, 27, 28), and the association of the TaqI polymorphism was examined in 6 studies (21–24, 26, 27). Table 1–3 summarize the characteristics and genotype frequencies of the included studies. With regard to the NOS, 2 studies were classified as high quality (21, 24), and the remaining studies were of moderate quality, with a mean score of 5.7 (Table 1). The evidence that contributes to these analyses is considered to be of moderate quality.
TABLE 1.
Summary characteristics of the studies included in the meta-analysis1
| Age, y | Sample size, n | Sex, % female | Genotyping methods | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Country | Ethnicity | Study design | Disease | Case | Control | Case | Control | Control source | SNPs | NOS | ||
| de Oliveira et al. (21) | Brazil | Caucasian | Case–control | MCI, AD | 74.60 ± 4.94 | 74.09 ± 7.17 | 15 | 24 | 24 (61.5) | Healthy elderly | PCR | ApaI, BsmI, FokI, TaqI | 7 |
| Keyimu et al. (30) | China | Asian | Case–control | MCI | 65.63 ± 7.46 | 64.44 ± 6.20 | 124 | 124 | 109 (44.0) | Patients who were without MCI, but have the same or similar life background, age, and gender as selected MCI patients | PCR | ApaI, BsmI | 6 |
| Abriz et al. (29) | China | Asian | Case–control | MCI | 77. 22 ± 6.59 | 76. 72 ± 5.71 | 100 | 145 | 127 (51.8) | Patients who were without MCI, but have the same or similar life background, age, and gender as selected MCI patients | PCR | ApaI, BsmI | 6 |
| Gezen-Ak et al. (27) | Turkey | Caucasian | Case–control | AD | 75.1 ± 5.7 | 73.6 ± 7.3 | 104 | 109 | NA | Age-matched controls without any neurodegenerative diseases | PCR | ApaI, BsmI, FokI, TaqI | 4 |
| Gezen-Ak et al. (25) | Turkey | Caucasian | Case–control | AD | 74 ± 4.2 | 75.2 ± 6.8 | 108 | 115 | NA | Controls without any neurodegenerative diseases | PCR | BsmI, FokI | 4 |
| Khorram et al. (24) | Iran | Asian | Case–control | AD | 78.5 ± 7.8 | 77.4 ± 7.0 | 145 | 162 | 190 (61.9) | Age-matched unrelated healthy controls | PCR | ApaI, TaqI | 8 |
| Lehmann et al. (26) | United Kingdom | Caucasian | Case–control | AD | NA | NA | 255 | 260 | 265 (51.5) | Elderly controls from the longitudinal cohort of the Oxford Project to Investigate Memory and Ageing | PCR | ApaI, TaqI | 5 |
| Łaczmański et al. (23) | Poland | Caucasian | Case–control | AD | 73.7 ± 8.6 | 64.5 ± 7.8 | 108 | 77 | 167 (90.3) | Healthy volunteers | PCR | ApaI, BsmI, FokI, TaqI | 6 |
| Luedecking-Zimmer et al. (28) | United States | Caucasian | Case–control | AD | 77.3 ± 6.4 | 76.8 ± 6.3 | 564 | 523 | NA | Controls were obtained from the same geographical area from which the patients were derived, and were found cognitively intact | PCR | FokI | 5 |
| Mun et al. (22) | South Korea | Asian | Case–control | AD | 79.82 ± 7.02 | 68.94 ± 6.10 | 144 | 335 | 285 (59.5) | Healthy elderly | PCR | ApaI, BsmI, FokI, TaqI | 6 |
Values are means ± SDs unless otherwise indicated. AD, Alzheimer disease; MCI, mild cognitive impairment; NA, not available; NOS, Newcastle-Ottawa Scale; SNP, single nucleotide polymorphism.
TABLE 2.
Genotype frequencies of vitamin D receptor gene polymorphisms in AD patients and matched controls1
| Genotype | HWE P value | |||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Authors | AD patients | Controls | |||||
| ApaI | AA | Aa | aa | AA | Aa | aa | ||
| Gezen-Ak et al. (27) | 35 | 63 | 6 | 55 | 43 | 11 | 0.55 | |
| de Oliveira et al. (21) | 14 | 15 | 3 | 9 | 13 | 2 | 0.37 | |
| Lehmann et al. (26) | 86 | 132 | 37 | 64 | 140 | 56 | 0.21 | |
| Łaczmański et al. (23) | 26 | 59 | 23 | 17 | 41 | 12 | 0.14 | |
| Mun et al. (22) | 3 | 62 | 79 | 12 | 129 | 188 | 0.07 | |
| Khorram et al. (24) | 29 | 65 | 51 | 28 | 78 | 56 | 0.93 | |
| BsmI | BB | Bb | bb | BB | Bb | bb | ||
| Gezen-Ak et al. (25) | 30 | 38 | 39 | 34 | 32 | 48 | 0.58 | |
| de Oliveira et al. (21) | 9 | 11 | 12 | 2 | 12 | 10 | 0.54 | |
| Łaczmański et al. (23) | 12 | 61 | 35 | 10 | 44 | 23 | 0.12 | |
| Mun et al. (22) | 0 | 19 | 125 | 1 | 34 | 294 | 0.99 | |
| FokI | FF | Ff | ff | FF | Ff | ff | ||
| Gezen-Ak et al. (25) | 52 | 46 | 10 | 51 | 51 | 10 | 0.58 | |
| de Oliveira et al. (21) | 15 | 14 | 3 | 12 | 11 | 1 | 0.43 | |
| Luedecking-Zimmer et al. (28) | 233 | 225 | 78 | 198 | 229 | 65 | 0.92 | |
| Łaczmański et al. (23) | 36 | 53 | 19 | 27 | 36 | 14 | 0.74 | |
| Mun et al. (22) | 43 | 77 | 24 | 129 | 148 | 53 | 0.53 | |
| TaqI | TT | Tt | tt | TT | Tt | tt | ||
| Gezen-Ak et al. (27) | 38 | 50 | 16 | 53 | 39 | 17 | 0.03 | |
| de Oliveira et al. (21) | 10 | 11 | 11 | 13 | 6 | 5 | 0.93 | |
| Lehmann et al. (26) | 68 | 136 | 51 | 101 | 117 | 42 | 0.41 | |
| Łaczmański et al. (23) | 42 | 55 | 11 | 31 | 38 | 8 | 0.46 | |
| Mun et al. (22) | 125 | 19 | 0 | 296 | 32 | 1 | 0.89 | |
| Khorram et al. (24) | 64 | 64 | 17 | 76 | 65 | 21 | 0.24 | |
1AD, Alzheimer disease; HWE, Hardy–Weinberg equilibrium; SNP, single nucleotide polymorphism.
TABLE 3.
Genotype frequencies of vitamin D receptor gene polymorphisms in MCI patients and matched controls1
| Genotype | HWE P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Authors | MCI patients | Controls | |||||||||
| ApaI | A | a | AA | Aa | aa | A | a | AA | Aa | aa | ||
| de Oliveira et al. (21) | 20 | 10 | 6 | 8 | 1 | 31 | 17 | 9 | 13 | 2 | 0.37 | |
| Keyimu et al. (30) | 121 | 127 | 29 | 63 | 32 | 92 | 156 | 17 | 58 | 49 | 0.98 | |
| Abriz et al. (29) | 79 | 121 | 20 | 39 | 41 | 75 | 215 | 13 | 49 | 83 | 0.15 | |
| BsmI | B | b | BB | Bb | bb | B | b | BB | Bb | bb | ||
| de Oliveira et al. (21) | 11 | 19 | 2 | 7 | 6 | 16 | 32 | 2 | 12 | 10 | 0.54 | |
| Keyimu et al. (30) | 185 | 63 | 69 | 47 | 8 | 211 | 37 | 89 | 33 | 2 | 0.59 | |
| Abriz et al. (29) | 184 | 16 | 84 | 16 | 0 | 279 | 11 | 135 | 9 | 1 | 0.07 | |
1HWE, Hardy–Weinberg equilibrium; MCI, mild cognitive impairment; SNP, single nucleotide polymorphism.
Meta-analysis results of VDR gene polymorphisms and MCI susceptibility
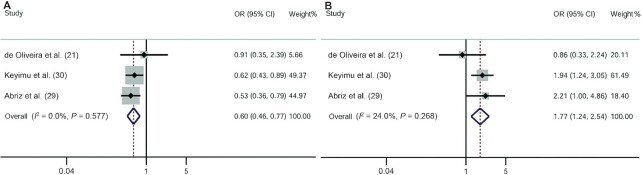
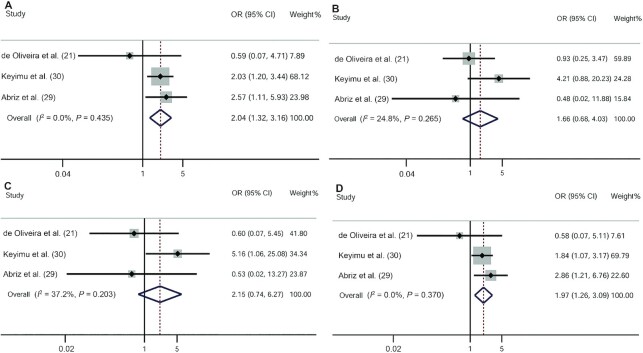
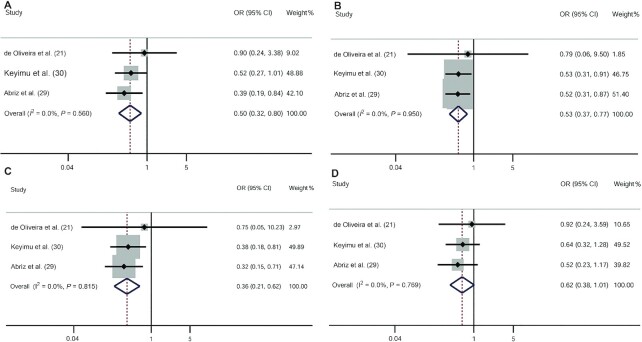
Three studies reported the associations between VDR gene polymorphisms and MCI risk (21, 29, 30). Two studies were conducted in Asia and 1 was conducted in the Americas. The fixed-effects model of the Mantel–Haenszel method was used because there was no heterogeneity. Overall, our meta-analysis indicated that the allelic model (OR: 1.77; 95% CI: 1.24, 2.54) (Figure 2B), the dominant model (OR: 2.04; 95% CI: 1.32, 3.16), and the heterozygote model (OR: 1.97; 95% CI: 1.26, 3.09) of the VDR BsmI polymorphism were associated with an increased risk of MCI (Figure 3A–D). In contrast, the VDR ApaI polymorphism was protective against MCI (allelic model: OR: 0.60; 95% CI: 0.46, 0.77; dominant model: OR: 0.50; 95% CI: 0.32, 0.80; recessive model: OR: 0.53; 95% CI: 0.37, 0.77; homozygote model: OR: 0.36; 95% CI: 0.21, 0.62; heterozygous model: OR: 0.62; 95% CI: 0.38, 1.01) (Figures 2A, 4A–D).
FIGURE 2.
ORs and 95% CIs of individual studies and pooled data for the allele associations between the vitamin D receptor ApaI (A) and BsmI (B) polymorphisms and mild cognitive impairment.
FIGURE 3.
ORs and 95% CIs of individual studies and pooled data for the associations between the vitamin D receptor BsmI polymorphism and mild cognitive impairment. (A) Dominant model (bb + Bb compared with BB); (B) recessive model (bb compared with Bb + BB); (C) homozygous model (bb compared with BB); (D) heterozygous model (Bb compared with BB).
FIGURE 4.
ORs and 95% CIs of individual studies and pooled data for the associations between the vitamin D receptor ApaI polymorphism and mild cognitive impairment. (A) Dominant model (aa + Aa compared with AA); (B) recessive model (aa compared with Aa + AA); (C) homozygous model (aa compared with AA); (D) heterozygous model (Aa compared with AA).
Meta-analysis results of VDR gene polymorphisms and AD susceptibility
Pooled ORs were calculated to determine the association between VDR gene polymorphisms and the odds of AD. The random-effects model of the DerSimonian–Laird method was used in some comparative groups with significant heterogeneity. Our analysis revealed no altered odds of AD based on the ApaI, BsmI, and FokI polymorphisms of the VDR gene in any genetic model. In contrast, the dominant model (OR: 1.44; 95% CI: 1.17, 1.79) and the homozygous model (OR: 1.43; 95% CI: 1.02, 2.00) revealed an association between the TaqI polymorphism of the VDR gene and increased odds of AD, with no heterogeneity found (Table 4).
TABLE 4.
Meta-analysis results for the association between vitamin D receptor gene polymorphisms and Alzheimer disease1
| Pooled estimate value | P-heterogeneity | Egger's P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | Comparison | n | Model | OR (95% CI) | Z | P value | I 2, % | ||
| ApaI | aa + Aa vs. AA | 6 | Random | 1.01 (0.65, 1.55) | 0.02 | 0.981 | 60.2 | 0.028 | 0.870 |
| aa vs. Aa + AA | Fixed | 0.86 (0.68, 1.08) | 1.31 | 0.187 | 0 | 0.468 | 0.140 | ||
| aa vs. AA | Fixed | 0.77 (0.55, 1.06) | 1.59 | 0.112 | 9.0 | 0.358 | 0.129 | ||
| Aa vs. AA | Random | 1.06 (0.67, 1.67) | 0.24 | 0.811 | 61.0 | 0.025 | 0.640 | ||
| BsmI | bb + Bb vs. BB | 4 | Fixed | 0.96 (0.61, 1.51) | 0.18 | 0.858 | 9.8 | 0.344 | 0.536 |
| bb vs. Bb + BB | Random | 1.14 (0.41, 3.12) | 0.25 | 0.805 | 88.9 | <0.001 | 0.663 | ||
| bb vs. BB | Fixed | 0.89 (0.54, 1.48) | 0.44 | 0.657 | 0 | 0.498 | 0.679 | ||
| Bb vs. BB | Fixed | 1.05 (0.64, 1.74) | 0.21 | 0.836 | 26.0 | 0.255 | 0.510 | ||
| FokI | ff + Ff vs. FF | 5 | Fixed | 1.01 (0.84, 1.21) | 0.07 | 0.944 | 20.5 | 0.284 | 0.501 |
| ff vs. Ff + FF | Fixed | 1.09 (0.84, 1.41) | 0.62 | 0.533 | 0 | 0.964 | 0.548 | ||
| ff vs. FF | Fixed | 1.10 (0.83, 1.46) | 0.65 | 0.516 | 0 | 0.888 | 0.395 | ||
| Ff vs. FF | Fixed | 0.98 (0.81, 1.20) | 0.15 | 0.877 | 32.7 | 0.203 | 0.720 | ||
| TaqI | tt + Tt vs. TT | 6 | Fixed | 1.44 (1.17, 1.79) | 3.37 | 0.001 | 0 | 0.463 | 0.803 |
| tt vs. Tt + TT | Fixed | 1.14 (0.84, 1.55) | 0.85 | 0.397 | 0 | 0.860 | 0.788 | ||
| tt vs. TT | Fixed | 1.43 (1.02, 2.00) | 2.09 | 0.037 | 0 | 0.609 | 0.704 | ||
| Tt vs. TT | Random | 1.59 (0.84, 3.01) | 1.43 | 0.152 | 86.0 | <0.001 | 0.229 | ||
1SNP, single nucleotide polymorphism.
Subgroup analysis based on stratification by ethnicity showed that the dominant model (OR: 1.60; 95% CI: 1.22, 2.08) and the homozygous model (OR: 1.61; 95% CI: 1.10, 2.35) of the TaqI polymorphism were associated with an increased risk of AD in Caucasians but not in Asians. Moreover, the recessive model (OR: 4.33; 95% CI: 2.39, 7.87) of the BsmI polymorphism and the heterozygous model (OR: 1.56; 95% CI: 1.00, 2.43) of the FokI polymorphism were associated with increased susceptibility to AD in Asians (Table 5).
TABLE 5.
Meta-analysis results for the association between vitamin D receptor gene polymorphisms and Alzheimer disease based on stratification by ethnicity1
| SNP, ethnicity | n | OR (95% CI) | P-interaction | OR (95% CI) | P-interaction | OR (95% CI) | P-interaction | OR (95% CI) | P-interaction |
|---|---|---|---|---|---|---|---|---|---|
| ApaI | 6 | aa + Aa vs. AA | aa vs. Aa + AA | aa vs. AA | Aa vs. AA | ||||
| Asian | 2 | 0.96 (0.47, 1.94) | 0.915 | 0.96 (0.71, 1.30) | 0.305 | 1.00 (0.56, 1.78) | 0.269 | 1.02 (0.48, 2.20) | 0.939 |
| Caucasian | 4 | 1.01 (0.55, 1.85) | 0.74 (0.50, 1.10) | 0.69 (0.43, 1.10) | 1.06 (0.56, 1.98) | ||||
| BsmI | 4 | bb + Bb vs. BB | bb vs. Bb + BB | bb vs. BB | Bb vs. BB | ||||
| Asian | 1 | 1.32 (0.59, 32.05) | 0.722 | 4.33 (2.39, 7.87)* | 0.036 | 1.28 (0.05, 31.60) | 0.828 | 1.70 (0.07, 43.66) | 0.580 |
| Caucasian | 3 | 0.90 (0.46, 1.78) | 0.73 (0.39, 1.39) | 0.89 (0.49, 1.60) | 0.92 (0.41, 2.11) | ||||
| FokI | 5 | ff + Ff vs. FF | ff vs. Ff + FF | ff vs. FF | Ff vs. FF | ||||
| Asian | 1 | 1.51 (0.99, 2.29) | 0.034 | 1.05 (0.62, 1.77) | 0.764 | 1.36 (0.75, 2.46) | 0.419 | 1.56 (1.00, 2.43)* | 0.023 |
| Caucasian | 4 | 0.91 (0.74, 1.12) | 1.10 (0.81, 1.48) | 1.03 (0.75, 1.42) | 0.88 (0.70, 1.09) | ||||
| TaqI | 6 | tt + Tt vs. TT | tt vs. Tt + TT | tt vs. TT | Tt vs. TT | ||||
| Asian | 2 | 1.20 (0.84, 1.72) | 0.207 | 0.89 (0.45, 1.73) | 0.413 | 0.95 (0.47, 1.92) | 0.196 | 3.23 (0.42, 24.79) | 0.265 |
| Caucasian | 4 | 1.60 (1.22, 2.08)* | 1.22 (0.87, 1.72) | 1.61 (1.10, 2.35)* | 1.00 (0.74, 1.36) | ||||
1SNP, single nucleotide polymorphism.
∗Significant association with AD, P < 0.05
Sensitivity analysis
Sensitivity analysis was performed to assess the impact of individual studies on the overall effect estimate. Deleting each study 1 at a time and reanalyzing the data set did not lead to significant changes in the pooled OR estimate, indicating the statistical stability of the results.
Publication bias
Egger's linear regression test and a visual funnel plot were used to estimate the publication bias between the studies involved in the meta-analysis (Table 4). These 2 testing methods showed that there was no evidence of publication bias in any of the genetic models that were compared.
Discussion
We comprehensively evaluated the correlations of 4 candidate SNPs (ApaI, BsmI, FokI, TaqI) of the VDR gene with susceptibility to MCI and AD from 10 selected studies. Our results are somewhat inconsistent with the previous study by Lee et al. (17). The ApaI and TaqI polymorphisms were demonstrated to be associated with a high risk of AD in the allelic, recessive, and homozygous gene models in Caucasians in their study, but the results were limited to only 2 studies. Regarding our results, 6 trials including Caucasians and Asians found that dominant genes and a homozygous gene model of the TaqI gene polymorphism may increase the risk of AD. This is the first meta-analysis that we know of to assess the relation between VDR gene polymorphisms and MCI susceptibility. The allele, dominant, and heterozygous gene models of the BsmI polymorphism are related to an increased risk of MCI. Conversely, the ApaI polymorphism may be protective. These results indicate that functional polymorphisms of VDR located near the 3' untranslated region may affect the function of vitamin D by regulating gene stability and protein translation, thereby interfering with the effect of vitamin D on cognitive function.
SNPs describe the DNA sequence polymorphism caused by single-nucleotide variation at the genomic level, affecting the transcription, translation, expression, and function of proteins to define discrepancies in individual genetic polymorphisms (31). Confounding factors and reverse causality lead to relatively limited observational research results (32). Genetic genes are used as an instrumental variable to reveal the relation between exposure and outcome while avoiding confounding factors and reverse causality because genetic genes are rarely affected by environmental factors (8, 33). 1,25-Dihydroxyvitamin D [1,25(OH)2D], the most active metabolic form of vitamin D, not only plays a vital role in bone metabolism and calcium homeostasis but also participates in nonendocrine effects, such as cardiovascular disease, immune function, diabetes, and cancer (34). The current view on the use of vitamin D in managing cognitive impairment is based on the hypothesis that low serum vitamin D concentration is associated with the prevalence and severity of cognitive decline. Therefore, the evaluation of serum vitamin D concentrations is part of the clinical management of cognitive impairment. Detecting its deficiency in a target population and replenishing it in a timely manner may help prevent or improve cognitive decline. In fact, vitamin D supplementation might play a critical role in the pathophysiology of neurodegenerative disease, including AD, multiple sclerosis, Parkinson disease, amyotrophic lateral sclerosis, and Huntington disease (35–39). VDR expression and nuclear activation are necessary for 1,25(OH)2D function, although the vitamin D needs to be metabolized in the liver and kidneys first (40). Therefore, genetic alteration of VDR genes may lead to abnormal gene activation, which could influence the biological effects of vitamin D. Multiple studies have reported the relation between VDR polymorphism and susceptibility to MCI and AD, but which SNPs are protective or dangerous has not been determined. Our results may provide clear evidence for this controversy.
The results of the stratified analysis showed that the dominant and homozygous models of the TaqI polymorphism were associated with an increased risk of AD in Caucasians, and the recessive model of the BsmI polymorphism and the heterozygous model of the FokI polymorphism were associated with increased susceptibility to AD in Asians. These results can be explained by ethnic differences because SNPs play a multifunctional role in cognitive impairment and vary between different ethnic groups, which indicates the influence of genetic and environmental factors. It is worth noting that the expression and effect of VDR are determined by not only genetics but also race and environment and involve complex interactions that can change the connection with disease.
This meta-analysis tested the strength of the association between 4 VDR gene polymorphisms and MCI or AD based on 5 gene models. The probability of random errors was reduced by grouping data from a single study. Most of the trials received ≥5 stars according to NOS criteria. Considering the sample size, inclusion criteria, and characteristics of patients and controls, our results may provide the strongest evidence to date. However, despite the strong statistical significance of these results, the current research still has some potential limitations. First, language and selection biases cannot be excluded without quantifying publication bias. Publications were limited to Chinese and English, and some research published in other languages may have been omitted. Moreover, a limited number of electronic databases were reviewed, and related research indexed in other electronic databases may have been ignored. Second, most studies did not report on genetic–environment interactions, limiting the scope of this topic. Third, significant heterogeneity may weaken the validity of the conclusions. Fourth, data on stratification by ethnicity are limited; thus, a large-scale case–control study needs to be carried out to provide strong evidence to verify our findings.
In conclusion, the current meta-analysis provides statistical evidence that VDR ApaI and BsmI polymorphisms may be associated with the risk of MCI, and TaqI polymorphisms may be associated with the risk of AD. Further studies with larger sample sizes are essential to reach clear conclusions.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—NL, TZ, and HL: made substantial contributions to the conception and design; LM and WW: acquired the data; ZL, JS, and XJ: performed the analysis; HP: performed the interpretation, drafted the manuscript, and revised it critically for important intellectual content; and all authors: read and approved the final manuscript.
Notes
Supported by China National Science and Technology Major Project for “Essential new drug research and development” grant 2018ZX09301038-003 (to HL). The funding source had no role in the study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AD, Alzheimer disease; MCI, mild cognitive impairment; NOS, Newcastle-Ottawa Scale; SNP, single nucleotide polymorphism; VDR, vitamin D receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Contributor Information
Nanyang Liu, Department of Geratology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
Tingting Zhang, College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Shandong, China.
Lina Ma, Department of Geratology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
Wei Wei, College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Shandong, China.
Zehui Li, College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Shandong, China.
Xuefan Jiang, Graduate School, Beijing University of Chinese Medicine, Beijing, China.
Jiahui Sun, Graduate School, Beijing University of Chinese Medicine, Beijing, China.
Hui Pei, Department of Geratology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
Hao Li, Department of Geratology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China; Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
References
- 1. Den H, Dong X, Chen M, Zou Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer's disease or mild cognitive impairment—a meta-analysis of randomized controlled trials. Aging. 2020;12:4010–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16:391–460. [Google Scholar]
- 3. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer's Disease International (ADI) . World Alzheimer Report 2019: attitudes to dementia. London, United Kingdom: ADI; 2019. [Google Scholar]
- 5. Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12:733–48. [DOI] [PubMed] [Google Scholar]
- 6. Andrews SJ, Fulton-Howard B, Goate A. Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. Lancet Neurol. 2020;19:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jayedi A, Rashidy-Pour A, Shab-Bidar S. Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response. Nutr Neurosci. 2019;22:750–9. [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Qiao Y, Zhang H, Zhang Y, Hua J, Jin S, Liu G. Circulating vitamin D levels and Alzheimer's disease: a Mendelian randomization study in the IGAP and UK Biobank. J Alzheimers Dis. 2020;73:609–18. [DOI] [PubMed] [Google Scholar]
- 9. Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F, Millet P. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer's disease. Mol Neurobiol. 2018;55:6463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino Det al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones N, Ougham H, Thomas H, Pasakinskiene I. Markers and mapping revisited: finding your gene. New Phytol. 2009;183:935–66. [DOI] [PubMed] [Google Scholar]
- 12. Fang N, Hu C, Sun W, Xu Y, Gu Y, Wu L, Peng Q, Reiter RJ, Liu L. Identification of a novel melatonin-binding nuclear receptor: vitamin D receptor. J Pineal Res. 2020;68:e12618. [DOI] [PubMed] [Google Scholar]
- 13. Mokhtar WA, Fawzy A, Allam RM, Amer RM, Hamed MS. Maternal vitamin D level and vitamin D receptor gene polymorphism as a risk factor for congenital heart diseases in offspring; an Egyptian case-control study. Genes Dis. 2019;6:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukhtar M, Sheikh N, Suqaina SK, Batool A, Fatima N, Mehmood R, Nazir S. Vitamin D receptor gene polymorphism: an important predictor of arthritis development. Biomed Res Int. 2019:8326246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nunes IFOC, Cavalcante AACM, Alencar MVOB, Carvalho MDF, Sarmento JLR, Teixeira NSCCA, Paiva AA, Carvalho LR, Nascimento LFM, Cruz MSPet al. Meta-analysis of the association between the rs228570 vitamin D receptor gene polymorphism and arterial hypertension risk. Adv Nutr. 2020;11:1211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mason DL, Assimon MM, Bishop JR, El-Fawal HAN. Nervous system autoantibodies and vitamin D receptor gene polymorphisms in hemodialysis patients. Hemodial Int. 2013;17:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YH, Kim J-H, Song GG. Vitamin D receptor polymorphisms and susceptibility to Parkinson's disease and Alzheimer's disease: a meta-analysis. Neurol Sci. 2014;35:1947–53. [DOI] [PubMed] [Google Scholar]
- 18. Moher DL; The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Oliveira ACR, Magalhães CA, Loures CMG, Fraga VG, Souza LCD, Guimarães HC, Cintra MTG, Bicalho MA, Sousa MCR, Silveira JNet al. BsmI polymorphism in the vitamin D receptor gene is associated with 25-hydroxy vitamin D levels in individuals with cognitive decline. Arq Neuropsiquiatr. 2018;76:760–6. [DOI] [PubMed] [Google Scholar]
- 22. Mun M-J, Kim M-S, Kim J-H, Jang W-C. A TaqI polymorphism of vitamin D receptor is associated with Alzheimer's disease in Korean population: a case-control study. Int J Clin Exp Med. 2016;9:19268–79. [Google Scholar]
- 23. Łaczmański Ł, Jakubik M, Bednarek-Tupikowska G, Rymaszewska J, Słoka N, Lwow F. Vitamin D receptor gene polymorphisms in Alzheimer's disease patients. Exp Gerontol. 2015;69:142–7. [DOI] [PubMed] [Google Scholar]
- 24. Khorram KH, Gozalpour E, Saliminejad K, Karimloo M, Ohadi M, Kamali K. Vitamin D receptor (VDR) polymorphisms and late-onset Alzheimer's disease: an association study. Iran J Public Health. 2013;42:1253–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Gezen-Ak D, Dursun E, Bilgiç B, Hanagasi H, Ertan T, Gürvit H, Emre M, Eker E, Ulutin T, Uysal Öet al. Vitamin D receptor gene haplotype is associated with late-onset Alzheimer's disease. Tohoku J Exp Med. 2012;228:189–96. [DOI] [PubMed] [Google Scholar]
- 26. Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer's disease. Neurosci Lett. 2011;504:79–82. [DOI] [PubMed] [Google Scholar]
- 27. Gezen-Ak D, Dursun E, Ertan T, Hanağasi H, Gürvit H, Emre M, Eker E, Oztürk M, Engin F, Yilmazer S. Association between vitamin D receptor gene polymorphism and Alzheimer's disease. Tohoku J Exp Med. 2007;212:275–82. [DOI] [PubMed] [Google Scholar]
- 28. Luedecking-Zimmer E, DeKosky ST, Nebes R, Kamboh MI. Association of the 3′ UTR transcription factor LBP-1c/CP2/LSF polymorphism with late-onset Alzheimer's disease. Am J Med Genet. 2003;117B:114–17. [DOI] [PubMed] [Google Scholar]
- 29. Abriz P, Keim K, Ajimu Z, Xiaohui Z. Correlation between vitamin D receptor gene polymorphism and serum 25(OH)D level in elderly patients with mild cognitive impairment in Han nationality in Xinjiang. Chin J Gerontol. 2019;39:5726–30. [Google Scholar]
- 30. Keyimu K, Zhou X-H, Miao H-J, Zou T. Relationship between vitamin D receptor gene polymorphism and mild cognitive impairment in elderly Uygur people. Int J Clin Exp Med. 2014;7:5282–8. [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuda K. PCR-based detection methods for single-nucleotide polymorphism or mutation: real-time PCR and its substantial contribution toward technological refinement. Adv Clin Chem. 2017;80:45–72. [DOI] [PubMed] [Google Scholar]
- 32. Adam D. The gene-based hack that is revolutionizing epidemiology. Nature. 2019;576:196–9. [DOI] [PubMed] [Google Scholar]
- 33. Larsson SC, Traylor M, Markus HS, Michaëlsson K. Serum parathyroid hormone, 25-hydroxyvitamin D, and risk of Alzheimer's disease: a Mendelian randomization study. Nutrients. 2018;10(9):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT. Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol. 2014;144:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Owusu JE, Islam S, Katumuluwa SS, Stolberg AR, Usera GL, Anwarullah AA, Shieh A, Dhaliwal R, Ragolia L, Mikhail MBet al. Cognition and vitamin D in older African-American women– Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc. 2019;67:81–6. [DOI] [PubMed] [Google Scholar]
- 36. Ates BE, Soysal P, Yavuz I, Kocyigit SE, Isik AT. Effect of vitamin D on cognitive functions in older adults: 24-week follow-up study. Am J Alzheimers Dis Other Demen. 2019;34(2):112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Koning EJ, Lips P, Penninx BWJH, Elders PJM, Heijboer AC, den Heijer M, Bet PM, van Marwijk HWJ, van Schoor NM. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. 2019;110:1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shih E-J, Lee W-J, Hsu J-L, Wang S-J, Fuh J-L. Effect of vitamin D on cognitive function and white matter hyperintensity in patients with mild Alzheimer's disease. Geriatr Gerontol Int. 2020;20:52–8. [DOI] [PubMed] [Google Scholar]
- 39. Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer's disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90:1347–52. [DOI] [PubMed] [Google Scholar]
- 40. Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]