Abstract
Surrogate markers of HIV central nervous system (CNS) persistence are needed because direct HIV measurements from the CNS require specialized protocols and are not always detectable or quantifiable. We analyzed paired plasma and CSF samples from people with HIV (PWH) on suppressive therapy (ART) with a validated HIV single copy RNA assay. Two potential markers of CNS persistence were measured (CXCL10 and sCD30). We then examined associations with CSF HIV RNA positivity in univariable and multivariable analyses. Among 66 individuals, 18.2% had detectable CSF HIV. Individuals who had detectable HIV in CSF had higher CSF CXCL10 concentrations (median 514 pg/ml versus median 317 pg/ml, p= 0.019), but did not have significantly different CSF sCD30 concentrations (median 7.5 ng/ml versus median 7.6 ng/ml, p=0.78). In the multiple logistic analysis, both higher CSF CXCL10 (p=0.038) and plasma HIV detectability (p=0.035) were significantly associated with detectable CSF HIV. Both sCD30 and CXCL10 correlated positively with NfL and NSE, two neuronal markers. This study demonstrates that CSF CXCL10 concentrations reflect low level HIV CNS persistence despite virologic suppression on ART. Given that it is readily detectable and quantifiable, this chemokine may be a promising biomarker to evaluate HIV eradication therapies that target the CNS.
Keywords: HIV, Central Nervous System, Cerebrospinal Fluid, Chemokine CXCL10
1. Introduction
Despite the dramatic successes of combination antiretroviral therapy (ART), HIV cure has proved extremely elusive (Hutter et al., 2009). Mounting evidence supports the hypothesis that the central nervous system (CNS) is a reservoir of HIV persistence and thus a barrier to viral eradication. HIV DNA is commonly found in brain tissue from HIV-infected individuals on suppressive ART (Lamers et al., 2016), which suggests that some CNS cells allow for reverse transcription and could be latently infected. However, given the lack of brain tissue availability from living people with HIV (PWH), more research is needed to identify biomarkers of CNS persistence that could be potentially used in studies of HIV cure efforts.
Given its direct communication with brain interstitial fluid (Ratner et al., 2017), the cerebrospinal fluid (CSF) is part of the CNS and a potential window into HIV brain persistence. Several CSF studies have provided important evidence that supports the HIV CNS persistence hypothesis. This includes multiple independent reports of CNS virologic escape, a condition in which CSF HIV RNA concentrations are elevated despite relative virologic suppression in the blood during ART (Canestri et al., 2010, Peluso et al., 2012). Additionally, viral genetic compartmentalization occurs in the CSF and may be associated with adverse clinical consequences (Joseph et al., 2015, Oliveira et al., 2017). Multiple studies have also demonstrated that HIV RNA can be detected at very low levels in CSF during suppressive ART, which provides more evidence of HIV CNS persistence (Anderson et al., 2017, Dahl et al., 2014). This low level presence of HIV in the CSF is also associated with increased markers of inflammation (Dahl, Peterson, 2014), and thus is potentially detrimental to the CNS. HIV DNA can also be found in CSF cells, and this finding was recently shown to be associated with neurocognitive impairment in individuals with HIV (Spudich et al., 2019).
While it is possible to detect HIV RNA and DNA from the CSF during ART, these markers are undetectable even with the use of highly sensitive techniques in 50% or more of PWH (Anderson, Munoz-Moreno, 2017, Spudich, Robertson, 2019). For the purposes of HIV cure studies, other markers that reflect HIV CNS persistence may be needed that are more readily quantifiable and can be measured without the need for advanced molecular techniques. CXCL10 is a chemokine induced by gamma interferon and is produced by macrophages, astrocytes, and other cells (Majumder et al., 1996). In the absence of virologic suppression, CSF CXCL10 concentrations correlate with CSF HIV RNA concentrations but not plasma HIV RNA concentrations, making this protein a potential indicator of CNS HIV replication (Cinque et al., 2005). Another potential marker of CNS HIV is soluble CD30 (sCD30). This is a cell surface marker that is shed from activated (but not resting) immune cells (Fernandez-Ruiz et al., 2017). In CSF, sCD30 is elevated in HIV-infected individuals despite suppressive ART (Peluso et al., 2020), and thus may also be a marker of HIV CNS persistence.
For the current study, we used CSF samples from a cross-sectional subset of individuals with HIV RNA previously measured by single copy assay (Anderson, Munoz-Moreno, 2017). We hypothesized that both CXCL10 and sCD30 would be associated with the presence of low level CSF HIV RNA in the setting of suppressive ART. We also hypothesized that these markers would be associated with markers of neuronal damage.
2. Methods
2.1. Study participants
As in the parent study (Anderson, Munoz-Moreno, 2017), inclusion criteria were: a) adults older than 18 years who had CSF and blood collected within one hour, b) on stable ART with HIV RNA ≤50 copies/ml in CSF and plasma, and c) single copy assay (SCA) results available. Participants with neuropsychiatric comorbid conditions significant enough to confound attribution of neurocognitive impairment (NCI) to HIV as per Frascati criteria (Antinori et al., 2007) were excluded. For the current analysis, participants were also only included if sufficient volume was available for the proposed new biomarkers. All participants provided written consent.
Neuropsychological (NP) performance was assessed using a comprehensive and standardized battery of tests, which has been described in detail elsewhere (Carey et al., 2004, Heaton et al., 2010). Briefly, the battery covers seven neurocognitive domains commonly affected by HIV: verbal fluency, executive functioning, processing speed, learning, delayed recall, attention/working memory, and fine motor skills (Heaton, Clifford, 2010) Raw scores of each NP test were converted to uncorrected normalized scale scores with a mean of 10 and standard deviation of 3. Scaled scores were then calculated to T scores (Mean= 50; Standard Deviation = 10) corrected for the effects of age, education, sex, and race/ethnicity on neurocognition (Heaton et al., Norman et al., 2011). In cases where participants had previous study visits with neuropsychological testing, adjustment for practice effects was made by using median practice effect data from previous work (Cysique et al., 2011). A global T score was computed by average of the individual T scores for each test. T scores were also used to categorize HAND status by Frascati criteria (Antinori, Arendt, 2007) in those individuals who had functional status testing results available (Lawton Brody Questionnaire and Personal Assessment of Own Functioning Inventory). Based on this, participants were classified in four different categories: 1. Not impaired; 2. Asymptomatic neurocognitive impairment (ANI); 3. Mild Neurocognitive Disorder (MND); and 4. HIV-associated dementia (HAD). Using these, participants were categorized as either impaired (Either ANI, MND, or HAD) or not impaired.
2.2. Laboratory investigation
For plasma/CSF HIV RNA concentrations by conventional testing, the Roche Amplicor platform was used (lowest level of quantification 50 copies/ml). Low level HIV-1 RNA was measured in CSF and plasma with the single copy assay (SCA, bioMONTR Labs Research Triangle Park, NC, USA) that has been validated and used in other CSF studies (Santos et al., 2013). This method is based on a proprietary protocol which is used in conjunction with a commercial HIV-1 RNA easyQ reagent kit (bioMerieux Inc, Lyon, France). Briefly, a specimen of up to 2 ml of human plasma or CSF is added to lysis buffer containing guanidine thiocyanate. HIV-1 RNA is extracted in combination with the easyMAG platform (bioMerieux, Inc). Eluates containing HIV-1 RNA are aliquoted into 0.5 mL reaction tubes and amplified using 3 enzymes: T7 RNA polymerase, avian myeloblastosis virus reverse transcriptase, and RNase H. Molecular beacons targeting the pol/gag region of HIV-1 RNA are used for amplification and detection by isothermal reactions at 41°C. HIV-1 RNA level is quantified in conjunction with the NucliSENS easyQ HIV-1 v2.0 Director software and a proprietary algorithm developed by bioMONTR Labs. The dynamic range of this HIV-1 assay is 1–5,000,000 copies/ml, with lowest quantitation being 1–2 copies/ml. Such values are reported as 1.5 copies/ml. sCD30 was measured by enzyme linked immunosorbent assay (ELISA, Thermo Fisher). CXCL10 was measured using single molecule digital ELISA (Quanterix corporation). Two neuronal markers were selected. The first, neurofilament light chain (NfL), is a major structural component of axons and thus a marker of neuronal injury. CSF concentrations of NFL are elevated in the setting of HIV-associated neuronal injury (Abdulle et al., 2007, Jessen Krut et al., 2014). Neuron specific enolase (NSE) is the dominant enolase isoenzyme found in neuronal and neuroendocrine tissue. CSF concentrations of NSE increase in the setting of disease states such as seizures (Correale et al., 1998). These two neuronal markers were also measured with single molecule digital ELISA (Quanterix corporation).
2.3. Statistical analysis
Statistical analyses were performed with SAS JMP version 13 as well as Graphpad Prism 6. Normality of continuous variables was assessed with the Shapiro-Wilk test. Based on lack of normality in most instances, continuous variables were compared using Wilcoxon rank sum test (with the exception of NP T scores, which were compared with independent samples t tests). Also due to lack of normality, Spearman’s rho was calculated for correlations. Specifically, Spearman’s rho was used to evaluate correlation between the four CSF biomarkers: CXCL10, sCD30, NFL, and NSE. Fisher’s exact test was used to evaluate categorical variables. For the outcome of detectable CSF HIV RNA by SCA, univariable logistic regression was first performed with each of the following variables: age, sex, nadir CD4+, current CD4+, months on current ART, CSF total protein, detectable plasma HIV RNA by SCA, CSF WBC, CSF sCD30, and CSF CXCL10. Multiple logistic regression was then performed incorporating variables associated with detectable CSF HIV RNA in the univariable analyses with p value < 0.1. Linear regression was also performed for the global T score variable with the same variables as the analysis of CSF HIV RNA (except that age, sex, and race/ethnicity were not evaluated separately given that they are incorporated in T scores). P value of <0.05 was considered statistically significant for all results.
3. Results
Paired CSF/plasma samples from 66 participants who had sufficient sample volumes for the planned biomarkers were analyzed. The majority of these particular participant visits were between the years of 2005 and 2011. All participants were on three drug ART with the most common regimen being efavirenz/tenofovir/emtricitabine followed by atazanavir/ritonavir/tenofovir/emtricitabine. As seen in Table 1, participants were mostly male with median current CD4+ of 483 cells/microliter and median nadir CD4+ of 98 cells/microliter. 33 participants out of 66 (50%) had positive plasma HIV RNA (plasmaHIV+) by SCA, while 12 out of 66 (18.2%) had detectable CSF HIV RNA (CSFHIV+) by SCA. Median RNA value in the 12 CSFHIV+ participants was 6.5 copies/ml (interquartile range 1.5–14). Median plasma value in the 33 plasmaHIV+ participants was 8 copies/ml (interquartile range 3–18). In the limited group with both detectable CSF HIV and plasma HIV (n=10), the Spearman rho correlation between CSF HIV copies/ml and plasma HIV copies/ml was −0.13 (p=0.73). Please see Table 2 for quantitative results by single copy assay in participants with detectable HIV RNA in either plasma or CSF.
Table 1:
Variables are reported as either median (interquartile range), mean [standard deviation] or number (%).
| Variable | All participants (n=66) | CSFHIV+ (n=12) | CSFHIVneg (n=54) | P value for difference |
|---|---|---|---|---|
| Age (year) | 45 (41–50) | 42 (39–49) | 46 (42–50) | 0.15 |
|
| ||||
| Male Sex | 60 (90.9%) | 10 (83.3%) | 50 (92.6%) | 0.3 |
|
| ||||
| Race | 0.82 | |||
|
| ||||
| White | 35 (53%) | 8 (66.7%) | 27 (50%) | |
| Black | 17 (25.8%) | 3 (25.0%) | 14 (25.9%) | |
| Other | 14 (21.2%) | 1 (8.3%) | 13 (24.1%) | |
|
| ||||
| Estimated years with HIV | 14 (8–18) | 13 (9–16) | 15 (7–18) | 0.7 |
|
| ||||
| Current CD4+ a | 483 (356–718) | 476 (359–752) | 500 (351–717) | 0.98 |
|
| ||||
| Nadir CD4+ a | 98 (21–231) | 88 (34–259) | 104 (14–226) | 0.61 |
|
| ||||
| Months on current ART regimen | 19 (9–36) | 15 (5–23) | 19 (10–37) | 0.19 |
|
| ||||
|
Proportion with plasma
HIV+ RNA by SCA |
33 (50%) | 10 (83%) | 23 (43%) | 0.023* |
|
| ||||
| NP Performance | ||||
| Global T score | 46.3 [6.5] | 47.7 [5.9] | 46.0 [6.6] | 0.43 |
| Impaired by | 30 (47.7%) | 4 (36%) | 26 (50%) | 0.51 |
| HAND status | ||||
|
| ||||
| Plasma HIV SCA Copies/ml (n=33 that were detected) | 8 (3–18) | 31 (6–65) | 7 (1.5–12) | 0.06 |
|
| ||||
| CSF HIV SCA Copies/ml (n=12 that were detected) | N/A | 6.5 (1–14) | N/A | N/A |
|
| ||||
| CSF results | ||||
| WBCa | 2 (1–4) | 4 (2–7) | 2 (1–3) | 0.035* |
| Total proteinb | 40 (33–51) | 39 (34–55) | 41 (32–51) | 0.99 0.019* |
| CXCL10c | 341 (218–541) | 514 (353–800) | 317 (213–480) | 0.78 |
| sCD30d | 7.6 (4.2–11.6) | 7.5 (4.2–10.0) | 7.6 (4.1–12.4) | 0.96 |
| NFLc | 572 (407–706) | 586 (433–669) | 572 (402–732) | 0.8 |
| NSEc | 11039 (8805–14178) | 12094 (9780–13623) | 10918 (8716–14701) | |
Denotes p value <0.05. NP= Neuropsychological; SCA= single copy assay; ml=milliliter; N/A= not applicable;
= measurement in cells/microliter;
= measurement in milligrams/deciliter;
= measurements in picograms/milliliter;
= measurement in nanograms/milliliter.
Table 2:
results from individuals with either plasma or CSF detectable (n=35).
| Plasma copy/ml | CSF copy/ml |
|---|---|
| 45 | 10 |
| 17 | 20 |
| 0 | 3 |
| 170 | 11 |
| 44 | 1.5 |
| 120 | 15 |
| 0 | 3 |
| 7 | 1.5 |
| 3 | 11 |
| 46 | 1.5 |
| 7 | 1.5 |
| 1.5 | 26 |
| 12 | 0 |
| 3 | 0 |
| 17 | 0 |
| 8 | 0 |
| 3 | 0 |
| 18 | 0 |
| 1.5 | 0 |
| 39 | 0 |
| 1.5 | 0 |
| 5 | 0 |
| 1.5 | 0 |
| 1.5 | 0 |
| 7 | 0 |
| 6 | 0 |
| 1.5 | 0 |
| 12 | 0 |
| 6 | 0 |
| 240 | 0 |
| 12 | 0 |
| 9 | 0 |
| 11 | 0 |
| 1.5 | 0 |
| 9 | 0 |
Ml=milliliter. 0 denotes not detectable in that compartment. 1–2 copies/ml is detectable at the lowest limit of detection and is denoted by the 1.5 value.
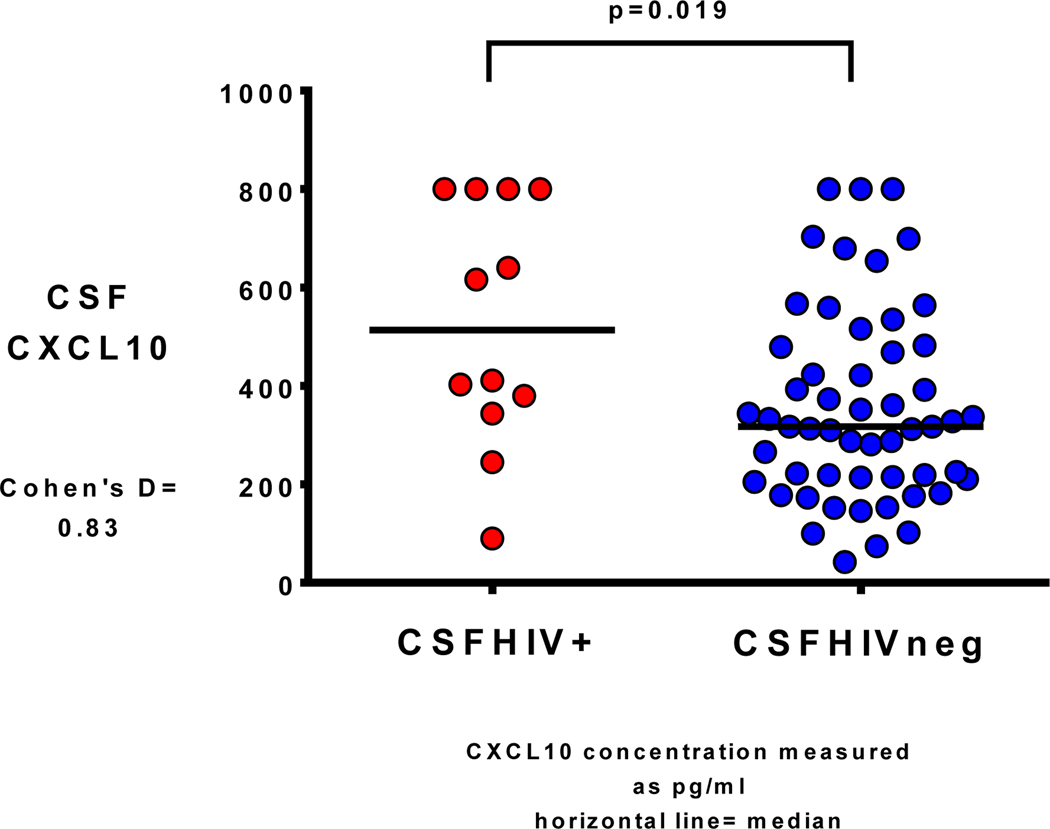
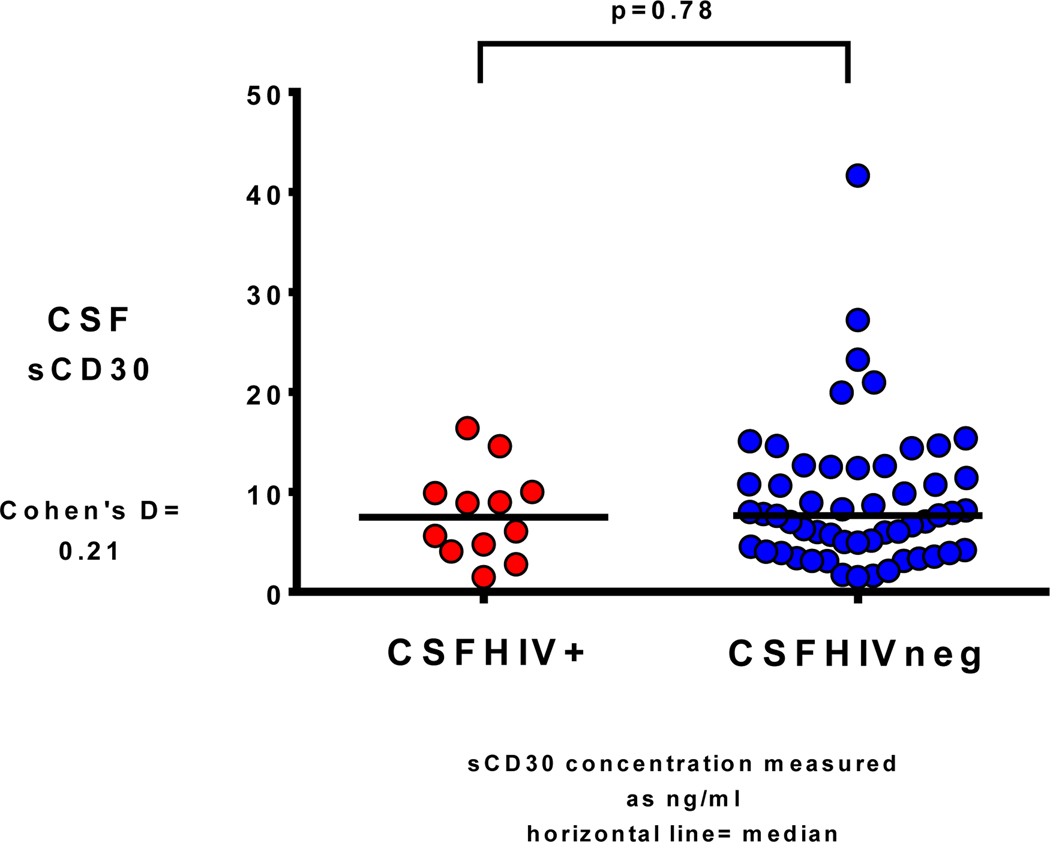
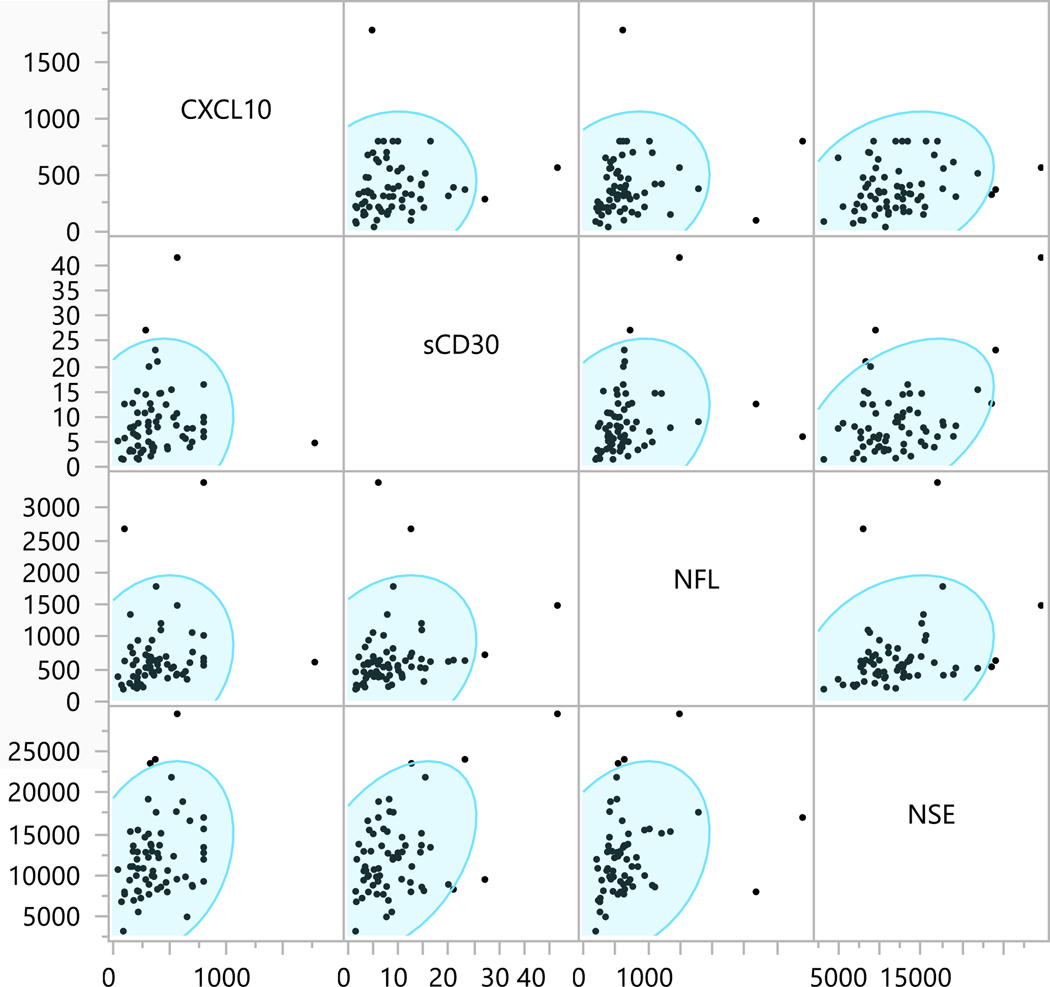
There were no significant differences between CSFHIV+ and CSFHIVnegative in terms of age, race, sex, current CD4+, CD4+ nadir, estimated duration of HIV infection or months on current ART regimen. However, CSFHIV+ participants were more likely to be plasmaHIV+ than CSFHIVnegative participants (83% versus 43%, p= 0.023). Additionally, CSFHIV+ participants had higher CSF CXCL10 concentrations (median 514 picograms/milliliter [pg/ml] versus median 317 pg/ml, p= 0.019 (Fig. 1 with Cohen’s d result, Pg= picograms; ml= milliliters), and higher CSF WBC concentrations (median 4 cells/microliter versus median 2 cells/microliter, p= 0.035). There were no concentration differences for NFL, NSE, or sCD30 (Fig. 2 with Cohen’s d result for sCD30, Pg= picograms; ml= milliliters) between CSFHIVnegative and CSFHIV+ groups. Between the four CSF biomarkers, there were positive correlations between NFL and NSE, the two neuronal markers (rho=0.31, p= 0.011) as well as between sCD30 and CXCL10, the two inflammatory markers (rho=0.23, p=0.067). There were also positive correlations between inflammatory and neuronal markers CXCL10 to NFL (rho=0.29, p=0.02), CXCL10 to NSE (rho=0.26, p=0.033), sCD30 to NFL (rho=0.34, p=0.005), and sCD30 to NSE (rho=0.25, p=0.048). See Figure 3 for correlation matrices.
Fig. 1.
CSF CXCL10 concentration in participants with detectable CSF HIV RNA (CSFHIV+) versus with undetectable CSF HIV RNA (CSFHIVneg)
Fig. 2.
CSF sCD30 concentration in participants with detectable CSF HIV RNA (CSFHIV+) versus with undetectable CSF HIV RNA (CSFHIVneg)
Figure 3.
Correlation matrices with density ellipses for the four CSF biomarkers: CXCL10, sCD30, NFL, and NSE. Units are in picograms/milliliter except for sCD30 which is nanograms/milliliter. Spearman’s rho and p values are: NFL to NSE: rho=0.31, p= 0.011; sCD30 to CXCL10: rho=0.23, p=0.067; CXCL10 to NFL: rho=0.29, p=0.02; CXCL10 to NSE: rho=0.26, p=0.033; sCD30 to NFL: rho=0.34, p=0.005; and sCD30 to NSE rho=0.25, p=0.048.
For NP performance, 65 participants (98.5%) had global T score results available (see Table 1). There was no difference in global T score between the two detectable CSF HIV groups (mean= 47.7, standard deviation= 5.9 for CSFHIV+ group and mean= 46.0, standard deviation= 6.6 for CSFHIVnegative group, p= 0.43). 63 participants had full HAND status available. Table 1 also shows no difference between the two groups in terms of impairment by HAND status (p=0.51). Of the 30 participants who were impaired by HAND status, the majority (77%) had ANI. For the univariable linear regression analysis with global T score in the cohort as a whole (n=65) as the outcome, none of the following variables were associated significantly: nadir CD4+, current CD4+, months on current ART regimen, plasma HIV RNA detectable by SCA, CSF HIV detectable by SCA, sCD30, CXCL10, NFL, and NSE (all p>0.1).
In the univariable logistic regression analyses with CSFHIVRNA+ as the outcome (top part of table 3), two variables were significantly associated with CSFHIVRNA+. Specifically, the presence of plasma HIV RNA and higher CSF CXCL10 both had statistically significant positive odds ratios in relationship to CSFHIVRNA+. There was no significant difference in CSF CXCL10 concentrations based on plasma HIV RNA detectability (median 373 pg/ml for plasma HIV+ versus median 313 pg/ml for plasma HIV negative, p=0.135). In the multivariable model using these two variables, both remained significantly associated with CSFHIVRNA+ (see bottom part of table 3). The P value for this model was statistically significant at 0.003. The odds ratio of detectable CSF HIV by SCA associated with detectable plasma HIV RNA by SCA was 5.91 (95% Confidence interval [CI]= 1.13–30.98). The odds ratio of detectable CSF HIV by SCA per unit increase of CXCL10 was 1.003, which translates to an odds ratio of 1.4 (95% CI= 1.02–1.93) per CXCL10 unit increase of 100. In the CSFHIVRNA+ group, the Spearman’s rho correlation between CSF HIV RNA and CXCL10 was 0.28 (p=0.38).
Table 3:
Results of logistic regression models for the outcome of detectable CSF HIV RNA by SCA.
| Variable | Odds ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Univariable Models | |||
| Age per year increase | 0.984 | 0.909–1.064 | 0.683 |
| Male Sex | 0.4 | 0.064–2.489 | 0.326 |
| Current CD4+ | 1.0 | 0.997– 1.002 | 0.779 |
| Nadir CD4+ | 1.0 | 0.995–1.007 | 0.766 |
| Months on current ART | 0.97 | 0.931–1.010 | 0.135 |
| Plasma HIV RNA detectable by SCA | 6.74 | 1.346–33.753 | 0.020* |
| CSF WBC | 1.064 | 0.954–1.186 | 0.266 |
| CSF total protein | 0.996 | 0.957–1.035 | 0.822 |
| CSF sCD30 | 0.964 | 0.861–1.078 | 0.517 |
| CSF CXCL10 | 1.004 | 1.001–1.007 | 0.017* |
| Multivariable Model | |||
| Plasma HIV RNA detectable by SCA | 5.91 | 1.129–30.977 | 0.035* |
| CSF CXCL10 | 1.003 | 1.0002–1.007 | 0.038* |
Denotes p value <0.05. Top part shows the results from univariable modeling, while bottom part shows the results for the multivariable model which only includes variables with p<0.1 in univariable analyses.
4. Discussion:
Despite suppressive ART, HIV persists and is associated with adverse clinical outcomes (Coghill et al., 2017). There is great interest in developing a cure for HIV, which if achieved would obviate the need for lifetime ART with its associated costs and toxicities. There is significant evidence that the CNS is an HIV reservoir and a barrier to HIV cure. Such evidence includes the finding of HIV DNA in brain tissue despite ART (Lamers, Rose, 2016) as well as the presence of HIV DNA in CSF cells and low level CSF HIV RNA during virologic suppression (Anderson, Munoz-Moreno, 2017, Spudich, Robertson, 2019). However, HIV DNA and RNA from CSF are often not detectable even with very sensitive methods such as single copy assay (Dahl, Peterson, 2014). Therefore, more research is needed to identify more markers of HIV CNS persistence that reflect the size of the HIV CNS reservoir. Ideal CNS HIV reservoir markers would be consistently detectable and quantifiable. Quantification of markers before and after an HIV cure intervention could potentially indicate how effective the intervention is in decreasing the HIV CNS reservoir.
In the current study, we examined two biomarkers that have shown promise as indicators of HIV in the CNS. CXCL10 is a chemokine induced by gamma interferon and is produced by macrophages, astrocytes, and other cells (Majumder, Zhou, 1996). This chemokine is one of the first inflammatory markers to become elevated in the acute HIV infection period and thus its production is very sensitive to the presence of HIV (Stacey et al., 2009). In the absence of virologic suppression, CSF CXCL10 concentrations correlate with CSF HIV RNA concentrations but not plasma HIV RNA concentrations, and therefore may be particularly valuable as an HIV CNS reservoir marker (Cinque, Bestetti, 2005) Another potential marker of CNS HIV is soluble CD30 (sCD30). The CD30 molecule is part of the tumor necrosis factor family and in healthy individuals is only rarely expressed on lymphocytes (Falini et al., 1995). In the case of PWH however, CD4+CD30+ cells are common and HIV is particularly enriched in these cells (Hogan et al., 2018). In CSF, the soluble form of the receptor (sCD30) is elevated in HIV-infected individuals despite suppressive ART (Peluso, Thanh, 2020). Therefore, sCD30 is another candidate marker of HIV CNS persistence.
In the current study, we evaluated these two markers in relationship to the presence of low level CSF HIV by SCA. We found that while CSF sCD30 was not associated with CSF HIV detectability, higher CSF CXCL10 was associated with CSF HIV detectability in both univariable and multivariable analyses. The fact that there was no relationship between the presence of HIV RNA in plasma and CSF CXCL10 supports the hypothesis that our findings were most likely driven by CNS HIV as opposed to peripheral HIV. Further investigation is indicated into CSF CXCL10 as a CNS HIV marker. For example, it could be measured from PWH whose CSF was tested for the presence of HIV DNA, or from pre-mortem CSF samples from PWH who then had post mortem brain tissue measurement of HIV DNA. The advantage to CSF CXCL10 compared to HIV nucleic acid tests is that it is readily detectable and quantifiable. Such a marker is potentially very valuable because concentrations could be measured before and after HIV cure interventions to better understand the effect of such interventions on the CNS HIV reservoir.
We also found positive correlations (all p=0.048 or less) between sCD30 and NFL, between NFL and NSE, and between NFL and CXCL10. The correlation between NFL and NSE is not unexpected in that both are markers of neuronal damage. A study involving individuals with traumatic brain injury and normal pressure hydrocephalus showed a positive correlation between NFL and NSE (rho=0.597, p<0.05) (Minta et al., 2020). Our study adds to this literature. Our study also adds to the literature that higher CSF sCD30 concentrations correlate with higher NFL concentrations (Peluso, Thanh, 2020). Given the extensive literature showing a relationship between CNS inflammation and neurotoxicity (Fritz-French and Tyor, 2012, Ransohoff, 2016), the correlations we found in this study between inflammatory markers and neuronal damage are not unexpected.
We acknowledge the limitations of the current study. Men made up the majority of the study population, so the inclusion of more women would have made the study more representative of PWH. The study was relatively small (n=66) and less than 20% of participants had detectable CSF HIV RNA by SCA. Therefore, it is possible that the absence of significant association between CSF sCD30 and the presence of CSF HIV was influenced by lack of statistical power. A larger study may have shown a significant association. While another study evaluating sCD30 from CSF in PWH showed that concentrations of this marker were higher in PWH on suppressive therapy compared to HIV-negative controls, this study did not involve a single copy assay (Peluso, Thanh, 2020). Somewhat counter-intuitively, CSF sCD30 concentrations were similar between PWH off ART and HIV-negative controls in that study.
The direction of causality for the relationship between low level CSF HIV and CXCL10 cannot be established with this study. It is possible that the presence of low level CNS virus causes increased production of CXCL10. It is also possible that CXCL10, with its chemotactic properties, could increase CD4+ T-cell trafficking in the CNS, which could then be a source of HIV via egress from the intracellular compartment. It is also possible that a third, unmeasured factor is driving both. One possible approach to understanding directionality would be administration of therapies that block CXCL10, followed by examining for low level HIV in the CSF. We did not find an association between the biomarkers that were measured and NP performance. However, given the relatively small size of the study and the fact that the majority of participants were not impaired, the study was likely underpowered to rigorously examine relationships with cognition.
Other biomarkers could also reflect low level HIV in the CNS and may merit further study as well. For example, another study of the presence of low level CSF HIV by SCA showed that neopterin, a marker of monocyte activation, was higher in individuals with detectable CSF HIV (Dahl, Peterson, 2014). Additionally, other inflammatory markers correlate with CSF HIV RNA levels in PWH who do not have full virologic suppression (Christo et al., 2009) and therefore could also reflect the HIV CNS reservoir. It would be interesting to include even more biomarkers in future studies to evaluate if a composite biomarker panel could be more reflective of HIV CNS persistence than individual biomarkers by themselves. This study demonstrates that CSF CXCL10 concentrations reflect low level HIV CNS persistence despite virologic suppression with ART. Given that it is readily detectable and quantifiable, CXCL10 may be a promising biomarker to evaluate HIV eradication therapies that target the CNS.
Highlights.
CSF HIV was measured by single copy assay as were two CSF markers of inflammation.
CXCL10 was significantly higher among those with detectable CSF HIV RNA.
By logistic regression, CXCL10 and plasma HIV RNA were associated with CSF HIV.
CXCL10 and sCD30 correlated with higher concentrations of two neuronal markers.
Acknowledgments
Study Sponsorship
National Institutes of Health R21 MH085610 (Principal Investigator: S. Letendre), R01 MH058076 (Principal Investigator: R. Ellis), R01 MH107345 (Co-Principal Investigators: R. Heaton, S. Letendre), K23 MH095679, R21 MH118092, R01 AG062387 (Principal Investigator: A. Anderson), and K24 MH097673 (Principal Investigator: S. Letendre). H. Zetterberg is a Wallenberg Scholar.
Declaration of interest/source of funding
K Blennow has served as a consultant or at advisory boards for Axon, Biogen, CogRx, Lilly, MagQu, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper.
Footnotes
A Anderson reports no conflicts
S Kundu reports no conflicts
B Tang reports no conflicts
F Vaida reports no conflicts
O Okwuegbuna reports no conflicts
D McClernon reports no conflicts
M Cherner reports no conflicts
R Deutsch reports no conflicts
D Cookson reports no conflicts
M Crescini reports no conflicts
I Grant reports no conflicts
H Zetterberg reports no conflicts
K Blennow reports no conflicts
M Gisslen reports no conflicts R Ellis reports no conflicts
S Letendre reports no conflicts
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulle S, Mellgren A, Brew BJ, Cinque P, Hagberg L, Price RW, et al. CSF neurofilament protein (NFL) -- a marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–32. [DOI] [PubMed] [Google Scholar]
- Anderson AM, Munoz-Moreno JA, McClernon DR, Ellis RJ, Cookson D, Clifford DB, et al. Prevalence and Correlates of Persistent HIV-1 RNA in Cerebrospinal Fluid During Antiretroviral Therapy. The Journal of infectious diseases. 2017;215:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:773–8. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18:234–48. [DOI] [PubMed] [Google Scholar]
- Christo PP, Vilela Mde C, Bretas TL, Domingues RB, Greco DB, Livramento JA, et al. Cerebrospinal fluid levels of chemokines in HIV infected patients with and without opportunistic infection of the central nervous system. Journal of the neurological sciences. 2009;287:79–83. [DOI] [PubMed] [Google Scholar]
- Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. Journal of neuroimmunology. 2005;168:154–63. [DOI] [PubMed] [Google Scholar]
- Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess Mortality among HIV-Infected Individuals with Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Rabinowicz AL, Heck CN, Smith TD, Loskota WJ, DeGiorgio CM. Status epilepticus increases CSF levels of neuron-specific enolase and alters the blood-brain barrier. Neurology. 1998;50:1388–91. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D Jr., Abramson I, Ellis RJ, Letendre S, Collier A, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. Journal of clinical and experimental neuropsychology. 2011;33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. Aids. 2014;28:2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- Fernandez-Ruiz M, Parra P, Lopez-Medrano F, Ruiz-Merlo T, Gonzalez E, Polanco N, et al. Serum sCD30: A promising biomarker for predicting the risk of bacterial infection after kidney transplantation. Transpl Infect Dis. 2017;19. [DOI] [PubMed] [Google Scholar]
- Fritz-French C, Tyor W. Interferon-alpha (IFNalpha) neurotoxicity. Cytokine & growth factor reviews. 2012;23:7–14. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsycho- logical norms for African American and Caucasian adults scoring program Lutz, FL.: Psychological Assessment Resources; 2004. [Google Scholar]
- Hogan LE, Vasquez J, Hobbs KS, Hanhauser E, Aguilar-Rodriguez B, Hussien R, et al. Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS pathogens. 2018;14:e1006856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine. 2009;360:692–8. [DOI] [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PloS one. 2014;9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Kincer L, Bowman N, Menezes P, Robertson K, Anderson A, et al. HIV-1 Replication in the CNS Is Associated With Increased Neurocognitive Impairment. Poster 440 Conference on Retroviruses and Opportunistic Infections February 2015, Seattle Washington2015. [Google Scholar]
- Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol. 2016;90:8968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Zhou LZ, Ransohoff RM. Transcriptional regulation of chemokine gene expression in astrocytes. J Neurosci Res. 1996;45:758–69. [DOI] [PubMed] [Google Scholar]
- Minta K, Brinkmalm G, Al Nimer F, Thelin EP, Piehl F, Tullberg M, et al. Dynamics of cerebrospinal fluid levels of matrix metalloproteinases in human traumatic brain injury. Sci Rep. 2020;10:18075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr., Cysique L, Ake C, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of clinical and experimental neuropsychology. 2011;33:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MF, Chaillon A, Nakazawa M, Vargas M, Letendre SL, Strain MC, et al. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS pathogens. 2017;13:e1006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. Aids. 2012;26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Thanh C, Prator CA, Hogan LE, Arechiga VM, Stephenson S, et al. Cerebrospinal fluid soluble CD30 elevation despite suppressive antiretroviral therapy in individuals living with HIV-1. J Virus Erad. 2020;6:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–83. [DOI] [PubMed] [Google Scholar]
- Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, et al. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. NeuroImage. 2017;152:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JR, Munoz-Moreno JA, Molto J, Prats A, Curran A, Domingo P, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PloS one. 2013;8:e70201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest. 2019;129:3339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]