Abstract
It is well recognized that decreased vascular endothelial growth factor A (VEGF-A) mRNA plays an important role in retinal vessel regression induced by hyperoxia. However, this concept has been challenged by increasing new evidence. Furthermore, VEGF-A strongly enhances Dll4 expression and inhibition of Dll4-Notch signaling leads to excessive sprouting angiogenesis. Recently, it is shown that inactivation of Dll4-Notch1 signaling reduce hyperoxia induced vessel regression. It is unknown whether sprouting angiogenesis contributes to the protective effect or not and further investigations are needed. Moreover, the expression of Dll4 or Notch1 activation in the regressing plexus remains elucidated.To determine the role of VEGF-A and Dll4-Notch1 signaling in hyperoxia induced vascular regression in the retina, we used mice at postnatal day 5 (P5) - P7. Hyperoxia induced massive vascular regression in the central plexus but not in the angiogenic plexus and had no effect on sprouting angiogenesis. Immunostaining showed that VEGF-A was significantly repressed in the angiogenic front region after hyperoxia exposure but not detectable in the central area of both normoxia and hyperoxia treated retinas. In contrast, Notch ligand Delta-like 4 (Dll4) and Notch1 intracellular domain (N1-ICD) expression were inhibited in the regressing capillaries of central retina but comparable in the angiogenic plexus after high oxygen treatment. Moreover, administration of Dll4 neutralizing antibody or γ-Secretase inhibitor DAPT significantly aggravated vessel regression induced by short-time hyperoxia administration. Our data show that repressed Dll4-Notch1 signaling pathway but not downregulation of VEGF-A expression are responsible for hyperoxia induced pervasive vessel regression.
Keywords: Vascular endothelial growth factor A, Vessel regression, Hyperoxia, Delta-like 4, Notch1
INTRODUCTION
Blood vessels are responsible for transportation of nutrients and oxygen throughout the whole body. Changes of oxygen level or metabolites lead to vascular network trimming (also called vessel pruning) or regression, an essential step for establishing a functional and hierarchical mature vascular network to meet the needs of various organs[1]. Physiological vessel regression occurs during development (such as hyaloid vessel regression) and in the adult (for example, luteolysis caused by vessel regression and rapid disassociation of the mature ovarian corpus luteum)[2]. Extensive vessel regression is an initial event of retinopathy of prematurity (ROP), a disease extremely premature babies suffer from[3–5]. This pathological vessel regression is characterized by central capillaries pruning in the developing retinal vasculature[4,6]. The mechanisms of vessel regression is comprehensively investigated[6–8]. Vascular endothelial growth factor A(VEGF-A), also known as VEGF, is recognized as the most important factor for retinal vessel regression induced by hyperoxia, especially in the immature vasculature[2,6,7]. For instance, Claxton et al show that VEGF mRNA in the central retina is repressed by hyperoxia, and vaso-obliteration is rescued by intraocular injection of recombinant VEGF[6]. Notwithstanding, the Ivan group demonstrate that the role of VEGF on the vessel regression induced by hyperoxia is rather modest[8]. Stringent VEGF-A inhibition by intravitreal (ITV) microinjecting VEGF Trap in the normal retina could not mimic hyperoxia pervasive regression[8]. Lobov et al argued that VEGF-A is a proximate cause of the massive vessel pruning induced by hyperoxia [8]. Therefore, whether VEGF-A is responsible for hyperoxia induced vessel regression or not needs further investigation.
In addition, as a major downstream target, Dll4 is induced by VEGF[9,10]. Dll4-Notch1 pathway is crucial for sprouting angiogenesis and inhibition of this pathway causes excessive sprouting angiogenesis in retina vasculature [11]. Recently, it was demonstrated that inhibition of Dll4-Notch1 signaling prevents hyperoxia induced vascular regression2,[8]. However, the effects of increased sprouting angiogenesis by Dll4-Notch1 inhibition in vessel regression is ignored. More meticulous studies should be carried out.
In our current study, we surprisingly found the mismatched VEGF-A protein location at regression vessels after hyperoxia treatment, implying that VEGF-A is not an indispensable factor in hyperoxia induced vessel regression. Besides, we demonstrated that repressed Dll4-Notch1 signaling in short-time significantly increased the vessel regression. Moreover, we showed that high oxygen did not affect sprouting angiogenesis in the angiogenic front.
MATERIALS AND METHODS
Detailed materials and methods are available on the supplementary data.
RESULTS
Hyperoxia stimulated massive vessel regression in the central plexus but not in the spouting front
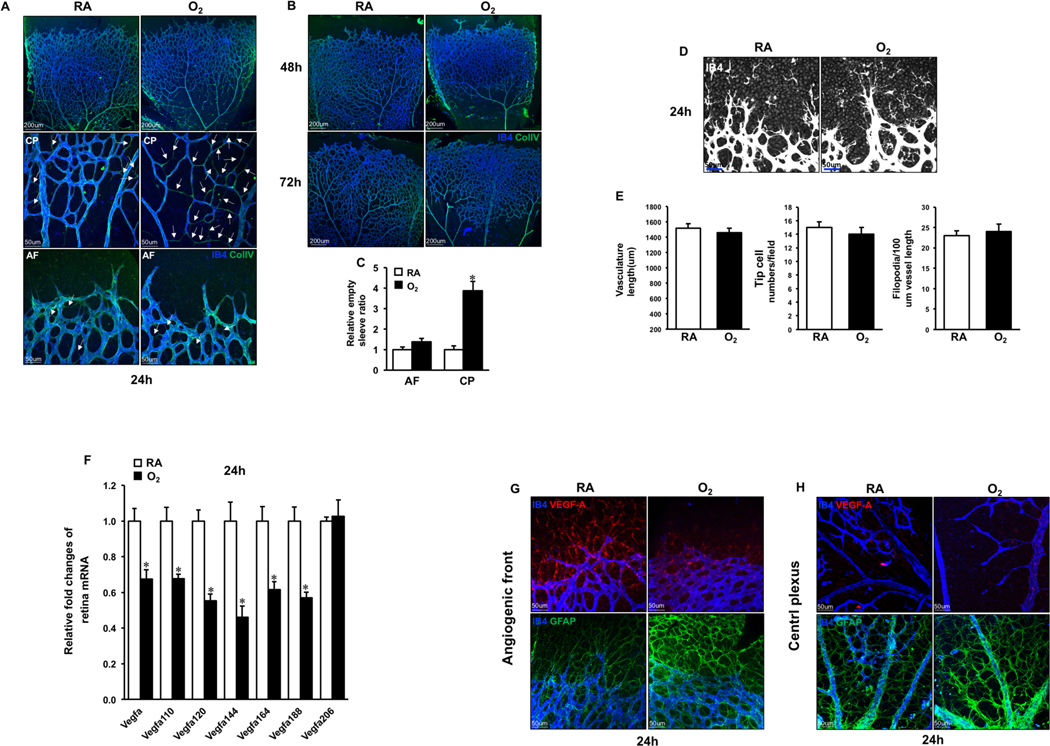
To visualize the vessel regression, neonates were exposed to RA or hyperoxia between P4 to P7 on which we performed retinal whole mount staining of IB4 (marker of endothelial cell (EC)) and collagen IV (matrix secreted by EC). Retinas from RA neonates showed colocalization of IB4 and collagen IV. In contrast, hyperoxia induced remarkable retinal vessel regression in the central plexus indicated by the following observations. a. After 24hrs hyperoxia exposure, IB4 and collagen IV co-staining demonstrated about 4 folds increased empty sleeves (IB4 negative but collagen IV positive) in the central plexus but not in the distal plexus at P5 (Fig. 1A, C). b. The prolonged exposure to hyperoxia for 48 or 72hrs expanded capillary-free spaces in proximal region at P6 and P7, and there were no capillaries left except arteries and veins. Even the matrix deposits’ collagen IV disappeared (Fig. 1B). However, ECs in the distal plexus were preserved at P5, even at P6–7 after long time high oxygen exposure (Fig. 1A–B). The sprouts and filopodia extension showed no significant changes compared with RA at P5 retinas (Fig.1D–E), as well as at P6 and P7 retinas (data not shown). Therefore, hyperoxia stimulated pervasive vessel regression in the central retina but not in the front region and did not affect sprouting angiogenesis in the leading edge.
Fig. 1. VEGF-A protein expression patterns in retina of RA or hyperoxia treated neonate for 24hrs.
A. P4 murine neonates were exposed to RA (room air) or O2 (hyperoxia) for 24hrs, and the retinas were harvested at P5, followed by staining of IB4 (blue) and Collagen IV (green). Retinal vasculature of high magnification was represented at central and bottom panels. White arrows indicate Collagen IV-positive and IB4-negative staining. B. Confocal images of IB4 (blue) and Collagen IV (green) stained retinal vasculature of neonates treated by RA or O2 for 48 and 72 hrs. C. Numbers of empty sleeves were quantified. *p< 0.05 compared with RA (mean ± SEM; n = 6). D. Whole-mount staining of IB4 (white) showing the angiogenic plexus after RA (room air) or O2 (hyperoxia) treatment for 24hrs. E. Quantification of vascular length, tip cells and filopodia in RA or O2 treated retinas for 24hrs. Data are presented as mean ± SEM (n=4). F. After RA (room air) or O2 (hyperoxia) exposure for 24hrs, the retina samples were collected and mRNA was extracted at P5, followed by RT-qPCR analysis of Vegfa and its splice variants. *p < 0.05 compared with RA (mean ± SEM; n = 4). G-H. Whole-mount double immunostaining for VEGF-A (red) and IB4 (blue) or GFAP (green) and IB4 (blue) at P5 retinas.
VEGF-A was not responsible for hyperoxia induced vessel regression
VEGF-A is mainly secreted by the astrocytes during retinal angiogenesis and haploinsufficiency of VEGF-A leads to embryonic lethal[14,15]. Claxton et al demonstrated by in situ hybridization that Vegfa mRNA expresses in the entire murine neonatal retina and is robustly decreased in central plexus after hyperoxia treatment[6]. Because we showed in Fig.1A that capillaries disappear after 2-day hyperoxia treatment, we performed the following experiments using retinas from P5 neonates treated with RA or hyperoxia for 1 day. Consistently, transcripts of Vegfa and its splice variants except Vegfa 206 in retinas were significantly reduced by hyperoxia (Fig. 1F). However, the VEGF-A protein expression, especially in the whole-mounted developing retina, was not reported before. Recently Ralf Adams’ group demonstrated an optimal antibody which recognizes the VEGF-A protein in the flat vascular superficial layer[13]. We performed the VEGF-A immunostaining together with IB4. Unexpectedly, in RA treated mice, we observed that VEGF-A was expressed in the distal avascular area and the angiogenic plexus but not in the central retina (Fig. 1G–H). In hyperoxia treated mice, no VEGF-A was detected in the central region (Fig. 1H). In contrast, there was a remarkable decrease of VEGF-A expression in distal region (Fig. 1G). In addition, it is known that hyperoxia increases Glial fibrillary acidic protein (GFAP) expression in astrocyte[15,16]. Accordingly, we found an enhanced GFAP expression throughout the whole retina in hyperoxia group compared to RA control (Fig. 1G–H).
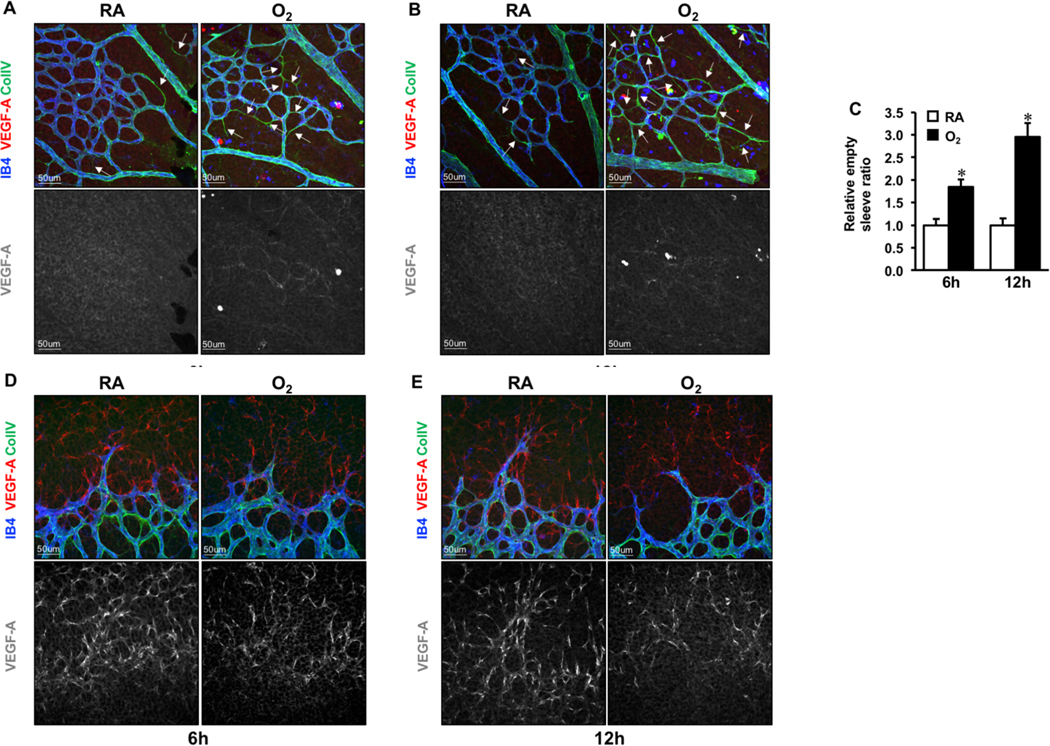
Because massive regression was induced and VEGF-A mRNA markedly decreased after 24hrs hyperoxia treatment, we are wondering whether vessel regression and VEGF-A protein level changes at early time points. Thus, neonates at P4 were exposed to hyperoxia for 6 or 12hrs (Fig. 2A–2B). Similar to what we observed after 24hrs, empty sleeves in the central retina increased by 1.8 and 2.9 folds at 6 or 12hrs respectively compared to RA groups (Fig. 2C), while no differences in the angiogenic front (Fig. 2D–E). VEGF-A expression patterns were the same as at 24hrs showed in Fig.1. Together, our findings don’t support the critical role of VEGF-A in hyperoxia induced central vessel regression in retina.
Fig. 2. VEGF-A protein expression patterns in retina of RA or hyperoxia treated neonate for 6 and 12hrs.
A-B. Triple immunostaining of VEGF-A (red), IB4 (blue) and Collagen IV (green) in the central region of P6 retinas exposed to RA or O2 for 6 and 12h. VEGF-A immune-signal was hardly measured in the proximal area of retinas. White arrows indicate empty sleeves (Collagen IV+ IB4-). C. Numbers of empty sleeves in the central plexus were quantified. *p < 0.05 compared with RA (mean ± SEM; n = 6). D-E. Confocal images of VEGF-A (red), IB4 (blue) and Collagen IV (green) staining.
Hyperoxia repressed Dll4-Notch1 signaling in the central plexus but not in the angiogenic front
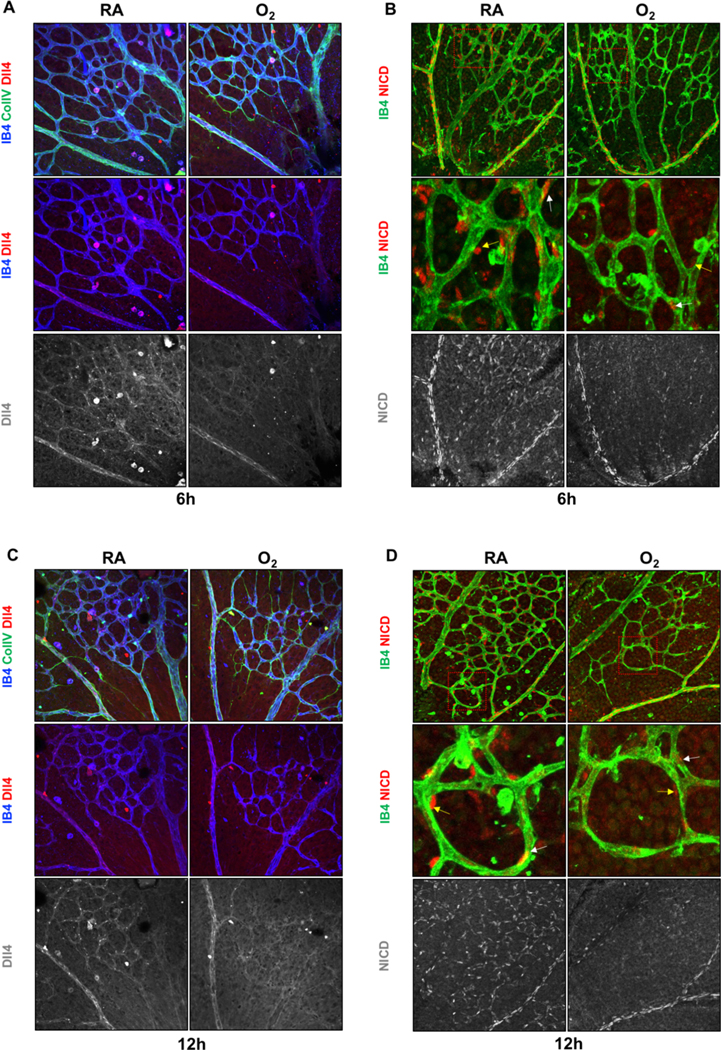
It has been reported that Dll4-Notch1 signaling is involved in post angiogenic vascular remodeling and oxygen induced vessel regression[8,17]. However, Dll4 expression or Notch1 activation (detected by specific antibody for notch1 intracellular domain, N1-ICD ) after hyperoxia exposure have not been investigated yet. Because of excessive vessel regression caused by long-time hyperoxia, we performed whole mount staining in retinas of same littermates at P5 or P6 after RA or hyperoxia treatment for 6 and 12 hours. Interestingly, Dll4 protein expression was substantially reduced in the central capillaries in the hyperoxia group compared with RA groups, but not in the arteries (Fig. 3A, C). The N1-ICD antibody (Val 1744) with high sensitivity and specificity is a useful tool to detect the Notch1 activity[18]. The N1-ICD and IB4 co-staining showed that the N1-ICD localized in ECs and perivascular cells and was significantly decreased in the central plexus region but not in the arteries (Fig. 3B–D). It is well established that Dll4 is enriched in the tip cells and sparse in the stalk cells[10,19,20]. Consistently, we observed similar phenomena in retinas of RA neonates (Fig. S1 A–B). The Dll4 and N1-ICD expression in sprouting fronts were comparable (Fig. S1 A–D). These results imply an important role of Dll4-Notch1 pathway in hyperoxia induced vessel regression.
Fig. 3. Dll4-Notch1 signaling was inhibited in the regressing central plexus induced by short-time hyperoxia treatment.
A, C. Representative images of Dll4 (red), IB4 (blue) and Collagen IV (green) staining of P6 retinas after 6 or 12hrs hyperoxia (O2) exposure compared with room air (RA). B, D. Whole-mounted P6 retinas stained by IB4 (green) and NICD (red). Higher magnification of boxed areas are showed in middle panels.
Short-time inhibition of Dll4–Notch1 signaling aggravated hyperoxia induced vascular regression
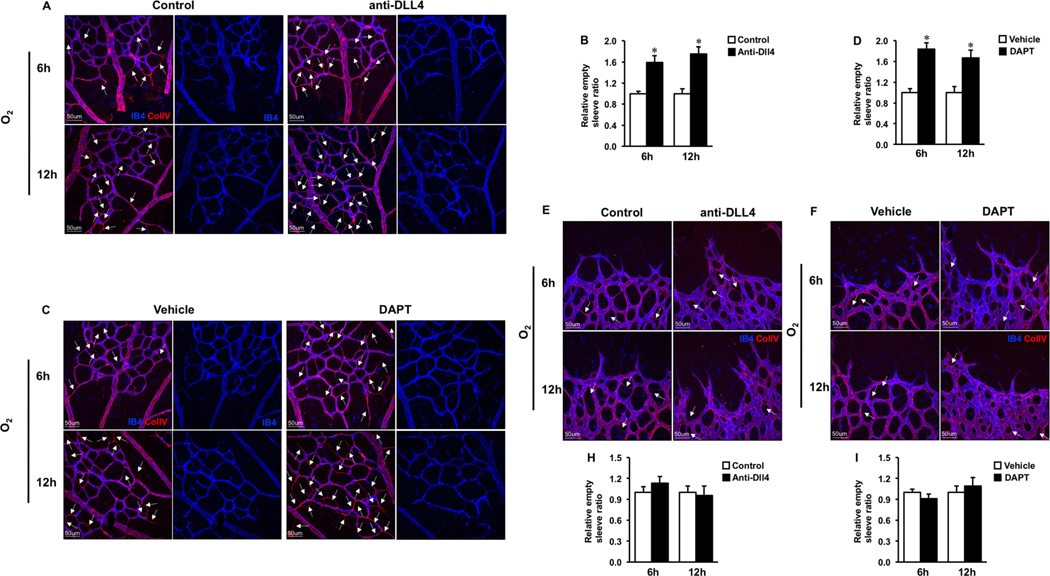
To determine the role of Dll4-Notch1 signaling in vessel regression, neonates at P5 or P6 were injected subcutaneously with Dll4 neutralizing antibodies or DAPT (γ-Secretase inhibitor ) twice (3hrs and right before hyperoxia exposure) and retinas were harvested after 6 and 12hrs hyperoxia or RA treatment. To confirm the inhibitory effects of Dll4 antibody or DAPT on Dll4-Notch1 signaling, we performed N1-ICD and IB4 co-staining on the 6-hour and 12-hour RA treated retinas (6-hour data not shown). As expected, N1-ICD expression in retinal central plexus was hardly detected after Dll4 neutralizing antibody or DAPT treatment (Fig. S2 A–B). Meanwhile, inactivation of Dll4-Notch1 signaling increased sprouting angiogenesis indicated by increased tip cell numbers and filopodia formation (Fig. S2 C–H). However, no vascular regression (empty sleeves) were observed (Fig. S2 I–L). In contrast, in hyperoxia treated neonates, we observed enhanced numbers of empty sleeves (increased by 1.6 and 1.8 folds in the Dll4 treated group, 1.8 and 1.7 folds in the DAPT treated group, at 6 or 12hrs respectively) in the central plexus (Fig. 4A–D) after the short-time (6–12hrs) inhibition of Dll4-Notch1 signaling. Whereas there were no overt changes in the distal capillary plexus (Fig. 4E–H), except enhancing sprouting angiogenesis (data not shown).
Fig.4. Short-time pharmacologic inhibition of Dll4-Notch1 signaling deteriorated the hyperoxia induced vessel regression.
A, E. Representative images of P6 retinas treated by control (IgG) and anti-Dll4 antibody for 3h, followed by 6h or 12h hyperoxia (O2) exposure, stained with IB4 (blue) and Collagen IV(red). The central plexus(A) and angiogenic plexus (E) were presented. C, F. Confocal images of IB4 (blue) and Collagen IV(red) stained retinas from P6 pups by pre administration of Vehicle and DAPT for 3h, followed by 6h or 12h of hyperoxia (O2) treatment. B, D, G, H The proximal (B) and distal capillaries (F) were demonstrated. White arrows indicate empty sleeves (Collagen IV+ IB4-) were quantified. *p < 0.05 (mean ± SEM; n = 4).
DISCUSSION
The major finding of our current study is that inactivation of Dll4-Notch1 signaling is critical for hyperoxia induced vessel regression in mouse retina, whereas the role of VEGF-A is dispensable. Our data demonstrated that VEGF-A was massively expressed in the distal avascular area but almost undetectable in the central retina in normal neonates. Hyperoxia induced substantial vessel regression in the central retina where there was no significant VEGF-A immunosignal changes compared with RA. We do observe significant decrease of VEGF-A protein expression in the distal region of retina where vessel regression was unaffected by hyperoxia. Inconsistent location of regression vessels and VEGF-A suggests that VEGF-A is not responsible for triggering pruning or regression induced by high oxygen. We also found that short-time(6–12h) hyperoxia treatment reduced Dll4 protein expression and Notch1 activation in the regressing central plexus. Inhibition of Dll4-Notch1 pathway exacerbated hyperoxia induced vessel regression in proximal plexus but not in the distal plexus. In addition, sprouting angiogenesis was not affected by hyperoxia.
Reduced VEGF-A mRNA expression is regarded as the most important cause of hyperoxia induced vessel regression[2,6,7]. Liu et al demonstrated that protein levels could not be predicted only by transcript levels[21], especially during dynamic transition like current scenario, e.g. immature vessel developing into functional and quiescent vasculature. Consistently, our data showed reduced VEGF-A protein expression induced by hyperoxia in the distal retina doesn’t increase the empty sleeves of angiogenic plexus compared with normoxia. The discrepancies are possibly caused by the spatial-temporal expression of various Vegfa transcripts or unavailability of resources for VEGF-A protein biosynthesis in the central retina.
We chose the P4-P7 pups for studying vessel regression while P7-P12 mice are commonly used for the traditional oxygen-induced retinopathy (OIR) model[3]. The reasons are: First, retinal vasculature grows from central to periphery in the first week and only superficial plexus layer is easily imaged and analyzed at high resolution by confocal microscope[22,23]. Second, investigation of murine expanding retinal vasculature in the first week might be more relative to the retinal vessel development of premature babies who are treated with hyperoxia[24]. Third, the retinal vasculature begins to penetrate into three dimension after P7 which makes it more complicated to investigate the role of VEGF-A or Dll4-Notch1 signaling pathway in the vessel regression[22,23].
Lobov et al demonstrates that genetic manipulation or pharmacological inhibition of Dll4-Notch1 signaling attenuates hyperoxia induced vessel regression[8]. In contrast, we observed decreased Dll4-Notch1 signaling in the regressing central plexus after hyperoxia exposure compared with RA. Inhibition of Dll4-Notch signaling did not rescue but deteriorated the central vascular regression after hyperoxia exposure. There are two explanations for these controversies. a. Different experimental duration. Lobov et al carried out the experiments under long-term hyperoxia exposure to induce retinopathy(P7-P12). It is well recognized that long-term inhibition of Notch signaling pathway results in excessive sprouting angiogenesis in the central retina under normoxia as well as after hyperoxia exposure[11]. Therefore, their observation is a combination of increased sprouting angiogenesis and decreased regression. Whereas in our study, neonates were treated with pharmacologic inhibitors of Dll4-Notch1 pathway and hyperoxia for a short time (6–12h). During this period, sprouting angiogenesis in central plexus is not initiated as we observed and other group reported before [11]. b. Different mouse ages. Most studies of vascular regression use neonate at P7–15 because the vasculature is regarded as mature vessel. However, during P7-P15 angiogenesis occurs in the perpendicular or deeper layer of retina and Dll4-Notch1 signaling pathway is pivotal for this process[11,19].
The mechanisms of how Dll4-Notch signaling regulates vessel regression is still unknown. Nevertheless, based on our and other groups’ findings, we speculate that EC and pericyte apoptosis could be potential mechanisms. Vessel constriction and capillary occlusion are important for hyperoxia induced vessel regression[6,8,25]. It has been proven that Dll4-Notch1 signaling could be activated by shear stress[26,27]. Schermuly group demonstrates depletion of Notch1 in EC increases cell apoptosis[28].Thus, we presume that decreased shear stress is responsible for reduced Dll4-Notch1 signaling after hyperoxia exposure in our study. Inactivation of Dll4-Notch1 signaling can lead to EC apoptosis and vessel regression. Moreover, It is well known that pericyte-EC attachment maintains vascular stability and prevents vessel regression[25,29,30]. Recently, several studies reveal loss of Notch signaling cause pericyte apoptosis. Consistently, we found marked decrease of Notch1 activity in perivascular cells (possible pericytes because capillary usually associates with pericyte) after hyperoxia or Dll4 neutralizing antibody treatment. These findings suggest EC-pericyte communication through Dll4-Notch1 signaling. Therefore, further investigation utilizing inducible pericyte specific Notch1 knockout mice is needed.
We observed significant decrease of VEGF-A expression in sprouting region but no change in the Dll4-Notch1 signaling. Compelling evidence shows that VEGF is essential for sprouting angiogenesis[31]. We observed significant decrease of VEGF expression in sprouting region. Unexpectly, sprouting angiogenesis and Dll4-Notch signaling are not affected by hyperoxia treament. It implies novel mechanisms responsible for maintaining Dll4-Notch signaling need exploring.
In conclusion, our results break the dogma that VEGF-A is responsible for hyperoxia induced vaso-obliteration. Moreover, we find inactivation of Dll4-Notch1 signaling in vessel regression region after hyperoxia exposure. Short-time administration of Dll4 neutralizing antibodies or DAPT accentuates the hyperoxia induced vascular regression. However, novel mechanisms related to vessel regression require further investigation. These studies will provide novel mechanisms and therapeutic strategies for hyperoxia related vascular diseases, such as ROP. More importantly, Dll4 neutralizing antibody is under clinical trial for cancer patients[32,33]. Our findings suggest a more cautious approach when using Dll4 neutralizing antibody as a treatment due to the vascular side effects observed in our study.
Supplementary Material
Acknowledgments
We thank Hongqiang Li for technical assistance.
Sources of funding
This study was supported by the National Natural Science Foundation of China (No. 81470514 and No. 91639109 to Jinjiang Pang)
Abbreviations:
- EC
endothelial cell
- VEGF-A
vascular endothelial growth factor A
- Dll4
Delta-like 4
- N1-ICD
Notch1 intracellular domain
- GFAP
Glial fibrillary acidic protein
Footnotes
Conflict of interest
The authors declare non-financial competing interests
REFERENCES
- [1].Hlushchuk R, Ehrbar M, Reichmuth P, Heinimann N, Styp-Rekowska B, Escher R, Baum O, Lienemann P, Makanya A, Keshet E, Djonov V, Decrease in VEGF expression induces intussusceptive vascular pruning, Arterioscler. Thromb. Vasc. Biol. 31 (2011) 2836–2844. doi: 10.1161/ATVBAHA.111.231811. [DOI] [PubMed] [Google Scholar]
- [2].Korn C, Augustin HG, Mechanisms of Vessel Pruning and Regression, Dev. Cell. 34 (2015) 5–17. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- [3].Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LEH, Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis, Nat. Protoc. 4 (2009) 1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geller S, Krowka R, Valter K, Stone J, Toxicity of hyperoxia to the retina: evidence from the mouse, Retin. Degener. Dis. 3841 (2006) 425–437. doi: 10.1007/0-387-32442-9_60. [DOI] [PubMed] [Google Scholar]
- [5].Gariano RF, Gardner TW, Retinal angiogenesis in development and disease, Nature. 438 (2005) 960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- [6].Claxton S, Fruttiger M, Role of arteries in oxygen induced vaso-obliteration, Exp. Eye Res. 77 (2003) 305–311. doi: 10.1016/S0014-4835(03)00153-2. [DOI] [PubMed] [Google Scholar]
- [7].Benjamin LE, Hemo I, Keshet E, A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF., Development. 125 (1998) 1591–1598. [DOI] [PubMed] [Google Scholar]
- [8].Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ, The DII4/notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow, Blood. 117 (2011) 6728–6737. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- [9].Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ, Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting, Proc. Natl. Acad. Sci. 104 (2007) 3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH, Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling, Nature. 483 (2012) 110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- [11].Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C, Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis, Nature. 445 (2007) 776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- [12].Majumder S, Zhu GF, Xu X, Senchanthisai S, Jiang D, Liu H, Xue C, Wang X, Coia H, Cui Z, Smolock EM, Libby RT, Berk BC, Pang J, G-Protein-Coupled Receptor-2-Interacting Protein-1 Controls Stalk Cell Fate by Inhibiting Delta-like 4-Notch1 Signaling, Cell Rep. 17 (2016) 2532–2541. doi: 10.1016/j.celrep.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D, Rocha SF, Langen UH, Stehling M, Nagasawa T, Ferrara N, Borggrefe T, Adams RH, Dll4 and Notch signalling couples sprouting angiogenesis and artery formation, Nat. Cell Biol. 19 (2017) 915–927. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- [14].Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risaut W, Nagy A, Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele, Nature. 380 (1996) 435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- [15].West H, Richardson WD, Fruttiger M, Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes, Development. 132 (2005) 1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- [16].Duan LJ, Pan SJ, Sato TN, Fong GH, Retinal Angiogenesis Regulates Astrocytic Differentiation in Neonatal Mouse Retinas by Oxygen Dependent Mechanisms, Sci. Rep. 7 (2017) 1–16. doi: 10.1038/s41598-017-17962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ehling M, Adams S, Benedito R, Adams RH, Notch controls retinal blood vessel maturation and quiescence, Development. 3061 (2013) 3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- [18].Del Monte G, Grego-Bessa J, González-Rajal A, Bolós V, De La Pompa JL, Monitoring Notch1 activity in development: Evidence for a feedback regulatory loop, Dev. Dyn. 236 (2007) 2594–2614. doi: 10.1002/dvdy.21246. [DOI] [PubMed] [Google Scholar]
- [19].Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH, The Notch Ligands Dll4 and Jagged1 Have Opposing Effects on Angiogenesis, Cell. 137 (2009) 1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- [20].Benedito R, Hellström M, Notch as a hub for signaling in angiogenesis, Exp. Cell Res. 319 (2013) 1281–1288. doi: 10.1016/j.yexcr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- [21].Liu Y, Beyer A, Aebersold R, On the Dependency of Cellular Protein Levels on mRNA Abundance, Cell. 165 (2016) 535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- [22].Fruttiger M, Development of the retinal vasculature, Angiogenesis. 10 (2007) 77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- [23].Pitulescu ME, Schmidt I, Benedito R, Adams RH, Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice, Nat. Protoc. 5 (2010) 1518–1534. doi: 10.1038/nprot.2010.113. [DOI] [PubMed] [Google Scholar]
- [24].Uno K, Merges CA, Grebe R, Lutty GA, Prow TW, Hyperoxia inhibits several critical aspects of vascular development, Dev. Dyn. 236 (2007) 981–990. doi: 10.1002/dvdy.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA, Oxygen-induced retinopathy in the mouse., Invest. Ophthalmol. Vis. Sci. 35 (1994) 101–111. [PubMed] [Google Scholar]
- [26].Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragón RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, De Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, Iruela-Arispe ML, NOTCH1 is a mechanosensor in adult arteries, Nat. Commun. 8 (2017) 1620. doi: 10.1038/s41467-017-01741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS, A non-canonical Notch complex regulates adherens junctions and vascular barrier function, Nature. 552 (2017) 258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dabral S, Tian X, Kojonazarov B, Savai R, Ghofrani HA, Weissmann N, Florio M, Sun J, Jonigk D, Maegel L, Grimminger F, Seeger W, Pullamsetti SS, Schermuly RT, Notch1 signalling regulates endothelial proliferation and apoptosis in pulmonary arterial hypertension, Eur. Respir. J. 48 (2016) 1137–1149. doi: 10.1183/13993003.00773-2015. [DOI] [PubMed] [Google Scholar]
- [29].Simonavicius N, Ashenden M, Van Weverwijk A, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM, Pericytes promote selective vessel regression to regulate vascular patterning, Blood. 120 (2012) 1516–1528. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]
- [30].Eilken HM, Diéguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH, Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1, Nat. Commun. 8 (2017) 1574. doi: 10.1038/s41467-017-01738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C, VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia, J. Cell Biol. 161 (2003) 1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McKeage MJ, Kotasek D, Markman B, Hidalgo M, Millward MJ, Jameson MB, Harris DL, Stagg RJ, Kapoun AM, Xu L, Hughes BGM, Phase IB Trial of the Anti-Cancer Stem Cell DLL4-Binding Agent Demcizumab with Pemetrexed and Carboplatin as First-Line Treatment of Metastatic Non-Squamous NSCLC, Target. Oncol. 13 (2017) 89–98. doi: 10.1007/s11523-017-0543-0. [DOI] [PubMed] [Google Scholar]
- [33].Couch JA, Zhang G, Beyer JC, Zuch De Zafra CL, Gupta P, Kamath AV, Lewin-Koh N, Tarrant J, Allamneni KP, Cain G, Yee S, Ross S, Cook R, Tsai SP, Ruppel J, Ridgway JB, Paluch M, Hass PE, Franklin J, Yan M, Balancing Efficacy and Safety of an Anti-DLL4 Antibody through Pharmacokinetic Modulation, Clin. Cancer Res. 22 (2016) 1469–1479. doi: 10.1158/1078-0432.CCR-15-1380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.