Abstract
Background -
Lipoprotein (a) [Lp(a)] levels are higher in individuals of African ancestry (AA) than in individuals of European ancestry (EA). We examined associations of genetically predicted Lp(a) levels with 1) atherosclerotic cardiovascular disease (ASCVD) subtypes: coronary heart disease (CHD), cerebrovascular disease (CVD), peripheral artery disease (PAD), and abdominal aortic aneurysm (AAA); and 2) non-ASCVD phenotypes, stratified by ancestry.
Methods -
We performed 1) Mendelian randomization (MR) analyses for previously reported cardiovascular associations, and 2) phenome-wide MR (MR-PheWAS) analyses for novel associations. Analyses were stratified by ancestry in electronic MEdical Records and GEnomics, United Kingdom Biobank, and Million Veteran Program cohorts separately and in a combined cohort of 804,507 EA and 103,580 AA participants.
Results -
In MR analyses using the combined cohort, a 1-standard deviation (SD) genetic increase in Lp(a) level was associated with ASCVD subtypes in EA – odds ratio and 95% confidence interval for CHD 1.28(1.16–1.41); CVD 1.14(1.07–1.21); PAD 1.22(1.11–1.34); AAA 1.28(1.17–1.40); in AA the effect estimate was lower than in EA and nonsignificant for CHD 1.11(0.99–1.24) and CVD 1.06(0.99–1.14) but similar for PAD 1.16(1.01–1.33) and AAA 1.34(1.11–1.62). In EA, a 1-SD genetic increase in Lp(a) level was associated with aortic valve disorders 1.34(1.10–1.62), mitral valve disorders 1.18(1.09–1.27), congestive heart failure 1.12(1.05–1.19), and chronic kidney disease 1.07(1.01–1.14). In AA no significant associations were noted for aortic valve disorders 1.08(0.94–1.25), mitral valve disorders 1.02(0.89–1.16), congestive heart failure 1.02(0.95–1.10), or chronic kidney disease 1.05(0.99–1.12). MR-PheWAS identified novel associations in EA with arterial thromboembolic disease, non-aortic aneurysmal disease, atrial fibrillation, cardiac conduction disorders, and hypertension.
Conclusions -
Many cardiovascular associations of genetically increased Lp(a) that were significant in EA were not significant in AA. Lp(a) was associated with ASCVD in four major arterial beds in EA but only with PAD and AAA in AA. Additional, novel cardiovascular associations were detected in EA.
Keywords: Mendelian randomization, lipoprotein, cardiovascular genomics, cardiovascular disease, PheWAS, ASCVD, Lipids and Cholesterol, Genetic, Association Studies, Race and Ethnicity, Atherosclerosis, Coronary Artery Disease
Introduction
Lipoprotein (a) [Lp(a)] is a causal risk factor for atherosclerotic cardiovascular disease (ASCVD)1, but its physiologic and pathophysiologic roles remain elusive.2 Lp(a) promotes atherothrombosis through multiple potential mechanisms including pro-inflammatory (carrier of oxidized phospholipids, stimulating cytokine release from leukocytes, etc.), pro-atherogenic (upregulation of endothelial cell adhesion molecules, smooth muscle cell proliferation, etc.), and pro-thrombotic (decreased plasminogen activation, decreased fibrin degradation, etc.).3 The association of measured circulating Lp(a) levels,3, 4 or genetically predicted Lp(a) levels by Mendelian randomization5, 6 (MR) with coronary heart disease (CHD), ischemic cerebrovascular disease (CVD), peripheral artery disease (PAD), and abdominal aortic aneurysm (AAA) is established primarily in individuals of European ancestry (EA). Various additional roles for Lp(a) have been speculated,7 but a systematic investigation on a phenome-wide basis, stratifying for ancestry, is lacking.
A unique aspect of Lp(a) biology is that individuals of African ancestry (AA) have up to 4-fold higher mean Lp(a) levels than EA,8–11 and tend to have smaller isoform size.10 Despite having higher levels than EA,8, 12 it is unclear whether Lp(a) levels are associated with ASCVD in AA.10–16 Previous studies in EA identified associations with CHD, CVD, PAD, AAA, aortic valve stenosis and regurgitation, mitral valve regurgitation, congestive heart failure, and chronic kidney disease,5, 6, 17, 18 but little or no information is available for possible associations in AA for most of these phenotypes.
We investigated differences in the associations of genetically predicted Lp(a) levels with EHR-derived phenotypes between EA and AA, within the electronic MEdical Records and GEnomics (eMERGE) network,19 United Kingdom (UK) Biobank, and the Million Veteran Program (MVP)20 datasets (combined sample size of 804,507 and 103,580 for EA and AA, respectively). We leveraged EHR-derived phenotypes linked to high-density genotype data21 and compared associations of genetically predicted Lp(a) levels with 1) CHD, CVD, PAD, and AAA using 2-sample MR analyses, 2) aortic valve disorders, mitral valve disorders, congestive heart failure, and chronic kidney disease using 2-sample MR analyses, and 3) the entire spectrum of EHR-derived phenotypes without previously known Lp(a) association in both ancestry groups using MR-PheWAS analyses.
Methods
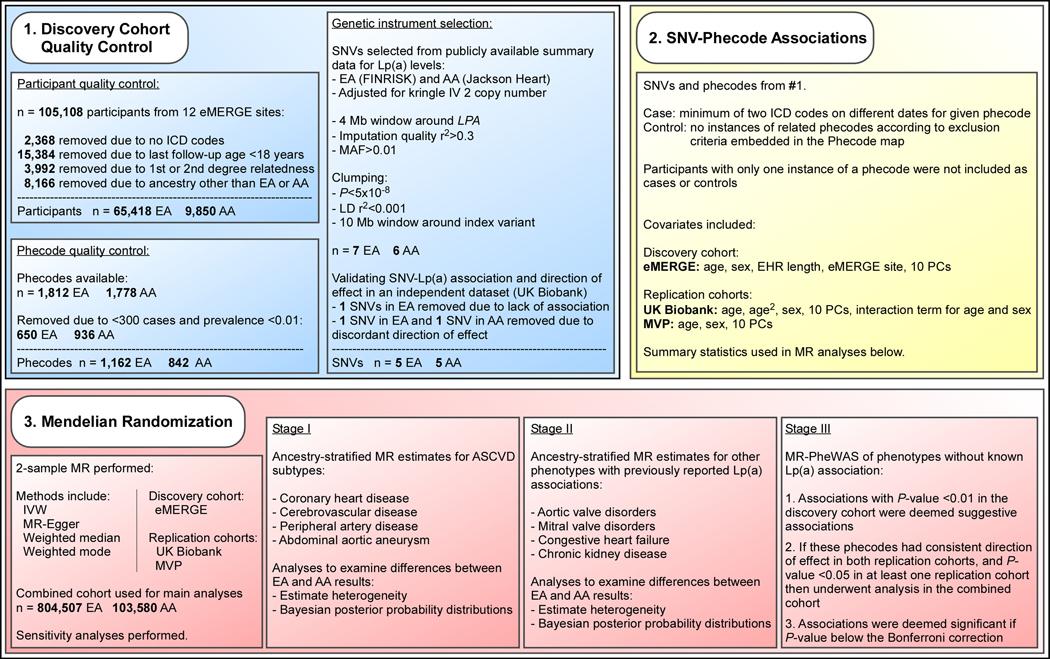
The data that support the findings of this study are available from the corresponding author upon reasonable request. Each study cohort obtained approval from its respective institutional review board and all participants gave informed consent. Demographics of the UK Biobank and MVP replication cohorts are summarized in Table 1. The methods are summarized in Figure 1. All methods are described in the supplemental material.
Table 1.
Demographic characteristics of participants in discovery and replication cohorts
| Discovery Cohort | Replication Cohorts | |||||
|---|---|---|---|---|---|---|
| eMERGE Network | UK Biobank | MVP | ||||
| Ancestry | European | African | European | African | European | African |
| n | 65,418 | 9,850 | 420,531 | 6,636 | 318,558 | 87,094 |
| Mean age ± SD (years) | 64±18* | 53±17* | 58±7† | 51±5† | 64±13† | 58±12† |
| Female (%) | 54 | 66 | 55 | 61 | 7 | 13 |
eMERGE: electronic MEdical Records and GEnomics, MVP: Million Veteran Program, SD: standard deviation, UK: United Kingdom
Age at last follow-up
Age at enrollment
Figure 1.
Overview of analyses exploring differences by ancestry in genetically predicted Lp(a) level-phenotype associations. AA: individuals of African ancestry, ASCVD: atherosclerotic cardiovascular disease, EA: individuals of European ancestry, EHR: electronic health record, eMERGE: electronic MEdical Records and GEnomics, ICD: international classification of diseases, IVW: inverse variance weighted, LD: linkage disequilibrium, Lp(a): lipoprotein(a), MAF: minor allele frequency, MR: Mendelian randomization, PC: principal component, SNV: single-nucleotide variant, UK: United Kingdom
Results
ASCVD Subtype MR Analyses
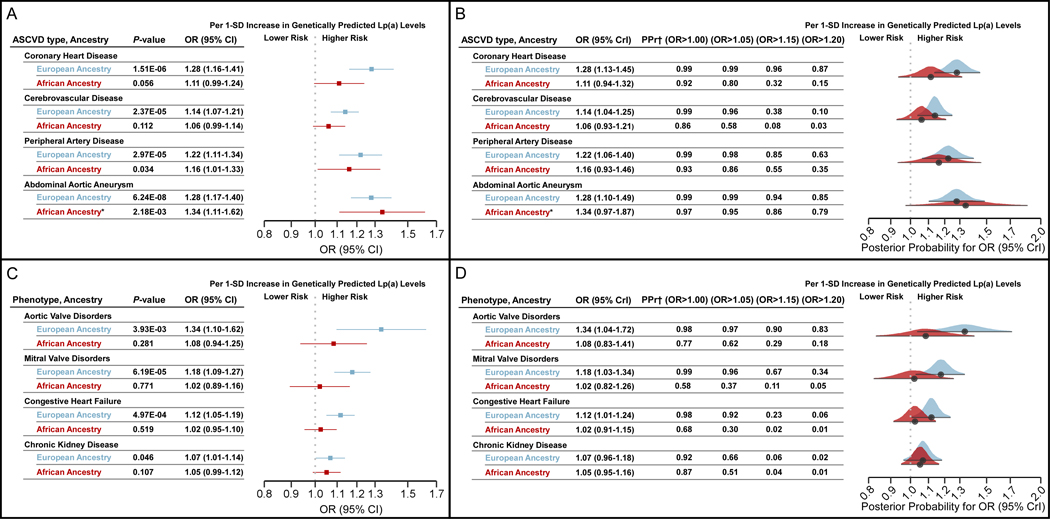
The genetic instruments that passed were selected included 5 SNVs in EA and 5 non-overlapping SNVs in AA that were strongly associated with Lp(a) levels across discovery22 and validation datasets (Table 2) and these explained 14% and 20% of the variation in Lp(a) levels in EA and AA, respectively. Results of the four MR methods for each individual cohort and the combined cohort are presented in Supplemental Excel File I. Only the results from the combined cohort are presented below (n=804,507 and 103,580 for EA and AA, respectively). Across all MR methods, a 1-standard deviation (SD) genetically predicted increase in Lp(a) level was associated with the four ASCVD subtypes in EA, consistent with previous reports6 (Figure 2A, blue). In AA, the effect sizes of a 1-SD genetically predicted increase in Lp(a) with CHD and CVD were smaller than in EA and non-significant (Figure 2A, red), and although the Bayesian posterior probability of effect was largely in favor of OR>1, the majority of this mass was concentrated at lower ranges than in EA (Figure 2B). This is in contrast to the PAD and AAA associations in AA which were significant and similar in magnitude to EA for both the frequentist (Figure 2A) and Bayesian (Figure 2B) analyses. The direction of effects and effect size differences in the ancestry-specific estimates were similar in other MR methods (Supplemental Excel File I). Power calculations based on the case and control numbers in the combined cohort analysis are presented in Supplemental Table I.
Table 2.
SNVs selected as genetic instruments for Lp(a) levels
| Zekavat et al 201822 | UK Biobank‡ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome 6, Position* | Variant | Effect allele | Non-effect allele | Beta | SE | P-value | MAF | Sample size | F-statistic | Beta | SE | P-value | MAF | Sample size | F-statistic |
| European Ancestry | |||||||||||||||
| 160416196 | rs73019695 | A | T | 0.298 | 0.049 | 1.28E-09 | 0.027 | 6440 | 36.84 | 0.208 | 0.008 | 3.57E-150 | 0.033 | 318922 | 681.16 |
| 160620141 | rs75885118 | C | T | −0.321 | 0.035 | 4.93E-20 | 0.054 | 6440 | 84.01 | −0.165 | 0.007 | 5.09E-135 | 0.051 | 318922 | 611.56 |
| 160765055 | rs572889 | G | A | −0.364 | 0.061 | 2.42E-09 | 0.018 | 6440 | 35.60 | −0.363 | 0.000 | NC† | 0.027 | 318922 | NC† |
| 161089307 | rs56393506 | T | C | 0.487 | 0.021 | 1.62E-124 | 0.187 | 6440 | 563.28 | 1.153 | 0.000 | NC† | 0.171 | 318922 | NC† |
| 161304242 | rs62435349 | T | A | −0.362 | 0.027 | 2.16E-42 | 0.104 | 6440 | 186.19 | −0.212 | 0.000 | NC† | 0.099 | 318922 | NC† |
| Chromosome 6, Position* | Variant | Effect allele | Non-effect allele | Beta | SE | P-value | MAF | Sample size | F-statistic | Beta | SE | P-value | MAF | Sample size | F-statistic |
| African Ancestry | |||||||||||||||
| 160901411 | rs114067707 | A | G | −0.621 | 0.077 | 1.08E-15 | 0.023 | 2832 | 64.28 | −0.214 | 0.050 | 1.90E-05 | 0.024 | 5140 | 18.30 |
| 160946747 | rs75143493 | G | T | 0.645 | 0.099 | 5.85E-11 | 0.013 | 2832 | 42.87 | 0.618 | 0.076 | 6.06E-16 | 0.009 | 5140 | 65.42 |
| 160986915 | rs6938647 | C | A | 0.463 | 0.059 | 4.32E-15 | 0.041 | 2832 | 61.55 | 0.281 | 0.048 | 5.39E-09 | 0.027 | 5140 | 34.04 |
| 161025797 | rs78347018 | C | T | −0.760 | 0.059 | 2.14E-38 | 0.041 | 2832 | 167.89 | −0.272 | 0.041 | 2.66E-11 | 0.031 | 5140 | 44.40 |
| 161087440 | rs41269135 | A | G | 0.785 | 0.051 | 1.28E-53 | 0.053 | 2832 | 237.65 | 0.587 | 0.037 | 1.02E-56 | 0.039 | 5140 | 251.81 |
NC: not calculated, SE: standard error, MAF: minor allele frequency, UK: United Kingdom
Position in human genome assembly hg19 (GRCh37)
Unable to calculate as the reported SE in Pan-UK Biobank was provided as 0 at the given precision level in the summary statistics
Figure 2.
Ancestry-stratified 2-sample MR estimates of ASCVD associations and other phenotypes previously known to be associated with Lp(a). (A) Forest plots of the combined (eMERGE, UK Biobank, and MVP) cohort MR estimates and (B) Bayesian posterior probability MR estimates for ASCVD subtypes. (C) Forest plots of combined cohort MR estimates and (D) Bayesian posterior probability MR estimates for other phenotypes with previously known associations. For all panels, results are for the inverse variance weighted method. For panels B and D the black circle represents the posterior mean and the interval represents the 95% highest posterior density. ASCVD: atherosclerotic cardiovascular disease, CI: confidence interval, CrI: credible interval, eMERGE: electronic MEdical Records and GEnomics, Lp(a): lipoprotein(a), MR: Mendelian randomization, MVP: Million Veteran Program, PPr: posterior probability, OR: odds ratio, SD: standard deviation, UK: United Kingdom.
*Results for abdominal aortic aneurysm in African ancestry are only for MVP cohort as the other two cohorts did not meet minimum case requirements.
†The Bayesian posterior probability for the indicated OR range.
To assess whether the association estimates differed by ancestry, we quantified heterogeneity when combining EA and AA results for each ASCVD phenotype using I2 and Cochran Q heterogeneity test (Table 3). I2 across the MR methods ranged between 0.62–0.90 in CHD and 0.45–0.92 in CVD, indicating moderate-to-high heterogeneity. In PAD and AAA, there was no measurable heterogeneity (except when using the MR-Egger method for PAD, I2=0.38). MR sensitivity analyses for ASCVD associations are presented in Supplemental Figures I–IV and Supplemental Excel File I.
Table 3.
Heterogeneity of MR estimates with combined results from both ancestry groups
| Phenotype (phecode) | MR Method | I 2 | Heterogeneity P-value |
|---|---|---|---|
| CHD (411) | IVW | 0.69 | 0.071 |
| MR-Egger | 0.83 | 0.015 | |
| Weighted median | 0.62 | 0.107 | |
| Weighted mode | 0.90 | 2.03E-03 | |
| CVD (433) | IVW | 0.54 | 0.139 |
| MR-Egger | 0.92 | 3.56E-04 | |
| Weighted median | 0.45 | 0.178 | |
| Weighted mode | 0.87 | 5.66E-03 | |
| PAD (443) | IVW | 0 | 0.549 |
| MR-Egger | 0.38 | 0.205 | |
| Weighted median | 0 | 0.588 | |
| Weighted mode | 0 | 0.693 | |
| AAA (442.11) | IVW | 0 | 0.648 |
| MR-Egger | 0 | 0.629 | |
| Weighted median | 0 | 0.406 | |
| Weighted mode | 0 | 0.766 | |
| Aortic valve disorders (395.2) | IVW | 0.64 | 0.095 |
| MR-Egger | 0.91 | 8.00E-04 | |
| Weighted median | 0 | 0.336 | |
| Weighted mode | 0.87 | 5.82E-03 | |
| Mitral valve disorders (395.1) | IVW | 0.70 | 0.069 |
| MR-Egger | 0.11 | 0.290 | |
| Weighted median | 0.64 | 0.096 | |
| Weighted mode | 0.59 | 0.120 | |
| Congestive heart failure (428) | IVW | 0.70 | 0.069 |
| MR-Egger | 0.58 | 0.125 | |
| Weighted median | 0.74 | 0.048 | |
| Weighted mode | 0.72 | 0.060 | |
| Chronic kidney disease (585.3) | IVW | 0 | 0.728 |
| MR-Egger | 0 | 0.921 | |
| Weighted median | 0 | 0.968 | |
| Weighted mode | 0 | 0.935 |
AAA: abdominal aortic aneurysm, CHD: coronary heart disease, CVD: cerebrovascular disease, IVW: inverse variance weighted, MR: Mendelian randomization, PAD: peripheral artery disease
MR Analyses of Other Previously Described Lp(a)-Associated Phenotypes
Additional associations have been previously demonstrated with genetically predicted Lp(a) levels in EA, but little is known about whether these associations are also present in AA. Therefore, we performed 2-sample MR analyses with these phenotypes (aortic valve disorders, mitral valve disorders, congestive heart failure, and chronic kidney disease) in both ancestry groups at the cohort level as well as with the combined cohort (Supplemental Excel File I). In the AA combined cohort analysis (Figure 2C, red), per 1-SD genetically predicted increase in Lp(a), associations with all phenotypes were weaker than in EA except chronic kidney disease, which although not significantly associated, had a similar magnitude of association as in EA (Figure 2C, blue); this was similar across other MR methods (Supplemental Excel File I). Power calculations based on the case and control numbers in the combined cohort analysis are given in Supplemental Table I.
As before, we quantified the heterogeneity when combining EA and AA results for these phenotypes (Table 3) and found I2 across all MR methods to range between 0.64–0.91 (except weighted median, which showed no heterogeneity) in aortic valve disorders, 0.11–0.70 in mitral valve disorders, and 0.58–0.74 for congestive heart failure, indicating moderate-to-high heterogeneity in these three phenotypes. In chronic kidney disease there was no measurable heterogeneity with any method. Most of the mass of the posterior probability distribution of ORs for the associations with aortic valve disorders, mitral valve disorders, and congestive heart failure was located in the higher ranges in EA than in AA. For chronic kidney disease, EA and AA had similar posterior probabilities of having a modest OR (Figure 2D). MR sensitivity analyses for these associations are presented in Supplemental Figures V–VIII and Supplemental Excel File I.
MR-PheWAS of Phenotypes without Known Lp(a) Associations
Of the 1,812 and 1,778 phecodes available in EA and AA, respectively, 1,162 in EA and 842 in AA met the minimum required number of cases or prevalence threshold in the discovery cohort.
European ancestry
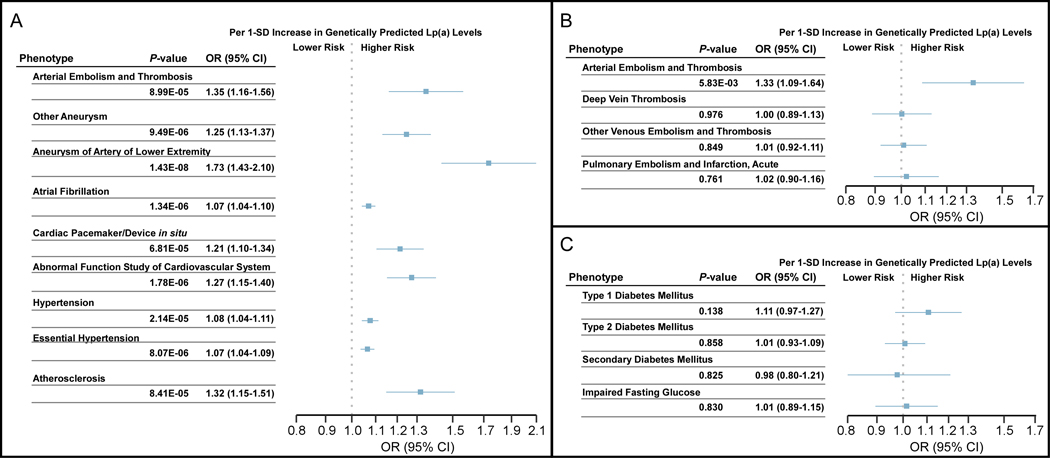
Among phenotypes not known to be associated with Lp(a), MR-PheWAS in the eMERGE discovery cohort identified 34 phecodes that had suggestive associations (P-value <0.01) with genetically predicted Lp(a) levels in EA. Of these, 18 were excluded from further analyses because either the P-value (IVW) was >0.05 in both replication cohorts, or the direction of effect was not consistent in one of the replication cohorts compared to the discovery cohort. The remaining 16 phecodes underwent analysis using the combined cohort where 15 of these phecode associations were deemed significant after Bonferroni correction. These included phecodes that fit into 6 categories: arterial thromboembolic disease, non-aortic aneurysmal disease, atrial fibrillation, cardiac conduction disorders, hypertension, and atherosclerosis-related traits (Figure 3A). Results of all four methods in each individual cohort as well as the combined cohort for the 9 significant phenotypes are in Supplemental Excel File II.
Figure 3.
MR-PheWAS estimates for novel associations, thromboembolic disease, and diabetes mellitus. Forest plots (A) of novel associations from the combined (eMERGE, UK Biobank, and MVP) European Ancestry cohort; (B) of arterial and venous thromboembolic disease phenotypes within the eMERGE cohort; and (C) diabetes phenotypes within the eMERGE cohort. For all panels, results are for the inverse variance weighted method. CI: confidence interval, eMERGE: electronic MEdical Records and GEnomics, Lp(a): lipoprotein(a), MR: Mendelian randomization, MVP: Million Veteran Program, OR: odds ratio, SD: standard deviation, UK: United Kingdom.
Although we detected an association with arterial thromboembolic disease, we did not find a significant association with any venous thromboembolism phenotypes using the IVW method (Figure 3B) or other MR methods (Supplemental Table II) in the eMERGE cohort. Given conflicting reports about possible associations between Lp(a) level and type 2 diabetes,23–26 we looked for, but did not find a significant association with any diabetes-related phecodes with the IVW method (Figure 3C) or other MR methods (Supplemental Table II) with the exception of type 1 diabetes with the weighted median method (P=0.032; OR 1.15 [1.01–1.32]).
African ancestry
MR-PheWAS in the eMERGE discovery cohort identified 7 phecodes that had suggestive associations (P-value <0.01) with genetically predicted Lp(a) levels in AA, however all 7 of these were excluded from further analyses because the P-value (IVW) was >0.05 in both replication cohorts. Power calculations for both EA and AA based on a range of prevalence from 0.01–0.20 in the eMERGE discovery cohort are presented in Supplemental Table III.
Discussion
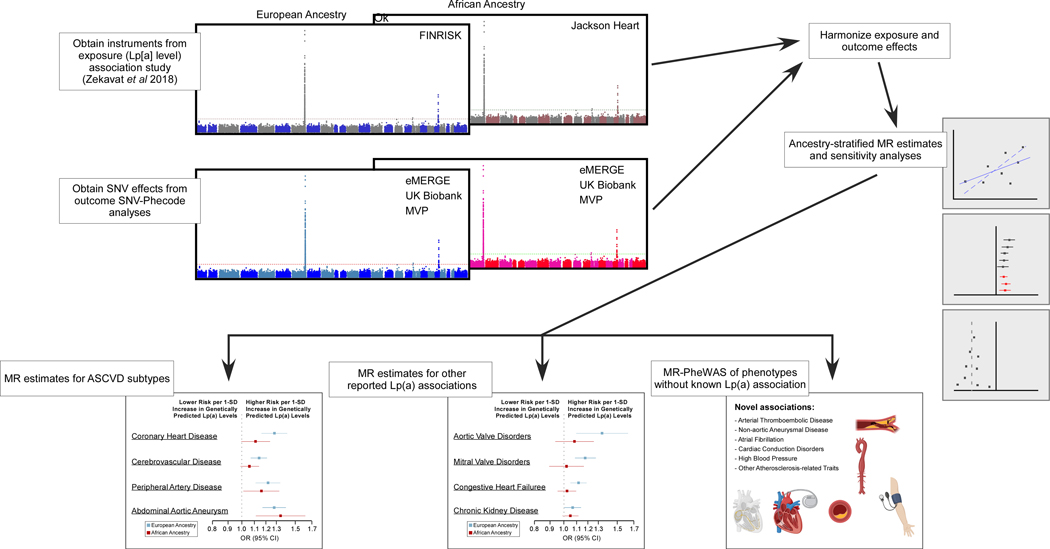
Ancestry-stratified MR analyses of 804,507 EA and 103,580 AA individuals demonstrated that for many associations of genetically mediated increase in Lp(a) levels with cardiovascular traits observed in EA, no significant association was observed in AA (summarized in Figure 4). In AA the effect estimates of CHD and CVD were less than in EA and nonsignificant, whereas the associations with PAD or AAA were of similar magnitude. Associations with disorders of aortic and mitral valves, congestive heart failure, and chronic kidney disease were absent in AA. We also identified several novel associations with additional cardiovascular traits in EA. These observations of a stronger association between genetically predicted Lp(a) levels and CHD in EA than AA may explain the descrepancies in prior studies where some found associations between Lp(a) levels and CHD in AA while others did not.10–16
Figure 4.
Summary of strategy and results from MR and MR-PheWAS analyses. ASCVD: atherosclerotic cardiovascular disease, CI: confidence interval, eMERGE: electronic MEdical Records and GEnomics, Lp(a): lipoprotein(a), MR: Mendelian randomization, MVP: Million Veteran Program, OR: odds ratio, SD: standard deviation, SNV: single-nucleotide variant, UK: United Kingdom. The images in the MR-PheWAS box were created with BioRender.com.
While Lp(a) was associated with ASCVD in four major arterial beds in EA, in AA the association was limited to PAD and AAA. Lp(a) level has been previously reported to be associated with AAA in EA and AA,18 but we are unaware of previous ancestry-stratified reports for association with PAD. Further investigation is needed to better understand the basis of this observation. Endothelial cell ‘phenotypes’ are known to vary across arterial beds27 and it can be speculated that such heterogeneity might influence effects of Lp(a) on different arterial beds in AA. Previous studies have identified differences in how endothelial cells in EA and AA respond to various stimuli28, 29 which results in varied responses to different classes of antihypertensive medications.30 It is possible such endothelial heterogeneity influences atherothrombotic effects of Lp(a) in different arterial beds.
The apolipoprotein (a) component of Lp(a) inhibits plasminogen activation and competes for binding of plasminogen to fibrin clots in vitro,31, 32 We detected a hitherto unreported association of genetically predicted Lp(a) level with arterial thromboembolic diseases in EA, supporting in vitro observations and suggesting that Lp(a) affects both atherogenesis and thrombosis in EA; however, we did not find an association with venous thromboembolism, consistent with prior studies.5, 33 These observations suggest differential effects of Lp(a) in arteries versus veins.
We confirmed previously reported associations in EA including aortic valve disorders (stenosis and regurgitation), mitral valve disorders (regurgitation), congestive heart failure, and chronic kidney disease.5, 6 However, these associations were not detected in AA. Pathways that mediate the association of Lp(a) level with aortic valve disorders (stenosis and regurgitation), congestive heart failure, or chronic kidney disease, are unclear although increased propensity to ASCVD may play a role.
Our agnostic phenome-wide analysis to identify associations of Lp(a) with the spectrum of EHR-derived phenotypes using a MR-PheWAS approach identified additional novel associations including aneurysmal disease in vascular beds other than the aorta, atrial fibrillation, cardiac conduction disorders, blood pressure disorders, and other atherosclerosis-related traits. While the association with arterial aneurysmal disease is likely mediated by ASCVD, the basis for the remaining associations is unclear at present. Associations with non-cardiovascular phenotypes were not detected in either ancestry group, suggesting that the pathophysiology of Lp(a) is mostly restricted to cardiovascular traits. In particular, we did not detect an association with type 2 diabetes.
Our findings could have potential clinical implications. The AHA/ACC guideline34 recommends measurement of Lp(a) levels in certain settings whereas the European Atherosclerosis Society recommends routine measurement in all individuals for risk stratification.35 However, modification of these recommendations may be warranted for AA; rather than using a uniform threshold for all ancestry groups, ancestry-specific thresholds might be more appropriate as has also been suggested by others.36 Additionally, new drugs that specifically lower Lp(a) are being evaluated in clinical trials and it is important that these studies include an adequate representation of AA to determine whether any benefit observed differs by ancestry. In a recent clinical trial demonstrating a marked reduction of Lp(a)37 with an antisense therapy, 97% of the participants were EA. Finally, medically lowered Lp(a) levels are unlikely to have unintended effects as associations with non-cardiovascular phenotypes were not detected in either ancestry group.
Strengths and Limitations
Our study examines the causal role of genetically predicted Lp(a) levels in ASCVD and other phenotypes in two ancestry groups from three large cohorts. For many of these phenotypes this is the first report in AA, while for others we examine results from a much larger AA cohort than in previously published studies. We also report results of the first PheWAS for Lp(a) in AA as well as the first PheWAS to examine the entire range of EHR phenotypes in either ancestry group.
Due to the difference in Lp(a) levels between EA and AA, we examined associations with a 1-SD change in ancestry-specific genetically predicted Lp(a) levels instead of absolute values. The differences in associations between the two ancestry groups could have been greater if we used absolute values since a 1-SD change in genetically predicted Lp(a) levels would correspond to a large change in absolute Lp(a) levels in AA. We did not have access to the individual level data for Lp(a) levels within the UK Biobank. In the PheWAS approach, power to detect weaker phenotypic associations may vary depending on the number of cases for a phenotype and, although comparatively large, the sample size of AA was smaller than EA. In both ancestry groups, particularly in AA, we were relatively underpowered to detect any weak phenotypic associations.
Conclusion
In contrast to robust associations of genetically predicted Lp(a) with CHD, CVD, aortic valve disorders, mitral valve disorders, and congestive heart failure, in EA, such associations were weaker or absent in AA. However, associations with PAD and AAA were significant and similar in both EA and AA. Additional novel cardiovascular associations in EA included arterial thromboembolic disease, aneurysmal disease of vascular beds other than the aorta, atrial fibrillation, cardiac conduction disorders, blood pressure disorders, and atherosclerosis-related traits. Our findings have potentially important clinical implications for the use of Lp(a) levels in ASCVD risk stratification in AA and highlight the need to test the effect of Lp(a)-lowering on ASCVD events in AA.
Supplementary Material
Acknowledgments:
We thank the investigators and participants of the eMERGE Network, UK Biobank, and MVP.
Sources of Funding: This work was supported by the National Human Genome Research Institute’s electronic Medical Records and Genomics Network through grants U01HG04599 and U01HG006379 (Mayo Clinic, Rochester, Minnesota), U01HG006378 (Vanderbilt University Medical Center, Nashville, Tennessee), U01HG04603 and U01HG006385 (Vanderbilt University Medical Center serving as the Coordinating Center), U01HG004608 (Marshfield Clinic, Marshfield, Wisconsin), U01HG006389 (Marshfield Clinic Research Foundation and Pennsylvania State University), U01HG006382 (Geisinger Clinic, Danville, Pennsylvania), U01HG004610 and U01HG006375 (Kaiser Permanente Washington Health Research Institute/University of Washington, Seattle, Washington), U01HG004609 and U01HG006388 (Northwestern University, Chicago, Illinois), U01HG006380 (Icahn School of Medicine at Mount Sinai, New York, New York), U01HG008680 (Columbia University, New York, New York), U01HG004438 (CIDR) and U01HG004424 (the Broad Institute) serving as Genotyping Centers. BAS is supported by the Mayo Clinic Clinician-Investigator Training Program. IJK is additionally supported by NIH grant K24HL137010. The Million Veteran Program is funded by Grant #I01BX003362 from the Department of Veterans Affairs Office of Research and Development. This publication does not represent the views of the Department of Veteran Affairs or the United States Government.
Nonstandard Abbreviations and Acronyms
- AA
African ancestry
- AAA
abdominal aortic aneurysm
- ACC
American College of Cardiology
- AHA
American Heart Association
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cerebrovascular disease
- EA
European ancestry
- EHR
electronic health record
- eMERGE
electronic MEdical Records and Genomics
- IVW
inverse variance weighted
- Lp(a)
Lipoproetin (a)
- MR
Mendelian randomization
- MVP
Million Veteran Program
- OR
odds ratio
- PAD
peripheral artery disease
- PheWAS
phenome-wide association study
- SD
standard deviation
- UK
United Kingdom
Footnotes
Disclosures: None.
Supplemental Material:
Supplemental Methods
Supplemental Tables I-IV
Supplemental Figures I-IX
VA Million Veteran Program: Core Acknowledgements for Publications
Supplemental Excel Files I-II
Publisher's Disclaimer: This article is published in its accepted form; it has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation: Genomic and Precision Medicine involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final published version.
References:
- 1.Kassner U, Schlabs T, Rosada A, Steinhagen-Thiessen E. Lipoprotein (a)–An independent causal risk factor for cardiovascular disease and current therapeutic options. Atheroscler Suppl. 2015;18:263–267. [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–21. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 4.Trinder M, Uddin MM, Finneran P, Aragam KG, Natarajan P. Clinical Utility of Lipoprotein (a) and LPA Genetic Risk Score in Risk Prediction of Incident Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson SC, Gill D, Mason AM, Jiang T, Bäck M, Butterworth AS, Burgess S. Lipoprotein (a) in Alzheimer, Atherosclerotic, Cerebrovascular, Thrombotic, and Valvular Disease: Mendelian Randomization Investigation. Circulation. 2020;141:1826–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emdin CA, Khera AV, Natarajan P, Klarin D, Won HH, Peloso GM, Stitziel NO, Nomura A, Zekavat SM, Bick AG, et al. Phenotypic Characterization of Genetically Lowered Human Lipoprotein(a) Levels. J Am Coll Cardiol. 2016;68:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsimikas S. In search of a physiological function of lipoprotein(a): causality of elevated Lp(a) levels and reduced incidence of type 2 diabetes. J Lipid Res. 2018;59:741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumitrescu L, Glenn K, Brown-Gentry K, Shephard C, Wong M, Rieder MJ, Smith JD, Nickerson DA, Crawford DC. Variation in LPA is associated with Lp(a) levels in three populations from the Third National Health and Nutrition Examination Survey. PLoS One. 2011;6:e16604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanktree MB, Anand SS, Yusuf S, Hegele RA; SHARE Investigators. Comprehensive analysis of genomic variation in the LPA locus and its relationship to plasma lipoprotein(a) in South Asians, Chinese, and European Caucasians. Circ Cardiovasc Genet. 2010;3:39–46. [DOI] [PubMed] [Google Scholar]
- 10.Pare G, Caku A, McQueen M, Anand SS, Enas E, Clarke R, Boffa MB, Koschinsky M, Wang X, Yusuf S, et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation. 2019;139:1472–1482. [DOI] [PubMed] [Google Scholar]
- 11.Marcovina SM, Albers JJ, Wijsman E, Zhang Z, Chapman NH, Kennedy H. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J Lipid Res. 1996;37:2569–85. [PubMed] [Google Scholar]
- 12.Guyton JR, Dahlen GH, Patsch W, Kautz JA, Gotto AM. Relationship of plasma lipoprotein Lp(a) levels to race and to apolipoprotein B. Arteriosclerosis. 1985;5:265–72. [DOI] [PubMed] [Google Scholar]
- 13.Moliterno DJ, Jokinen EV, Miserez AR, Lange RA, Willard JE, Boerwinkle E, Hillis LD, Hobbs HH. No association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis in African-Americans. Arterioscler Thromb Vasc Biol. 1995;15:850–5. [DOI] [PubMed] [Google Scholar]
- 14.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ. Associations between lipoprotein (a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R. Race is a key variable in assigning lipoprotein (a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–24. [DOI] [PubMed] [Google Scholar]
- 17.Wei WQ, Li X, Feng Q, Kubo M, Kullo IJ, Peissig PL, Karlson EW, Jarvik GP, Lee MTM, Shang N, et al. LPA Variants Are Associated With Residual Cardiovascular Risk in Patients Receiving Statins. Circulation. 2018;138:1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota Y, Folsom AR, Ballantyne CM, Tang W. Lipoprotein (a) and abdominal aortic aneurysm risk: The Atherosclerosis Risk in Communities study. Atherosclerosis. 2018;268:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, Li R, Masys DR, Ritchie MD, Roden DM, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–23. [DOI] [PubMed] [Google Scholar]
- 21.Safarova MS, Satterfield BA, Fan X, Austin EE, Ye Z, Bastarache L, Zheng N, Ritchie MD, Borthwick KM, Williams MS, et al. A phenome-wide association study to discover pleiotropic effects of PCSK9, APOB, and LDLR. NPJ Genom Med. 2019;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamstrup PR, Nordestgaard BG. Lipoprotein (a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2013;1:220–227. [DOI] [PubMed] [Google Scholar]
- 24.Ye Z, Haycock PC, Gurdasani D, Pomilla C, Boekholdt SM, Tsimikas S, Khaw K-T, Wareham NJ, Sandhu MS, Forouhi NG. The association between circulating lipoprotein (a) and type 2 diabetes: is it causal? Diabetes. 2014;63:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein (a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, Willeit J, Kiechl S, Willeit P. Lipoprotein (a) and incident type-2 diabetes: results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc Diabetol. 2017;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aird WC. Endothelial cell heterogeneity and atherosclerosis. Curr Atheroscler Rep. 2006;8:69–75. [DOI] [PubMed] [Google Scholar]
- 28.Pienaar P, Micklesfield L, Gill J, Shore A, Gooding K, Levitt N, Lambert E. Ethnic differences in microvascular function in apparently healthy South African men and women. Exp Physiol. 2014;99:985–994. [DOI] [PubMed] [Google Scholar]
- 29.Muniz JJ, Izidoro-Toledo TC, Metzger IF, Sandrim VC, Tanus-Santos JE. Interethnic differences in the distribution of clinically relevant vascular endothelial growth factor genetic polymorphisms. DNA Cell Biol. 2009;28:567–572. [DOI] [PubMed] [Google Scholar]
- 30.Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calciumblockers and diuretics than to ACE inhibitors and β-adrenergic blockers? Asystematic review. BMC Med. 2013;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buechler C, Ullrich H, Ritter M, Porsch-Oezcueruemez M, Lackner KJ, Barlage S, Friedrich SO, Kostner GM, Schmitz G. Lipoprotein (a) up-regulates the expression of the plasminogen activator inhibitor 2 in human blood monocytes. Blood. 2001;97:981–986. [DOI] [PubMed] [Google Scholar]
- 32.Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, Broze GJ Jr, Simari RD. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98:2980–2987. [DOI] [PubMed] [Google Scholar]
- 33.Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, Jones GT, van Rij AM, Eapen DJ, Baas AF, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–9. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart Journal. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 36.Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, Khera AV. Lp (a)(Lipoprotein [a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights From a Large National Biobank. Arterioscler Thromb Vasc Biol. 2021;41:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 38.Stanaway IB, Hall TO, Rosenthal EA, Palmer M, Naranbhai V, Knevel R, Namjou-Khales B, Carroll RJ, Kiryluk K, Gordon AS, et al. The eMERGE genotype set of 83,717 subjects imputed to ~40 million variants genome wide and association with the herpes zoster medical record phenotype. Genet Epidemiol. 2019;43:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter-Zinck H, Shi Y, Li M, Gorman BR, Ji S-G, Sun N, Webster T, Liem A, Hsieh P, Devineni P. Genotyping Array Design and Data Quality Control in the Million Veteran Program. Am J Hum Genet. 2020;106:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biobank P-U. https://pan.ukbb.broadinstitute.org/docs/study-design/index.html#how-did-you-decide-what-ancestry-groups-to-include-how-did-you-assign-individuals-to-each-ancestry-groupLast accessed November 15, 2020. 2020. [Google Scholar]
- 44.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei WQ, Bastarache LA, Carroll RJ, Marlo JE, Osterman TJ, Gamazon ER, Cox NJ, Roden DM, Denny JC. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS One. 2017;12:e0175508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll RJ, Bastarache L, Denny JC. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. 2014;30:2375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slob EA, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44:313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Team S. Stan Modeling Language Users Guide and Reference Manual Version 2.21.0. 2020. [Google Scholar]
- 54.Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925–1926. [DOI] [PubMed] [Google Scholar]
- 55.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. 2019;10:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess S, Foley CN, Allara E, Staley JR, Howson JM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N,Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36:1783–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.