Abstract
Introduction:
Hepatic fibrosis is wound-healing response that is the result of hepatic stellate cell (HSC) activation and subsequent excess extracellular matrix deposition. HSCs can be activated by a variety of inflammatory stimuli as well as through the signal transducer and activator of transcription 3 (STAT3) pathway. HJC0416 is a novel, orally bioavailable small-molecule inhibitor of STAT3 that was developed by our team using a fragment-based drug design approach. Previously, our team has shown that HJC0416 has antifibrogenic effects in activated HSCs. Recently, increasing evidence suggests that nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) plays an important role in the activation of HSCs.
In the present study, we examined the role of NF-κB inhibition of HSC activation by HJC0416.
Methods:
LX-2 (human) and HSC-T6 (rat) cell lines were used. Expression levels of extracellular proteins, NF-κB and STAT3 expression and DNA binding, and inflammatory cytokine levels were determined using Western Blot, ELISA, and immunofluorescence assay.
Results:
HJC0416 decreased cell viability in a dose dependent manner in both cell lines and arrested the cell cycle at the S phase. Increased apoptosis was seen in LX-2 cells through Yo-Pro-1 and Propidium Iodide immunofluorescent stating. HJC0416 significantly decreased expression of fibronectin and collagen I as well as markedly decreased α-SMA and laminin. HJC0416 inhibited the STAT3 pathway by decreasing phosphorylation of STAT3, as well as signal transduction pathway activation. Notably, HJC0416 also inhibited the classic and alternative pathways of NF-κB activation. HJC0416 inhibited LPS-induced p65 nuclear translocation and DNA binding, as well as prevented phosphorylation and degradation of inhibitory protein IκBα. HJC0416 also prevented phosphorylation of Serine residue 536 on p65.
Conclusion:
HJC0416, an inhibitor of STAT3, was found to have anti-fibrogenic properties in activated hepatic stellate cell lines. Additionally, HJC0416 was found to inhibit the NF-κB pathway. Due to this double effect, HJC0416 demonstrates promise for in-vivo experimentation as an antifibrosis treatment.
Keywords: Hepatic fibrosis, NF-κB, STAT3
Introduction
Sustained inflammatory signaling in hepatocytes can lead to an aberrant wound-healing response, resulting in hepatic fibrosis.1,2 Hepatic fibrosis typically occurs in response to chronic liver disease and can ultimately lead to cirrhosis.1 Hepatic fibrosis is a reversible dynamic process, but cirrhosis more permanently alters hepatic structure and function.3 Prevention of progression from advanced fibrosis to cirrhosis or inducing regression of advanced hepatic fibrosis is a therapeutic target for agents to treat cirrhosis.4,5
Hepatic fibrosis is a result of hepatic stellate cell (HSC) activation and the deposition of excess extracellular matrix (ECM). In the quiescent state, HSCs have a variety of functions, including ECM homeostasis and vasoregulation.6 However, HSC activation leads to over-expression of multiple cytokines and overexpression of α-smooth muscle actin (α-SMA) as well as ECM proteins collagen I, fibronectin and laminin.7–11 Activation of the signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) pathways promotes HSC activation and proliferation, thereby promoting hepatic fibrogenesis.12–14 Previous work from our lab and others has demonstrated that STAT3 and NF-κB inhibition results in HSC apoptosis and accelerated recovery from hepatic fibrosis.4,15–17
Fragment-based drug design allows for synthesis of new molecules with enhanced properties by chemically merging privileged fragments from small molecule libraries.18,19 Using fragment-based drug design, our group has designed and synthesized a family of STAT3 specific inhibitors, which have been shown to have potent anti-cancer effects.20–23 Given the previous demonstrated success of inhibiting STAT3 in the treatment of hepatic fibrosis, we hypothesized that this family of compounds could successfully attenuate fibrosis in activated HSCs. After screening of the molecules for efficacy, HJC0123 and HJC0416 were both identified to have potent anti-fibrotic effects.16 However, HJC0416 was noted to have additional anti-inflammatory properties which we sought to further characterize in the current study.
Methods
Experiments/Assays were done in triplicate unless otherwise stated in results.
Cell Culture
Cell culturing was done as previously published30, briefly: Immortalized HSC lines: LX-2 and HSC-T6 (Mount Sinai Medical Center, New York – Dr. Scott Friedman) were cultured at 37° C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM), a high glucose concentration (4.5 g/L), and 5% fetal bovine serum (FBS), 1% penicillin/streptomycin.
Reagents
HJC0146 was obtained from the department of pharmacology and toxicology at UTMB. HJC0146 was created via a fragment-based drug design approach as previously described, stored at −4°C and administered in DMSO.16,20 LPS and Stattic were obtained from Sigma-Aldrich (St. Louis, MO) and stored according the manufacturer’s instructions.
Cell Cycle
2 X 106 cells were trypsinized, washed with PBS, and resuspended in a low-salt solution for staining (3% polyethylene glycol 8000, 50 μg of propidium iodide per mL, 0.1% Triton X-100, 4 mM sodium citrate, 180 units of RNase A per mL). They were then incubated for 20 minutes at 37°C; an equal volume higher salt containing solution was added (3% polyethylene glycol 8000, 50 μg of propidium iodide per ml, 0.1% Triton X-100, and 400 mM sodium chloride). Propidium iodide stained cells were stored at 4°C for 3 hours before the fluorescence-activated cell sorter analysis (BD FACSCanto II flow cytometer: Becton, Dickinson and Company, Franklin Lakes, NJ). We used ModFit LT for Win32 software for data analysis (Verity Software House, Inc., Topsham, ME).
Western Immunoblotting
Whole cell extracts were prepared as previously described.31 Protein (10–30 μg) was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Life Technologies Corporation, Carlsbad, CA) under denaturing conditions and transferred to a polyvinylidene fluoride (PVDF) membrane. After 1 hour of blocking buffer incubation (LI-COR Inc., Lincoln, NE), the membrane was probed with each antibody for 2 hours at room temperature. Membranes were then washed three times with phosphate buffered saline, 0.1% Tween 20 (PBST), and incubated with infrared fluorescent dye: IRDye 680-conjugated anti-mouse or IRDye 800-conjugated anti-rabbit antibodies (LI-COR, Inc.) for 1 hour. Finally, the membranes were washed with PBST, and signals were visualized using Odyssey Infrared Imaging System (LICOR, Inc., Lincoln, NE).
Immunofluorescence Staining
0.25×105 cell/well were grown in chamber slides with supplemented medium. After 24 hours of incubation, the cells were treated as indicated for 48 hours. Cells were fixed with 4% formaldehyde, diluted in PBS for 15 minutes at room temperature, and again washed with PBS. After incubating for 60 minutes with blocking buffer and 2 hours of incubation at room temperature with the corresponding antibody cells were rinsed with PBS 3x for 5 minutes, followed by another 1 hour incubation period with each corresponding secondary antibody at room temperature in the dark. Immunofluorescence was determined by Nikon Eclipse Ti confocal microscope at 20x or 60x magnification (Nikon Instruments, Inc, Melville, New York). Using Image J (v1.50b, NIH), mean corrected cellular fluorescence was equalized and calculated. Stains included: Hoechst33342 (Catalog #62249,Thermofisher Scientific Waltham, Massachusetts) for dsDNA binding, ab34471 AND ab34710 (Abcam Biotechnology, Cambridge, United Kingdom); corresponding manufacturer’s instructions were utilized.
Yo-Pro-1 and Propidium Iodide Staining
For the detection of apoptosis, cells were seeded in 24-well plates with 0.25×105 cells/well. Next day, cells were treated with 1μM of HJC0416 for 24 hours. After being washed with PBS, cells were incubated with 1 μM of Yo-Pro-1 (Life Technologies Corporation, Grand Island, NY) or propidium iodide (PI) (Life Technologies Corporation, Grand Island, NY) for 1 hour. Yo-Pro-1 and PI uptake were determined by confocal microscope (Nikon Instruments Inc. Melville, NY).
Alamar Blue/Cell Viability Assay
3×103 cells/well of LX-2 cells and 4×103 cells/well of HSC-T6 cells were seeded into 96 well plates; after 24 hours incubation once cells reached 50–60% confluence, fresh medium was given and treatment with the indicated concentrations and time with HJC0416. Alamar blue solution (Life Technologies Corporation, Grand Island, NY) was diluted into culture medium at 1:1 with a volume of 25μL/well, creating a 10% concentration. Four more hours of incubation was done, then fluorescence was monitored with SpectraMax M2 microplate reader (Molecular Devices, LLC, Sunnyvale, CA) with excitation and emission wavelengths set at 540 and 590 nm.
ELISA
Cells were treated with 1.0μM HJC0416 pretreatment for 1 hour, LPS 0.5μg/mL for 24 hours, then the culture supernatant was collected for measurement of cytokine secretion. IL-6 ELISA kit (#KHC0061; Life Technologies, Frederick, MD) was used according manufacturer’s instructions and normalized to the total amount of protein in the cell pellets.
Nuclear Protein Extraction
Nuclear protein was isolated with NE-PER nuclear and cytoplasmic extraction reagent (Pierce Biotechnology; Rockford, IL) according to the instructions of the manufacturer.
NK-κB p65 DNA Binding and STAT3 Signal Transduction Activation Assay
The Trans-AM NF-κB p65 Transcription Factor kit was used to assess DNA binding (Active Motif North America, Carlsbad, CA) and the manufacturer’s instructions were followed. For STAT3 signal transduction pathway activation, a Qiagen Assay with STAT3 responsive/luciferase reporter plasmid (CCS-9028L, Hilden, Germany) was used following manufacturer’s instructions.
Data and statistical analysis
Statistical analysis was performed using GraphPad Prism 7.03 (GraphPad Software Inc. La Jolla, CA). The results are representative of independent experiments. Data was compared using paired t-test for Western Immunoblotting and non-paired t-tests/Mann-Whitney tests or one-way ANOVA/Kruskal-Wallis tests for measurement of immunofluorescence and ELISA studies depending on normality. Data is presented as mean ± standard deviation in results, and graphs are given as mean ± 95% confidence interval; level of significance was set as p <0.05.
Results
HJC0416 inhibits LX-2 and HSC-T6 cell viability and causes cell cycle arrest
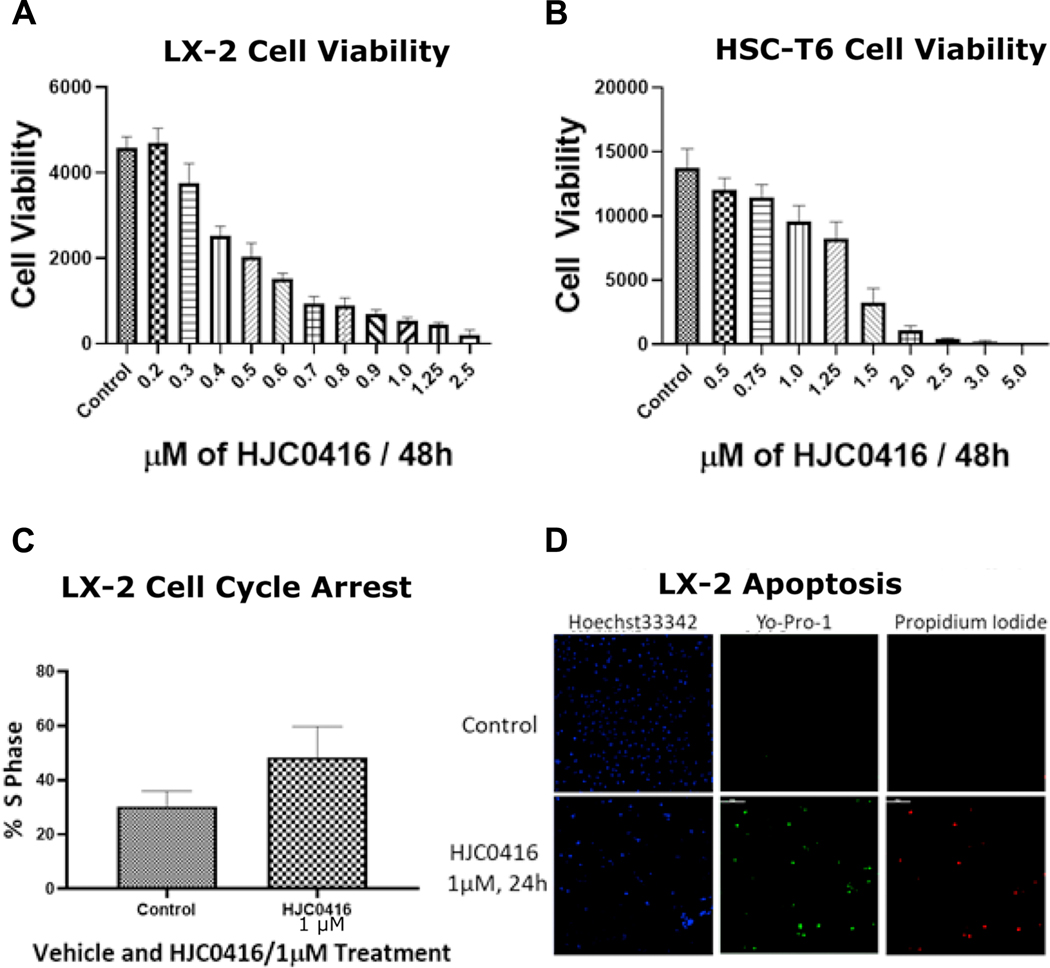
HJC0416 decreased LX-2 cell viability in a dose-dependent manner (Figure 1A) with an IC50 of 0.43 μM. HJC0416 decreased HSC-T6 cell viability in a dose-dependent manner (Figure 1B) with an IC50 of 1.3 μM.
Figure 1:
HJC0416 inhibits LX-2 and HSC-T6 cell viability and causes cell cycle arrest
(A) LX-2 and (B) HSC-T6 cells were treated with a series of concentrations of HJC0416 for 48 hours, and cell viability was determined using alamar blue assay. (C) HJC0416 induced cell cycle arrest at the S phase in LX-2 cells. (D) HJC0416 decreased Hoechst dsDNA staining, and increased staining for Yo-Pro-1 and Propidium Iodide, markers of apoptosis. The results are representative of at least three different experiments.
HJC0416 induced cell cycle arrest at the S phase in LX-2 cells (30.3% ± 0.6% vs. 48.5 % ± 1.25%, p=0.01, Figure 1C). HJC0416 induced apoptosis in LX-2 cells; decreases were seen in Hoescht33342, a dsDNA stain, (12.7 ± 0.4 vs. 9.4 ± 0.2, p<0.001) while Yo-Pro-1 and propidium iodide, a markers of apoptosis, were increased (2.1 ± 0.09 vs. 4.6 ± 0.3, p<0.001; 0.006 ± 0.003 vs. 0.25 ± 0.1, p<0.001, Figure 1D).
HJC0416 decreased extracellular matrix protein expression and α-SMA expression
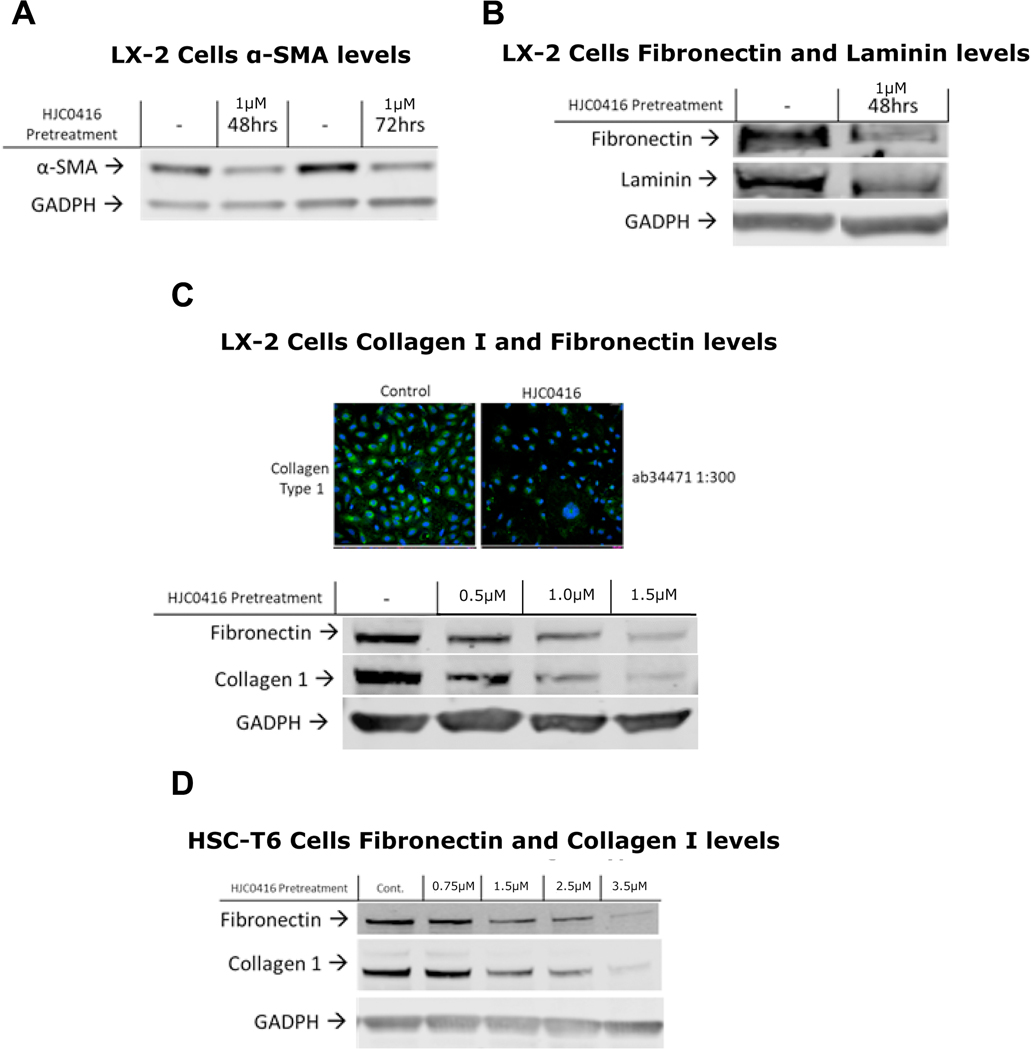
Next we examined ECM proteins as a surrogate for HSC activation. α-SMA, a key marker of HSC activation, was markedly decreased after HJC0416 administration (Figure 2A). Treatment with HJC0416 demonstrated a significant decrease in fibronectin (Figures 2B, 2C, 2D) and collagen I (Figures 2C, 2D) in both LX-2 and HSC-T6 cells. Additionally, laminin was significantly decreased in LX-2 cells after HJC0416 administration (Figure 2B). Immunofluorescence staining for collagen I confirmed significant decrease in collagen I expression (Figure 2C).
Figure 2:
HJC0416 decreased extracellular matrix protein expression and α-SMA expression
(A) HJC0416 decreases α-SMA expression in LX-2 cells after 48 and 72 hours. (B) HJC0416 decreases fibronectin and laminin expression in LX-2 cells after 48 hours. (C) HJC0416 decreases collagen I immunofluorescence and decreases fibronectin and collagen I in LX-2 cells in a dose-dependent manner. (D) HJC0416 decreases fibronectin and collagen I in HSC-T6 cells in a dose-dependent manner. The results are representative of at least three different experiments.
HJC0416 inhibited the STAT3 pathway
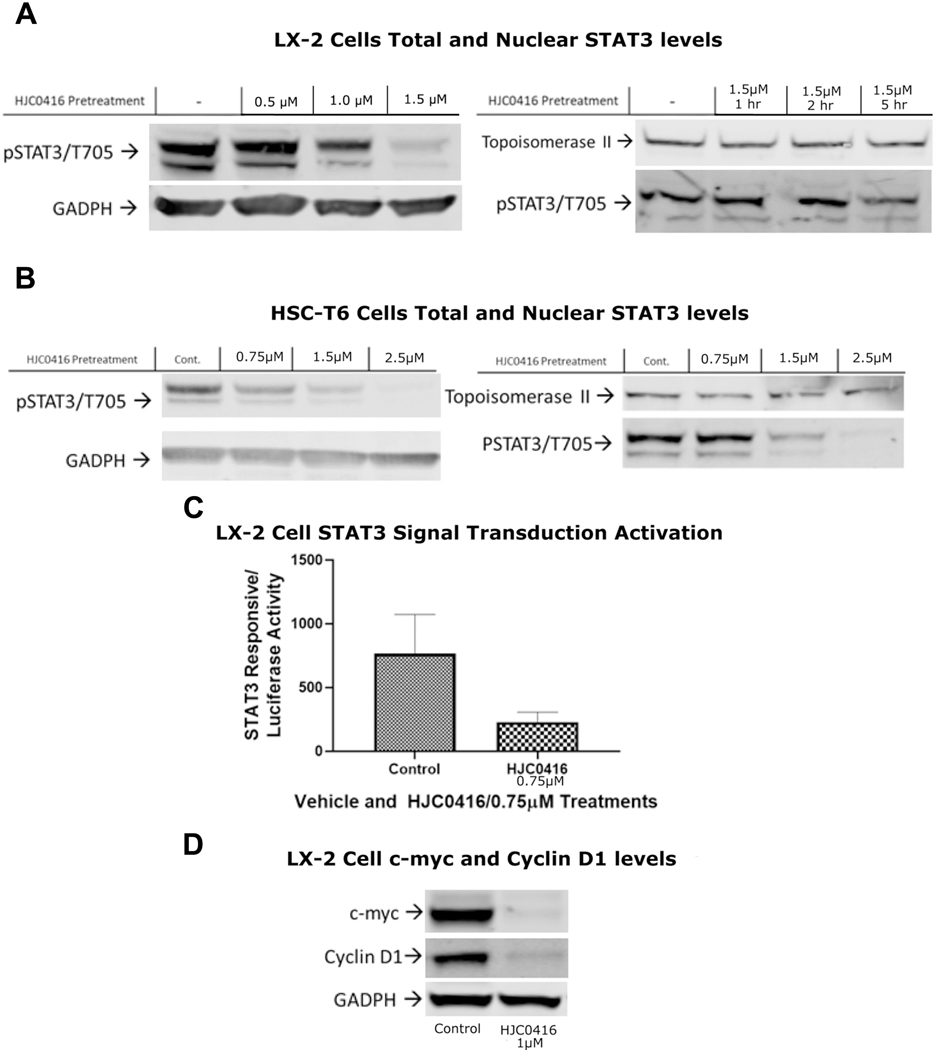
HJC0416 was designed to be a STAT3 pathway inhibitor and we confirmed its effects on the STAT3 pathway in activated HSCs. HJC0416 decreased phosphorylated STAT3 (Tyrosine 705) in LX-2 and HSC-T6 total cell lysate expression in a dose dependent manner, with an up to 20-fold decrease in expression (Figures 3A, 3B). HJC0416 decreased the nuclear expression of STAT3 in LX-2 cells by 2-fold and in HSC-T6 cells by up to 20-fold (Figures 3A, 3B). STAT3 signal transduction pathway activation had a significant decrease in after HJC0416 treatment (Figure 3C). Downstream target of STAT3, cyclin D1, was decreased in LX-2 cells after HJC0416 administration (Figure 3D). Similarly, another downstream target of STAT3, c-myc, is decreased in LX-2 cells after HJC0416 administration. (Figure 3D).
Figure 3:
HJC0416 inhibited the STAT3 pathway
(A) HJC0416 decreased total and nuclear levels of phosphorylated STAT3 in LX-2 cells. (B) HJC0416 decreased total and nuclear levels of phosphorylated STAT3 in HSC-T6 cells. (C) HJC0416 decreases STAT3 signal transduction pathway activation. (D) STAT3 downstream targets c-myc and cyclin D1 expression are decreased after HJC0416 treatment. The results are representative of at least three different experiments.
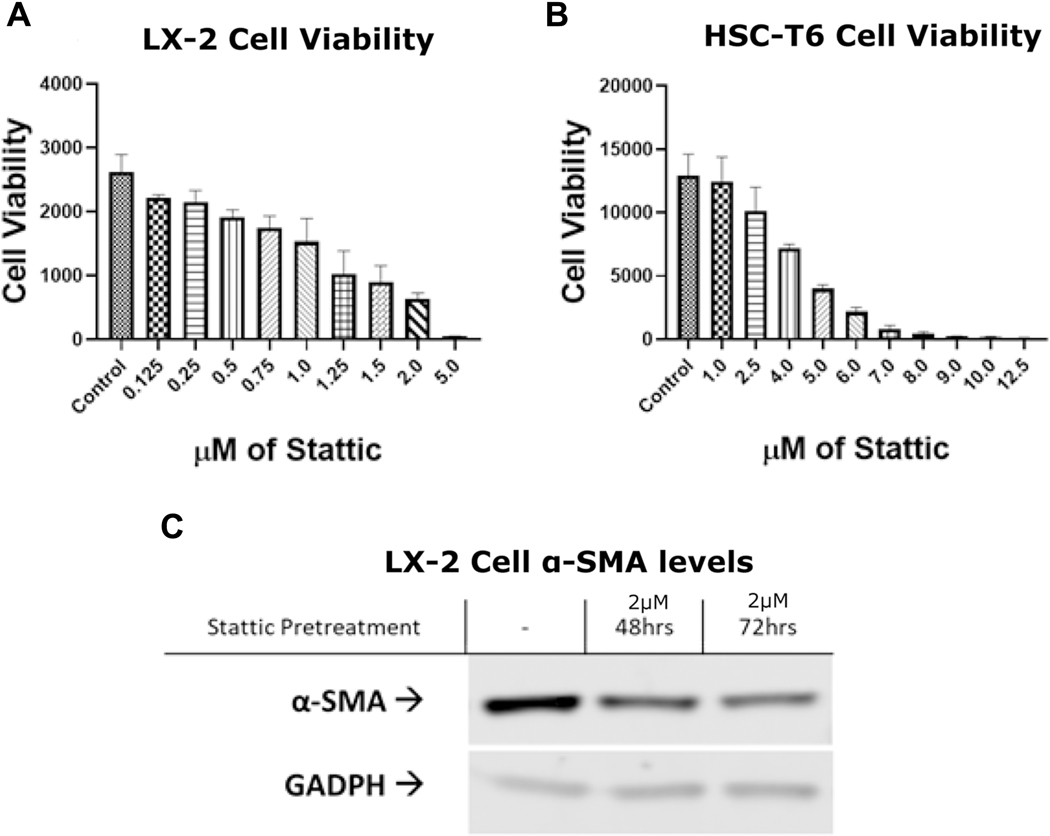
To confirm the role of STAT3 pathway in activated HSC fibrogenesis, we used commercially available STAT3 specific inhibitor Stattic. Stattic demonstrated similar decreases in cell viability in Alamar Blue assays in the LX-2 cell line (Figure 4A) and the HSC-T6 cell line (Figure 4B). Stattic also demonstrated a marked decrease in α-SMA expression (Figure 4C).
Figure 4:
Chemical inhibitor Stattic confirms the role of STAT3 pathway in activated HSCs
(A) LX-2 and (B) HSC-T6 cells were treated with a series of concentrations of Stattic and cell viabilitywas determined using alamar blue assay. (C) Stattic decreases α-SMA expression in LX-2 cells after 48 and 72 hours. The results are representative of at least three different experiments.
HJC0416 inhibited pro-inflammatory cytokines and the NF-κB pathway
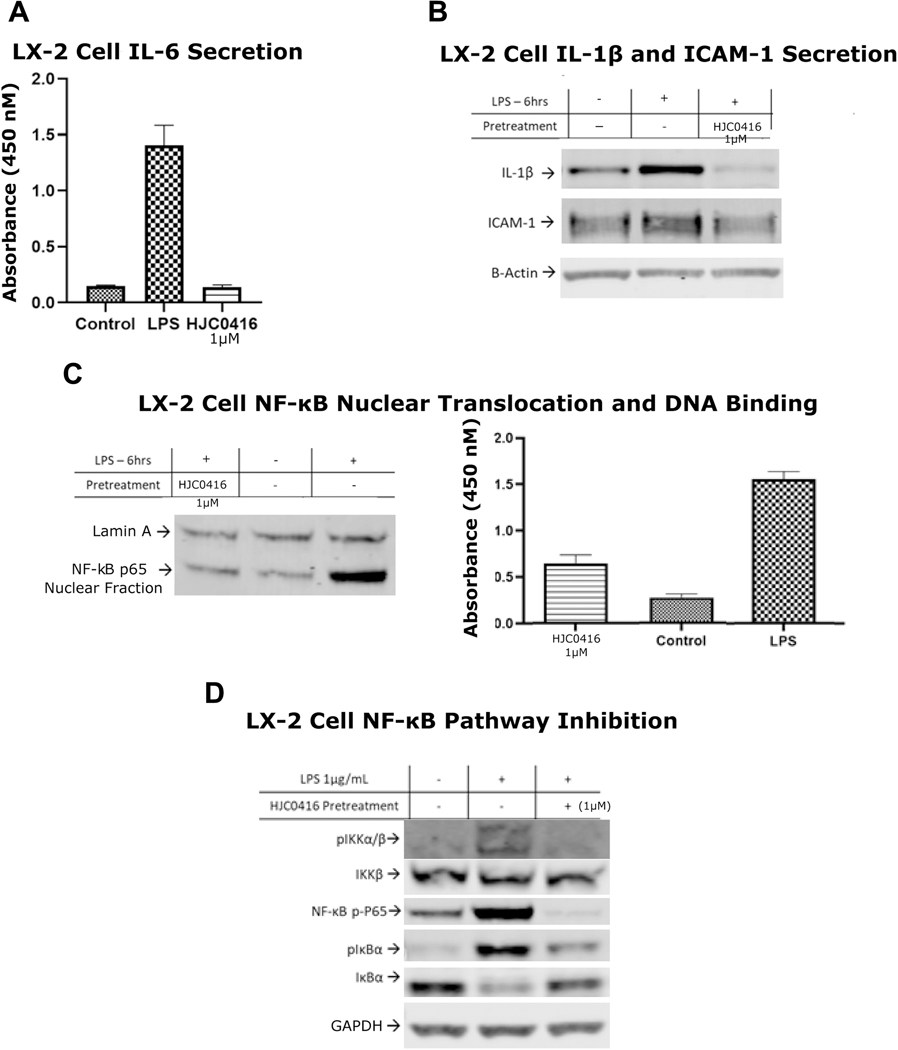
Hepatic fibrosis is tied to inflammatory signaling, therefore we sought to test the role of HJC0416 on inflammation. LPS-stimulated IL-6 secretion in LX-2 cells demonstrated a 10-fold decrease after HJC0416 treatment (Figure 5A). HJC0416 also decreased LPS-stimulated expression of IL-1ß and ICAM-1 (Figure 5B).
Figure 5:
HJC0416 inhibited pro-inflammatory cytokines and the NF-κB pathway
(B) ELISA for (A) IL-6 antibodies demonstrates that HJC0416 significantly inhibits LPS-induced expression. (B) IL-1ß and ICAM-1 expression is decreased after treatment with HJC0416. (C) HJC0416 decreases LPS-induced NF-κB nuclear translocation and DNA binding. (D) HJC0416 decreases LPS-induced NF-κB pathway activation. The results are representative of at least three different experiments.
NF-κB pathway activation is known to regulate these pro-inflammatory cytokines, and we hypothesized that HJC0416 may affect the NF-κB pathway in addition to the STAT3 pathway. HJC0416 prevented the phosphorylated and degradation of NF-κB inhibitory protein IκBα, and decreased phosphorylation of IκBα kinase IKKα/ß (Figure 5D). HJC0416 significantly inhibited LPS-induced NF-κB p65 nuclear translocation and DNA binding activity in LX-2 cells (Figure 5C). Additionally, HJC0416 prevented the alternative pathway of NF-κB activation by significantly decreasing Serine 536 residue phosphorylation of p65 (Figure 5D).
Discussion
Our results demonstrate that HJC0416 is a novel inhibitor of both the STAT3 and NF-κB pathways with potential for in vivo testing as an anti-fibrogenic agent. HJC0416 induced apoptosis as well as decreased cell viability in two activated HSC cell lines. HJC0416 attenuated the fibrogenic activity of activated HSCs by decreasing expression of fibrosis-related ECM proteins collagen I, fibronectin, and laminin as well as myofibroblast marker α-SMA. We confirmed that HJC0416 inhibited the STAT3 pathway by measuring the levels of total phosphorylated STAT3 and nuclear STAT3 expression, as well as STAT3 downstream targets c-myc and cyclin D1. The role of the STAT3 was confirmed by a chemical STAT3 inhibitor, Stattic, which decreased cell viability and α-SMA levels. HJC0416 also inhibited hepatic inflammation by inhibiting the classic and alternative pathways of NF-κB activation as well as NF-κB regulated pro-inflammatory cytokines IL-6, IL-1ß, and ICAM-1. HJC0416 acts as both an anti-fibrotic and anti-inflammatory agent through dual inhibition of the STAT3 and NF-κB pathways. This is unique to its family of STAT3 inhibitors and makes HJC0416 a particularly attractive agent for in vivo testing as a treatment for hepatic fibrosis.
When activated, HSCs deposit excess ECM in the liver parenchyma. The ECM is a hydrated gel which is composed of collagens, elastins, fibronectins, laminins, and proteoglycans.24 Previous studies have demonstrated that apoptosis of HSCs accelerates recovery from hepatic fibrosis.17 Our study demonstrates that HJC0416 decreases cell viability in both activated HSC lines, as well as increases cell-cycle arrest and apoptosis, which could possibly represent induction of senescence. The senescent form of activated HSCs is characterized by decreased cellular mitosis and increased apoptosis.25 While it is unclear why HSCs eventually enter senescence, senescence of activated HSCs provides a barrier which limits liver fibrosis.26
Senescent activated HSCs also downregulate genes encoding ECM components and upregulate ECM-degrading enzymes, such as matrix metalloproteinases.26 Our results demonstrated that HJC0416 administration resulted in decreased expression of ECM proteins. Collagen I, fibronectin, and laminin are all key ECM proteins expressed by activated HSCs.7–11 In addition, treatment with HJC0416 resulted in decreased expression of α-SMA. α-SMA is a surrogate marker of HSC activation.27 Attenuation of these key proteins establishes the anti-fibrotic properties of HJC0416.
STAT3 is an important factor in HSC activation and represents a therapeutic target for anti-fibrotic treatment.14,28,29 HJC0416 treatment resulted in inhibition of the STAT3 pathway. Typically, the STAT3 pathway is activated by phosphorylation of the tyrosine 705 residue of STAT3.30 However, dimers of non-phosphorylated STAT3 can also exist and be active and phosphorylation of other sites such as Serine 727 can result in STAT3 transcriptional activity.31,32 HJC0416 inhibits both phosphorylation of the tyrosine 705 residue of STAT3 as well as decreases STAT3 nuclear translocation, theoretically blocking multiple pathways of activation. Additionally, HJC0416 decreases expression of STAT3 regulated genes c-myc and cyclin D1. Downregulation of cyclin D1 is associated with G1 phase arrest and could be contributing to the increased apoptosis and cell cycle arrest seen after HJC0416 administration.33,34
HJC0416 also inhibited pro-inflammatory cytokine expression and the NF-κB pathway, effects which are unique to it within its family of STAT3 inhibitors.20–23 HJC0416 decreased expression of Il-6, IL-1ß, and ICAM-1. IL-6 is a driving force of hepatic fibrogenesis due to its activation of the STAT3 pathway as well as its role in a positive feedback circuit which lead to further activation of HSCs.29 IL-1ß has been implicated in the transformation of steatosis to steatohepatitis and liver fibrosis.35–37 ICAM-1 expression has been linked to HSC activation, hepatic inflammation and hepatic fibrogenesis.38 Since IL-6, IL-1ß and ICAM-1 are NF-κB regulated genes, we then sought to further define the molecular mechanism by which HJC0416 exerted its anti-inflammatory effects. HJC0416 decreased the phosphorylation and activation of IKKα/ß, the kinase responsible for the phosphorylation and degradation of NF-κB inhibitory protein IκBα. Similarly, HJC0416 prevented phosphorylation and degradation of IκBα as well as NF-κB p65 DNA binding activity. Finally, HJC0416 inhibited the alternative pathway of NF-κB activation by attenuating phosphorylation of Serine 536 on subunit p65. Unlike the phosphorylation of IKKα/ß, which results in equal expression of all NF-κB associated genes, activation through p65 phosphorylation has gene-specific effects. Phosphorylation of p65 is the result of at least 5 different kinases and is also involved in the recruitment of components of the basal transcriptional machinery.39,40
The future direction of our work will include in vivo testing of HJC0416 in a murine hepatic fibrosis model. While we cannot predict our future results, other studies have demonstrated that in vivo inhibition of the STAT3 and NF-κB pathways ameliorates hepatic fibrosis. IL-22, an anti-inflammatory cytokine, has been shown to induce cellular senescence in a murine model of hepatic fibrosis via the STAT3 pathway.41 STAT3 modulation additionally has a theoretical benefit to modulating hepatic fibrosis due its activation by the pro-inflammatory cytokines which elicit fibrogenic cell behavior in HSCs.42–44 Similalry, the NF-κB pathway has been shown to both inhibit fibrogenesis in in vivo models as well as have a theoretical anti-fibrotic benefit.45 In addition, NF- κB target genes, such as IL-6, activate the STAT3 pathway, allowing for cross-talk between these two pathways.46,47,48
Conclusions
In activated human and rat HSCs, HJC0416 has demonstrated a significant ability to inhibit hepatic fibrogenesis. While HJC0416 was designed for STAT3 inhibition, it additionally demonstrates the ability to inhibit the NF-κB pathway. This dual effect makes HJC0416 a promising future candidate for in vivo testing as an anti-fibrosis agent.
Acknowledgments
Christian Sommerhalder and Claire B. Cummins were supported by a training grant from the National Institutes of Health (T32 GM008256). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Povero D, Busletta C, Novo E, et al. Liver fibrosis: a dynamic and potentially reversible process. Histol Histopathol. 2010;25(8):1075–1091. [DOI] [PubMed] [Google Scholar]
- 4.Cummins CB, Wang X, Xu J, et al. Antifibrosis Effect of Novel Oridonin Analog CYD0618 Via Suppression of the NF-kappaB Pathway. J Surg Res. 2018;232:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123(5):1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alter G, Heckerman D, Schneidewind A, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476(7358):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832(7):948–954. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wang Z, Kwong SQ, et al. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55(3):612–625. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Seo GS, Park YN, Yoo TM, Sohn DH. Effects and regulation of osteopontin in rat hepatic stellate cells. Biochem Pharmacol. 2004;68(12):2367–2378. [DOI] [PubMed] [Google Scholar]
- 10.Ramm GA, Shepherd RW, Hoskins AC, et al. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology. 2009;49(2):533–544. [DOI] [PubMed] [Google Scholar]
- 11.Fang S, Yuan J, Shi Q, et al. Downregulation of UBC9 promotes apoptosis of activated human LX-2 hepatic stellate cells by suppressing the canonical NF-kappaB signaling pathway. PLoS One. 2017;12(3):e0174374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang JX, Mikami K, Venugopal S, Li Y, Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51(1):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parola M, Marra F. Adipokines and redox signaling: impact on fatty liver disease. Antioxid Redox Signal. 2011;15(2):461–483. [DOI] [PubMed] [Google Scholar]
- 14.Su TH, Shiau CW, Jao P, et al. Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc Natl Acad Sci U S A. 2015;112(23):7243–7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins CB, Wang X, Nunez Lopez O, et al. Luteolin-Mediated Inhibition of Hepatic Stellate Cell Activation via Suppression of the STAT3 Pathway. Int J Mol Sci. 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunez Lopez O, Bohanon FJ, Wang X, et al. STAT3 Inhibition Suppresses Hepatic Stellate Cell Fibrogenesis: HJC0123, a Potential Therapeutic Agent for Liver Fibrosis. RSC Adv. 2016;6(102):100652–100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10(5):927–939. [DOI] [PubMed] [Google Scholar]
- 18.Leach AR, Hann MM. Molecular complexity and fragment-based drug discovery: ten years on. Curr Opin Chem Biol. 2011;15(4):489–496. [DOI] [PubMed] [Google Scholar]
- 19.Erlanson DA. Fragment-based lead discovery: a chemical update. Curr Opin Biotechnol. 2006;17(6):643–652. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Yang Z, Ding C, et al. Fragment-based drug design and identification of HJC0123, a novel orally bioavailable STAT3 inhibitor for cancer therapy. Eur J Med Chem. 2013;62:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Yang Z, Ding C, et al. Discovery of potent anticancer agent HJC0416, an orally bioavailable small molecule inhibitor of signal transducer and activator of transcription 3 (STAT3). Eur J Med Chem. 2014;82:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Wu M, Xu Z, et al. HJC0152, a novel STAT3 inhibitor with promising anti-tumor effect in gastric cancer. Cancer Manag Res. 2018;10:6857–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Li H, Wu X, et al. HJC0152 suppresses human non-small-cell lung cancer by inhibiting STAT3 and modulating metabolism. Cell Prolif. 2020:e12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018;68–69:452–462. [DOI] [PubMed] [Google Scholar]
- 25.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37(3):653–664. [DOI] [PubMed] [Google Scholar]
- 26.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpino G, Franchitto A, Morini S, Corradini SG, Merli M, Gaudio E. Activated hepatic stellate cells in liver cirrhosis. A morphologic and morphometrical study. Ital J Anat Embryol. 2004;109(4):225–238. [PubMed] [Google Scholar]
- 28.Kagan P, Sultan M, Tachlytski I, Safran M, Ben-Ari Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS One. 2017;12(5):e0176173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang DM, Sun W, Ning BF, et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67(9):1704–1715. [DOI] [PubMed] [Google Scholar]
- 30.Wake MS, Watson CJ. STAT3 the oncogene - still eluding therapy? FEBS J. 2015;282(14):2600–2611. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Iness A, Yoon J, et al. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn’s disease. J Immunol. 2015;194(7):3422–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Qiu J, Dong S, et al. Stat3 isoforms, alpha and beta, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3beta mapping to its unique C-terminal end. J Biol Chem. 2007;282(48):34958–34967. [DOI] [PubMed] [Google Scholar]
- 33.Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. 2014;44(4):1032–1040. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Wang J, Wu M, Zhang X, Zhang H. The inhibition of activated hepatic stellate cells proliferation by arctigenin through G0/G1 phase cell cycle arrest: persistent p27(Kip1) induction by interfering with PI3K/Akt/FOXO3a signaling pathway. Eur J Pharmacol. 2015;747:71–87. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64(3):955–965. [DOI] [PubMed] [Google Scholar]
- 36.Kamari Y, Shaish A, Vax E, et al. Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55(5):1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier RPH, Meyer J, Montanari E, et al. Interleukin-1 Receptor Antagonist Modulates Liver Inflammation and Fibrosis in Mice in a Model-Dependent Manner. Int J Mol Sci. 2019;20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellerbrand Wang SC, Tsukamoto H, Brenner DA, Rippe RA. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24(3):670–676. [DOI] [PubMed] [Google Scholar]
- 39.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279(53):55633–55643. [DOI] [PubMed] [Google Scholar]
- 40.Hochrainer K, Racchumi G, Anrather J. Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential NF-kappaB and RNA polymerase II promoter recruitment. J Biol Chem. 2013;288(1):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong X, Feng D, Wang H, et al. Interleukin-22 Induces Hepatic Stellate Cell Senescence and Restricts Liver Fibrosis. Hepatology. 2012;56(3):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casini A, Ceni E, Salzano R, et al. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology. 1997;25(2):361–367. [DOI] [PubMed] [Google Scholar]
- 43.Canbay A, Friedman S, Gores GJ. Apoptosis: The Nexus of Liver Injury and Fibrosis. Hepatology. 2004;39(2):273–278. [DOI] [PubMed] [Google Scholar]
- 44.Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21(3):417–426. [DOI] [PubMed] [Google Scholar]
- 45.Luedde T, Schwabe RF. NF- κB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2)108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 on immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. [DOI] [PubMed] [Google Scholar]
- 48.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:793–506. [DOI] [PMC free article] [PubMed] [Google Scholar]