This editorial refers to ‘Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR’, by J. Escaned et al., http://dx.doi.org/10.1093/eurheartj/ehab702.
Graphical Abstract.
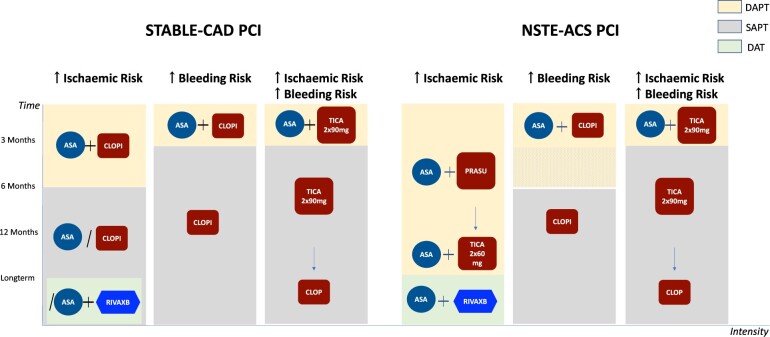
Antithrombotic strategies after PCI according to the risk stratification.* ASA, aspirin; ACS, acute coronary syndrome; CAD, coronary artery disease; CLOPI, clopidogrel; DAPT, dual antiplatelet therapy; DAT, dual antithrombotic therapy; PCI, percutaneous coronary intervention; PRASU, prasugrel; RIVAXB, rivaroxaban; SAP, single antiplatelet therapy; TICA, ticagrelor. *The authors’ suggestion.
To win without risk is to triumph without glory.
–Le Cid, Pierre Corneille, 1636
The journey from monotherapy to dual therapy
In contrast to primary prevention,1,2 the status of aspirin in the treatment of vascular diseases caused by atherothrombosis is unquestionable and unshakable. In addition to its undeniable efficiency, its affordability is a significant extra benefit. However, preventing thrombosis after iatrogenic disruption of the inner surface of a vessel wall by implantation of an intracoronary stent needs more than the antithrombotic efficacy of aspirin.
Many advances in coronary intervention were made possible by extending single antiplatelet therapy (APT) to dual antiplatelet therapy (DAPT), which was done best by adding a P2Y12 receptor inhibitor. Inhibition of platelet haemostatic function by suppressing the two pathways of platelet activation, subsequent platelet adhesion, and aggregation decreases the risk of thrombotic events but increases simultaneously the risk of bleeding, whether spontaneous or iatrogenic.
The availability of P2Y12 inhibitors with different potency, onset of action, doses, and route of administration allows APT to be adjusted according to the risk profile of individuals and the acuity of the situation. DAPT antithrombotic protection can be modulated, i.e. escalated or de-escalated using different drugs or doses of these drugs but also by extending or reducing the duration of DAPT.
Aspirin has been perceived as the cornerstone antithrombotic therapy. If it is necessary to discontinue DAPT prematurely due to bleeding risk, the most common path forward is to continue with aspirin monotherapy, or switch to clopidogrel monotherapy, which may be associated with a lower bleeding risk.3,4 In the absence of high bleeding risk (HBR), aspirin has reinforced its position with a new possible strategy combining a low dose of rivaroxaban with a low dose of aspirin to better control the ischaemic risk.
The full antithrombotic effect of aspirin, which is to inhibit cyclooxygenase-1 and suppress thromboxane A2 production, is achieved at low doses. Increasing the dose of aspirin leads to inhibition of cyclooxygenase-2 and suppression of prostacyclin synthesis with proven inhibition of platelet aggregation and vasodilatory properties. Therefore, while providing little (if any) additional antiplatelet effect at high doses, aspirin may induce a prothrombotic state that adversely affects the cardiovascular system and can cause side effects such as gastric mucosa irritation or renal toxicity.5 The risk of severe gastrointestinal bleeding or, more rarely, intracranial haemorrhage may occur at any dose; however, the risk of bleeding from a peptic ulcer increases with the dose of aspirin.
P2Y12 inhibitors prevent platelet activation via ADP, and, like aspirin, they also reduce the aggregatory response of platelets to thromboxane A2 and sensitize platelets to endogenous prostacyclin, which further inhibits platelet activation.
Pharmacodynamic studies performed in patients after percutaneous coronary intervention (PCI) showed that overall thrombogenicity is substantially and similarly suppressed by ticagrelor monotherapy or combined therapy using ticagrelor plus aspirin. The aspirin’s role in inhibiting platelet aggregation was measured in patients receiving a potent P2Y12 inhibitor.6,7 This suggests that ticagrelor monotherapy may not be universally applicable to patients at high ischaemic risk, but could be a strategy for those at HBR.
The challenge of double risk stratification
The first difficulty is that predictors of ischaemic and bleeding risk evolve continuously throughout the life of individual patients. The second difficulty is that patients in the prothrombotic state are often at HBR, e.g. patients with diabetes, nephropathy, and anaemia.8 These patients frequently undergo PCI because cardiovascular disease is the most common cause of death in these situations.
As cancer survivability increases, close to 1 in 10 patients undergoing PCI now have a cancer diagnosis.9 Many patients also undergo PCI with unrecognized malignancies. The risk of stent thrombosis is much higher in patients with active cancer, and these patients may develop anaemia or need surgery while on DAPT after stent implantation. PCI in patients with cancer is a good example where ischaemic and bleeding risks are high, requiring individualization of antithrombotic therapy, with challenging decisions to be taken.
Another challenge is age, as the number of risk factors and the general complexity of PCI patients increases with age. At the same time, an exponential trend in the comorbidity index has been noted and associated with the increasing prevalence of atrial fibrillation and anaemia.10 All of these factors have made the management of long-term antiplatelet/antithrombotic therapy post-PCI more demanding.
A critical step in preventing treatment adjustment due to adverse events [major adverse cardiovascular events (MACE)/bleeding] is choosing the right antithrombotic strategy immediately after PCI. The choice must be based on the patient’s clinical and angiographic (i.e. the extent of coronary artery disease, lesion/lesions characteristics, and coronary intervention procedural results) profile. The risk of an early recurrent ischaemic event is higher in shock, acute coronary syndrome (ACS), diabetic, or multivessel disease patients. In these situations, there is, in general, a benefit of escalating DAPT using more potent P2Y12 inhibitors.
Patients at HBR are usually excluded or under-represented in clinical trials evaluating APT in PCI. Management of antithrombotic therapy in patients at HBR (and especially if simultaneously at high thrombotic risk) has represented a difficult challenge evaluated now in multiple trials with varying strategies. Prerequisites for generalizable evidence from randomized studies are methodological requirements and, for example, to have consensual definitions of bleeding events and bleeding risk, and consistency across trial designs.
P2Y12 inhibitor monotherapy in high bleeding risk
The TWILIGHT trial tested the safety of DAPT de-escalation to ticagrelor monotherapy starting after an adverse event-free 3 month period following PCI in patients at high thrombotic and/or bleeding risk according to a list of clinical and angiographic criteria. Patients on oral anticoagulation, with a platelet count <100 000 mm3, on dialysis, with a prior stroke, or with an ‘extreme risk’ for major bleeding, as well as those undergoing primary or salvage PCI were excluded, representing, however, the most serious potential bleeders in whom the management is the most difficult. In this issue of the European Heart Journal published the TWILIGHT-HBR pre-specified subgroup analysis of patients with at least a 4% risk of BARC bleeding 3 (= overt bleeding plus hemoglobin drop at least 3 g per dL, or cardiac tamponade, or intraocular bleed compromising vision, or need of any transfusion, intravenous vasoactive agents, surgical intervention to stop bleeding) or 5 (fatal bleeding) or at least a 1% risk of intracranial bleeding within 12 months after the index PCI is adding consistency in the challenge of selecting an antiplatelet therapy strategy in patients well defined for their HBR.11
In ARC-HBR patients, consistent with the main study results, ticagrelor monotherapy compared with DAPT lowered the risk of clinically relevant BARC bleeding [hazard ratio (HR) 0.53, 95% confidence interval (CI) 0.35–0.82] without increasing the occurrence of major thrombotic events (death, myocardial infarction, or stroke) (HR 1.16, 95% CI 0.71–1.90). The TWILLIGHT-HBR analysis was underpowered to detect clinically relevant differences in ischaemic events, and findings must be seen as hypothesis-generating.
ARC-HBR patients experienced higher bleeding rates and ischaemic events than non-HBR patients and there was a progressive increase in the risk of bleeding complications relative to the number of HBR criteria present, thus validating the ARC-HBR criteria within TWILIGHT. The relative effect of ticagrelor monotherapy was similar in both HBR and non-HBR patients, confirming the global TWILIGHT results in these two subsets of the population. The absolute reduction in major bleeding complications associated with ticagrelor monotherapy was more pronounced in ARC-HBR vs. non-HBR patients. The Kaplan–Meier curves showing the benefit of bleeding reduction using ticagrelor monotherapy over standard DAPT had a steeply divergent trend relative to time since PCI in the ARC-HBR group.
However, in the TWILIGHT study, the approach involves paradoxically a de-escalation strategy to ticagrelor monotherapy for chronic therapy following an escalation with the initial 3 months of DAPT combining ticagrelor and aspirin, in high-risk but stable or stabilized patients. Recently, the ALPHEUS study12 showed that ticagrelor-based DAPT was not superior to clopidogrel-based DAPT in reducing periprocedural myocardial infarction or myocardial injury within 48 h of high-risk PCI in stable patients without HBR; and there was an increase in the risk of minor bleeding at 30 days.
Unresolved issues
The TWILIGHT-HBR analysis raises new questions in the field of antithrombotic therapy management. The choice of ticagrelor monotherapy after PCI in the non-ST segement elevation ACS setting is not consistent with the current ESC guidelines, where the duration of DAPT is longer except in the case of HBR and where a prasugrel-based strategy is preferred due to its better efficacy/safety profile. How should we deal with long-term (>15 months) APT after PCI? Should we continue with ticagrelor monotherapy, or rather switch to clopidogrel?4 or aspirin? Or even aspirin plus rivaroxaban? Before deciding, it is essential that the patients’ risk profile is re-evaluated (Graphical Abstract). Long-term treatment with ticagrelor in a PCI population with heterogeneous risks has not shown clear benefits over standard of care.13 There is also an adherence issue related to the dyspnoea side effect of ticagrelor which manifests in almost a quarter of treated patients. It should also be emphasized that carefully considering the patient’s preference, whose compliance with treatment fundamentally affects its benefits, is crucial when deciding on the long-term treatment plan.14
Funding
This paper was supported by the Cardiovascular Research Program of the Charles University ‘Progres Q38’.
Conflict of interest: Z.M. reports consulting or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Amgen, and Pfizer. G.M. reports consulting or speaker fees from Abbott, AIM Group, Amgen, Actelion, American College of Cardiology Foundation, AstraZeneca, Axis-Santé, Bayer, Boston Scientific, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Fréquence Médicale, ICOM, Idorsia, Elsevier, Fédération Française de Cardiologie, Fréquence Médicale, ICAN, Lead-Up, Menarini, Medtronic, MSD, Novo Nordisk, Pfizer, Quantum Genomics, Sanofi Aventis, SCOR Global Life, Servier, and WebMD.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Cleland JG. Is aspirin useful in primary prevention? Eur Heart J 2013;34:3412–3418. [DOI] [PubMed] [Google Scholar]
- 2. Raber I, McCarthy CP, Vaduganathan M, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 2019;393:2155–2167. [DOI] [PubMed] [Google Scholar]
- 3. Committee CAPRIE Steering. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 4. Koo BK, Kang J, Park KW, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021;397:2487–2496. [DOI] [PubMed] [Google Scholar]
- 5. Warner TD, Nylander S, Whatling C.. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol 2011;72:619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baber U, Zafar MU, Dangas G, et al. Ticagrelor with or without aspirin after PCI: the TWILIGHT platelet substudy. J Am Coll Cardiol 2020;75:578–586. [DOI] [PubMed] [Google Scholar]
- 7. Johnson TW, Baos S, Collet L, et al. Pharmacodynamic comparison of ticagrelor monotherapy versus ticagrelor and aspirin in patients after percutaneous coronary intervention: the TEMPLATE (Ticagrelor Monotherapy and Platelet Reactivity) randomized controlled trial. J Am Heart Assoc 2020;9:e016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stauffer ME, Fan T.. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014;9:e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potts JE, Iliescu CA, Lopez Mattei JC, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J 2019;40:1790–1800. [DOI] [PubMed] [Google Scholar]
- 10. Alkhouli M, Alqahtani F, Kalra A, et al. Trends in characteristics and outcomes of hospital inpatients undergoing coronary revascularization in the United States, 2003–2016. JAMA Netw Open 2020;3:e1921326. [DOI] [PubMed] [Google Scholar]
- 11. Escaned J, Cao D, Baber U, et al. Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR. Eur Heart J 2021;42:doi:10.1093/eurheartj/ehab702. [DOI] [PubMed] [Google Scholar]
- 12. Silvain J, Lattuca B, Beygui F, et al.; ALPHEUS investigators. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet 2020;396:1737–1744. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt DL. Aspirin—still the GLOBAL LEADER in antiplatelet therapy. Lancet 2018;392:896–897. [DOI] [PubMed] [Google Scholar]
- 14. Motovska Z. Stent thrombosis after ACS-PCI: does adherence to antiplatelet therapy involve more than its intensity? Rev Esp Cardiol 2019;7:282–284. [DOI] [PubMed] [Google Scholar]