ABSTRACT
Background
Intake of a single meal containing herbs and spices attenuates postprandial lipemia, hyperglycemia, and oxidative stress, and improves endothelial function. There has been limited investigation of the effect of longer-term intake of mixed herbs and spices on risk factors for cardiometabolic diseases.
Objectives
The objective was to assess the effect of an average American diet containing herbs and spices at 0.5 (low-spice diet; LSD), 3.3 (moderate-spice diet; MSD), and 6.6 (high-spice diet; HSD) g · d−1 · 2100 kcal−1 on lipids and lipoproteins as well as other risk factors for cardiometabolic diseases in at-risk adults.
Methods
A 3-period, randomized, crossover, controlled-feeding study with 71 participants was conducted at the Pennsylvania State University. Each diet was consumed for 4 wk with a minimum 2-wk washout period. Outcomes were assessed at baseline and the end of each diet period.
Results
No between-diet effects were observed for LDL cholesterol, the primary outcome. Between-diet differences were observed for mean 24-h systolic (P = 0.02) and diastolic (P = 0.005) ambulatory blood pressure. The HSD lowered mean 24-h systolic blood pressure compared with the MSD (−1.9 mm Hg; 95% CI: −3.6, −0.2 mm Hg; P = 0.02); the difference between the HSD and LSD was not statistically significant (−1.6 mm Hg; 95% CI: −3.3, 0.04 mm Hg; P = 0.058). The HSD lowered mean 24-h diastolic blood pressure compared with the LSD (−1.5 mm Hg; 95% CI: −2.5, −0.4 mm Hg; P = 0.003). No differences were detected between the LSD and MSD. No between-diet effects were observed for clinic-measured blood pressure, markers of glycemia, or vascular function.
Conclusions
In the context of a suboptimal US-style diet, addition of a relatively high culinary dosage of mixed herbs and spices (6.6 g · d−1 · 2100 kcal−1) tended to improve 24-h blood pressure after 4 wk, compared with lower dosages (0.5 and 3.3 g · d−1 · 2100 kcal−1), in adults at elevated risk of cardiometabolic diseases.
This trial was registered at clinicaltrials.gov as NCT03064932.
Keywords: herbs, spices, lipids/lipoproteins, blood pressure, cardiovascular disease, pulse wave velocity, flow-mediated dilation, controlled feeding study
Introduction
Cardiometabolic diseases, including cardiovascular disease (CVD), stroke, and type 2 diabetes, are leading contributors to the disease burden globally and in the United States (1). Dyslipidemia (particularly elevated LDL cholesterol), hypertension, and dysglycemia are the primary targets for prevention of cardiometabolic diseases (2–5). Previous research has shown incorporation of herbs and spices into a single meal attenuates postprandial lipemia, hyperglycemia, and oxidative stress, and improves endothelial function (6–12). Because humans spend ∼18 h/d in the postprandial state, repeated exposure to herbs and spices at meal/snack times may improve postprandial metabolic responses, which may lower cardiometabolic disease risk. However, no randomized controlled trials have examined the effect of repeated exposure to (i.e., longer-term intake of) mixed herbs and spices on risk factors for cardiometabolic diseases.
Dietary guidelines in many countries including the United States, United Kingdom, and Australia recommend flavoring foods with herbs and spices as a strategy to reduce salt intake (13–15). Anderson et al. (16) showed that a behavioral intervention emphasizing herbs and spices lowered urinary sodium excretion (−957 mg/d; 95% CI: −1539, −375 mg/d) after 20 wk compared with the control group. Sensory research suggests spices may also be an acceptable replacement for added sugars (17). In addition, seasoning vegetables with herbs and spices has been shown to increase selection of vegetables in university cafeteria settings (18–20) and increase vegetable intake in school cafeterias (21, 22) compared with unseasoned vegetables. Thus, use of herbs and spices may be a strategy to improve diet quality, which may indirectly lower cardiometabolic disease risk. However, whether the consumption of herbs and spices in quantities that can be feasibly incorporated into recommended or commonly consumed dietary patterns directly affects risk factors for cardiometabolic diseases remains relatively unexplored.
To determine whether dietary intake of herbs and spices is an efficacious strategy for cardiometabolic disease risk reduction, clinical trials examining incorporation of mixed herbs and spices into a dietary pattern representative of usual intake, on major risk factors for cardiometabolic diseases, are needed. Therefore, the aim of the present trial was to assess the dose-response effect of incorporating herbs and spices into an average American diet on lipids and lipoproteins as well as other risk factors for cardiometabolic diseases in adults at elevated risk of CVD.
Methods
Trial design
A 3-period, randomized, crossover, controlled-feeding study (NCT03064932) was conducted at the Pennsylvania State University, University Park, PA to examine the dose-response effects of including herbs and spices in a diet representative of average American macronutrient intake. The test diets contained the following quantities of herbs and spices (incorporated on a g/kcal basis): 1) low-dosage, 0.5 g/d at 2100 kcal (low-spice diet; LSD); 2) moderate-dosage, 3.3 g/d at 2100 kcal (moderate-spice diet; MSD); and 3) high-dosage, 6.6 g/d at 2100 kcal (high-spice diet; HSD). Each diet was consumed for 4 wk with a minimum 2-wk washout period (median break: 19 d; range: 14–99 d). A crossover within-subject design was used to optimize the sample size; the major phenolics in herbs and spices have a relatively short excretion half-life (1.3–20 h) and therefore the probability of carryover effects was deemed low (23). Eligible individuals were randomly assigned immediately before baseline testing in a 1:1 ratio using a computer-generated 6-sequence scheme (randomization.com) that contained blocks of 6 sequences. The randomization code was held by the metabolic kitchen managers and the outcome assessors were blinded to participant random assignment. Participants were not told the order in which the test diets were provided, although true blinding could not be achieved because of the difference in taste profiles of the diets. Outcomes were assessed at baseline and the end of each diet period. The Institutional Review Board at Pennsylvania State University approved the protocol, and all participants gave informed consent.
Participants
Participants were recruited between January 2017 and September 2019 from the State College, PA area using StudyFinder (https://studyfinder.psu.edu/); advertisements in local newspapers, circulars, and on the radio; and emails to campus listservs. Flyers were also placed on campus and in the community, and the study was advertised on our research group's webpage. Eligible individuals were aged 30–75 y, had a BMI (in kg/m2) ≥25 and ≤35, a waist circumference greater than the International Diabetes Federation (24) Europid cutoff for abdominal obesity (men ≥94 cm and women ≥80 cm), and ≥1 other risk factor for CVD. Risk factors were defined as the following: 1) elevated glucose (≥100 and ≤126 mg/dL); 2) low HDL cholesterol (men <40 mg/dL and women <50 mg/dL); 3) elevated triglycerides (≥150 mg/dL and ≤300 mg/dL); 4) high blood pressure (≥130/85 mm Hg and ≤160/100 mm Hg); 5) elevated LDL cholesterol (>130 mg/dL); and 6) elevated high-sensitivity C-reactive protein (hs-CRP; >1 mg/L). Exclusion criteria were current or recent (≤6 mo) use of tobacco products; >10% change in body weight in the previous 6 mo; use of medications or over-the-counter products that lower blood pressure, cholesterol, or glucose; or consumption of >14 alcoholic beverages per week. In addition, individuals who had a history of CVD, type 1 or type 2 diabetes, liver disease, cancer, or inflammatory conditions (e.g., gastrointestinal disorders, rheumatoid arthritis) were not eligible. Individuals with thyroid disease were eligible if they were on a stable dosage of medication for ≥6 mo. Women who had been pregnant or breastfeeding within the previous 12 mo were ineligible for the study. Individuals taking oral steroids and those with allergies, intolerance, or aversions to foods included in the study menu were excluded from participating in the study.
Interested individuals completed a telephone screening to assess initial eligibility. Individuals meeting the inclusion criteria were scheduled for a clinic screening at the Pennsylvania State University Clinical Research Center (CRC). After a 12-h fast and avoidance of alcohol and over-the-counter medication for 12 h, height, weight, waist circumference, and blood pressure were measured, and a blood sample was drawn by trained research nurses. Waist circumference was measured at the iliac crest by 2 nurses while participants were standing with their feet shoulder-width apart with clothing removed from their waistline. Two measurements were taken to 0.1 cm and averaged; if measurements differed by >0.5 cm, a third measurement was taken. Weight and height were measured in light clothing after removal of shoes, and blood pressure was measured in triplicate using a validated sphygmomanometer after a 5-min rest. Women of childbearing potential were asked to provide a urine sample for pregnancy testing. Screening blood samples were sent to a commercial laboratory (Quest Diagnostics) for screening assays (biochemical analysis, complete blood count, lipids/lipoproteins, fasting glucose, and hs-CRP).
Dietary intervention
Table 1 shows the nutrient profile of the background diet. Twenty-four different herbs and spices (Supplemental Table 1) were incorporated into this diet to create the 3 levels (low, moderate, high) of herb/spice intake; Table 2 shows the herb/spice composition at 2100 kcal. Because herbs/spices were incorporated into recipes, the quantity of spices increased with increasing caloric intake (Supplemental Table 2), although all caloric levels within a spice dose were approximately matched on a per 1000 kcal basis (Supplemental Table 3). The only difference between the 3 test diets was the amount of herbs/spices included in the recipes; the foods remained the same across the 3 diets (Supplemental Table 4). The 7-d test menu was developed by chefs at McCormick Science Institute using Food Processor® (ESHA Research). Herbs and spices were weighed to 0.001 g.
TABLE 1.
Nutrient composition of the background study diet1
| Nutrient | |
|---|---|
| Carbohydrate2 | 50 |
| Protein2 | 17 |
| Total fat2 | 33 |
| Saturated fat2 | 11 |
| Monounsaturated fat2 | 11 |
| Polyunsaturated fat2 | 8 |
| Fiber, g/d | 22 |
| Sodium, mg/d | 3023 |
Nutrient composition was estimated using Food Processor® (ESHA Research) and based on the 2100-kcal diet.
Percentage of total calories.
TABLE 2.
The herb/spice composition of each study diet at 2100 kcal1
| Herbs/spices, g/d (% of total dose) | LSD | MSD | HSD |
|---|---|---|---|
| Cinnamon | 0.099 (18.55) | 0.595 (18.51) | 1.190 (18.51) |
| Coriander | 0.069 (12.98) | 0.417 (12.96) | 0.833 (12.96) |
| Ginger | 0.055 (10.23) | 0.328 (10.21) | 0.656 (10.21) |
| Cumin | 0.045 (8.42) | 0.270 (8.40) | 0.540 (8.40) |
| Parsley | 0.041 (7.72) | 0.238 (7.42) | 0.477 (7.42) |
| Black pepper | 0.039 (7.36) | 0.239 (7.45) | 0.479 (7.45) |
| Garlic | 0.028 (5.25) | 0.174 (5.42) | 0.348 (5.42) |
| Turmeric | 0.026 (4.88) | 0.156 (4.87) | 0.313 (4.87) |
| Onion powder | 0.026 (4.85) | 0.156 (4.85) | 0.311 (4.85) |
| Paprika | 0.020 (3.80) | 0.122 (3.79) | 0.244 (3.79) |
| Chili powder | 0.014 (2.67) | 0.086 (2.67) | 0.171 (2.67) |
| Rosemary | 0.013 (2.41) | 0.080 (2.50) | 0.161 (2.50) |
| Cilantro | 0.013 (2.38) | 0.076 (2.38) | 0.153 (2.38) |
| Oregano | 0.013 (2.35) | 0.075 (2.35) | 0.151 (2.35) |
| Basil | 0.011 (2.13) | 0.068 (2.13) | 0.137 (2.13) |
| Red pepper | 0.009 (1.59) | 0.051 (1.59) | 0.102 (1.59) |
| Thyme | 0.008 (1.50) | 0.050 (1.57) | 0.101 (1.57) |
| Bayleaf | 0.006 (1.21) | 0.040 (1.25) | 0.080 (1.25) |
| Cardamom | 0.004 (0.76) | 0.024 (0.76) | 0.049 (0.76) |
| Sesame seeds | 0.002 (0.44) | 0.014 (0.44) | 0.029 (0.44) |
| Sage | 0.002 (0.33) | 0.011 (0.33) | 0.021 (0.33) |
| Poppy seeds | 0.001 (0.22) | 0.007 (0.22) | 0.014 (0.22) |
| Dill weed | <0.001 (0.08) | 0.003 (0.08) | 0.005 (0.08) |
| Allspice | <0.001 (0.08) | 0.003 (0.08) | 0.005 (0.08) |
| Total | 0.547 | 3.285 | 6.571 |
Values are the mean composition of the 7-d menu. HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
All of the food was prepared in the metabolic kitchens at the Pennsylvania State University. Participants were provided with food daily Monday through Friday; on Friday they were given all of their weekend food. Energy requirements were calculated using the Harris–Benedict equation (25); weight was monitored at the daily food pickups and adjustments to caloric intake were made to keep participants’ weight approximately stable. Compliance was monitored by the metabolic kitchen managers using daily checklists. Participants were allowed to consume noncaloric beverages ad libitum, but were limited to <1184 mL/d of caffeinated beverages (5 cups/d) and ≤2 alcoholic drinks per week.
Outcomes
Testing was conducted on 2 separate days at baseline and at the end of each diet period. After a 12-h fast and avoidance of alcohol and over-the-counter medication for 48 h, participants attended the CRC for testing. On both test days weight was measured, and a fasting blood draw was taken for analysis of lipids and lipoproteins, glucose, insulin, and hs-CRP. On 1 of the test days, vascular testing was performed and waist circumference was measured. Waist circumference was measured using the same protocol as described for screening.
Specimen collection and assay methods
Blood was collected into serum separator tubes, left to clot at room temperature for ∼30 min, and then centrifuged at 1590 × g (± 90) for 15 min at room temperature. Blood collected into EDTA-coated tubes was centrifuged immediately upon collection at 1590 × g (± 90) for 15 min at room temperature. Aliquots of plasma and serum were frozen at −80°C and analyzed in a single batch at the completion of the trial.
Serum total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, insulin, and hs-CRP were measured in samples from both test days by the Pennsylvania State University Biomarker Core Lab (BCL) using a Cobas c311 chemistry analyzer (Roche Diagnostics); fructosamine was measured in samples from 1 test day only at each time point. In addition, glucose was measured in EDTA plasma collected on both test days at the BCL. For analytes measured in duplicate at each time point, values were averaged for data analysis. Lipoprotein particle and subparticle concentration and size as well as glycoprotein acetylation (GlycA) were measured in EDTA samples taken on 1 d at each time point by NMR spectroscopy (LabCorp) according to the method described by Jeyarajah et al. (26). Oxidized LDL was measured, in 1 EDTA plasma sample taken at each time point, by Quest Diagnostics using ELISA. Cholesterol efflux capacity was measured by VascularStrategies LLC using serum collected on 1 d at each time point (Supplemental Methods).
Concentrations of 15-series (8-iso-prostaglandin-F2α) and 5-series (isoprostane-F2α-I and 8,12-iso-isoprostane-F2α-VI) F2-isoprostanes were measured, using EDTA plasma collected on 1 d at each time point, by the Vanderbilt University Eicosanoid Core Laboratory. Briefly, plasma (200 uL) was diluted with 200 uL H2O:methanol (95:5) containing 2 ng [2H4]-8-iso-PGF2α (m/z 357 → 197) and then treated with 400 uL 1 N KOH. The mixture was incubated at 37°C for 30 min. The samples were then purified using an Oasis HLB uElution plate (Waters Corporation) before analysis by LC/MS. 15-Series-F2-isoprostanes were monitored with the transition m/z 353 → 193, whereas the 5-series were monitored by m/z 353 → 115. The instrumentation used was a Waters Acquity I-Class UPLC coupled to a Waters Xevo TQ-XS triple quadrupole mass spectrometer.
Vascular testing
Central and peripheral blood pressure and augmentation index
The SphygmoCor® XCEL (AtCor Medical) pulse wave analysis (PWA) system was used to measure central and peripheral blood pressure and augmentation index. The measurement was performed in the seated position after a 5-min rest period; 3 measurements were taken and the mean of the last 2 measurements was used for data analysis. If brachial systolic blood pressure was inconsistent (i.e., >10 mm Hg difference) a fourth measurement was taken.
Carotid-femoral pulse wave velocity
Arterial stiffness was assessed using the SphygmoCor® XCEL (AtCor Medical) carotid-femoral pulse wave velocity system (PWV). This measurement was performed in the supine position immediately after the PWA measurement. All of the measurements were performed by a trained operator. Briefly, carotid and femoral arterial pressure waveforms were assessed simultaneously by applanation tonometry of the carotid artery and a femoral cuff. Three measurements within 0.5 m/s were taken and averaged for data analysis.
Ambulatory blood pressure
Participants wore an ambulatory blood pressure monitor for a 24-h period at baseline and the end of each treatment period. Before baseline and during the last week of each treatment period, participants were provided with the monitor (Mortara Instrument Inc.) and given instructions for how to fit the monitor on the nondominant arm and record accurate measurements. The monitor was programmed to take a measurement every 20 min from 06:00 to 22:00 and then every 30 min from 22:00 to 06:00. This protocol was burdensome for participants and therefore to improve compliance with the outcome measure we reduced the frequency of measurements to hourly for the whole 24-h period. Twenty-one outcome assessments were completed using the original protocol and 248 were completed with the modified protocol. Data are reported for participants that had ≥1 blood pressure recording for the 24-h period at a time point that met the inbuilt quality control standards of the blood pressure monitor; machine malfunction and/or incorrect wearing of the monitor resulted in some participants having no valid readings for a time point. Participants reported their bed-time and wake-up time for the 24-h ambulatory blood pressure monitoring period and this was used to classify measurements as occurring during wakefulness or sleep.
Flow-mediated dilation
Flow-mediated dilation (FMD) was only performed on participants that were men or postmenopausal women because hormonal changes during the menstrual cycle affect FMD (27), and testing premenopausal women in a standardized phase of the menstrual cycle was deemed infeasible given the study design and timing of measurements. FMD was measured in the supine position after the participant had rested in a darkened room for 5 min. All of the ultrasound examinations were completed by a single sonographer using a GE Logiq e (General Electric Company) ultrasound imaging system with a 10-MHz linear array transducer. Continuous, longitudinal, images of the brachial artery at 5–10 cm above the elbow on the right arm were recorded at 5 frames/s during baseline (1 min), occlusion (5 min), and postdeflation (2 min). Occlusion was induced by inflation of a blood pressure cuff on the forearm (distal to the target artery) to 250 mm Hg using an automated device (D. E. Hokanson, Inc.).
Automated edge detection software (Brachial Analyzer; MIA) was used to measure artery diameter continuously throughout the recording by 2 trained scorers. Baseline diameter was defined as the mean of all of the images collected over the 1-min baseline recording period. Peak artery diameter was determined as the largest diameter recorded in the first 2 min of the postdeflation period. Percentage change in brachial diameter at peak dilation compared with baseline was calculated. The mean of the 2 scorers’ values was used for analysis; if the 2 scorers’ FMD values differed by >2 percentage points, a third person scored the scan and the mean of the 2 values within 2 percentage points was used for analysis. Flow velocity was measured using duplex-pulsed Doppler with the ultrasound beam at 2 time points: at the beginning of baseline and immediately after cuff release. Flow (mL/min) was calculated, based on the mean of 5 cardiac cycles at each time point, using the following equation: velocity time integral × cross-sectional area of the vessel [π*(brachial artery diameter at baseline/2)2] × heart rate. Reactive hyperemia was calculated as the change in flow after cuff release and was calculated as (peak flow − baseline flow)/baseline flow × 100.
Diet satisfaction
A Qualtrics survey was developed to measure diet satisfaction. The survey consisted of questions related to feelings of healthiness, energy levels, hunger, fullness, deprivation, and thirst during the diet period. In addition, participants were asked about their liking of the diet, the ease of consuming foods in the diet, the taste of foods in the diet, their liking of the amount of herbs/spices in the diet, and the saltiness of the diet. Finally, participants were asked how much they would like to continue consuming the diet and if their liking of the diet increased from the beginning of the diet period. Participants were emailed a link to the Qualtrics survey at the completion of diet periods 1, 2, and 3. All of the questions were answered on a Likert scale ranging from not at all (assigned value 0) to extremely (assigned value 100). The participants were blinded to the numerical value of their response.
Participants who completed the study were given an exit survey to assess whether they could identify which test diet they consumed in each diet period. In addition, they were asked to rank the diet periods in terms of their overall preference for continuing to consume each diet tested.
Statistical analysis
Sample size calculations showed that completion of 63 participants would provide 90% power to detect a 12.5-mg/dL (SD: 25.9 mg/dL) between-diet difference in LDL cholesterol (⍺ = 0.05) based on the findings of previously conducted trials (10, 28–32). LDL cholesterol was the primary outcome. All other outcomes were secondary.
All of the statistical analyses were performed with SAS version 9.4 (SAS Institute). All available data from randomly assigned participants were included in data analyses consistent with intent-to-treat principles. Data from participants who withdrew from the study were included when endpoint measures were obtained. The mixed-models procedure does not perform listwise deletion preserving the df, therefore this analytical approach allows inclusion of participants with ≥1 missing data point. The normality of the residuals was assessed using univariate analysis (PROC UNIVARIATE) to quantitatively evaluate skewness and to visually inspect the distribution and normal probability (Q–Q) plots. Nonnormally distributed variables were log transformed for analysis. Homogeneity of residual variance was examined using Levene's test. The assumption of equal residual variance was met for all variables except the ambulatory blood pressure outcomes. Satterthwaite df approximation was used to correct for unequal residual variance for the ambulatory blood pressure variables. Linearity was examined by plotting model residuals against the outcome of interest; for all variables the assumption of linearity was met. The mixed-models procedure (PROC MIXED) was used to examine the effect of diet on each outcome. Participant nested within randomization sequence was modeled to account for the repeated-measures crossover design. Study visit, randomization sequence, and diet were modeled as fixed effects. To account for potential carryover effects, all models included carryover covariates consistent with the approach described by Kuehl (33). Sex was included as a fixed effect to assess sex differences in diet response. For outcomes where no sex × diet effects were observed, sex was removed from the final model because it did not change the diet main effect. In addition, for each outcome the baseline value was included as a covariate and results remained unchanged (data not presented). Selection of model covariance structures was based on optimizing fit statistics (evaluated as the lowest Bayesian information criterion). In the primary analyses, the between-diet difference in mean values for each outcome was assessed. Secondary analyses assessed within- and between-diet change from baseline for all outcome variables (PROC MIXED). When a main effect of diet or a diet-by-sex interaction was detected, post hoc pairwise comparisons were conducted and the Tukey–Kramer method was used to adjust for multiple comparisons; data from post hoc testing are presented as the pairwise mean difference and 95% CI with the Tukey–Kramer adjusted P value. Statistical significance was set at P < 0.05.
Results
Participants
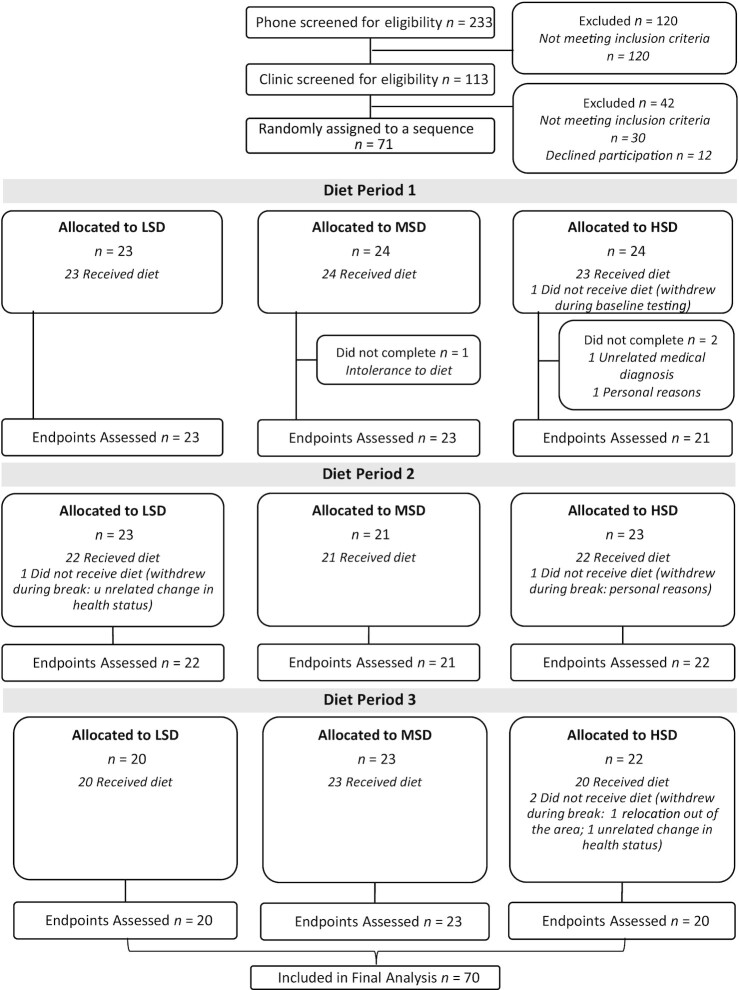
A total of 233 individuals were assessed for eligibility and 71 individuals were randomly assigned (Figure 1). Of the 71 individuals randomly assigned, 1 failed to complete baseline testing and therefore 70 participants received the first allocated diet. Three participants did not complete the first diet period and 2 participants withdrew during the first washout period, resulting in 65 participants commencing diet period 2. All 65 participants completed data collection at the end of diet period 2, although 2 participants withdrew during the second washout period. All 63 participants who commenced diet period 3 completed data collection. In total, 7 participants who received study diets withdrew during the trial; 1 subject was intolerant to the diet, 3 participants had an unrelated change in health status/medical diagnosis precluding them from further participation, 1 participant relocated out of the area, and 2 discontinued participation for personal reasons. Thus, 89% of the randomly assigned participants completed the trial.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
Table 3 presents baseline characteristics of the total sample and by randomization sequence. The cohort was 55% female and had a mean ± SD age of 44 ± 11 y, BMI of 29.7 ± 2.9 kg/m2, and waist circumference of 102.4 ± 7.3 cm.
TABLE 3.
Baseline characteristics of the sample overall and by randomization sequence1
| Characteristics | All (n = 71) | LSD-MSD-HSD (n = 11) | LSD-HSD-MSD (n = 12) | MSD-LSD-HSD (n = 12) | MSD-HSD-LSD (n = 12) | HSD-LSD-MSD (n = 12) | HSD-MSD-LSD (n = 12) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 32 (45) | 5 (45) | 6 (50) | 5 (42) | 5 (42) | 5 (42) | 6 (50) |
| Female | 39 (55) | 6 (55) | 6 (50) | 7 (58) | 7 (58) | 7 (58) | 6 (50) |
| Menopausal status | |||||||
| Premenopausal | 23 (59) | 3 (50) | 2 (33) | 4 (57) | 6 (86) | 4 (57) | 4 (67) |
| Postmenopausal | 16 (41) | 3 (50) | 4 (67) | 3 (43) | 1 (14) | 3 (43) | 2 (33) |
| Age, y | 44 ± 11 | 44 ± 12 | 48 ± 10 | 41 ± 10 | 39 ± 8 | 51 ± 10 | 43 ± 11 |
| Weight, kg | 88.9 ± 12.3 | 83.9 ± 6.4 | 92.9 ± 14.2 | 90.3 ± 12.9 | 91.2 ± 15.0 | 82.5 ± 11.0 | 92.4 ± 10.4 |
| Height, m | 1.73 ± 0.09 | 1.70 ± 0.09 | 1.77 ± 0.10 | 1.73 ± 0.11 | 1.71 ± 0.12 | 1.72 ± 0.07 | 1.73 ± 0.07 |
| BMI, kg/m2 | 29.7 ± 2.9 | 29.0 ± 2.6 | 29.6 ± 3.0 | 30.0 ± 3.1 | 30.9 ± 3.3 | 28.0 ± 2.4 | 30.8 ± 2.6 |
| ≥25 to <30 | 39 (55) | 8 (73) | 7 (58) | 6 (50) | 4 (33) | 9 (75) | 5 (42) |
| ≥30 to ≤35 | 32 (45) | 3 (27) | 5 (42) | 6 (50) | 8 (67) | 3 (25) | 7 (58) |
| Waist circumference, cm | 102.4 ± 7.3 | 100.9 ± 4.4 | 103.7 ± 7.5 | 101.3 ± 6.1 | 104.8 ± 9.3 | 100.1 ± 9.3 | 103.2 ± 6.4 |
| Male | 103.4 ± 6.9 | 98.3 ± 2.3 | 104.0 ± 5.7 | 100.4 ± 5.6 | 107.0 ± 11.2 | 104.9 ± 8.0 | 105.2 ± 5.5 |
| Female | 101.6 ± 7.7 | 103.1 ± 4.7 | 103.5 ± 9.5 | 102.0 ± 6.8 | 103.3 ± 8.2 | 96.6 ± 9.1 | 101.3 ± 7.2 |
| Total cholesterol, mg/dL | 193 ± 34 | 188 ± 33 | 176 ± 21 | 213 ± 42 | 203 ± 26 | 189 ± 28 | 187 ± 39 |
| LDL cholesterol, mg/dL | 126 ± 28 | 122 ± 29 | 114 ± 15 | 142 ± 37 | 131 ± 21 | 121 ± 30 | 126 ± 30 |
| HDL cholesterol, mg/dL | 48 ± 12 | 50 ± 10 | 47 ± 13 | 52 ± 14 | 48 ± 10 | 50 ± 14 | 43 ± 10 |
| Triglycerides, mg/dL | 106 [79–149] | 96 [67–113] | 92 [72–119] | 107 [77–167] | 105 [87–178] | 108 [91–182] | 112 [88–149] |
| Glucose, mg/dL | 100 ± 7 | 100 ± 6 | 98 ± 6 | 101 ± 8 | 98 ± 9 | 100 ± 6 | 101 ± 10 |
| hs-CRP, mg/L | 2.3 [0.8–4.0]2 | 1.8 [0.7–3.2] | 2.0 [0.4–4.6] | 2.3 [0.8–3.5]3 | 2.5 [1.0–4.1]3 | 1.5 [0.7–3.9]3 | 3.2 [1.8–5.2] |
| Systolic blood pressure, mm Hg | 129 ± 14 | 126 ± 12 | 130 ± 10 | 128 ± 16 | 128 ± 7 | 134 ± 21 | 130 ± 12 |
| Diastolic blood pressure, mm Hg | 81 ± 11 | 76 ± 11 | 83 ± 10 | 82 ± 11 | 81 ± 9 | 85 ± 13 | 80 ± 11 |
Values are mean ± SD, n (%), or median [IQR]. hs-CRP, high-sensitivity C-reactive protein; HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
Values >10 mg/L were excluded from analysis, n = 68.
n = 11.
On 94% of study days participants self-reported consuming all of the provided study foods and adherence was comparable across the diet periods (93%, diet period 1; 94%, diet period 2; 95%, diet period 3) and the diets (94% for LSD, MSD, and HSD).
Lipids/lipoproteins and markers of glycemic control and inflammation
Table 4 presents the endpoint-to-endpoint comparisons for lipids and lipoproteins and markers of glycemic control and inflammation. No main effect of diet was observed for the primary outcome, LDL cholesterol (P = 0.08). Main effects of diet were observed for total cholesterol (P = 0.005) and non-HDL cholesterol (P = 0.005). Post hoc testing showed that compared with the HSD, total cholesterol was lower after the MSD (−5.9 mg/dL; 95% CI: −10.2, −1.5 mg/dL; P = 0.005); no other pairwise comparisons were significant. Similarly, non-HDL cholesterol was lower after the MSD than after the HSD (−6.0 mg/dL; 95% CI: −10.3, −1.7 mg/dL; P = 0.004) and no other pairwise differences were observed. Compared with baseline, the MSD lowered total cholesterol (−7.4 mg/dL; 95% CI: −11.9, −2.9 mg/dL; P = 0.002) and non-HDL cholesterol (−5.0 mg/dL; 95% CI: −9.4, −0.6 mg/dL; P = 0.03); these endpoints were unchanged from baseline with the LSD and HSD. No between-diet differences were observed for HDL cholesterol, triglycerides, oxidized LDL, glucose, insulin, fructosamine, hs-CRP, GlycA, or F2 isoprostanes. Analysis of triglyceride-rich lipoprotein particles, LDL particles, HDL particles and subparticles, and apoA1 as well as global, ATP-binding cassette transporter A1 (ABCA1), and non-ABCA1 cholesterol efflux showed no between-diet differences (Supplemental Table 5). A main effect of diet was observed for apoB (P = 0.047); post hoc testing showed no significant pairwise differences between the diets.
TABLE 4.
The effect of the study diets on lipids, lipoproteins, and markers of glycemia, inflammation, and oxidative stress1
| Outcome | Baseline2 | LSD | MSD | HSD | P value |
|---|---|---|---|---|---|
| Total cholesterol, mg/dL | 193 ± 4.0 | 190 ± 3.9a,b | 186 ± 3.9a,* | 192 ± 3.9b | 0.005 |
| LDL cholesterol, mg/dL | 126 ± 3.4 | 126 ± 3.3 | 121 ± 3.3§ | 125 ± 3.4 | 0.08 |
| HDL cholesterol, mg/dL | 48 ± 1.4 | 47 ± 1.4# | 46 ± 1.4§ | 47 ± 1.4§ | 0.64 |
| Non-HDL cholesterol, mg/dL | 145 ± 3.9 | 143 ± 3.8a,b | 140 ± 3.8a,# | 146 ± 3.8b | 0.005 |
| Triglycerides, mg/dL | 106 (96, 117) | 103 (92, 116) | 100 (90, 113) | 108 (97, 122) | 0.09 |
| Oxidized LDL, U/L | 39 (37, 42) | 38 (36, 41) | 38 (36, 41) | 39 (37, 42) | 0.73 |
| Glucose, mg/dL | 100 ± 0.9 | 99 ± 0.9 | 99 ± 0.9 | 98 ± 0.9# | 0.97 |
| Insulin, µIU/mL | 10.49 (9.39, 11.70) | 10.80 (9.49, 12.30) | 10.07 (8.94, 11.47) | 9.78 (8.67, 11.13) | 0.16 |
| Fructosamine, µmol/L | 226 ± 2.1 | 225 ± 2.3 | 227 ± 2.3 | 228 ± 2.3 | 0.34 |
| hs-CRP,3 mg/L | 1.9 (1.5, 2.4) | 2.0 (1.6, 2.5) | 1.8 (1.5, 2.3) | 1.8 (1.4, 2.3)# | 0.23 |
| GlycA, µmol/L | 384 (369, 399) | 380 (369, 395) | 388 (372, 403) | 376 (361, 392)# | 0.12 |
| 8-iso-prostaglandin-F2α | 0.27 (0.24, 0.30) | 0.27 (0.24, 0.31) | 0.28 (0.25, 0.32) | 0.27 (0.23, 0.30) | 0.67 |
| Isoprostane-F2α-I and 8,12-iso-isoprostane-F2α-VI | 0.72 (0.62, 0.84) | 0.67 (0.57, 0.78) | 0.68 (0.58, 0.80) | 0.65 (0.56, 0.76) | 0.46 |
Values are least-squares means ± SEMs or geometric least-square means (95% CIs) unless otherwise stated. Baseline, n = 70; LSD, n = 65; MSD, n = 67; HSD, n = 63; unless otherwise stated. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on each outcome measure, the P values represent the main effect for diet. When a main effect was detected, post hoc tests were conducted and adjusted for multiple comparisons using the Tukey–Kramer method. Means in the same row without a common letter differ, P < 0.05. The MIXED procedure was also used to assess change from baseline; within-diet changes are denoted as follows: *P ≤ 0.001 compared with baseline; §P < 0.01 compared with baseline; #P < 0.05 compared with baseline. GlycA, glycoprotein acetylation; hs-CRP, high-sensitivity C-reactive protein; HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
Values are arithmetic means ± SEMs or geometric means (95% CIs).
Individuals with values >10 mg/L at a time point were excluded from analysis: baseline, n = 68; LSD, n = 63; MSD, n = 61; HSD, n = 59.
Adiposity, blood pressure, and vascular function
Table 5 shows the endpoint-to-endpoint comparisons for the adiposity, blood pressure, and vascular function outcomes. No between-diet differences in weight, waist circumference, peripheral or central blood pressure, augmentation index, PWV, or FMD were observed (all P > 0.05). Relative to baseline, weight was reduced after all 3 diets (LSD: −0.8 kg; 95% CI: −1.3, −0.2 kg; MSD: −1.1 kg; 95% CI: −1.6, −0.6 kg; HSD: −0.9 kg; 95% CI: −1.4, −0.3 kg; all P < 0.01); no between-diet differences were observed (P = 0.37). Waist circumference was lower after the LSD (−0.9 cm; 95% CI: −1.8, −0.003 cm; P = 0.049), MSD (−1.1 cm; 95% CI: −2.0, −0.3 cm; P = 0.01), and HSD (−1.1 cm; −2.0, −0.2 cm; P = 0.02) than at baseline with no between-diet differences (P = 0.79). Brachial systolic blood pressure was lower after the LSD (−4.8 mm Hg; 95% CI: −7.4, −2.3 mm Hg; P < 0.001), MSD (−3.8 mm Hg; 95% CI: −6.3, −1.3 mm Hg; P = 0.003), and HSD (−4.3 mm Hg; 95% CI: −6.9, −1.7 mm Hg; P = 0.001) than at baseline; no between-diet differences were detected (P = 0.79). Similarly, relative to baseline, central systolic blood pressure was reduced after the LSD (−3.7 mm Hg; 95% CI: −5.9, −1.5 mm Hg; P = 0.001), MSD (−2.7 mm Hg; 95% CI: −4.9, −0.5 mm Hg; P = 0.02), and HSD (−3.1 mm Hg; 95% CI: −5.4, −0.9 mm Hg; P = 0.01) with no between-diet differences (P = 0.73). Compared with baseline, an increase in FMD was observed after the HSD only (0.96%; 95% CI: 0.16%, 1.76%; P = 0.02). For all other outcomes, no within-diet differences were observed.
TABLE 5.
The effect of the study diets on adiposity, blood pressure, and vascular function1
| Outcome | Baseline2 | LSD | MSD | HSD | P value |
|---|---|---|---|---|---|
| Weight, kg | 88.9 ± 1.5 | 87.7 ± 1.5§ | 87.3 ± 1.5* | 87.6 ± 1.5§ | 0.32 |
| Waist circumference, cm | 102.4 ± 0.9 | 101.4 ± 0.9# | 101.1 ± 0.9# | 101.1 ± 0.9# | 0.79 |
| Brachial systolic blood pressure, mm Hg | 129 ± 1.6 | 124 ± 1.6* | 125 ± 1.6§ | 124 ± 1.6§ | 0.79 |
| Brachial diastolic blood pressure, mm Hg | 81 ± 1.3 | 79 ± 1.2 | 80 ± 1.2 | 79 ± 1.3 | 0.38 |
| Central systolic blood pressure, mm Hg | 118 ± 1.5 | 114 ± 1.4§ | 115 ± 1.4# | 114 ± 1.4§ | 0.73 |
| Central diastolic blood pressure, mm Hg | 82 ± 1.3 | 80 ± 1.3 | 81 ± 1.2 | 80 ± 1.3 | 0.35 |
| Central augmentation pressure, mm Hg | 9 ± 0.5 | 9 ± 0.6 | 9 ± 0.6 | 9 ± 0.6 | 0.64 |
| Augmentation index,3 % | 21 ± 1.4 | 22 ± 1.6 | 23 ± 1.6 | 23 ± 1.6 | 0.88 |
| Heart rate, bpm | 66 ± 1.2 | 65 ± 1.3 | 65 ± 1.2 | 65 ± 1.3 | 0.78 |
| Pulse wave velocity, m/s | 7.9 ± 0.2 | 7.7 ± 0.2 | 7.8 ± 0.2 | 7.7 ± 0.2 | 0.60 |
| Pulse transit time, m/s | 64 ± 1.2 | 65 ± 1.3 | 64 ± 1.3 | 65 ± 1.3 | 0.43 |
| Resting brachial artery diameter,4 mm | 4.71 ± 0.12 | 4.65 ± 0.13 | 4.68 ± 0.13 | 4.54 ± 0.13 | 0.19 |
| Peak brachial artery diameter,4 mm | 4.99 ± 0.12 | 4.95 ± 0.13 | 4.99 ± 0.13 | 4.85 ± 0.13 | 0.27 |
| Peak dilation,4 mm | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.65 |
| Flow-mediated dilation,4 % | 6.18 ± 0.32 | 6.58 ± 0.37 | 6.73 ± 0.36 | 7.10 ± 0.37# | 0.39 |
| Resting blood flow,4,5 mL/min | 250 (209, 299) | 247 (202, 302) | 265 (219, 321) | 257 (213, 314) | 0.73 |
| Peak blood flow,4,5 mL/min | 1394 (1236, 1556) | 1330 (1176, 1495) | 1380 (1224, 1556) | 1300 (1153, 1466) | 0.37 |
| Reactive hyperemia,4,5 % | 446 (388, 513) | 428 (365, 503) | 412 (354, 478) | 399 (340, 469) | 0.75 |
Values are least-square means ± SEMs unless otherwise stated. Baseline, n = 70; LSD, n = 65; MSD, n = 67; HSD, n = 63 unless otherwise stated. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on each outcome measure, the P values represent the main effect for diet. The MIXED procedure was also used to assess change from baseline; within-diet changes are denoted as follows: *P ≤ 0.001 compared with baseline; §P < 0.01 compared with baseline; #P < 0.05 compared with baseline. HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
Values are mean ± SEMs unless otherwise stated.
Standardized to a heart rate of 75 bpm.
Baseline, n = 47; LSD, n = 43; MSD, n = 44; HSD, n = 42.
Baseline values are geometric means (95% CIs) and endpoint values are least-square means (95% CIs).
Significant between-diet differences were observed for mean 24-h systolic (P = 0.02) and diastolic (P = 0.005) ambulatory blood pressure, and mean systolic (P = 0.02) and diastolic (P = 0.03) blood pressure during awake hours (Table 6). Post hoc testing showed mean 24-h systolic (−1.9 mm Hg; 95% CI: −3.6, −0.2 mm Hg; P = 0.02) blood pressure and awake systolic (−2.3 mm Hg; 95% CI: −4.2, −0.4 mm Hg; P = 0.01) blood pressure were lower after the HSD than after the MSD. Mean 24-h systolic blood pressure (−1.6 mm Hg; 95% CI: −3.3, 0.04 mm Hg; P = 0.058) tended to be lower after the HSD than after the LSD, but this difference was not statistically significant. Mean 24-h diastolic (−1.5 mm Hg; 95% CI: −2.5, −0.4 mm Hg; P = 0.003) blood pressure and mean awake diastolic (−1.3 mm Hg; 95% CI: −2.5, −0.1 mm Hg; P = 0.03) blood pressure were lower after the HSD than after the LSD; no other pairwise comparisons were statistically significant. No main effect of diet was observed for mean asleep systolic or diastolic blood pressure.
TABLE 6.
The effect of the study diets on ambulatory blood pressure1
| Outcome | Measurements2 | Baseline | Measurements2 | LSD | Measurements2 | MSD | Measurements2 | HSD | P value |
|---|---|---|---|---|---|---|---|---|---|
| Mean systolic blood pressure | 24 ± 12 | 118 ± 1.2 | 19 ± 11 | 117 ± 1.3a,b | 19 ± 7 | 117 ± 1.3a | 19 ± 10 | 115 ± 1.3b,* | 0.02 |
| Mean diastolic blood pressure | 24 ± 12 | 76 ± 0.7 | 19 ± 11 | 76 ± 0.8a | 19 ± 7 | 75 ± 0.8a,b | 19 ± 10 | 74 ± 0.8b,* | 0.005 |
| Mean awake systolic blood pressure | 17 ± 10 | 123 ± 1.2 | 14 ± 9 | 120 ± 1.4a,b,§ | 13 ± 6 | 121 ± 1.4a | 14 ± 7 | 119 ± 1.4b,* | 0.02 |
| Mean awake diastolic blood pressure | 17 ± 10 | 79 ± 0.8 | 14 ± 9 | 78 ± 0.9a | 13 ± 6 | 78 ± 0.9a,b | 14 ± 7 | 77 ± 0.9b,* | 0.03 |
| Mean asleep systolic blood pressure | 7 ± 3 | 108 ± 1.5 | 6 ± 3 | 108 ± 1.6 | 6 ± 2 | 108 ± 1.6 | 6 ± 3 | 106 ± 1.6 | 0.22 |
| Mean asleep diastolic blood pressure | 7 ± 3 | 70 ± 0.8 | 6 ± 3 | 70 ± 0.9 | 6 ± 2 | 69 ± 0.9 | 6 ± 3 | 68 ± 0.9* | 0.08 |
Values are least-square means ± SEMs unless otherwise stated. Baseline, n = 67; LSD, n = 61; MSD, n = 61; HSD, n = 60. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on each outcome, the P values represent the main effect for diet. When a main effect was detected, post hoc tests were conducted and adjusted for multiple comparisons using the Tukey–Kramer method. Means in the same row without a common letter differ, P < 0.05. The MIXED procedure was also used to assess change from baseline; within-diet changes are denoted as follows: *P ≤ 0.001 compared with baseline; §P < 0.01 compared with baseline; #P < 0.05 compared with baseline. HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet.
Mean ± SD number of blood pressure measurements taken per participant at the respective time point.
Compared with baseline, the HSD reduced mean 24-h systolic (−2.7 mm Hg; 95% CI: −4.3, −1.1 mm Hg; P < 0.001) and diastolic (−1.6 mm Hg; 95% CI: −2.6, −0.5 mm Hg; P < 0.001) blood pressure and mean awake systolic (−3.6 mm Hg; 95% CI: −5.3, −1.8 mm Hg; P < 0.001) and diastolic (−1.8 mm Hg; 95% CI: −2.9, −0.6 mm Hg; P < 0.001) blood pressure; no change in asleep systolic or diastolic blood pressure was observed with the HSD (Table 6). In addition, the LSD (−2.4 mm Hg; 95% CI: −4.1, −0.7 mm Hg; P = 0.002) lowered awake systolic blood pressure compared with baseline. No other changes from baseline were observed.
Diet-by-sex interactions were observed for 24-h systolic (P = 0.002) and diastolic (P < 0.001) blood pressure, awake systolic (P = 0.001) and diastolic (P < 0.001) blood pressure, and asleep systolic blood pressure (P = 0.04). Post hoc testing showed diet effects did not differ between sexes; however, deviations in diet effects were observed by sex (Supplemental Table 6). In women, 24-h (−2.8 mm Hg; 95% CI: −5.3, −0.3 mm Hg; P = 0.02) and awake (−2.9 mm Hg; 95% CI: −5.7, −0.2 mm Hg; P = 0.03) systolic blood pressure were lower after the HSD than after the LSD. In men, 24-h (3.1 mm Hg; 95% CI: 0.04, 6.1 mm Hg; P = 0.043) and awake (3.9 mm Hg; 95% CI: 0.6, 7.2 mm Hg; P = 0.01) systolic blood pressure were higher after the MSD than after the LSD. No other significant pairwise effects were observed for systolic blood pressure outcomes for either sex. In women, 24-h diastolic blood pressure was lower after the HSD (−2.1 mm Hg; 95% CI: −3.7, −0.5 mm Hg; P = 0.002) and MSD (−1.9 mm Hg; 95% CI: −3.6, −0.3 mm Hg; P = 0.01) than after the LSD. Awake diastolic blood pressure was also lower after the HSD (−2.2 mm Hg; 95% CI: −4.0, −0.5 mm Hg; P = 0.005) and MSD (−2.2 mm Hg; 95% CI: −4.1, −0.4 mm Hg; P = 0.008) than after the LSD. No other significant pairwise comparisons were observed in women. In men, 24-h (−1.9 mm Hg; 95% CI: −3.8, −0.01 mm Hg; P = 0.04) and awake (−2.2 mm Hg; 95% CI: −4.4, −0.1 mm Hg; P = 0.03) diastolic blood pressure were lower after the HSD than after the MSD. Awake diastolic blood pressure was higher after the MSD than after the LSD in men (2.2 mm Hg; 95% CI: 4.3, 0.04 mm Hg; P = 0.04). No other significant post hoc tests were observed in men.
Diet satisfaction
Participants’ self-reported feelings of healthiness, energy levels, hunger, fullness, deprivation, and thirst did not differ between the diets (Supplemental Table 7). Similarly, liking of the diet, ease of consuming the foods in the diet, taste of the foods in the diet, and perceived saltiness were not different between the diets. Self-reported liking of the amount of herbs/spices differed by diet (P = 0.005). Post hoc testing showed that compared with the LSD, liking of the amount of herbs/spices was higher for the MSD (14 points; 95% CI: 4, 24 points; P = 0.003); no other pairwise comparisons were statistically significant. No between-diet differences in preference to continue diet consumption or a change in liking from the beginning of the diet period were reported.
Of the participants that completed the study (n = 63), in the exit survey 26 (41%) reported that they would prefer to continue consuming the diet period corresponding to the HSD and 23 (37%) reported preferring to continue with the diet corresponding to the MSD; 7 participants (11%) preferred the diet period corresponding to the LSD and 7 participants (11%) indicated no difference between the diet periods (Supplemental Table 8). At the end of the study, 40 (63% of) participants ranked the diet period in which they consumed the HSD as the diet period containing the greatest amount of herbs/spices, 31 (50% of) participants rated the diet period in which they consumed the MSD as having the second greatest herb/spice quantity, and 36 (58% of) participants ranked the diet period in which they consumed the LSD as the lowest herb/spice-containing diet. No participant correctly identified which diet periods corresponded to all 3 herb/spice dosages.
Discussion
To our knowledge, this is the first controlled feeding study to examine the effect of incorporating mixed herbs and spices into a US-style dietary pattern on risk factors for cardiometabolic diseases. In this study, the HSD (6.6 g/2100 kcal) tended to improve 24-h blood pressure compared with the MSD (3.3 g/2100 kcal) and the LSD (0.5 g/2100 kcal) after 4 wk in adults at risk of cardiometabolic diseases. In addition, the MSD improved total cholesterol and non-HDL cholesterol compared with the HSD. We did not observe any effect of the diets on the primary outcome, LDL cholesterol, or on clinic-measured blood pressure, markers of glycemia, vascular function, or oxidative stress. This study provides evidence that mixed herb/spice intake may benefit 24-h ambulatory blood pressure, a stronger predictor of all-cause and CVD mortality than clinic blood pressure measurement (34, 35).
In 2013–2016, 46% of US adults had hypertension (defined as blood pressure ≥130/80 mm Hg or taking antihypertensive medication) (36). At baseline, our cohort, on average, had elevated blood pressure and was close to the hypertensive range (baseline mean blood pressure: 129/81 mm Hg) and therefore it represents individuals that are targeted for blood pressure reduction. In the whole cohort the HSD lowered 24-h systolic and diastolic blood pressure by a margin likely to confer clinical benefit (−1.6–1.9/−1.3 mm Hg) compared with the other test diets. Based on data from a registry-based, multicenter Spanish cohort, the reduction in 24-h systolic blood pressure that we observed is associated with an ∼5% reduction in cardiovascular mortality risk (34). However, we did see divergent diet effects by sex. In women, mean 24-h and awake systolic and diastolic blood pressure were lower after the HSD than after the LSD; mean 24-h and awake diastolic blood pressure were also lower after the MSD than after the LSD in women. These effects differ from the diet responses observed in men. For mean 24-h and awake systolic and diastolic blood pressure, values tended to be higher after the MSD than after the other diets. It is unclear why different effects were observed in men and women, although cautious interpretation is warranted given these are post hoc findings. The sex differences may be in part attributable to the number of measurements taken and statistical power. In men, the number of measurements taken across the 24-h period was less than in women.
Although we did not detect between-diet differences in clinic-measured central or peripheral blood pressure, this is not unexpected given the sample size and the within-individual variation inherent to clinic blood pressure measurement. Brachial and central systolic blood pressure were lowered from baseline with all diets; this finding should be cautiously interpreted because of regression to the mean and temporal effects, but directionally aligns with the detected 24-h blood pressure effects. These 24-h blood pressure effects are likely because of the bioactives in herbs and spices, which preclinically have been shown to increase NO bioavailability, inhibit angiotensin I–converting enzyme, and regulate vascular smooth-muscle cell signaling pathways to modulate blood pressure (37).
Despite improvements in blood pressure, the spice-containing diets did not affect markers of vascular function including PWV, augmentation index, or FMD. A trend toward improvement in FMD was observed with the HSD compared with baseline; this within-diet effect should be cautiously interpreted. The study might have been too short for vascular remodeling to occur, which likely explains the lack of effect on arterial stiffness, measured by PWV. Previously, improvements in endothelial function, measured by FMD or reactive hyperemia peripheral artery tonometry, have been observed in the postprandial phase with consumption of meals containing 6 g, 11.25 g, and 14.5 g herbs/spices (8, 10, 12). The daily doses of herbs/spices tested in the present study were well below the amounts tested in the aforementioned acute meal studies, which may explain the discordance. In addition, the bioactives present in herbs/spices may only elicit acute effects on the vasculature that are not detected in the fasting state, although this requires further investigation.
The primary hypothesis tested was that herbs and spices would lower LDL cholesterol. This was based on in vitro evidence showing dose-dependent inhibition of digestive enzymes, phospholipase A2 (PLA2) and pancreatic lipase (PL), with a herb/spice blend containing many of the spices tested in the present study (7). We, therefore, hypothesized that reductions in lipid absorption would occur with herbs and spices resulting in blood cholesterol–lowering, particularly LDL cholesterol, analogous to the effects of orlistat, a gastrointestinal lipase inhibitor (38). We did not observe diet effects on LDL cholesterol, which may be because herb/spice-induced inhibition of pancreatic digestive enzymes does not result in clinically significant effects in vivo when administered in the dosages we tested. The findings of the lipoprotein particle analysis are consistent with this interpretation, particularly the lack of change in triglyceride-rich lipoproteins. We did observe a reduction in total cholesterol and non-HDL cholesterol with the MSD compared with the HSD; however, these results are somewhat difficult to interpret because no difference was observed between the LSD and MSD, or the LSD and HSD. Therefore, dosage-dependent effects were not evident, which would be expected if the mechanism was related to reduced lipid absorption via inhibition of digestive enzymes. It is also possible that the dosages of herbs/spices were too low to modulate lipid metabolism given the saturated fat load (11% of kcal) in the background diet, which is greater than recommended for general health (14) and lipid/lipoprotein-lowering (39, 40). The majority of clinical trials to date have studied single herbs or spices in supplement form and therefore are not directly comparable with our study. In support of our assertion that the dosages tested in our study were below the threshold needed for metabolic benefit, a recent meta-analysis of 12 randomized controlled trials showed supplementation with turmeric or curcuminoids (dose: 66.3–1795 mg/d) lowered lipids and lipoproteins, but subgroup analysis showed low dosages (<200–300 mg/d) did not have an effect (41). At 2100 kcal, the HSD provided 313 mg of turmeric.
In this study, we sought to determine the efficacy of mixed herbs/spices to lower cardiometabolic disease risk in the context of a background diet representative of the macronutrient intake of the US adult population, which is suboptimal and does not align with dietary guidance. Although this provides evidence that is broadly generalizable, it is possible that we did not observe consistent improvements in the cardiometabolic risk factors measured because intake of these dosages of herbs/spices could not mitigate the metabolic effects of the unhealthy background dietary pattern. This suggests that increasing intake of herbs and spices cannot be unequivocally recommended in the context of a poor-quality dietary pattern to lower risk of cardiometabolic diseases. However, it is plausible that divergent effects would be observed if the background diet was aligned with recommendations for healthy dietary patterns. It is recognized that constituents of healthy dietary patterns have interactive and synergistic effects (42) and, therefore, incorporation of herbs and spices into a healthy dietary pattern abundant in fruits, vegetables, whole grains, nuts, and legumes may have additive benefits and yield different findings to those observed in the current study. Further research on incorporation of herbs and spices into healthy dietary patterns is needed and would provide evidence that informs recommendations for healthy dietary patterns.
To our knowledge, this is the first randomized, controlled feeding study to examine the effect of repeated exposure to mixed herbs and spices over a 4-wk period on major risk factors for cardiometabolic diseases. Strengths of the study include the controlled feeding design that enabled known dosages of herbs and spices to be provided in the context of a well-defined and unchanged background diet, therefore minimizing confounding from other dietary factors. Self-reported adherence was high (>93%), and the study was adequately powered for the primary endpoint.
The study is limited by the inclusion of 24 different herbs and spices, which precludes conclusions about the effects of individual herbs and spices. In addition, each day in the 7-d menu included different combinations and amounts of the 24 herbs and spices. Although this is representative of the way herbs and spices are consumed as part of a dietary pattern, experimentally this inconsistent exposure introduces the possibility that outcomes may have been influenced by the herb/spice composition of the days more proximal to testing because herbs and spices have a relatively short-half life (23). This is likely a source of variance and may have reduced our power to detect effects on the outcomes measured. This study is also limited by the lack of data about habitual herb/spice intake at baseline; therefore, we are unable to make inferences about how the dosages of herbs/spices tested compare with habitual intake or any heterogeneity in observed effects by baseline intake. We also acknowledge that the lack of chemical analysis of the test herbs/spices is a limitation. Particularly, the coumarin composition of the cinnamon used is unknown. Coumarin has been shown to have hepatotoxic effects and the European Food Safety Authority established a tolerable daily intake level of 0.1 mg · kg body weight−1 · d−1 (43). The dose of cinnamon provided in the HSD (1.190 g) could have contained ∼3.57 mg of coumarin if the cinnamon was Cassia cinnamon (Cinnamomun cassia) (nil if true cinnamon, Cinnamomum verum) (44); this amount is well below the tolerable daily intake level, which equates to 6 mg/d for a 60-kg adult. Finally, a large number of statistical tests were conducted for the secondary endpoints, which increases the risk of type 1 statistical errors.
In conclusion, incorporation of a relatively high culinary dosage of mixed herbs and spices (6.6 g · d−1 · 2100 kcal−1) into a US-style dietary pattern tended to improve 24-h blood pressure compared with lower intakes (0.5 and 3.3 g · d−1 · 2100 kcal−1) after 4 wk in adults at elevated risk of cardiometabolic diseases. These effects were more pronounced in women. Intake of these dosages of herbs/spices did not change lipids and lipoproteins, clinic-measured central or peripheral blood pressure, markers of glycemic control, vascular function, or oxidative stress. Further research is needed to determine whether inclusion of herbs and spices in a healthy dietary pattern augments diet-induced health benefits.
Supplementary Material
Acknowledgments
We thank Cyndi Flanagan, Christa Oelhaf, Filamena Martin, Lori Gray, and all the staff at the Penn State Clinical Research Center for their assistance with data collection. In addition, we thank Amy Ciccarella, Marcella Smith, and all of the staff in the metabolic kitchens for preparing the test meals, and Paul Wagner for conducting the FMD procedure. We also thank Danette Teeter, Jennifer Fleming, Alyssa Tindall, Emily Johnston, Valarie Sullivan, Stacey Meily, Keally Haushalter, Megan Brunermer, Hannah Testa, Hannah Morris, Megan Kostek, Tara Greenwood, and Kunal Gupta for their help with the study. We thank the Biomarker Core Lab in the Department of Biobehavioral Health, Penn State for biological sample analysis. We also thank the Vanderbilt University Eicosanoid Core Laboratory for conducting the isoprostane analyses.
The authors’ responsibilities were as follows—KSP, CJR, DNP, SGW, and PMK-E: designed the research (project conception and development of the overall research plan); KSP and KMD: conducted the research (hands-on conduct of the study and data collection); KSP: performed the statistical analyses, wrote the paper, and had primary responsibility for the final content; and all authors: critically reviewed the manuscript and read and approved the final manuscript. CJR, DNP, SGW, and PMK-E received funding from McCormick Science Institute for the research reported in this article. SGW has received honoraria and travel expenses from McCormick Science Institute. All other authors report no conflicts of interest.
Notes
Supported by McCormick Science Institute (to PMK-E, DNP, CJR, and SGW). The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014. McCormick Science Institute and the NIH had no role in study design, data collection, data analysis, data interpretation, or preparation, writing, review, or approval of the manuscript. Chefs from McCormick and Company, Inc. designed the menus based on nutrient targets defined by the investigators. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Tables 1–8 and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ABCA1, ATP-binding cassette transporter A1; BCL, Biomarker Core Lab; CRC, Clinical Research Center; CVD, cardiovascular disease; FMD, flow-mediated dilation; GlycA, glycoprotein acetylation; hs-CRP, high-sensitivity C-reactive protein; HSD, high-spice diet; LSD, low-spice diet; MSD, moderate-spice diet; PWA, pulse wave analysis; PWV, pulse wave velocity.
Contributor Information
Kristina S Petersen, Department of Nutritional Sciences, Texas Tech University, Lubbock, TX, USA; Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, USA.
Kristin M Davis, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA, USA.
Connie J Rogers, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, USA.
David N Proctor, Department of Kinesiology, Pennsylvania State University, University Park, PA, USA.
Sheila G West, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, USA; Department of Biobehavioral Health, Pennsylvania State University, University Park, PA, USA.
Penny M Kris-Etherton, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, USA.
Data availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim Aet al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DEet al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DWet al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020;43(Supplement 1):S32–6. [DOI] [PubMed] [Google Scholar]
- 6. Skulas-Ray AC, Kris-Etherton PM, Teeter DL, Chen C-YO, Vanden Heuvel JP, West SG. A high antioxidant spice blend attenuates postprandial insulin and triglyceride responses and increases some plasma measures of antioxidant activity in healthy, overweight men. J Nutr. 2011;141(8):1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCrea CE, West SG, Kris-Etherton PM, Lambert JD, Gaugler TL, Teeter DL, Sauder KA, Gu Y, Glisan SL, Skulas-Ray AC. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: results of a randomized crossover study and in vitro experiments. J Transl Med. 2015;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Henning SM, Zhang Y, Rahnama N, Zerlin A, Thames G, Tseng CH, Heber D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with type 2 diabetes mellitus. Diabet Med. 2013;30(5):590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Z, Henning SM, Zhang Y, Zerlin A, Li L, Gao K, Lee R-P, Karp H, Thames G, Bowerman Set al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am J Clin Nutr. 2010;91(5):1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakayama H, Tsuge N, Sawada H, Masamura N, Yamada S, Satomi S, Higashi Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial. Nutr J. 2014;13(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haldar S, Chia SC, Lee SH, Lim J, Leow MK-S, Chan ECY, Henry CJ. Polyphenol-rich curry made with mixed spices and vegetables benefits glucose homeostasis in Chinese males (Polyspice Study): a dose–response randomized controlled crossover trial. Eur J Nutr. 2019;58(1):301–13. [DOI] [PubMed] [Google Scholar]
- 12. Petersen KS, Rogers CJ, West SG, Proctor DN, Kris-Etherton PM. The effect of culinary doses of spices in a high-saturated fat, high-carbohydrate meal on postprandial lipemia and endothelial function: a randomized, controlled, crossover pilot trial. Food Funct. 2020;11(4):3191–200. [DOI] [PubMed] [Google Scholar]
- 13. National Health and Medical Research Council (NHMRC). Australian Dietary Guidelines. Canberra (Australia): NHMRC; 2013. [Google Scholar]
- 14. US Department of Health and Human Services (DHHS), USDA. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington (DC): US DHHS and USDA; 2015. [Google Scholar]
- 15. Public Health England. The Eatwell Guide. [Internet]. London (United Kingdom): Public Health England; 2016; [cited 2020 Jun 8]. Available from: https://www.nhs.uk/live-well/eat-well/the-eatwell-guide/. [Google Scholar]
- 16. Anderson CAM, Cobb LK, Miller ER III, Woodward M, Hottenstein A, Chang AR, Mongraw-Chaffin M, White K, Charleston J, Tanaka Tet al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: results of the SPICE randomized clinical trial. Am J Clin Nutr. 2015;102(3):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peters JC, Marker R, Pan Z, Breen JA, Hill JO. The influence of adding spices to reduced sugar foods on overall liking. J Food Sci. 2018;83(3):814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manero J, Phillips C, Ellison B, Lee S-Y, Nickols-Richardson SM, Chapman-Novakofski KM. Influence of seasoning on vegetable selection, liking and intent to purchase. Appetite. 2017;116:239–45. [DOI] [PubMed] [Google Scholar]
- 19. Luu L, Manero J, Lee S-Y, Nickols-Richardson S(S), Chapman-Novakofski K. Role of seasoning vegetables on consumer behavior: purchase, intake, liking, and intention to pay for larger servings. Food Qual Preference. 2020;82:103890. [Google Scholar]
- 20. Luu L, Lee S-Y, Nickols-Richardson S(S), Chapman-Novakofski K. Larger serving size and seasoning's role in consumer behaviors toward vegetables. Food Qual Preference. 2021;88:104105. [Google Scholar]
- 21. D'Adamo CR, Parker EA, McArdle PF, Trilling A, Bowden B, Bahr-Robertson MK, Keller KL, Berman BM. The addition of spices and herbs to vegetables in the National School Lunch Program increased vegetable intake at an urban, economically-underserved, and predominantly African-American high school. Food Qual Preference. 2021;88:104076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fritts JR, Bermudez MA, Hargrove RL, Alla L, Fort C, Liang Q, Cravener TL, Rolls BJ, D'Adamo CR, Hayes JEet al. Using herbs and spices to increase vegetable intake among rural adolescents. J Nutr Educ Behav. 2019;51(7):806–16.e1. [DOI] [PubMed] [Google Scholar]
- 23. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1):230S–42S. [DOI] [PubMed] [Google Scholar]
- 24. Alberti K, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62. [DOI] [PubMed] [Google Scholar]
- 25. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–70. [DOI] [PubMed] [Google Scholar]
- 27. Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher Tet al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40(30):2534–47. [DOI] [PubMed] [Google Scholar]
- 28. Zare R, Heshmati F, Fallahzadeh H, Nadjarzadeh A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement Ther Clin Pract. 2014;20(4):297–301. [DOI] [PubMed] [Google Scholar]
- 29. Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25(2):144–50. [DOI] [PubMed] [Google Scholar]
- 30. Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65(4):515–20. [DOI] [PubMed] [Google Scholar]
- 31. Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SEJ. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food Sci Nutr. 2013;64(6):682–6. [DOI] [PubMed] [Google Scholar]
- 32. Khan A, Zaman G, Anderson RA. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J Clin Biochem Nutr. 2009;44(1):52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuehl RO. Design of experiments: statistical principles of research design and analysis. 2nd ed. Pacific Grove (CA): Duxbury/Thomson Learning; 2000. [Google Scholar]
- 34. Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378(16):1509–20. [DOI] [PubMed] [Google Scholar]
- 35. Yang W-Y, Melgarejo JD, Thijs L, Zhang Z-Y, Boggia J, Wei F-F, Hansen TW, Asayama K, Ohkubo T, Jeppesen Jet al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322(5):409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FNet al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. [DOI] [PubMed] [Google Scholar]
- 37. Jiang TA. Health benefits of culinary herbs and spices. J AOAC Int. 2019;102(2):395–411. [DOI] [PubMed] [Google Scholar]
- 38. Muls E, Kolanowski J, Scheen A, Van Gaal L. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled, multicentre study. Int J Obes. 2001;25(11):1713–21. [DOI] [PubMed] [Google Scholar]
- 39. Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Miller NHet al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25_suppl_2):S76–99. [DOI] [PubMed] [Google Scholar]
- 40. Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PBet al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9(6):S1–S122.e1. [DOI] [PubMed] [Google Scholar]
- 41. Yuan F, Dong H, Gong J, Wang D, Hu M, Huang W, Fang K, Qin X, Qiu X, Yang Xet al. A systematic review and meta-analysis of randomized controlled trials on the effects of turmeric and curcuminoids on blood lipids in adults with metabolic diseases. Adv Nutr. 2019;10(5):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dietary Guidelines Advisory Committee. Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington (DC): USDA Agricultural Research Service; 2020. [Google Scholar]
- 43. European Food Safety Authority (EFSA). Coumarin in flavourings and other food ingredients with flavouring properties - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008;6(10):793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. German Federal Institute for Risk Assessment. FAQ on coumarin in cinnamon and other foods. [Internet]. Berlin (Germany): Bundesinstitut für Risikobewertung; 2012; [cited 2021 Jun 10]. Available from: https://www.bfr.bund.de/en/faq_on_coumarin_in_cinnamon_and_other_foods-8487.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.