ABSTRACT
Background
Adipose tissue radiodensity may have prognostic importance for colorectal cancer (CRC) survival. Lower radiodensity is indicative of larger adipocytes, while higher radiodensity may represent adipocyte atrophy, inflammation, or edema.
Objectives
We investigated associations of adipose tissue radiodensity and longitudinal changes in adipose tissue radiodensity with mortality among patients with nonmetastatic CRC.
Methods
In 3023 patients with stage I–III CRC, radiodensities of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were quantified from diagnostic computed tomography (CT) images. There were 1775 patients with follow-up images available. Cox proportional hazards models and restricted cubic splines were used to examine associations of at-diagnosis values and of longitudinal changes in VAT and SAT radiodensities with risks of death after adjusting for potential confounders, including body size and comorbidities.
Results
VAT and SAT radiodensities were linearly associated with all-cause mortality: the HRs for death per SD increase were 1.21 (95% CI, 1.11–1.32) for VAT radiodensity and 1.18 (95% CI, 1.11–1.26) for SAT radiodensity. Changes in adipose tissue radiodensity had curvilinear associations with risks of death. The HR for an increase in VAT radiodensity of at least 1 SD was 1.53 (95% CI, 1.23–1.90), while the HR for a decrease of at least 1 SD was nonsignificant at 1.11 (95% CI, 0.84–1.47) compared with maintaining radiodensity within 1 SD of baseline. Similarly, increases (HR, 1.88; 95% CI, 1.48–2.40) but not decreases (HR, 1.20; 95% CI, 0.94–1.54) in SAT radiodensity significantly increased the risk of death compared with no change in radiodensity.
Conclusions
In patients with nonmetastatic CRC, adipose tissue radiodensity is a novel risk factor for total mortality that is independent of BMI and changes in body weight.
Keywords: adipose tissue, radiodensity, mortality, colorectal cancer, prognosis
Introduction
The 5-year mortality rates are 10%–30% for local and regional colorectal cancer (CRC) (1), which underscores the need for prognostic markers to identify patients with nonmetastatic disease at high risk for early mortality. Computed tomography (CT) imaging is the standard of care for CRC diagnoses and surveillance, opening the possibility of using imaging data to find novel prognostic markers. Accumulating evidence demonstrates the importance of body composition—quantity, distribution, and quality of skeletal muscle and adipose tissue—in CRC. Multiple studies show that a higher skeletal muscle mass is associated with improved survival after CRC (2–4). In addition, the size of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) depots is associated with survival (2, 5, 6).
CT images offer the opportunity to examine the “quality” of skeletal muscle and adipose tissue, above and beyond quantity and distribution. Tissue quality has been studied mostly in the context of skeletal muscle, where lower skeletal muscle radiodensity, indicative of lipid deposition within muscle, is associated with worse survival (7, 8). While the quality of adipose tissue may also have prognostic importance, this has been examined in only a handful of studies, mostly in the context of cardiovascular disease (9–11); to our knowledge, no prior study has examined longitudinal changes in adipose tissue radiodensity.
The radiodensity of adipose tissue is not constant, as morphology and composition of the tissue change over time. For example, the size of the adipocytes increases with weight gain (12), and higher adipocyte weight is associated with lower radiodensity (13). Thus, lower radiodensity can be interpreted as a measure of adipocyte hypertrophy and higher lipid content. In patients without cancer, lower adipose tissue radiodensity has been associated with adverse biomarkers and cardiometabolic risks (10, 14). Conversely, higher adipose tissue radiodensity has been associated with higher risks of death among the elderly (9) and in patients with advanced cancer, including hepatocellular carcinoma (15), metastatic CRC (16), and extremity sarcoma (17). To expand the limited knowledge regarding the prognostic importance of adipose tissue radiodensity, the aim of the current study was to assess whether adipose tissue radiodensity and longitudinal changes in radiodensity after CRC diagnosis were associated with all-cause and CRC-specific mortality.
Methods
Study design
This analysis was conducted with data from the Colorectal Cancer Sarcopenia and Near-Term Survival (C-SCANS) cohort (2), a cohort study within the Kaiser Permanente Northern California (KPNC) integrated health-care system. It included all patients diagnosed at KPNC from 2006–2011 with stage I–III CRC who had a surgical resection (n = 4465). The C-SCANS study excluded patients who did not have an abdominal or pelvic CT image collected as part of the routine staging workup (n = 693), did not have a valid weight measure around the time of the CT image (n = 411), or who had unreadable images (n = 99), resulting in a sample of 3262 patients. Excluded participants were more likely to be older, to have colon rather than rectal cancer, and to have stage I compared with stage II or III disease. For the current analysis, we excluded 239 patients for whom a valid assessment of adipose tissue radiodensity from the CT image was not possible due to streaking or notable graininess on the image, resulting from metal implants or the patient's abdomen touching the scanner. The mean BMI (41.4 kg/m2; SD, 7.7 kg/m2) was higher for the 239 patients whose CT images were excluded than for those patients included in the analysis (27.6 kg/m2; SD, 5.3 kg/m2), but all other baseline characteristics were similar. Our final analytic sample included 3023 patients with usable at-diagnosis CT images. These baseline CT images represent the body composition at diagnosis. For this reason, we chose the scan closest to diagnosis, but prior to any chemotherapy or radiotherapy treatment. On average, baseline images were taken 1 week after diagnosis, with an SD of 9 days. Of those 3023 patients, 1775 patients had a follow-up CT image available: those follow-up images were used to assess longitudinal changes in adipose tissue radiodensity (for a flowchart, see Supplemental Figure 1). Follow-up images were annual surveillance scans, which are the standard of care in colorectal cancer; those standard surveillance scans were available for a subset of patients who received follow-up care within the KPNC health system. Follow-up images represent the body composition in the postdiagnosis period, once treatment was complete; these images were taken on average 15 months after diagnosis, with an SD of 4 months.
The study was approved by the KPNC and the University of Alberta Institutional Review Boards, with a waiver of written informed consent.
Body composition and adipose tissue radiodensity
Body composition was assessed from a single transverse CT image at the level of the third lumbar vertebra (18) using Slice-O-Matic software (V5.0; TomoVision). Adipose tissue depots were segmented to distinguish VAT and SAT. Cross-sectional areas in cm2 were demarcated using anatomic knowledge and tissue-specific Hounsfield Unit (HU) ranges of −150 to −50 HU for VAT, −190 to −30 HU for SAT, and −29 to 150 HU for skeletal muscle (19, 20). Visceral, subcutaneous, and skeletal muscle tissue radiodensities were defined as the average radiodensity (in HU) of the tissue area of interest. All CT images for this study were manually segmented using Slice-O-Matic software by raters with years of experience who underwent training at the University of Alberta. A randomly selected subsample of 50 CT images was analyzed by 2 raters blinded to the outcome, and the remaining CT images were analyzed by a single rater. The inter‐rater coefficients of variation were 2.7% and 1.1% for VAT and SAT, respectively (2). CT images in our data set were primarily (90%) contrast-enhanced images; a sensitivity analysis considering contrast status did not appreciably alter the results (data not shown).
Covariates
KPNC electronic data sources, including patients' electronic medical record and the Cancer Registry, provided information on disease stage, tumor site (rectum compared with colon), treatment (receipt of chemotherapy and/or radiation), demographic factors (age, self-reported race/ethnicity, sex), smoking history, and comorbid conditions (Charlson Comorbidity Index). Height and weight, measured at the clinical visit closest to the diagnosis CT image, were used to calculate BMI. Body weights are collected virtually every time a patient visited the clinic. For our study sample, on average, valid weights were taken 8 days prior to the CT scan, with an SD of 30 days.
Outcomes
The primary study outcome was all-cause mortality and the secondary outcome was CRC-specific mortality. Deaths were identified from the California state death registry, the National Death Index using Social Security Administration data, and KPNC mortality files through 31 May 2020. Deaths were classified as CRC-specific if CRC was documented as an underlying or contributing cause of death. Follow-up began from the date of the diagnostic CT in the analyses focusing on at-diagnosis values of adipose tissue radiodensity, whereas follow-up began from the date of follow-up CT for analyses of changes in radiodensity. For analyses of all-cause mortality, patients were followed until death from any cause or 31 May 2020. For analyses of CRC-specific mortality, patients were followed until death from CRC, censoring due to death from another cause, or 31 May 2020.
Statistical analysis
We grouped the baseline (at-diagnosis) values of VAT radiodensity and SAT radiodensity into 3 groups according to whether radiodensities of adipose tissues were within 1 SD of the mean, >1 SD higher than the mean, or >1 SD lower than the mean (SDs, 8 HU for VAT radiodensity and 9 HU for SAT radiodensity). For the patients with a follow-up image, we computed the difference in continuous HU between follow-up and baseline images. We grouped these changes into 3 categories according to whether patients maintained adipose tissue radiodensity within 1 SD of the baseline scan, increased in radiodensity >1 SD compared with baseline, or decreased in radiodensity >1 SD compared with baseline.
We used restricted cubic splines to examine the associations of the continuous baseline values of VAT radiodensity and, in separate models, SAT radiodensity with risks of death. In all models, we used a data-driven approach developed by Spiegelman et al. (21) to fit a spline with 4 knots, with the median value as the reference, and to test for nonlinearity using a likelihood ratio test to compare the flexible model to the more parsimonious, linear model. Finding no evidence of nonlinearity, as a complement to the graphic presentation of the splines, we computed the HR for the continuous (per SD) association of at-diagnosis VAT and SAT radiodensities with risks of death from any cause and from CRC.
In the subset of patients with follow-up images, we used restricted cubic splines to examine the associations of continuous changes in VAT radiodensity and, in separate models, SAT radiodensity with risks of death. Finding evidence of nonlinearity, we also computed the HR for the categorical association of VAT and SAT radiodensities (increased or decreased radiodensity compared with no change) with risks of death.
All models were adjusted for baseline values of potential confounders selected a priori: age at diagnosis, sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, or mixed/other), tumor site (colon compared with rectum), cancer stage (I, II, or III), Charlson Comorbidity Index, smoking status (current, former, or never), and receipt of chemotherapy and/or radiation, distinguishing between neoadjuvant and adjuvant treatment. Models for baseline radiodensity were additionally adjusted for the continuous BMI and for the adipose tissue area of interest (e.g., VAT area in analyses of VAT radiodensity), whereas models for a change in radiodensity were adjusted for the square of the continuous change in BMI between images and for a change in the adipose tissue area of interest, as well as for the number of days between images. Covariates were selected a priori due either to their potential confounding relationship with both radiodensity and outcomes (e.g., age) or because they have known associations with mortality (e.g., receipt of chemotherapy), and therefore controlling for them improves statistical efficiency. We examined possible effect modifications by sex and stage at diagnosis through the use of product terms of our exposures with these covariates; finding no evidence of an interaction, we present the results overall.
As a sensitivity analysis, we excluded 25 patients who died within 30 days of colorectal resection, as these deaths were likely due to surgical complications. In addition, we considered an adjustment for skeletal muscle area as a covariate. Further, in models for changes in radiodensity, we restricted analyses to weight-stable patients who maintained a body weight within 5% between images, in addition to adjusting for the continuous change in weight.
All analyses were performed using SAS (Statistical Analysis Software 9.4, SAS Institute Inc.).
Results
Population characteristics
Patients with higher VAT radiodensities had similar ages and comorbidity statuses as patients with lower VAT radiodensities, but had lower BMIs, had higher stages at diagnosis, and were more likely to be female (Table 1). The VAT radiodensity was inversely correlated with the VAT area (Pearson correlation coefficients for radiodensity and area were −0.69 for VAT and −0.48 for SAT). In addition, the radiodensities of VAT and SAT were correlated (Pearson correlation coefficient, 0.70), but the mean radiodensity of SAT was lower (−96.6 HU; SD, 9.3 HU) than that of VAT (−87.1 HU; SD, 8.0 HU).
TABLE 1.
Descriptive characteristics of our cohort of early stage colorectal cancer patients, shown overall and by category of baseline VAT radiodensity (n = 3023)1
| Characteristic | Overall | Low density, >1 SD lower than mean, n = 404 | Within 1 SD of mean, n = 2132 | High density, >1 SD higher than mean, n = 487 |
|---|---|---|---|---|
| Age in years, mean (SD) | 62.5 (11.5) | 62.9 (10.6) | 62.5 (11.3) | 62.5 (12.9) |
| BMI, kg/m2, mean (SD) | 27.6 (5.3) | 30.5 (4.6) | 28.2 (5.0) | 22.6 (3.8) |
| VAT area, cm2, mean (SD) | 152.4 (107.4) | 269.0 (103.9) | 156.0 (91.5) | 40.0 (45.7) |
| SAT area, cm2, mean (SD) | 203.5 (106.7) | 238.6 (102.0) | 216.6 (103.6) | 116.7 (75.5) |
| Skeletal muscle tissue area, cm2, mean (SD) | 139.9 (37.6) | 154.1 (37.4) | 140.9 (37.5) | 123.4 (31.6) |
| Neutrophil/lymphocyte ratio, mean (SD) | 4.8 (5.3) | 4.8 (5.7) | 4.7 (5.2) | 5.6 (5.7) |
| Subcutaneous adipose density, HU, mean (SD) | −96.6 (9.3) | −102.6 (4.8) | −98.4 (5.9) | −83.6 (12.5) |
| Visceral adipose density, HU, mean (SD) | −87.1 (8.0) | −97.8 (2.3) | −88.2 (4.2) | −73.4 (4.8) |
| Charlson Comorbidity Index, mean (SD) | 0.8 (1.3) | 0.9 (1.4) | 0.8 (1.3) | 0.8 (1.4) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 1954 (64.6%) | 275 (68.1%) | 1353 (63.5%) | 326 (66.9%) |
| Black/African American | 215 (7.1%) | 17 (4.2%) | 157 (7.4%) | 41 (8.4%) |
| Hispanic/Latino | 337 (11.1%) | 53 (13.1%) | 253 (11.9%) | 31 (6.4%) |
| Asian/Pacific Islander | 500 (16.5%) | 55 (13.6%) | 357 (16.7%) | 88 (18.1%) |
| Other | 17 (0.6%) | 4 (1.0%) | 12 (0.6%) | 1 (0.2%) |
| Sex, n (%) | ||||
| Male | 1515 (50.1%) | 262 (64.9%) | 1069 (50.1%) | 184 (37.8%) |
| Female | 1508 (49.9%) | 142 (35.1%) | 1063 (49.9%) | 303 (62.2%) |
| Smoking history, n (%) | ||||
| Never | 1418 (46.9%) | 187 (46.3%) | 998 (46.8%) | 233 (47.8%) |
| Former | 1241 (41.1%) | 186 (46.0%) | 876 (41.1%) | 179 (36.8%) |
| Current | 364 (12.0%) | 31 (7.7%) | 258 (12.1%) | 75 (15.4%) |
| Stage, n (%) | ||||
| I | 917 (30.3%) | 159 (39.4%) | 626 (29.4%) | 132 (27.1%) |
| II | 945 (31.3%) | 119 (29.5%) | 666 (31.2%) | 160 (32.9%) |
| III | 1161 (38.4%) | 126 (31.2%) | 840 (39.4%) | 195 (40.0%) |
| Cancer site, n (%) | ||||
| Rectum | 885 (29.3%) | 136 (33.7%) | 615 (28.8%) | 134 (27.5%) |
| Colon | 2138 (70.7%) | 268 (66.3%) | 1517 (71.2%) | 353 (72.5%) |
| Receipt of radiation therapy, n (%) | ||||
| No | 2552 (84.4%) | 344 (85.1%) | 1793 (84.1%) | 415 (85.2%) |
| Yes | 471 (15.6%) | 60 (14.9%) | 339 (15.9%) | 72 (14.8%) |
| Receipt of chemotherapy, n (%) | ||||
| No | 1440 (47.6%) | 216 (53.5%) | 985 (46.2%) | 239 (49.1%) |
| Yes, adjuvant | 1206 (39.9%) | 140 (34.7%) | 868 (40.7%) | 198 (40.7%) |
| Yes, neoadjuvant | 377 (12.5%) | 48 (11.9%) | 279 (13.1%) | 50 (10.3%) |
HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Baseline characteristics according to the category of SAT radiodensity showed similar patterns overall to those described above for VAT radiodensity. Patients with higher SAT radiodensities were similar in terms of stage but appeared to be slightly older and less likely to be female (Supplemental Table 1).
Radiodensities of VAT and SAT and mortality.
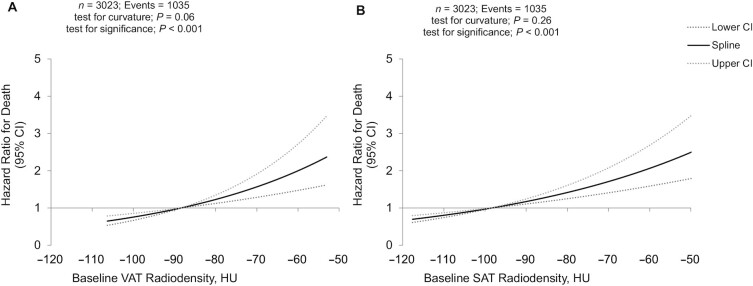
The VAT radiodensity was significantly, linearly associated with all-cause mortality (Figure 1A; Table 2): for each SD increase in VAT radiodensity, the HR for all-cause mortality increased by 1.21 (95% CI, 1.11–1.32). Results for CRC-specific death were similar: the HR per SD increase in VAT radiodensity was 1.22 (95% CI, 1.08–1.37). SAT radiodensity was also significantly, linearly associated with all-cause mortality (Figure 1B; Table 2): per SD increase in SAT radiodensity the HR for all-cause mortality was 1.18 (95% CI, 1.11–1.26). Results for CRC-specific death were similar (HR, 1.19; 95% CI, 1.09–1.31).
FIGURE 1.
Higher VAT and SAT radiodensities are associated with higher risks of death. Restricted cubic spline for the associations of (A) VAT and (B) SAT radiodensities with mortality in our cohort of early stage colorectal cancer patients. HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 2.
HRs for associations of baseline adipose tissue radiodensity and mortality after colorectal cancer in the cohort of early stage colorectal cancer patients1
| Overall mortality (events=1035) | CRC-specific mortality (events=560) | |||
|---|---|---|---|---|
| Exposure | HR (95% CI) | P value | HR (95% CI) | P value |
| VAT radiodensity, per SD (8 HU) | 1.21 (1.11–1.32) | <0.0001 | 1.22 (1.08–1.37) | 0.001 |
| SAT radiodensity, per SD (9 HU) | 1.18 (1.11–1.26) | <0.0001 | 1.19 (1.09–1.31) | 0.0002 |
Data are over a median follow-up of 9.9 years (minimum, 0.01 years; maximum, 14.4 years). The models are adjusted for age, sex, race/ethnicity, colon compared with rectum cancer, cancer stage, Charlson Comorbidity Index, smoking status, receipt of chemotherapy and/or radiation, BMI at baseline scan squared, and adipose tissue area. CRC, colorectal cancer; HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Changes in adipose tissue radiodensity: baseline characteristics.
The mean time between images was 1.25 years (SD, 0.32 years) for the subset of patients that had a follow-up image available. This subset had similar ages and BMIs as the total cohort but was more likely to have stage III compared with stage I disease (Supplemental Table 2). While on average the adipose tissue radiodensity did not change between images, there was extensive between-patient variation: the mean change in VAT radiodensity was 1.13 HU (SD, 6.87 HU) and the mean change in SAT radiodensity was -0.01 HU (SD, 8.89 HU). Between images, 428 (24%) of participants lost at least 5% of body weight, 395 (22%) gained at least 5%, and 952 (54%) maintained a stable weight within 5% of baseline. The mean change in BMI was −0.14 kg/m2 (SD, 2.45 kg/m2).
Patients who increased in VAT radiodensity were more likely to be female, to have rectal compared with colon cancer, to have a lower BMI, and to have a higher stage at diagnosis relative to patients whose VAT radiodensity remained stable or decreased (Supplemental Table 2). Increases in VAT and SAT radiodensity were inversely correlated with changes in body weight (Pearson correlation coefficients of −0.47 and −0.41, respectively) and positively correlated with each another (Pearson correlation coefficients r = 0.73).
Changes in VAT radiodensity and mortality.
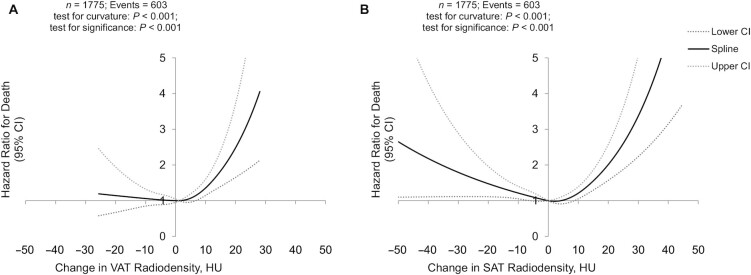
The association of changes in VAT radiodensity following CRC diagnosis and risks of all-cause mortality was curvilinear (Figure 2A). In a categorical analysis, an increase in VAT radiodensity of 1 SD was associated with a higher risk of all-cause mortality relative to remaining a stable radiodensity (HR, 1.53; 95% CI, 1.23–1.90), while a decrease in VAT radiodensity was not significantly associated with mortality (Table 3). Meanwhile, both increases and decreases in VAT radiodensity were strongly associated with CRC-specific death compared to maintaining radiodensity within 1 SD of the baseline value (Table 3). These results were independent of changes in BMI and in the adipose tissue area between images.
FIGURE 2.
Changes in visceral and subcutaneous radiodensities are associated with higher risks of death. Restricted cubic spline for the association of changes in (A) VAT and (B) SAT radiodensities with mortality in our cohort of early stage colorectal cancer patients. HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 3.
HRs for associations of change in adipose tissue radiodensity and mortality after colorectal cancer in the subset of patients that had follow-up images available (n = 1775)1
| Overall mortality | CRC-specific mortality | |||||
|---|---|---|---|---|---|---|
| Exposure | n/events | HR (95% CI) | P value | n/events | HR (95% CI) | P value |
| Change in VAT radiodensity between CT images, per SD (8 HU) | ||||||
| Increase > 1 SD | 234/113 | 1.53 (1.23–1.90) | 0.002 | 234/73 | 1.56 (1.19–2.06) | 0.002 |
| No change < 1 SD | 1398/431 | REF | 1398/257 | REF | ||
| Decrease > 1 SD | 143/59 | 1.11 (0.84–1.47) | 0.45 | 143/40 | 1.44 (1.02–2.02) | 0.036 |
| Change in SAT radiodensity between CT images, per SD (9 HU) | ||||||
| Increase > 1 SD | 165/93 | 1.88 (1.48–2.40) | <0.0001 | 165/61 | 2.00 (1.48–2.70) | <0.0001 |
| No change < 1 SD | 1426/ 435 | REF | 1426/260 | REF | ||
| Decrease > 1 SD | 184/75 | 1.20 (0.94–1.54) | 0.15 | 184/49 | 1.36 (1.00– 1.86) | 0.05 |
Data are over a median follow-up time after the second CT image of 8.6 years (minimum, 0.002 years; maximum 13.4 years). The models were adjusted for age, sex, race/ethnicity, colon compared with rectum cancer, cancer stage, Charlson Comorbidity Index, smoking status, receipt of chemotherapy and/or radiation, and days between the at-diagnosis and follow-up CT images, as well as changes in BMI squared and in adipose tissue area between images. CRC, colorectal cancer; HU, Hounsfield Unit; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Changes in SAT radiodensity and mortality.
The association of changes in SAT radiodensity and all-cause mortality was curvilinear (Figure 2B). In a categorical analysis, increases (HR, 1.88; 95% CI, 1.48–2.40) but not decreases in SAT radiodensity were associated with higher risks of all-cause mortality relative to remaining a stable radiodensity. Associations of increases and decreases in SAT radiodensity with CRC-specific mortality followed a similar pattern (Table 3). These results were independent of changes in BMI and the adipose tissue area between images.
Sensitivity analyses.
Excluding patients who died within 30 days of colorectal resection (n = 25) did not impact the results, nor did adjustment for the skeletal muscle tissue area. Analyses restricted to patients who maintained a stable body weight between images had reduced statistical power due to a smaller number of events (285 deaths, 110 from CRC). However, the point estimates were similar in magnitude and direction. That is, changes in VAT radiodensity were associated with increased risks of all-cause mortality, independent of the continuous change in BMI, visceral adipose tissue area, and other confounders: the HRs were 1.35 (95% CI, 0.88–2.06) for increases and 1.40 (95% CI, 0.93–2.11) for decreases in VAT radiodensity. For SAT radiodensity, the HRs were 1.73 (95% CI, 1.09–2.75) for increases and 1.38 (95% CI, 0.95–2.00) for decreases.
Discussion
In a cohort of patients with nonmetastatic CRC, higher radiodensities of VAT and of SAT at diagnosis increased the risk of death. Results were independent of BMI, adipose tissue area, and other confounders, including the comorbidity burden and disease stage, suggesting that adipose tissue radiodensity offers novel information regarding a patient's prognosis. In addition, we are the first to report that the VAT and SAT radiodensities change in the months following diagnosis. These changes had a curvilinear relationship with mortality: independent of changes in body weight and in adipose tissue area, patients with a stable radiodensity had the lowest risk of death.
Our findings that higher-radiodensity adipose tissue is associated with increased mortality is in line with findings of the few existing studies in advanced cancer populations (15–17). We can only speculate on the possible mechanisms underlying the relationship of adipose tissue radiodensity with mortality after cancer; further insight could come from studies that collect adipose tissue samples during surgery or clinical follow-up. In part, higher VAT or SAT radiodensities can be explained as a lower lipid content of adipocytes, potentially due to weight loss, a hallmark of progressive disease. However, we found similar results when we adjusted our analyses for continuous weight change between images or restricted our analyses to patients who maintained a stable body weight between images. Thus, a higher VAT or SAT radiodensity is not exclusively an indication of weight loss and reduction in adipocytes size, but may also reflect greater adipose tissue inflammation or other occult pathological processes.
With respect to longitudinal changes in adipose tissue radiodensity, the most notable increase in mortality risk was among patients whose VAT or SAT radiodensity increased after diagnosis. We speculate that 3 factors may explain the association of increases in radiodensity with higher mortality: inflammation, browning of adipose tissue, and edema. First, the increased radiodensity may be a sign of more inflammation, ultimately leading to higher mortality rates: in a study of 40 patients undergoing cardiac surgery (22), higher radiodensity was highly correlated with 18F fluorodeoxyglucose uptake in positron emission tomography–CT, which is indicative of inflammation (23). Second, others (15) have speculated that among patients with cachexia, an increase in radiodensity may represent browning of the white adipose tissue (24), indicative of lipid utilization and wasting of energy, leading to higher mortality. Whether browning of adipose tissue is also relevant in nonmetastatic disease remains to be elucidated. The third factor that may explain the association with mortality may be edema. Accumulation of protein-rich fluid in the abdominal cavity (ascites) will result in an increase in radiodensity of the adipose tissue; this accumulation may be the result of progressing disease, which can explain the higher mortality.
Decreases in adipose tissue radiodensity over time were also associated with higher risks of mortality, though these achieved statistical significance only for SAT radiodensity. It is possible that patients have decreased adipose tissue radiodensity due to increased adipocyte size during weight gain, perhaps leading to increased cardiometabolic morbidity, as has been observed in the Framingham Heart and Multi-Ethnic Studies (10, 14). Though our results were independent of changes in weight and the adipose tissue area, patients who gained at least 5% of body weight between images had the largest decreases in adipose tissue radiodensity.
While overall the patterns of association between radiodensities of VAT and SAT and mortality after cancer diagnosis were similar, there are anatomical and physiological differences between VAT and SAT which bear mention. Generally, VAT will have smaller adipocytes, higher vascularization, and a higher extent of inflammation (25–27), explaining the higher average radiodensity of this depot compared to SAT. Meanwhile, the size of the adipocytes in SAT is determined by the size of the lipid droplets inside the adipocyte, and thus upon the amount of lipid that is stored (28). When the capacity of the SAT depot to store lipid is disturbed, lipid will also be deposited in VAT and in organs such as the liver and muscle (27).
Strengths and limitations
As with any observational study, we cannot exclude the possibility of residual confounding. We adjusted our models for changes in BMI and for other potential confounding factors, but changes in adipose tissue radiodensity could be secondary to other, unmeasured physiological changes leading to an increase in mortality. Another limitation is that we had to exclude CT images where radiodensity could not be assessed due to imaging considerations such as photon starvation: these images were more often from patients with a very high BMI, and therefore patients with a very high BMI were more often excluded. Nevertheless, we still had large variability in BMI in our cohort, ensuring generalizability. We used a single CT image at the level of the third lumbar vertebrae to estimate the average adipose tissue radiodensities of VAT and SAT. We assume that this is representative for total adipose tissue radiodensity of those depots, but variability of radiodensity across depots of adipose tissue requires further investigation. In addition, our cohort derives from real-world electronic medical record data; as such, our results are highly generalizable, but unfortunately we do not have all the technical parameters of the CT scanners available. Prior research has shown that variations in contrast phase and other parameters introduce small variations in the quantification of adipose tissue area and radiodensity (29, 30), but these parameters are unlikely to be related to prognosis; thus, the effect of these small differences based on the specifics of the scanning protocol is most likely to introduce random variability, potentially weakening the observed associations (bias towards the null). Strengths of this work are the inclusion of a large patient population from a community setting with repeated measures of body composition. To the best of our knowledge, this is the first study with sequential CT images that has examined longitudinal changes in radiodensity.
Conclusion
We observed that higher radiodensity of adipose tissue was associated with increased mortality after a diagnosis of nonmetastatic CRC. In addition, changes in radiodensity of adipose tissue, while correlated with BMI and weight change, had prognostic importance independent of body weight. Thus, changes in radiodensity may reflect biological processes that occur before changes in weight are noted clinically. Our results suggest that measures of body composition—including adipose tissue quality—are powerful prognostic indicators that deserve more attention in clinical practice.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows – EMCF, RMW: project conception, research plan, and data analysis; and all authors: contributed to data interpretation, editing, and a critical review and read and approved the final manuscript.EMCF was supported by National Cancer Institute grants K01CA226155 and R01CA240394. All other authors report no conflicts of interest.
Notes
EMCF was supported by National Cancer Institute grants K01CA226155 and R01CA240394; data collection was supported by National Cancer Institute grant R01CA175011.
Supplemental Figure 1 and Supplemental Tables 1–2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
EMCF and RMW contributed equally to this work.
Abbreviations used: CRC, colorectal cancer; C-SCANS, Colorectal Cancer Sarcopenia and Near-Term Survival; CT, computed tomography; HU, Hounsfield Unit; KPNC, Kaiser Permanente Northern California; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Contributor Information
Elizabeth M Cespedes Feliciano, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Renate M Winkels, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands.
Jeffrey A Meyerhardt, Dana Farber Cancer Institute, Boston, MA, USA.
Carla M Prado, Department of Agricultural, Food and Nutritional Science, Faculty of Agricultural, Life and Environmental Sciences, University of Alberta, Canada.
Lydia A Afman, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands.
Bette J Caan, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Data Availability
Data described in the article, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The requirement for informed consent was waived by the institutional review board. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were all treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteed.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DRet al. . SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute. 2019. [Google Scholar]
- 2. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan MLet al. . Explaining the obesity paradox: The association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hopkins JJ, Reif R, Bigam D, Baracos VE, Eurich DT, Sawyer MM. Change in skeletal muscle following resection of stage I–III colorectal cancer is predictive of poor survival: A cohort study. World J Surg. 2019;43(10):2518–26. [DOI] [PubMed] [Google Scholar]
- 4. Pring ET, Malietzis G, Kennedy RH, Athanasiou T, Jenkins JT. Cancer cachexia and myopenia–Update on management strategies and the direction of future research for optimizing body composition in cancer–A narrative review. Cancer Treat Rev. 2018;70:245–54. [DOI] [PubMed] [Google Scholar]
- 5. Brown JC, Caan BJ, Prado CM, Cespedes Feliciano EM, Xiao J, Kroenke CH, Meyerhardt JA. The association of abdominal adiposity with mortality in patients with stage I–III colorectal cancer. J Natl Cancer Inst. 2020;112(4):377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Baar H, Winkels RM, Brouwer JGM, Posthuma L, Bours MJL, Weijenberg MP, Boshuizen HC, van Zutphen M, van Duijnhoven FJB, Kok DEet al. . Associations of skeletal muscle mass, fat mass and mortality among men and women with stage I–III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(5):956–65. [DOI] [PubMed] [Google Scholar]
- 7. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, Kroenke CH, Castillo A, Kwan ML, Prado CM. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I–III colorectal cancer: A population-based cohort study (C-SCANS). J Cachexia Sarcopenia Muscle. 2018;9(4):664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Baar H, Beijer S, Bours MJL, Weijenberg MP, van Zutphen M, van Duijnhoven FJB, Slooter GD, Pruijt JFM, Dronkers JJ, Haringhuizen Aet al. . Low radiographic muscle density is associated with lower overall and disease-free survival in early-stage colorectal cancer patients. J Cancer Res Clin Oncol. 2018;144(11):2139–47. [DOI] [PubMed] [Google Scholar]
- 9. Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield Set al. . Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(1):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah RV, Allison MA, Lima JAC, Abbasi SA, Eisman A, Lai C, Jerosch-Herold M, Budoff M, Murthy VL. Abdominal fat radiodensity, quantity and cardiometabolic risk: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26(2):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, Murabito JM, Hoffmann U, Fox CS. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100(1):227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15(3):139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Côté JA, Nazare J-A, Nadeau M, Leboeuf M, Blackburn L, Després J-P, Tchernof A. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte. 2016;5(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6(7):762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebadi M, Moctezuma-Velazquez C, Meza-Junco J, Baracos VE, Dunichandhoedl AR, Ghosh S, Sarlieve P, Owen RJ, Kneteman N, Montano-Loza AJ. Visceral adipose tissue radiodensity is linked to prognosis in hepatocellular carcinoma patients treated with selective internal radiation therapy. Cancers. 2020;12(2):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charette N, Vandeputte C, Ameye L, Bogaert CV, Krygier J, Guiot T, Deleporte A, Delaunoit T, Geboes K, Van Laethem JLet al. . Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: A post hoc analysis of two non-randomized phase II trials. BMC Cancer. 2019;19(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veld J, Vossen JA, De Amorim Bernstein K, Halpern EF, Torriani M, Bredella MA. Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol. 2016;26(12):4649–55. [DOI] [PubMed] [Google Scholar]
- 18. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 19. Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–75. [DOI] [PubMed] [Google Scholar]
- 20. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22. [DOI] [PubMed] [Google Scholar]
- 21. Hertzmark E, Li R, Hong B, Spiegelman D. The SAS GLMCURV9 macro. [Internet]. Harvard, Boston MA: Channing Laboratory; 2014. Available from: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2014/10/glmcurv9.pdf. [Google Scholar]
- 22. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli A-M, Akoumianakis Iet al. . Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):eaal2658. [DOI] [PubMed] [Google Scholar]
- 23. Reijrink M, de Boer SA, Antunes IF, Spoor DS, Heerspink HJL, Lodewijk ME, Mastik MF, Boellaard R, Greuter MJW, Benjamens Set al. . [18F]FDG uptake in adipose tissue is not related to inflammation in type 2 diabetes mellitus. Mol Imaging Biol. 2021;23(1):117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson Get al. . A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–47. [DOI] [PubMed] [Google Scholar]
- 25. Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes Rev. 2010;11(1):11–8. [DOI] [PubMed] [Google Scholar]
- 26. Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit Rev Clin Lab Sci. 2015;52(6):301–13. [DOI] [PubMed] [Google Scholar]
- 27. Tchernof A, Després J-P. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93(1):359–404. [DOI] [PubMed] [Google Scholar]
- 28. Gillian EW, Paolo M, Roberta R, Gianni B, Flavia P. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm Mol Biol Clin Investig. 2014;19(1):57–74. [DOI] [PubMed] [Google Scholar]
- 29. Paris MT, Furberg HF, Petruzella S, Akin O, Hotker AM, Mourtzakis M. Influence of contrast administration on computed tomography-based analysis of visceral adipose and skeletal muscle tissue in clear cell renal cell carcinoma. JPEN J Parenter Enteral Nutr. 2018;42(7):1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Troschel AS, Troschel FM, Fuchs G, Marquardt JP, Ackman JB, Yang K, Fintelmann FJ. Significance of acquisition parameters for adipose tissue segmentation on CT images. Am J Roentgenol. 2021;217(1):177–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available because this was a retrospective cohort study using electronic medical records. The requirement for informed consent was waived by the institutional review board. Consequently, study subjects did not explicitly consent for their data to be shared publicly. Moreover, study subjects were all treated within a single health system for an uncommon disease, and our ability to preserve subject anonymity cannot be guaranteed.