Abstract
The neural mechanisms that underlie selective attention in children are poorly understood. By administering a set-shifting task to children with intracranial electrodes stereotactically implanted within anterior cingulate cortex (ACC) for epilepsy monitoring, we demonstrate that selective attention in a set-shifting task is dependent upon theta-band phase resetting immediately following stimulus onset and that the preferred theta phase angle is predictive of reaction time during attentional shift. We also observe selective enhancement of oscillatory coupling between the ACC and the dorsal attention network and decoupling with the default mode network during task performance. When transient focal epileptic activity occurs around the time of stimulus onset, phase resetting is impaired, connectivity changes with attentional and default mode networks are abolished, and reaction times are prolonged. The results of the present work highlight the fundamental mechanistic role of oscillatory phase in ACC in supporting attentional circuitry and present novel opportunities to remediate attention deficits in children with epilepsy.
Keywords: ADHD, Attention, oscillations, phase resetting
The neural mechanisms subserving human attention are complex and incompletely understood. Rhythmic oscillatory activity of neural assemblies supports a variety of neurocognitive functions, including attention (Fiebelkorn and Kastner 2019, 2020). At the neuronal level, oscillation of membrane potentials imposes precise temporal windows for integration and transmission of synaptic inputs (Volgushev et al. 1998). Inputs received in synchrony with membrane oscillations are selectively propagated, whereas others are suppressed (Izhikevich et al. 2003). Oscillatory activity modulates neuronal interactions and the phase of oscillations mechanistically alters connectivity among neural assemblies, thereby facilitating selective attention (Womelsdorf and Fries 2007). Precise phase relations precede brain activity and neural network connectivity diminishes when synaptic inputs arrive at random phases (Womelsdorf and Fries 2007). For instance, pre-stimulus phase can predict suppression of object perception depending on when the stimulus is presented relative to the phase of oscillation (Mathewson et al. 2009; Mazaheri et al. 2009; Sierra et al. 2015).
The anterior cingulate cortex (ACC) is a region of the brain where the precision of oscillatory activity is critical for the maintenance and sustainment of attention (Voloh et al. 2015). Along with the insular cortices, the ACC comprises a major hub of the salience network (Seeley et al. 2007) involved in detecting and filtering salient stimuli. The ACC is recruited to focus attention on behaviourally-relevant stimuli and to regulate conflict between an object of attention and distractors during goal-directed, sustained attention (Kerns et al. 2004; Weissman et al. 2005; Roelofs et al. 2006). Modulation of ACC activity affects processing speed and provides a continuously updated prediction of expected cognitive demands in order to adapt future behavioral responses (Sheth et al. 2012). It has been shown that reduced ACC activity accounts for attention lapses (Weissman et al. 2006) and lesions of the ACC impair sustained attention regardless of the sensory input modality (Wu et al. 2017).
Phase resetting represents a mechanism by which the salience of attention-demanding stimuli may be modulated in/by the ACC. This phenomenon refers to the expression of a concurrent change in oscillatory phase in temporal association with the perception of a stimulus (Canavier 2015). Phase resetting serves several critical purposes. First, realignment of the phase of an oscillation to a specific reference point allows phasic information to be consistently decoded. Second, resetting allows a periodic stimulus to control the frequency and phase of a neural oscillation to provide an appropriate time frame for encoding and decoding. Finally, consistency in phase shifts may support the integration of information flow across a large-scale network. The degree to which attention is dependent upon phase resetting within the ACC has not been characterized in humans.
Attention deficits are disproportionately prevalent among children with epilepsy (Cohen et al. 2013; Reilly et al. 2014; Besag et al. 2016). While 4%–12% of the general population of school-aged children manifest attention deficits, one-third of those with epilepsy exhibit these impairments, which contribute to academic difficulties, psychosocial isolation and minimized future potential (Brown et al. 2001). It is thought that such deficits in epileptic patients are in part related to dysfunction of the neurological mechanisms supporting attention, rather than the effects of medications or chronic seizures, as significant behavioral problems and attention deficits often predate the onset of seizures and the diagnosis of epilepsy (Austin et al. 2001).
Momentary lapses in attention, also known as transient cognitive impairment, are proposed to be the substrate of attention deficits in affected children (Aircardi 1996) and have been linked to ongoing interictal epileptic activity (Binnie 1993). Children with attention deficits but without a known seizure disorder also exhibit epileptic-like discharges on electroencephalography (EEG) (Silvestri et al. 2007; Frye et al. 2010; Attila et al. 2013). Interictal epileptiform activity at the time of stimulus presentation has been reported to disrupt task performance (Aarts et al. 1984) and the frequency of interictal epileptiform activity has been associated with lowered performance on attention tasks (Nicolai et al. 2012). The administration of antiepileptic treatment has been associated with both reductions in epileptiform activity and improved psychological function (Marston et al. 1993). However, there remains a critical gap in knowledge regarding the mechanisms that link interictal epileptiform activity to transient cognitive impairment (momentary lapses) and attention deficits.
Given the converging evidence for (i) the importance of phase precision of oscillatory neural activity in supporting neurocognitive functions; (ii) the role of the ACC in maintaining and modulating attention; and (iii) transient attention deficits as a result of epileptiform activity, we sought to study phase resetting as a mechanism of attention and its potential breakdown during momentary lapses thereof. Unique intracranial recordings within the ACC in children with drug resistant epilepsy were studied during a set-shifting task that required alternating attention to shift from one task rule to another. Findings revealed that oscillatory phase precision was correlated with processing speed, which is highly associated with measures of inattentive behavior (Kalff et al. 2005) and functional limitations (Cook et al. 2018) in children with attention deficits.
The current work presents novel understanding of fundamental mechanisms of attention using intracranial recordings in children with epilepsy. Given the critical role of oscillatory activity in the formation of neural networks, we provide evidence for a phase-based neural syntax subserving attention in the ACC. We highlight the breakdown of oscillatory interactions within the ACC during momentary lapses in attention related to epileptic events, thus providing further support for the mechanistic importance of fluctuations in neural activity in maintaining normative function. These data provide a framework to study the neurophysiological underpinnings of attention and further, a potential means to rescue attention deficit through targeted neuromodulation.
Materials and Methods
Study Population
Thirteen children with drug resistant epilepsy underwent stereoelectroencephalography (SEEG) with stereotactic placement of intracranial depth electrodes (0.86 mm diameter; AdTech, USA) directly into the ACC. The participants’ detailed clinical demographics including sex, seizure onset location, pathology, and focality are presented in Supplementary Table S1. In all cases, using presurgical data, it was hypothesized that the ACC was part of the seizure propagation zone but not the zone of seizure onset. The ACC was targeted anatomically using preoperative 3 T magnetic resonance imaging (MRI). The surgical trajectory to the ACC was planned to be the most orthogonal while avoiding cortical veins and sulci. On the day of the procedure, under general anesthesia, a Leksell stereotactic frame was applied and computed tomography-angiography (CTA) was performed. Fusion of the CTA to the preoperative MRI with the planned targets allowed for frame-based stereotactic placement of ACC electrodes. Additional electrodes were placed in the presumed epileptogenic zone, as individualized for each patient. One patient underwent electrode implantation using a frameless stereotactic approach. Imaging performed after implantation confirmed the locations of all electrodes.
All participants underwent neuropsychological assessment prior to invasive monitoring, which included measures of learning abilities. During a rote learning task, learning was indexed as the combined recall score of the final three trials for a given list of words, which is impacted by sustained attention (Talley 1993). A parent report of attention difficulties was also obtained for all participants using the Child Behavior Checklist (Achenbach 1991).
Children were prospectively recruited into the study. The Research Ethics Board of the Hospital for Sick Children approved the protocol, which complies with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Localization of Electrodes in Common Space
The locations of the electrodes were marked in CT space. The CT was linearly registered with the pre-operative MRI, and the pre-operative MRI was non-linearly registered with the ICBM152 MNI template. Registration was performed using FSL 5.0.9–5.
Each electrode was further assigned to a particular resting state brain network when overlaid with an atlas modeling the 7-network cortical parcellation template introduced by Yeo and colleagues (Thomas Yeo et al. 2011). Regions of interest of 5 mm diameters were created at the location of recording electrodes from the ACC as well as the epileptogenic lesion. All electrodes were confirmed to be localized within the ACC based on concordant atlas labels using both the Automated Anatomical Labelling and Harvard-Oxford Cortical atlases (Tzourio-Mazoyer et al. 2002; Makris et al. 2006) (Fig. S2) In all cases, the ACC electrode chosen was outside the seizure-onset zone. Lesional electrodes were chosen by experienced clinical neurophysiologists (A.O., H.O.) as the most active electrodes interictally and the site of seizure onset.
Attentional Set Shifting Task and the Anterior Cingulate
A set shifting task (Oh et al. 2014) was performed by all participants while clinical recordings were taking place from the intracranial electrodes (see Fig. 1b). Set shifting is a measure of cognitive flexibility that relies on complex cognitive processes including attention shifting, inhibition and salience detection (Dajani and Uddin 2015). During set shifting, subjects select responses based on an implicit rule that shifts every few trials. Selective attention is essential to efficiently track these shifts and accurately adapt one’s response; slowed reaction times and errors indicate a lapse in attention. A trial concluded when a button press was registered or after 4000 ms. Each trial was presented with a jittered interstimulus interval between 800–1200 ms (mean 1000 ms).
Figure 1 .

Localization of intracranial electrodes and results of the attentional set-shifting task. (a) The location of the stereotactic electrodes recordings from the lesion (red) and ACC (blue) in normalized MNI brain space for all subjects combined. (b) Time-frequency decomposition time-locked to HFO occurrences in both the lesion and the anterior cingulate (P < 0.05 FDR-controlled). HFOs are localized to the lesion and are not significant in the anterior cingulate. (c) Schematic of the set shifting attention-demanding task, demonstrating non-shift, intradimensional shift, and extradimensional shift trials. Shift trials are followed by three or more non-shift trials for acclimatization. (d) Time-frequency decomposition showing relative change in power from baseline and demonstrating theta and alpha synchronization and beta desynchronization in shift trials. Regions with significant differences (P < 0.05 FDR-controlled) relative to the −0.75 to 0 s baseline interval are shown with full opacity, non-significant time-frequency regions are masked. (e) Time-frequency decomposition with highlighted regions demonstrating significant relationship to reaction time. Relative increase of peri-stimulus beta power was related to slowed reaction time in shift trials (P < 0.05 FDR-controlled).
The neuroimaging literature has highlighted the role of attention in successful completion of this task and the critical role of the ACC in these processes. Whereas multiple brain regions demonstrate functional activation during set shifting (Buchsbaum et al. 2005; Dunkley et al. 2015), the operational task of set-shifting is an attention-dependent process that is critically reliant upon the ACC (Dajani and Uddin 2015). Indeed, cognitive flexibility is often described in the context of processes requiring shifts in attention, including attentional flexibility (Vilgis et al. 2015), attention switching (Casey et al. 2004), and attentional set-shifting (Owen et al. 1991). Numerous studies involving multiple non-invasive modalities have identified activations in attentional set-shifting within the ACC (Monchi et al. 2001; Periáñez et al. 2004; Smith et al. 2004; Kim et al. 2012). Activity within the ACC prior to stimulus onset also reliably predicted cognitive flexibility, in keeping with its attentional role within the recruited executive function network (Leber et al. 2008). Other frontal and parietal brain regions implicated in executive control are also activated by set-shifting tasks (Oh et al. 2014). To date, direct recordings from the ACC during set-shifting tasks have not been reported in humans.
The primary outcome measure was processing speed, as indexed by the trial reaction time. This was selected because this measure is the most predictive of inattentive behavior and is superior to task accuracy (i.e., error rates) at distinguishing children with attention deficits from pathological and healthy controls (Kalff et al. 2005). Second, processing speed has been previously used to monitor response to medical treatments aimed at improving attention deficits (Adalio et al. 2018; Thorsen et al. 2018). Finally, reaction time strongly correlates with clinical and functional limitations that are prevalent in affected children, including academic skills, adaptive behavior, self-reported anxiety and social competence (Cook et al. 2018).
Signal Acquisition and Oscillation Analysis
Participants underwent digitally recorded intracranial video-EEG (iEEG) using a Natus Quantum 256-channel amplifier (Natus Medical Inc., Pleasanton, CA, USA) with a sampling rate of 2048 Hz and an anti-aliasing filter at 512 Hz (−6 dB/oct) applied prior to sampling. The recordings were exported as European Data Format Plus (EDF+) files (Kemp and Olivan 2003) and imported into MATLAB for subsequent analysis (R2018a, The MathWorks, Natick, MA, USA). A notch-filter was applied to remove 60 Hz line noise and harmonics. The data were then band-pass filtered to 1–500 Hz (4th-order Butterworth). The electrode data were digitally re-referenced to a common average reference.
For each source and frequency range the analytic signal, S(t), was then obtained from the filtered signal, 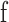 (t), and its Hilbert transform,
(t), and its Hilbert transform,  (t), for each frequency band as follows:
(t), for each frequency band as follows:
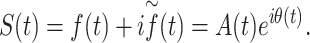 |
(1) |
From this result, A(t) and θ(t) were derived, representing the timeseries of the instantaneous envelope amplitude and instantaneous phase, respectively.
Detection of Epileptic High Frequency Oscillations
While participants performed the task, the lesional electrode within the epileptogenic zone often registered coincidental epileptic activity. In order to determine the presence of epileptic activity within the lesion relative to the timeframe of the stimulus, we actively determined the occurrence of pathological high-frequency oscillations (HFOs) using an open-source framework (Wong et al. 2021). High frequency oscillations are transient events that are a robust marker of epileptogenicity (Ochi et al. 2007; Akiyama et al. 2011) and were identified on the basis of accepted definitions (Engel et al. 2009). In presurgical evaluation, the detection of HFOs in EEG can be used to identify the seizure onset zone (SOZ) (Crépon et al. 2010; Chua et al. 2011) and predict clinical outcomes following resection (Quitadamo et al. 2018). HFOs appear to be more specific to the epileptogenic zone (Worrell et al. 2004; Jacobs et al. 2008) than the classical biomarker: the epileptic spike; and appear to reflect the epileptogenicity of the tissue better, increasing in frequency immediately prior to seizure onset (Jacobs et al. 2010) and with reduction of anti-epileptic medications (Zijlmans et al. 2011). Importantly, these epileptic dynamics are not typically detected on noninvasive electromagnetic recordings and can only be reliably gleaned from invasive monitoring with intracranial electrodes. A detailed summary of the HFO detection procedure we utilize has been previously described (Wong et al. 2021).
Induced Oscillatory Power
The spectral properties of the signal were measured using an induced analysis. Induced oscillatory power was computed relative to stimulus onset. This was performed through FieldTrip toolbox’s ft_freqanalysis function with 7-cycle Morlet wavelets (Oostenveld et al. 2011). The data were epoched relative to the stimulus marker, and for HFOs. Time-and-frequency-resolved oscillatory power was computed through the wavelet transform. FDR-controlled correlations between the time-frequency power and reaction times were also computed.
Phase Resetting
Phase properties of the signals from the ACC were analyzed to investigate oscillatory dynamics occurring during attentional set-shifting. Phase resetting was calculated within the ACC during attention tasks by defining a z-score for a Rayleigh test at each time point prior to and after presentation of the stimulus. The Rayleigh test z-score (Rayleigh Z) is a measure of consistency of phase at a given time point (Berens 2009). If random phase is present, uniform distribution of instantaneous phases would be expected relative to the reference point of the stimulus presentation, indicating a low Rayleigh Z. The presence of a preferred phase denotes phase resetting, which is indexed by an elevated score (Fig. 2b). A random inter-stimulus interval was introduced to prevent the periodic nature of oscillations from imposing falsely elevated Rayleigh Z.
Figure 2 .

a: Experimental set-up. The filtered signal from the ACC is computed during the set-shifting task. The oscillatory phase is then derived from the Hilbert transform. At each sample, Rayleigh Z is computed across trials, providing a measure of the consistency or randomness of signal phase, which results in high and low Rayleigh Z, respectively. b/c: Phase resetting in shift and non-shift trials. Rayleigh Z, a measure of asymmetry of the phase distribution, is computed at each time point in shift (b) and non-shift trials (c) in theta. The shading indicates 95% confidence intervals derived from bootstrap sampling. Trials without associated HFOs are shown in blue; those with HFOs in orange. The circular-linear correlation coefficient between the trial reaction time and the phase angle in the ACC is shown below. d: Mean reaction time of each subject in trials dichotomized by alignment to the preferred phase or the antiphase. Trials with proper phase alignment are attended faster than trials with phase aligned opposite to the preferred phase (P = 0.006). e: Reaction time distribution by alignment to preferred phase or to the antiphase. Trials within each subject were binned into quartiles and shown by whether the instantaneous phase was aligned to the preferred phase or to the antiphase. In-phase trials tend to be faster than antiphase trials.
To measure associations between oscillatory phase and reaction time, for every sample, the circular-linear Pearson’s correlation coefficient was computed between phase and eventual reaction time (Fig. 2b and c) using the provided function from the circstat toolbox (Berens 2009).
With regards to preferred phase analysis, phase oscillations at the time of maximal phase resetting were binned into six regions of π/3 radians each. Instantaneous phases deviating by the preferred phase by more than π/6 radians were therefore deemed to be in another more distant phase bin. Multivariate mixed effects analysis adjusting for patient age was used to investigate associations between instantaneous phase and reaction time.
Inter-Regional Time-Resolved Connectivity
In order to assess inter-regional connectivity of larger-scale networks, time-resolved phase synchrony between electrodes implanted in the ACC and canonical resting-state networks (i.e., ACC-dorsal attention network or ACC-default mode network) was computed using the PDD (Breakspear et al. 2004; Thomas Yeo et al. 2011). PDD estimates the inter-trial likelihood of inter-regional phase synchrony at each time point and was selected for its ability to estimate time-resolved phase-synchrony-based functional connectivity without the need to temporally window the data. PDD is particularly suited for analysis of transient network impairments caused by epileptiform activity resulting in desynchronous bursts in an otherwise synchronous network (Breakspear et al. 2004).
Briefly, instantaneous phase estimates from the canonical frequency bands, θ(t), were computed from the Hilbert transform. The derivative of the phase difference between electrode pairs was computed using the PDD, defined as
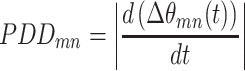 |
(2) |
where 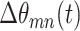 is the modulus of the phase difference between electrodes m and n. Pairs of electrodes in phase synchrony will have phases that evolve in synchrony, resulting in a derivative of the phase difference approximately near zero. Otherwise, the value of Eq. 2 will be bounded by some number k < 2π, where k can be used selectively as a threshold to define phase synchrony.
is the modulus of the phase difference between electrodes m and n. Pairs of electrodes in phase synchrony will have phases that evolve in synchrony, resulting in a derivative of the phase difference approximately near zero. Otherwise, the value of Eq. 2 will be bounded by some number k < 2π, where k can be used selectively as a threshold to define phase synchrony.
We used frequency-adaptive thresholds, as previously described (Breakspear et al. 2004), by examining individual phase timeseries. The derivative timeseries thresholded to 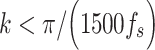 rad s−1. Subthreshold (i.e., in synchrony) clusters of time points with duration
rad s−1. Subthreshold (i.e., in synchrony) clusters of time points with duration 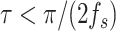 seconds were considered spurious. Remaining subthreshold clusters with sufficient duration indicated true phase synchrony at that sample and in that trial. The threshold-binarized clusters were averaged over trials to obtain the proportion of trials with phase synchrony and was used as an estimate of synchronization likelihood.
seconds were considered spurious. Remaining subthreshold clusters with sufficient duration indicated true phase synchrony at that sample and in that trial. The threshold-binarized clusters were averaged over trials to obtain the proportion of trials with phase synchrony and was used as an estimate of synchronization likelihood.
To evaluate PDD across subjects, the PDD timeseries were then z-scored relative to the −750 to −500 ms baseline (i.e., subtracting the baseline mean and dividing by baseline standard deviation). To further determine the significance of the resultant PDD z-scores, a one-sample t-test was computed at each timepoint in the timeseries and corrected for multiple comparisons. Differences between trial types (i.e., trials with HFOs and trials without HFOs) were calculated using permutation testing and corrected for multiple comparisons. Correction for multiple comparisons was performed using a point-by-point false discovery rate, which controls the overall rate of type I error across all comparisons. A widely accepted threshold of q < 0.05 was used (Benjamini and Hochberg 1995).
To visualize significant PDD synchronization in trials without HFOs over time, each ACC-network PDD timeseries was segmented into five 250-ms time-windows, and within each time-window, the highest z-score from trials without HFOs was extracted and plotted onto the respective glass brain as a function of electrode size (Fig. S9).
Statistical Analyses
Behavioral data analysis was performed using a mixed-effects multivariate hierarchical analysis using the lmer package in R (R Core Team 2019). Results of these analyses are presented as means with 95% confidence intervals unless otherwise stated. Differences between states were calculated through permutation tests and corrected for multiple comparisons, where appropriate. Correction for multiple comparisons were performed using a point-by-point false discovery rate, whereby regions of the timeseries that reached q < 0.05 were viewed as significant. Circular data are analyzed as described above. All calculations were performed in MATLAB software (R2020a, The MathWorks, Natick, MA, USA), unless otherwise specified.
Results
Behavioral Correlates of Reaction Time
Thirteen children undergoing invasive epilepsy monitoring with stereotactic EEG (sEEG) with at least one intracranial electrode in ACC were included in the study. Figure 1a and b provides an overview of intracranial electrode localization and experimental paradigm for the set-shifting task. Across all patients, the mean reaction time for the set-shifting task was 0.817 ± 0.290 s (mean ± standard deviation), with significantly shorter reaction times in trials that did not require attentional shifting (β = −0.07, t = −3.4, P < 0.01). In shift trials, on univariable mixed-effects analysis, there was a significant association between child age and reaction time, whereby older children were able to complete the attentional shift trials of the task with shorter reaction times (Table S3; β = −0.07, t = −2.6, P = 0.03). Similarly, during non-shift trials, older children with higher scores on the general abilities index (GAI) achieved faster reaction times (Table S3; β = −0.01, t = −2.4, P = 0.03).
Increased Theta and Alpha Power Following Stimulus Presentation Are Not Associated with Reaction Time
Induced analysis of mean signal power is provided in Figure 1c and d. This demonstrated event-related synchronization in the theta band and desynchronization in beta band following stimulus presentation in attentional shift trials; a less robust theta synchronization is seen in trials without attentional shift (Fig. 1c). In shift trials, there was no correlation between theta or alpha band power increases and reaction time. Only increases in beta-band power at the time of stimulus presentation were associated with slower reaction times for shift trials (Fig. 1e, P < 0.05 FDR-controlled). No spectral changes in induced analysis were associated with reaction time for non-shift trials.
Phase Resetting within Anterior Cingulate Cortex Indexes Attentional Shifts and Precision of Oscillatory Phase Angle Is Predictive of Reaction Time
Following stimulus presentation, several brain regions demonstrated resetting of oscillatory phase in theta, alpha, and beta bands as demonstrated by non-uniform phase distributions and therefore an elevated Rayleigh Z within the combined 560 recording electrodes (Fig. S3). These included regions within the frontal and temporal lobes as well as peri-Rolandic cortex. In ACC, post-stimulus phase-resetting responses were robust and unique to the theta band (Fig. S3). In both shift and non-shift trials, ACC consistently demonstrated elevated post-stimulus theta-band Rayleigh Z (Fig. 2b and c). These findings are recapitulated at the individual patient level (Fig. S4). We did not observe significant phase resetting in the beta band within the ACC (Fig. S5). Furthermore, when shift trials were analyzed by type of shift (intradimensional vs. extradimensional), no significant differences were identified between the two conditions.
The extent of phase resetting of theta oscillations was significantly associated with reaction time for both trial types (Fig. 2c). To investigate the influence of phase precision on reaction time, a preferred phase was defined during the period of maximal phase resetting based on the Rayleigh Z distribution. Anti-phase was defined by a deviation of π radians from the preferred phase angle, effectively a phase angle at the opposing side of the unit circle. On multivariable mixed effects analysis, trials that were in-phase with the preferred phase angle demonstrated significantly faster reaction times compared to those that were anti-phase to the preferred phase angle exclusively for the attentional shift condition (Table S4 and Fig. 2d, e; β = −0.09, t = −2.7, P = 0.006). When the reaction times were categorized by quartiles, attentional shift trials with preferred theta phase demonstrated greater proportion of faster reaction times (Fig. 2e). No significant effect of preferred phase when compared to the antiphase oscillation was observed in trials without attentional shift (t = −0.52, P = 0.56).
Epileptic Activity Impairs Phase Resetting and Prolongs Reaction Time
High frequency oscillations (HFOs) gleaned through spectral analysis of intracranial EEG data are robust biomarkers of epileptogenicity, which occurred spontaneously within the epileptogenic foci during task performance (Fig. 1d). The distribution of HFOs per trial is shown in Figure S6 and the total number of events captured per patient is shown in Table S5.
In trials requiring attentional shift that were coincident with epileptic HFOs, no significant phase resetting was evident (Fig. 2b). Similarly, during trials that did not require attentional shifts, there was significant attenuation of the post-stimulus theta phase resetting in trials coincident with HFOs (Fig. 2c). A greater number of HFO events and spikes were associated with prolonged reaction time in attentional shift trials (P = 0.003 for HFOs, P = 0.02 for spikes) and non-shift trials (P = 0.003 for HFOs, P < 0.001 for spikes), after adjusting for child age. HFO events were randomly distributed throughout trials (Figs S7 and S8). A multivariable analysis incorporating age showed that attentional shift trials with coincident events occurring in the pre- (−0.75 to −0.25) and peri-stimulus period (−0.25 to 0.25 s) were significantly more likely to have prolonged reaction time (Table S6; P = 0.04).
Coordinated Interactions between ACC and Internal/External Intrinsic Connectivity Networks Facilitate Attentional Behavior and Are Impaired by Epileptic Dynamics
A critical role of the ACC in attentional processing relates to its coordinated interactions with large-scale resting-state networks (Kumfor et al. 2015; Voloh et al. 2015; Chand and Dhamala 2017). When the functional connectivity between two brain regions was indexed using the phase difference derivative (PDD), coordinated interactions were identified between the ACC and attention-salient resting-state networks (namely dorsal and ventral attention [DAN/VAN], frontoparietal [FPN], somatomotor, and default-mode networks [DMN]) across several canonical frequency bands (Fig. S9). Of these, significant differences were identified in the DAN and DMN in the theta band between trials with and without HFOs.
Eight patients in our series had coverage of nodes within the DAN (Fig. 3a), which critically subserves external attention (Dixon et al. 2018). In the absence of HFOs, an increase in functional connectivity was observed between the ACC and the DAN in the theta band (thick blue lines in Fig. 3b and c; P < 0.05, q < 0.05). In trials with coincident HFOs, these coordinated interactions between the ACC and the DAN were not observed (red lines in Fig. 3b and c). Rather, during coincident HFOs, a paradoxical decrease in theta connectivity was evident following stimulus presentation (thick red line in Fig. 3b).
Figure 3 .

Theta-band inter-regional functional connectivity between the anterior cingulate (ACC) and the dorsal attention network (DAN) and default mode network (DMN). a: ACC-DAN electrode locations. Electrodes recording from the ACC and key regions of the ipsilateral DAN were classified using the 7-network cortical parcellation atlas introduced by Yeo and colleagues (Thomas Yeo et al. 2011) (n = 8). b/c: ACC-DAN connectivity in shift and non-shift trials. Shift (b) and non-shift (c) trials without HFOs demonstrate increased post-stimulus connectivity between the ACC and the DAN (thick blue lines; P < 0.05, q < 0.05). Shift (b) trials with HFOs are associated with decreased post-stimulus connectivity between the ACC and the DAN (thick red line; P < 0.05, q < 0.05). Non-shift (c) trials with HFOs are not associated with change in ACC-DAN synchronization following stimulus onset (red line). ACC-DAN post-stimulus connectivity in shift (b) and non-shift (c) trials differs significantly between trials with HFOs and trials without HFOs (black lines with asterisks indicate significance; P < 0.05 using permutation testing). Shaded regions denote 95% confidence intervals. d: ACC-DMN electrode locations Electrodes recording from the ACC and key regions of the ipsilateral DMN were classified using the 7-network cortical parcellation atlas introduced by Yeo and colleagues (Thomas Yeo et al. 2011) (n = 11). e/f: ACC-DMN connectivity in shift and non-shift trials. Shift trials (e) demonstrate decreased post-stimulus connectivity between the ACC and the DMN (thick blue line and red lines; P < 0.05, q < 0.05). Non-shift (f) trials demonstrate increased post-stimulus connectivity between the ACC and the DMN (thick blue line; P < 0.05, q < 0.05). This trend was not statistically significant for trials with HFOs. Shaded regions denote 95% confidence intervals.
Eleven patients had electrode coverage that sampled nodes of the default-mode network (DMN; Fig. 3d), which is involved in internal mental processing (Dixon et al. 2018). Trials requiring attentional shifts (Fig. 3e) demonstrated decreased peri-stimulus functional connectivity between the ACC and the DMN in the theta band (thick blue and red lines in Figure 3e; P < 0.05, q < 0.05), whereas non-shift trials did not (Fig. 3f). During shift trials with coincident HFOs, the peri-stimulus functional disconnection between the ACC and DMN network was attenuated and delayed.
Notably, local connectivity between individual DAN/DMN nodes and the ACC were also analyzed. Functional interactions between ACC and DAN were most related to activity in pre-central and supramarginal nodes of the DAN. Regarding interactions with DMN, the most contributory nodes were the frontal and parietal cortices. Further analysis of shift connectivity by the extradimensional and intradimensional conditions revealed no significant differences between the two trial types, although this stratification reduced analytical power.
Discussion
The current report leverages stereotactic intracranial recordings of oscillatory activity in human ACC to better understand the underpinnings of childhood attention. Four main findings are reported: (i) phase resetting consistently occurs in human ACC in relation to attentional set shifting, and is associated with processing speed; (ii) the preferred phase angle of theta oscillations is predictive of reaction time; (iii) epileptic activity diminishes phase resetting when the activity occurs near the time of stimulus presentation, resulting in longer reaction times, and; (iv) in the absence of epileptic activity, we find enhanced functional connectivity between the ACC and the DAN, as well as reduced connectivity between the ACC and the DMN. Given that processing speed is strongly associated with inattentive behavior (Kalff et al. 2005) and its subsequent functional limitations (Cook et al. 2018), these findings provide unique insights into the fundamental mechanisms of attention in children and the effects that epileptic activity may have on them.
Set shifting is a complex cognitive process that relies on a combination of attentional processes that include attention shifting, inhibition and salience detection (Dajani and Uddin 2015). Although multiple brain regions are engaged by attentional shift (Buchsbaum et al. 2005; Dunkley et al. 2015), the cognitive process of set shifting heavily depends on attentional processing in ACC (Dajani and Uddin 2015). Here we propose that the precision of oscillatory phase within human ACC serves as one critical component of the neural syntax by which selective attention may be focused on relevant stimuli.
The phase of neural oscillations allows some information to be selectively propagated while other information is selectively suppressed depending on the preferred phase at critical time windows of stimulus processing. Selective communication of attended stimuli is mediated by gamma-band activity that is modulated by synchronized inter-regional theta rhythms (Canolty et al. 2006). Theta rhythmic resetting results in the modulation of gamma activity and synchronization strength (Canolty et al. 2006). In addition, theta rhythmic resetting corresponds to the termination of attentional selection and a potential shift of attention to another stimulus (Fries 2015). One such example is gaze shift during visual exploration routines (i.e., saccades) which occur naturally with a 7 Hz theta rhythm (Otero-Millan et al. 2008). During fixation, theta phase modulates the strength of gamma-band synchronization, a means of implementing selective attention (Bosman et al. 2009; Fries 2015). Several studies have established the critical role of oscillatory activity within the ACC for attentional set-shifting (Monchi et al. 2001; Periáñez et al. 2004; Smith et al. 2004; Kim et al. 2012).
Attentional sampling occurs through a moment-to-moment reweighting of attentional priorities based on object properties and mediated by rhythmic patterns at the theta frequency (Tsujimoto et al. 2006; Fiebelkorn et al. 2013; Landau et al. 2015; Voloh et al. 2015). Indeed, work in non-human primates has demonstrated the role of theta-band oscillatory activity in ACC and attentional shifts (Voloh et al. 2015). Using stereotactic intracranial recordings, the present work demonstrates the role of theta phase resetting in ACC and its influence on attentional behavior in human children. In concordance with animal studies, we find that stimulus presentation elicits robust theta phase resetting during shift and non-shift trials. Importantly, we also report that the consistency of theta phase angle at the time of peak phase resetting is strongly associated with reaction time during attentional set shifting.
The current report also presents direct electrophysiological evidence of enhanced theta coupling between the ACC and the DAN in the absence of epileptic activity and during attentional shifts, providing a putative mechanism for the large-scale coordination of stimulus monitoring, selective attention, and executive control. The DAN comprises the bilateral superior parietal lobules, intraparietal sulci, and frontal eye fields and is implicated in goal-directed, top-down attentional processing (Corbetta and Shulman 2002). The DAN activates during orienting and visual search (Corbetta and Shulman 2002; Vossel et al. 2014) and is thought to facilitate the selection of sensory stimuli based on internal goals or expectations and link these selections to appropriate motor responses (Corbetta et al. 2008). Given the role of the DAN in selective attention and the role of the ACC in executive attention (Weissman et al. 2005; Raz and Buhle 2006), enhanced theta connectivity between the ACC and the DAN would likely facilitate the executive control of selective attention, such as the focusing of attentional resources towards a goal-relevant stimulus. Conceivably, these processes would be interrupted by HFO activity, and indeed, we do not observe a post-stimulus enhancement in ACC-DAN theta synchrony in the presence of HFOs. Notably, the extent of decreased functional connectivity within DAN regions in epilepsy has previously been associated with impaired performance on a task designed to probe visual attention and task switching (Zhang et al. 2009).
Furthermore, we report decreased theta coupling between the ACC and the DMN during attentional shifts, providing a putative mechanism for the large-scale suppression of irrelevant mental processes during externally directed attention. The DMN comprises the medial prefrontal cortex, posterior cingulate cortex, superior and inferior frontal gyri, medial and lateral temporal lobes and the posterior aspect of the inferior parietal lobule (52). This network is responsible for internally directed mental processing, such as self-referential cognition, and is characteristically anti-correlated with DAN activity (53). Indeed, the onset of an attentional task typically deactivates the DMN (28) and this suppression of the DMN has been linked to successful goal-directed attention (54). As such, increased theta desynchronization between the ACC and the DMN would likely suppress goal-irrelevant mental processes, and by extension, facilitate externally directed attention. Importantly, a lesser degree of task-induced deactivation of the DMN has previously been associated with momentary lapses in attention (28).
The current report highlights putative mechanisms by which epilepsy, and specifically interictal epileptic dynamics may interfere with oscillatory activity supporting attention. Attention deficits are the most common neuropsychiatric comorbidity of epilepsy, evident in one-third of affected children (Cohen et al. 2013; Reilly et al. 2014; Besag et al. 2016). Such deficits are also commonplace within the general population, affecting 4%–12% of school-aged children (Brown et al. 2001). The association between transient epileptic activity and attention deficits is highlighted by the finding that children with attention deficits but without a known seizure disorder manifest epileptic-like activity associated with their functional impairments.
While the causal relations of these dynamics is unknown, focal, multifocal or generalized interictal discharges have all been identified on EEG of children with attention deficits, but without a known seizure disorder (Silvestri et al. 2007; Frye et al. 2010; Attila et al. 2013). Interestingly, trial use of antiepileptic drugs in order to suppress these discharges resulted in an improvement in attentional deficits in children who were refractory to standard educational interventions (Frye et al. 2010). In concordance with these findings, we presently show that trials occurring coincidentally with HFOs were associated with significantly longer reaction times during attentional set-shifting. Taken together, these data support the notion that suppression of these dynamics could rescue neurocognitive function.
Indeed, it has been shown that epileptic HFOs disrupt network function in animal models with epilepsy. Recently, epileptic HFOs occurring in the hippocampus were found to disrupt spatial coding in the behaving rat (Ewell et al. 2019). These effects include reduction in the spatial precision of place cells that were activated by the pathological oscillation. The associations identified between pathological HFOs, higher network sparsity, and a decrease in information coding could be mechanistically explained by disruption of phase alignment, which serves as a neural syntax for information processing and propagation (Womelsdorf et al. 2007).
Our finding of a critical time window for phase resetting following stimulus presentation, which is associated with processing speed provides context to prior reports of impaired attention in epilepsy. In remarkable consistency with our findings, Aarts and colleagues (Aarts et al. 1984) reported that focal interictal epileptiform activity at the time of stimulus presentation disrupted task performance, whereas activity occurring during the response period was without demonstrable effect. These findings further support a causal role for phase resetting during critical windows for cognitive processing in directing attention. Importantly, HFOs cannot be reliably gleaned from non-invasive electromagnetic recordings such as scalp EEG or magnetoencephalography. It is likely that all previous studies, which have largely relied on the detection of epileptic activity through non-invasive recordings have underestimated the prevalence of ongoing epileptic activity.
Of note, this work does not necessarily differentiate whether HFOs are causally responsible for network dysfunction or whether network dysfunction results in release of epileptic activity. We have previously shown that large-scale network reorganization precedes the expression of epileptic activity (Ibrahim et al. 2014a). This may serve as mechanism to isolate the epileptogenic focus from the moderating influence of the large-scale brain network, or one mechanism for expression of epileptic events, such as seizures and interictal discharges (Ibrahim et al. 2013; Ibrahim et al. 2014b). During the preictal period, which is characterized by particularly active epileptic discharges, reduced inter-regional phase synchrony is observed between the seizure focus and surrounding brain regions (Ibrahim et al. 2013; Martire et al. 2019).
The current findings may inform the controversy regarding the extent to which the goal of medical and surgical treatment is to abort interictal epileptiform activity. Currently, the goal of these treatments is to prevent clinical seizures, and thus seizure-freedom remains the benchmark by which the success of interventions is measured. The association between interictal discharges, network changes and neurocognitive outcomes suggests that it is of clinical importance to suppress discharges to foster more typical brain network function in children with focal epilepsy. Previous works have also suggested a detrimental role for epileptiform discharges on cognitively-salient intrinsic connectivity networks (Fahoum et al. 2013; Ibrahim et al. 2014a).
We speculate that one reason why some children have persistent attention deficits following seizure-freedom, while others demonstrate remarkable improvements, relates to subclinical epileptiform dynamics that may disrupt oscillatory phenomena, such as phase resetting. Furthermore, children often present with attention deficits before the onset of seizures (Austin et al. 2001), which may be the result of subclinical epileptiform discharges that precede clinical seizure activity. Our findings support the view that the clinical phenotype of “attention deficits” may be at least in part the result of cumulative momentary lapses in attention that are the result of disrupted phase encoding within salient neural circuitry, such as the ACC. This has been previously termed transient cognitive impairment (Aircardi 1996).
The current work also offers new avenues by which to rescue attention deficits by synchronizing rhythmic brain circuitry that is disrupted in childhood epilepsy. Closed-loop therapies aimed at modulating rhythmic activity have shown promise in a variety of conditions characterized by impaired normative oscillatory activity, including memory impairment (Reinhart and Nguyen 2019).
In this study, we included thirteen participants with intracranial electrodes. Although this is a small number, these are relatively rare recordings, and the individual results did not deviate from the main patterns of phase resetting we describe (Fig. S9). Second, the participants also possessed varying levels of attention deficits, which correlated with their task performance. Therefore, while implanted case series such as ours are by their nature clinically heterogeneous, the correlations we observe further underscore the relevance of the proposed phase resetting mechanism to individual attentional performance. While the expression of HFOs was uniformly associated with worse performance across all participants, heterogeneity in neuropsychological outcome should be considered in larger samples. Finally, the ACC is a large brain region comprised of heterogeneous neuronal populations that likely subserve different cognitive functions. All participants included in this study had electrodes implanted in the ACC as it was a putative area of propagation of the seizure network. It is not clear which subdivision of the ACC is most vulnerable to the effects of epileptic activity. Future work aimed at physiological segmentation of the ACC may yield more accurate targets for putative neuromodulation.
Funding
This work was funded by a Canadian Institutes of Health Research CGS-M scholarship to Simeon Wong as well as a research grant from the American Association of Neurological Surgeons/Congress of Neurological Surgeons Pediatric Section.
Notes
Conflict of Interest: The authors have no competing interests to declare.
Author Contributions
S.M.W. made substantial contributions to the design of the study, acquired the data, analyzed and interpreted the data and drafted the work; O.N.A made substantial contributions to data acquisition, interpretation, and analysis of the phase-difference derivative; N.M.W made substantial contributions to data analysis, interpretation, critical review, and revisions of this work; E.P. made substantial contributions to the conception of the study, interpreted the data and substantially revised the work; E.K. acquired and interpreted the data and substantially revised the work; M.L.S. acquired and interpreted the data and substantially revised the work; B.D. substantially reviewed the work; A.O. acquired and interpreted the data and substantially revised the work; H.O. acquired and interpreted the data and substantially revised the work; E.D. acquired and interpreted the data and substantially revised the work; O.C.S. acquired and interpreted the data and substantially revised the work; G M.I. made substantial contributions to the conception and design of the study, interpretation of the data and substantially revised the work; All authors approved the submitted version; All authors have agreed both to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the authors was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature.
Supplementary Material
Contributor Information
Simeon M Wong, Institute of Biomedical Engineering, University of Toronto, 164 College St Room 407, Toronto, ON, M5S 3G9, Canada; Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada.
Olivia N Arski, Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada; Institute of Medical Science, University of Toronto, 27 King’s College Circle, Toronto, Ontario, M5S 1A1, Canada.
Nebras M Warsi, Institute of Biomedical Engineering, University of Toronto, 164 College St Room 407, Toronto, ON, M5S 3G9, Canada; Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada; Division of Neurosurgery, The Hospital for Sick Children, Department of Surgery, University of Toronto, Toronto, Canada.
Elizabeth W Pang, Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada; Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
Elizabeth Kerr, Department of Psychology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada; Department of Psychology, University of Toronto, Toronto, M5G 1X8, Canada.
Mary Lou Smith, Department of Psychology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada; Department of Psychology, University of Toronto, Toronto, M5G 1X8, Canada.
Benjamin T Dunkley, Institute of Biomedical Engineering, University of Toronto, 164 College St Room 407, Toronto, ON, M5S 3G9, Canada.
Ayako Ochi, Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
Hiroshi Otsubo, Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
Roy Sharma, Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
Puneet Jain, Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
Elizabeth Donner, Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada.
O Carter Snead, Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada; Division of Neurology, The Hospital for Sick Children, 555 University Ave., Toronto, Ontario, M5G 1X8, Canada; Institute of Medical Science, University of Toronto, 27 King’s College Circle, Toronto, Ontario, M5S 1A1, Canada.
George M Ibrahim, Institute of Biomedical Engineering, University of Toronto, 164 College St Room 407, Toronto, ON, M5S 3G9, Canada; Program in Neuroscience and Mental Health, The Hospital for Sick Children Research Institute, 686 Bay St., Toronto, Ontario, M5G 0A4, Canada; Division of Neurosurgery, The Hospital for Sick Children, Department of Surgery, University of Toronto, Toronto, Canada; Institute of Medical Science, University of Toronto, 27 King’s College Circle, Toronto, Ontario, M5S 1A1, Canada.
References
- Aarts JHP, Binnie CD, Smit AM, Wilkins AJ. 1984. Selective cognitive impairment during focal and generalized epileptiform eeg activity. Brain. 107:293–308. [DOI] [PubMed] [Google Scholar]
- Achenbach T. 1991. Attention problems from the Child Behavior Checklist (CBLC). Burlington, VT: University of Vermont, Dept of Psychiatry. [Google Scholar]
- Adalio CJ, Owens EB, McBurnett K, Hinshaw SP, Pfiffner LJ. 2018. Processing speed predicts Behavioral treatment outcomes in children with attention-deficit/hyperactivity disorder predominantly inattentive type. J Abnorm Child Psychol. 46:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aircardi J. 1996. Epilepsy as a non-paroxysmal disorder. Acta Neuropediatr. 2:249–257. [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC, Rutka JT et al. 2011. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 52:1802–1811. [DOI] [PubMed] [Google Scholar]
- Attila A, Altunel E, Sever A. 2013. Electroencephalogram in attention deficit hyperactivity disorder: spike and wave paroxysms, foci and seizures. Clin EEG Neurosci. 30:357–361. [DOI] [PubMed] [Google Scholar]
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. 2001. Behavior problems in children before first recognized seizures. Pediatrics. 107:115–122. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. [Google Scholar]
- Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw. 31:1–21. [Google Scholar]
- Besag F, Aldenkamp A, Caplan R, Dunn DW, Gobbi G, Sillanpää M. 2016. Psychiatric and behavioural disorders in children with epilepsy: an ILAE task force report. Epileptic Disord. [DOI] [PubMed] [Google Scholar]
- Binnie CD. 1993. Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev. 15:23–30. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, Fries P. 2009. A microsaccadic rhythm modulates gamma-band synchronization and behavior. J Neurosci. 29:9471–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M, Williams LM, Stam CJ. 2004. A novel method for the topographic analysis of phase dynamics in neural systems reveals formation and dissolution of dynamic cell assemblies. J Comput Neurosci. 16:49–68. [DOI] [PubMed] [Google Scholar]
- Brown R, Freeman W, Perrin J, Stein M, Amler R, Feldman H, Pierce K, Wolraich M. 2001. Prevalence and assessment of attention- deficit/hyperactivity disorder in primary care settings. Pediatrics. 107:E43. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. 2005. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavier CC. 2015. Phase-resetting as a tool of information transmission. Curr Opin Neurobiol. 31:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbare NM, Knight RT. 2006. High gamma power is phase-locked to theta oscillations in human neocortex. Science (80-). 313:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Davidson MC, Hara Y, Thomas KM, Martinez A, Galvan A, Halperin JM, Rodríguez-Aranda CE, Tottenham N. 2004. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 7:534–542. [DOI] [PubMed] [Google Scholar]
- Chand GB, Dhamala M. 2017. Interactions between the anterior cingulate-insula network and the fronto-parietal network during perceptual decision-making. Neuroimage. 152:381–389. [DOI] [PubMed] [Google Scholar]
- Cohen R, Senecky Y, Shuper A, Inbar D, Chodick G, Shalev V, Raz R. 2013. Prevalence of epilepsy and attention-deficit hyperactivity (ADHD) disorder: a population-based study. J Child Neurol. 28:120–123. [DOI] [PubMed] [Google Scholar]
- Cook NE, Braaten EB, Surman CBH. 2018. Clinical and functional correlates of processing speed in pediatric attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Child Neuropsychol. 24:598–616. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ. 2015. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 38:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, Christoff K. 2018. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A. 115:E1598–E1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Sedge PA, Doesburg SM, Grodecki RJ, Jetly R, Shek PN, Taylor MJ, Pang EW. 2015. Theta, mental flexibility, and post-traumatic stress disorder: connecting in the parietal cortex. PLoS One. 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Bragin A, Staba R, Mody I. 2009. High-frequency oscillations: what is normal and what is not? Epilepsia. 50:598–604. [DOI] [PubMed] [Google Scholar]
- Ewell LA, Fischer KB, Leibold C, Leutgeb S, Leutgeb JK. 2019. The impact of pathological high-frequency oscillations on hippocampal network activity in rats with chronic epilepsy. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahoum F, Zelmann R, Tyvaert L, Dubeau F, Gotman J. 2013. Epileptic discharges affect the default mode network--FMRI and intracerebral EEG evidence. PLoS One. 8:e68038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2019. A rhythmic theory of attention. Trends Cogn Sci. 23:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2020. Functional specialization in the attention network. Annu Rev Psychol. 71:221–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, Kastner S. 2013. Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr Biol. 23:2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. 2015. Rhythms for cognition: communication through coherence. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Butler I, Strickland D, Castillo E, Papanicolaou A. 2010. Electroencephalogram discharges in atypical cognitive development. J Child Neurol. 25:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim GM, Anderson R, Akiyama T, Ochi A, Otsubo H, Singh-Cadieux G, Donner E, Rutka J, Snead OC, Doesburg SM. 2013. Neocortical pathological high frequency oscillations are associated with frequency-dependent alterations in functional network topology. J Neurophysiol. 110:2475–2483. [DOI] [PubMed] [Google Scholar]
- Ibrahim GM, Cassel D, Morgan BR, Lou SM, Otsubo H, Ochi A, Taylor M, Rutka JT, Snead OC, Doesburg S. 2014a. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. 137:2690–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim GM, Wong SM, Anderson RA, Singh-Cadieux G, Akiyama T, Ochi A, Otsubo H, Okanishi T, Valiante TA, Donner E et al. 2014b. Dynamic modulation of epileptic high frequency oscillations by the phase of slower cortical rhythms. Exp Neurol. 251:30–38. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC. 2003. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26:161–167. [DOI] [PubMed] [Google Scholar]
- Kalff AC, de Sonneville LMJ, Hurks PPM, Hendriksen JGM, Kroes M, Feron FJM, Steyaert J, van Zeben TMCB, Vles JSH, Jolles J. 2005. Speed, speed variability, and accuracy of information processing in 5 to 6-year-old children at risk of ADHD. J Int Neuropsychol Soc. 11:173–183. [DOI] [PubMed] [Google Scholar]
- Kemp B, Olivan J. 2003. European data format “plus” (EDF+), an EDF alike standard format for the exchange of physiological data. Clin Neurophysiol. 114:1755–1761. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. 2004. Anterior cingulate conflict monitoring and adjustments in control. Science (80-). 303:1023–1026. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Gold BT. 2012. Common and distinct neural mechanisms of attentional switching and response conflict. Brain Res. 1469:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor X, Dermody N, Irish M. 2015. Considering the impact of large-scale network interactions on cognitive control. J Neurosci. 35:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Schreyer HM, Van Pelt S, Fries P. 2015. Distributed attention is implemented through theta-rhythmic gamma modulation. Curr Biol. 25:2332–2337. [DOI] [PubMed] [Google Scholar]
- Leber AB, Turk-Browne NB, Chun MM. 2008. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 105:13592–13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. 2006. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- Marston D, Besag F, Binnie CD, Fowler M. 1993. Effects of transitory cognitive impairment on psychosocial functioning of children with epilepsy: a therapeutic trial. Dev Med Child Neurol. 35:574–581. [DOI] [PubMed] [Google Scholar]
- Martire D, Wong S, Mikhail M, Ochi A, Otsubo H, Snead OC, Donner E, Ibrahim GM. 2019. Thalamocortical dysrythmia in intraoperative recordings of focal epilepsy. J Neurophysiol. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. 2009. To see or not to see: Prestimulus α phase predicts visual awareness. J Neurosci. 29:2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis ILC, Van Dijk H, Jensen O. 2009. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp. 30:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. 2001. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 21:7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J, Ebus S, Biemans DPLJJG, Arends J, Hendriksen J, Vles JSH, Aldenkamp AP. 2012. The cognitive effects of interictal epileptiform EEG discharges and short nonconvulsive epileptic seizures. Epilepsia. 53:1051–1059. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT et al. 2007. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 48:286–296. [DOI] [PubMed] [Google Scholar]
- Oh A, Vidal J, Taylor MJ, Pang EW. 2014. Neuromagnetic correlates of intra- and extra-dimensional set-shifting. Brain Cogn. 86:90–97. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. 2008. Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J Vis. 8:1–18. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. 1991. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 29:993–1006. [DOI] [PubMed] [Google Scholar]
- Periáñez JA, Maestú F, Barceló F, Fernández A, Amo C, Ortiz Alonso T. 2004. Spatiotemporal brain dynamics during preparatory set shifting: MEG evidence. Neuroimage. 21:687–695. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2019. A Language and Environment for Statistical Computing. R Found Stat Comput. [Google Scholar]
- Raz A, Buhle J. 2006. Typologies of attentional networks. Nat Rev Neurosci. 7:367–379. [DOI] [PubMed] [Google Scholar]
- Reilly C, Atkinson P, Das KB, Chin RFMC, Aylett SE, Burch V, Gillberg C, Scott RC, Neville BGR. 2014. Neurobehavioral comorbidities in children with active epilepsy: a population-based study. Pediatrics. 133:1586–1503. [DOI] [PubMed] [Google Scholar]
- Reinhart RMG, Nguyen JA. 2019. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 22:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs A, Van Turennout M, Coles MGH. 2006. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proc Natl Acad Sci U S A. 103:13884–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra H, Cordova M, Chen CSJ, Rajadhyaksha M. 2015. Confocal imaging-guided laser ablation of basal cell carcinomas: an ex vivo study. J Invest Dermatol. 135:612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri R, Gagliano A, Calarese T, Aricò I, Cedro C, Condurso R, Germanò E, Vita G, Tortorella G. 2007. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 75:130–137. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. 2004. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 21:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley J. 1993. Children’s audtory verbal learning test-2. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen AL, Meza J, Hinshaw S, Lundervold AJ. 2018. Processing speed mediates the longitudinal association between ADHD symptoms and preadolescent peer problems. Front Psychol. 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. 2006. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol. 95:2987–3000. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Vilgis V, Silk TJ, Vance A. 2015. Executive function and attention in children and adolescents with depressive disorders: a systematic review. Eur Child Adolesc Psychiatry. 24:365–384. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chistiakova M, Singer W. 1998. Modification of discharge patterns of neocortical neurons by induced oscillations of the membrane potential. Neuroscience. 83:15–25. [DOI] [PubMed] [Google Scholar]
- Voloh B, Valiante TA, Everling S, Womelsdorf T. 2015. Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc Natl Acad Sci U S A. 112:8457–8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. 2014. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. 2005. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 15:229–237. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. 2006. The neural bases of momentary lapses in attention. Nat Neurosci. 9:971–978. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. 2007. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 17:154–160. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. 2007. Modulation of neuronal interactions through neuronal synchronization. Science. 316:1609–1612. [DOI] [PubMed] [Google Scholar]
- Wong SM, Arski ON, Workewych AM, Donner E, Ochi A, Otsubo H, Snead OC, Ibrahim GM. 2021. Detection of high-frequency oscillations in electroencephalography: a scoping review and an adaptable open-source framework. Seizure. 84:23–33. [DOI] [PubMed] [Google Scholar]
- Wu D, Deng H, Xiao X, Zuo Y, Sun J, Wang Z. 2017. Persistent neuronal activity in anterior cingulate cortex correlates with sustained attention in rats regardless of sensory modality. Sci Rep. 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, Chen Z, Shi J, Liu Y. 2009. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett. 458:97–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


