Abstract
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infection worldwide, but reports of temporal changes in the risk of transmission among close contacts has been scarce. This study aimed to examine an association between the viral load trajectory and transmission risk to develop a better control strategy for the disease spread. We conducted a household-based prospective cohort study in Biliran Province, the Philippines, and enrolled 451 participants to observe the development of acute respiratory infection. Including the cases found at the health-care facility, we analyzed the data of viral loads with symptom records obtained from 172 followed participants who had household member positive for RSV with a rapid test during an RSV outbreak in 2018–2019. We developed a model estimating a temporal change in the viral shedding from the infection and evaluated transmission dynamics. We found that most transmission events occurred within approximately 7 days of the household exposure, including potential presymptomatic transmissions. The inferred risk of infection among those younger than 5 years was 3.5 times higher than that of those older than 5 years. This finding suggested that the initial week after the household exposure is particularly important for preventing RSV spread.
Keywords: infectious disease transmission, mathematical model, respiratory syncytial virus, viral load
Abbreviations
- ARI
acute respiratory infection
- CI
confidence interval
- RSV
respiratory syncytial virus
Respiratory syncytial virus (RSV) causes acute lower respiratory tract infections, with an estimated annual burden of 25 million episodes and 76,600 deaths, 54% of which occur in children younger than 5 years worldwide (1). Various vaccines considering age-related clinical and epidemiologic features have been under development, and there is ongoing discussion about which populations would benefit most from RSV vaccination to protect those at risk of severe RSV disease (2, 3). The high-risk populations include young infants (0–6 months), due to their physical and immunological immaturity and difficulty of direct immunization (4, 5); older infants and young children (6–24 months), whose maternal passive transferred immunity is waning but among whom disease burden is highest (6, 7); and adults over age 65 years (8). However, because the correlation of protection against clinically relevant RSV infections is unclear, the development of a vaccine has been challenging, and none has yet been commercialized (9).
An ideal strategy should prevent severe disease in at-risk populations by reducing person-to-person transmission (10, 11). Because all ages are susceptible to RSV reinfection, transmission frequently occurs from various age groups, including older children and adults to infants, particularly in households (12–14). A study that monitored RSV infection in children recruited at birth in rural Kenya over 3 epidemic periods reported that after primary RSV infection, neutralizing antibody responses declined rapidly over about 3 months to before-infection levels (15). Nor does immunity last a long time in adults: In a volunteer challenge study at 2, 4, 8, 14, 20, and 26 months after natural infection, measured immunological responses showed that within 26 months 73% had 2 or more infections (16). Nosocomial outbreaks have also been highlighted, specifically in neonatal intensive care, hematology, transplant, and oncology units (17). To prevent RSV infection in such vulnerable populations, understanding of transmission risk is essential.
An understanding of viral shedding kinetics and changes in infection risk over time will inform prevention of disease spread. Viral loads after diagnosis have been studied with particular emphasis on hospitalized cases to examine the correlation between viral loads and outcome of RSV illness (18–20). However, studies of the course of viral shedding at earlier stages of infection, including prior to onset of symptoms, are scarce, given that the exposure to the source of infection is generally difficult to observe.
This study aimed to identify typical viral shedding dynamics from the point of infection and evaluate the risk of transmission specifically in household settings. We conducted a prospective household-based cohort study to collect epidemiologic data and clinical specimens of persons infected with RSV in various age groups in the Philippines. The data on viral loads, including some before onset of secondary cases, were used to develop a mathematical model of changes in viral shedding over time, from the point at which patients were infected with RSV. Based on the estimated time of infection, we evaluated the risk of acquiring infection to account for the transmission dynamics in the household setting.
METHODS
Study population
All children in the present study were a part of the original Biliran cohort study targeting children under 5 years of age in Biliran Province, which has been described previously (21, 22). Since 2011, this study has been conducted in municipalities Caibiran and Kawayan, in Biliran Province, the Philippines. All households with children aged less than 5 years were visited, and children living there whose guardian consented to participation in the study were enrolled as a cohort. Since 2018 the study area has focused on 5 smaller districts in Caibiran.
Given the situation that the highest burden of RSV was found in infants aged 3–5 months, while the burden was also substantial in the children aged 12–20 months in the study area (23), we targeted not only infants, but also children aged 1–4 years and their household members, who were a part of the original cohort. Thus, households with at least 1 child younger than 5 years and their household members were enrolled in the enhanced cohort.
Collection of clinical specimen and data during the RSV outbreak
We collected nasal swabs to detect respiratory viruses at the primary health-care facility when any children enrolled in the original cohort visited there for consultation for respiratory symptom. In the present study, RSV was screened using a rapid test, Quick Navi-Flu+RSV (Denka, Niigata, Japan), to capture RSV activity in the study area. Once an RSV outbreak started, nurses launched visits of households enrolled in the enhanced cohort every 3 to 4 days, and acute respiratory symptoms were recorded. When any symptoms of cough, coryza, or difficulty breathing were present, 2 nasal swabs were collected from the household members and RSV infection was tested for by a rapid test with one of swabs. If the rapid test was positive for RSV, a nasal swab was collected from the rest of members of the household regardless of symptoms, and this day became day 0 of a follow-up period (Web Figure 1A, available at https://doi.org/10.1093/aje/kwab181). On the following 3 visits during the follow-up period, a nasal swab for laboratory confirmation was collected from the household members through day 10. If the rapid test was negative for RSV, the follow-up period did not begin; however, the other nasal swab was kept for the laboratory confirmation.
Additionally, when a child with RSV was found through the screening at the primary health-care facility, the households were invited to join in the study, and the data collection for the follow-up period was performed (Web Figure 1B). More details are available in Web Appendix 1.
Laboratory procedure
The collected specimens were stored at 4°C in a refrigerator and transported with ice packs to the Research Institute for Tropical Medicine in Manila twice a week. Quantitative reverse-transcription polymerase chain reaction was conducted to estimate viral loads. Details are described in Web Appendix 2 and Web Table 1.
Identification of RSV-associated acute respiratory infections
Acute respiratory infection (ARI) episodes were determined using symptom recording data. Symptoms including cough, coryza, and self-reported fever were recorded for all ages of household members. In addition, difficulty of breathing was also observed and recorded for children under 5 years old. The definition of ARI was previously described (24). In short, ARI was defined as an acute onset of cough, and for children under 5 years old, either cough or difficulty of breathing. Each ARI episode was distinguished by an at least 7-day asymptomatic period since the last episode, and had duration less than 28 days. RSV-ARI was defined as an ARI episode with laboratory-confirmed RSV. Duration from the ARI onset to date of RSV-positive testing had to be 14 days or less.
Model for estimating the date of infection
We focused on the data and specimens collected during follow-up of the household members. The estimated viral load of specimens collected from 7 days before to 7 days after the follow-up period was included in the analysis given that we had collected specimens even before the start of follow-up because of the way we define time 0. For households with a participant whose polymerase chain reaction result was RSV-positive before the date of the first positive rapid test, the data and specimens collected prior to the follow-up period were also included. Date of onset of RSV-ARI determined by data analysis of the daily symptom records was also used.
The estimated viral load of 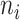 specimens collected from individual
specimens collected from individual 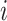 were used in this model.
were used in this model.
The difference between the time point at which each specimen was collected (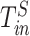 for the nth sample for individual
for the nth sample for individual 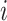 , and in total
, and in total 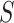 specimens were collected from
specimens were collected from 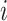 ) and the (unknown) time of infection (
) and the (unknown) time of infection (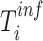 ), at which we assumed the person was infected and started to shed virus, was defined as
), at which we assumed the person was infected and started to shed virus, was defined as 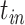 :
:
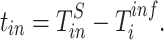 |
The true viral load 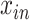 was assumed to follow a 1-sided truncated normal distribution:
was assumed to follow a 1-sided truncated normal distribution:
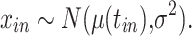 |
where σ is a standard deviation. Censoring is assumed to occur at 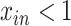 .
.
We searched for a mathematical representation of the temporal changes in viral load by graphing the estimated viral load obtained through polymerase chain reaction for all individuals to identify suitable functional forms, leading to the following equation for 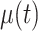 :
:
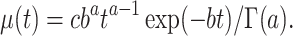 |
The parameter vector was estimated with Markov chain Monte Carlo methods. Details are available in Web Appendix 3 and Web Table 2. The code used can be found at GitHub (25).
Statistical analysis
We then compared 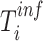 among the household members. The index case in the household was defined to be the individual whose inferred time of infection (
among the household members. The index case in the household was defined to be the individual whose inferred time of infection (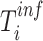 ) was earliest among the household members. To identify associations between model-based viral loads and potential risk factors, we tested their correlation by using generalized estimating equations. We calculated residuals as the difference between the model-based mean viral load and the estimated viral load at each time point of specimen collection and used them as a dependent variable. An exchangeable correlation structure was assumed. In addition, we investigated the association of the duration of RSV-ARI with age by using a linear regression.
) was earliest among the household members. To identify associations between model-based viral loads and potential risk factors, we tested their correlation by using generalized estimating equations. We calculated residuals as the difference between the model-based mean viral load and the estimated viral load at each time point of specimen collection and used them as a dependent variable. An exchangeable correlation structure was assumed. In addition, we investigated the association of the duration of RSV-ARI with age by using a linear regression.
An extended Cox proportional hazards model was used to evaluate risks of household transmission as a function of viral load of the index case. We assumed that household members were exposed to RSV when one of the household members acquired RSV infection. The household exposure was therefore defined as the estimated infection time of the index case in the household. If the time between the inferred infection time of the index and the secondary case was within a day, the households were excluded from this analysis. The proportion infected in the households according to the time elapsed from the household exposure was also computed using the Kaplan-Meier method. Details can be found in Web Appendix 4.
All analysis was performed with R, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) (26).
Data availability
Anonymized data can be found at GitHub (25).
Ethics statement
The study protocol was approved by the ethics committee of Tohoku University Graduate School of Medicine, Japan, and the institutional review board of the Research Institute for Tropical Medicine, the Philippines. Informed consent was obtained from individuals aged 18 years or older. Informed assent was obtained from children aged 12–17 years together with consent by their guardians. For individuals younger than 18 years, parental informed consent was obtained.
RESULTS
Enrolled participants and RSV cases
In total, 451 individuals in 101 households were enrolled and were eligible for household visits. An RSV epidemic was confirmed to start in October 2018. During the outbreak, 55 individuals in 14 households were further enrolled through the RSV rapid kit screening at the primary health-care facility. In total, 42 households had at least 1 positive test according to rapid test and entered follow-up. The demographic information of 172 participants residing in the followed households is shown in Table 1.
Table 1.
Demographic Information of Households Evaluated for Respiratory Syncytial Virus, Caibiran, Biliran Province, the Philippines, 2018–2019
| No. of Participants | ||||||
|---|---|---|---|---|---|---|
| Characteristic | RSV Not Detected (n = 76) | RSV Detected (n = 96) | Total (n = 172) | |||
| Sex | No. | % | No. | % | No. | % |
| Female | 54 | 71 | 49 | 51 | 103 | 60 |
| Male | 22 | 29 | 47 | 49 | 69 | 40 |
| Age group, years | ||||||
| <1 | 1 | 1 | 24 | 25 | 25 | 15 |
| 1–4 | 10 | 13 | 35 | 36 | 45 | 26 |
| 5–39 | 53 | 70 | 29 | 30 | 82 | 48 |
| ≥40 | 12 | 16 | 8 | 8 | 20 | 12 |
| No. in household | ||||||
| ≤3 | 21 | 28 | 35 | 36 | 56 | 33 |
| 4–6 | 36 | 47 | 33 | 34 | 69 | 40 |
| ≥7 | 19 | 25 | 28 | 29 | 47 | 27 |
Abbreviation: RSV, respiratory syncytial virus.
RSV cases and collected specimens
From 7 days before to 7 days after the follow-up period, 617 specimens were collected (Table 2). Among the followed participants, 96 were infected (56%, 96/172) with, in all, 191 RSV-positive specimens. Among the positive specimens, 153 belonging to 90 cases were classified into RSV-B, 38 belonging to 6 cases were not successfully classified by nucleotide sequences possibly due to lower concentration of viral RNA or other reasons. There were no specimens classified into RSV-A. The specimens in which viral load was more than a censoring threshold were 177 (Table 2). Three RSV cases whose duration from ARI onset to the date of RSV-positive testing was 14 days or more were excluded from the model of viral load, and the household members of those cases were excluded from the further statistical analysis. In total, 93 cases with 344 specimens were included in the model, and 81% (75/93) exhibited ARI symptoms. The collected specimen data is summarized in Web Figure 2 and Web Appendix 5.
Table 2.
Summary of Collected Respiratory Syncytial Virus Specimens, Caibiran, Biliran Province, the Philippines, 2018–2019
| No. of Specimens | ||||
|---|---|---|---|---|
| Age Group, years | RSV Detecteda (n = 177) | Overall (n = 617) | ||
| No. | % | No. | % | |
| <1 | 57 | 32 | 100 | 16 |
| 1–4 | 67 | 38 | 164 | 27 |
| 5–39 | 44 | 25 | 288 | 47 |
| ≥40 | 9 | 5 | 65 | 11 |
Abbreviation: RSV, respiratory syncytial virus.
a The specimens in which viral load was more than a censoring threshold.
Modeled viral load trajectories
The mean of model-based viral load fit the estimated viral load reasonably well (Figure 1 and Web Figure 3). The median of calculated residuals was 1.05 (interquartile range, –0.14 to 1.76). The generalized estimating equations analysis showed that younger ages had significantly higher viral loads than expected (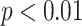 ). In contrast, it was worth noting that there was no significant association of whether the case had RSV-ARI with the viral load trajectories.
). In contrast, it was worth noting that there was no significant association of whether the case had RSV-ARI with the viral load trajectories.
Figure 1.
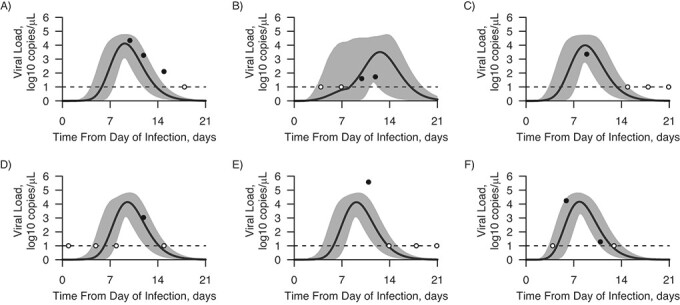
Fitting estimated viral load to model-based viral load for 6 arbitrary instances from the inferred time of respiratory syncytial virus infection, Caibiran, Biliran Province, the Philippines, 2018–2019. Solid lines represent the mean value of the model-based viral load, and shading represents the 95% credible interval for the mean. The black circles describe the estimated viral load for the positive samples and the white circles the negative samples at the assumed limit of detection. A) Participant 1, 0–5 years old; B) participant 2, 25–30 years old; C) participant 3, 5–10 years old; D) participant 4, 0–5 years old; E) participant 5, 0–5 years old; F) participant 6, 5–10 years old.
Based on the comparison of the time of infection among household members, we identified the index case in the household (n = 42). Among the index cases, 38 cases were less than 5 years old (90%), including 15 infants, and 40 cases (95%) showed RSV-ARI. There was also no significant association of whether the case was an index case in the household with the viral load trajectories.
Features of overall estimated viral load and respiratory symptoms
Maximal viral shedding averaged 4.4 log10 copies/μL (95% credible interval: 3.9, 4.9) and took 6.2 days to reach its peak (Figure 2A). At day 4, approximately 90% of RSV-ARI cases developed either or both of cough and coryza, and 23% were febrile, followed by a smaller percentage with difficulty breathing (Figure 2B). The model-based viral load at the onset of most cases was 3.7 log10 copies/μL (95% credible interval: 3.0, 4.3). The mean duration of viral shedding was 14.1 days, and approximately 80% of symptomatic cases became asymptomatic (Figure 2C). Median duration of RSV-ARI in under-5-year-olds was 8.5 days (interquartile range, 6–11), and among those aged 5 years or older it was 6 days (interquartile range, 5–9). With linear regression, duration of RSV-ARI was negatively correlated with age (P < 0.05). However, a comparison of the empirical and modeled viral load estimates suggested that the model adequately characterized the majority of cases’ viral kinetics, with no evidence of systematic deviation in the tails that might reflect prolonged shedding within some groups, such as younger cases.
Figure 2.
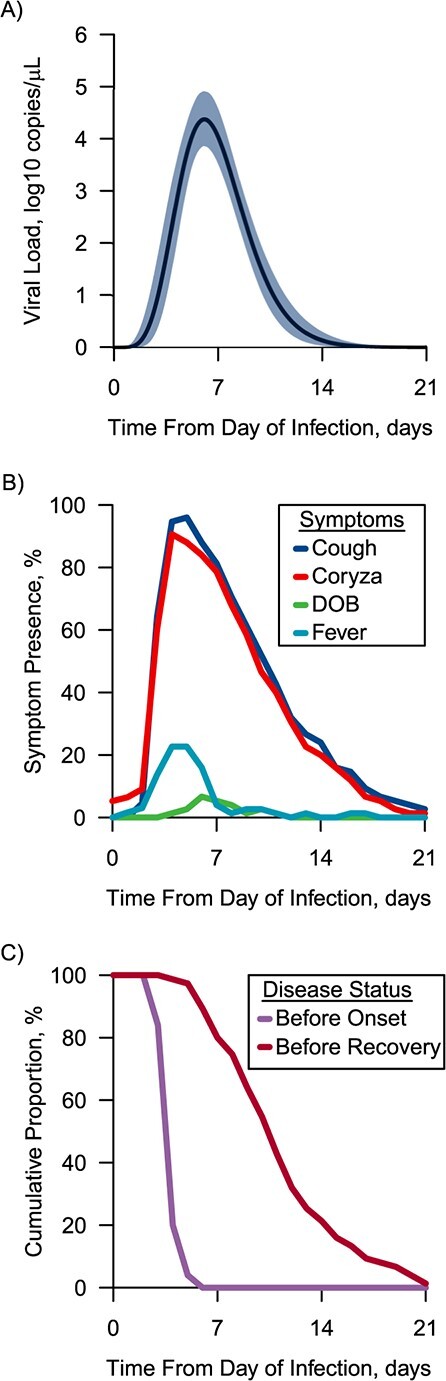
Features of overall model-based viral load and respiratory symptoms of respiratory syncytial virus (RSV), Caibiran, Biliran Province, the Philippines, 2018–2019. A) The mean of the model-based viral load from the inferred time of infection for all cases. The solid line represents the mean value of the model-based viral load, and the blue shade represents the 95% credible interval; B) the presence of each symptom (cough, coryza, difficulty breathing (DOB), self-reported fever) in cases with RSV acute respiratory infection (ARI) from inferred time of infection to 21 days later as a fraction of the active participants; C) cumulative proportion of before-onset cases and before-recovery cases of RSV-ARI from inferred time of infection. The interval between the lines is the symptomatic period.
Risk of transmission
Next, we compared the estimated time of infections among the household members to identify the inferred index case. We further excluded 5 households in which the time between the inferred time of infection of the index case and the secondary case was less than 1 day. We assessed the risk of transmission among the at-risk household members (n = 92) residing in 35 households. Thirty-one of the at-risk household members (34%) were confirmed as positive for RSV. The mean interval from the household exposure to infection was 5.1 (standard deviation, 3.1) days. Among the secondary cases of symptomatic index cases (n = 30), the inferred time of infection for 30% was estimated to have been before the onset of the index case.
In the extended Cox model, the age group of at-risk household members and time-varying presence of cough in the index case were analyzed as explanatory variables. Household members under age 5 years had a significantly higher risk for acquiring infection (hazard ratio = 3.5; 95% confidence interval (CI): 1.7, 7.1). The presence of cough in the index case was not found to be a significant risk factor (hazard ratio = 1.3; 95% CI: 0.5, 3.4). The proportion infected in household members by day 7 was 52% (95% CI: 27, 68) for those under age 5 years and 19% (95% CI: 10, 27) for those 5 years or older, respectively (Figure 3). The corresponding quantities on day 14 were 63% (95% CI: 36, 79) and 25% (95% CI: 14, 34).
Figure 3.
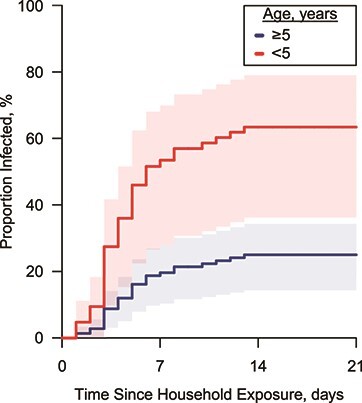
The proportion infected with respiratory syncytial virus, Caibiran, Biliran Province, the Philippines, 2018–2019. The lines denote the change in the proportion of infected from exposure to the household with their 95% confidence intervals, calculated using the Kaplan-Meier method.
DISCUSSION
We conducted a prospective cohort study to clarify the risk of RSV transmission in Biliran the Province, the Philippines. We estimated the viral load kinetics from the time of RSV infection in various age groups to determine the association between amount of viral discharge and disease. In addition, we inferred the risk of infection to evaluate transmission dynamics at the household level. The study revealed that most transmission events occurred within approximately 7 days of household exposure. The risk of infection among those under 5 years of age was about 3.5 times higher than that of those older than 5 years, which we postulate to be due to the higher susceptibility of RSV reinfection or infection in that age.
We found that the younger age was associated with higher viral load and longer durations of RSV-ARI. This result supports previous work of Munywoki et al. (27) in a prospective cohort study of infants and their household members in Kenya. In addition, this association between the higher viral load and prolonged episodes was also reported in previously healthy infants hospitalized by RSV (28). This delay of viral clearance at younger age might be attributed to immunological mechanisms. Although there is limited supporting evidence, recent studies imply a role for CD8 T cells in the elimination of respiratory viruses in adults (29). However, in infants hospitalized for RSV, no correlation between RSV-specific T cell numbers and disease severity has been found, and it appears unlikely they play a crucial role in immune pathology (30). Such differences by age have also been found in neutralizing antibody responses (4, 31). Hence, age-dependent variability in the immunological reaction could explain our finding.
This study also revealed that cases younger than age 5 years were infected earlier than those older than 5 years, suggesting that younger children can be infected by a lower dose of exposure than other age groups. In animal models, peak RSV viral load was reduced by the lower dose of intranasal inoculation in adult mice but not in neonatal mice (32), suggesting that viral replication in neonatal mice was less dependent on the initial dose than in adults of the same strain. In an experimental challenge study of adults, more participants were infected at the higher dose although the sample size might not be enough to discuss with statistical significance (33). Another possibility to explain this result is the difference in behavior between children and adults. Previous studies have suggested that all contact and physical contact between children is more frequent than that between other ages (34, 35). More frequent physical contact might promote faster transmission.
We also found that 34% of household members were infected after exposure by the end of the follow-up period. Similar findings were also reported in prospective studies of household members triggered by infant RSV hospitalization (13) and in a childcare facility in which one child was infected (36). In addition, we have added the finding that approximately half of those under 5 years of age were infected in 7 days, and viral shedding could start 3–4 days before the onset of symptoms and continue for at least 14 days. Moreover, it is also worth noting that approximately 30% of the observed secondary cases might have been infected prior to the onset of the index case. This suggests that those who do not yet have symptoms can infect others and that the infection might already have spread when the first case is discovered. This finding highlighted that the early detection of secondary cases and isolation of cases could be important in the design of control strategies.
We did not find a clear association between viral load and the presence of symptoms. Because we collected specimens after the household exposure, most of the cases we observed were infected through close contacts in the household, and it is thought that they had exposure to high doses of viral shedding. As a result, the exposed participants might have been more likely to have been symptomatic, resulting in the smaller proportion of asymptomatic cases. A previous study reported that 42% of RSV infection episodes were asymptomatic, when specimens were regularly collected regardless of symptoms (27), while 18% of followed family members of a hospitalized RSV case were asymptomatically infected (13).
Our study has several limitations. First, we did not collect specimens from asymptomatic participants other than in the follow-up. Because adults and older children frequently experience asymptomatic infection of RSV, it is possible that they were infected asymptomatically and cleared before the follow-up period. Therefore, the proportion infected aged 5 years or older in the Kaplan-Meier analysis could be an underestimate. In addition, a previous longitudinal study conducted for infants hospitalized because of RSV-associated pneumonia found that 6 out of 31 infants had detectable viral loads a month or longer after symptom onset (37). Longer follow-up would be necessary to clarify the transmissibility of such cases. Further studies with regular specimen collection would therefore be helpful.
Second, we speculate that we missed an opportunity to collect data for the follow-up period given that we employed rapid tests to find the case who triggered the follow-up period. The sensitivity of rapid tests might be lower than that of polymerase chain reaction. Considering that viral load was lower in older age groups, this trend might be strongest in cases where adults were infected first and children were not. As above, evaluation by regular specimen collection is warranted.
Finally, because the clinical specimens collected were nasal swabs, the detection rate might be underestimated. To reduce uncertainty, more invasive methods, such as nasopharyngeal aspiration, are better suited to assess viral load. However, a previous study suggested that nasal swabs are less sensitive than nasal aspirates but are not related to age in children (38). Therefore, although the viral load might have been underestimated, the degree of underestimation is likely to be constant, and the effect on the results of the generalized estimating equations analysis was considered to be minor.
Despite these limitations, the observed and modeled change in viral shedding from the estimated time of infection, together with the risk of transmission at the household setting, show that the initial week after the exposure to an RSV case is particularly important for preventing RSV spread.
Supplementary Material
ACKNOWLEDGMENTS
Author Affiliations: Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore, Singapore (Hirono Otomaru, Alex R. Cook); Department of Virology, Tohoku University Graduate School of Medicine, Sendai, Japan (Hirono Otomaru, Taro Kamigaki, Michiko Okamoto, Mariko Saito-Obata, Mayuko Saito, Hitoshi Oshitani); Research Institute for Tropical Medicine, Metro Manila, the Philippines (Johanna Beulah T. Sornillo, Marianette T. Inobaya, Edelwisa Segubre-Mercado, Portia P. Alday, Veronica L. Tallo, Beatriz P. Quiambao); and Research Institute for Tropical Medicine–Tohoku Collaborating Research Center on Emerging and Re-emerging Infectious Diseases, Metro Manila, the Philippines (Samantha Louise P. Bado).
This research was supported by Singapore’s National Medical Research Council under the Centre Grant Programme–Singapore Population Health Improvement Centre (grant NMRC/CG/C026/2017_NUHS); the Leading Young Researcher Overseas Visit Program under the Ministry of Education, Culture, Sports, Science and Technology, Japan; the Japan Initiative for Global Research Network (J-GRID) from the Japan Agency for Medical Research and Development (AMED) (grants JP19fm0108013 and JP20wm0125001); and by JSPS KAKENHI (grants JP19K24250, JP16H02642, JP19H01072, and JP19KK0204).
We thank the staff members of the Research Institute for Tropical Medicine–Tohoku Collaborating Research Center on Emerging and Re-emerging Infectious and Department of Virology, Tohoku University Graduate School of Medicine for dedicated work on this project in terms of field investigation, data collection, and laboratory work.
Conflict of interest: none declared.
REFERENCES
- 1. GBD 2016 Lower Respiratory Infections Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson LJ, Dormitzer PR, Nokes DJ, et al. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(suppl 2):B209–B215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyoglu-Barnum S, Chirkova T, Anderson LJ. Biology of infection and disease pathogenesis to guide RSV vaccine development. Front Immunol. 2019;10:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32(37):4726–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crowe JE, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr Respir Rev. 2003;4(2):112–119. [DOI] [PubMed] [Google Scholar]
- 6. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanco JCG, Boukhvalova MS, Morrison TG, et al. A multifaceted approach to RSV vaccination. Hum Vaccines Immunother. 2018;14(7):1734–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(suppl 7):S577–S583. [DOI] [PubMed] [Google Scholar]
- 9. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18(10):e295–e311. [DOI] [PubMed] [Google Scholar]
- 10. Yamin D, Jones FK, DeVincenzo JP, et al. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2016;113(46):13239–13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham BS. Protecting the family to protect the child: vaccination strategy guided by RSV transmission dynamics. J Infect Dis. 2014;209(11):1679–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209(11):1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heikkinen T, Valkonen H, Waris M, et al. Transmission of respiratory syncytial virus infection within families. Open Forum Infect Dis. 2015;2(1):ofu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacoby P, Glass K, Moore HC. Characterizing the risk of respiratory syncytial virus in infants with older siblings: a population-based birth cohort study. Epidemiol Infect. 2017;145(2):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sande CJ, Mutunga MN, Okiro EA, et al. Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Med Virol. 2013;85(11):2020–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall CB, Walsh EE, Long CE, et al. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–698. [DOI] [PubMed] [Google Scholar]
- 17. French CE, McKenzie BC, Coope C, et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respi Viruses. 2016;10(4):268–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chemaly RF, Dadwal SS, Bergeron A, et al. A phase 2, randomized, double-blind, placebo-controlled trial of Presatovir for the treatment of respiratory syncytial virus upper respiratory tract infection in hematopoietic-cell transplant recipients. Clin Infect Dis. 2020;71(11):2777–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin ET, Kuypers J, Heugel J, et al. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62(4):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houben ML, Coenjaerts FEJ, Rossen JWA, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kosai H, Tamaki R, Saito M, et al. Incidence and risk factors of childhood pneumonia-like episodes in Biliran island, Philippines—a community-based study. PloS One. 2015;10(5):e0125009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamaki R, Tallo VL, Tan AG, et al. Comprehensive etiological and epidemiological study on acute respiratory infections in children: providing evidence for the prevention and control of childhood pneumonia in the Philippines. J Disaster Res. 2018;13(4):740–750. [Google Scholar]
- 23. Ueno F, Tamaki R, Saito M, et al. Age-specific incidence rates and risk factors for respiratory syncytial virus-associated lower respiratory tract illness in cohort children under 5 years old in the Philippines. Influenza Other Respi Viruses. 2019;13(4):339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furuse Y, Tamaki R, Okamoto M, et al. Association between preceding viral respiratory infection and subsequent respiratory illnesses among children: a prospective cohort study in the Philippines. J Infect Dis. 2019;219(2):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otomaru H. RSV_Viralload. 2021. https://github.com/hiro-oto/RSV_viralload. Accessed June 7, 2021.
- 26. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 27. Munywoki PK, Koech DC, Agoti CN, et al. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect. 2015;143(4):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El Saleeby CM, Bush AJ, Harrison LM, et al. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204(7):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heidema J, Lukens MV. Maren WWC van, et al. CD8+ T cell responses in Bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179(12):8410–8417. [DOI] [PubMed] [Google Scholar]
- 31. Capella C, Chaiwatpongsakorn S, Gorrell E, et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis. 2017;216(11):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruckwardt TJ, Malloy AMW, Gostick E, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7(12):e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee FE-H, Walsh EE, Falsey AR, et al. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63(3):191–196. [DOI] [PubMed] [Google Scholar]
- 34. Prem K, Cook AR, Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput Biol. 2017;13(9):e1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horby P, Pham QT , Hens N, et al. Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS ONE. 2011;6(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chu HY, Kuypers J, Renaud C, et al. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J Clin Virol. 2013;57(4):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brint ME, Hughes JM, Shah A, et al. Prolonged viral replication and longitudinal viral dynamic differences among respiratory syncytial virus infected infants. Pediatr Res. 2017;82(5):872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stensballe LG, Trautner S, Kofoed PE, et al. Comparison of nasopharyngeal aspirate and nasal swab specimens for detection of respiratory syncytial virus in different settings in a developing country. Trop Med Int Health. 2002;7(4):317–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data can be found at GitHub (25).


