Abstract
Three touchdown enzyme time release (TETR)-PCR assays were used to amplify different DNA sequences in the variable regions of the 16S and 16S-23S spacer rRNA genes specific for Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci as improved tests for sensitive diagnosis and rapid species differentiation. The TETR-PCR protocol used 60 cycles of amplification, which provided improved analytical sensitivity (0.004 to 0.063 inclusion-forming unit of Chlamydia species per PCR). The sensitivity of TETR-PCR with primer set CTR 70-CTR 71 was 96.7%, and the specificity was 99.6%, compared to those of the AMPLICOR PCR for the detection of C. trachomatis in vaginal swab samples. TETR-PCR for C. pneumoniae with primer set CPN 90-CPN 91 was 90% sensitive and 93.3% specific compared with a nested PCR with primer set CP1/2-CPC/D for clinical respiratory samples. TETR-PCR for C. psittaci with primer set CPS 100-CPS 101 showed substantial agreement with cell culturing (κ, 0.78) for animal tissue samples. Primer sets were then combined into a single multiplex TETR-PCR test. The respective 315-, 195-, and 111-bp DNA target products were precisely amplified when DNA from each of the respective Chlamydia species or combinations of them was used. Multiplex chlamydia TETR-PCR correctly identified one strain of each of the 15 serovars of C. trachomatis, 22 isolates of C. pneumoniae, and 20 isolates of C. psittaci. The primer sets were specific for each species. No target products were amplified when DNA from C. pecorum or a variety of other microorganisms was tested for specificity. TETR-PCR with primers selected for specific sequences in the 16S and 16S-23S spacer rRNA genes is a valuable test that could be used either with individual primers or in a multiplex assay for the identification and differentiation of Chlamydia species from culture isolates or for the detection of chlamydiae in clinical samples.
Three species in the genus Chlamydia can cause disease in humans: C. pneumoniae, C. trachomatis, and C. psittaci. Diseases caused by chlamydiae include trachoma; respiratory infection, including pneumonia; and sexually transmitted infection of reproductive organs, including cervicitis, pelvic inflammatory disease, urethritis, and epididymitis (24, 34, 38). Pneumonia in adults can be caused by both C. pneumoniae and C. psittaci (psittacosis), and that in newborn children can be caused by C. trachomatis (19). Recent isolations of Chlamydia species in cardiovascular tissue have also been reported, suggesting a broader range of diseases and syndromes (36).
Chlamydia species are intracellular bacteria that require tissue culture techniques to be isolated (16, 28). Identification of species depends on phenotype differences and immunoreactivity to species-specific monoclonal antibodies (37). Also, molecular amplification techniques based on genomic sequences have been used for the differentiation of Chlamydia species (32, 40). In addition, various techniques to detect and differentiate Chlamydia species have been developed; they include DNA hybridization with genomic DNA probes (3), restriction fragment polymorphism analysis of PCR-amplified products (20, 31, 43), and nested PCR (5, 32). Recently, molecular amplification techniques have been demonstrated to have improved sensitivity compared to culturing and other diagnostic assays for the detection of chlamydia infection in urine, endocervix, throat and urethral swabs, sputa, bronchoalveolar lavage fluids, and eye secretions (4, 5, 27, 35, 40, 41).
In this study, three primer sets targeting the 16S and 16S-23S spacer rRNA genes were designed for the detection of C. trachomatis, C. pneumoniae, and C. psittaci. Primers were designed to target DNA sequences that were most heterogeneous in the 16S and 16S-23S spacer rRNA genes of the three Chlamydia species (11, 15, 33). The three amplified products were designed to be sufficiently different in size so that they could be easily discriminated when detected by gel electrophoresis. An improved PCR protocol was also developed. Four characteristics distinguish our touchdown enzyme time release (TETR)-PCR: a hot start polymerase enzyme to avoid artifacts before amplification, a touchdown protocol for annealing temperatures to improve the specificity of primer binding, an enzyme time release protocol to allow 60 cycles of amplification for improved analytical sensitivity, and multiplex target coamplification capabilities.
The sensitivity, specificity, and agreement of TETR-PCR with various primer sets used to detect DNA of C. trachomatis in vaginal swab samples, DNA of C. pneumoniae in clinical respiratory specimens, and DNA of C. psittaci in animal tissue samples were measured. TETR-PCR with primer set CTR 70-CTR 71 (CTR 70/71) for the detection of C. trachomatis was compared to a commercial PCR test (AMPLICOR Chlamydia trachomatis test; Roche Diagnostics Systems, Branchburg, N.J.). TETR-PCR with primer set CPN 90-CPN 91 (CPN 90/91) for the detection of C. pneumoniae was compared to a nested PCR with primer sets CP1-CP2 and CPC-CPD (CP1/2-CPC/D) (5, 40). TETR-PCR with primer set CPS 100-CPS 101 (CPS 100/101) for the detection of C. psittaci was compared to tissue culturing. In addition, the three primer sets were combined into a multiplex TETR-PCR to detect and identify C. pneumoniae, C. trachomatis, or C. psittaci tissue culture isolates in a single test.
MATERIALS AND METHODS
Clinical samples.
Vaginal samples for the detection of C. trachomatis were obtained as part of another study designed to assess the utility of vaginal swab samples for the molecular detection of sexually transmitted diseases (29). This study was approved by the institutional review boards of The Johns Hopkins University and Fort Bragg Womack Army Medical Center. Consecutive active-duty military women, 18 to 59 years old and attending the Epidemiology and Disease Control Clinic at the Womack Army Medical Center, Fort Bragg, N.C., were invited to participate in the study from March to September 1997. During enrollment, 89% (265 of 298) consented to participate. Vaginal swab samples collected by clinicians were placed in 1 ml of specimen transport medium (AMPLICOR) and kept at 4°C to prevent DNA degradation until arrival at the laboratory.
Fifty respiratory specimens were obtained in 1994 during an outbreak of C. pneumoniae respiratory infection in northern Sweden (5). These included sputum (n = 17), nasopharyngeal (n = 17), and throat (n = 16) samples, which were processed by a modified version of a method described previously (5, 39, 40). Specimens were vortexed for 1 min and transferred to 15-ml tubes. The sputum was digested in an equal volume of freshly prepared 6% N-acetyl-l-cysteine (Mucomyst; Draco, Lund, Sweden) for 30 min during vortexing. The throat and nasopharyngeal specimens were digested in an equal volume of 2 or 4% N-acetyl-l-cysteine, depending on their viscosity. Digested specimens were washed with twice their volume of cold phosphate-buffered saline (pH 7.2) by centrifugation at 3,000 × g for 15 min. The supernatant was discarded, the pellet was resuspended in an equal volume of 0.01 M Tris HCl buffer (pH 8.2), and the preparation was stored frozen at −70°C.
Twenty minced liver or spleen tissue samples from 20 birds in which psittacosis was suspected were kindly provided by Arthur Andersen (National Animal Disease Center, Ames, Iowa). Samples were ground in 0.01 M Tris HCl buffer (pH 8.2), and 50 μl was used for DNA preparation.
Organisms.
A strain of each of 15 serovars of C. trachomatis, 22 strains of C. pneumoniae, 20 strains of C. psittaci, and 2 strains of Chlamydia pecorum were obtained from our freezer repository to be tested by multiplex TETR-PCR (Table 1). Twenty-nine other bacteria, protozoa, and fungi used to test the specificity of the primers were obtained from the Clinical Microbiology Laboratory, The Johns Hopkins Hospital, Baltimore, Md., and the American Type Culture Collection, Manassas, Va.
TABLE 1.
Isolates (n = 59) tested with multiplex TETR-PCR for the identification of C. trachomatis (primer set CTR 70/71), C. pneumoniae (primer set CPN 90/91), and C. psittaci (primer set 100/101)a
| C. pneumoniae isolate | Result obtained with the following primer set (PCR product, bp)
|
C. trachomatis isolateb | Result obtained with the following primer set (PCR product, bp)
|
C. psittaci isolate | Result obtained with the following primer set (PCR product, bp)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR 70/71 (315) | CPN 90/91 (195) | CPS 100/101 (111) | CTR 70/71 (315) | CPN 90/91 (195) | CPS 100/101 (111) | CTR 70/71 (315) | CPN 90/91 (195) | CPS 100/101 (111) | |||||
| AR39c | − | + | − | VR 571 ser. Ac | + | − | − | 6BCc | − | − | + | ||
| AR388c | − | + | − | VR 573 ser. Bc | + | − | − | SM006h | − | − | + | ||
| TW183c | − | + | − | VR 347 ser. Bac | + | − | − | AW-17-9-6i | − | − | + | ||
| VR1310c | − | + | − | VR 572 ser. Cc | + | − | − | B577-23i | − | − | + | ||
| 2023d | − | + | − | VR 885 ser. Dc | + | − | − | FC-GRW#97-5i | − | − | + | ||
| 2043d | − | + | − | VR 348 ser. Ec | + | − | − | FITZ-65-5i | − | − | + | ||
| BAL15d | − | + | − | VR 346 ser. Fc | + | − | − | JP-I-757-4i | − | − | + | ||
| BAL16d | − | + | − | VR 878 ser. Gc | + | − | − | LW613-11i | − | − | + | ||
| BAL37d | − | + | − | VR 879 ser. Hc | + | − | − | LW646-4i | − | − | + | ||
| T2364d | − | + | − | VR 880 ser. Ic | + | − | − | LW679-5i | − | − | + | ||
| CMIe | − | + | − | VR 886 ser. Jc | + | − | − | 11245 PAj | − | − | + | ||
| CWL011e | − | + | − | VR 887 ser. Kc | + | − | − | 18331 Cj | − | − | + | ||
| FML16e | − | + | − | VR 901 ser. L1c | + | − | − | 21339j | − | − | + | ||
| AO3f | − | + | − | VR 902 ser. L2c | + | − | − | 27256j | − | − | + | ||
| AL-1g | − | + | − | VR 903 ser. L3c | + | − | − | 95-28189 Nebraskaj | − | − | + | ||
| IOL 1515g | − | + | − | 95-42776 #3j | − | − | + | ||||||
| T 45 953g | − | + | − | 95-42776 #8j | − | − | + | ||||||
| U1092g | − | + | − | 95-42776 #9j | − | − | + | ||||||
| U127g | − | + | − | Akronj | − | − | + | ||||||
| U1271g | − | + | − | Donald parakeetj | − | − | + | ||||||
+, positive; −, negative. Results for C. pecorum VR 628 and VR 629 (from the American Type Culture Collection) were negative.
ser., serovar.
American Type Culture Collection.
Provided by Margaret R. Hammerschlag (SUNY Health Science Center, Brooklyn, N.Y.).
Provided by Carolyn M. Black (Centers for Disease Control and Prevention, Atlanta, Ga.).
Provided by Jim T. Summersgill (University of Louisville, Louisville, Ky.).
Provided by Jens Boman (University Hospital of Northern Sweden).
Isolated in the Chlamydia Laboratory, The Johns Hopkins University.
Provided by J. Storz (Louisiana State University, Baton Rouge).
Provided by Trudy O. Messmer (Centers for Disease Control and Prevention.
Culturing.
Culturing was performed as described previously for C. trachomatis and C. pneumoniae (9, 16). Chlamydia strains (200 μl) were inoculated in triplicate into shell vials containing monolayers of buffalo green monkey kidney (BGMK) cells or HEp-2 cells. Inoculated shell vials were centrifuged (800 × g) for 60 min at 35°C. Following aspiration of the supernatant, 1 ml of inoculation medium containing 1.0 μg of cycloheximide per ml was added. After 72 h (48 h for C. trachomatis) of incubation at 35°C, a second passage was performed. The contents of a shell vial were fixed with 90% methanol, stained with genus-specific fluorescein-conjugated monoclonal antibody (Kallestad, Chaska, Minn.) as well as species-specific fluorescein-conjugated monoclonal antibody for C. trachomatis (Behring Diagnostics Inc., Cupertino, Calif.) or for C. pneumoniae (Washington Research Foundation, Seattle), and read for the presence of inclusion bodies with an epifluorescence microscope (Axioplan, Zeiss, Germany); organisms from two shell vials were stored at −70°C. Culturing of C. psittaci from animal tissues was performed at the reference laboratory (National Animal Disease Center). Titration of chlamydia stock cultures to determine the titers of organisms was performed with 96-well microtiter plates containing monolayers of BGMK cells. One hundred microliters of 10-fold dilutions of the chlamydia cultures was inoculated, incubated, fixed, and stained as described above in duplicate wells. The number of inclusion bodies counted under the epifluorescence microscope for each well was multiplied by the dilution factor to calculate the number of inclusion-forming units (IFU) per milliliter in the chlamydia stock cultures.
DNA extraction.
DNAs from chlamydia cultures, processed respiratory samples, minced tissue samples, and other microorganisms were prepared by the Chelex method (42). Mixtures of 50 μl of specimen and 200 μl of a 5% suspension of chelating resin (Chelex 100; Sigma, St. Louis, Mo.) in Tris HCl buffer (0.01 M, pH 8.0) were incubated at 56°C for 15 to 30 min. The chelating resin was kept in suspension by continuous stirring with a magnetic bar while it was added to the specimens. After incubation, preparations were mixed gently and heated at 100°C for 8 to 10 min. After being mixed again, preparations were stored at −70°C. Immediately before testing, preparations were centrifuged for 30 s in a microcentrifuge (11,000 × g), and 10 μl of the supernatant was used for PCR.
Vaginal swab samples were processed by the AMPLICOR sample processing method. For specimens transported in specimen transport medium, an equal volume of specimen diluent (Roche) was added, mixed, and incubated at room temperature for 10 min. The DNA extract was stored at −70°C until testing.
Primer design.
Primer sets specific for each of the three species were designed based on the DNA sequences of the 16S rRNA and 16S-23S spacer rRNA genes (11, 15, 33) for C. trachomatis (CTR 70/71), C. pneumoniae (CPN 90/91), and C. psittaci (CPS 100/101). Oligonucleotides were synthesized and purified after synthesis on a DNA synthesizer (380; Applied Biosystems, Norwalk, Conn.) at The Johns Hopkins Genetic Core Laboratory. The sequences were as follows: for CTR 70, 5′ GGC GTA TTT GGG CAT CCG AGT AAC G 3′; for CTR 71, 5′ TCA AAT CCA GCG GGT ATT AAC CGC CT 3′; for CPN 90, 5′ GGT CTC AAC CCC ATC CGT GTC GG 3′; for CPN 91, 5′ TGC GGA AAG CTG TAT TTC TAC AGT T 3′; for CPS 100, 5′ CCC AAG GTG AGG CTG ATG AC 3′; and for CPS 101, 5′ CAA ACC GTC CTA AGA CAG TTA 3′. The sizes of the PCR products predicted with these primers and their corresponding Chlamydia species were 315 bp with primer set CTR 70/71, 197 bp with primer set CPN 90/91, and 111 bp with primer set CPS 100/101.
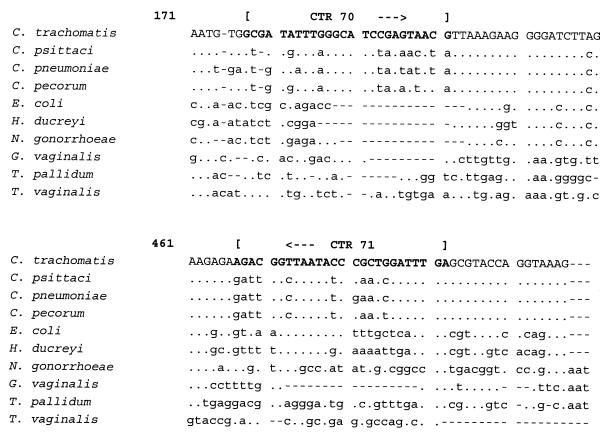
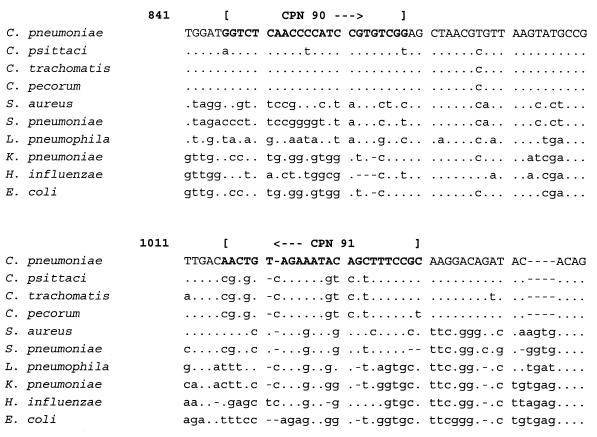
To check for the specificity of the primer design, the targeted DNA sequence of primer set CTR 70/71 was aligned with the 16S rRNA sequences of other microorganisms present in vaginal samples (GenBank accession numbers): C. trachomatis (D85722), Escherichia coli (M25588), Haemophilus ducreyi (M75084), Neisseria gonorrhoeae (X07714), Gardnerella vaginalis (M58744), Treponema pallidum (M88726), and Trichomonas vaginalis (U17510) (Fig. 1). The targeted DNA sequence of primer set CPN 90/91 was aligned with the 16S rRNA sequences of other bacteria present in respiratory samples (GenBank accession number): C. pneumoniae (L06108), Staphylococcus aureus (L37597), Streptococcus pneumoniae (Z22807), Legionella pneumophila (M59157), Klebsiella pneumoniae (X87276), Haemophilus influenzae (X87976), and E. coli (M25588) (Fig. 2). In the same way, the targeted DNA sequence of primer set CPS 100/101 was aligned with the 16S and 16S-23S spacer rRNA sequences of other bacteria (GenBank accession numbers): C. psittaci (U73108 and U68447), C. pecorum (D85716 and U68434), Simkania negevensis (U68460), S. aureus (U39769), Mycobacterium shimoidei (AJ005005), E. coli (U55308), H. influenzae (L31410), and L. pneumophila (AF000654) (Fig. 3).
FIG. 1.
Alignment of the target sequences of primer set CTR 70/71 in the 16S rRNA gene of C. trachomatis with the 16S rRNA genes of other vaginal pathogens. A dot indicates the same base, and a letter indicates a base different from that in C. trachomatis.
FIG. 2.
Alignment of the target sequences of primer set CPN 90/91 in the 16S rRNA gene of C. pneumoniae with the 16S rRNA genes of other respiratory pathogens. A dot indicates the same base, and a letter indicates a base different from that in C. pneumoniae. A dash indicates a deletion.
FIG. 3.
Alignment of the target sequences of primer CPN 100 in the 16S rRNA gene of C. psittaci and primer CPN 101 in the 16S-23S spacer rRNA gene of C. psittaci with the 16S and 16S-23S spacer rRNA genes of other bacteria. A dot indicates the same base, and a letter indicates a base different from that in C. psittaci. A dash indicates a deletion.
TETR-PCR.
The chlamydia TETR-PCR was performed with 50-μl vaginal swab samples in STM processed with AMPLICOR specimen diluent, 10-μl processed respiratory specimens, or 10-μl tissue samples processed by the Chelex method in a total volume of 100 μl of PCR mixture overlaid with 1 drop of mineral oil. The final mixture contained 25 pmol of each primer, 0.25 mM deoxynucleosides triphosphates (dNTPs), PCR buffer, and 2 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Branchburg, N.J.). Since 1.0 mM MgCl2 is a component of AMPLICOR specimen buffers, 1.5 mM MgCl2 was added to the reaction mixture when vaginal swabs were tested; otherwise, 2.5 mM MgCl2 was used when cultures or tissue or respiratory samples were tested as recommended by the manufacturer of the polymerase (Perkin-Elmer). A touchdown method for thermal cycling (10) combined with an enzyme time release protocol was used with a DNA thermal cycler (480; Perkin-Elmer Cetus, Norwalk, Conn.). Cycling times were 75 s at 95°C (to activate a small fraction of the heat-activated DNA polymerase), followed by 60 cycles of denaturation at 94°C for 45 s, annealing beginning at 62°C and ending at 52°C for 45 s, and extension at 72°C for 1 min. The annealing temperature was lowered 1°C every four cycles until it reached 52°C; this annealing temperature was kept until the end of the cycling process. Progressive release of the heat-activated DNA polymerase occurred during the thermal cycling process. The DNA polymerase was gradually activated at each cycle during denaturation to extend its activity to 60 cycles of DNA amplification. PCR products (20 μl) were separated by electrophoresis in 12% polyacrylamide gels (7 by 10 cm) at 80 mA for 30 min with Tris-borate-EDTA buffer (pH 8.3) and visualized with ethidium bromide (0.5 μg/ml) (37).
Analytical sensitivity.
The analytical sensitivities of TETR-PCR with the various primer sets and nested CP1/2-CPC/D PCR were determined by extracting DNA from fourfold serial dilutions of stock cultures of C. trachomatis VR 348 serovar E, C. pneumoniae AO3, and C. psittaci SM006. Chlamydia stock cultures of known titer were diluted in culture media containing tissue culture cells to keep the level of background DNA constant. The analytical sensitivities of primer sets CTR 70/71, CPN 90/91, and CPS 100/101 were also tested with vaginal, nasopharyngeal, and mouse liver specimens spiked with fourfold serial dilutions of the corresponding Chlamydia species. For comparison purposes, the analytical sensitivities of primer sets CTR 70/71, CPN 90/91, and CPS 100/101 were also determined with a previously described PCR protocol (14) which did not use a TETR protocol. In addition, analytical sensitivity with spiked clinical specimens were tested by use of the TETR-PCR protocol described above but with an alternative DNA polymerase (2 U of HotStarTaq DNA polymerase; Qiagen, Valencia, Calif.) and 1.5 mM MgCl2.
Multiplex TETR-PCR.
Multiplex TETR-PCR was performed with 5-μl chlamydia culture DNA preparations (∼25 IFU/PCR) processed by the Chelex method. The three primer sets, CTR 70/71, CPN 90/91, and CPS 100/101, were combined for the simultaneous detection and identification of the three Chlamydia species. The optimal concentration of primers was obtained by checkerboard titration with 1 IFU each of C. trachomatis, C. pneumoniae, and C. psittaci per PCR. Concentrations of primers ranging from 10 to 30 pmol were tested. Optimal primer concentrations were 10 pmol for primers CTR 70, CTR 71, and CPN 91, 25 pmol for CPN 90, and 30 pmol for CPS 100 and CPS 101. The amplification and thermal cycling conditions were the same as for the TETR-PCR described above except for the amount of primers (a total of 115 pmol of primers per reaction in the multiplex TETR-PCR).
To avoid product carryover, PCRs were set up in an area physically separate from all activities involving amplified target sequences, including thermal cycling, PCR product storage, and the running of gels. A separate set of pipettes was devoted to the setup of PCRs, and aerosol barrier pipette tips were used. Negative controls, including uninoculated cell cultures, were used throughout the specimen preparation, DNA extraction, and PCR processes. A low concentration of DNA (from 10 IFU of Chlamydia per PCR) was used as a positive control and was included in every PCR run.
C. trachomatis comparison commercial PCR test.
The AMPLICOR Chlamydia trachomatis test was performed by following the manufacturer's instructions. Briefly, 50 μl of processed vaginal samples or controls was added to a PCR tube containing 50 μl of master mix, including biotin-labeled primers targeting a 207-bp fragment in the cryptic plasmid of C. trachomatis, Taq polymerase, uracil N-glycosylase, and dNTPs. PCR amplification was carried out for 30 cycles with a GeneAMP 9600 thermal cycler (Perkin-Elmer Cetus). After amplification, 100 μl of denaturation solution (Roche) was added to inactivate uracil N-glycosylase (included in the reaction to prevent contamination of new clinical specimens with previously amplified DNA) (26). PCR products were detected in a colorimetric assay with avidin-horseradish peroxidase as a conjugate and tetramethylbenzidine as a substrate. The optical density of the reactions was measured at 450 nm. A sample/cutoff ratio of greater than 0.5 was considered positive (35).
C. pneumoniae comparison nested PCR test.
Respiratory samples were tested for the detection of C. pneumoniae with a nested PCR as described earlier (5, 40). Respiratory specimens processed with N-acetyl-l-cysteine were diluted 1/10 in PCR buffer (10 mM Tris HCl [pH 9.0], 50 mM KCl, 2.0 mM MgCl2, 0.1% Triton X-100) and digested with proteinase K (2 mg/ml) at 55°C for 1 h in an Eppendorf thermomixer (5436; Fisher Scientific, Pittsburgh, Pa.) at 700 rpm. After digestion, proteinase K was inactivated by heating at 95°C for 10 min. DNA was extracted by the Chelex method described above. Ten-microliter samples processed with Chelex were subjected to PCR with 50 pmol of each outer primer (CP1 and CP2) targeting the major outer membrane protein gene of chlamydia (omp1) and 1 U of Taq polymerase (Promega Corp., Madison, Wis.) in a total volume of 100 μl as described previously (10, 40).
Ten microliters of PCR products amplified by the outer primers was transferred to a new 100-μl PCR mixture for a second amplification with the inner nested primers (CPC and CPD). Another outer primer set (CP1-CP2) can amplify a 333-bp product from both C. pneumoniae and C. psittaci omp1 genes but not from the C. trachomatis omp1 gene. Internal primer CPC is specific for C. pneumoniae; therefore, only the first-stage product from C. pneumoniae can be amplified in this nested PCR, giving a 207-bp target product (40).
Analysis of discrepant results.
Vaginal swab samples with discrepant results between the TETR-PCR with primer set CTR 70/71 and the AMPLICOR PCR for the detection of C. trachomatis were resolved by an additional PCR targeting the omp1 gene, which encodes the major outer membrane protein of C. trachomatis, with primer set CT 0005-CT 0006 as described previously (3, 4). This expanded reference standard (e.g., for samples found negative by CTR 70/71 TETR-PCR but positive by AMPLICOR PCR, with omp1 PCR being judged true positive) has been used in the past to help resolve discrepancies between test results, particularly among tests that might be more sensitive than culturing (1, 8, 22, 25, 34).
Respiratory samples with discrepant results between the TETR-PCR with primer set CPN 90/91 and the nested PCR with primer set CP1/2-CPC/D were resolved by an additional nested PCR with outer primer set CPN A-CPN B (CPN A/B) (14) and inner primer set pTW 50-pTW 51 (pTW 50/51) (17), which targets a 270-bp DNA product in a region of the 16S rRNA gene different from the region targeted by primer set CPN 90/91.
Statistical analysis.
The sensitivity and specificity of CTR 70/71 TETR-PCR with vaginal swab samples were calculated by using as a reference gold standard samples that were detected as positive by at least two PCRs, AMPLICOR, CTR 70/71, or omp1. In the same way, the reference gold standard used for true positive for CPN 90/91 TETR-PCR with respiratory samples was defined as samples that were found positive by at least two PCR tests, CPN 90/91 TETR-PCR, nested PCR with CP1/2-CPC/D, or nested PCR with CPN A/B-pTW 50/51. Agreement between the TETR-PCR tests with the various chlamydia primer sets and the AMPLICOR PCR for C. trachomatis, the nested CP1/2-CPC/D PCR for C. pneumoniae, and culturing for C. psittaci was calculated with the kappa test (13). Agreement was qualified with the following kappa values: <0, poor; 0 to 0.2, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and >0.80, excellent (12, 30).
RESULTS
The predicted 315-, 195-, and 111-bp targeted DNA products were successfully amplified by TETR-PCR with each primer set and the corresponding Chlamydia species DNA (CTR 70/71 for C. trachomatis, CPN 90/91 for C. pneumoniae, and CPS 100/101 for C. psittaci DNAs, respectively). No amplified DNA products were detected when DNA from 29 other microorganisms were tested for specificity (Acinetobacter baumannii 10292, Bordetella pertussis, Candida albicans 71, Chilomastix sulcatus ATCC 50562, Cryptococcus neoformans 116, Enterococcus cloacae 10174, Enterococcus faecalis 9385, E. coli, Giardia intestinalis ATCC 30888, K. pneumoniae 10288, L. pneumophila, Macrococcus caseolyticus 3345, Mycobacterium tuberculosis 14323, Mycoplasma pneumoniae, N. gonorrhoeae ATCC 19424, Porphyromonas gingivalis ATCC 53878, Proteus mirabilis 10954, Pseudomonas aeruginosa 10173, S. aureus 3513, Staphylococcus epidermidis 3578, Staphylococcus chromogenes 3262, Streptococcus agalactiae 2152, Streptococcus oralis 2373, S. pneumoniae 3096, Streptococcus salivarius 3233, Streptococcus sanguinis 2536, T. vaginalis P, Trichomonas gallinae ATCC 30002, and Trichomonas tenax ATCC 30207).
Analytical sensitivity.
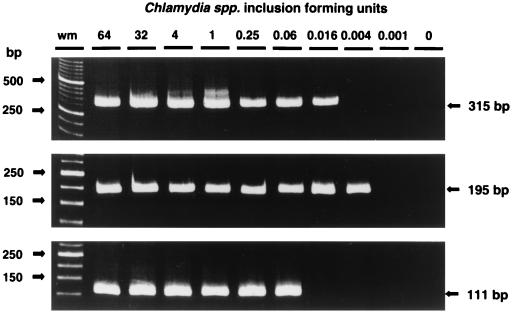
The analytical sensitivity of the PCR assays was determined with DNA extracted from fourfold serial dilutions of chlamydia cultures. The analytical sensitivity of TETR-PCR with primer set CTR 70/71 was 0.016 C. trachomatis IFU per PCR, that with primer set CPN 90/91 was 0.004 IFU of C. pneumoniae per PCR, and that with primer set CPS 100/101 was 0.063 IFU of C. psittaci per PCR (Fig. 4). As a comparison, when we used a conventional (35-cycle) PCR protocol (14) which did not use a touchdown and enzyme time release protocol, the analytical sensitivities achieved with primer sets CTR 70/71, CPN 90/91, and CPS 100/101 were lower: 1 IFU for C. trachomatis, 3 IFU for C. pneumoniae, and 4 IFU for C. psittaci, respectively (data not shown). The analytical sensitivity of nested PCR with primer set CP1/2-CPC/D was 0.063 C. pneumoniae IFU per PCR. The analytical sensitivity of TETR-PCR with primer set CTR 70/71 for a spiked vaginal sample was 0.016 C. trachomatis IFU per PCR, that with primer set CPN 90/91 for a spiked nasopharyngeal specimen was 0.063 IFU of C. pneumoniae per PCR, and that with primer set CPS 100/101 for a spiked mouse liver sample was 0.004 IFU of C. psittaci per PCR. Similar levels of analytical sensitivity were obtained when HotStarTaq DNA polymerase was used in place of AmpliTaq Gold DNA polymerase with primer sets CTR 70/71, CPN 90/91, and CPS 100/101 for spiked clinical samples: 0.004 IFU for C. trachomatis, 0.063 IFU for C. pneumoniae, and 0.063 IFU for C. psittaci, respectively.
FIG. 4.
Analytical sensitivities of C. trachomatis TETR-PCR (primer set CTR 70/71) (top), C. pneumoniae TETR-PCR (primer set CPN 90/91) (middle), and C. psittaci TETR-PCR (primer set CPS 100/101) (bottom). The number of IFU is indicated at the top. Base-pair sizes of weight markers (wm) and PCR products are indicated in the margins. DNA from C. trachomatis VR 348 serovar E, C. pneumoniae AO3, and C. psittaci SM006 was amplified.
C. trachomatis.
A total of 265 vaginal swab samples were tested by TETR-PCR with primer set CTR 70/71 and by the AMPLICOR PCR for the diagnosis of C. trachomatis infection. Twenty-nine samples were found positive and 233 were found negative by both tests (Table 2). The agreement between the two tests was excellent (κ, 0.94). There were three discrepant results (one TETR-PCR-positive sample was AMPLICOR negative, and two AMPLICOR-positive samples were TETR-PCR negative). One of the three discrepant specimens (AMPLICOR positive) was confirmed as true positive by the omp1 PCR (4), and the other two discrepant specimens were not confirmed and were considered false positive (one TETR-PCR-positive specimen and one AMPLICOR-positive specimen). After discrepant samples were resolved, 30 of 265 (11%) of the samples were judged true positive. The sensitivity of TETR-PCR with primer set CTR 70/71 was 96.7% (29 of 30), and the specificity was 99.6% (234 of 235). The AMPLICOR Chlamydia trachomatis test was 100% (30 of 30) sensitive and 99.6% (234 of 235) specific.
TABLE 2.
Comparison of TETR-PCR with primer set CTR 70/71 and AMPLICOR PCR for the detection of C. trachomatis in 265 vaginal swabsa
| No. of samples | Result obtained by:
|
||
|---|---|---|---|
| CTR 70/71 TETR-PCR | AMPLICOR PCR | omp1 PCR | |
| 29 | + | + | NT |
| 1 | − | + | + |
| 1 | − | + | − |
| 1 | + | − | − |
| 233 | − | − | NT |
Discrepant results were resolved by a third PCR targeting the omp1 gene of C. trachomatis (4). +, positive; −, negative; NT, not tested.
C. pneumoniae.
Fifty respiratory clinical samples were tested by TETR-PCR with primer set CPN 90/91 and by nested PCR with primer set CP1/2-CPC/D. Sixteen specimens were found positive and 28 were found negative by both PCR methods. Six samples had discrepant results (Table 3). Four samples with discrepant results (two TETR-PCR positive and two nested CP1/2-CPC/D PCR positive) were judged true positive by an additional nested PCR with primer set CPN A/B-pTW 50/51 (14, 17). Two additional TETR-PCR-positive discrepant specimens were not confirmed as true positive. After resolution of discrepant results, 40% (20 of 50) specimens were judged true positive. The sensitivity of TETR-PCR with primer set CPN 90/91 was 90% (18 of 20), and the specificity was 93.3% (28 of 30). Nested CP1/2-CPC/D PCR was 90% (18 of 20) sensitive and 100% (30 of 30) specific.
TABLE 3.
Comparison of TETR-PCR with primer set CPN 90/91 and nested CP1/2-CPC/D PCR (40) for the detection of C. pneumoniae in 50 respiratory samplesa
| No. of samples | Result obtained by:
|
||
|---|---|---|---|
| CPN 90/91 TETR-PCR | Nested CP1/2-CPC/D PCR | Nested CPN A/B-pTW 50/51 PCR | |
| 16 | + | + | NT |
| 2 | + | − | + |
| 2 | − | + | + |
| 2 | + | − | − |
| 28 | − | − | NT |
C. psittaci.
Thirty-five percent (7 of 20) of the animal tissue samples had a previously positive culture reported by the reference laboratory (National Animal Disease Center). TETR-PCR with primer set CPS 100/101 detected 7 of 20 (35%) positive samples. However, one sample was culture positive and TETR-PCR negative, and one sample was TETR-PCR positive and culture negative. The agreement between the two tests was substantial (κ, 0.78). No resolution of discrepant results was performed.
Multiplex TETR-PCR.
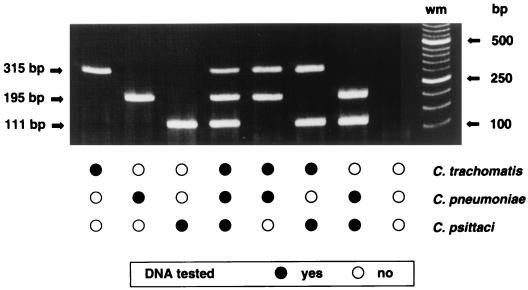
Primer sets CTR 70/71, CPN 90/91, and CPS 100/101 were combined into a multiplex TETR-PCR for the identification of chlamydia tissue culture isolates in a single test. Titration of the primer concentration was performed to achieve the simultaneous amplification of all three target products. Only predicted target products were amplified by each species-specific set of primers when DNA equivalent to 1 IFU of a Chlamydia species was tested alone or in combinations of two or three Chlamydia species by multiplex TETR-PCR (Fig. 5). Multiplex TETR-PCR correctly identified all 15 different serovars of C. trachomatis (315-bp DNA product), 22 of 22 isolates of C. pneumoniae (195-bp DNA product), and 20 of 20 isolates of C. psittaci (111-bp DNA product). The primer sets were specific for the respective species, only predicted DNA products were amplified, and no targeted products were amplified when DNA from two C. pecorum strains was tested (Table 1).
FIG. 5.
Multiplex TETR-PCR products obtained for Chlamydia species with species-specific primer sets CTR 70/71, CPN 90/91, and CPS 100/101 targeting the 16S and 16S-23S spacer rRNA genes. One IFU per PCR was tested individually and in combinations. Base-pair sizes of weight markers (wm) and PCR products are indicated in the margins. DNA from C. trachomatis VR 348 serovar E, C. pneumoniae BAL 37, and C. psittaci SM006 was amplified.
DISCUSSION
Species-specific TETR-PCR for the detection of chlamydia DNA in the 16S and 16S-23S spacer rRNA genes is a practical and useful method that could be easily implemented in laboratories currently performing molecular amplification techniques with basic laboratory reagents and infrastructure. The three sets of primers used individually or in a multiplex assay detected and differentiated C. trachomatis, C. pneumoniae, and C. psittaci.
With the use of a touchdown method, TETR-PCR showed improved annealing conditions for the primers, especially in the multiplex TETR-PCR, where three set of primers with different optimal annealing temperatures were used simultaneously. Successful coamplification of multiple targeted DNA products was achieved when this protocol was used with the three primer sets, CTR 70/71, CPN 90/91, and CPS 100/101. The targeted DNA products were of different sizes and were easy to identify by means of gel electrophoresis (315 bp for C. trachomatis, 197 bp for C. pneumoniae, and 111 bp for C. psittaci). In addition, multiplex TETR-PCR did not require dimethyl sulfoxide, which has been previously used for coamplification of multiple DNA targets (7) but is reported to inhibit Taq polymerase (18). The enzyme time release protocol of TETR-PCR allowed 60 cycles of amplification, which improved the analytical sensitivity of the test, allowing detection of 0.004 to 0.063 IFU of Chlamydia species per PCR compared to 0.1 to 4 IFU when a conventional PCR protocol with 35 cycles was used (14). These levels of analytical sensitivity are better than those reported for conventional PCR protocols (6, 14, 32) and PCR-enzyme immunoassays (3, 17, 21, 41) and are equivalent to those of nested PCR assays (0.063 IFU for nested CP1/2-CPC/D PCR) (2, 40). No reduction in the analytical sensitivity was noted when the TETR-PCRs were tested with spiked clinical samples (0.004 to 0.063 IFU). In addition, similar analytical sensitivities (0.004 to 0.063 IFU) were obtained when HotStarTaq DNA polymerase was used in place of AmpliTaq Gold DNA polymerase, suggesting that HotStarTaq DNA polymerase could be used as an alternative enzyme for TETR-PCR.
The DNA sequences of primer sets CTR 70/71 and CPN 90/91 and of primer CPS 100 were selected from the 16S rRNA gene. Since the 16S rRNA gene is very conserved within Chlamydia species (15, 33), very few regions are distinctive for C. psittaci. Therefore, a target DNA sequence for primer CPS 101 was selected in the contiguous 16S-23S spacer rRNA gene, which has more variable regions between Chlamydia species that are unique for C. psittaci (11). The correct primer selection provided specificity for the TETR-PCRs. One primer targeting a unique chlamydia DNA sequence was sufficient to confer specificity to a primer set. Adequate specificity of the primer sets was supported by the lack of amplified products when DNAs from C. pecorum and other microorganisms were tested. The alignment of the target sequences of primer sets CTR 70/71, CPN 90/91, and CPS 100/101 with the 16S rRNA gene and/or 16S-23S spacer rRNA gene sequences from a selection of other pathogens and normal flora that could be present in clinical samples demonstrated that the selected primer sets, CTR 70/71, CPN 90/91, and CPS 100/101, were significantly different from the 16S rRNA gene sequences of the other organisms, thus confirming their specificity.
The 96.7% sensitivity and 99.6% specificity achieved by TETR-PCR with primer set CTR 70/71 for the detection of C. trachomatis in vaginal specimens were comparable to the sensitivity and specificity of the commercially available AMPLICOR Chlamydia trachomatis test, which includes a colorimetric hybridization assay for the detection of amplified PCR products and has been reported to have high sensitivity and specificity compared with culturing (23, 35). The agreement between the two PCR tests in this study was excellent (κ, 0.94). In addition, TETR-PCR with primer set CPN 90/91 for the detection of C. pneumoniae in respiratory samples was as sensitive (90%) as the nested PCR described by Tong and Sillis, which uses two consecutive PCRs and has been reported to have a high sensitivity for detecting C. pneumoniae DNA in respiratory specimens (40). TETR-PCR with primer set CPS 100/101 for the detection of C. psittaci showed substantial agreement (κ, 0.78) with culturing when evaluated with animal tissue samples.
We evaluated our set of primers combined into a multiplex TETR-PCR for the identification of Chlamydia species from tissue culture isolates only and not from clinical specimens. The 16S rRNA and the 16S-23S rRNA spacer genes are highly conserved (11, 15, 33), and primers based on these regions provided for the correct identification of all 57 chlamydia isolates tested by the multiplex TETR-PCR. The actual testing of the many strains of C. pneumoniae, C. psittaci, and C. trachomatis corroborated that the differences between strains in the 16S rRNA genes where the primers were targeted were indeed conserved differences, as there were no strains that failed to be amplified by multiplex TETR-PCR with their respective primer sets. Multiplex TETR-PCR is more difficult to perform than single-target PCR. To avoid competition, purification of primers after synthesis and careful titration of the primer concentration tested with a small number of targeted DNA copies are required to prevent reduction of the analytical sensitivity.
Multiplex TETR-PCR can potentially be used for clinical samples. Messmer et al. (32) described a nested multiplex PCR targeting a different region of the 16S rRNA gene. Messmer et al. (32) were able to detect 13 specimens positive for C. psittaci among 75 bird tissue or feces samples (4 confirmed by culturing). One human tissue sample detected by nested multiplex PCR for C. psittaci was also reported (32). Multiplex TETR-PCR may also be useful for the identification of tissue culture isolates of chlamydiae from respiratory specimens, where any of the three species of chlamydiae may cause disease. The analytical sensitivity can be reduced slightly when multiple primer sets are combined in a single PCR. Competition of the primers for dNTPs and DNA polymerase enzyme, the increased probability of amplification of spurious DNA products, and the increased chance for primer-dimer formation are the problems to circumvent with good primer design and PCR techniques.
We extracted the DNA by boiling the cultures with a suspension containing chelating resin as described previously (42). This method is simple, inexpensive, and easy to perform and requires minimal sample manipulation, which may reduce the chances for contamination (29). TETR-PCR being a single amplification assay provided fewer chances for carryover contamination than nested PCR, in which amplified DNA products of the first amplification are transferred to a second PCR amplification.
In conclusion, we have developed a sensitive and specific TETR-PCR method for the identification of all Chlamydia species that can cause disease in humans by using the 16S rRNA and 16S-23S spacer rRNA genes. This single-step TETR-PCR method combines (i) a DNA polymerase activated at 95°C, simulating a hot start to prevent DNA synthesis before thermal cycling; (ii) a touchdown protocol that, by reducing the annealing temperature from high to low, improved the specificity of the primers; (iii) the gradual activation of the DNA polymerase enzyme during the thermal cycling, allowing 60 amplification cycles for improved analytical sensitivity; and (iv) a range of annealing temperatures (62 to 52°C) that allowed specific coamplification of multiple DNA targets with primers with different annealing temperatures. These four characteristics provided for sensitivities and specificities comparable to those of the AMPLICOR Chlamydia trachomatis test with vaginal swab samples and the nested PCR for C. pneumoniae with respiratory samples and substantial agreement with animal tissue culturing for C. psittaci.
ACKNOWLEDGMENTS
This study was partially supported by grant DAMD17-96-1-6309, U. S. Army Medical Research and Material Command, Fort Detrick, Md.
We thank Arthur Andersen, Anne Rompalo, Kelly T. McKee, Jr., Marie Tenant, J. Storz, and Katherine Kacena for collaboration and assistance with specimens; Carolyn M. Black, Jim T. Summersgill, Trudy O. Messmer, and Margaret R. Hammerschlag for assistance with chlamydia strains; Dien Pham and Kimberly Crotchfelt for laboratory assistance; and M. Atenas for assistance with the preparation of the manuscript.
REFERENCES
- 1.Bauwens J G, Clark A M, Loeffelholtz M J, Herman S A, Stamm W E. Diagnosis of Chlamydia trachomatis urethritis in men by polymerase chain reaction assay of first-catch urine. J Clin Microbiol. 1993;31:3013–3016. doi: 10.1128/jcm.31.11.3013-3016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black C M, Fields P I, Messmer T O, Berdal B P. Detection of Chlamydia pneumoniae in clinical specimens by polymerase chain reaction using nested primers. Eur J Clin Microbiol Infect Dis. 1994;13:752–756. doi: 10.1007/BF02276060. [DOI] [PubMed] [Google Scholar]
- 3.Bobo L, Coutlee F, Yolken R H, Quinn T C, Viscidi R. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with enzyme immunoassay. J Clin Microbiol. 1990;28:1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobo L, Munos B, Viscidi R, Quinn T C, Mkocha H, West S. Diagnosis of Chlamydia trachomatis eye infection in Tanzania by polymerase chain reaction/enzyme immunoassay. Lancet. 1991;338:847–850. doi: 10.1016/0140-6736(91)91502-l. [DOI] [PubMed] [Google Scholar]
- 5.Boman J, Allard A, Persson K, Lundborg M, Juto P, Wadell G. Rapid diagnosis of respiratory Chlamydia pneumoniae infection by nested touchdown polymerase chain reaction compared with culture and antigen detection by EIA. J Infect Dis. 1997;175:1523–1526. doi: 10.1086/516492. [DOI] [PubMed] [Google Scholar]
- 6.Campbell L A, Melgosa M P, Hamilton D J, Kuo C C, Grayston J T. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992;30:434–439. doi: 10.1128/jcm.30.2.434-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain J S, Gibbs R A, Ranier J E, Nguyen P N, Caskey C T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988;16:11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernesky M, Castriciano S, Sellor J, Stewart I, Cunningham I, Landis S, Seidelman W, Grant L, Devlin C, Mahony J. Detection of Chlamydia trachomatis antigens in urine as an alternative to swabs and cultures. J Infect Dis. 1990;161:124–126. doi: 10.1093/infdis/161.1.124. [DOI] [PubMed] [Google Scholar]
- 9.Crotchfelt K A, Pare B, Gaydos C A, Quinn T C. Detection of Chlamydia trachomatis by Gen-Probe AMPLIFIED Chlamydia trachomatis Assay (AMP CT) in urine specimens from men and women and endocervical specimens from women. J Clin Microbiol. 1998;36:391–394. doi: 10.1128/jcm.36.2.391-394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Don R H, Cox P T, Wainwright B J, Baker K, Mattick J S. “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett K D E, Andersen A A. The ribosomal intergenic spacer and domain I of the 23S rRNA gene are phylogenetic markers for Chlamydia spp. Int J Syst Bacteriol. 1997;47:461–473. doi: 10.1099/00207713-47-2-461. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein A. Clinical epidemiology. Philadelphia, Pa: The W. B. Saunders Co.; 1985. [Google Scholar]
- 13.Fleiss J L. Statistical methods for rates and proportions. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 14.Gaydos C A, Quinn T C, Eiden J J. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992;30:796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaydos C A, Palmer L, Quinn T C, Falkow S, Eiden J. Phylogenetic relationship of Chlamydia pneumoniae to Chlamydia psittaci and Chlamydia trachomatis as determined by analysis of 16S ribosomal DNA sequences. Int J Syst Bacteriol. 1993;43:610–612. doi: 10.1099/00207713-43-3-610. [DOI] [PubMed] [Google Scholar]
- 16.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaydos C A, Fowler C L, Gill V J, Eiden J J, Quinn T C. Detection of Chlamydia pneumoniae by polymerase chain reaction-enzyme immunoassay in an immunocompromised population. Clin Infect Dis. 1993;17:718–723. doi: 10.1093/clinids/17.4.718. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand D H, White T J. Thermostable DNA polymerases. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols, a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 129–141. [Google Scholar]
- 19.Hammerschlg M R. Chlamydia trachomatis in children. Pediatr Ann. 1994;23:349–353. doi: 10.3928/0090-4481-19940701-08. [DOI] [PubMed] [Google Scholar]
- 20.Holland S M, Gaydos C A, Quinn T C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990;162:984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- 21.Jantos C A, Roggendorf R, Wuppermann F N, Hegemann J H. Rapid detection of Chlamydia pneumoniae by PCR-enzyme immunoassay. J Clin Microbiol. 1998;36:1890–1894. doi: 10.1128/jcm.36.7.1890-1894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaschek G, Gaydos C A, Welsh L E, Quinn T C. Direct detection of Chlamydia trachomatis in urine specimens from symptomatic men using a rapid polymerase chain reaction assay. J Clin Microbiol. 1993;31:1209–1212. doi: 10.1128/jcm.31.5.1209-1212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay I D, Palladino S, Alexander R, Leahy B J, Pearman J W. Evaluation of commercial polymerase chain reaction assay for the detection of Chlamydia trachomatis. Diagn Microbiol Infect Dis. 1997;27:75–79. doi: 10.1016/s0732-8893(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C C, Jackson L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Chernesky M, Schachter J, Burczak J, Andrews W, Muldoon S, Leckie G, Stamm W. Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet. 1995;345:213–216. doi: 10.1016/s0140-6736(95)90221-x. [DOI] [PubMed] [Google Scholar]
- 26.Logo M C, Beringer M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 27.Maass M, Dalhoff K. Comparison of sample preparation methods for detection of Chlamydia pneumoniae in bronchioalveolar lavage fluid by PCR. J Clin Microbiol. 1994;32:2616–2619. doi: 10.1128/jcm.32.10.2616-2619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maass M, Dalhoff K. Transport and storage conditions for cultural recovery of Chlamydia pneumoniae. J Clin Microbiol. 1995;33:1793–1796. doi: 10.1128/jcm.33.7.1793-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madico G, Quinn T C, Rompalo A, McKee K T, Jr, Gaydos C. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36:3205–3210. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madico G, Gilman R H, Jabra A, Rojas L, Hernandez H, Fukuda J, Bern C, Steinhoff M. The role of pulse oximetry. Its use as an indicator of severe respiratory disease in Peruvian children living at sea level. Arch Pediatr Adolesc Med. 1995;149:1259–1263. doi: 10.1001/archpedi.1995.02170240077012. [DOI] [PubMed] [Google Scholar]
- 31.Meijer A, Kwakkel G J, de Vries A, Schouls L M, Ossewaarde J M. Species identification of chlamydia isolates by analyzing restriction fragment length polymorphism of the 16S-23S rRNA spacer region. J Clin Microbiol. 1997;35:1179–1183. doi: 10.1128/jcm.35.5.1179-1183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messmer T O, Skelton S K, Moroney J F, Daugharty H, Fields B S. Application of a nested, multiplex PCR to psittacosis outbreaks. J Clin Microbiol. 1997;35:2043–2046. doi: 10.1128/jcm.35.8.2043-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pudjiatmoko, Fukushi H, Ochiai Y, Yamaguchi T, Hirai K. Phylogenetic analysis of the genus Chlamydia based on 16S rRNA gene sequences. Int J Syst Bacteriol. 1997;47:425–431. doi: 10.1099/00207713-47-2-425. [DOI] [PubMed] [Google Scholar]
- 34.Quinn T C. Recent advances in the diagnosis of sexually transmitted diseases. Sex Transm Dis. 1994;21(Suppl. 2):S19–S27. [PubMed] [Google Scholar]
- 35.Quinn T C, Welsh L, Lentz A, Crotchfelt K, Zenilman J, Newhall J, Gaydos C A. Diagnosis by AMPLICOR PCR of Chlamydia trachomatis infection in urine samples from women and men attending sexually transmitted disease clinics. J Clin Microbiol. 1996;34:1401–1406. doi: 10.1128/jcm.34.6.1401-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez J A the Chlamydia pneumoniae/Atherosclerosis Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schlossberg D. Chlamydia psittaci (psittacosis) In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1693–1696. [Google Scholar]
- 39.Sillis M, White P, Caul E Q, Paul I D, Treharne J D. The differentiation of Chlamydia species by antigen detection in sputum specimens from patients with community-acquired acute respiratory infections. J Infect. 1992;25:77–86. doi: 10.1016/0163-4453(92)92130-b. [DOI] [PubMed] [Google Scholar]
- 40.Tong C Y W, Sillis M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J Clin Pathol. 1993;46:313–317. doi: 10.1136/jcp.46.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toye B, Peeling R W, Jessamine P, Claman P, Gemmill I. Diagnosis of Chlamydia trachomatis infections in asymptomatic men and women by PCR assay. J Clin Microbiol. 1996;34:1396–1400. doi: 10.1128/jcm.34.6.1396-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh P S, Metzger D A, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 43.Wilson P A, Phipps J, Samuel D, Saunders N A. Development of a simplified polymerase chain reaction-enzyme immunoassay for the detection of Chlamydia pneumoniae. J Appl Bacteriol. 1996;80:431–438. doi: 10.1111/j.1365-2672.1996.tb03239.x. [DOI] [PubMed] [Google Scholar]