Abstract
The combat against the Corona virus disease of 2019 (COVID-19), has created a chaos among the healthcare institutions and researchers, in turn accelerating the dire need to curtail the infection spread. The already established entry mechanism, via ACE2 has not yet successfully aided in the development of a suitable and reliable therapy. Taking in account the constant progression and deterioration of the cases worldwide, a different perspective and mechanistic approach is required, which has thrown light onto the cluster of differentiation 147 (CD147) transmembrane protein, as a novel route for SARS-CoV-2 entry. Despite lesser affinity towards COVID-19 virus, as compared to ACE2, this receptor provides a suitable justification behind elevated blood glucose levels in infected patients, retarded COVID-19 risk in women, enhanced susceptibility in geriatrics, greater infection susceptibility of T cells, infection prevalence in non-susceptible human cardiac pericytes and so on. The manuscript invokes the title role and distribution of CD147 in COVID-19 as an entry receptor and mediator of endocytosis-promoted entry of the virus, along with the “catch and clump” hypothesis, thereby presenting its Fundamental significance as a therapeutic target for potential candidates, such as Azithromycin, melatonin, statins, beta adrenergic blockers, ivermectin, Meplazumab etc. Thus, the authors provide a comprehensive review of a different perspective in COVID-19 infection, aiming to aid the researchers and virologists in considering all aspects of viral entry, in order to develop a sustainable and potential cure for the 2019 COVID-19 disease.
Keywords: COVID-19, ACE2, CD147, Receptor, Catch and clump, Melatonin
Graphical abstract
1. Introduction
The global population is currently facing a significant threat by the uncontrollable spread of the severe corona virus disease of 2019 (COVID-19), wrecking a havoc worldwide. The 2019 outbreak of corona virus disease began from Wuhan (China), transforming into a leading pandemic, posing an immense threat to the global population. Gradual progress in identification, diagnosis, clinical studies and management of this virus became a major concern.
The corona virus tropism is significantly determined by the spike (S) protein, which facilitates the infection by the virus, by aiding in its binding to the host cell surface (Hulswit et al., 2016). One of the important host cell membrane receptors are angiotensin-converting enzyme-2 (ACE2), which identifies the viral S protein and mediates its infection (Lan et al., 2020; Hoffmann et al., 2020). This receptor is significantly expressed stomach, colon, liver, kidney, lungs and ileum, but its concentration levels are lower, primarily in the lungs (M.Y. Li et al., 2020). The homotrimeric spike (S) glycoprotein is embedded in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which interacts with the respective receptors on the host cells, triggering multiple events, which results in fusion of the membranes of the cell and virus, aiding in its entry (Wrobel et al., 2020), followed by manipulation of the Raf/MEK/ERK signalling pathway in the host and regulation of gene transcription and viral replication in the host cell (Ghasemnejad-Berenji and Pashapour, 2020). On account of extensive infection spread and risk of COVID-19, it is considered that the viral infection may depend on other significant receptors, to mediate its infection. Neuropilin-1 has been recognized as a co-factor, associated with SARS-CoV-2 infection, mediated by ACE2 (Daly et al., 2020; Cantuti-Castelvetri et al., 2020).
Among the significant claims for existence of another receptor, mediating severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) infection, cluster of differentiation 147 (CD147) has been identified as the binding receptor for the viral S protein, along with functional importance in viral entry (Chan et al., 2016; Chu et al., 2018). The elevated susceptibility to failed respiratory system and poor detection, worsens the condition in case of middle-aged patients and geriatric patients (Gorbalenya et al., 2020). CD147 contributes to the COVID-19 symptoms on account of its expression in the inflammatory, infected and tumour cells (Chen et al., 2017), therefore forming the basis of the possible COVID-19 treatment.
Cluster of differentiation 147 (CD147), also referred to as basigin or extracellular matrix metalloproteinase inducer (EMMPRIN), is a transmembrane protein, which contributes to the development of tumour, invasion of Plasmodium and infection mediated by bacteria or viruses (Lu et al., 2018; Pushkarsky et al., 2001, Pushkarsky et al., 2001; Zhang et al., 2018; Zhao et al., 2011; Bernard et al., 2014). Multiple investigations have portrayed the significant role of CD147 in mediating SARS-CoV-2 infection, and anti-viral effect of CD147 antagonist peptide-9, as a consequence (Chen et al., 2005). CD147 has been found to be involved in the indirect interaction between cyclophilin A and viral spike protein, while itself binds directly to the viral S protein, with a significantly high affinity, as per the results of surface plasmon resonance, co-immunoprecipitation and enzyme-linked immunosorbent assay (ELISA) (Shilts et al., 2021). The role of CD147 as a significant therapeutic target has already been established, on account of its role as a receptor for the invasion of malaria-causing protozoan, Plasmodium falciparum (Crosnier et al., 2011; Zenonos et al., 2015). CD147 was previously recognized as an epithelial cell receptor for measles virus (Watanabe et al., 2010, Watanabe et al., 2010). Furthermore, CD147 significantly mediates the SARS-CoV-2-caused cardiovascular damage.
The authors invoke the significance of CD147 as an alternative receptor for SARS-CoV-2, with evidences displaying its critical involvement in the COVID-19 disease, thereby suggesting it to be used as an optimum target for the development of a therapeutic regimen. In addition to this, the authors portray the significance of CD147 receptor, in providing suitable explanations behind the elevated blood sugar levels, in patients, infected with COVID-19, more susceptibility of T cells towards COVID-19 infection, alleviated infection risks in women and greater susceptibility in geriatrics and middle-aged population, prevalence of COVID-19 infection in non-susceptible human cardiac pericytes etc. Thus, the manuscript provides a comprehensive data based upon the highlights if COVID-19 disease, entry mechanisms via ACE2 and CD147, as a novel route, role of CD147 as a receptor for malaria-causing plasmodium, its involvement in mediating viral entry via endocytosis, the catch and clump hypothesis, and its significance as a target for suitable therapeutic candidates, like melatonin, statins, meplazumab, ivermectin, azithromycin, and so on.
2. Getting a grasp of the COVID-19 disease
The COVID-19 infected patients depict greater WBC number, enhanced plasma levels of pro-inflammatory cytokines and irregular respiratory findings. A case report elaborated cough, heavy breathing sounds and a body temperature of 102° Fahrenheit from 5 days, along with sputum samples of the patient depicting positive real time PCR findings, evidently supporting COVID-19 infection (Lei et al., 2020). The laboratory profile showed high PCR and D-dimer accompanied with 16.16 mg/L of blood G-reactive protein levels (Lei et al., 2020). The analysis suggested condition of leucopenia in the patients (Lei et al., 2020). Recent results have reported the occurrence of thromboembolism in serious COVID-19 patients (Srivastava and Kumar, 2021). RNAemia and pneumonia are associated with this virus, with clinical results elaborating ground glass opacities and acute cardiac injury (Huang et al., 2020). Moreover, the patients exhibited high levels of cytokines and chemokines in blood and a few with enhanced levels of pro-inflammatory cytokines, contributing to the severity of the disease. Clinical features revealed grand – glass opacities in the sub – pleural regions of the lungs of infected patients (Lei et al., 2020). This leads to an immune response mediated inflammatory event in the infected patient. Infiltration of upper part of lungs was observed after chest radiography in patients, leading to hypoxemia and dyspnoea (Phan et al., 2020). The COVID-19 disease is caused by SARS CoV – 2, which expresses genetic similarity with SARS HCoV-1. SARS-CoV – 2 is a beta coronavirus (coronaviridae family) possessing a ssRNA sequence (Lu et al., 2020; COVID-19 Current Challenges and Future Perspectives, 2021).
Talking about SARS-CoV-1, it binds to the ACE - 2 enzyme via 14 binding residues, 8 of which have been detected in novel coronavirus (Fehr and Perlman, 2015). Such a resemblance between SARS-CoV-1 and SARS-CoV-2 can depict a more detailed picture of pathophysiology of the virus, which is not yet completely illustrated due to pending trials, and provide a valid explanation for the inflammatory response and pneumonia occurrence in infected patients (Fehr and Perlman, 2015). The pathogenetic events in SARS and Middle East respiratory syndrome-corona virus-2 (MERS-CoV) infected models reveal the generation of significant inflammatory and immune responses, activating a “cytokine storm”, promoting endothelial and epithelial cell apoptosis, aberrant T cell response, vascular leakage, induction of acute lung injury/acute respiratory diseases (ALI/ARDS) or other alterations leading to death (Zhang et al., 2018). The COVID-19 infected patients also experience the cytokine storm similar to the previous members of corona virus family, along with an exceptional increase in the levels of interleukins, interferons, interferon inducible protein 10 and monocyte chemoattractant protein 1, unlike SARS and MERS-CoV forms (Zhang et al., 2018). The immune function is disoriented in COVID-19 infected patients, accompanied with lymphopenia, neutropenia, excessive inflammation, retarded cluster of differentiation 8 (CD8) +T cell levels and hypo-albuminemia (Huang et al., 2020). Dendritic and epithelial cells are activated in the initial stages of infection, mediating the release of pro-inflammatory cytokines and chemokines, overproduction of which aids in disease progression (Cheung et al., 2005). T-helper-2 produces interleukin-10 (IL-10), whose production decreases in an infected individual on account of its anti-viral property (Fehr et al., 2016). However, in some patients the infection might enhance IL-10 levels (Huang et al., 2020). A clinical approach is essential to study the “behind the scenes” events of the infection. Other than the common pneumonia and cough symptoms, many patients complaint about unusual adverse effects like, lethargy, nausea, diarrhoea, vomiting, headache, confused state of mind, sneezing, rhinorrhoea, nasal congestion, pleurisy and sore throat (N. Chen et al., 2020; Y. Chen et al., 2020). A group of middle aged 41 Chinese patients proclaimed the appearance of symptoms like, cough (76%), dyspnoea (55%), fever (98%), headache (8%), diarrhoea (3%), haemoptysis (5%) and fatigue (44%) (Huang et al., 2020). Gastrointestinal symptoms like anorexia, stomach pain, vomiting and diarrhoea are observed in a case study of 204 patients, out of which 7 patients were only experiencing GIT symptoms and no respiratory problems (Pan et al., 2020). A temperature cut off of 100 degrees Fahrenheit is recommended by U.S. Centres for Disease Control and Prevention, in order to facilitate rapid identification of infected patients (CDC, 2019). Acute respiratory distress syndrome (ARDS) may occur in about 17–29% patients (Huang et al., 2020), and various other problems, like heart injury, secondary bacterial infection, septic shock, coagulation dysfunction, multiple organ failure and ventilator-associated pneumonia, may occur simultaneously (N. Chen et al., 2020; Y. Chen et al., 2020). Even in children infected with the virus, certain adverse effects were observed like, lowered blood pressure, mental regression, tachypnoea, oliguria and body temperature variation (WHO, 2020). The physical examination results of COVID-19 infected patients focused on pulmonary and hemodynamic indications of the disease progression. Irregular respirations and tachypnoea are associated with severe cases of infection.
3. Environmental impact of the COVID-19 strengthens the need to find a suitable treatment
Amidst the health effects, the COVID-19 disease has exerted, the disease has also exhibited environmental effects, which make it extremely necessary to retard the infection progression. Since the outbreak, there has been a significant increase in the production of medical waste, posing critical threat to environment as well as human health (Rume and Ul Islam, 2020). The diagnosis, treatment and infection procedures result in the generation of tremendous amount of waste materials from the hospitals (Somani et al., 2020; Zambrano-Monserrate et al., 2020). For instance, in Wuhan, about 240 metric tons of medical was generated every day, which exceeded the normal limit by 190 metric tons (Zambrano-Monserrate et al., 2020).
Safety equipment, such as face masks, hand gloves etc., is being used at a global level, which also adds on to the medical waste (Rume and Ul Islam, 2020). It has been revealed that the trash amount has been elevated in the USA, due to the elevated domestic use of PPE (Calma, 2020). Also, the use of plastic-PPE has been increased across the world (Singh et al., 2020), which has accelerated the environmental pollution and is the prime source of microplastic fibres in the environment (Fadare and Okoffo, 2020). The Tyvek used in gloves, face shields and protective suits, as well as polypropylene used in the production of N-95 masks, release dioxin, on account of its long persistence in the environment, thereby releasing toxic chemicals (Singh et al., 2020). Lack of knowledge of disposal of infectious waste has promoted the dumping of medical waste in open areas and neighbourhoods (Rahman et al., 2020), leading to water way clogging and lang pollution (Singh et al., 2020; Zambrano-Monserrate et al., 2020).
Furthermore, the elevated use of disinfectants, to alleviate the risk of infection, might cause damage to non-targeted beneficial species, thereby disrupting the ecological balance (Rume and Ul Islam, 2020). Also, viral particles were detected in faeces of patients, as well as municipal waster water (Ahmed et al., 2020; Nghiem et al., 2020; Mallapaty, 2020). This establishes a dire need of proper waste management and development of a suitable treatment to terminate the pandemic across the globe. Therefore, this intensifies the need to develop a cure to terminate the disease spread, as the disastrous impact of the virus on the human population is already a Herculean task to control, thus environmental deterioration would make things more worse.
4. Host cell entry of SARS-CoV-2
One of the key aspects of the study designs of COVID-19 is the detailed understanding of the entry mechanism of novel corona virus (COVID-19 Current Challenges and Future Perspectives, 2021). The corona viruses belong to the category of RNA viruses, which are not segmented, resulting in enzootic infections, especially in mammals and birds, which is extremely dangerous to the human kind (Wang et al., 2020a). The four structural proteins that the virus encompasses of are envelope (E), membrane (M), nucleocapsid (N) and spike (S) proteins, where the last one is a fundamental component to aid in the entry of SARS-CoV-2 into the host cell (Rota et al., 2003; Hulswit et al., 2016), via ACE-2, expressed in multiple tissues (Donoghue et al., 2000; Harmer et al., 2002). The spike (S) protein of SARS-CoV-2 is responsible for the viral entry into the host cell, where it is activated by proteases, mainly transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al., 2020). The spike (S) glycoproteins of SARS-CoV-2 comprises of protrusions, which confer their specificity for some receptors of the host cell (Rodrigues-Diez et al., 2020). It comprises of 2 subunits, namely S1, containing receptor binding domain (RBD) which identifies receptors at the cell surface, and S2, significant for fusion of membrane (Rodrigues-Diez et al., 2020).
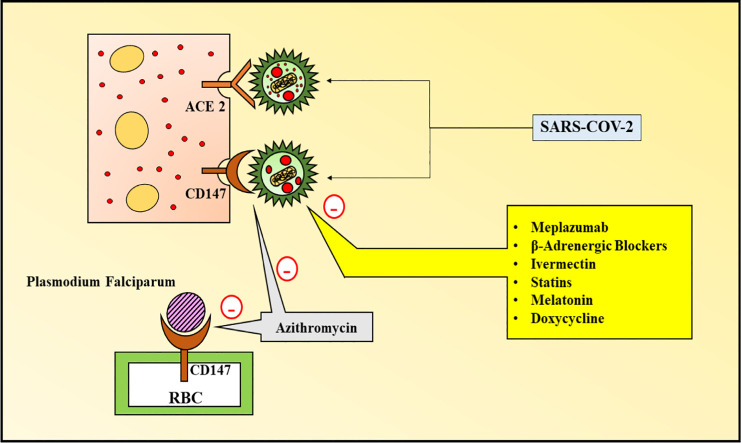
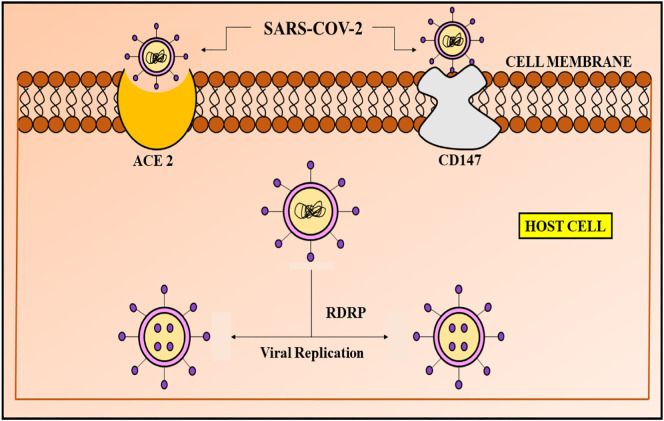
The virus has been reported to interact directly with cluster of differentiation 147 (CD147) (H. Ulrich and Pillat, 2020), a type 1 transmembrane protein, which belongs to the immune-globulin superfamily, playing a critical role in the development of tumour, entry of plasmodium and infection mediated by viruses (Kuba et al., 2010). Pulmonary edema and vascular penetrability is elevated by CD147 and ACE-2 in lung disorders, activating the renin-angiotensin-aldosterone system (RAAS), aiding in destruction of the lungs (Kuba et al., 2010). Therefore, ACE-2 and CD147 are the two receptors, via which SARS-CoV-2 enters the host cell (Wang et al., 2020b; Yan et al., 2020) (Fig. 1 ). The virus invades, when its spike protein binds to CD147 or ACE2 on the host cell, followed by dissemination of the virus. The common similarity between SARS-CoV-1 and SARS-CoV-2 is same structure of spike proteins in both and the binding receptor, i.e. ACE-2 (Wang et al., 2020a; Yan et al., 2020). Recently, it has been demonstrated that besides ACE-2, CD147 is another receptor to which the SARS-CoV-2 virus binds and enters into the host cell (Wang et al., 2020a). In a study, Meplazumab (an anti-CD147 humanized antibody), has been used along with, ELISA, immune electron microscopy and co-immunoprecipitation, to portray the novel route of viral entry, via CD147-spike protein. This, thereby provides a prime target for development of drugs to treat the COVID-19 infection (Ulrich and Pillat, 2020).
Fig. 1.
Entry receptors of SARS-CoV-2: ACE2 and CD147. The ACE-2 receptor on the cell surface binds to the S-protein of the viral genome, thereby inducing conformational changes in the protein, which induces fusion of the viral envelope to the host cell membrane, followed by release of RNA into the host cell which undergoes translation and produces viral replicase polyproteins, cleaved to form proteinases. Irregular transcription and translation events lead to the formation of subgenomic mRNAs via RNA-dependent RNA polymerase (RDRP) enzyme, followed by viral proteins production. Like ACE-2, CD147 has also been identified as the binding site for the viral spike protein, elevated by angiotensin 2 and cyclophilins. Cyclophilin A and B bind to and activate CD147, thereby aiding in the process of interaction between viral S protein and CD147. SARS-CoV-2 enters the host cell through CD147 mediated endocytosis.
The Raf/MEK/ERK signalling pathway is activated by the S protein in the host cell (Ghasemnejad-Berenji and Pashapour, 2020). Manipulation of the ERK1/2 signalling pathway in the host supports the replication of the virus (Ghasemnejad-Berenji and Pashapour, 2020) and cyclooxygenase-2 (COX-2) induction, aiding in inflammation (Liu et al., 2007). The viral inhibition is inhibited by blockage of ERK1/2, via siRNAs (Cai et al., 2007) Therefore, the drug which hinders the viral proteins and their interaction to cell receptors are suitable candidates for COVID-19 treatment.
The ORF1 downstream regions of all forms of corona virus consists of special protein encoding genes for spikes and nucleotide production and viral replication (Van Boheemen et al., 2012). The virus attaches itself to the host cell surface via glycoprotein spikes on the outer membrane, and may attaché to multiple hosts on account of the loosely linked receptor-binding domain (Raj et al., 2013). SARS-CoV-1 and MERS – CoV forms of corona virus recognize exopeptidases enzymes as primary receptors favouring viral entry into the human cell, unlike other forms which mainly recognize carbohydrates and aminopeptidases for the same (Wang et al., 2013). The cellular proteases like cathepsins, human airway trypsin like protease and transmembrane protease serine 2, significantly regulate the entry mechanisms of the virus via inducing penetration favouring alterations in the virus structure (Wu et al., 2020).
4.1. ACE 2 mediated entry of COVID-19 virus
Dipeptidyl peptidase 4 (DPP4) and ACE2 are the key receptors for MERS and SARS-CoV-1 respectively (Wang et al., 2013). The life cycle of novel corona virus begins with ACE-2 receptor binding of S-protein of the viral genome leading to S-protein conformational alteration mediated fusion of envelope of the viral entity with the host cell membrane via endosomal pathway (Adnan Shereen et al., 2020). This fusion is followed by release of the viral generic material (RNA) in the host cell, which undergoes translation and produces viral replicase polyproteins ppa1 and 1ab (Adnan Shereen et al., 2020). The polyproteins are then cleaved to form proteinases. Irregular transcription and translation events lead to the formation of sub-genomic messenger RNA (mRNAs) from polymerase enzyme, followed by viral proteins production (Adnan Shereen et al., 2020). These proteins and RNA genome are accumulated into virions in endoplasmic reticulum and golgi apparatus, followed by transportation and release as vesicles (Adnan Shereen et al., 2020). The protein encoding genes include spike (S), envelope (E), membrane (M) and nucleocapsid (N) genes (Adnan Shereen et al., 2020). Pp1ab protein and 15 nsps are encoded by the largest gene in COVID-19, i.e. orf1ab (Adnan Shereen et al., 2020). The orf1a gene encodes for 10 nsps containing pp1ab protein (N. Chen et al., 2020; Y. Chen et al., 2020). Significant alterations are observed in SARS-CoV-2 genome, like amino acid fluctuation in 8b and 3c protein as well as absence of 8a protein (Wu et al., 2020). The SARS-CoV-2 spike glycoprotein is the association of bat SARS-CoV-1 and an unknown beta coronavirus entity, and is considered to have been undergone modification via homologous recombination (B. Li et al., 2020). Even the entry mechanism of SARS-CoV-2 inside the host cell is similar to SARS-CoV-1, i.e., using ACE2 receptor for attachment, as confirmed by a fluorescent study (Gralinski andMenachery, 2020). The SARS-CoV-2 is considered to exhibit a single N501T mutation, that might be responsible for its enhanced affinity towards ACE2 receptors (Wan et al., 2020). Therefore, this data accounts for the fact that ACE-2 is an important receptor, which promotes viral entry into the host organism.
5. CD147 and SARS-CoV-2
Belonging to the immunoglobulin superfamily, CD147 (a highly glycosylated transmembrane protein) functions as a significant upstream regulator of matrix metalloproteinases (MMPs), which may be elevate in its concentration level, in case of diabetic and asthmatic complications (Ulrich and Pillat, 2020). The concentration of MMPs and CD147 is mostly elevated during inflammation and tumours (Kong et al., 2014).
A study investigated the distribution of CD147 along with its transmembrane components and extracellular agonists in adaptive and innate immune cells in the periphery and the lungs, which facilitates their further study in the spread and infection by the virus (Radzikowska et al., 2020, Radzikowska et al., 2020). Also, CD147 is expressed in high amounts in the epithelial tissues. Furthermore, monocytes, T cells, epithelial cells, B cells, macrophages, innate lymphoid and natural killer cells can be infected in lungs, or can carry the virus from epithelial cells, infected with virus, via CD147, and contribute to the systemic and local spread of the virus, along with its extended immune responses (Peng et al., 2020).
CD147 is found be greatly expressed in the bronchial biopsy and human bronchial epithelial cells (HBECs) of chronic obstructive pulmonary disease (COPD) individuals, and blood of patients with obesity, which is one of the significant comorbidities associated with COVID-19 infection (Radzikowska et al., 2020). In addition to this, the extracellular ligands and transmembrane components of CD147 have been found to aid in other viral infections (Pushkarsky et al., 2001, Pushkarsky et al., 2001). Viruses combine cyclophilin A and B in their virions, followed by binding via CD147 or activation of CD147-mediated signalling (Chen et al., 2005; Pushkarsky et al., 2001, Pushkarsky et al., 2001; Watanabe et al., 2010, Watanabe et al., 2010). Therefore, all these findings suggest the possible involvement of CD147 and its associated ligands in the pathogenesis of COVID-19, primarily in patients with diseases. CD147 is present throughout the body, including the lungs, cell membrane of endothelial and epithelial cells and T cells (Jouneau et al., 2011; The Human Protein Atlas, 2020; Moheimani et al., 2018, Moheimani et al., 2018; Hu et al., 2010). Preferentially, CD147 can localise to the basal domain of polarised cells, on account of its role in acting as a chaperone of the carboxylate transporters, to basal surface of epithelial cells.
More hyaluronan is produced as a result of greater expression of CD147 on cancer stem cell surface, which lead to lipid raft stabilization and elevate the expression of ABC drug transporters in MMPs, promoting increased resistance and survival (Toole, 2019). Greater levels of MMP-9 have been reported to be present in the sputum of asthmatic patients (Mattos et al., 2002). Other names for CD147 are extracellular matrix metalloproteinase inducer (EMMPRIN) and Basigin (Ulrich and Pillat, 2020). CD147 is produced in multiple types of cells and is extensively distributed to varying tissues, like liver, brain, intestines, lungs, kidneys and spleen (Miyauchi et al., 1990). The various ligands and receptors to which CD147 binds are mono-carboxylate carrier 1 and 4 (MCT-1, MCT-4), S100A9, CD44, glycoprotein 4, CD98, E-selectin, CD43, apolipoprotein D (ApoD), annexin-2, nucleotide-binding oligomerization domain-containing protein 2 (NOD2), syndecan-1, caveolin-1, integrin α 3β1 and α 6β1 (Kirk et al., 2000; Yurchenko et al., 2001; Slomiany et al., 2009). The inflammation and disease severity is retarded when CD147 is targeted, by affecting the ligands in multiple sclerosis, asthmatic pulmonary inflammation and myocardial ischemia (Marchiq et al., 2015; Xiong et al., 2014, Xiong et al., 2014; Wang et al., 2011). Furthermore, the activity of matrix metalloproteinase (MMP) is also found to be elevated by CD147, which is promoted by angiotensin 2, bound to MAPKs (Bukrinsky, 2015; Li et al., 2015; Yu et al., 2019). In addition, CD147 contributes to inflammation, via production of pro-inflammatory cytokines, like interferon-gamma (INF-γ), interleukin-6 (IL-6), monocyte-chemoattractant protein (MCP-1) and tumour necrosis factor-α (TNF-α) (Zhai et al., 2016). Recently, certain investigations have reported the role of SARS-CoV-2 infection in elevating the cytokine expression, creating cytokine storm (hypercytokinemia), which intensifies the disease condition (Saghazadeh and Rezaei, 2020; K. Liu et al., 2020). This condition of hypercytokinemia is uncontrolled release of cytokines, which is observed in cases of infectious and non-infectious disorders, facilitating a hyper-inflammatory state in the host organism (Mahmudpour et al., 2020).
5.1. CD147 as a receptor for Plasmodium infection: forming a basis for SARS-CoV-2
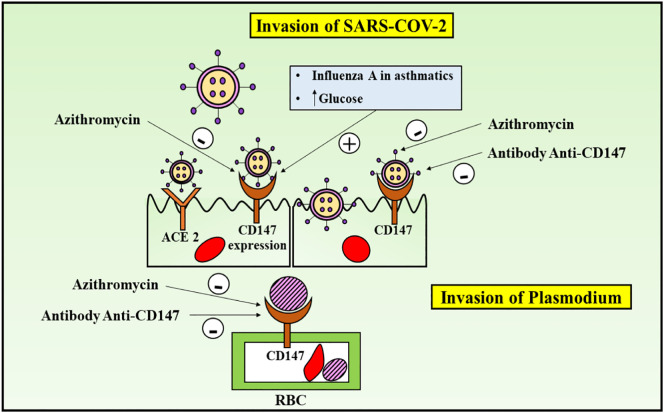
This is a red blood cell (RBC) receptor for Plasmodium falciparum, which is a malaria causing protozoan in humans (Crosnier et al., 2011), where the Plasmodium falciparum reticulocyte binding-like homologue 5 (PfRH5) complex of the membrane of the parasite binds CD147 to the RBC, followed by entry of all the tested strains in vitro (Crosnier et al., 2011) (Fig. 2 ). PfRH5-CD147 interaction inhibition hindered the RBC invasion and prevented the infection in humanized mouse model, as reported in investigations with recombinant chimeric antibody (Ab-1), directed against CD147 (Zenonos et al., 2015). Azithromycin also functioned as the blocker of this RBC invasion (Zenonos et al., 2015; Wilson et al., 2015; Muralidharan and Striepen, 2015), by hindering the significant initial step of formation of tight junctions, as evidently portrayed by invasion inhibition assays and experiments of live video microscopy (Wilson et al., 2015). However, azithromycin loose the property of inhibition of viral invasion, upon removing the two significant glycan groups (Wilson et al., 2015). Moreover, there are other possible mechanisms, such as the interference of azithromycin in ligand/receptor (PfRH5/CD147) interaction should be considered (Muralidharan and Striepen, 2015), thereby providing possible therapeutic action of azithromycin against other foreign invaders like, SARS-CoV-2 (Fig. 2). Upon investigating other macrolide antibiotics, many erythromycin and azithromycin derivatives were reported to exert invasion blocking actions (Wilson et al., 2015). A study investigated the possible reason behind fewer cases of COVID-19 in Sub-Saharan Africa (Kalungi et al., 2021). Besides the reasons such as early lockdown, population socio-demographics and possible under reporting provisions, the authors presented an interesting hypothesis of co-infection, associated with CD147. Since both the SARS-CoV-2 and Plasmodium falciparum merozoites interact with the CD147 immunoglobulin, the immunological memory, against the malaria-causing protozoan, primes the cells infected with SARS-CoV-2, for early phagocytosis, thereby providing protection to the individuals, who exhibited recent falciparum infection, against the COVID-19 infection (Kalungi et al., 2021). Therefore, this data provides a possibility of use of CD147-targeting anti-malarial drugs in the COVID-19 disease, taking in account the involvement of the immunoglobulin in infection.
Fig. 2.
CD147 functioning as a receptor for malaria-causing Plasmodium falciparum and COVID-19, presenting the possible role of Azithromycin in COVID-19, on account of its potential in ameliorating malaria, by interfering with CD147 receptor. Anti-CD147 antibody therapy hinders invasion of SARS-CoV-2 and plasmodium falciparum, however, the impact of azithromycin on SARS-CoV-2 is yet to be investigated further. The concentration of interferons and interferon-stimulated proteins is elevated and viral replication and release id retarded, via which the anti-viral responses are induced in the endothelial cells.
5.2. CD147 aids in the viral entry
Unlike ACE2, which exhibits its role as a receptor for both binding and permeation of the SARS-CoV-2 virus into the host cell (H. Zhang et al., 2020; Bourgonje et al., 2020), CD147 receptor might permit the virus to exhibit destruction in the host cell, by binding alone (Scheim, 2020, Scheim, 2020).
Along with ACE2, CD147 has also been identified as the binding site for the viral spike protein (Wang et al., 2020a). Angiotensin 2 and cyclophilins are reported to be significant proteins, which elevate the expression and function of CD147, in case of SARS-CoV-2 infection. Interestingly, these proteins are involved in the endothelial dysfunction, associated with heart problem, hypertension and diabetes (Zhang et al., 2017). Cyclophilin A and B bind to and activate CD147, thereby aiding in the process of interaction between viral S protein and CD147 (Radzikowska et al., 2020, Radzikowska et al., 2020). Therefore, during infection mediated by SARS-CoV-2, the CD147 functions by interaction by cyclophilin A (CyPA) (C. Liu et al., 2020). Even though, the binding affinity of the viral S protein to CD147 is 12 folds than that of ACE2 (Kini and Kundu, 2020), yet the CD147 surface density on host cells permits the formation of various bonds with necessary combined affinity (Kini and Kundu, 2020). The interaction between S protein and CD147 was confirmed by surface plasmon resonance, using Biacore analysis (Hui et al., 2020). The HEK293 expressing CD147 has been reported to attach to the SARS-CoV-2, with high affinity, thereby indicating the significance of CD147 in the host organism (Chen et al., 2005). On account of the relationship between the COVID-19 infection and metabolic disorders, as well as the interaction between CD147 and virus entry, CD147 forms a significant platform for endothelial cell infection and viraemia of SARS-CoV-2 in the host cell (Saltos and Saltos, 2020). Also, a direct interaction has been reported between RBD region of S1 subunit of SARS-CoV-2 and CD147, as per co-immunoprecipitation assay and surface plasmon resonance (Rodrigues-Diez et al., 2020).
Furthermore, a study reported the participation of CD147-cyclophilin A axis in pathogenesis of kidney injury in COVID-19 patients (Su et al., 2021).
IL-6 has been reported to facilitate CD147 expression, which is the key receptor for SARS-CoV-2 entry (Hu et al., 2016; Arendt et al., 2012; Dai et al., 2012). In the host, this might be a normal adaptive process, as its level, in combination with cyclophilins, aids in immune cell recruitment to inflammation sites, via chemokine-like activity (Yurchenko et al., 2006). CD147 may abet IL-6, with intravascular presence of plasminogen activator inhibitor-1, Von Willebrand factor and vitronectin, which creates the prothrombotic phenotype in multiple cases of SARS (Wu e al., 2006; Jin et al., 2017). However, a study indicated the absence of evidence, supporting the role of CD147 as a direct SARS-CoV-2 spine binding receptor (Shilts et al., 2021). Very weak interactions were reported between the recombinant forms of SARS-CoV-2 spike and CD147 on human cell surfaces, which was confirmed by using specialized assays. Therefore, this supported their finding that no direct interaction of viral spike protein was found with any of the two CD147 isoforms. Also, no change was observed in the susceptibility towards SARS-CoV-2 infection, when CD147 was removed from the surface of the lung epithelial cells. This puts the scientists in a major conundrum about the involvement of CD147 in COVID-19 infection (Shilts et al., 2021). However, still, it does not rule out the evidences obtained by other studies about the role of CD147 in COVID-19 disease, cited in the manuscript.
5.3. SARS-CoV-2 enters the host cell through CD147 mediated endocytosis
Endocytosis and membrane fusion are observed to be the two major entry pathways for viral infection (Harrison, 2015; Slonska et al., 2016). A sequential endocytosis of SARS-CoV-2 in Vero E6 cells is reported by an electron microscope, where CD147 is observed to invade the cells, via endocytosis, independent of clathrin (Maldonado-Baez et al., 2013; Eyster et al., 2009). Endocytosis is reported to be significantly regulated by Rab5, which is located at early endosome (Saitoh et al., 2017). The study detected co-localization of spike CD147 and Rab5 in BHK-21-CD147 cells and lung tissues, isolated from COVID-19 infected patients, which indicated that virions and CD147 exhibited endocytosis, thereby supporting the entry of SARS-CoV-2 into the host cell, via CD147-mediated endocytosis (Wang et al., 2020b). This, therefore, strengthens the involvement of CD147 in SARS-CoV-2 entry, and take the researchers a step further towards this entry route.
5.4. The “Catch and Clump” hypothesis
A model has been explained to portray the dual role of the receptors, facilitating viral entry and infection in the respiratory and circulatory systems, by distributing both CD147 and ACE2 on the respective tissues, where both these receptors are found in the endothelial lining of vasculature and lungs (Hamming et al., 2004; Radzikowska et al., 2020, Radzikowska et al., 2020; Bian et al., 2020; Yurchenko et al., 2006; Muramatsu, 2016). However, not ACE2, but only CD147 is found in the white and red blood cells (Radzikowska et al., 2020, Radzikowska et al., 2020; Aguiar et al., 2020). The expression of CD147 on the reticulocytes (1 to 2% of all red blood cells) is decreased as the cells mature (Manchanda, 2020; Sparrow et al., 2006; Malleret et al., 2013), however, their levels on the mature red blood cells (1695 CD147 receptors/cell) remains plenty (Odièvre et al., 2008). On account of CD147 receptor being an adhesion molecule, its extensive distribution on blood cells and its interaction with SARS-CoV-2 S protein, further investigation and research is required in the area (Odièvre et al., 2008; Telen, 2000; Yurchenko et al., 2006). The clump and catch hypothesis focus on the development of two forms of interactions, clumps and catches. Bothe the platelets and RBCs are extensively present in the capillary blood, and both express CD147 receptors (Von Ungern-Sternberg et al., 2018; Schmidt et al., 2008; Mock et al., 2011). These cells form virus linked clusters, where they are connected to each other via the virus particle (mutual virus interaction) to form clumps. A virus particle joined to the endothelial cell lining of the capillary or to another RBC, is referred to as catch (Scheim, 2020, Scheim, 2020).
These “clumps and catches” have the tendency to impede the blood flow. Even in the absence of the virus, RBCs can form aggregates, connected by plasma macromolecules, under low blood flow shear rates (Maeda et al., 1988). However, this type of aggregation can be reversed, by disconnecting the RBCs at higher shear rates (Maeda et al., 1988). On the other hand, in the presence of the viral infection, even moderate floe of the blood through the capillaries can form clumps and catches, possessing sufficient binding affinity to induce some drag on the flow in the capillaries affected (Scheim, 2020, Scheim, 2020). Retarding the flow would aid in aggregation, thereby producing a series of viral-mediated clumps and catches (Scheim, 2020, Scheim, 2020). Comprehensive investigation into the working of the catch and clump hypothesis can provide a detailed understanding of the infection mediated by COVID-19 from a different perspective.
5.5. Evidence for the role of CD147 in COVID-19 infection
The interaction between CD147 and SARS-CoV-2 S protein was investigated in a study, by using enzyme-linked immune-sorbent assay (ELISA) and surface plasmon resonance (SPR), which portrayed direct interaction between the viral S protein and CD147 (Wang et al., 2020a). As per the electron microscopy results, the virions in Vero E6 cells, infected with SARS-CoV-2. Further, the co-localization of CD147 and viral S proteins, were found in the Vero E6 cells and kidney and lung tissues, infected with SARS-CoV-2, which verified the interaction between viral S protein and CD147, further mediating the infection in the host cells. In order to confirm the role of CD147, as a receptor for the entry of SARS-CoV-2 in the host cell, the Vero E6-shCD147 and BEAS-2B-ssCD147 (CD147 knockdown cells) were infected with SARS-CoV-2 for 48 h, where the virus copy number was reported to be reduced in this group, as observed after quantitative polymerase chain reaction (PCR) analysis (Wang et al., 2020b). Opposing to this, the group over-expressing CD147 in BEAS-2B cells portrayed SARS-CoV-2 infection. A very significant observation was that the virus particles were reported to enter the non-susceptible BHK-21 cells, as a result of expression of human CD147. Therefore, CD147 introduction changes the virus tropism towards BHK-21 cells, where initially they could not be infected with SARS-CoV-2, as confirmed in the study. Also, the destructive impact of SARS-CoV-2 infection was reported to be blocked by anti-CD147 antibody, meplazumab in a dose dependent manner, which also retarded the virus copy number and replication, which showed the role of CD147, as a receptor for the entry of SARS-CoV-2 virus. Furthermore, the study also reported the infection of SARS-CoV-2 pseudo-virus in T cells, defiant of ACE2, where CD147 was overexpressed, thereby facilitating the viral infection (Wang et al., 2020b). Moreover, the CD147 expression was found to be increased in the inflammatory alveolar epithelial cells (Barth et al., 2006) and ACE2 level is decreased by viral infection (Kuba et al., 2005). Meanwhile the concentration of CD147 is greater than ACE2, in Vero E6 cells and ACE2 is not at detected in the BEAS-2B cells. An interesting observation of the immunofluorescent assay results depicted that there was no co-localization of ACE2 and CD147 in the lung tissues in patients infected with COVID-19, along with no interaction in detected cells (Wang et al., 2020b). Therefore, the expression of ACE2 and CD147 are significantly independent in the single lung cell, which shows that these two might be two complimentary receptors in promoting the viral infection. In order to study the infection ability of SARS-CoV-2, hCD147 mice were constructed, where the lungs of these mice, infected with virus, were found to be loaded with virus particles, which was not the case of infected wild-type mice, after 48 h of infection. The mice stained with eosin and haematoxylin were found to develop pneumonia, along with serofluid exudation, thickening of the alveolar septum, formation of hyaloid membrane, infiltration of inflammatory cells and pulmonary consolidation, without any histopathological alterations. However, the wild-type mice did not experience any histopathological alterations. These results indicated that the SARS-CoV-2-susceptible mouse model is successfully developed and established the significant involvement of CD147, as a receptor for the infection, mediated by the SARS-CoV-2. This functions as an alternative virus receptor for ACE2-defiant cells (Wang et al., 2020b). Despite the contradictory outcomes in studies, the previous data aims to provide a substantial evidence and platform to evaluate the role of CD147 in COVID-19 disease, which might unfold the mysteries of the COVID-19 disease.
5.6. Superiority of CD147 over ACE2 in COVID-19
CD147 can explain two things, which ACE2 receptor alone cannot, where the first one being risk of elevated levels of blood glucose in prognosis of COVID-19, and the second one being susceptibility of T-cells towards COVID-19. Individuals with type-2 diabetes, with poor blood glucose regulation, exhibited greater mortality rate, as compared to those individuals where the blood glucose level was well maintained (Yong, 2020). This was explained by the fact that the increase in the blood glucose level elevated the expression of CD147 receptor on the immune cells.
Furthermore, investigations have identified T-cell death, mediated by SARS-CoV-2, which has been reported to be caused by CD147 pathway, and not ACE2, because the expression levels of hACE2 is very low in T cells, unlike CD147 (Yong, 2020).
5.7. CD147 in other comorbidities
CD147 is a type 1 integral membrane receptor, which interacts with many ligands, like integrins, cyclophilins and Plasmodium falciparum reticulocyte binding-like homologue 5 (PfRh5) (Xiong et al., 2014, Xiong et al., 2014). CD147 contributes to development of tumour and invasion of plasmodium ad virus (Tseng et al., 2010; Li, 2016; Schoeman and Fielding, 2019). It plays a fundamental role in infection of multiple viruses, like HBV, HIB, KSHV and HCV (C. Liu et al., 2020). The CD147 expression are also reported to be increased by infection with influenza A virus, in cells of asthmatic patients (Moheimani et al., 2018, Moheimani et al., 2018). Additionally, CD147 expression is also reported to be induced by elevated concentration of glucose in monocytes (Bao et al., 2010). Expression of MMP-9 and MMP-1 is elevated by increased in vitro expression of glucose, resulting in retarded expression of CD147, by small interfering RNA (siRNA) and anti-CD147 antibody (Ulrich and Pillat, 2020).
Interestingly, SARS-CoV-2 has been hypothesized to exert a potential impact on the reproduction, via CD147, on account of the expression of this receptor in the uterus and its significant role in female and male reproduction, embryo implantation and normal fertility (Mahdian et al., 2020). Thus, it is recommended for the couple to not attempt conception, if infected with COVID-19, until they have been treated (Mahdian et al., 2020).
Moreover, unlike ACE2, CD147 is also present in retinal ganglionic cells (Hamashima et al., 2020), where it mediates disruption of neurovascular barrier, promoted in vitro by proinflammatory cytokines and damages the function of blood-retinal barrier in mice with diabetes, induced by streptozocin (Arima et al., 2016). Therefore, CD147 possible promotes the retinal invasion of SARS-CoV-2 in diabetic patients, by breaking down the blood retinal barrier. However, future investigations are required to confirm this action of CD147 (Raony and De Figueiredo, 2020). Therefore, CD147 inhibition might play a beneficial role in the treatment of diabetic complications, which involve inflammation (Bao et al., 2010), along with severe acute respiratory syndrome, triggered by SARS-CoV-2. However, there is a need for more investigations, to study the relationship between diabetes mellitus and CD147, in clinical complications, mediated by infection by SARS-CoV-2.
-
A)
CD147 as a driver for SARS-CoV-2 in disruption of human cardiac pericytes
Studies have confirmed that the cardiac pericytes are not infected by SARS-CoV-2 infection. Even though the cardiac pericytes express the ACE2 miRNA transcript, as confirmed by single cell sequencing investigations, yet there is an absence of viral infection in the cardiac pericytes, due to significantly lower amount of TMPRSS2 and ACE2 in these cells (Avolio et al., 2020). Therefore, this depicted that in vitro lower concentration of ACE2 in myocardial stromal cells lead to lower risk to viral infection (Amendola et al., 2021), whereas, the mesenchymal stromal cells were not infected due to absence of TMPRSS2 and ACE2 (Avanzini et al., 2021). However, studies confirmed the presence of an alternative receptor, CD147 (basilin), an oligomannosidic glycan-associated plasma membrane protein, as a novel receptor of SARS-CoV-2 entry (Avolio et al., 2020). Not ACE2, but CD147 has been recognized to lead S signalling in cardiac pericytes (Avolio et al., 2020). The inhibition of CD147, by gene silencing or a neutralising antibody, hindered the S protein from carrying out the phosphorylation of ERK1/2 and protected other multiple functional characteristics of cardiac pericytes from destruction by the S protein, like generation of pro-inflammatory mediators contributing to cytokine storm, elevated migration, production of cell-death mediating pro-apoptotic factors and retarded ability to support the formation of endothelial cell network on Matrigel (Avolio et al., 2020). Furthermore, inhibition of CD147 provide protection to CAECs from paracrine apoptotic impact of S protein-pried cardiac pericytes (Avolio et al., 2020).
-
B)
Expression levels of CD147, TMPRSS2 and ACE2
A study investigated the expression levels of CD147 and ACE2 receptors, as well as serine protease TMPRSS2 in mouse brain and human cell lines, using western blot and qRT-PCR. As per the results their concentration levels in different tissues varied from that of lungs (Qiao et al., 2020). The mouse brain cell lines and tissues expressed lower ACE2 and greater CD147 levels, unlike the lung tissue and cell line, whereas TMPRSS2 exhibited a consistent expression both in brain cell lines and mouse lung tissues (Qiao et al., 2020). The central nervous system (CNS) is more like to be infected with the COVID-19 disease, via CD147 receptors and TMPRSS2 protease, and not ACE2 receptors (Qiao et al., 2020). Owing to the availability of both CD147 and TMPRSS2, neuron and microglia exhibited the possibility of SARS-CoV-2 infection, however, astrocyte is less likely to be infected (Qiao et al., 2020). Greater expression of TMPRSS2 and CD147 were found in cortex, cerebellum and hypophysis of mouse brain. Also, it has been revealed that S protein of SARS-CoV-2 has lower affinity towards CD147, as compared to ACE2, which explains the reason behind lower viral infection in the brain (Qiao et al., 2020).
-
C)
CD147 increases with age in endothelial cells
As per the recent investigations, CD147 has been identified as the receptor for SARS-CoV-2 infection in the endothelial cells (Saltos and Saltos, 2020), and its positive relationship with age is only observed in the endothelial cells and skin (Li et al., 2011) However, the reason behind no age-related increase in CD147 levels is not yet clear. The studies reveal that CD147 might be involved behind greater mortality and infection risks of COVID-19 in patients greater than 40 years of age. These findings provide evidential support aiding in the development of CD147 as a target for COVID-19 treatment (Ahmetaj-Shala et al., 2020). The expression data of CD147 is considered to be as an aiding component in COVID-19 treatment, however requiring further investigation (Ahmetaj-Shala et al., 2020). Furthermore, CD147 is elevated in other disorders, including the ones which are associated with the COVID-19 infection, such as diabetes, pulmonary hypertension, obesity and renal disease (Ahmetaj-Shala et al., 2020). Also, CD147 induces extracellular matrix metalloproteinases (MMPs), which may be significant while considering the role vasculature, primarily endothelial dysfunction, in promoting pulmonary fibrosis, one of the most detrimental COVID-19 complications (Ahmetaj-Shala et al., 2020).
A detailed understanding of the interaction between CD147 and SARS-CoV-2 will provide a comprehensive insight and identify suitable therapeutic targets for COVID-19 treatment (Ahmetaj-Shala et al., 2020).
6. CD147 as a target: accelerating the therapeutic regimen
Owing to the role of CD147, it has been identified as a suitable target in the treatment of inflammatory disorders (Zhu et al., 2014). A study explored the effect of small molecules in CD147 blockage, by using a CD147-derived pharmacophore model, where the results indicated the ability of the molecule to specifically damage dimerization of CD147 and block the movement and entry of hepatocellular carcinoma cells (Fu et al., 2016). Thus, small molecule-mediated CD147 inhibition plays a significant role in the treatment of viral infections and cancer patients, including the COVID-19 infection (Mahdian et al., 2021). However, till now, no small molecule inhibitors have been created as FDA-approved candidates. The COVID-19 patients, under mechanical ventilation, experience physico-pathological states, like myocardial injury and refractory hypoxemia, for the treatment of acute respiratory distress syndrome (ARDS), caused by oxidative stress-induced damage to the erythrocytes, mediated by SARS-CoV-2 (Ramos et al., 2021). The SARS-CoV-2 targets the erythrocytes, via binding onto CD147 receptors (Ramos et al., 2021). Furthermore, changes in the CD147-cyclophilin A signalling process and impairment of calcium homeostasis have promoted inflammatory injury and thrombosis in heart failure and lungs (Loh, 2020). Thus, CD147 can be used as a suitable therapeutic target to retard inflammation and progression of the disease (Ramos et al., 2021).
The free binding energy of estradiol benzoate and CD147 is the most favourable, as indicated by the outcomes of MD simulation (Mauvais-Jarvis et al., 2020). Estradiol benzoate is the estradiol (naturally occurring hormone) prodrug, which functions as a major sex hormone in women (Scheim, 2020, Scheim, 2020). Studies have indicated that the COVID-19 incidence is more in men as compared to women, the reason considered to be elevated estradiol levels in women (Molina et al., 2020; Magagnoli et al., 2020). The animal studies have suggested blockage of inflammatory events by estrogen, which has resulted in improvement in COVID-19 patients (Rosenberg et al., 2020). Furthermore, irinotecan and olaparib also possess a suitable free binding energy with CD147 (Mauvais-Jarvis et al., 2020). Despite the optimised results, parenteral administration, non-availability in market as well as improper knowledge and data limits its use as therapeutic candidate for COVID-19 treatment.
A tetracycline analogue, doxycycline, ameliorates the levels of CD147 in gallbladder cell line (Wang et al., 2017; Emingil et al., 2008). Moreover, azithromycin can be considered as a possible candidate for mitigating the effects of SARS-C0V-2, owing to its role in interfering with CD147 receptor, and preventing the invasion of malaria-causing Plasmodium falciparum (Ulrich and Pillat, 2020).
6.1. A humanized anti-CD147 antibody: Meplazumab
A novel investigation examined the effectiveness and safety portfolio of Meplazumab, which is a humanized anti-CD147 antibody, where patients with SARS-CoV-2 pneumonia were examined (Mahdian et al., 2021). This candidate effectively improved the condition and recovery rate of the patients infected with COVID-19 pneumonia, simultaneously maintaining the safety profile (Ulrich and Pillat, 2020). The role of Meplazumab for treating COVID-19 pneumonia, is under phase 2 clinical trial in China, which aims to hinder viral infection by preventing the binding of its spike proteins to CD147, which is blocked by monoclonal antibody (https://clinicaltrials.gov/ct2/show/NCT04275245). The virologic clearance rate monitors this trial, via real-time PCR of samples of respiratory tract, to investigate the impact of Meplazumb (Ulrich and Pillat, 2020). Also, Meplazumad was administered to 17 hospitalized patients with COVID-19, where significant improvement in the condition of the patients was observed in the treated group (Scheim, 2020, Scheim, 2020).
6.2. Beta adrenergic blockers
Beta-adrenergic blockers have also reported to exert beneficial impact on COVID-19 infection, where these drug candidates retard the viral entry of the host cell (Vasanthakumar, 2020). They negatively regulate the juxtaglomerular cells in the kidney, which ameliorate the activity of RAAS pathway, thus it might retard the expression levels of ACE2, contributing to the reduction in the viral cellular entry (Vasanthakumar, 2020). It has been reported that beta-adrenergic blocker curbs the CD147 expression (Vasanthakumar, 2020). Thus, these drugs can inhibit the cellular entry of SARS-CoV-2, by ameliorating the levels of entry receptors, both ACE2 and CD147.
6.3. Ivermectin: promoting non-availability of CD147
The RBC located transmembrane receptor, CD147, functions as a significant binding site for the viral S protein (Zaidi and Dehgani-Mobaraki, 2021). Besides internalising into the RBC, such interactions can form clumps and catches, as described earlier in the manuscript (Scheim, 2020, Scheim, 2020). Ivermectin binds to the viral S protein, thereby decreasing its availability to CD147. This might be effective in advanced cases of patients infected with COVID-19 (Zaidi and Dehgani-Mobaraki, 2021). Interestingly, in the parasitic diseases, ivermectin exerts of cytotoxic effect and kills the microfilaria, thereby generating an irreversible response (Scheim, 2020, Scheim, 2020). However, in COVID-19, ivermectin does not have a cytotoxic action, but exerts a cytostatic one, where SARS-CoV-2 might resume it's replication after the plasma level of the drug drops below the threshold, potentiating the risk of infection (Scheim, 2020, Scheim, 2020).
6.4. The family of statins: downregulating CD147
Another suitable candidate is the family of statins, which regulate the level of CD147, and induce changes in the structure, function and expression of these receptors, by blocking N-glycosylation and isoprenylation of the proteins (Sasidhar et al., 2017). The translocation of CD147 to the cell surface was unpaired in cultured THP-1 monocytes, after pre-treatment with pravastatin, fluvastatin and ayorvastatin, decreasing the activity of MMP and blocking differentiation of THP-1 to macrophages, post-treatment with phorbol-12-myristate-13 acetate (Sasidhar et al., 2017). The CD147 levels were retarded by ayorvastatin, along with attenuation of plaque susceptibility in mice models with experimental atherosclerosis (Liang et al., 2017). Such investigations depict the role of statins in decreasing the level of CD147 in human cells, including lung tissues, resulting in impairment of the cuts ability to infect and disrupt the host cells, and used as a co-adjuvant treatment therapy for COVID-19 management.
6.5. Hindering the CD147-mediated signalling pathway by melatonin
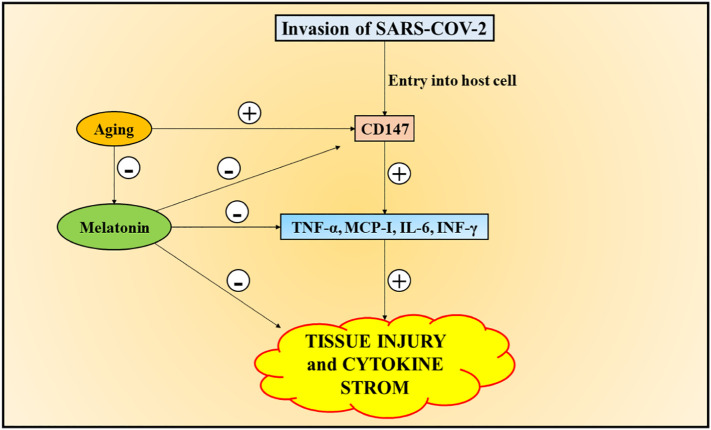
Generally, the immunity-inflammatory aspects regulate the extent of viral infections, which comprise of elevated oxidative stress conditions as well as harmful impact on different organs (Lu et al., 2019). Melatonin does not possess viricidal properties, but exhibits indirect antiviral actions, via potentiating immunity, antioxidant effects and anti-inflammatory actions (R. Zhang et al., 2020). Melatonin has been reported to suppress viral infections before (Lu et al., 2019; Bonilla et al., 2004), and prevent viral mediated stroke and death, along with alleviation of viral potency, by regulating the concentrations of IL-6 and interferon-gamma (INF-γ) during Venezuela equine encephalomyelitis (VEE) SARS-CoV-2 infection in mice (Negrette et al., 2001). The lung damage in models of respiratory syncytial virus has been found to be ameliorated by melatonin, via hindering oxidative stress and release of proinflammatory cytokines. Certain investigations have revealed the role of CD147-spike protein in mediating the entry of SARS-CoV-2 into the host cell (Wang et al., 2020b). Melatonin has been reported to inhibit CD147-mediated signalling pathway and exert a protective effect, by mediating antioxidant actions in models of cardiac hypertrophy, induced by angiotensin 2 (Su et al., 2016). The melatonin levels are retarded, due to induction of processes, which hinders the formation of free radicals, during conditions of viral infections, toxicity and stress, in old age and deteriorated immune system states (Lee et al., 2018). This, in turn, explains the greater risk of respiratory failure and poor detection in the middle-aged patients and geriatrics. Despite non-availability of investigations portraying the role of melatonin in treating COVID-19, promising and reliable results have been observed as a result of its administration, via suppression of circulating cytokines in multiple disorders and conditions of enhanced inflammation states (Zhou et al., 2020; Shneider et al., 2020; R. Zhang et al., 2020). Furthermore, the clinical and experimental investigations have reported the role of melatonin in retarding serum TNF-α, MCP-1, MMP, C-reactive protein (CRP), IL-6 and INF-γ levels (Savtekin et al., 2018; de Farias et al., 2019), which exhibit a significant involvement in inflammation, mediated by CD147. In addition, melatonin has also been reported to exert anti-diabetic effect, as its role in induction of production of insulin growth factor and phosphorylation of insulin receptor tyrosine has been reported in various experimental studies. Melatonin has been observed to restore the insulin resistance and glucose intolerance, caused as a result of disrupted internal circadian system (Sharma et al., 2015; Karamitri and Jockers, 2019; Song et al., 2017). Also, cellular apoptosis has been retarded, as a result of administration of melatonin, along with stimulation of antioxidants in diabetes (Mok et al., 2019). Therefore, it can be considered that CD147 is involved in mediating the effects of melatonin in diabetic patients infected with SARS-CoV-2. The safety of administration of melatonin should be considered significantly, for its use in COVID-19 treatment. The short-term administration of melatonin has been found to be safe, even at high doses (Kucukakin et al., 2008; Sanchez-Lopez et al., 2018). In addition, in COVID-19 treatment, melatonin can be used in combination with other drugs as well as elevate their potency and retard the occurrence of side effects (Adikwu et al., 2020). Furthermore, melatonin inhibits the CD147 signalling pathway and mitigate the cardiac damage (Su et al., 2016), thereby playing a crucial role in COVID-19 patients with diabetes (Sehirli et al., 2020, Sehirli et al., 2020). Fig. 3 portrays the role of melatonin in ameliorating SARS-CoV-2 infection.
Fig. 3.
Role of melatonin in minimizing the SARS-CoV-2 – mediated infection in host cell. Melatonin has been reported to inhibit CD147-mediated signalling pathway and retards serum cytokine (TNF-α, MCP-1, IL-6 and INF-γ) levels, mediated by CD147, which exhibit a significant involvement in inflammation, thereby retarding tissue injury and cytokine storm. [CD147 – cluster of differentiation 147; SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2; TNF-α – tumour necrosis factor; MCP-1 – monocyte chemoattractant protein 1; IL-6 – interleukin-6; INF-γ – interferon-gamma].
7. Conclusion
The manuscript provides a comprehensive data on the significance of CD147 receptor, as a novel route for the entry of SARS-CoV-2, besides the already established ACE2. The CD147 receptor has not only been found to be associated with the viral entry, but is associated with the related comorbidities, like asthma, diabetes, obesity etc. Irrespective of lesser affinity of CD147 to the viral S glycoprotein, as compared to ACE2, this receptor, provides a suitable explanation behind the elevated blood glucose levels in COVID-19 patients, greater susceptibility of T cells towards SARS-CoV-2 infection, lower infection risks in women, greater vulnerability of geriatrics towards SARS-CoV-2, occurrence of COVID-19 infection in non-susceptible human cardiac pericytes and so on. The manuscript details the basics of the COVID-19 disease and the established entry mechanisms and findings of SARS-CoV-2, mediated by ACE2, followed by the role of CD147 in the body and its already known importance in functioning as a receptor for malaria-causing Plasmodium falciparum protozoan, explaining the possible role of Azithromycin in COVID-19. The entry of SARS-CoV-2 via endocytosis, mediated by CD147 has also been explained, along with the catch and clump hypothesis of the SARS-CoV-2 action on the RBCs and the blood capillaries. The role of CD147 in other comorbidities provides a clear picture of its role in the body, followed by its expression levels and distribution throughout.
All this data provides a platform for the identification of CD147 as a target for the action of suitable therapeutic candidates, including, a humanized anti-CD147 antibody, Meplazumab; beta adrenergic blockers; ivermectin, which promotes non-availability of CD147 towards SARS-CoV-2; CD147-downregulating statins; Melatonin, hindering the CD147-mediated signalling pathway; doxycycline; Azithromycin etc., thereby providing a critical opportunity for the global researchers and virologists to observe the viral entry process from a different perspective, simultaneously obtaining answers to many other questions. A study, however, reveals the lack of evidence supporting the role of CD147 receptor in SARS-CoV-2 entry and identifies weak spike-receptor interactions, yet does not rule out the possibility of the possible significant involvement of the receptor as an alternative viral entry mechanism. There is a stringent need of development of more investigations and studies, to evaluate clearly the spike-receptor relationship, which, the authors believe, would definitely provide reliable evidence in favour of the relationship, to confirm the potential role of CD147 in COVID-19 infection and associated comorbidities. The manuscript aims to provide a comprehensive approach towards the involvement of CD147 in COVID-19 disease, aiming to combat with the prevailing infection and put an end to it. Despite the already established entry mechanism, via ACE-2, the CD147 mediated entry provides a valid explanation regarding the prevalence of infection in individuals with other comorbidities, and identifies the link between them. The ultimate goal of finding a cure cannot be achieved without in-depth analysis of the problem and identification of suitable connections, that might create a reliable pathway, leading towards an appropriate therapeutic regimen. These findings would possibly aid the researchers in the development of a suitable therapy, however, still many investigations are required to properly establish the role of this receptor in COVID-19 treatment. Still the proper events encircling the entry of the virus are unclear, along with the long-term impact of the infection, which should be investigated in the future studies.
CRediT authorship contribution statement
Conceptualisation and wrote the initial Draft: TB, IK and SBU.
Literature Review: TB, LA and SS.
Figure Work: AS, NS and SB.
Proof Read: AAH and SBU.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Damia Barcelo
References
- Adikwu E., Ebinyo N.C., Bokolo B. Melatonin and alpha lipoic acid attenuate methotrexate/cisplatin-induced kidney toxicity in albino rats. J. Nephropharmacol. 2020;9 doi: 10.15171/npj.2020.17. [DOI] [Google Scholar]
- Adnan Shereen M., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar J.A., Tremblay B.J.-M., Mansfield M.J., et al. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. bioRxiv. 2020 doi: 10.1101/2020.04.07.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brier J.W. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmetaj-Shala B., Vaja R., Atanur S.S., et al. Cardiorenal tissues express SARS-CoV-2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC: basic to translational. Science. 2020;5(11):1111–1123. doi: 10.1016/j.jacbts.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola A., et al. Human cardiosphere-derived stromal cells exposed to SARS-CoV-2 evolve into hyper-inflammatory/pro-fibrotic phenotype and produce infective viral particles depending on the levels of ACE2 receptor expression. Cardiovasc. Res. 2021;117(6):1557–1566. doi: 10.1093/cvr/cvab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt B.K., et al. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: role in regulation of myeloma cell proliferation. Leukemia. 2012;26(10):2286–2296. doi: 10.1038/leu.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima M., Cui D., Kimura T., Sonoda K.H., Ishibashi T., Matsuda S., et al. Basigin can be a therapeutic target to restore the retinal vascular barrier function in the mouse model of diabetic retinopathy. Sci. Rep. 2016;6:38445. doi: 10.1038/srep38445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini M.A., et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021;10(4):636–642. doi: 10.1002/sctm.20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio E., Carrabba M., Milligan R., et al. The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147-receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. BioRxiv. 2020 doi: 10.1101/2020.12.21.423721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Min D., Twigg M., et al. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am. J. Physiol. Cell Physiol. 2010 doi: 10.1152/ajpcell.00228.2010. [DOI] [PubMed] [Google Scholar]
- Barth K., Blasche R., Kasper M. Lack of evidence for caveolin-1 and CD147 interaction before and after bleomycin-induced lung injury. Histochem. Cell Biol. 2006;5:563–573. doi: 10.1007/s00418-006-0192-3. [DOI] [PubMed] [Google Scholar]
- Bernard S.C., et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 2014;7:725–731. doi: 10.1038/nm.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H., Zheng Z.-H., Wei D., et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.21.20040691. [DOI] [Google Scholar]
- Bonilla E., Valero N., Chacin-Bonilla L., Medina-Leendertz S. Melatonin and viral infections. J. Pineal Res. 2004;36:73–79. doi: 10.1046/j.1600-079x.2003.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. Extracellular cyclophilins in health and disease. Biochim. Biophys. Acta. 2015;1850:2087–2095. doi: 10.1016/j.bbagen.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., et al. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J. Virol. 2007;81(2):446–456. doi: 10.1128/JVI.01705-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calma J. The COVID-19 pandemic is generating tons of medical waste. The Verge, Mar. 26:2020. 2020. https://www.theverge.com/2020/3/26/21194647/the-covid-19-pandemic-is-generating-tons-of-medical-waste
- Cantuti-Castelvetri L., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;6518:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Novel Coronavirus (2019-nCoV) Healthcare Infection Prevention and Control FAQs for COVID-19. Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-prevention-controlfaq.html Published February 11, 2020.
- Chan C.M., et al. Carcinoembryonic antigen-related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of middle east respiratory syndrome coronavirus. J. Virol. 2016;90:9114–9127. doi: 10.1128/JVI.01133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;5:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Peng C., Lei L., et al. Nuclear envelope-distributed CD147 interacts with and inhibits the transcriptional function of RING1 and promotes melanoma cell motility. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0183689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L.M., Ng I.H.Y., et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;679:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J. Biol. Chem. 2018;293:11709–11726. doi: 10.1074/jbc.RA118.001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Current Challenges and Future Perspectives . 2021. Singapore: Bentham Science Publishers. [Google Scholar]
- Crosnier C., Bustamante L.Y., Bartholdson S.J., et al. Basigin is a receptor essential for erythrocyte invasion by plasmodium falciparum. Nature. 2011;480(7378):534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., et al. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int. J. Cancer. 2012;131(4):834–843. doi: 10.1002/ijc.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;6518:861–865. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Farias T.S.M., da Paixao R.I., Cruz M.M., et al. Melatonin supplementation attenuates the pro-inflammatory adipokines expression in visceral fat from obese mice induced by a high-fat diet. Cells. 2019;8:1041. doi: 10.3390/cells8091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., et al. A novel angioten sin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Emingil G., Atilla G., Sorsa T., et al. The effect of adjunctive subantimicrobial dose doxycycline therapy on gcf emmprin levels in chronic periodontitis. J. Perinatol. 2008;2008 doi: 10.1902/jop.2008.070165. [DOI] [PubMed] [Google Scholar]
- Eyster C.A., et al. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;5:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Channappanavar R., Jankevicius G., et al. The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. MBio. 2016;7 doi: 10.1128/mBio.01721-16. Published February 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.G., Wang L., et al. A novel small-molecule compound targeting CD147 inhibits the motility and invasion of hepatocellular carcinoma cells. Oncotarget. 2016;7(8) doi: 10.18632/oncotarget.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnejad-Berenji M., Pashapour S. SARS-CoV-2 and the possible role of Raf/MEK/ERK pathway in viral survival: is this a potential therapeutic strategy for COVID 19? Pharmacology. 2020:1–3. doi: 10.1159/000511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species severe acute respiratory syndrome-related coronavirus: classi fying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamashima K., Gautam P., Lau K.A., Khiong C.W., Blenkinsop T.A., Li H., Loh Y.H. Potential modes of COVID-19 transmission from human eye revealed by single-cell atlas. BioRxiv. 2020 doi: 10.1101/2020.05.09.085613. [Cited 2020 May 29] [DOI] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Harrison S.C. Viral membrane fusion. Virology. 2015;479–480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;2:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Dang N., Yao H., et al. Involvement of HAb18G/CD147 in T cell activation and immunological synapse formation. J. Cell. Mol. Med. 2010;14:2132–2143. doi: 10.1111/j.1582-4934.2010.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., et al. Interleukin-6 drives multiple myeloma progression by up-regulating of CD147/emmprin expression. Blood. 2016;128(22):5632. [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Madani T.A., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit R.J., De Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R., et al. Inhibition of CD147 (cluster of differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. Stroke. 2017;48(12):3356–3365. doi: 10.1161/STROKEAHA.117.018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouneau S., Khorasani N., Souza P.D., et al. EMMPRIN (CD147) regulation of MMP-9 in bronchial epithelial cells in COPD. Respirology. 2011;16:705–712. doi: 10.1111/j.1440-1843.2011.01960.x. [DOI] [PubMed] [Google Scholar]
- Kalungi A., Kinyanda E., Akena D.H., et al. Less severe cases of COVID-19 in sub-saharan Africa: could co-infection or a recent history of plasmodium falciparum infection be protective? Front. Immunol. 2021 doi: 10.3389/fimmu.2021.565625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitri A., Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019;15(105–125) doi: 10.1038/s41574-018-0130-1. [DOI] [PubMed] [Google Scholar]
- Kini R.M., Kundu S. 2020. Infection-GENOMICS of COVID-19: Are Some Communities Resistant? Preprints.org 2020040310. [DOI] [Google Scholar]
- Kirk P., Wilson M.C., Heddle C., et al. CD147 is tightly asso ciated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.M., Liao C.G., Zhang Y., et al. A regulatory loop involving miR-22, Sp1, and c-myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-3555. [DOI] [PubMed] [Google Scholar]
- Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;8:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Tril ogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukakin B., Lykkesfeldt J., Nielsen H.J., et al. Utility of melatonin to treat surgical stress after major vascular surgery—a safety study. J. Pineal Res. 2008;44:426–431. doi: 10.1111/j.1600-079X.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- Lan J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;7807:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lee F.-Y., Sun C.-K., Sung P.-H., et al. Daily melatonin protects the endothelial lineage and functional integrity against the aging process, oxidative stress, and toxic environment and restores blood fow in critical limb ischemia area in mice. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12489. [DOI] [PubMed] [Google Scholar]
- Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;200236 doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xie H., Yi M., et al. Expression of cyclophilin a and CD147 during skin aging. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:203–211. doi: 10.3969/j.issn.1672-7347.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Li J., Huang Q., Long X., et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/ mTOR/SREBP1c and P38/PPARalpha pathways. J. Hepatol. 2015;63:1378–1389. doi: 10.1016/j.jhep.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Li B., Si H.R., Zhu Y., Yang X.L., Anderson D.E., Shi Z.L., et al. Discovery of bat coronaviruses through surveillance and probe capture-based next-generation sequencing. mSphere. 2020;5(1) doi: 10.1128/mSphere.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Yang L.X., Guo R., Shi Y., Hou X., Yang Z., et al. Atorvastatin attenuates plaque vulnerability by downregulation of EMMPRIN expression via COX-2/PGE2 pathway. Exp. Ther. Med. 2017;13:835–844. doi: 10.3892/etm.2017.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., et al. Spike protein of SARS-CoV stimulates cyclooxygenase-2 expression via both calcium-dependent and calcium-independent protein kinase C pathways. FASEB J. 2007;21(7):1586–1596. doi: 10.1096/fj.06-6589com. [DOI] [PubMed] [Google Scholar]
- Liu C., Brunn A.V., Zhu D. Cyclophilin a and CD147: novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 2020;7 doi: 10.1016/j.medidd.2020.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;9:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh D. The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 2020;3:380–416. [Google Scholar]
- Lu M., et al. Basolateral CD147 induces hepatocyte polarity loss by E-cadherin ubiquitination and degradation in hepatocellular carcinoma progress. Hepatology. 2018;1:317–332. doi: 10.1002/hep.29798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Liu Z., Shao Y., et al. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide dependent pathway. Virol. J. 2019;16:141. doi: 10.1186/s12985-019-1228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Seike M., Kon K., et al. Erythrocyte aggregation as a determinant of blood flow: effect of pH, temperature and osmotic pressure. Adv. Exp. Med. Biol. 1988;222:563–570. doi: 10.1007/978-1-4615-9510-6_68. [DOI] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F., et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdian S., Shahhoseini M., Moini A. COVID-19 mediated by basigin can affect male and female fertility. IntJ Fertil Steril. 2020;14(3):262–263. doi: 10.22074/ijfs.2020.134702. Published online 2020 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdian S., Zarrabi M., Panahi Y., Dabbagh S. Repurposing FDA-approved drugs to fight COVID-19 using in silico methods: targeting SARS-CoV-2 RdRp enzyme and host cell receptors (ACE2, CD147) through virtual screening and molecular dynamic simulations. Informatics Med. Unlocked. 2021;23 doi: 10.1016/j.imu.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmudpour M., Roozbeh J., Keshavarz M., et al. COVID 19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Baez L., Cole N.B., Kramer H., Donaldson J.G. Microtubuledependent endosomal sorting of clathrin-independent cargo by Hook1. J. Cell Biol. 2013;2:233–247. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Malleret B., Xu F., Mohandas N., et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda N. In: Rodak's Hematology. Sixth edition. Keohane E.M., Otto C.N., Walenga J.M., editors. Saunders; St. Louis (MO): 2020. 16 - anemias: Red blood cell morphology and approach to diagnosis; pp. 251–263. [Google Scholar]
- Marchiq I., Albrengues J., Granja S., et al. Knock out of the BASIGIN/CD147 chaperone of lactate/H+ symporters dis proves its pro-tumour action via extracellular matrix metallo proteases (MMPs) induction. Oncotarget. 2015;6(24636–24648) doi: 10.18632/oncotarget.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos W., Lim S., Russell R., et al. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest. 2002 doi: 10.1378/chest.122.5.1543. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., et al. Estradiol, progesterone, immunomodulation and COVID-19 outcomes. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T., Kanekura T., Yamaoka A., et al. Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta-chain of major histocompatibility complex class II antigen. J. Biochem. 1990;107:316–323. doi: 10.1093/oxfordjournals.jbchem.a123045. [DOI] [PubMed] [Google Scholar]
- Mock D.M., Matthews N.I., Zhu S., et al. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51(1):148–157. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani F., Koops J., Williams T., et al. Influenza a virus infection dysregulates the expression of microRNA-22 and its targets; CD147 and HDAC4, in epithelium of asthmatics. Respir. Res. 2018;2018 doi: 10.1186/s12931-018-0851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani F., Koops J., Williams T., et al. Influenza a virus infection dysregulates the expression of microRNA-22 and its targets; CD147 and HDAC4, in epithelium of asthmatics. Respir. Res. 2018;19:145. doi: 10.1186/s12931-018-0851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok J.X., Ooi J.H., Ng K.Y., et al. A new prospective on the role of melatonin in diabetes and its complications. Horm. Mol. Biol. Clin. Investig. 2019 doi: 10.1515/hmbci-2019-0036. [DOI] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Six of the 11 study subjects had cancer or HIV, and all but one were on oxygen at the start of the trial. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan V., Striepen B. Teaching old drugs new tricks to stop malaria invasion in its tracks. BMC Biol. 2015;13:72. doi: 10.1186/s12915-015-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016;159(5):481–490. doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrette B., Bonilla E., Valero N., et al. Melatonin treatment enhances the efciency of mice immunization with venezuelan equine encephalomyelitis virus TC-83. Neurochem. Res. 2001;26:767–770. doi: 10.1023/a:1011645400123. [DOI] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odièvre M.-H., Bony V., Benkerrou M., et al. Modulation of erythroid adhesion receptor expression by hydroxyurea in children with sickle cell disease. Haematologica. 2008;93:502–510. doi: 10.3324/haematol.12070. [DOI] [PubMed] [Google Scholar]
- Pan L., Mu M., Ren H.G., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study, Published March 5. 2020. https://journals.lww.com/ajg/Documents/COVID_Digestive_Symptoms_AJG_Preproof.pdf Available at. [DOI] [PMC free article] [PubMed]
- Peng L., Liu J., Xu W., et al. SARS-CoV-2 can be detected in urine, blood, anal swabs and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarsky T., et al. CD147 facilitates HIV-1 infection by interacting with virus associated cyclophilin a. Proc. Natl. Acad. Sci. U. S. A. 2001;11:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarsky T., Zybarth G., Dubrovsky L., et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin a. Proc. Natl. Acad. Sci. U. S. A. 2001;98(11):6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., et al. 2020. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U., Ding M., Tan G., et al. Distribution of ACE2, CD147, cyclophilins, CD26 and other SARS-CoV-2 associated molecules in human tissues and immune cells in health and disease. bioRxiv. 2020 doi: 10.1101/2020.05.14.090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Eur. J. Allergy Clin. Immunol. 2020 doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Bodrud-Doza M., Griffiths M.D., Mamun M.A. 2020. Biomedical waste amid COVID-19: perspectives from Bangladesh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E., López-Muñoz F., Gil-Martín E., Egea J., et al. The coronavirus disease 2019 (COVID-19): key emphasis on melatonin safety and therapeutic efficacy. Antioxidants. 2021;10(7):1152. doi: 10.3390/antiox10071152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raony I., De Figueiredo C.S. De figueiredo CS (2020) retinal outcomes of COVID-19: possible role of CD147 and cytokine storm in infected patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2020;165 doi: 10.1016/j.diabres.2020.108280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Diez R.R., Tejera-Muñoz A., Marquez-Exposito L., et al. Statins: could an old friend help in the fight against COVID-19? Br. J. Pharmacol. 2020;177(21):P4873–P4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., Udo T., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Rume T., Ul Islam D. Environmental effects of COVID-19 pandemic and potential strategies of sustainability. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e04965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus—a perspective. Expert. Rev. Clin. Immunol. 2020;16:465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S., et al. Rab5-regulated endocytosis plays a crucial role in apical extrusion of transformed cells. Proc. Natl. Acad. Sci. U. S. A. 2017;12:E2327–E2336. doi: 10.1073/pnas.1602349114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltos F.A., Saltos A.A. Entry of SARS-CoV2 through the basal surface of alveolar endothelial cells—a proposed mechanism mediated by CD147 in COVID-19. 2020. https://www.preprints.org/manuscript/202005.0359/v1 Available at:
- Sanchez-Lopez A.L., Ortiz G.G., Pacheco-Moises F.P., et al. Efcacy of melatonin on serum pro-infammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch. Med. Res. 2018;49:391–398. doi: 10.1016/j.arcmed.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Sasidhar M.V., et al. Downregulation of monocytic differentiation via modulation of CD147 by 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0189701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtekin G., Serakinci N., Erzik C., et al. Effects of circadian rhythm hormones melatonin and 5-methoxytryptophol on COXs, Raf-1 and STAT3. IntJ Pharmacol. 2018;14(787–795) doi: 10.3923/ijp.2018.787.795. [DOI] [Google Scholar]
- Scheim D. SSRN; 2020. Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-threshold Doses Via Hypothesized Alleviation of CD147-mediated Vascular Occlusion. [Cited 2020 May 29] [DOI] [Google Scholar]
- Scheim D.E. Ivermectina for COVID 19 treatment clinical response at quasi-threshold doses via hypothesized alleviation of CD147 mediated vascular occlusive. Pharmacol. Rep. 2020;73:736–749. doi: 10.1007/s43440-020-00195-y. [DOI] [Google Scholar]
- Schmidt R., Bültmann A., Fischel S., et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 2008;102(3):302–309. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehirli A.O., Sayiner S., Serakinci N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster diferentiation 147 (CD147) Mol. Biol. Rep. 2020 doi: 10.1007/s11033-020-05830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehirli A.O., Sayiner S., Serakinci N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147) Mol. Biol. Rep. 2020;47:8229–8233. doi: 10.1007/s11033-020-05830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Singh H., Ahmad N., et al. The role of melatonin in diabetes: therapeutic implications. Arch. Endocrinol. Metab. 2015;59:391–399. doi: 10.1590/2359-3997000000098. [DOI] [PubMed] [Google Scholar]
- Shilts J., Crozier T.W.M., Greenwood E.J.D., et al. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci. Rep. 2021;11:413. doi: 10.1038/s41598-020-80464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- Singh N., Tang Y., Ogunseitan O.A. Environmentally sustainable management of used personal protective equipment. Environ. Sci. Technol. 2020;54:8500–8502. doi: 10.1021/acs.est.0c03022. [DOI] [PubMed] [Google Scholar]
- Slomiany M.G., Dai L., Tolliver L.B., et al. Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin. Cancer Res. 2009;15:7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonska A., Cymerys J., Banbura M.W. Mechanisms of endocytosis utilized by viruses during infection. Postepy Hig. Med. Dosw. (Online) 2016:572–580. doi: 10.5604/17322693.1203721. [DOI] [PubMed] [Google Scholar]
- Somani M., Srivastava A.N., Gummadivalli S.K., Sharma A. Indirect implications of COVID-19 towards sustainable environment: an investigation in Indian context. Biores. Technol. Rep. 2020;11 doi: 10.1016/j.biteb.2020.100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Whitcomb D.J., Kim B.C. The role of melatonin in the onset and progression of type 3 diabetes. Mol. Brain. 2017;10:35. doi: 10.1186/s13041-017-0315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow R.L., Healey G., Patton K.A., et al. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin a and the release of annexin V. Transfus. Apher. Sci. 2006;34(1):15–23. doi: 10.1016/j.transci.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Kumar A. Use of aspirin in reduction of mortality of COVID-19 patients: a meta-analysis. Int. J. Clin. Pract. 2021;00 doi: 10.1111/ijcp.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Li J., Chen T., et al. Melatonin attenuates angiotensin II-induced cardiomyocyte hypertrophy through the CyPA/CD147 signaling pathway. Mol. Cell Biochem. 2016;422:85–95. doi: 10.1007/s11010-016-2808-9. [DOI] [PubMed] [Google Scholar]
- Su H., Wan C., Wang Z.D., et al. Expression of CD147 and cyclophilin a in kidneys of patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2021;16(4):618–619. doi: 10.2215/CJN.09440620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M.J. Red blood cell surface adhesion molecules: their possible roles in normal human physiology and disease. Semin. Hematol. 2000;37(2):130–142. doi: 10.1016/s0037-1963(00)90038-6. [DOI] [PubMed] [Google Scholar]
- The Human Protein Atlas BSG protein expression- Summary [WWW Document] 2020. https://www.proteinatlas.org/ENSG00000172270-BSG
- Toole B.P. The CD147-hyaluronan axis in cancer. Anat. Rec. 2019 doi: 10.1002/ar.24147. [DOI] [PubMed] [Google Scholar]
- Tseng Y.T., Wang S.M., Huang K.J., et al. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J. Biol. Chem. 2010;285(17):12862–12872. doi: 10.1074/jbc.M109.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested efects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3(6) doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar N. Beta-adrenergic blockers as a potential treatment for COVID-19 patients. BioEssays. 2020 doi: 10.1002/bies.202000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ungern-Sternberg S.N.I., Zernecke A., Seizer P. Extracellular matrix metalloproteinase inducer EMMPRIN (CD147) in cardiovascular disease. Int. J. Mol. Sci. 2018;19(2):507. doi: 10.3390/ijms19020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-H., Dai J.-Y., Wang L., et al. Expression of CD147 (EMMPRIN) on neutrophils in rheumatoid arthritis enhances chemotaxis, matrix metalloproteinase production and invasive ness of synoviocytes. J. Cell Mol. Med. 2011;15:850–860. doi: 10.1111/j.1582-4934.2010.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu C., Liu X., et al. Effects of matrix metalloproteinase inhibitor doxycycline and CD147 antagonist peptide-9 on gallbladder carcinoma cell lines. Tumor Biol. 2017;2017 doi: 10.1177/1010428317718192. [DOI] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhou Y.-S., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.J., Chen R., Zhang H., Wang B., Zhu Y.M., Nan G., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., et al. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 2010;84(9):4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Yoneda M., Ikeda F., Terao-Muto Y., Sato H., Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 2010;84(9):4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance. WHO website. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected Updated March 13, 2020.
- Wilson D.W., Goodman C.D., Sleebs B.E., et al. Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 2015;13:52. doi: 10.1186/s12915-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel A.G., et al. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020;27(8):763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Edwards C.K., 3rd, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 2014;15:17411–17441. doi: 10.3390/ijms151017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Edwards C.K., Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 2014;15:17411–17441. doi: 10.3390/ijms151017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., et al. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020 doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S.J. Overlooked receptors in Covid-19: what ACE2 alone cannot explain. Microbial Instincts. https://medium.com/microbial-instincts/overlooked-receptors-could-explain-quirks-of-covid-19-that-ace2-alone-cannot-9470817f59d0. 2020 [Google Scholar]
- Yu Q., Yang D., Chen X., Chen Q. CD147 increases mucus secretion induced by cigarette smoke in COPD. BMC Pulm. Med. 2019;19:29. doi: 10.1186/s12890-019-0791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko V., O’Connor M., Dai W.W., et al. CD147 is a signaling receptor for cyclophilin B. Biochem. Biophys. Res. Commun. 2001;288:786–788. doi: 10.1006/bbrc.2001.5847. [DOI] [PubMed] [Google Scholar]
- Yurchenko V., Constant S., Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117(3):301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A.K., Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2: an evidence-based clinical review article. J. Antibiot. (Tokyo) 2021;1–13 doi: 10.1038/s41429-021-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Monserrate M.A., Ruanob M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenonos Z.A., et al. Basigin is a druggable target for host-oriented antimalarial interventions. J. Exp. Med. 2015;212:1145–1151. doi: 10.1084/jem.20150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Wu B., Li J., et al. CD147 promotes IKK/Ikap paB/NF-kappaB pathway to resist TNF-induced apoptosis in rheumatoid arthritis synovial fbroblasts. J. Mol. Med. (Berl) 2016;94:71–82. doi: 10.1007/s00109-015-1334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Fan Q., Xie H., et al. Elevated serum cyclophilin B levels are associated with the prevalence and severity of metabolic syndrome. Front. Endocrinol. 2017;8 doi: 10.3389/fendo.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.Y., et al. Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium falciparum. Blood. 2018;10:1111–1121. doi: 10.1182/blood-2017-08-802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensiv. Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang X., Ni L., et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., et al. HAb18G/CD147 promotes cell motility by regulating annexin IIactivated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;6:2012–2024. doi: 10.1002/hep.24592. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(1054–1062) doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Song Z., Zhang S., Nanda A., Li G. CD147: a novel modulator of inflammatory and immune disorders. Curr. Med. Chem. 2014;21(19) doi: 10.2174/0929867321666131227163352. [DOI] [PubMed] [Google Scholar]