Abstract
Background
Healthcare-acquired COVID-19 has been an additional burden on hospitals managing increasing numbers of patients with SARS-CoV-2. One acute hospital (W) among three in a Scottish healthboard experienced an unexpected surge of COVID-19 clusters.
Aim
To investigate possible causes of COVID-19 clusters at Hospital W.
Methods
Daily surveillance provided total numbers of patients and staff involved in clusters in three acute hospitals (H, M and W) and care homes across the healthboard. All clusters were investigated and documented, along with patient boarding, community infection rates and outdoor temperatures from October 2020 to March 2021. Selected SARS-CoV-2 strains were genotyped.
Findings
There were 19 COVID-19 clusters on 14 wards at Hospital W during the six-month study period, lasting from two to 42 days (average, five days; median, 14 days) and involving an average of nine patients (range 1–24) and seven staff (range 0–17). COVID-19 clusters in Hospitals H and M reflected community infection rates. An outbreak management team implemented a control package including daily surveillance; ward closures; universal masking; screening; restricting staff and patient movement; enhanced cleaning; and improved ventilation. Forty clusters occurred across all three hospitals before a January window-opening policy, after which there were three during the remainder of the study.
Conclusion
The winter surge of COVID-19 clusters was multi-factorial, but clearly exacerbated by moving trauma patients around the hospital. An extended infection prevention and control package including enhanced natural ventilation helped reduce COVID-19 clusters in acute hospitals.
Keywords: COVID-19, SARS-CoV-2, Outbreak, Healthcare, Patient boarding, Genotyping
Introduction
NHS Lanarkshire (NHSL) is a semi-rural health board situated between Edinburgh and Glasgow. Acute services, including Emergency and Critical Care, Medicine and Surgery, are delivered by all three hospitals, with centralization of vascular surgery and ophthalmic services at Hospital H (500 beds), infectious diseases, haematology and renal services at Hospital M (490 beds) and paediatric, maternity, and orthopaedic trauma at Hospital W (630 beds). NHSL experienced the third highest rate of COVID-19 infection in Scotland after Glasgow and Edinburgh during winter 2020–2021.
The prevalence of COVID-19 was noted to be higher at Hospital W than the other two hospitals in early December 2020. There was a sudden escalation of COVID-19 clusters involving patients and staff on wards that had been designated low to medium risk for COVID-19. These clusters caused substantial disruption to routine clinical services, with elective surgery curtailed and patients transferred elsewhere. Clusters in all three hospitals contributed towards COVID-19-related morbidity and mortality in NHS Lanarkshire.
This article provides a summary of policies implemented to manage COVID-19 at all three hospitals, with an additional focus on possible reasons for escalating clusters at Hospital W. The six-month study period began on 1st October 2020 and ended on 31st March 2021. We hope that this report might benefit other similar hospitals in the future.
Overview of outbreak
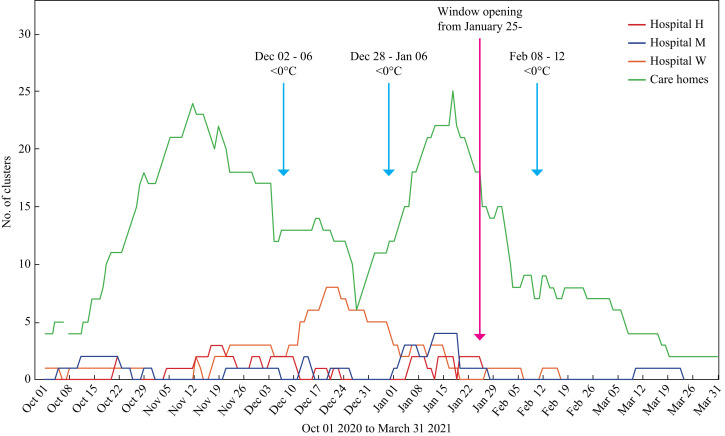
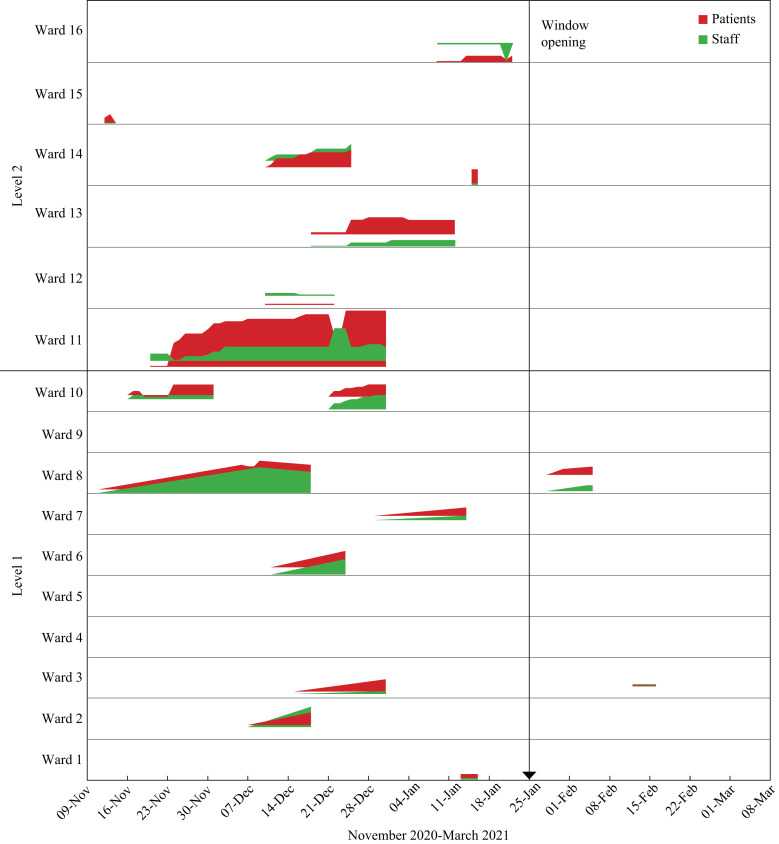
COVID-19 clusters on non-COVID-19 wards suddenly increased from three to six during the second week in December at Hospital W, with a further three wards closed before the end of December (Figure 1, Figure 2 ). Clusters at Hospitals M and H remained at low levels (0–2) throughout this month. A cluster was defined as two or more patients and/or staff with laboratory-confirmed PCR tests clearly linked in time and space (https://www.nss.nhs.scot/browse/antimicrobial-resistance-and-healthcare-associated-infection). National and local infection rates were decreasing at this time, along with community care home closures (Supplementary Figure S1). However, patient admissions remained high, with Scotland and NHSL experiencing a biphasic second pandemic wave of COVID-19, attributed to national COVID-19 policies.
Figure 1.
COVID-19 clusters in care homes and three acute hospitals in one Scottish health board.
Figure 2.
Ward-based COVID-19 clusters (staff and patients) at Hospital W from November 2020 to March 2021.
From 7th October 2020, all licensed premises in the central belt area closed, with the exception of takeaway services. Outdoor live events, indoor recreational facilities and adult contact sports were stopped and shops introduced 2-m physical distancing. Workplace masking was implemented from 15th October, including senior schools from 30th October. Travel restrictions were imposed on 7th November, with a 14-day quarantine for overseas arrivals. By 20th December, no travel between Scotland and England was permitted, although restrictions were lifted for Christmas Day. Complete lockdown was instituted across Scotland on 26th December.
The first sub-zero temperatures of winter occurred on 28th November, and again during early December (Figure 1). Both early January and early February 2021 also saw sustained low temperatures (minimum -8 °C).
Methods
An outbreak management team was convened to control the COVID-19 clusters. Probable hospital onset was defined as the first positive specimen on days 8–14 of admission, and definite hospital onset was defined as a first positive specimen date 15 or more days after hospital admission.
Wards identified a ‘contact tracer’ to improve communication with any positive or symptomatic staff member. This was organized by service managers with support from Infection Prevention & Control (IP&C). All staff testing positive for COVID-19 were requested to self-isolate for 14 days. Physical distancing was implemented in staff rooms and offices during handover times and in tea rooms. Posters and leaflets were used to improve compliance with patient masking.
Patient management
Patients with newly confirmed COVID-19 were isolated or cohorted in bays. Once transmission was suspected, the ward was closed to new admissions and asymptomatic patients were reviewed and screened. Complex operational management began on 17th December in order to minimize admissions: (1) all General Surgery cases were diverted to the other two hospitals; (2) all elective surgery was halted (inpatient, day case and endoscopy); (3) general practitioner (GP) referrals for Medicine were diverted to the other two hospitals; (4) the neighbouring health board accepted fracture neck of femur transfers; (5) other health boards supported cancer surgery; (6) reduced movement of patients was implemented; and (7) clinical staff remained in designated clinical areas.
Personal protective equipment
Staff wore surgical masks at all times, except when eating and drinking in staff rooms or canteen. Masking policies followed national guidance, which mandated universal masking along with guidance for changing wet or time-expired masks. If staff needed to perform aerosol-generating procedures for patients known, or suspected to have COVID-19, then they were fit tested and issued with FFP3 respirator masks. These were worn with face shield, gloves and aprons [1,2].
Visitors
A phased re-introduction of visiting commenced in July 2020 whereby in-patients could receive one named visitor at a scheduled slot. This stopped and reverted back to essential visitors only when the second wave became apparent. These restrictions were generally maintained in all three hospitals.
Cleaning
UK Four Nations Guidance advised cleaning of frequently touched points (door handles, etc.) a minimum of twice daily using chlorine-based products in April 2020. This was introduced for all three hospitals. Following the increase in clusters at Hospital W, the cleaning regimen increased to twice daily cleaning of affected wards with additional touch-point cleans. Once a cluster was declared over, this reverted to once daily cleaning with continued touch-point cleans.
Ventilation
NHSL hospitals are naturally ventilated, with mechanical systems in critical care, Infectious Diseases and theatres (10 AC/h). Following concern over hospital-acquired COVID-19, a ventilation review took place at Hospital W during November 2020. This was performed according to guidance set out in SHTM 01–03 Part A and B, Specialised Ventilation for Health Care Premises. The Institute of Occupational Medicine (IOM) measured the supply, extract, and pressures in assessed wards and associated rooms. Airflow measurements are taken at diffuser grilles within each ward/utility room using a balometer hood. Air change rates were calculated by multiplying the measured volumetric airflow rate by the measured volume of the room. Pressure differentials between rooms were taken using a micromanometer with a Pitot tube placed under the door to the neighbouring room.
An ‘Open The Window’ policy was launched on 25th January, requesting staff to open all windows in clinical areas for 15 min three times per day [3,4]. This policy emanated from amalgamated guidelines published by the European Centre of Disease Control (ECDC) (https://www.ecdc.europa.eu/sites/default/files/documents/Heating-ventilation-air-conditioning-systems-in-the-context-of-COVID-19-first-update.pdf). Windows in corridors and canteens were also opened in order to improve air change rates throughout the hospitals [5]. Window opening in winter, when the outside air is colder than the indoor air, encourages the flow of contaminated air towards the window due to thermal buoyancy effects [6]. This policy was widely adopted for general wards in the acute hospitals.
Screening
Patient admissions were screened using Roche LIATs and Biofire FilmArray platforms at each hospital and, if negative, repeated on day 5 from 27th November. Routine testing of asymptomatic patient-facing staff commenced on 1st December using the Biomerieux EasyMag or QIAgen QIAcubeHT for extraction, followed by Roche LC480 for amplification. This was fully implemented by 31st December. Point-of-care testing (POCT) began on 17th December for patients admitted through Accident & Emergency. Staff were screened by use of the Innova Lateral Flow SARS-CoV-2 Antigen rapid qualitative test from 21st December.
Immunization
The SARS-CoV-2 vaccination programme for care home patients and staff, followed by hospital front-line staff, began on 13th December. Two doses of the Pfizer vaccine were offered with an eight-week interval between doses.
Staff toilets
While the risk of SARS-CoV-2 transmission from toilets remains unknown, IP&C advised staff to wear masks during bathroom visits, replace the toilet lid (if present) before flushing, stagger toilet visits, and wash and dry hands using paper towels rather than automated driers [[7], [8], [9]].
Findings
Forty clusters were confirmed in all three hospitals between 1st October and 25th January, with three occurring after window opening until 31st March (Figure 1). Of these 40, 19 COVID-19 clusters involving 14 non-COVID-19 wards occurred at Hospital W, lasting from two to 42 days (average, 5 days; median, 14 days) (Figure 2). Each ward-based cluster involved an average of nine patients (range 1–24) and seven staff (range 0–17) (Supplementary Table S1). Six affected wards were designated Orthopaedic and/or Care of the Elderly. Hospitals H and M also experienced COVID-19 clusters but to a lesser extent; these reflected community infection rates (Supplementary Figure S1).
Following window opening, two further clusters occurred at Hospital W: the first was two days after window opening, lasted nine days and involved 13 patients and four staff, and the second occurred three weeks after window opening, lasted four days and involved six staff and seven patients. There was one new cluster at Hospital M six weeks after window opening; this involved six patients only and lasted 12 days. There were no new clusters at Hospital H after window opening.
Key factors
There were a number of key factors that may have encouraged the clusters. Firstly, there was a high community prevalence of COVID-19 which affected staff living locally. Staff testing was not introduced until 21st December and contact tracing was difficult to implement. Staff came to work with mild or no symptoms before discovering they were positive [10]. Because transmission risk increases during the early stages of infection when viral load is highest, staff-to-staff transmission may have occurred while working on the same ward and/or sharing bathroom and other facilities [11,12].
Secondly, there were high hospital occupancy rates, with increased patient boarding. The lack of single rooms severely restricted isolation of newly identified COVID-19 patients [13]. Infected patients were accommodated in shared rooms, which facilitated transmission to non-infected patients [14]. A minority of patients were reluctant to wear masks and challenging them was difficult. Unfortunately, this was not audited at the time.
Thirdly, most wards are naturally ventilated and it is unlikely that windows remained open once outside temperatures plummeted [15]. This would have exacerbated inadequate air change in clinical areas, thus impeding removal of any infectious aerosols [[16], [17], [18]].
Fourthly, surgical masks might have been insufficient for protecting patients and staff [[19], [20], [21]]. Despite screening, it was not possible to identify pre-symptomatic or asymptomatic individuals. These persons would have unwittingly transmitted the virus when donning/doffing masks, through poorly fitting masks, and after removing masks for eating or drinking.
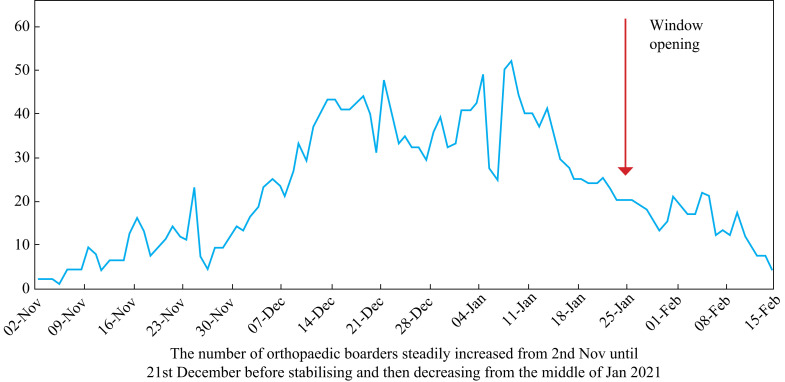
Finally, centralizing trauma cases at Hospital W led to an abundance of immobile elderly patients needing close personal care. Many were boarded on other wards, which led to increased throughput of medical, nursing and other staff (Figure 3 ). The upward trend in boarding mirrors the increase in hospital clusters at Hospital W during December. The average number of patients boarding per day in November was eight (maximum 23); 33 in December (maximum 48); and 30 in January (maximum 52). This may have encouraged the spread of infection due to increased movement between wards.
Figure 3.
Number of orthopaedic patients boarding out with orthopaedic wards.
Ventilation review
The ventilation review revealed generally inadequate air changes in most rooms tested. Aside from fully occupied wards, restricted staff areas and toilets were shared by many people with limited fresh air ventilation. Most rooms relied on natural ventilation rather than mechanical supply and extract. National regulations specify 6 AC/h for general ward areas and 10 AC/h for toilets and treatment rooms [22]. Only one toilet achieved the recommended air change rate; three other toilets achieved 3, 8 and 9 AC/h; four patient rooms achieved <6 AC/h and a treatment room had 1 AC/h.
Shielding patients
An audit revealed shielding patients in bays with non-shielding patients at Hospital W during summer 2020. Staff utilized single rooms for shielded patients in a similar way to Hospitals H and M, but were not using bays to shield patients alone nor were they cohorting shielded patients. While this audit was carried out before the study period, there were no policy changes instigated on acute sites and it is possible that these practices were still relevant during the winter.
Mortality rates
Table I shows the number of patients that died from SARS-CoV-2, whether contracted prior to, or during, hospital admission. It also shows the mortality rates for both total and hospital-acquired COVID-19 according to admissions. NHSL reported a total of 685 deaths during the study period, with the highest number (177: 26%) occurring in January. Hospital W experienced the greatest number of deaths overall (266: 39%) but the highest mortality rate (2.81 deaths/admissions) occurred at Hospital M in January 2021. Hospital-acquired COVID-19 rates varied from 0 to 0.897 hospital-acquired deaths/admissions over the study period, with the highest rate at Hospital H in November. Hospital W had a higher hospital-acquired death rate due to COVID-19 than the other two hospitals in December, possibly linked to the surge in clusters.
Table I.
Patient deaths, admissions and COVID-19 mortality rates for three acute hospitals from October 2020 to March 2021
| Month | Hospital H |
Hospital M |
Hospital W |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | Admissions | Rate | Deaths | Admissions | Rate | Deaths | Admissions | Rate | |
| Oct 2020 | 28 (5) | 2140 | 1.31 (0.234) | 17 (5) | 3106 | 0.55 (0.161) | 17 (0) | 3960 | 0.43 (0.000) |
| Nov 2020 | 56 (19) | 2117 | 2.64 (0.897) | 29 (3) | 2877 | 1.01 (0.104) | 67 (23) | 3530 | 1.90 (0.652) |
| Dec 2020 | 28 (5) | 2268 | 1.23 (0.220) | 31 (7) | 2957 | 1.05 (0.237) | 52 (19) | 3139 | 1.66 (0.605) |
| Jan 2021 | 47 (13) | 1860 | 2.53 (0.699) | 75 (18) | 2669 | 2.81 (0.674) | 55 (16) | 3188 | 1.72 (0.502) |
| Window Opening from 25th Jan | |||||||||
| Feb 2021 | 32 (0) | 1903 | 1.68 (0.000) | 46 (6) | 2494 | 1.84 (0.241) | 62 (2) | 3229 | 1.92 (0.062) |
| Mar 2021 | 16 (0) | 2501 | 0.64 (0.000) | 14 (2) | 3141 | 0.45 (0.064) | 13 (1) | 3018 | 0.43 (0.033) |
| Totals | 207 (42) | 12789 | 1.62 (0.33) | 212 (41) | 17244 | 1.23 (0.238) | 266 (61) | 20064 | 1.33 (0.304) |
Figures in brackets are patient deaths and rates attributable to hospital acquisition.
Discussion
This article describes a six-month investigation of hospital-acquired COVID-19 in one Scottish health board, with focus on an unexpected surge of clusters at one hospital during December 2020. While many factors contributed towards this surge, it is felt that the main driver was excess boarding of elderly trauma cases (Figure 3). Certainly, the overall mortality rate due to hospital-acquired COVID-19 in Hospital W was higher among these patients than for any other specialty. Trauma patients require more hands-on care, leading to longer periods of close contact between staff and patient. Prolongation, and even enhancement, of clusters may also have been encouraged by freezing weather inhibiting window opening [15]. This would exacerbate the transmission risk in naturally ventilated wards. The same weather conditions would have contributed towards slips, trips and falls in the community, increasing admissions for orthopaedic trauma. Because this service is centralized at Hospital W, such pressures were not evident at the other two hospitals. Furthermore, SARS-CoV-2 survives better at low temperatures, along with enhanced transmission in winter [15,[23], [24], [25]].
Wintry conditions may also have affected care home clusters during the six-month study. For example, the number of care home clusters stabilized in early December (after sub-zero temperatures) rather than continuing the decreasing trend previously demonstrated (Figure 1). Likewise, the increasing trend in care home clusters accelerated in January, at the same time as all three hospitals were experiencing clusters during freezing weather. Then, in early February, care home clusters stabilized again instead of following a decreasing trend during a week of exceptionally low temperatures. The ‘Open the Window’ policy was made available to care homes but it is unclear how many (if any) adopted the advice given to acute hospitals.
NHSL hospitals are naturally ventilated and thus there was a risk of airborne transmission in wards where windows remained closed [26,27]. While surgical masks reduce the risk of transmission, they are not as effective as FFP3-type respirator masks [28,29]. Nosocomial COVID-19 infections have been reported among staff wearing surgical masks and eye protection [[19], [20], [21]]. Potential transmission among staff may have occurred in areas where they believed that they could safely remove personal protective equipment (PPE) (e.g., changing rooms, offices, staff rooms), but ventilation in these areas, along with other controls such as cleaning, may not have been as rigorous as in patient areas [12].
Despite multiple confounders, window opening might have influenced cluster occurrence. In Hospital W, there were 17 clusters between 1st October and 25th January compared with just two from 25th January until 31st March. Overall, 40 clusters reduced to three after window opening. Furthermore, there was a sudden drop in hospital-acquired COVID-19 mortality rates during February–March 2021 (Table 1). This occurred despite higher COVID-19 rates in NHSL at the time of window opening and more care home clusters than documented during December. High admission rates continued at Hospital W, with more COVID-19 patients in the hospitals when window opening began than during December (Supplementary Table S2). All new admissions were screened for COVID-19 throughout the study and staff screening began on 21st December without any discernible impact on January clusters.
Further support comes from the reported association between freezing temperatures and new clusters. While this may have been the case in early December and January for all three hospitals, no association was seen after window opening despite a week of very cold weather during the second week in February (Figure 1). In addition, there was no evidence for Alpha (B.1.1.7) or Delta (B.1617.2) variants from a snapshot of 64 isolates sent for genotyping between mid-November and late December 2020. The dominant lineage in Hospital W resembled the successful European summer lineage.
Increasing numbers of patients boarding at Hospital W appeared to mirror the increase in hospital clusters in December. This was gradually curtailed throughout January, which might have contributed towards the sudden decrease in clusters. However, clusters at the other two hospitals also decreased, without any change in patient boarding rates.
Points against any window opening effect include the fact that the overall rate of COVID-19 was already slowing following the nationwide ‘lock down’ on 26th December. This was reflected in the number of care home clusters, which peaked on 19th January (N = 25), and then gradually decreased over the following three months. In addition, care home and frontline staff vaccination began in early December and this may have influenced the number of clusters seen after window opening. However, vaccinated individuals would not have received the second dose of the vaccine until well after window opening began.
Increasing fresh air on the wards may have reduced the risk of further COVID-19 transmission, without compromising staff and patient well-being. Such practices are not solely COVID-19 themed; they represent another level of infection prevention that would have an impact on other airborne pathogens such as influenza; respiratory viruses; norovirus; and Clostridioides difficile (because the latter produces airborne spores which take 48 h to settle) [5,6]. Furthermore, meticillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and coliforms have also been found in hospital air, and efforts to improve indoor ventilation might benefit infection prevention strategies against these pathogens [6,30].
In conclusion, the increase in COVID-19 clusters affecting staff and patients in NHSL was probably due to asymptomatic and pre-symptomatic staff introducing the virus from the community despite universal masking. SARS-CoV-2 spread to patients on low-risk wards, who transferred it elsewhere when moved around the hospital. Seasonal influences almost certainly exacerbated transmission. Many hospitals and care homes rely upon natural ventilation, which fails to optimize air exchange, particularly in cold weather. It is plausible that normally minimal levels of infectious respirable aerosols attained a threshold where classic airborne transmission became significant [27,31]. While future research is needed to confirm this scenario, hospitals should try to eliminate physical, environmental, and administrative risk factors to protect frontline workers [32]. The role of ventilation in indoor transmission of viruses has been neglected for years [27,28]. It has taken a pandemic to focus attention on airborne pathogens but now that it has, we should embrace the opportunity and explore all possibilities for protecting staff and patients now, and for the future.
Acknowledgements
We wish to acknowledge Professor M. Holden and his team at Public Health Scotland for providing WGS data, and NHSL staff and laboratories for all their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.11.019.
Conflict of interest statement
None to report.
Funding sources
This study was funded by NHS Lanarkshire.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.WHO. Scientific Brief . World Health Organisation; 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Google Scholar]
- 2.Respirator masks only for AGPs . 2020. Public Health England. 6. COVID-19 infection prevention and control guidance: aerosol generating procedures.https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infectionprevention-and-control/covid-19-infection-prevention-and-controlguidance-aerosol-generating-procedures Available at: (last accessed November 2021) [Google Scholar]
- 3.Stockwell R.E., Ballard E.L., O'Rourke P., Knibbs L.D., Morawska L., Bell S.C. Indoor hospital air and the impact of ventilation on bioaerosols: a systematic review. J Hosp Infect. 2019;103(2):175–184. doi: 10.1016/j.jhin.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobday R.A., Dancer S.J. Roles of sunlight and natural ventilation for controlling infection: historical and current perspectives. J Hosp Infect. 2013;84(4):271–282. doi: 10.1016/j.jhin.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang J.W., Li Y., Eames I., Chan P.K., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.L., et al. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. Sci Tot Environ. 2020;753:141710. doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott C.V., Alicic R.Z., Harden N., Cox E.J., Scanlan J.M. Put a lid on it: are faecal bio-aerosols a route of transmission for SARS-CoV-2? J Hosp Infect. 2020;105(3):397–398. doi: 10.1016/j.jhin.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dancer S.J., Li Y., Hart A., Tang J.W., Jones D.L. What is the risk of acquiring SARS-CoV-2 from the use of public toilets? Sci Total Environ. 2021;792:148341. doi: 10.1016/j.scitotenv.2021.148341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illingworth C.J.R., Hamilton W.L., Warne B., Routledge M., Popay A., Jackson C., et al. Superspreaders drive the largest outbreaks of hospital onset COVID-19 infections. Elife. 2021;10 doi: 10.7554/eLife.67308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temkin E., Schwaber M.J., Vaturi A., Nadir E., Zilber R., Barel O., et al. Effect of a national policy of universal masking and uniform criteria for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) exposure on hospital staff infection and quarantine. Infect Control Hosp Epidemiol. 2021 May 3:1–7. doi: 10.1017/ice.2021.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon CL, Trubiano JA, Holmes NE, Chua KYL, Feldman J, Young G, et al. Staff to staff transmission as a driver of healthcare worker infections with COVID-19. Infect Dis Health. 2021;26(4):276–283. doi: 10.1016/j.idh.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read J.M., Green C.A., Harrison E.M., Docherty A.B., Funk S., Harrison J., et al. Hospital-acquired SARS-CoV-2 infection in the UK's first COVID-19 pandemic wave. Lancet. 2021;12 doi: 10.1016/S0140-6736(21)01786-4. S0140-6736(21)01786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karan A., Klompas M., Tucker R., Baker M., Vaidya V., Rhee C., CDC Prevention Epicenters Program The Risk of SARS-CoV-2 Transmission from Patients with Undiagnosed Covid-19 to Roommates in a Large Academic Medical Center. Clin Infect Dis. 2021:ciab564. doi: 10.1093/cid/ciab564. [DOI] [PubMed] [Google Scholar]
- 15.Quilodrán C.S., Currat M., Montoya-Burgos J.I. Air temperature influences early Covid-19 outbreak as indicated by worldwide mortality. Sci Total Environ. 2021;792:148312. doi: 10.1016/j.scitotenv.2021.148312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Man P., Paltansing S., Ong D.S.Y., Vaessen N., van Nielen G., Koeleman J.G.M. Outbreak of COVID-19 in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis. 2020:ciaa1270. doi: 10.1093/cid/ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingotti N., Grogono D., Dello Ioio G., Curran M., Barbour K., Taveira M., et al. The impact of hospital-ward ventilation on airborne-pathogen exposure. Am J Respir Crit Care Med. 2021;203(6):766–769. doi: 10.1164/rccm.202009-3634LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen P.L., Yam R., Yung R., Choy K.L. Fast-track ventilation strategy to cater for pandemic patient isolation surges. J Hosp Infect. 2012;81:246–250. doi: 10.1016/j.jhin.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klompas M., Baker M.A., Griesbach D., Tucker R., Gallagher G.R., Lang A.S., et al. Transmission of SARS-CoV-2 from asymptomatic and presymptomatic individuals in healthcare settings despite medical masks and eye protection. Clin Infect Dis. 2021:ciab218. doi: 10.1093/cid/ciab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D., et al. A SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021;174(6):794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg L., Levinsky Y., Marcus N., Hoffer V., Gafner M., Hadas S., et al. SARS-CoV-2 infection among health care workers despite the use of surgical masks and physical distancing-the role of airborne transmission. Open Forum Infect Dis. 2021;8(3):ofab036. doi: 10.1093/ofid/ofab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASHRAE . American Society of Heating, Refrigerating and Air-Conditioning Engineers; Atlanta, GA: 2019. Standard 62.1.2019. Ventilation for acceptable indoor air quality. [Google Scholar]
- 23.Romero Starke K., Mauer R., Karskens E., Pretzsch A., Reissig D., Nienhaus A., et al. The effect of ambient environmental conditions on COVID-19 mortality: a systematic review. Int J Environ Res Public Health. 2021;18(12):6665. doi: 10.3390/ijerph18126665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellicour S., Linard C., Van Goethem N., Da Re D., Artois J., Bihin J., et al. Investigating the drivers of the spatio-temporal heterogeneity in COVID-19 hospital incidence: Belgium as a study case. Int J Health Geogr. 2021;20(1):29. doi: 10.1186/s12942-021-00281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClymont H., Hu W. Weather variability and COVID-19 transmission: a review of recent research. Int J Environ Res Public Health. 2021;18(2):396. doi: 10.3390/ijerph18020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J.W., Marr L.C., Li Y., Dancer S.J. Covid-19 has redefined airborne transmission. BMJ. 2021;373:n913. doi: 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- 28.Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Hosp Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Improve Ventilation of Care Settings & Upgrade Respiratory Personal Protective Equipment - Open Letter. https://docs.google.com/forms/d/e/1FAIpQLSejgqHBTsCjfOG3BdZRHsBzx4cMxZEEnarozhztDo4tdc4zdQ/viewform Available at: (last accessed November 2021)
- 30.Morawska L., Allen J., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. A paradigm shift to combat indoor respiratory infection. Science. 2021;372(6543):689–691. doi: 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- 31.Milton D.K. A Rosetta Stone for Understanding Infectious Drops and Aerosols. J Pediat Infect Dis Soc. 2020;9(4):413–415. doi: 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.