Abstract
Objective:
The chemokine CXCL12, together with its specific receptor, CXCR4, have been shown to mediate invasiveness and metastatic behaviour in pancreatic cancer cells. The expression of CXC12/CXCR4 has not been previously examined in pancreatic intraepithelial neoplasias (PanIN), the accepted precursor lesions to pancreatic duct cancer.
Design:
In this study we sought to characterise the expression of CXCL12 and CXCR4 during the progression of PanIN using both a murine model and human tissues.
Results:
These studies reveal that both CXCL12 and CXCR4 are expressed in PanIN and that the frequency increases during PanIN progression (0% CXCR4 expression in normal mouse and human ducts vs 100% in mouse PanIN 3 and 77% in human PanIN 3). Next we demonstrate a dose-dependent increase in the proliferation of murine PanIN cells when exposed to CXCL12. Finally, we show that expression of CXCR4 in murine PanIN cells is partially dependent on mitogen-activated protein kinase (MAPK) signalling and that the effect of CXCL12 on PanIN proliferation can be abrogated by an MAPK inhibitor.
Conclusions:
Together these results demonstrate that CXCL12/CXCR4 expression begins in the pre-invasive stages of pancreatic neoplasia, and suggest that the presence of an autocrine loop that is at least partially regulated by MAPK signalling. Further studies that define the role of CXCR4 signalling in PanIN progression will determine if CXCR4 could serve as a novel target for chemoprevention and early stage therapy in pancreatic cancer.
Pancreatic cancer is a devastating disease that is most often diagnosed when the disease is disseminated thereby precluding any hope for cure. During the last 10 years, the precursor lesions to invasive pancreatic cancer have been defined and validated by murine models that recapitulate the histological progression hypothesised to occur in humans. It remains of great interest and importance that we gain an improved understanding of the signalling pathways critical to the progression of these pancreatic intraepithelial neoplasias (PanIN) to invasive cancer. Recent evidence has suggested that certain chemokine signalling pathways, most notably chemokine receptor CXCR4, may play a role in pancreatic cancer migration, invasion, angiogenesis and apoptosis.1-4 Several studies have documented the expression of CXCR4 in primary cancers and metastases.5-8 Tail vein injection of nude mice with CXCR4-expressing pancreatic cancer cells resulted in a significant increase in liver and lung metastases as compared to the non-CXCR4-expressing controls.3 In this study CXCR4 was not found to regulate cell proliferation. While the evidence for CXCR4 as a mediator of metastatic behaviour continues to accumulate, it remains unknown as to whether the CXCL12/CXCR4 axis plays any role in the development or progression of PanIN.
In this report, we demonstrate that CXCR4 is highly expressed in PanIN cells derived from a Kras-driven mouse model of pancreatic cancer and that both CXCL12 and CXCR4 are differentially expressed in tissue samples from this mouse model as well as in human PanIN. We also demonstrate that exposure to CXCL12 results in PanIN cell proliferation that is abrogated by the concurrent inhibition of the mitogen-activated protein kinase (MAPK) signalling pathway. Finally, we demonstrate that downregulation of MAPK signalling decreases expression of CXCR4 in cultured PanIN cells. These data demonstrate that CXCL12–CXCR4 signalling may play a role, not only in the acquisition of metastatic potential, but earlier during the progression of PanIN to invasive pancreatic cancer.
MATERIALS AND METHODS
Cell lines and maintenance
The murine PanIN cell line as well as the murine pancreatic cancer cells lines 4964PDA, 4964LM, 5143PDA and 5143LM were derived from the PdxCre/LSL-KrasG12D and PdxCre/LSL-KrasG12D/p53−/− mouse model of pancreatic cancer as previously described.9-11 The PanIN cell line was derived from high-grade PanIN lesions from a PdxCre/LSL-KrasG12D mouse; no invasive cancer was present within the pancreas of this mouse. Unless stimulated with growth factor, these cells are poorly motile and minimally invasive in a modified Boyden–Chamber assay.11 In addition, the PanIN cell line is non-tumorigenic in nude mice (data not shown). HeLa cells were obtained from the American Type Culture Collection (Manassas, Virginia, USA). All murine pancreatic cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, Utah, USA), HeLa cells were maintained in minimal essential medium (MEM), and both were supplemented with 10% fetal bovine serum (FBS) and 2% penicillin–streptomycin unless otherwise noted. Cells were grown in a humidified incubator at 37°C in 5% CO2.
Immunoblot analysis
Cells were lysed with 2× sodium dodecyl sulfate (SDS) lysis buffer (125 mmol/l Tris, 20% glycerol, 4% SDS, pH 6.8) and quantification of protein concentrations was determined utilising the Micro BCA Protein Assay Kit (Pierce, Rockford, Illinois, USA). Cell lysates prepared for the analysis of phosphorylated proteins were performed utilising the 2× SDS lysis buffer with the addition of sodium fluoride (50 mmol/l) and sodium orthovanadate (1 mmol/l). Cell lysates were resolved on a 10% SDS–polyacrylamide gel and transferred to a polyvinylidine difluoride membrane (Millipore, Bedford, Massachusetts, USA) or BioTrace NT nitrocellulose membrane (Pall Corporation, East Hills, New York, USA), when phosphorylated proteins were to be analysed, at 4°C overnight. Membranes were blocked in blocking buffer (1 × Tris-buffered solution (TBS) +0.05% Tween + 5% milk) for 1 h and then probed with primary antibody. Primary antibody was diluted in blocking buffer unless directed against phosphorylated protein in which case it was diluted in 1 × TBS + 0.05% Tween + 5% bovine serum albumin. After probing with horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA), incubation of blots with Amersham ECL Plus Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, UK) was performed to visualise immunoblot bands. Immunoblot membranes were probed with antibodies to either goat anti-CXCR4 (1:1000) (Alexis Corporation, Lausen, Switzerland), rabbit anti-phospho-extracellular signal-related kinase (ERK) (1:1000), or rabbit anti-ERK (1:1000) (Cell Signaling. Beverly, Massachusetts, USA). Detection of α-actin (1:10 000, Sigma) or α-tubulin (1:500) (Santa Cruz Biotechnology) was used as a loading control. When necessary, membranes were stripped with Restore Western Blot Stripping Buffer (Pierce) according to the manufacturer’s specifications, re-blocked, and re-probed with primary antibody.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) cancer specimens were obtained from 10 patients who underwent curative resection for pancreatic duct adenocarcinoma at City of Hope National Medical Center. FFPE specimens were also obtained from PdxCre/LSL-KrasG12D mice. The archived specimens were reviewed by a surgical pathologist to confirm the diagnosis of invasive pancreatic cancer and PanIN. FFPE clinical specimens were assessed to determine the expression patterns of CXCR4 and CXCL12. Tissue blocks were sectioned (5 μm) and mounted onto positively charged slides. They were deparaffinised with xylene, washed with distilled water, and treated with hydrogen peroxide. Antigen retrieval was performed at 105°C for 30 min in a sodium citrate buffer solution. The sections were incubated at 4°C with a polyclonal anti-CXCR4 antibody (Abcam, Cambridge, Massachusetts, USA) at 1:50 dilution for 60 min and with a polyclonal anti-SDF-1a (CXCL12) antibody (US Biological, Swampscott, Massachusetts, USA) at 1:200 dilution for 30 min. Then, the sections were labelled with a secondary antibody (Envision+; Dako, Carpinteria, California, USA), developed with diaminobenzidine (DAB), counterstained in 50% Mayer’s haematoxylin and examined at ×200 and ×400 magnifications. In order to determine the incidence of CXCR4 expression in PanIN lesions, separate slides from 10 mouse and 10 human specimens were examined and scored.
In order to elucidate concurrent CXCR4/CXCL12 expression in mouse tissue samples, immunohistochemical staining was performed on 5-μm thick sections prepared from FFPE tissue. Tissue sections were deparaffinised in xylene followed by 100% ethanol. Samples were then quenched in 3% hydrogen peroxide and treated to promote antigen retrieval by placing slides in a pressure cooker with citrate buffer at 105° for 30 min. Then the slides were washed in distilled water and a protein block was added to them for 10 min. The sections were incubated with the anti-CXCR4 antibody for 30 min at room temperatures and a secondary antibody (rabbit polymer) for 30 min. After washing in buffer, the chromogen DAB was added for 10 min followed by an additional wash in distilled water for 10 min. After re-incubation in buffer, the anti-SDF-1 (CXCL12) antibody was added for 30 min at room temperature. Slides were then washed in buffer and then incubated with the secondary antibody (rabbit polymer) for 30 min. The AEC+ substrate chromogen was added for 10 min, washed in distilled water for 5 min, counterstained in 50% Mayer’s haematoxylin for 2 min, washed in distilled water for 10 min, then the slides were covered with cover slips using aqueous base media.
The presence of CXCR4 expression was based on the intensity of staining and each duct was scored based on a 0 to 3+ scoring system. Pancreatic islets were used a positive control for 3+ staining. A score of 2+ was chosen as the cut-off for positive or negative CXCR4 staining. The number of positive ducts divided by total number of ducts for each different PanIN grade was calculated to determine the incidence of CXCR4 staining within each PanIN grade or normal duct.
PanIN proliferation assay
The mouse PanIN cell line was plated at a density of 5 × 103 cells per well in a 96-well plate in 200 μl of growth media supplemented with 1% FBS and allowed to adhere overnight. The cells were washed three times with PBS and the media was changed to serum-free media with increasing amounts of mouse CXCL12 (R&D Systems, Minneapolis, Minneapolis, USA). The cells were incubated for various times (0, 12, 24, 48 and 72 h) under these conditions. Two hours prior to the designated time point, Alamar blue (Biosource, Camarillo, California, USA) was added to a final vol/vol concentration of 10% and incubated for 2 h. An automated fluorescence plate reader at excitation/emission wavelengths of 530/590 nm was used to measure the proliferating cell population. Alamar blue added at the time that the media was changed to serum-free media served as the 0 h time point. Cells grown in media +10% FBS served as a positive control whereas serum-free media with 10% vol/vol Alamar blue served as the blank which was averaged and subtracted from the fluorescence value obtained for each well. Results were expressed as units representing fold increase in fluorescence, in proportion to changes in PanIN cell proliferation. Inhibitory studies performed with the specific CXCR4 inhibitor, AMD310012,13 (5 mg/ml, Sigma–Aldrich, St Louis, Missouri, USA), or the MEK1/2 inhibitor, U0126 (100 μmol/l; Invivogen, San Diego, California, USA), were performed as above except that upon changing the media to serum-free media, the respective inhibitor was added and the cells were allowed to incubate for 1 h. At this time, the media was again changed to serum-free media to include both the respective inhibitor as well as CXCL12. It was this point that was designated the 0 h time point. Each experiment was done in triplicate and repeated as three independent experiments.
Statistical analyses
Analysis of variance with Dunnett’s multiple comparison tests was used to compare treatment groups to the untreated control using GraphPad Prism software (version 3.03). A p value of ⩽ 0.05 was considered statistically significant.
RESULTS
CXCR4 is expressed in cell lines derived from PdxCre/LSL-KrasG12D Mice
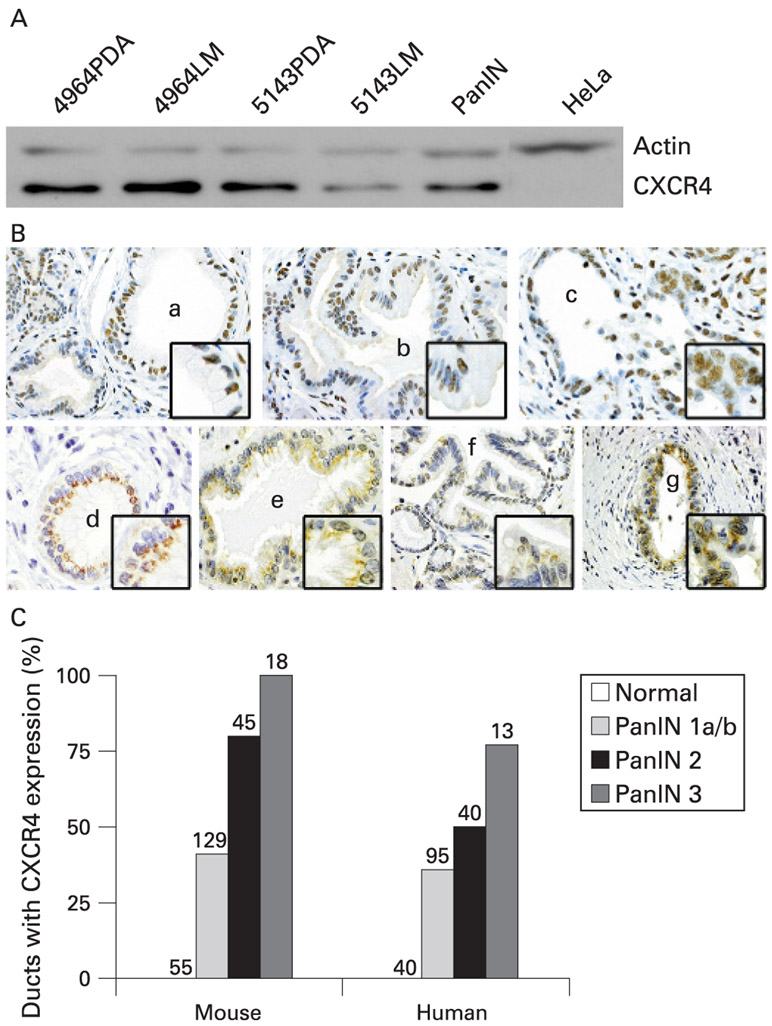
Although previous work has demonstrated that CXCR4 is expressed in pancreatic neoplasia, there are no reports documenting its expression in pancreatic cancer precursors. As a first step to examine this question, we studied CXCR4 expression in murine cell lines derived from PdxCre/LSL-KrasG12D and PdxCre/LSL-KrasG12D/p53 mutant strains using immunoblot. A single cell line derived from PanIN as well as two each derived from primary and metastatic pancreatic carcinoma cell lines were examined. Figure 1A shows that CXCR4 is highly expressed in all cell lines, including the PanIN line. These findings demonstrate that in this murine model, CXCR4 expression begins in the early stages of pancreatic carcinogenesis and is maintained during progression to invasive cancer and metastatic disease.
Figure 1.
The chemokine receptor CXCR4 is expressed in murine and human pancreatic intraepithelial neoplasia. (A) Immunoblotting of cell lysates derived from PdxCre/LSL-KrasG12D mice included two primary pancreatic ductal adenocarcinoma lines (4964PDA, 5143PDA), two metastatic lines (4964LM, 5143LM), and a pancreatic intraepithelial neoplasia (PanIN) cell line which all demonstrate strong expression of CXCR4. (B) Mouse (mPanIN) and human (hPanIN) pancreatic intraepithelial neoplasia demonstrate increased expression of CXCR4 with progression from PanIN 1A to PanIN 3 with an absence of expression in normal duct. Representative samples are shown and include (a) mPanIN 1A, (b) mPanIN 1B, (c) mPanIN 3, (d) hPanIN 1A, (e) hPanIN 1B, (f) hPanIN 2, and (g) hPanIN 3. (C), Ten independent immunohistochemistry (IHC) specimens from murine and human pancreata were evaluated for CXCR4 incidence in PanIN lesions and demonstrate increased CXCR4 expression with progression from normal duct to PanIN3. The number of PanIN lesions (1A-3) that stained positive for CXCR4 was divided by the total number of each particular PanIN subtype present to obtain the % positive. The total number of PanIN examined is indicated by the number above each bar.
CXCR4 is increasingly expressed during the progression of mouse and human pancreatic intraepithelial neoplasia
After demonstrating the expression of CXCR4 in a PanIN cell line, we next assessed both CXCR4 and CXCL12 expression in murine and human pancreatic tissue samples containing PanIN. Figure 1B depicts representative images of CXCR4 expression from these tissue samples, while fig 1C demonstrates the incidence of CXCR4 expression in the various PanIN grades. Of 55 normal mouse and 40 normal human ducts analysed, 0% expressed CXCR4, but expression is present in increasing frequency and intensity during the progression from PanIN 1A to PanIN 3. In mouse and human, more than 33% of PanIN1A–1B lesions express CXCR4, an incidence that rises to greater than 50% for PanIN-2. In the mouse, all PanIN-3 lesions (n = 18) expressed CXCR4, while it was expressed in 77% of human PanIN-3 cells (n = 13). We observed that CXCR4 was expressed at the cell membrane as well as in the cytoplasm in both mouse and human tissues. Finally, it was apparent that even in the less genetically complex murine PanIN, all duct cells did not express CXCR4 to an equal extent. Both murine and human CXCR4 immunohistochemistry (IHC) samples were analysed in order to correlate the incidence of CXCR4 expression amongst various PanIN grades.
We next questioned whether PanIN cells themselves might express CXCL12, thus creating the opportunity for autocrine signalling to occur. To test this hypothesis, we performed IHC on the same cohort of tissue samples against CXCL12 (fig 2). These studies revealed that CXCL12, much like CXCR4, was expressed in mouse and human PanIN. In contrast to CXCR4, for CXCL12 we did not detect a clear pattern of progressive expression from early to late grade PanIN as we found that CXCL12 was often highly expressed in early PanIN lesions. These findings demonstrate that most PanIN express both CXCR4 and its ligand CXCL12, thereby creating the potential for autocrine signalling early in the course of pancreatic duct neoplasia as shown in the CXCR4/CXCL12 double staining in fig 2B.
Figure 2.
The chemokine CXCL12 is expressed in murine and human pancreatic intraepithelial neoplasia. Murine and human pancreata were examined for CXCL12 expression via immunohistochemistry (IHC). CXCL12 expression was present in all pancreatic intraepithelial neoplasia (PanIN) grades but there was no statistical difference in expression patterns between low grade and high grade PanIN. Panels designate varying degrees of PanIN. (A) mPanIN 1A (single asterisk) and mPanIN 1B (double asterisk). (B) Double staining of CXCR4/CXCL12 in a mouse PanIN 1B with CXCL12 in brown and CXCR4 in green, demonstrates concurrent expression of ligand and receptor, suggesting the possibility of an autocrine PanIN activation. (C), hPanIN 1B, and (D) hPanIN 2.
CXCL12/CXCR4 signalling induces proliferation in mouse PanIN cells
After demonstrating that both CXCL12 and CXCR4 are expressed in mouse and human PanIN, we sought to explore the potential biological significance of this finding. Some investigators have reported that CXCR4 mediates cell proliferation, while other authors have noted effects only on metastatic properties. To explore this question in PanIN cells, we exposed serum-starved murine PanIN to increasing doses of CXCL12 and monitored cell proliferation using an Alamar blue assay. As depicted in fig 3A, CXCL12 resulted in a dose-dependent increase in PanIN cell proliferation as seen by a 32%, 38% and 47% difference in fold increase in fluorescence at 72 h between 0 ng/ml CXCL12 compared to the 25, 100 and 250 ng/ml CXCL12 treatment groups, respectively. The specificity of this effect for CXCR4 was examined using a CXCR4 inhibitor AMD3100. Figure 3B reveals that co-incubation with AMD3100, abrogated the CXCL12-induced proliferation.
Figure 3.

Stimulation of the chemokine receptor CXCR4 leads to increased proliferation of pancreatic intraepithelial neoplasias (PanIN) in vitro which is abrogated by concominant MEK1/2 inhibition. (A) Alamar blue proliferation assays were performed in PanIN cells with increasing concentration of the CXCR4 ligand, CXCL12. Fluorescence measurements were performed at baseline as well as 12, 24, 48 and 72 h post-CXCL12 administration. PanIN cells demonstrated a dose-dependent increase in proliferation with CXCL12 administration compared to control and was statistically significant for all concentrations at all time points (p<0.05) with a 47% difference in fluorescence at 72 h between PanIN stimulated with 250 ng/ml CXCL12 and untreated control. (B) Pre- and concomitant incubation of the PanIN cells with the CXCR4 specific inhibitor AMD3100 abrogated the increased proliferation seen with CXCL12 administration alone. (C) Further decrease in proliferation when PanIN cells were co-incubated with the MEK1/2 inhibitor U0126. (D) Incubation with AMD3100 and U0126 was similar to U0126 alone, indicating that CXCL12 mediated PanIN proliferation is mitogen-activated protein kinase (MAPK) dependent.
CXCL12-induced PanIN proliferation is MEK dependent
After determining that CXCR4 stimulation increased PanIN proliferation, we wished to explore putative mechanisms underlying this response. In other cell systems, CXCR4 has been shown to signal through the MAPK pathway.14 To determine if CXCL12-induced PanIN proliferation was dependent on MAPK signalling, we exposed PanIN cells to CXCL12 in the presence or absence of the MEK1/2 specific inhibitor U0126. We noted that CXCL12-mediated PanIN proliferation was significantly reduced by U0126 (fig 3C). In fact, it appeared that U0126 inhibited proliferation to a greater degree than did AMD3100. Given this, we next sought to determine if concurrent exposure to U0126 and AMD3100 would be synergistic in inhibiting CXCL12-dependent proliferation. As shown in fig 3D, the concurrent administration of U0126 and AMD3100 was no more effective in reducing proliferation than U0126, suggesting that CXCL12/CXCR4 induced proliferation is dependent on MAPK signalling. As CXCR4 is not expressed in normal pancreatic ducts, and given that activated Kras is the sole initiating genetic event in PdxCre/LSL-KrasG12D mice, one possible explanation for our findings is that CXCR4 expression is regulated by MAPK signalling. To investigate this, we examined expression of CXCR4 following treatment of PanIN cells with U0126. As CXCL12 can downregulate CXCR4 expression, these experiments were performed without addition of exogenous CXCL12. Following exposure to U0126, CXCR4 expression was downregulated in PanIN cells, a finding that persisted at 16 and 48 h post-stimulation. Densitometry revealed 83% and 62% downregulation of CXCR4 at 16 h and 48 h, respectively. To confirm inhibition of MAPK signalling, we examined expression of phospho-ERK at the same time points and observed a near-complete abrogation of its expression without any change in total ERK levels (fig 4). These experiments reveal that, in vitro, PanIN cells proliferate in response to CXCL12, and that this proliferative response is blocked by inhibition of the MAPK pathway. The effects on proliferation appear to be at least partially mediated by expression of CXCR4 itself, which is downregulated by inhibition of MEK1/2.
Figure 4.

Inhibition of MEK1/2 with U0126 leads to decreased expression of the chemokine receptor CXCR4 in pancreatic intraepithelial neoplasia (PanIN) cells. Immunoblotting of PanIN lysates that were treated with 100 μmol/l of U0126 revealed an 83% and 62% downregulation of CXCR4 at 16 h and 48 h, respectively when compared with untreated controls by densitometry. The inhibition of MEK1/2 was confirmed by the downregulation of phospho-extra-cellular signal-related kinase (ERK) (pERK), the direct downstream target of MEK signalling. Tubulin and total-ERK (tERK) served as loading controls.
DISCUSSION
Pancreatic cancer is a devastating disease which will account for an estimated 33 000 deaths in 2007, reflective of a mortality rate that nearly matches its incidence.15 The deadly nature of this disease makes it imperative that we gain an improved understanding of the pathways that facilitate the progression of PanIN to invasive pancreatic cancer as these could serve as targets for chemoprevention or early stage treatment. Recently, chemokines and their respective receptors have been implicated in the progression of numerous tumours, including pancreatic cancer.16-18 CXCR4 expression has been demonstrated in numerous primary and metastatic human pancreatic cancer cell lines and in human tissue samples. To our knowledge, the expression status of CXCR4 and its ligand CXCL12 in pancreatic precursor lesions has not been previously characterised. This may be due to the fact that CXCR4 has been primarily implicated in mediating pancreatic cancer invasion and metastases, events not thought of as associated with PanIN. In this report, we demonstrate that CXCR4 is expressed in murine PanIN cells derived from a Kras-driven mouse model of pancreatic cancer. More importantly, we demonstrate that murine and human PanIN express CXCR4 and its ligand, CXCL12, even at their earliest recognisable stages and that expression of CXCR4 becomes increasingly common during PanIN progression. Expression of CXCL12 has been demonstrated in a minority of pancreatic cancer cell lines, but is generally present in abundance within invasive pancreatic cancers. These studies suggest that most CXCL12 in human pancreatic cancer may be derived from tumour-associated stroma. Our immunostaining demonstrates abundant CXCL12 expression in PanIN cells, prompting the hypothesis that autocrine signalling may occur in the early stages of pancreatic neoplasia prior to the development of tumour-associated stroma. The CXCR4 expression pattern we observed is remarkably consistent between the murine model and the human disease, yet it is insufficient evidence to conclude that the CXCL12/CXCR4 axis contributes to PanIN progression. Genetic manipulation of CXCR4 and CXCL12 expression within the murine pancreas will be required to test this hypothesis directly. As we observed in PanIN, Hermann et al19 recently demonstrated that distinct subpopulations of CD133+ pancreatic cancer stem cells express CXCR4. CXCR4 expressing cells appeared to mediate pancreatic cancer metastasis, provoking the hypothesis that CXCR4 expressing PanIN could be the progenitors of this CXCR4 expressing cancer cells.
Our work shows that, in vitro, CXCL12/CXCR4 signalling mediates PanIN proliferation, a finding which suggests it could be relevant to in vivo PanIN progression. Studies of CXCL12/CXCR4 signalling and pancreatic cancer cell proliferation have varied in their findings. In one report, CXCL12 enhanced proliferation of the CXCR4-expressing AsPC-1 and Hs766T cells in serum-free media. In contrast, Saur et al3 demonstrated that murine pancreatic cancer cells (TD-2) stably transfected with CXCR4 had increased metastatic potential without effecting proliferation. In our studies, CXCL12 induced PanIN cell proliferation was abrogated by administration of AMD3100 or U0126, specific CXCR4 or MEK 1/2 inhibitors, respectively. Importantly, the addition of AMD3100 did not diminish proliferation below the level seen with U0126 alone. These findings suggest that CXCL12 stimulated PanIN cell proliferation is MAPK dependent. This finding is consistent with that of other investigators who have demonstrated that CXCL12/CXCR4 activates multiple signalling pathways including MAPK, PI3K/AKT, and nuclear factor kappa B (NF-κB), and distinct pathways may regulate specific oncogenic phenotypes.14
Mutant Kras is the earliest known genetic alteration in human pancreatic cancer and we demonstrate that murine PanIN cells containing solely this mutation express CXCR4. This suggested to us that pathways downstream of Kras may be responsible for regulation of CXCR4 expression. By inhibition of the MEK1/2 pathway with U0126, we have shown that the MAPK signalling does in fact regulate CXCR4 expression. Kras-regulated MAPK signalling results in the upregulation of various transcription factors including c-Myc, ELK and STAT1/3. Consensus binding sites for each of these transcription factors are present within the CXCR4 promoter region and CXCR4 has previously been shown to be upregulated by c-Myc.20-25 As previously mentioned, evidence exists for CXCR4-dependent ERK1/2 activation, nuclear translocation, and subsequent ELK phosphorylation.26 Taken together, it is reasonable to propose that as Kras and CXCR4 each activate MAPK, a positive feedback loop is created which serves to promote CXCR4 expression and further amplify MAPK signalling. This hypothesis is consistent with our finding that CXCR4 expression increases during PanIN progression both in genetically complex human PanIN and in murine PanIN driven solely by expression of mutant Kras.
In summary, our findings reveal that both CXCR4 and its ligand CXCL12 are expressed in the earliest stages of pancreatic neoplasia. CXCL12/CXCR4 signalling regulates the in vitro proliferation of PanIN derived from PdxCre/LSL-KrasG12D mice via the MAPK signalling pathway which also serves to regulate CXCR4 expression. The biological contribution of CXCR4 signalling to the progression of PanIN to invasive carcinoma deserves further investigation as this chemokine receptor represents a potential target for chemoprevention and early therapy.
Funding:
This work was supported by NIH CA89403 (AML), a University of Cincinnati Cancer Center Pilot Award (AML), a National Pancreas Foundation Research Grant (AML), Hirshberg Foundation for Pancreatic Cancer Research (JK), and NIH T32 DK64581 (RMT).
Footnotes
Competing interests: None.
Ethics approval: The Institutional Review Board of the City of Hope National Medical Center, Duarte, California, approved the human portions of this study on 20 December 2006.
REFERENCES
- 1.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: A possible role for tumor progression. Clin Cancer Res 2000;6:3530–5. [PubMed] [Google Scholar]
- 2.Marchesi F, Monti P, Leone BE, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res 2004;64:8420–7. [DOI] [PubMed] [Google Scholar]
- 3.Saur D, Seidler B, Schneider G, et al. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005;129:1237–50. [DOI] [PubMed] [Google Scholar]
- 4.Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther 2004;3:29–37. [PubMed] [Google Scholar]
- 5.Scotton CJ, Wilson JL, Milliken D, et al. Epithelial cancer cell migration: A role for chemokine receptors? Cancer Res 2001;61:4961–5. [PubMed] [Google Scholar]
- 6.Phillips RJ, Burdick MD, Lutz M, et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 2003;167:1676–86. [DOI] [PubMed] [Google Scholar]
- 7.Schimanski CC, Bahre R, Gockel I, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer 2006;95:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett 2006;238:30–41. [DOI] [PubMed] [Google Scholar]
- 9.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. [DOI] [PubMed] [Google Scholar]
- 11.Thomas R, Earley K, Revelo P, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res 2007;67:6075–82. [DOI] [PubMed] [Google Scholar]
- 12.Fricker SP, Anastassov V, Cox J, et al. Characterization of the molecular pharmacology of AMD3100: A specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol 2006;72:588–96. [DOI] [PubMed] [Google Scholar]
- 13.Hatse S, Princen K, Bridger G, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 2002;527:255–62. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Cancer Facts & Figures 2007. Atlanta, Georgia: American Cancer Society, 2007. [Google Scholar]
- 15.Ganju RK, Brubaker SA, Meyer J, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 1998;273:23169–75. [DOI] [PubMed] [Google Scholar]
- 16.Balkwill F The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol 2004;14:171–9. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Bai Z, Srinoulprasert Y, et al. Chemokines in tumor progression and metastasis. Cancer Sci 2005;96:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JM, Deng X, Gong W, et al. Chemokines and their role in tumor growth and metastasis. J Immunol Methods 1998;220:1–17. [DOI] [PubMed] [Google Scholar]
- 19.Hermann PC, Huber SL, Herrier T, et al. Distinct subpopulations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–323. [DOI] [PubMed] [Google Scholar]
- 20.Sears RC. The life cycle of C-myc: From synthesis to degradation. Cell Cycle 2004;3:1133–7. [PubMed] [Google Scholar]
- 21.Yeh E, Cunningham M, Arnold H, et al. A signalling pathway controlling c-myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol 2004;6:308–18. [DOI] [PubMed] [Google Scholar]
- 22.Whitmarsh AJ, Shore P, Sharrocks AD, et al. Integration of MAP kinase signal transduction pathways at the serum response element. Science 1995;269:403–7. [DOI] [PubMed] [Google Scholar]
- 23.Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995;82:241–50. [DOI] [PubMed] [Google Scholar]
- 24.Moriuchi M, Moriuchi H, Turner W, et al. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol 1997;159:4322–9. [PubMed] [Google Scholar]
- 25.Moriuchi M, Moriuchi H, Margolis DM, et al. USF/c-myc enhances, while yin-yang 1 suppresses, the promoter activity of CXCR4, a coreceptor for HIV-1 entry. J Immunol 1999;162:5986–92. [PubMed] [Google Scholar]
- 26.Zhao M, Discipio RG, Wimmer AG, et al. Regulation of CXCR4-mediated nuclear translocation of extracellular signal-related kinases 1 and 2. Mol Pharmacol 2006;69:66–75. [DOI] [PubMed] [Google Scholar]