Abstract
Objective:
Adult intensive care unit (ICU) survivors that experience delirium are at high risk for developing new functional disabilities and mental health disorders. We sought to determine if individual motoric subtypes of delirium are associated with worse disability, depression, and/or post-traumatic stress disorder (PTSD) in ICU survivors.
Design:
Secondary analysis of a prospective multicenter cohort study
Setting:
Academic, community, and Veteran Affairs hospitals
Patients:
Adult ICU survivors of respiratory failure and/or shock
Interventions:
We assessed delirium and level of consciousness using the Confusion Assessment Method-ICU and Richmond Agitation and Sedation Scale (RASS) daily during hospitalization. We classified delirium as hypoactive (RASS ≤ 0) or hyperactive (RASS > 0). At 3 and 12 months post-discharge, we assessed for dependence in Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADLs), symptoms of depression, and symptoms of PTSD. Adjusting for baseline and in-hospital covariates, multivariable regression examined the association of exposure to delirium motoric subtype and long-term outcomes.
Measurements and Main Results:
In our cohort of 556 adults with a median age of 62y, hypoactive delirium was more common than hyperactive (68.9% vs. 16.8%). Dependence in ADLs was present in 37% at 3 months and 31% at 12 months, while dependence in IADLs was present in 63% at 3 months and 56% at 12 months. At both time points, depression and PTSD rates were constant at 36% and 5%, respectively. Each additional day of hypoactive delirium was associated with higher IADL dependence at 3 months only [0.24 points (95% CI: 0.07, 0.41, p=0.006)]. There were no associations between motoric delirium subtype and ADL dependence, depression, or PTSD.
Conclusions:
Longer duration of hypoactive delirium, but not hyperactive, was associated with a minimal increase in early IADL dependence scores in adult survivors of critical illness. Motoric delirium subtype was neither associated with early or late ADL functional dependence or mental health outcomes, nor late IADL functional dependence.
Keywords: Critical care, delirium, limitation of activity, quality of life, depression, post-traumatic stress disorder
INTRODUCTION
Each year, millions of patients survive hospitalization for critical illness; many are left with new disability and prolonged recovery of Post-Intensive Care Syndrome (PICS).(1) Two pillars of PICS are functional dependence, which may impair patients’ abilities to live and function independently, and mental health impairments including depression and post-traumatic stress disorder (PTSD).(2–4) Delirium is a state of acute brain dysfunction that has been associated with worse functional outcomes across multiple cohorts(5–8) and with more depression and lower mental health quality of life at 12 months after discharge.(4)
Delirium may be classified by psychomotor presentation(9–11) as hyperactive (characterized by agitation, restlessness, or combativeness) or hypoactive (characterized by lethargy, somnolence, and reduced awareness). These subtypes are suggested to result from different pathophysiologic mechanisms, and evolving data support a difference in outcomes between the subtypes. Hypoactive delirium is more common than hyperactive(12, 13) and associated with worse short-term outcomes including longer mechanical ventilation and ICU length of stay, increased mortality, and development of pressure ulcers.(14–18)
Hypoactive delirium has been described as an “acute apathy syndrome” with elements of diminished action, will, and communication that shares similarities to major depressive disorder.(19) This state may lead to less mobility during critical illness, less participation in activity and rehabilitation, and may prime patients for depressed mental health during recovery. We, therefore, hypothesized that hypoactive delirium, but not hyperactive delirium, in the ICU would be associated with worse long-term functional dependence, depression, and PTSD in adult survivors of critical illness. We performed a post-hoc analysis of a prospective cohort study of civilian and veteran adults with critical illness that assessed delirium in the ICU and physical and mental health outcomes at 3 and 12 months after hospital discharge.
METHODS AND MATERIALS
Population and Setting
We performed a post-hoc analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) (NCT00392795) and Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU) (NCT00400062) studies. We conducted these prospective cohort parallel studies with identical protocols at Vanderbilt University Medical Center and Saint Thomas Hospital in Nashville, TN, USA (BRAIN-ICU) and the Tennessee Valley Healthcare System Nashville VA Medical Center (Nashville, TN, USA), George E. Wahlen Department of VA Medical Center in VA Salt Lake City Health Care System (Salt Lake City, UT, USA) and Seattle Division of the VA Puget Sound Health Care System (Seattle, WA, USA) (MIND-ICU). We enrolled patients from 2007 to 2010, and results have been published previously,(4, 20, 21) though the hypotheses and analyses presented in this manuscript are original.
We included adult medical and surgical ICU patients ≥18 years old with respiratory failure or shock, unless they met prespecified exclusion criteria. These criteria included >72 hours of organ failure, life expectancy <24 hours, and chronic critical illness including ICU admission lasting >5 days in the previous month or mechanical ventilation in the previous 2 months. We also excluded patients who were unable to participate in assessments owing to deafness or inability to speak English, those at high risk for preexisting cognitive deficits in the setting of severe dementia, neurodegenerative disease, concern for anoxic brain injury, or cardiac surgery within 3 months, and those for whom informed consent could not be reliably obtained or who would not be able to reliably attend follow-up visits. For this analysis, we included patients form the BRAIN-ICU and MIND-ICU studies who survived hospitalization and completed at least one follow-up evaluation at 3 or 12 months after discharge.
We obtained informed consent for all participants, primarily from authorized surrogates as many participants did not have capacity to consent. When consent was obtained from a surrogate, we re-consented patients once they regained decision-making capacity. Each center’s institutional review board approved the study protocol.
Procedure and Outcomes
Trained research nurses evaluated patients twice each day while in the ICU and once daily on the wards for up to 30 days of hospitalization. Assessments included level of consciousness using the Richmond Agitation and Sedation Scale (RASS)(22) and delirium evaluation using the Confusion Assessment Method for the ICU (CAM-ICU).(23) A RASS score −4 to −5 was designated coma and those patients were unable to be assessed for delirium. We determined motoric subtype of delirium using previously published criteria where hypoactive delirium was defined as a positive CAM-ICU with a corresponding RASS −3 to 0, and hyperactive delirium defined as a positive CAM-ICU with a corresponding RASS +1 to +4. Participants may qualify as having both a day with hypoactive and a day with hyperactive delirium if the above criteria were met in different assessments on the same day. While the presence of both hypoactive and hyperactive delirium in the same day has been classified as “mixed” delirium, we chose to allow participants to qualify as both hypoactive and hyperactive in the same day in order to collect the most granular data on presence and duration of delirium subtype, as pure hyperactive delirium is rare. Patients who were experiencing delirium on the day of ICU discharge were categorized as ICU or non-ICU delirium based on their location at the time of the assessment. We defined duration of delirium as the total number of days with each subtype of delirium during the hospitalization or up to 30 days from enrollment. For patients discharged from the hospital with ongoing delirium, any days after discharge were considered days without delirium, regardless of delirium status at the time of discharge. Participants that never developed delirium during the study were assigned a delirium duration of 0.
Trained neuropsychology professionals blinded to hospital course data and mental status assessments evaluated participants at 3 and 12 months after discharge for functional dependence, depression, and PTSD using a battery including the Katz Activities of Daily Living (ADL) questionnaire,(24) Pfeffer Functional Activities Questionnaire(25) for Instrumental Activities of Daily Living (IADLs), the Beck Depression Inventory-II (BDI-II),(26) and the Post Traumatic Stress Checklist – Specific Version (PCL-S).(27) ADLs include the basic functions necessary for self-care such as eating, bathing, dressing, and toileting, and participants score 1 point for dependence in any of the 6 categories. A score >0 is consistent with functional dependence and at least partial disability. The Pfeffer Questionnaire for IADLs assesses performance of 10 tasks needed for independent living including managing medications, arranging for transportation, managing finances, and shopping for home necessities. Tasks are scored 0 for “complete independence”, 1 for “difficulty but can complete without assistance,” 2 for “difficulty requiring assistance,” and 3 for “complete dependence” for a total score ranging from 0 – 30. Any score >1 indicates functional dependence, whereas a score >8 indicates disability. The BDI-II is comprised of 21 questions scored 0–3 addressing a range of cognitive and somatic symptoms of depression. Increasing scores correlate to an increased severity of depression, where 0–13 is minimal, 14–19 is mild depression, 20–28 is moderate depression, and 29–63 is severe depression. Finally, the PCL-S is a 17-question survey, scored 1–5 for each question, that is based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition(28) criteria for PTSD where higher scores indicate a higher likelihood of PTSD and 17–29 is little or no risk of PTSD, 28–29 is mild severity of PTSD, 30–44 is moderate severity of PTSD, and 45–85 is a high severity of PTSD.
Covariates
We collected baseline demographic data at enrollment and hospital data from admission until death or 30 days after enrollment. We selected all covariates a priori with consideration of their possible confounding effects on the association between motoric subtype of delirium and long-term functional and neuropsychiatric outcomes. We included the following baseline covariates: age, gender, race, education, Charlson comorbidity index,(29) preexisting cognitive impairment identified by the Short Form Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF),(30) pre-admission ADLs and IADLs, hospital site, and history of depression. We included the following covariates collected during the hospitalization: days of severe sepsis, days of coma, days on mechanical ventilation, and mean modified daily sequential organ failure (SOFA) score(31) excluding the neurological component as mental status was accounted for separately. We also collected daily exposure to medications that may impact motoric presentation of delirium including benzodiazepines (midazolam equivalents), propofol, dexmedetomidine, opioids (fentanyl equivalents), and haloperidol reported as mean 24-hour dose.
Statistical Analysis
We summarized patient demographics and clinical characteristics using the median (interquartile range) for continuous variables, and percentages for categorical variables. We used proportional odds and linear modeling, fitted as appropriate, to study the association of total number of days with hypoactive delirium and total number of days with hyperactive delirium during hospitalization with functional dependence (ADL and IADL scores), depression (BDI-II score), and PTSD (PCL-S score) with separate models for 3- and 12-month assessments, adjusting for the aforementioned covariates. Continuous variables were not restricted to a linear relationship and were modeled using restricted cubic splines with knots at the 10th, 50th, and 90th percentiles. We examined the potential interaction between hypoactive delirium days and hyperactive delirium days to evaluate if fluctuation between subtypes affected outcomes; however, this was removed from the model due to lack of evidence of an interaction (Appendix, Supplemental Table - ST 1).
When handling missing data, we used single imputation to impute an overall status (normal, hypoactive, hyperactive, or comatose) based on the status of the days immediately prior to and after the missing day on which a patient had no mental status assessment. For participants with missing data of other covariates, we used the multiple imputation method where 10 “complete” datasets were generated, and model parameters were estimated using each “complete” dataset, and then were combined into the final model parameter estimates using the Hmis(32) and rms(33) packages in R. A summary of missing outcomes data is reported in Supplemental Table 2.
We performed all analyses using R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). We used a significance level of 0.05 for statistical inference.
RESULTS
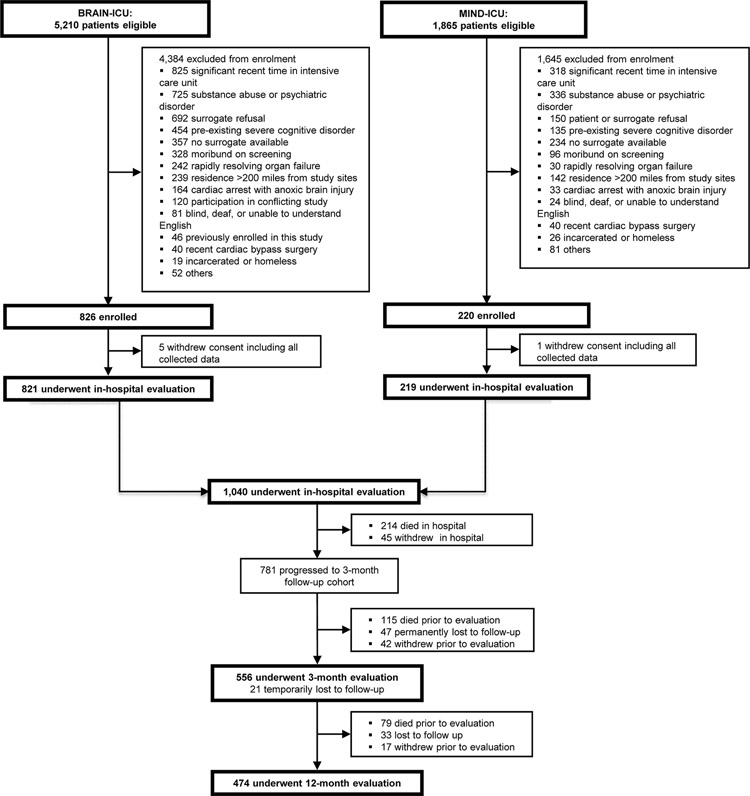
We included 556 adult survivors of critical illness who underwent assessment at 3 months and 474 who underwent assessment at 12 months. A flowchart of participants is displayed in Figure 1; data regarding screened and excluded patients for this cohort were previously reported.(4, 20, 21) In this cohort, the median age was 62 (52–71) and 60% were male. Patients demonstrated a moderate level of baseline functional impairment with 28% reporting some dependence in Katz ADL (ADL Score >0) and 31% reporting some dependence in IADL (FAQ Score >1) at enrollment. Per patient or proxy report, 37% of patients had a history of depression and 6% had a history of PTSD prior to admission. Remaining baseline data is in Table 1 and demonstrate a cohort with high severity of illness. We observed small, but potentially meaningful, differences in baseline characteristics between participants with and without complete outcome data. Those that did not complete all four evaluations (ADL, FAQ, BDI-II, and PCL-S) were older with a higher comorbidity burden and had higher baseline dependence in ADLs. They also had more days of severe sepsis in the ICU. A complete comparison of baseline data is included in Supplemental Table 3.
Figure 1.
Enrollment and Follow Up Flow Chart
Table 1:
Patient Characteristics
| Characteristic | Patients (N=556)a |
|---|---|
| Age (years) | 62 (52–71) |
|
| |
| Sex (%) | |
| Male | 60 |
| Female | 40 |
|
| |
| Race (%) | |
| Caucasian | 90 |
| African American | 10 |
|
| |
| Education (years) | 12 (12–14) |
|
| |
| Charlson score | 2 (1–4) |
|
| |
| Katz ADL at enrollmentb | 0 (0–1) |
| Score = 0 | 72% |
| Score > 0 | 28% |
|
| |
| FAQ Score (IADL) at enrollmentc | 0 (0–2) |
| FAQ 0–1 | 69% |
| FAQ > 1 | 31% |
|
| |
| History of depression (%) | 37% |
|
| |
| History of PTSD (%) | 6% |
|
| |
| IQCODE-SF at enrollment | 3 (3.0–3.19) |
|
| |
| Employment at Enrollment | 138 (25%) |
| • Full Time | 120 (22%) |
| • Part Time | 18 (3%) |
| • Unemployed or Retired | 418 (75%) |
|
| |
| AHRQ Socioeconomic Score at Enrollment | 49.8 (47.3–52.8) |
|
| |
| Mean Modified SOFA in the ICU | 4.7 (3.5–6.5) |
|
| |
| Length of mechanical ventilation (days) | 2.2 (0.9–5.8) |
|
| |
| Days severely septic in the ICU | 2 (0–5) |
|
| |
| ICU length of stay | 5 (3–10) |
|
| |
| Hospital length of stay | 10 (6–17) |
|
| |
| Mean 24hr dose of benzodiazepines in the ICUd | 0.62 (0–6.63) |
|
| |
| Mean 24hr dose of opiates in the ICUe | 268.3 (1.1–944.6) |
|
| |
| Mean 24hr dose of propofol in the ICU | 0 (0–578) |
|
| |
| Mean 24hr dose of haloperidol in the ICU | 0 (0–0) |
|
| |
| Mean 24hr dose of dexmedetomidine in the ICU | 0 (0–0) |
|
| |
| Delirium | 394 (71%) |
| Hypoactivef delirium prevalence | 390 (69%) |
| Days with hypoactive delirium among those exposed | 3 (2–7) |
| Hyperactiveg delirium prevalence | 95 (17%) |
| Days with hyperactive delirium among those exposed | 1 (1–3) |
| Both hypoactive and hyperactive delirium in the same day (prevalence) | 52 (9%) |
| Days with both types of delirium among those exposed | 1 (1–2) |
Abbreviations: ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; ICU, intensive care unit; IQCODE-SF, Informant Questionnaire on Cognitive Decline in the Elderly Short Form; PTSD, post-traumatic stress disorder; SOFA, Sequential Organ Failure Assessment.
Median (25th – 75th percentile) unless otherwise noted
The Katz ADLs are scored on a scale of 0 to 6. Patients are assigned a score of 1 for each of 6 total activities (e.g., bathing, dressing, feeding) that they require assistance to complete. A score >0 indicates partial disability in ADLs.
The FAQ assessment of IADLs surveys patients on their ability to complete 10 tasks that support independent living (e.g., managing money, arranging transportation). Each activity is assigned a scaled score of 0–2 where 0 indicates participants can complete the task independently, 1 indicates that the participant requires some help to complete the task, and 2 indicates complete dependence on someone else to complete the task. All scores are totaled together and a score >2 indicates dependence in IADLs, whereas a score >8 indicates disability in IADLs.
Measured in midazolam equivalents
Measured in fentanyl equivalents
Hypoactive delirium is defined as a positive CAM-ICU assessment with a corresponding RASS score of −1 to −3.
Hyperactive delirium is defined as a positive CAM-ICU assessment with a corresponding RASS score >0.
In our cohort, 68.9% of patients developed hypoactive delirium that persisted for a median (interquartile range) of 3 (2–7) days. Hyperactive delirium was observed in 16.8% of the cohort lasting for a median 1 (1–3) day. Additional details of delirium subtype and hospital location (i.e., ICU, floor) are reported in Supplemental Tables 4 and 5. At long-term follow up, 37% of participants reported some dependence in ADLs at the 3-month assessment, which decreased only slightly to 31% at the 12-month assessment (Table 2). Some dependence in IADLs was found in 63% and 56% of participants at 3 and 12 months, respectively, with 26% meeting criteria for disability at 3 months and 24% at 12 months. Rates of depression and PTSD remained consistent at both 3 and 12 months, with 36% of participants scoring for moderate to severe depression and 5% demonstrating PTSD (Table 2).
Table 2:
Outcomes
| 3 Month Outcomes | |
|
| |
| Activities of Daily Living1 | |
| ▪ Median Katz Total Score | 0 (0–1.2) |
| ▪ No Dependence (ADL = 0) | 63% |
| ▪ Some Dependence (ADL >0) | 37% |
|
| |
| Instrumental Activities of Daily Living2 | |
| ▪ Median FAQ Total Score | 3 (0–9) |
| ▪ No Dependence (FAQ = 0–1) | 37% |
| ▪ Some Dependence (FAQ >1) | 63% |
| ▪ Disability (FAQ >8) | 26% |
|
| |
| Depression3 | |
| ▪ Median BDI-II Total Score | 10 (5–17) |
| ▪ Little or no depression | 64% |
| ▪ Mild depression | 15% |
| ▪ Moderate depression | 11% |
| ▪ Severe depression | 10% |
|
| |
| Post-Traumatic Stress Disorder4 | |
| ▪ Median PCL-S Total Score | 23 (19–30) |
| ▪ No PTSD | 95% |
| ▪ Probable PTSD | 5% |
|
| |
| 12 Month Outcomes | |
|
| |
| Activities of Daily Living | |
| ▪ Median Katz Total Score | 0 (0–1) |
| ▪ No Dependence (ADL = 0) | 69% |
| ▪ Some Dependence (ADL >0) | 31% |
|
| |
| Instrumental Activities of Daily Living | |
| ▪ Median FAQ Total Score | 2 (0–8) |
| ▪ No Dependence (FAQ = 0–1) | 64% |
| ▪ Some Dependence (FAQ > 1) | 56% |
| ▪ Disability (FAQ >8) | 24% |
|
| |
| Depression | |
| ▪ Median BDI-II Total Score | 10 (4–17) |
| ▪ Little or no depression | 67% |
| ▪ Mild depression | 12% |
| ▪ Moderate depression | 14% |
| ▪ Severe depression | 8% |
|
| |
| Post-Traumatic Stress Disorder | |
| ▪ Median PCL-S Total Score | 22 (19–29) |
| ▪ No PTSD Probable PTSD | 95% 5% |
The Katz ADLs are scored on a scale of 0 to 6. Patients are assigned a score of 1 for each of 6 total activities (e.g., bathing, dressing, feeding) that they require assistance to complete. A score >0 indicates partial disability in ADLs.
The FAQ assessment of IADLs surveys patients on their ability to complete 10 tasks that support independent living (e.g., managing money, arranging transportation). Each activity is assigned a scaled score of 0–2 where 0 indicates participants can complete the task independently, 1 indicates that the participant requires some help to complete the task, and 2 indicates complete dependence on someone else to complete the task. All scores are totaled together and a score >2 indicates dependence in IADLs, whereas a score >8 indicates disability in IADLs.
The BDI-II surveys participants on 21 questions related to symptomatology of depression. Each item is rated on a severity scale of 0–3 where 0 indicates no presence of the symptom and 3 indicates the most extreme form of each symptom. The scores are totaled and classified as follows: 0–13 is minimal, 14–19 is mild depression, 20–28 is moderate depression, and 29–63 is severe depression.
The PCL-S is a 17-item self-report measure reflecting DSM-IV symptoms of PTSD. Each item/symptom is rated from 1–5 based on increasing severity where 1 indicates no presence of the symptom at all and 5 indicates extreme severity of the symptom. Scores are totaled together ranging from 17–85 and are classified as follows: 17–29 is little or no risk of PTSD, 28–29 is mild severity of PTSD, 30–44 is moderate severity of PTSD, and 45–85 is a high severity of PTSD.
In multivariable analyses, hypoactive delirium was associated with more dependence in IADLs at 3 months (p = 0.006). After adjusting for potential baseline and in-hospital confounders, the IADL score at 3 months increased by 0.24 points (95% CI: 0.07, 0.41) with each additional day with hypoactive delirium (Table 3). Thus, on average, a patient with 8 days with hypoactive delirium would be expected to have one new dependence in IADLs compared to a patient without hypoactive delirium. This association was not modified by the presence of hyperactive delirium. Hypoactive delirium, however, was not significantly associated with dependence in IADLs at 12 months (p=0.24) or with dependence in ADLs at 3 months (p=0.07) or 12 months (p=0.08). Hyperactive delirium was not significantly associated with IADL dependence at 3 months (p=0.49) or 12 months (P=0.72) or with ADL dependence at 3 months (p=0.44) or 12 months (p=0.25).
Table 3:
Motoric Subtypes vs. Functional Outcomes
| 3-month follow up (N=556) | ADL Odds Ratio (95% CI) | IADL-FAQ Difference (95% CI) |
| Hypoactive Delirium | 1.05 (1.00, 1.12) | 0.24 (0.07, 0.41) |
| Hyperactive Delirium | 0.94 (0.80, 1.10) | 0.17 (−0.31, 0.66) |
| 12-month follow up (N=474) | ADL Odds Ratio (95% CI) | IADL-FAQ Difference (95% CI) |
| Hypoactive Delirium | 1.06 (1.00, 1.12) | 0.11 (−0.07, 0.29) |
| Hyperactive Delirium | 0.89 (0.73, 1.08) | −0.09 (−0.58, 0.40) |
After adjusting for potential baseline and in-hospital confounders including age, gender, race, education, comorbidities, baseline cognitive and functional impairment, history of depression, severity of illness, and days of coma and mechanical ventilation, hypoactive delirium was associated with worse disability in IADLs at 3 months with a 0.24 point increase in FAQ score for each additional day of hypoactive delirium, or 1 additional disability for every 4 days of hypoactive delirium. We did not find a significant association between hypoactive or hyperactive delirium and the odds of developing a new disability in ADLs at 3 or 12 months, nor did we find an association between IADLs and hypoactive delirium at 12 months or hyperactive delirium at 3 or 12 months.
Regarding depression and PTSD, we did not find any significant associations with hypoactive or hyperactive delirium (Table 4). Hypoactive delirium was not independently associated with depression score at 3 months (p=0.43) or 12 months (P=0.83) or with PTSD score at 3 months (p=0.36) or 12 months (p=0.23). Likewise, hyperactive delirium was not independently associated with depression score at 3 months (p=0.11) or 12 months (P=.052) or with PTSD score at 3 months (p=0.052) or 12 months (p=0.10).
Table 4:
Motoric Subtypes vs. Mental Health Outcomes
| 3-month follow up (N=556) | BDI-II Difference (95% CI) | PCL-S Difference (95% CI) |
| Hypoactive Delirium | 0.11 (−0.16. 0.38) | −0.13 (−0.42, 0.15) |
| Hyperactive Delirium | 0.59 (−0.14, 1.32) | 0.78 (0.00, 1.55) |
| 12-month follow up (N=474) | BDI-II Difference (95% CI) | PCL-S Difference (95% CI) |
| Hypoactive Delirium | 0.03 (−0.27, 0.34) | 0.18 (−0.11, 0.47) |
| Hyperactive Delirium | 0.28 (−0.57, 1.12) | 0.67 (−0.13, 1.46) |
After adjusting for potential baseline and in-hospital confounders including age, gender, race, education, comorbidities, baseline cognitive and functional impairment, history of depression, severity of illness, and days of coma and mechanical ventilation, we did not find any significant association between days of hypoactive or hyperactive delirium and symptoms of depression or PTSD at 3 or 12 months after discharge from hospitalization for critical illness.
DISCUSSION
In this analysis of a large multicenter prospective study, we found that an increasing duration of hypoactive delirium during critical illness is independently associated with a minimal increase in IADL dependence at 3 months after discharge after controlling for potential baseline and hospital confounders including baseline functional impairment, severity of illness, and duration of mechanical ventilation. This finding, though statistically significant, is not likely clinically significant as each additional day with hypoactive delirium was associated with 0.24 points on the FAQ scale, which translates to 1 new functional dependence for every 8 days with hypoactive delirium or 1 new complete disability for every 12 days with hypoactive delirium. We did not find a significant association between hypoactive delirium and late IADL dependence, early or late ADL dependence, depression or PTSD, nor did we find evidence that hyperactive delirium is associated with functional dependence, depression, or PTSD after critical illness.
Our study provided a novel investigation in examining the association between hypoactive delirium and long-term functional and mental health recovery in survivors of critical illness. Unlike previous studies of short-term outcomes, however, we did not find a poorer prognosis associated with hypoactive delirium. Multiple studies have demonstrated an increased risk of mortality for patients with hypoactive delirium, (12, 14, 34–36) as well as longer durations and more severe symptoms of delirium, longer length of hospital stay,(37) and longer duration of mechanical ventilation.(38) The mechanistic differences between hypoactive and hyperactive delirium and their resulting long-term prognoses remain unknown. Some hypothesize that only individuals with adequate fitness and reserve during illness could manifest the physical symptoms of hyperactive delirium.(10) In concordance with this theory, hypoactive delirium is more common with increasing age and higher levels of frailty.(14) Schieveld and Strick postulate that hypoactive delirium is more aptly named “Acute Apathy Syndrome” and differs from its counterpart hyperactive delirium in both mechanism and treatment strategy.(19) In this depressed or apathetic neurocognitive state, patients with hypoactive delirium may be less likely or less able to participate in activities shown to reduce the burden of disability such as early mobility and physical therapy.
Newly acquired functional impairment after critical illness is highly prevalent and impacts a substantial number of survivors for months to years after discharge.(3, 4, 39) In previous studies, delirium has been associated with long-term disability in postoperative patients requiring an ICU after major surgery,(5) and the risk of developing functional disability increased with increasing duration of delirium.(40) Another major risk factor for developing functional impairment is immobility,(41) a prominent feature of hypoactive delirium. We predicted that the features of hypoactive delirium that contribute to immobility would lead to worse functional outcomes; however, our data did not support this conclusion. It is possible that because hypoactive delirium is associated with higher mortality, those patients do not survive to develop new functional dependence. It remains unclear whether preventing or shortening delirium can lead to improved functional outcomes, but early mobility programs have been shown to improve both delirium and functional recovery after critical illness, including preserved muscle fiber cross-sectional area, enhanced recovery of muscle force and functional exercise capacity at discharge, and improved functional mobility.(42–44)
A striking number of critical illness survivors will also experience long-term mental health disorders including anxiety, depression, and PTSD. Multiple studies have demonstrated depression in 35–40% of critical illness survivors up to 1-year after discharge.(4, 45, 46) PTSD is less common than depression with prevalence cited at 7–20% in the year following critical illness; however, up to half of patients will experience PTSD symptoms of hyperarousal and avoidance without meeting full criteria for diagnosis.(4, 47, 48) Risk factors for developing psychological conditions after discharge include pre-existing diagnosis of depression and PTSD, psychiatric symptoms during ICU stay, and memories of delusions or frightening experiences during the critical illness; ICU length of stay, severity of illness, and delirium have not been associated with long-term mental health outcomes.(4, 47–49) Delirium, however, has not previously been associated with developing depression or PTSD(4) and, similarly, we did not find an association between hypoactive or hyperactive delirium and mental health outcomes. Methods to reduce depression and PTSD after critical illness currently include ICU diaries during hospitalization(50) and coordinated post-ICU clinics.(51) Whether additional ICU management strategies can impact these outcomes remains to be seen.
This study has notable strengths and limitations to consider. Despite the low prevalence of hyperactive delirium, we classified motoric subtype per assessment as opposed to labeling a day with both hypoactive and hyperactive delirium as mixed. We were, therefore, able to analyze more episodes of hyperactive delirium and better assess their effects than in studies that used the mixed delirium classification. We have included a large cohort with a wide range of diagnoses that were treated in a community hospital, a major academic center, and 3 different Veterans Affairs hospitals which allow for increased generalizability of our results. We were able to control for a significant number of confounders given the large sample size including age, comorbidity burden, functional impairment at enrollment, pre-existing depression and PTSD, severity of illness, and sedative medications received. In collecting the prospective data, trained research personnel completed delirium assessments twice daily. However, as data was not collected by the bedside care team, we may not have captured all episodes of delirium, and hyperactive delirium episodes, for example, may have been treated with sedating medications. Owing to the low prevalence of hyperactive delirium, we had decreased power to detect an association between hyperactive delirium and our outcomes. Further, given our interest in long-term outcomes, there is a potential for survivor bias as only those surviving to follow-up evaluation were included in the analysis. In addition, we were unable to perform functional status and mental health assessments prior to hospitalization and relied on patient, or more often proxy, report of baseline status. In this study, we did not measure institutional differences in delirium care, such as the ABCDEF bundle, but our centers have demonstrated high (>90%) compliance in recent studies.(52) Finally, this is an observational study and we are, therefore, unable to determine causation.
In conclusion, our study found a statistically significant association between increasing duration of hypoactive delirium and increased dependence in IADLs at 3 months; however, this is not likely clinically significant. We did not find evidence that hyperactive delirium was associated with functional dependence after critical illness in our cohort, and it did not modify the association between hypoactive delirium and functional dependence. Neither hypoactive delirium nor hyperactive delirium were associated with ADL dependence, depression or PTSD after discharge. Further work is needed to identify potential mechanisms that drive the different delirium subtype presentations, understand the mechanism of acquired disability and poor mental health after critical illness, and develop interventions to improve recovery after critical illness.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge our research coordinators Leanne Boehm, PhD, RN, ACNS-BC; Brenda Truman Pun, RN, MSN; and Cayce Strength, RSN, BSN who performed project management, data acquisition, and data management. We acknowledge our neuropsychology staff Amy Kiehl, MA, LPC-MHSP who performed neuropsychological outcome testing.
This project was supported by the National Institute of Health (AG027472) and a Merit Review Grant from the Department of Veterans Affairs (Washington D.C., USA). In addition, Dr. Boncyk received support from the National Institute of Health (5T32GM108554); Dr. Patel received support from the National Institutes of Health (AG058639, HL111111, GM120484) and the Vanderbilt Faculty Research Scholars Program; Dr. Brummel received support from the National Institutes of Health TR00046 and AG040549 and by the Vanderbilt Clinical and Translational Scholars Program; Dr. Ely received support from the National Institutes of Health (AG058639, AG027472, AG035117, HL111111, GM120484), the VA Clinical Science Research and Development Service, and the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center; Dr. Pandharipande received support from the National Institutes of Health (AG027472, HL111111, GM120484); Dr. Hughes received support from American Geriatrics Society Jahnigen Career Development Award and the National Institutes of Health (HL111111, AG045085, GM120484). We used REDCap, a secure online database, supported in part by the National Institutes of Health TR 000445.
Copyright Form Disclosure: Dr. Rengel’s institution received funding from the National Institute of Health (NIH) and the Department of Veterans Affairs. Drs. Jackson, Boncyk, Patel, Brummel, and Ely received support for article research from the NIH. Dr. Patel’s institution received funding from NIH R01 GM120484, NIH R01 AG058639, and VA Merit, RRD, ORD, and he received funding from NIH Center for Scientific Review, OLOL Trauma Symposium, Haemonetics, and from the Eastern Association for the Surgery of Trauma, Board of Directors. Dr. Brummel’s institution received funding from NIH, and he received funding from Merck, Inc. Dr. Ely received funding from Pfizer, Masimo, Kohler, and NIH/VA. Dr. Pandharipande received a research grant from Pfizer (previously from Hospira) in collaboration with NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Needham DM, Davidson J, Cohen H, Hopkins RO, et al. : Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509 [DOI] [PubMed] [Google Scholar]
- 2.Ehlenbach WJ, Larson EB, Curtis JR, Hough CL: Physical Function and Disability After Acute Care and Critical Illness Hospitalizations in a Prospective Cohort of Older Adults. J Am Geriatr Soc 2015; 63(10):2061–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, et al. : Functional trajectories among older persons before and after critical illness. JAMA Intern Med 2015; 175(4):523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, et al. : Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abelha FJ, Luis C, Veiga D, Parente D, et al. : Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care 2013; 17(5):R257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, Bogardus ST Jr., Charpentier PA, Leo-Summers L, et al. : A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340(9):669–676 [DOI] [PubMed] [Google Scholar]
- 7.Noriega FJ, Vidan MT, Sanchez E, Diaz A, et al. : Incidence and impact of delirium on clinical and functional outcomes in older patients hospitalized for acute cardiac diseases. Am Heart J 2015; 170(5):938–944 [DOI] [PubMed] [Google Scholar]
- 8.Rudolph JL, Inouye SK, Jones RN, Yang FM, et al. : Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010; 58(4):643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipowski ZJ: Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry 1983; 140(11):1426–1436 [DOI] [PubMed] [Google Scholar]
- 10.Liptzin B, Levkoff SE: An empirical study of delirium subtypes. Br J Psychiatry 1992; 161:843–845 [DOI] [PubMed] [Google Scholar]
- 11.Girard TD, Thompson JL, Pandharipande PP, Brummel NE, et al. : Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 2018; 6(3):213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meagher DJ, Leonard M, Donnelly S, Conroy M, et al. : A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res 2011; 71(6):395–403 [DOI] [PubMed] [Google Scholar]
- 13.Pandharipande P, Cotton BA, Shintani A, Thompson J, et al. : Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med 2007; 33(10):1726–1731 [DOI] [PubMed] [Google Scholar]
- 14.Avelino-Silva TJ, Campora F, Curiati JAE, Jacob-Filho W: Prognostic effects of delirium motor subtypes in hospitalized older adults: A prospective cohort study. PLoS One 2018; 13(1):e0191092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Kim SW, Kim JM, Shin IS, et al. : Differential Associations Between Delirium and Mortality According to Delirium Subtype and Age: A Prospective Cohort Study. Psychosom Med 2015; 77(8):903–910 [DOI] [PubMed] [Google Scholar]
- 16.Stransky M, Schmidt C, Ganslmeier P, Grossmann E, et al. : Hypoactive delirium after cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth 2011; 25(6):968–974 [DOI] [PubMed] [Google Scholar]
- 17.Peterson JF, Pun BT, Dittus RS, Thomason JW, et al. : Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 2006; 54(3):479–484 [DOI] [PubMed] [Google Scholar]
- 18.Robinson TN, Raeburn CD, Tran ZV, Brenner LA, et al. : Motor subtypes of postoperative delirium in older adults. Arch Surg 2011; 146(3):295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19,Schieveld JNM, Strik J: Hypoactive Delirium Is More Appropriately Named as “Acute Apathy Syndrome”. Crit Care Med 2018; 46(10):1561–1562 [DOI] [PubMed] [Google Scholar]
- 20.Hughes CG, Patel MB, Jackson JC, Girard TD, et al. : Surgery and Anesthesia Exposure Is Not a Risk Factor for Cognitive Impairment After Major Noncardiac Surgery and Critical Illness. Ann Surg 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandharipande PP, Girard TD, Jackson JC, Morandi A, et al. : Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, et al. : The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166(10):1338–1344 [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Inouye SK, Bernard GR, Gordon S, et al. : Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286(21):2703–2710 [DOI] [PubMed] [Google Scholar]
- 24.Katz S, Ford AB, Moskowitz RW, Jackson BA, et al. : Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963; 185:914–919 [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, et al. : Measurement of functional activities in older adults in the community. J Gerontol 1982; 37(3):323–329 [DOI] [PubMed] [Google Scholar]
- 26.Beck ATS RA; & Brown G: Manual for the Beck Depression Inventory-II. San Antonio, TX, Psychological Corporation, 1996 [Google Scholar]
- 27.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA: Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996; 34(8):669–673 [DOI] [PubMed] [Google Scholar]
- 28.Association AP: Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC., American Psychiatric Association, 2013 [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 30.Jorm AF: A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24(1):145–153 [DOI] [PubMed] [Google Scholar]
- 31.Ferreira FL, Bota DP, Bross A, Melot C, et al. : Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286(14):1754–1758 [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE DC, et al. : Hmisc: Harrell Miscellaneous. 4.4–0 ed. R Foundation for Statistical Computing, 2019, p^pp [Google Scholar]
- 33.Harrell FE Jr.: rms: Regression Modeling Strategies. 5.1–4 ed. R Foundation for Statistical Computing, 2019, p^pp [Google Scholar]
- 34.Bellelli G, Carnevali L, Corsi M, Morandi A, et al. : The impact of psychomotor subtypes and duration of delirium on 6-month mortality in hip-fractured elderly patients. Int J Geriatr Psychiatry 2018 [DOI] [PubMed] [Google Scholar]
- 35.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER: Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci 2007; 62(2):174–179 [DOI] [PubMed] [Google Scholar]
- 36.Yang FM, Marcantonio ER, Inouye SK, Kiely DK, et al. : Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009; 50(3):248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang HW, Lee M, Shin JW, Jeong HS, et al. : Outcome Differences by Delirium Motor Subtype in Patients with Ischemic Stroke. Psychiatry Investig 2019; 16(11):852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Hu W, Shen M, Ye X, et al. : Profiles of delirium and the clinical outcomes of patients who underwent coronary artery bypass grafting: a prospective study from China. J Clin Nurs 2016; 25(5–6):631–641 [DOI] [PubMed] [Google Scholar]
- 39.Herridge MS, Tansey CM, Matte A, Tomlinson G, et al. : Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304 [DOI] [PubMed] [Google Scholar]
- 40.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, et al. : Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med 2014; 42(2):369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill TM, Allore HG, Gahbauer EA, Murphy TE: Change in disability after hospitalization or restricted activity in older persons. JAMA 2010; 304(17):1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burtin C, Clerckx B, Robbeets C, Ferdinande P, et al. : Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009; 37(9):2499–2505 [DOI] [PubMed] [Google Scholar]
- 43.Hickmann CE, Castanares-Zapatero D, Deldicque L, Van den Bergh P, et al. : Impact of Very Early Physical Therapy During Septic Shock on Skeletal Muscle: A Randomized Controlled Trial. Crit Care Med 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaller SJ, Anstey M, Blobner M, Edrich T, et al. : Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016; 388(10052):1377–1388 [DOI] [PubMed] [Google Scholar]
- 45.Hatch R, Young D, Barber V, Griffiths J, et al. : Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Crit Care 2018; 22(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, et al. : Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Crit Care Med 2016; 44(5):954–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, et al. : Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 2015; 43(5):1121–1129 [DOI] [PubMed] [Google Scholar]
- 48.Patel MB, Jackson JC, Morandi A, Girard TD, et al. : Incidence and Risk Factors for Intensive Care Unit-related Post-traumatic Stress Disorder in Veterans and Civilians. Am J Respir Crit Care Med 2016; 193(12):1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikayin S, Rabiee A, Hashem MD, Huang M, et al. : Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones C, Backman C, Capuzzo M, Egerod I, et al. : Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care 2010; 14(5):R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt K, Worrack S, Von Korff M, Davydow D, et al. : Effect of a Primary Care Management Intervention on Mental Health-Related Quality of Life Among Survivors of Sepsis: A Randomized Clinical Trial. JAMA 2016; 315(24):2703–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girard TD, Exline MC, Carson SS, Hough CL, et al. : Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 2018; 379(26):2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.