Abstract
Substance use and addiction are prominent global health concerns and are associated with abnormalities in reward sensitivity. Reward sensitivity and approach motivation are supported by a fronto-striatal neural circuit including the orbitofrontal cortex (OFC), ventral striatum (VS), and dorsal striatum (DS). Although research highlights abnormalities in reward neural circuitry among individuals with problematic substance use, questions remain about whether such use arises from excessively high, or excessively low, reward sensitivity. This study examined whether reward-related brain function predicted subsequent substance use course. Participants were 79 right-handed individuals (Mage = 21.52, SD = 2.19 years), who completed a Monetary Incentive Delay fMRI task, and follow-up measures assessing substance use frequency and impairment. The average duration of the follow-up period was 9.1 months. Regions-of-interest analyses focused on the reward anticipation phase of the MID. Decreased activation in the VS during reward anticipation predicted increased substance use frequency at follow-up. Decreased DS activation during reward anticipation predicted increased substance use frequency at follow-up, but this finding did not pass correction for multiple comparisons. Analyses adjusted for relevant covariates, including baseline substance use and the presence or absence of a lifetime substance use disorder prior to MRI scanning. Results support the reward hyposensitivity theory, suggesting that decreased reward-related brain function is a risk factor for increased substance use. Results have implications for understanding the pathophysiology of problematic substance use and highlight the importance of the fronto-striatal reward circuit in the development and maintenance of addiction.
Keywords: substance use, reward, monetary incentive delay, fronto-striatal circuit, reward deficiency model
General Scientific Summary
Findings from the current study suggest that decreased reward-related neural activity to secondary rewards confers risk for greater substance use frequency. These findings support the reward hyposensitivity theory of addiction.
Introduction
Substance use disorders (SUDs) are a growing global health concern and have high 12-month prevalence rates in the U.S. [12% for alcohol and 2–3% for drugs; (Merikangas & McClair, 2012)]. Investigating the mechanisms involved in the course and maintenance of problematic substance use is key to prevention and treatment. Reward sensitivity, the level of one’s approach motivation towards goals and rewards, is associated with problematic substance use and the onset and course of SUDs (Alloy et al., 2009; Dawe, Gullo, & Loxton, 2004; Dawe & Loxton, 2004); however, the nature of the relationship between reward sensitivity and excessive substance use is unclear (Nusslock & Alloy, 2017). The current study employs a prospective design to examine what specific profile of reward sensitivity confers risk for greater substance use frequency and impairment.
Reward Neural Circuitry and Substance Use
Processing and responding to rewards are central drivers of human behavior. Reward sensitivity describes the value individuals place on potential rewards, the perceived probability of receiving those rewards, and the mechanisms by which one processes rewards or goal-relevant cues in their environment. The processing of rewards has been connected to a fronto-striatal neural circuit involving the striatum [divided into the ventral striatum (VS), including the nucleus accumbens, and the dorsal striatum (DS), including the caudate nucleus and putamen], along with higher-level structures like the orbitofrontal cortex [OFC; (Haber & Knutson, 2010)]. These brain structures are involved in processing both primary (e.g., sex, food) and secondary (e.g., money) rewards, and are activated when using substances, and implicated in SUDs (Volkow, Koob, & McLellan, 2016; Volkow & Morales, 2015). The striatum, in particular, has been implicated in the transition from recreational substance use to chronic and compulsive drug-seeking behaviors (Yager, Garcia, Wunsch, & Ferguson, 2015). The VS encodes emotional and motivational aspects of rewards (e.g., hedonic drug value), and the DS regulates goal-directed and habitual behaviors [e.g., drug habit (Berke & Hyman, 2000; Pennartz, Ito, Verschure, Battaglia, & Robbins, 2011)]. The OFC is associated with outcome expectancy and its role in problematic substance use reflects a shift from flexible decision-making to compulsive drug-seeking (Moorman, 2018; Schoenbaum & Shaham, 2008). By facilitating anticipatory and consummatory reward processes, this fronto-striatal circuit drives motivation, goal-striving, and approach behavior to reward-relevant cues (Berridge & Robinson, 1998, 2003) and, when dysregulated, is implicated in SUDs. Along with reward processing dysfunction, problematic substance use also is linked to increased impulsivity, impaired behavioral control, particularly during intense positive and negative emotions, and impaired decision-making (Hariri et al., 2006; Zapolski, Cyders, & Smith, 2009). Importantly, the maturation of these neural circuits involved in the development of cognitive control and regulation of behavior are context-dependent on reward and the fronto-striatal circuit (Strang & Pollak, 2014).
Theoretical Models of Reward Sensitivity and Substance Use
Two theoretical models of reward sensitivity guide the literature concerning reward function and problematic substance use; the reward deficiency (hyposensitivity) model and the reward hypersensitivity model. The hyposensitivity model (Blum et al., 2000; Bowirrat & Oscar-Berman, 2005; Volkow, Fowler, & Wang, 2003) posits that addictive drugs activate reward regions by increasing dopamine, but once addicted, drugs trigger smaller dopamine increases in reward-related structures. The reward system becomes desensitized over time to both drug and non-drug related cues as substance use increases. Indeed, studies report that people who have current SUDs, or are prone to return to use after a period of remission, show attenuated responses in the reward circuit during reward-based fMRI tasks (Hyatt et al., 2012; May, Stewart, Migliorini, Tapert, & Paulus, 2013; Stewart et al., 2014; Tanabe et al., 2013). Although this prior research suggests reward hyposensitivity is associated with substance use once addiction has set in, the hyposensitivity model also proposes that low reward-related brain function may be a risk factor for engaging in problematic substance use in the first place. For example, individuals who have low reward-related brain function may attempt to compensate for reduced reward signaling by consuming substances to decrease dysphoria and increase pleasure (Blum et al., 2000; Bowirrat & Oscar-Berman, 2005; Volkow et al., 2003).
Conversely, the reward hypersensitivity model suggests that people with heightened reward function engage in excessive approach behavior towards rewards (Dawe et al., 2004; Dawe & Loxton, 2004). The inability to delay gratification is associated with an increased risk of consuming substances, and hyperactivity in the VS underlies a preference for immediate over delayed rewards (Hariri et al., 2006). Furthermore, drugs stimulate reward regions, which over time become hypersensitized, leading to increased approach motivation towards substances (Baskin-Sommers & Foti, 2015; Di Chiara et al., 2004). Thus, from this perspective, reward circuit hyperresponsivity may underlie a propensity for excessive motivation towards substances (McClure, Laibson, Loewenstein, & Cohen, 2004).
To probe reward network sensitivity, monetary reward tasks assess neural responses to secondary rewards, which are dysregulated in individuals with SUDs (Asensio et al., 2010; Lubman et al., 2009). However, there are differences in reward circuit reactivity to drug-related and monetary rewards. Overall, cue-reactivity research shows that substance-addicted individuals have increased reward-related brain activation to drug-related cues (MacNiven et al., 2018); however, literature involving neural responses to secondary rewards is less conclusive (Chase, Eickhoff, Laird, & Hogarth, 2011). For example, although a recent review of research on SUDs using the monetary incentive delay (MID) task found that substance use typically is associated with blunted VS activity during reward anticipation, discrepancy persists among studies (Balodis & Potenza, 2015). Thus, our understanding of how neural responses to secondary rewards (e.g., money) relates to problematic substance use warrants further examination. Importantly, it is possible for an individual to show heightened neural reward activity to drug cues and lower activity to non-drug cues. That is, once addiction has set in, an individual’s reward circuit may become “hijacked” by substances of abuse such that there are lower neuronal resources available for non-substance related rewards. Thus, reward hypersensitivity to substance-related cues and reward hyposensitivity to non-substance related reward cues may both serve to maintain problematic substance use.
Reward-related neural activity also may be predictive of future substance use. A longitudinal meta-analysis examining relapse found higher activation in reward regions to non-drug cues during reward anticipation predicted better outcomes [e.g., longer time to return to use; (Moeller & Paulus, 2018)]. Other prospective studies showed increased anticipatory activity in the caudate in cocaine-dependent individuals (Jia et al., 2011), and increased VS activity in cannabis users (Nestor, Hester, & Garavan, 2010) during monetary reward anticipation was associated with relapse. Recent work has examined neural markers of first substance use onset through longitudinal MRI studies in adolescents. Increased activation in the nucleus accumbens, a subnucleus of the VS, during reward anticipation on the MID task among adolescents was associated with increased alcohol-related problems 3–6 years later (Heitzeg et al., 2014). Likewise, heightened activation in the nucleus accumbens during reward anticipation predicted early substance use initiation in substance-naïve adolescents (Cope, Martz, Hardee, Zucker, & Heitzeg, 2019). In contrast, Büchel et al. (2017) found decreased VS activation during reward anticipation on the MID at age 14 predicted problematic substance use at age 16. And, using the same large, longitudinal sample, Whelan et al. (2014) found that reduced reward-related brain function during reward anticipation predicted future binge drinking. Thus, questions remain as to whether the profile of reward processing that is most strongly associated with risk for problematic substance use reflects hyper- or hypo-reward sensitivity.
The Current Study
Although the aforementioned studies shed light on the neural mechanisms involved in substance use, imaging studies of prospective substance use are sparse and inconsistent. Furthermore, the profile of risk for increased substance use over time remains unclear, with different studies reporting increased (hypersensitivity) and decreased (hyposensitivity) neural activation to secondary rewards. The current study adds to the few prospective studies examining neural activity during secondary reward processing as a predictor of prospective substance use and impairment and aims to clarify these discrepant findings. Additionally, we examine prospective substance use and impairment during the transition to adulthood, which is a period associated with heightened risk for problematic substance use (Stone, Becker, Huber, & Catalano, 2012). We focus our analyses on reward anticipation because drug expectancy is important in the onset and course of addictive behaviors due to its role in craving and attentional bias towards drug cues (Jędras, Jones, & Field, 2013), and to minimize multiple comparisons. We control for neural activation during the anticipation of a potential loss of reward, in order to examine the predictive influence of reward anticipation on substance use and impairment above and beyond loss. We also control for mood symptoms at time of scan, the duration of the prospective follow-up period, age at time of scan, gender, recruitment (i.e., reward risk) group status, substance use frequency at time of scan, the presence or absence of a lifetime substance use disorder prior to MRI scanning (as a proxy for impairment), and whether or not participants were taking psychotropic medication at time of scan.
Our sample included individuals with and without a lifetime history of SUD who completed the MID and follow-up visits to assess prospective substance use frequency and impairment (the average duration of the follow-up period was 9.1 months). We control for prior history of SUD in all prospective analyses in order to predict changes in substance use during the follow-up period above and beyond prior substance use and diagnoses. We examined whether reward-related neural activation in the VS, DS, and OFC prospectively predicts substance use frequency and impairment. Given research supporting both sides of the debate, we did not make predictions about whether reward hypo- or hypersensitivity underlies the course of substance use. Results from the present study will inform this ongoing debate.
Methods and Measures
Participants
Participants were selected from a larger longitudinal study [Project TEAM; (Alloy et al., 2012)] examining adolescent risk for bipolar disorders. Participants were classified as moderate reward sensitive (MRew) or high reward sensitive (HRew) based on the Behavioral Inhibition System (BIS)/Behavioral Activation System (BAS) Scale (Carver & White, 1994) and the Sensitivity to Punishment (SP)/Sensitivity to Reward (SR) Questionnaire (Torrubia, Ávila, Moltó, & Caseras, 2001). This recruitment approach was relevant to the overall aims of Project TEAM (Alloy et al., 2012). Further details regarding screening and eligibility criteria have been described elsewhere (Alloy et al., 2012). We focus on individual differences in reward-related brain function as predictors of substance use, the variance of which is increased because the larger TEAM recruitment was based on self-reported reward sensitivity.
fMRI data were available for 120 participants. Twenty-four were excluded due to excessive head motion (>3mm) and four due to MID acquisition errors. Of the remaining 92 participants, two were excluded because they were ambidextrous, 10 because they did not complete a follow-up visit, and one for missing data to control for mood symptoms at time of scan. Thus, the final sample consisted of 79 right-handed participants, including 26 MRew and 53 HRew participants (54% identified as female, 56% White, 24% Black, 10% Asian, 6% Bi/Multiracial, 4% Other/Unknown, and Mage at scan = 21.52, SD = 2.19 years). Participants who were excluded were slightly younger (Mage = 20.73) than those included (Mage = 21.52) at the time of scan [t(101.39) = 2.05, p = .04]. However, included and excluded participants did not differ on gender [χ2(1) = .35, p = .56), race [χ2(5) = 3.80, p = .58], reward risk group [χ2(1) = .02, p = .89], BAS total score [t(118) = .97, p = .34], or SR score [t(118) = .56, p = .58]. Furthermore, included and excluded participants did not differ on self-reported measures of substance use frequency at time of scan [t(117) = −.27, p = .79], or on whether they had a history of SUD prior to the scan [χ2(1) = .06, p = .81]. To increase power, we did not exclude participants who had a lifetime history of SUD prior to the MRI scan (16.5%). Of these 13 participants with a lifetime SUD, 10 had alcohol abuse, and 3 had alcohol dependence, based on DSM-IV-TR criteria. The present study was approved by the Temple University Institutional Review Board (IRB #11022). All participants provided written informed consent prior to participation.
Procedures
Following the MRI scan in which we administered the MID task to assess reward-related brain function, participants attended follow-up visits where they completed interviews and questionnaires, including self-report measures of substance use and impairment. For the present study, we used data on substance use frequency and impairment from the first follow-up assessment that occurred at least 30 days after the MRI scan (we required a minimum of 30 days for the follow-up period so that the time window covered by our assessments of substance use and impairment did not overlap with the date of the MRI scan). The follow-up assessment for the present study occurred, on average, 9.1 months after the MRI (SD = 8.6, range = 40.7 months).
Assessment of Substance Use and Substance Use Disorders
The Adolescent Alcohol and Drug Involvement Scale [AADIS; (Moberg, 2003)] is a two-part (drug and alcohol) self-report measure assessing the frequency of use of alcohol and twelve other drugs (marijuana, cocaine, hallucinogens, etc.). The measure was modified for use in the present longitudinal study. Participants rated how frequently they used alcohol and substances in the past 30 days on a 6-point scale (0=Never Used to 6=Several Times a Day). The AADIS was completed both at the MRI visit and at the post-MRI follow-ups. Reliability in our baseline TEAM sample was acceptable (α=.71).
The Short Inventory of Problems [SIP; (Blanchard, Morgenstern, Morgan, Labouvie, & Bux, 2003)] is a 15-item self-report measure assessing problems/impairment associated with alcohol and drug use over the past month (“I have gotten into trouble because of my drinking or drug use”). The original questionnaire includes yes/no responses to these 15 questions; however, we modified our version to utilize a 4-point Likert scale to increase variation across responses (0=never, 1=1–2 times/month, 3=twice a week, 4=daily/almost daily). The SIP was administered at post-MRI follow-ups, but not at the time of the MRI scan. Reliability in our baseline TEAM sample was excellent (α=.91).
Finally, an expanded version of the Schedule for Affective Disorders and Schizophrenia – Lifetime interview [exp-SADS-L (Alloy et al., 2008; Endicott & Spitzer, 1978)] was administered by trained diagnosticians at the time of the MRI visit. We used a yes/no coding scheme (yes = 1, no = 0) to indicate if participants met criteria for a SUD at any point up to, and including the day of, the MRI scan. Of note, no participant was in a SUD episode on the day of the MRI scan. Thus, a code of 1 indicates that a participant had a lifetime SUD at some point before the MRI scan. We use SUD history as a covariate in order to assess whether reward-related brain function predicted substance use course, above and beyond a lifetime history of SUD. Interrater reliability in our lab for the SADS interviews was κ>.80 (Alloy et al., 2008).
Assessment of Mood Symptoms
We controlled for mood symptoms at the MRI scan with two self-report measures: the Beck Depression Inventory [BDI; (Beck, Rush, Shaw, & Emery, 1979)] and the Altman Self Rating Mania Scale [ASRM; (Altman, Hedeker, Peterson, & Davis, 1997)]. The 21-item BDI assesses current depressive symptoms in affective, cognitive, motivational, and somatic domains. The 5-item ASRM assesses current (hypo)mania symptoms (elevated mood, psychomotor agitation, increased self-confidence, decreased need for sleep, and pressured speech). Both measures have good validity, internal and retest reliability (Altman, Hedeker, Peterson, & Davis, 2001; Beck, Steer, & Garbin, 1988). Average BDI score at scan was M=4.9, SD=6.2, and average ASRM score was M=4.0, SD=4.0. Reliability in our TEAM sample for the BDI and ASRM ranged from good to acceptable (α=.87, α=.75, respectively).
Functional MRI Task
Participants completed the MID task [(Samanez-Larkin et al., 2007); Figure 1]. First, a circle cue signaling a reward trial (the participant has the opportunity to Win $0.00, Win $1.50, or Win $5.00) or a square cue indicating a loss trial (the participant might Lose $0.00, Lose $1.50, or Lose $5.00) was presented for 2s. Then, a jittered fixation was presented followed by a solid white square. Participants were instructed to make a button response when the solid white square was still on the screen to either win money (reward trials) or avoid losing money (loss trials). Participants were presented with feedback detailing the amount of money won or lost on each trial for 2s. Finally, a jittered fixation cross was presented for 2s, 4s, or 6s as an intertrial interval. The initial target duration was calculated from each participant’s mean hit reaction time on a MID practice run completed before entering the scanner. The target duration dynamically updated during the MID task to maintain task difficulty so that participants accurately hit the target on 66% of trials, calculated separately for each trial type (i.e., Win $0.00, Win $1.50, Win $5.00, Lose $0.00, Lose $1.50, Lose $5.00). The six trial types each were presented 8 times in random order, totaling 96 trials, across two MID runs.
Figure 1. The (A) trial structure and (B) reward and loss cues of the monetary incentive delay (MID) task used to examine reward and loss anticipation and consumption.
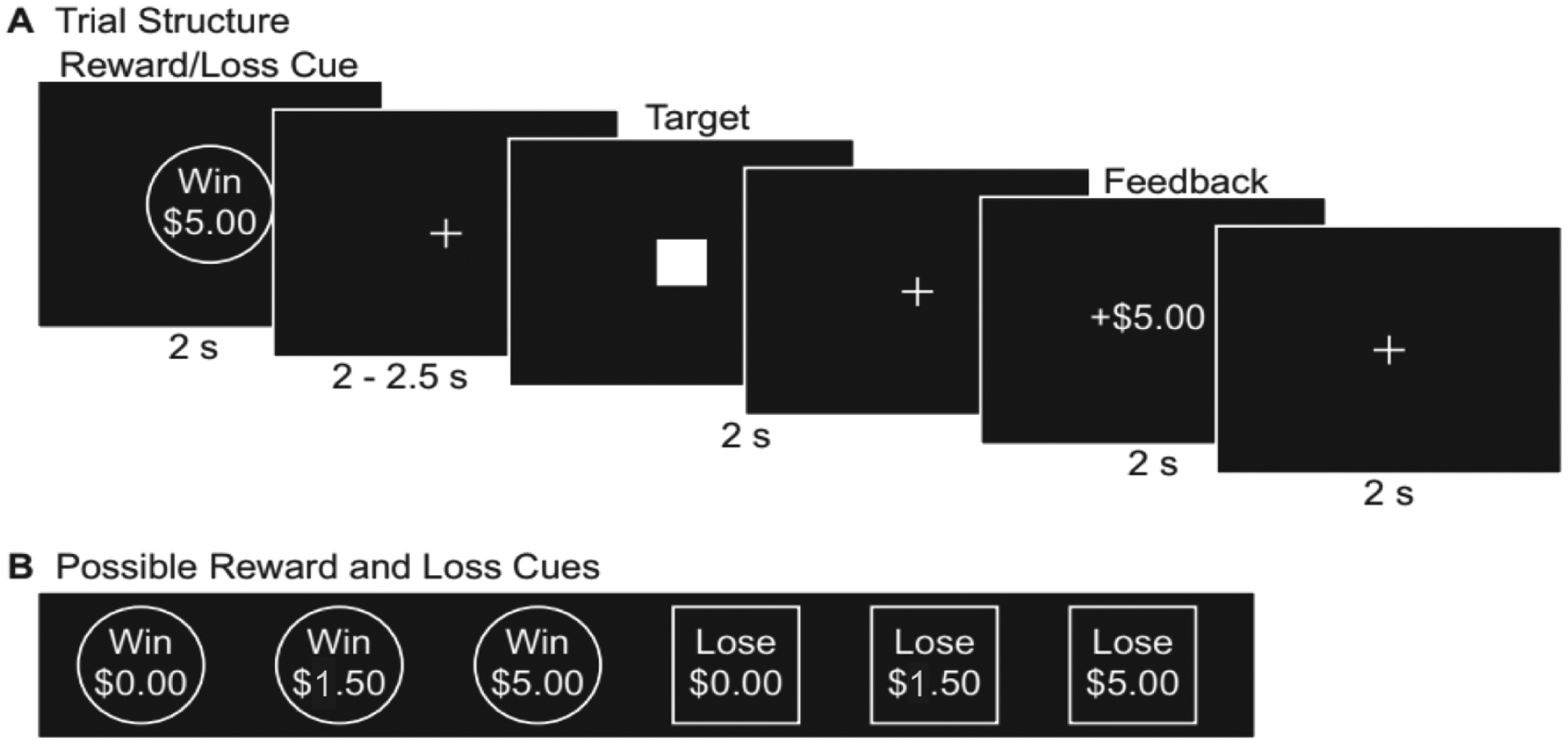
(adapted from: Young & Nusslock, Positive mood enhances reward-related neural activity, Social Cognitive and Affective Neuroscience, 2016, 11(6), 934–44, by permission of Oxford University Press)
Recent research raises some questions about the test-retest reliability of task-based fMRI measures for assessing individual differences (Elliott et al., 2020). However, in comparison to other task-based measures, there is evidence that the MID in particular displays good 15-day (ICCs = 0.52, 0.63 for left and right VS/nucleus acumbens), and 2.5 year (ICCs = 0.43, 0.68 for left and right VS/Nacc) test-retest reliability (Plichta et al., 2012; Wu, Samanez-Larkin, Katovich, & Knutson, 2014). In addition, results from a very small pilot project that we conducted (N = 4) using identical fMRI methods as the present study also indicate that the MID has good to moderate 2-day test-retest reliability for the reward anticipation phase (ICC = .54 for DS, .68 for VS, and .84 for OFC).
fMRI Data Acquisition and Analysis
Neuroimaging data were collected using a 3.0 Tesla Siemens Verio wide-bore MRI scanner with a standard 12-channel head coil at Temple University Medical Center. Structural 3D MPRAGE scans were collected in the sagittal plane using the following parameters: voxel size=0.5×0.5×1.0mm, TR=1600ms, TE=2.46ms, FOV=252, Flip Angle=9°, 176 volumes. Functional BOLD scans were collected using the following parameters: coverage=36 axial slices, 4mm thick (FOV=236mm), matrix=64×64, voxel size=3.7×3.7×4.0mm, TR=2000, TE=25ms, Flip Angle=70°, acquisition volumes=292.
Data were analyzed using a general linear model carried out in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Functional images were realigned and corrected for errors in slice-timing. Images then were spatially normalized to MNI space and smoothed using a 6 mm full width at half maximum (FWHM) Gaussian kernel. Motion correction of functional images in each participant was conducted based on calculations for translational (mm) and rotational (degree) movement.
The hemodynamic signal was deconvolved using a general linear model identifying the six trial types during the MID anticipation and consumption phase. The MID anticipation phase was defined as the period after presentation of the cue indicating the possibility to win or lose money but prior to presentation of the target square (2–2.5s). Six variables of no interest for motion were included. First-level voxel-wise t-statistics were generated for each participant contrasting reward (i.e., Win $1.50, Win $5.00) vs. non-reward (i.e., Win $0.00) trials to calculate reward anticipation and consumption, and loss (i.e., Lose $1.50, Lose $5.00) vs. non-loss (i.e., Lose $0.00) trials to calculate loss anticipation and consumption (Samanez-Larkin et al., 2007). Small and large rewards (i.e. $1.50, $5.00) were combined into one reward vs. non-reward contrast, and similarly, small and large losses were combined into one loss vs. non-loss contrast.
We extracted parameter estimates (beta-weights) from predefined regions-of-interest (ROIs) for reward and loss anticipation and consumption, and exported the averaged parameter value from across the entire ROI into SPSS for analyses. A functionally derived ROI for the bilateral VS was defined as two 8mm spheres based on MNI coordinates (right: x=9, y=9, z=−8; left: x=−9, y=9, z=−8) from a previous meta-analysis (Di Martino et al., 2008). We used anatomically defined ROIs for the bilateral OFC (using the Harvard Oxford Atlas) and bilateral DS (defined as the bilateral caudate and putamen using the Wake Forest Toolbox PickAtlas Talairach template), which are standard anatomical masks used in the literature. We used the Harvard Oxford OFC mask because it maximizes the balance between Type II and Type I error. For example, using multiple OFC ROIs to detect effects in smaller regions would increase risk of Type I error. On the other hand, using a mask that covers the entire OFC would require an especially large effect to observe significant associations, and thus, result in increased risk for Type II error. Although this mask excludes portions of the supramedial OFC, it does cover a relatively large portion of the OFC, while at the same time limiting risk for false negative findings. Finally, prior studies examining substance use and reward-related associations have typically found effects in more lateral portions of the OFC (Baker et al., 2019; Forbes, Rodriguez, Musselman, & Narendran, 2014; Nestor, McCabe, Jones, Clancy, & Garavan, 2018). Thus, the regions that the Harvard Oxford OFC mask covers are relevant to studies of reward and addiction.
Statistical Analyses
In separate analyses, we examined whether neural activation in the VS, DS, and OFC during reward anticipation predicted substance use frequency and substance use abuse during the follow-up period. We conducted multiple comparison correction for these six primary analyses using False Discovery Rate (FDR) via the Benjamini-Hochberg procedure set at .05.
Substance Use Frequency.
In the first set of analyses, we conducted three separate hierarchical linear regressions to determine whether beta-weights from each of the VS, DS, and OFC ROIs during reward anticipation predicted substance use frequency, as measured by the AADIS. We included the following covariates in the first step: Age at scan, gender, time to follow-up assessment, reward risk group status (MRew vs. HRew), medication status at scan (on versus off psychiatric medication), mood symptoms at scan, AADIS at time of scan, and lifetime history of SUD prior to the MRI. In the second step, we added the ROI beta-weight for loss anticipation for VS, DS, or OFC, respectively, to examine the predictive influence of reward neural activity above and beyond loss neural activity. In the third step we added the ROI beta-weight for reward anticipation for the VS, DS, or OFC, respectively.
Substance Use Impairment.
In our second set of analyses, we conducted three separate hierarchical linear regressions to determine whether beta-weights from each of the VS, DS, and OFC ROIs during reward anticipation predicted substance use impairment, as measured by the SIP. The following covariates were included in the first step: Age at scan, gender, time to follow-up, reward risk group status (MRew vs. HRew), medication status at scan, mood symptoms at scan, AADIS at time of scan, and lifetime history of SUD prior to the MRI. We did not administer the SIP at the time of the MRI scan, thus baseline SIP scores were not included as a covariate. In the second step, we added the ROI beta-weight for loss anticipation for VS, DS, or OFC, respectively. In the third step we added the ROI beta-weight for reward anticipation for the VS, DS, or OFC, respectively. All analyses used SPSS version 24.
Exploratory Analyses:
In addition to these primary analyses, we conducted three separate sets of exploratory analyses: 1) We conducted analyses with the reward consumption phase of the MID task if FDR-corrected analyses with the reward anticipation phase were significant to assess whether the relationships between reward processing and substance use and impairment are specific to reward anticipation. 2) We conducted follow-up analyses in which we removed the 13 participants with a lifetime history of SUD prior to the MRI scan in order to examine the relationship between reward processing and substance use frequency and impairment among individuals who do not have a history of problematic substance use. We were unable to exclude participants with a lifetime SUD in our primary analyses because we would not have sufficient statistical power with the smaller sample. Because these exploratory analyses already were underpowered due to removing 13 participants with prior SUD, we did not control for multiple comparisons in these exploratory analyses as we did in the primary analyses. 3) Finally, to account for potential confounding effects of the five individuals who were in a mood disorder episode at the time of the MRI scan (two were in a major depressive episode, one had subthreshold major depression, and two were in a hypomanic episode), we conducted follow-up analyses removing these participants.
Results
Demographics and Clinical Characteristics
See Table 1 for demographics. We examined demographic differences in AADIS scores at the MRI scan and SIP scores at the follow-up assessment (as we did not administer the SIP at the MRI scan) using one-way analyses of variance (ANOVA) and independent t-tests. AADIS and SIP scores were higher among men [t(77) = 2.792, p = .007; t(44.985) = 2.595, p = .013]. AADIS and SIP scores did not differ by race [F(5, 73) = 2.135, p=.071; F(5, 73) = 1.376, p = .243]. AADIS and SIP scores also did not differ by reward risk group [t(77) = −.932, p = .354; t(77) = −.820, p = .414].
Table 1.
Sample Characteristics by Risk Group
| HRew (N = 53) | MRew (N = 26) | Total (N = 79) | |
|---|---|---|---|
| Age at scan (years) | 21.37 ± 2.23 | 21.82 ± 2.12 | 21.52 ± 2.19 |
| Female (%) | 50.94 | 61.54 | 54.43 |
| Race/ethnicity (%) | |||
| White | 54.72 | 57.70 | 55.70 |
| Black | 18.87 | 34.62 | 24.05 |
| Asian | 13.21 | 3.85 | 10.13 |
| Bi-/Multiracial | 7.55 | 3.85 | 6.33 |
| Other/Unknown | 5.66 | 0.00 | 3.80 |
| Reward Measures | |||
| BAS Total Score | 46.31 ± 3.22 | 38.12 ± .99 | 43.61 ± 4.72 |
| SR Score | 18.30 ± 2.99 | 10.96 ± 1.61 | 15.89 ± 4.34 |
| Baseline Measures | |||
| BDI at scan | 4.92 ± 6.45 | 4.98 ± 5.85 | 4.94 ± 6.23 |
| ASRM at scan | 4.44 ± 4.58 | 3.08 ± 2.42 | 3.99 ± 4.03 |
| SUD Hx at scan (%) | 33.96 | 34.62 | 34.18 |
| AADIS at MRI | 6.79 ± 5.34 | 5.73 ± 4.60 | 6.44 ± 5.10 |
| Follow-Up Measures | |||
| AADIS | 7.28 ± 5.98 | 6.04 ± 4.57 | 6.87 ± 5.56 |
| SIP | 17.68 ± 5.24 | 16.77 ± 2.98 | 17.38 ± 4.62 |
Note. HRew = High Reward Group; MRew = Moderate Reward Group; BAS = Behavioral Activation Scale; SR = Sensitivity to Reward subscale; BDI = Beck Depression Inventory; ASRM = Altman Self-Rating Mania Scale; SUD Hx = Lifetime history of substance use disorder prior to the MRI scan; AADIS = Adolescent Alcohol and Drug Involvement Scale; SIP = Short Inventory of Problems; HRew and MRew groups did not significantly differ on any variables except BAS Total and SR scores.
See Table 2 for detailed descriptions of reported use on the AADIS and SIP scores. Specifically, we note what specific drugs were used during the follow-up period, and how many participants reported “light” (tried only once or twice or several times a month), “moderate” (weekends only or several times a week), or “heavy” (daily) use. As noted in Table 2, the majority of participants were light to moderate users; however, a meaningful percentage did report heavy use. Bivariate correlations between primary study variables are displayed in Table 3.
Table 2.
Description of Follow-Up Substance Use in Study Sample
| Substance | % Sample Reporting Use | Light Use (N) | Moderate Use (N) | Heavy Use (N) |
|---|---|---|---|---|
| Alcohol | 92.4 | 25 | 43 | 5 |
| Marijuana | 54.4 | 20 | 16 | 7 |
| Tobacco | 41.8 | 18 | 4 | 11 |
| LSD | 17.7 | 14 | 0 | 0 |
| Amphetamines | 17.7 | 12 | 2 | 0 |
| Cocaine | 16.5 | 11 | 2 | 0 |
| Valium | 11.4 | 9 | 0 | 0 |
| Inhalants | 7.6 | 5 | 1 | 0 |
| Opiates | 3.8 | 2 | 1 | 0 |
| Barbiturates | 1.3 | 1 | 0 | 0 |
| Crack | 1.3 | 1 | 0 | 0 |
| Number of Substances Used* | N (% of total sample) | AADIS M(SD) | SIP M(SD) |
|---|---|---|---|
| Abstainers | 6(27.6) | -- | -- |
| Alcohol Only | 19(24.1) | 2.5(1.2) | 15.2(0.6) |
| Alcohol + 1 other Substance | 18(22.8) | 4.7(1.6) | 16.1(1.6) |
| Alcohol + 2 other Substances | 17(21.5) | 9.2(3.6) | 18.2(3.6) |
| Alcohol + 3 or more Substances | 19(24.1) | 13.4(5.3) | 20.8(7.5) |
Note. Light Use = Report of 1 (Tried Once or Twice) or 2 (Several Times a Month) on AADIS; Moderate Use = Report of 3 (Weekends Only) or 4 (Several Times a Week) on AADIS; Heavy Use = Report of 5 (Daily) or 6 (Several Times a Day) on AADIS; AADIS = Adolescent Alcohol and Drug Involvement Scale; SIP = Short Inventory of Problems;
Reported frequency of alcohol use was higher among the groups reporting alcohol + 2 other substances and alcohol +3 or more substances than the group reporting alcohol only [F(3,69) = 3.048, p = .034).
Table 3.
Bivariate Correlations of Primary Study Variables
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||||||||
| 2. Gender | .171 | ||||||||||||||
| 3. Rew. Group | −.096 | −.100 | |||||||||||||
| 4. Medication | .006 | .043 | −.057 | ||||||||||||
| 5. AADIS MRI | −.137 | −.303* | .099 | .178 | |||||||||||
| 6. SUD Hx | .202 | .132 | .093 | −.066 | .083 | ||||||||||
| 7. BDI MRI | −.050 | .076 | −.005 | .288* | .275* | .060 | |||||||||
| 8. ASRM MRI | −.314* | −.160 | .160 | −.099 | .283* | .035 | .106 | ||||||||
| 9. VS rew ant | −.044 | −.007 | −.012 | .014 | −.210† | −.038 | −.124 | .135 | |||||||
| 10. DS rew ant | .058 | −.050 | .081 | .178 | −.186 | −.190† | −.032 | .065 | .707* | ||||||
| 11. OFC rew ant | .044 | −.077 | .182 | .160 | −.066 | .037 | .110 | .098 | .613* | .682* | |||||
| 12. VS loss ant | −.062 | −.143 | −.004 | −.040 | .042 | −.088 | −.051 | −.051 | −.307* | .129 | .159 | ||||
| 13. DS loss ant | −.091 | −.093 | .182 | −.018 | .079 | −.159 | .136 | .120 | −.264* | .290* | .264* | .703* | |||
| 14. OFC loss ant | −.122 | −.120 | .099 | −.013 | −.007 | −.130 | .044 | −.049 | .136 | .148 | .216† | .693* | .731* | ||
| 15. AADIS f/u | −.270* | −.214† | .106 | .119 | .669* | .202† | .235* | .155 | −.295* | −.272* | −.117 | .068 | .029 | .055 | |
| 16. SIP f/u | −.214† | −.301* | .093 | .035 | .564* | .119 | .273* | .189† | −.236* | −.153 | −.076 | .025 | .102 | .029 | .699* |
Note.
p< .10,
p<.05.
Rew. Group = Reward Risk Group; Medication = Taking psychotropic medication at time of scan; AADIS = Adolescent Alcohol and Drug Involvement Scale; SUD Hx = Lifetime history of substance use disorder prior to the MRI scan; BDI = Beck Depression Inventory; ASRM = Altman Self Rating Mania scale; VS = ventral striatum; DS = dorsal striatum; OFC = orbitofrontal cortex; rew. ant. = reward anticipation; loss ant = loss anticipation; f/u = follow up; SIP = Short Inventory of Problems
Primary Analyses
Substance Use Frequency
Lower activation in the VS and DS during reward anticipation predicted higher AADIS scores at follow-up, controlling for age at scan, gender, reward risk group, medication status at scan, mood symptoms at scan, AADIS at time of scan, lifetime SUD history prior to the MRI scan, and neural activation during loss anticipation (see Table 4 for results and Figures 2 and 3). However, after controlling for multiple comparisons, the DS no longer significantly predicted AADIS scores. There was also a non-significant trend for OFC activation during reward anticipation predicting higher AADIS scores at follow-up.
Table 4.
Hierarchical Linear Regressions of Fronto-Striatal Activation during Reward Anticipation as Predictors of Substance Use Frequency (AADIS) at next follow-up
| Ventral Striatal Activation | Dorsal Striatal Activation | Orbitofrontal Cortex Activation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | β | t | p | β | t | p | β | t | p |
| Intercept | 3.279 | .002 | 2.805 | .007 | 2.724 | .008 | |||
| Age | −.245 | −2.972 | .004 | −.220 | −2.573 | .012 | −.228 | −2.636 | .010 |
| Gender | −.035 | −.424 | .673 | −.052 | −.606 | .547 | −.050 | −.575 | .567 |
| Rew Risk Group | .002 | .023 | .982 | .036 | .447 | .657 | .034 | .420 | .676 |
| Medication | −.006 | −.077 | .939 | .001 | .011 | .991 | −.005 | −.060 | .953 |
| Follow-up time | .254 | 3.105 | .003 | .266 | 3.060 | .003 | .239 | 2.777 | .007 |
| Hx of SUD | .232 | 2.901 | .005 | .185 | 2.214 | .030 | .234 | 2.804 | .007 |
| AADIS MRI | .527 | 5.723 | .000 | .551 | 5.840 | .000 | .571 | 6.080 | .000 |
| BDI MRI | .061 | .732 | .466 | .091 | 1.046 | .299 | .099 | 1.132 | .262 |
| ASRM MRI | −.026 | −.293 | .771 | −.053 | −.598 | .552 | −.068 | −.756 | .453 |
| Loss Ant. | .072 | .876 | .384 | −.050 | −.569 | .571 | .024 | .280 | .780 |
| Rew. Ant. | −.260 | −2.971 | .004* | −.183 | −2.014 | .048 | −.161 | −1.804 | .076 |
| R 2 | .618 | .598 | .588 | ||||||
| Adjusted R 2 | .556 | .533 | .520 | ||||||
Note.
primary result survives FDR correction at .05;
AADIS = Adolescent Alcohol and Drug Involvement Scale; Age = Age at MRI; Rew Risk Group = High Reward vs. Moderate Reward group; Medication = taking psychotropic medications at time of scan; Follow-up time = time from MRI to follow-up assessment; Hx of SUD = Lifetime history of substance use disorder prior to the MRI scan; BDI MRI = Beck Depression Inventory score at time of scan; ASRM MRI = Altman Self Rating Mania score at time of scan; Loss Ant. = Loss (i.e., Lose $1.50, Lose $5.00) vs. Non-Loss (i.e., Lose $0.00) trials during the MID anticipation period; Rew. Ant. = Reward (i.e., Win $1.50, Win $5.00) vs. Non-Reward (i.e., Win $0.00) trials during the MID anticipation period.
Figure 2. Ventral Striatal Activation during Reward Anticipation as a Predictor of Substance Use Frequency, as Assessed by the AADIS at Follow-up.
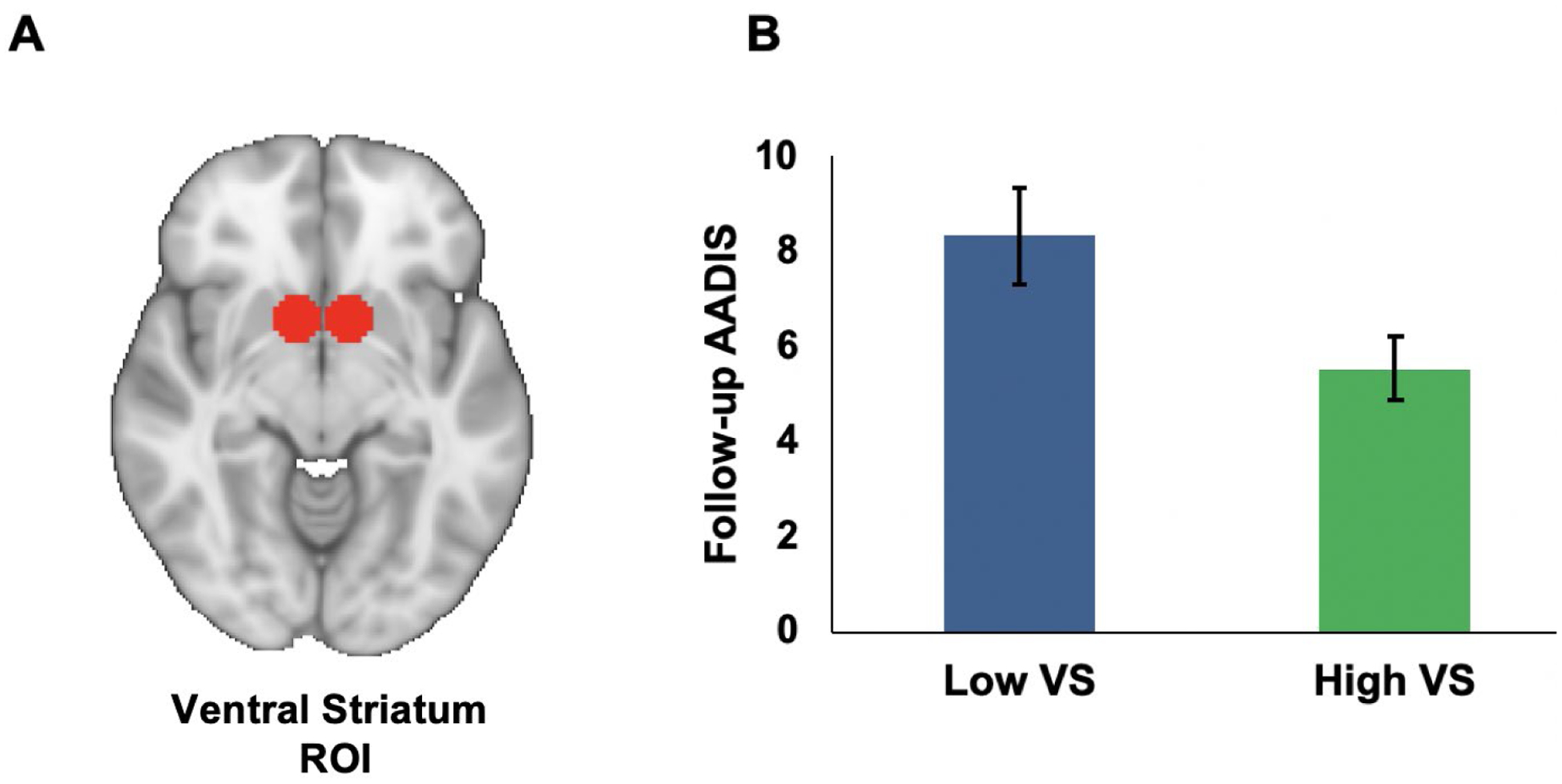
Note. A) Region-of-interest (ROI) for the bilateral ventral striatum defined as two 8 mm spheres based on MNI coordinates (right: x = 9, y = 9, z = −8; left: x = −9, y = 9, z = −8) from a previous meta-analysis (Di Martino et al., 2008). B) Bar Graph depicting low and high ventral striatum groups (graphed using median split; error bars represent standard error) and AADIS scores at follow-up. VS = ventral striatum; AADIS = Adolescent Alcohol and Drug Involvement Scale.
Figure 3. Dorsal Striatal Activation during Reward Anticipation as a Predictor of Substance Use Frequency, as Assessed by the AADIS at Follow-up.
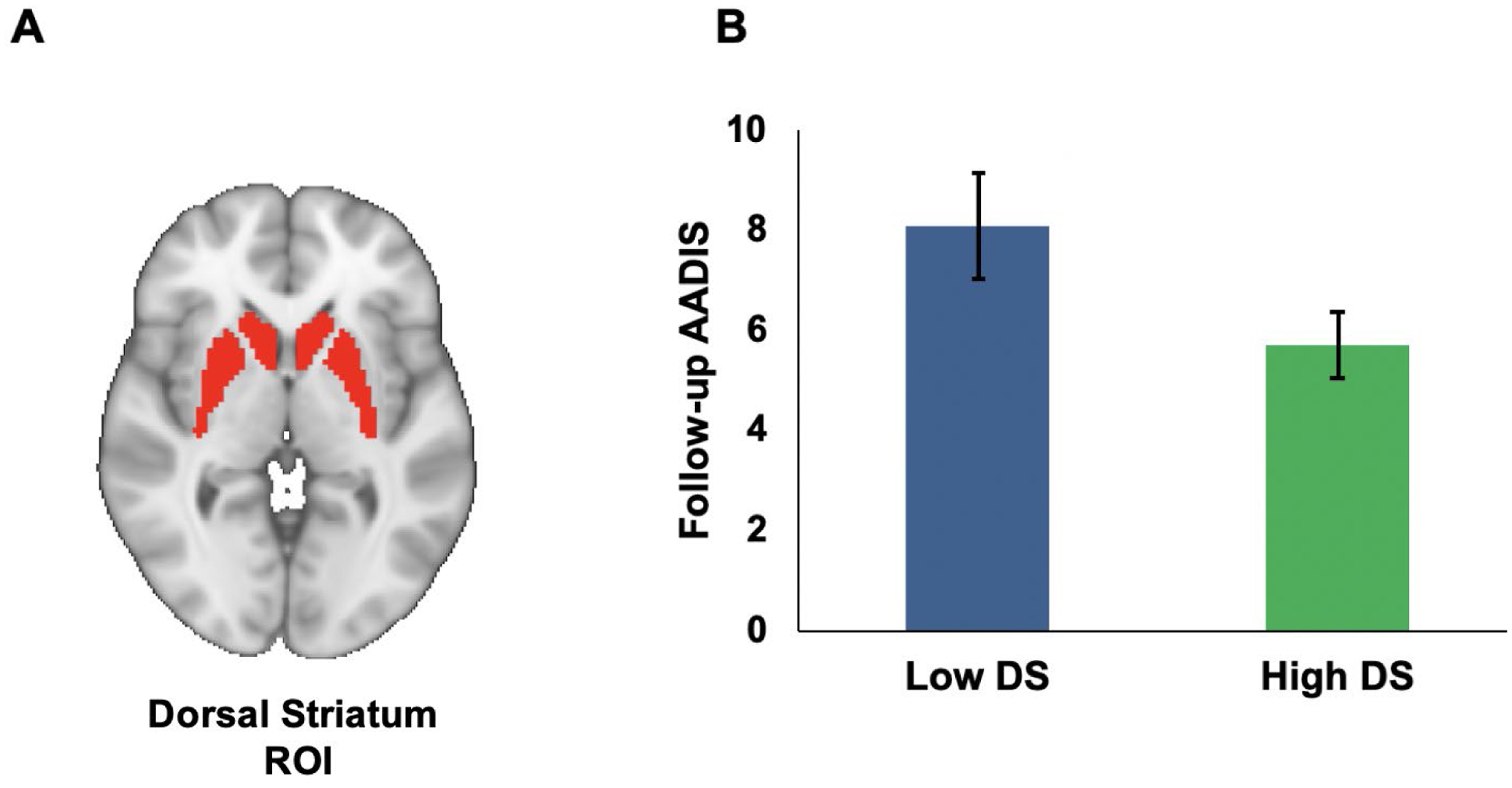
Note. A) Region-of-interest (ROI) for the bilateral dorsal striatum defined with Wake Forest Pick Atlas template. B) Bar Graph depicting low and high dorsal striatum groups (graphed using median split; error bars represent standard error) and AADIS scores at follow-up. DS = dorsal striatum; AADIS = Adolescent Alcohol and Drug Involvement Scale. As indicated on page 18, this analysis does not pass correction for multiple comparisons.
Substance Use Impairment
Although activation in the VS, DS, and OFC during reward anticipation did not significantly predict SIP scores at follow-up, there was a non-significant trend for lower activation in the VS during reward anticipation predicting higher SIP scores at follow-up (see Table 5 for results).1
Table 5.
Hierarchical Linear Regressions of Fronto-Striatal Activation during Reward Anticipation as Predictors of Substance Use Impairment (SIP) at next follow-up
| Ventral Striatal Activation | Dorsal Striatal Activation | Orbitofrontal Cortex Activation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | β | t | p | β | t | p | β | t | p |
| Intercept | 4.483 | .000 | 4.165 | .000 | 4.132 | .000 | |||
| Age | −.146 | −1.470 | .146 | −.135 | −1.309 | .195 | −.135 | −1.326 | .189 |
| Gender | −.176 | −1.752 | .084 | −.175 | −1.707 | .092 | −.186 | −1.815 | .074 |
| Rew Risk Group | −.003 | −.032 | .974 | .011 | .109 | .914 | .026 | .266 | .791 |
| Medication | −.093 | −.932 | .355 | −.091 | −.869 | .388 | −.090 | −.884 | .380 |
| Follow-up time | .198 | 2.005 | .049 | .170 | 1.628 | .108 | .188 | 1.852 | .068 |
| Hx of SUD | .136 | 1.410 | .163 | .119 | 1.181 | .242 | .137 | 1.393 | .168 |
| AADIS MRI | .392 | 3.523 | .000 | .425 | 3.745 | .000 | .418 | 3.779 | .000 |
| BDI MRI | .179 | 1.780 | .080 | .192 | 1.840 | .070 | .212 | 2.050 | .044 |
| ASRM MRI | .018 | .172 | .864 | −.013 | −.124 | .902 | −.011 | −.099 | .921 |
| Loss Ant. | .007 | .067 | .947 | .0002 | .020 | .984 | −.020 | −.199 | .843 |
| Rew. Ant. | −.186 | −1.760 | .083 | −.080 | −.726 | .470 | −.121 | −1.152 | .253 |
| R 2 | .442 | .419 | .427 | ||||||
| Adjusted R 2 | .351 | .324 | .333 | ||||||
Note. SIP = Short Inventory of Problems; Age = Age at MRI; Rew Risk Group = High Reward vs. Moderate Reward group; Medication = taking psychotropic medications at time of scan; Follow-up time = time from MRI to next follow-up assessment; Hx of SUD = Lifetime history of substance use disorder prior to the MRI scan; BDI MRI = Beck Depression Inventory score at time of scan; ASRM MRI = Altman Self Rating Mania score at time of scan; Loss Ant. = Loss (i.e., Lose $1.50, Lose $5.00) vs. Non-Loss (i.e., Lose $0.00) trials during the MID anticipation period; Rew. Ant. = Reward (i.e., Win $1.50, Win $5.00) vs, Non-Reward (i.e., Win $0.00) trials during the MID anticipation period
Exploratory Analyses
There were no significant relationships between neural activation during the consumption phase of the MID task and either AADIS or SIP scores at follow-up (ps > .20), indicating that the relationship between reward-related brain function and substance use and impairment was specific to the anticipation phase. Next, removing the 13 participants who had a lifetime history of a SUD prior to the MRI scan did not weaken the reported effects. Specifically, lower activation in the VS (B = −.348, p = .001), DS (B = −.214, p = .045), and OFC (B = −.221; p = .034) during reward anticipation predicted higher AADIS scores at follow-up. Finally, removing the five participants who were in a mood disorder episode at the time of the scan did not weaken the reported effects for AADIS scores at follow-up for the VS (B = −.269, p = .004), although the DS now showed a non-significant trend in predicting AADIS scores at follow-up (B = −.174, p = .071). There also was an additional significant finding that lower activation in the OFC during reward anticipation predicted higher AADIS scores (B = −.216, p = .021).
Discussion
The current study examined neural activation to secondary reward cues (i.e., monetary stimuli) as a prospective predictor of substance use frequency and substance use impairment. Results indicate that lower activation in the VS during reward anticipation prospectively predicted greater substance use frequency at a follow-up assessment that occurred, on average, 9.1 months after the MRI scan. Lower activation in the DS predicted higher substance use frequency at follow-up, although this finding did not survive correction for multiple comparisons. These findings were observed after controlling for age at scan, gender, time to follow-up assessment, reward risk group status, psychiatric medication status at the time of the scan, mood symptoms at the time of the scan, and a lifetime history of SUD prior to the MRI. These findings also were maintained after removing participants in a mood disorder episode at the time of the scan, and participants with a lifetime history of a SUD prior to the scan. Finally, these associations were specific to the anticipation phase, and were significant after controlling for loss anticipation, suggesting that the relationship between frontal-striatal activation and substance use frequency is unique to reward processing. Collectively, these results suggest that lower anticipatory reward-related brain function is a risk factor for future substance use engagement.
The study did not reveal unique effects of neural reward activation in predicting future substance use impairment, although there was a non-significant trend for lower activation in the VS predicting higher substance use impairment scores at follow-up. Given the high correlation between substance use frequency at baseline and follow-up impairment scores (see Table 3), frequency of substance use may be a confounding variable that explains why we were unable to detect significant effects in our models predicting to substance use impairment. Alternatively, substance use frequency may serve as a mediator between neural reward function and future problematic substance use. Given the current study design, we were unable to test this hypothesis; however, investigating the role of substance use frequency as a potential explanatory variable is an important future direction.
The debate regarding the reward hypo- versus hypersensitivity models of addictive behaviors is ongoing, with support for both perspectives in the literature. Our findings support the reward hyposensitivity model of addiction, which suggests people with blunted reward signaling may pursue exogenously (e.g., through the use of substances) what they lack endogenously in order to increase positive affect and attenuate dysphoria. These results are in line with a recent meta-analysis of largely cross-sectional studies that found blunted neural activation during reward anticipation on the MID is related to SUDs (Luijten, Schellekens, Kühn, MacHielse, & Sescousse, 2017). Although we did not directly compare the predictive strength of different regions within the fronto-striatal circuit, it is curious we did not find that all brain regions examined (VS, DS, and OFC) predicted each substance use outcome. Results suggest that hedonic processing within the VS, which helps encode the emotional and motivational value of rewards, is most strongly associated with substance use. Furthermore, results were specific to the reward anticipation phase of the MID, supporting the theory that reward anticipation (e.g., drug expectancy) is particularly important in the onset and course of SUDs, due to its role in craving and attentional bias towards drug cues (Jędras et al., 2013).
Most of the evidence in the reward hypo- vs. hypersensitivity debate is drawn from cross-sectional research, which limits conclusions about whether reward processing abnormalities are a pre-existing risk factor or a corollary of problematic substance use, and highlights the importance of longitudinal studies in resolving this debate (Büchel et al., 2017; Martz et al., 2016; Whelan et al., 2014). The current study extends the extant work by utilizing a prospective design, and suggests that a low sensitivity to rewarding stimuli is a risk factor for a worsening course of substance use frequency and impairment. This claim is strengthened by the fact that we controlled for baseline substance use and the presence of a lifetime SUD prior to MRI scanning, as well as other relevant clinical and demographic variables. These findings are consistent with other prospective studies reporting decreased reward-related brain function as a risk factor for SUD relapse (Moeller & Paulus, 2018) and initial onset of problematic substance use (Büchel et al., 2017). Taken together, the growing prospective literature suggests that reward hyposensitivity is a pre-existing risk factor for the initial onset of problematic substance use, and a worsening course of substance use and SUDs.
To elucidate the mechanisms involved in problematic substance use and addiction, it is important to integrate the literature on secondary reward processing (e.g., money) and substance use with research examining neural reactivity to substance-related cues (i.e., drug cue reactivity). Cue reactivity paradigms suggest that individuals with a SUD display an excessive increase in reward-related brain function to substance-related cues (Chase et al., 2011; MacNiven et al., 2018), which is associated with heightened clinical measures of SUDs (including craving), particularly in the presence of multisensory drug-cues (Yalachkov, Kaiser, & Naumer, 2012). However, because substances of abuse carry such a high reward value, they can disrupt regulated signaling in brain regions associated with incentive salience [e.g., VS; (Volkow, Fowler, Wang, Swanson, & Telang, 2007)], and drug habit [e.g., DS; (Everitt & Robbins, 2016)]. Our findings suggest that individuals on the path to more problematic substance use may have limited resources for non-substance related cues. This is a major component of the incentive sensitization theory, which suggests people with SUDs develop drug-induced sensitization of the dopaminergic reward circuit (Berridge & Robinson, 2016). Consequently, this network becomes desensitized to primary rewards and other non-drug related-cues (Robinson & Berridge, 2008), such as money. Combining the current findings with cue reactivity research suggests that sensitization to substance-related cues, along with desensitization to non-substance related-rewards are important for understanding the course and maintenance of addictive behaviors.
In addition to our primary findings, the results yielded interesting covariate effects of demographics and mood. In our substance use frequency models, age was negatively associated with substance use frequency at follow-up. Although the majority of our participants were in their early twenties and transitioning to early adulthood, which is a period associated with increased use of substances (Stone et al., 2012), we did have a wide age range (aged 19–29) with over half the sample above the US legal drinking age. Thus, it is possible that this effect may reflect some individuals maturing out of substance use in our sample. We also saw trend-level associations of gender and baseline depression scores with substance use impairment, such that identifying as male and having higher baseline depression tended to be associated with increased problematic substance use at follow-up. Although research on sex and gender differences in addiction has shown that women develop problematic SUDs more rapidly and experience more functional impairment than men, given our relatively young sample, this trend effect may indicate that men also tend to initiate substance use at an earlier age than women (McHugh et al., 2018). Additionally, these trend-level findings also provide support that substance misuse often occurs as a way to cope with negative mood (McHugh & Kneeland, 2019).
This study had several limitations. Despite its prospective design, the current study included some participants with a previous history of SUD. Thus, we cannot fully rule out the possibility that the initial onset of substance use in our participants was driven by reward hypersensitivity. From this perspective, reward hypersensitivity may play a role in initial pursuit of substances, which, over time, cause neuroadaptive changes in the reward circuit resulting in reduced reward-related brain function, which then serves to maintain addictive behaviors. Indeed, prospective studies of substance use initiation still report contradictory findings, with some suggesting reward hypersensitivity drives initial onset, and others suggesting hyposensitivity does (Büchel et al., 2017; Whelan et al., 2014). Given this limitation, the current study provides support for the potential of reward hyposensitivity in worsening the course of substance use, but the role of reward processing in initial onset requires further exploration. However, removing the 13 participants with a lifetime SUD prior to the MRI did not change the strength or direction of our results. This suggests that reward hyposensitivity may, in fact, be a pre-existent risk factor for substance use. However, future research using multi-wave longitudinal designs that track reward-related brain function and substance use behaviors during developmental periods involving first exposure to substances is needed to more fully examine whether reward hyper- or hyposensitivity is the primary risk factor for the initial onset of problematic substance use.
Another limitation is Project TEAM’s recruitment method. The absence of a low reward risk group restricted the range of reward responsivity in our sample, and so future work is needed that recruits along the full dimension of reward sensitivity. Additionally, because of the aims of Project TEAM, we were limited in terms of our sample characteristics for the present analyses. To fully assess neural mechanisms involved in the first onset of addictive behaviors, however, it may be necessary for future research to focus on younger samples who have not yet initiated substance use. Although we attempted to control for many baseline sample characteristics (e.g., reward risk group status, mood symptoms), the findings should be interpreted in the context of the heterogeneity of our sample. For example, because the sample was not specifically recruited based on substance use criteria, we had considerable variation in substance use frequency and impairment (see Table 2). Future research is needed to more fully examine whether reward-related brain function prospectively predicts very severe levels of substance use. Next, because we did not administer the SIP at the MRI scan, we were unable to control for baseline levels of substance impairment in our follow-up SIP analyses. However, we suggest that controlling for a history of SUD accounts, in part, for baseline levels of impairment, given that a SUD history implies there was impairment. Finally, given the modest sample size and the good-to-moderate test-retest reliability of the MID task, we may have been underpowered. Future research is needed to replicate and extend these findings in larger samples.
Overall, the current study provides support for the reward hyposensitivity theory of SUDs. These findings were maintained while controlling for neural activation during loss anticipation, which underscores the specific importance of reward anticipation in processes involved in SUDs. Additionally, the prospective design provides insight regarding the mechanisms and pathophysiology contributing to worsening SUD course. Finally, our findings of lower brain-related activity to non-drug related rewards suggests hypoactivity to primary and secondary rewards combined with hyperactivity to drug-related cues are mechanisms important to the onset, maintenance, and worsening of SUD course.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH077908 to Dr. Lauren B. Alloy, and a research fellowship award from the Civic Foundation to Corinne P. Bart.
Footnotes
Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
Due to the skewed nature of the SIP data, we re-ran analyses on the SIP outcome variable after removing one outlier (i.e. +/− 2 SDs from mean). Similar to the primary analyses, there were no significant effects in any of the ROIs during reward anticipation predicting SIP scores at follow-up. Additionally, a non-parametric Spearman’s rank-order correlation indicated that there was a negative correlation between VS activation during reward anticipation and follow-up SIP score (rs(77) = −.275, p = .014).
References
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, … Hogan ME (2008). Behavioral approach system (BAS) and behavioral inhibition system (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders, 10(11), 310–322. 10.1111/j.1399-5618.2007.00547.x [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, … Harmon-Jones E (2009). Bipolar Spectrum-Substance Use Co-Occurrence: Behavioral Approach System (BAS) Sensitivity and Impulsiveness as Shared Personality Vulnerabilities. Journal of Personality and Social Psychology, 97(3), 549–565. 10.1037/a0016061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, … Abramson LY (2012). High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology, 121(2), 339–351. 10.1037/a0025877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, & Davis JM (1997). The altman self-rating Mania scale. Biological Psychiatry, 42(10), 948–955. 10.1016/S0006-3223(96)00548-3 [DOI] [PubMed] [Google Scholar]
- Altman E, Hedeker D, Peterson JL, & Davis JM (2001). A comparative evaluation of three self-rating scales for acute mania. Biological Psychiatry, 50(6), 468–471. 10.1016/S0006-3223(01)01065-4 [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, … Romero FJ (2010). Altered neural response of the appetitive emotional system in cocaine addiction: An fMRI Study. Addiction Biology, 15(4), 504–516. 10.1111/j.1369-1600.2010.00230.x [DOI] [PubMed] [Google Scholar]
- Baker TE, Castellanos-Ryan N, Schumann G, Cattrell A, Flor H, Nees F, … Conrod P (2019). Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychological Medicine. 10.1017/S0033291718001459 [DOI] [PubMed] [Google Scholar]
- Balodis IM, & Potenza MN (2015). Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biological Psychiatry, 77(5), 434–444. 10.1016/j.biopsych.2014.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2 Pt 2), 227–239. 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, & Emery G (1979). Cognitive therapy of depression. New York, NY: Guilford Press. [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–10. [Google Scholar]
- Berke JD, & Hyman SE (2000). Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron, 25(3), 515–532. 10.1016/S0896-6273(00)81056-9 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28(3), 309–369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71(8), 670–679. 10.1037/amp0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard KA, Morgenstern J, Morgan TJ, Labouvie EW, & Bux DA (2003). Assessing Consequences of Substance Use: Psychometric Properties of the Inventory of Drug Use Consequences. Psychology of Addictive Behaviors, 17(4), 328–331. 10.1037/0893-164X.17.4.328 [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VI, Miller D, … Comings DE (2000). The reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. Journal of Psychoactive Drugs, 32 Suppl(i–iv), 1–112. 10.1080/02791072.2000.10736099 [DOI] [PubMed] [Google Scholar]
- Bowirrat A, & Oscar-Berman M (2005). Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics - Neuropsychiatric Genetics, 132B(1), 29–37. 10.1002/ajmg.b.30080 [DOI] [PubMed] [Google Scholar]
- Büchel C, Peters J, Banaschewski T, Bokde ALW, Bromberg U, Conrod PJ, … Knutson B (2017). Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nature Communications, 8, 14140. 10.1038/ncomms14140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, & Hogarth L (2011). The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biological Psychiatry, 70(8), 785–793. 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LM, Martz ME, Hardee JE, Zucker RA, & Heitzeg MM (2019). Reward activation in childhood predicts adolescent substance use initiation in a high-risk sample. Drug and Alcohol Dependence, 194, 318–325. 10.1016/j.drugalcdep.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, & Loxton NJ (2004). Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors, 29(7), 1389–1405. 10.1016/j.addbeh.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Dawe S, & Loxton NJ (2004). The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Reviews, 28(3), 343–351. 10.1016/j.neubiorev.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, … Lecca D (2004). Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology, 47 Suppl 1, 227–241. 10.1016/j.neuropharm.2004.06.032 [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, … Milham MP (2008). Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex, 18(12), 2735–1747. 10.1093/cercor/bhn041 [DOI] [PubMed] [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Ramrakha S, Sison ML, … Hariri AR (2020). What is the test-retest reliability of common task-fMRI measures ? New empirical evidence and a meta-analysis. Psychological Science, 31(7), 792–806. 10.1177/0956797620916786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, & Spitzer RL (1978). A diagnostic interview: The Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry, 35(7), 837–844. 10.1001/archpsyc.1978.01770310043002 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2016). Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. SSRN, 67, 23–50. 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, & Narendran R (2014). Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS ONE. 10.1371/journal.pone.0094640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, & Manuck SB (2006). Preference for Immediate over Delayed Rewards Is Associated with Magnitude of Ventral Striatal Activity. Journal of Neuroscience, 26(51), 13213–13217. 10.1523/JNEUROSCI.3446-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, & Zucker RA (2014). Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence, 141, 51–57. 10.1016/j.drugalcdep.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt CJ, Assaf M, Muska CE, Rosen RI, Thomas AD, Johnson MR, … Pearlson GD (2012). Reward-related dorsal striatal activity differences between former and current cocaine dependent individuals during an interactive competitive game. PLoS ONE, 7(5), e34917. 10.1371/journal.pone.0034917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jędras P, Jones A, & Field M (2013). The role of anticipation in drug addiction and reward. Neuroscience & Neuroeconomicss, 1, 1–10. 10.2147/NAN.S35917 [DOI] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, & Potenza MN (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–560. 10.1016/j.biopsych.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JWL, Scaffidi A, MacKenzie T, Simmons JG, & Allen NB (2009). Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Archives of General Psychiatry, 66(2), 205–212. 10.1001/archgenpsychiatry.2008.522 [DOI] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, MacHielse MWJ, & Sescousse G (2017). Disruption of reward processing in addiction: An image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry, 74(4), 387–398. 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- MacNiven KH, Jensen ELS, Borg N, Padula CB, Humphreys K, & Knutson B (2018). Association of Neural Responses to Drug Cues With Subsequent Relapse to Stimulant Use. JAMA Network Open, 1(8), e186466. 10.1001/jamanetworkopen.2018.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, & Heitzeg MM (2016). Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry, 73(8), 838–844. 10.1001/jamapsychiatry.2016.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, & Paulus MP (2013). Methamphetamine Dependent Individuals Show Attenuated Brain Response to Pleasant Interoceptive Stimuli. Drug and Alcohol Dependence, 131(3), 238–246. 10.1016/j.drugalcdep.2013.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, & Cohen JD (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306(5695), 503–507. 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- McHugh RK, & Kneeland ET (2019). Affective vulnerability in substance use disorders. Curr Opin Psychol, 30, 54–58. 10.1016/j.copsyc.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Sugarman DE, & Greenfield SF (2018). Sex and gender differences in substance use disorders. Clinical Psychology Review, 66, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, & McClair VL (2012). Epidemiology of substance use disorders. Human Genetics, 131(6), 779–789. 10.1007/s00439-012-1168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg DP (2003). Screening for Alcohol and Other Drug Problems using the Adolescent Alcohol and Drug Involvement Scale (AADIS). Madison, WI: Center for Health Policy and Program Evaluation, University of Wisconsin-Madison, 1–5. [Google Scholar]
- Moeller SJ, & Paulus MP (2018). Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 80(Pt B), 143–154. 10.1016/j.pnpbp.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE (2018). The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87(Pt A), 85–107. 10.1016/j.pnpbp.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Hester R, & Garavan H (2010). Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage, 49(1), 1133–1143. 10.1016/j.neuroimage.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, McCabe E, Jones J, Clancy L, & Garavan H (2018). Smokers and ex-smokers have shared differences in the neural substrates for potential monetary gains and losses. Addiction Biology. 10.1111/adb.12484 [DOI] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Ito R, Verschure PFMJ, Battaglia FP, & Robbins TW (2011). The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends in Neurosciences, 34(10), 548–559. 10.1016/j.tins.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, … Meyer-Lindenberg A (2012). Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage. 10.1016/j.neuroimage.2012.01.129 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3137–3146. 10.1016/B978-0-12-811986-0.00013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GRS, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, & Knutson B (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–791. 10.1038/nn1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, & Shaham Y (2008). The Role of Orbitofrontal Cortex in Drug Addiction: A Review of Preclinical Studies. Biological Psychiatry, 63(3), 256–262. 10.1016/j.biopsych.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Connolly CG, May AC, Tapert SF, Wittmann M, & Paulus MP (2014). Cocaine dependent individuals with attenuated striatal activation during reinforcement learning are more susceptible to relapse. Psychiatry Research - Neuroimaging, 223(2), 129–139. 10.1016/j.pscychresns.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AL, Becker LG, Huber AM, & Catalano RF (2012). Review of risk and protective factors of substance use and problem use in emerging adulthood. Addictive Behaviors, 37(7), 747–775. 10.1016/j.addbeh.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Strang NM, & Pollak SD (2014). Developmental continuity in reward-related enhancement of cognitive control. Developmental Cognitive Neuroscience. 10.1016/j.dcn.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, & Banich MT (2013). Reduced neural tracking of prediction error in Substance-dependent individuals. American Journal of Psychiatry, 170(11), 1356–1363. 10.1176/appi.ajp.2013.12091257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, & Caseras X (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Volkow ND, Fowler JS, & Wang GJ (2003). The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation, 111(10), 1444–1451. 10.1172/JCI18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, & Telang F (2007). Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Archives of Neurology, 64(11), 1575–1579. 10.1001/archneur.64.11.1575 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine, 374, 363–371. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Morales M (2015). The Brain on Drugs: From Reward to Addiction. Cell, 162(4), 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, … Garavan H (2014). Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature, 512(7513), 185–189. 10.1038/nature13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, & Knutson B (2014). Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 10.1016/j.neuroimage.2013.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, & Ferguson SM (2015). The ins and outs of the striatum: Role in drug addiction. Neuroscience, 301, 529–541. 10.1016/j.neuroscience.2015.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, & Naumer MJ (2012). Functional neuroimaging studies in addiction: Multisensory drug stimuli and neural cue reactivity. Neuroscience and Biobehavioral Reviews, 36(2), 825–835. 10.1016/j.neubiorev.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Zapolski TCB, Cyders MA, & Smith GT (2009). Positive Urgency Predicts Illegal Drug Use and Risky Sexual Behavior. Psychology of Addictive Behaviors. 10.1037/a0014684 [DOI] [PMC free article] [PubMed] [Google Scholar]


