Abstract
Introduction: The recommendation of exercise programs in the senior population may benefit inactive and sedentary individuals and improve and help to treat specific health conditions. The purpose of this review is to summarize the published evidence from RCT studies of aerobic exercise interventions for mental health in older adults over the last 20 years.
Methods: A literature search was conducted using electronic databases including Web of Science, PubMed/Medline, and ProQuest.
Results: A total of 15 studies met the inclusion criteria. The subjects of these studies were aged 60 years or older and had various physical health statuses. In 15 studies, the mean effect size for the experimental outcome was 0.56 ± 0.39 (95%CI: 0.36–0.76). One-way ANOVA indicated no significant differences in the intervention duration [F(2,15) = 0.919, p = 0.420], subject category [F(2,15) = 0.046, p = 0.955], or measurement category [F(3,14) = 0.967, p = 0.436]. However, there were significant differences in exercise frequencies [F(2,15) = 6.03, p = 0.012].
Conclusion: The available evidence suggests that aerobic exercise is beneficial for improving the mental health of adults aged 60 years and older. The intervention effect can be achieved regardless of the type of subject and the duration of the intervention. Further, the present study indicates that low-frequency, long-term and regular aerobic exercise is more effective for older adults. Therefore, we recommend that older adults to exercise at a low frequency depending on their physical condition.
Keywords: mental health, aerobic exercise, MCI, dementia, depression, cognition, older adults
Introduction
The World Health Organization (WHO) has published several cross-national comparisons of the prevalence, severity, and treatment progression of mental disorders (1–4). Studies have concluded that the 12-month prevalence of any mental disorder is highly variable. However, most countries have no access to timely treatments for mild or moderate mental disorders (5). For example, the median delay in seeking treatment for anxiety disorders is 3 years in Israel and 30 years in Mexico (5). In addition, seeking treatment for mental illness does not mean that individuals are optimally treated, and the mortality rates are higher for those with chronic or recurrent mental illnesses (6), while the morbidity is higher when depression occurs in combination with physical illnesses such as diabetes or cardiovascular disease (7). Data show that those with mental disorders die 10–15 years earlier than the general population, and major contributing factors include preventable cardiovascular diseases that are caused by poor lifestyle choices, such as a lack of physical activity (8).
Most people know that exercise and physical activity are critical for maintaining physical health; however, what about mental health? According to the U.S. Department of Health and Human Services, exercise can be defined as “physical or mental exertion, especially to train or improve health,” while, according to the U.S. Department of Health and Human Services, physical activity is “any physical exercise that exercises muscles and requires more energy than rest.” Physical activity is defined by the NIH as “any physical exercise that builds muscle and requires more energy than rest” (9). According to the U.S. DHHS, mental health can be defined as “our emotional, psychological, and social well-being. It helps determine how we handle stress, how we relate to others, and how we make choices” (Mental Health). However, it is still considered taboo to discuss mental health in the public arena. A study of 2,000 people conducted by The Guardian UK found that 30% of people found it “difficult to admit publicly that they have a mental illness” and that “admitting to a mental health condition is harder than admitting to having an alcohol problem, being broke or being gay” (9). People “are four times more likely to break up if their partner is diagnosed with major depression than if they have a physical disability” (10). Mental health has become a major “enemy” of people's health. Especially in the elderly population, the decline of various body functions due to physical decline directly affects their physical and mental health. However, physical activity is a simple and effective form of exercise, so it could play a more prominent role.
Numerous recent epidemiological studies have reviewed the relationship between physical activity and mental health (11). A meta-analysis of prospective studies including nearly 267,000 individuals showed that higher levels of PA were associated with lower odds of developing depression. In another meta-analysis including more than 80,000 people, PA was also associated with elevated odds of experiencing anxiety symptoms but lower odds of anxiety disorders (12). The data showed that, the higher the amount of PA, the lower the risk of mental health problems. There appears to be a dose–response relationship between increased PA and mental health and functioning across exercise modalities (13). Aerobic and resistance exercise proved to be of additional benefit to health (14). In conclusion, the epidemiological evidence supports the idea that more habitual PA is associated with better mental health and functioning (15).
The current research generally agrees that exercise has beneficial effects on a range of mental health outcomes. Some studies have observed that exercise improves mental health in various ways (16–18). For instance, neurobiological theories are used to explain the mechanisms by which aerobic exercise improves mental health in middle-aged and older age groups (18–20). Of these, the conceptual model of neurobiological and behavioral learning mechanisms (NBLMs) and the three overarching mechanistic hypotheses (TOMHs) are widely popular. The NBLM model assumes that exercise improves the neurobiological system of adaptive learning, as well as affective and cognitive control processes, reinforcing a virtuous circle and synergistically improving the regulation of cognitive and affective responses (20). The TOMHs comprise three hypotheses: (a) mental health is associated with the physical effects of exercise, (b) exercise improves mental health through neurobiological mechanisms, and (c) exercise is a vehicle for developing mechanisms of behavioral change (e.g., self-regulatory skills and self-efficacy). Smith et al. confirmed that the TOMHs were useful for constructing hypotheses about treatment improvements (15). However, the evidence for a dose–response effect of exercise is less robust than the observations. Although the frequency of exercise required for therapeutic mental health benefits appears to vary by population and exercise modality (21), interestingly, few studies have linked the degree of improvement to the frequency or duration of exercise (19).
The primary purpose of the current study was to review the randomized controlled trials studying the effects of aerobic exercise on older adults' mental health over the past 20 years and to analyze the effects of aerobic exercise (and their differences) on the effectiveness of mental health interventions in older adults, to provide scientific assurance that older adults should participate in aerobic exercise.
Methods
Search Strategy
The literature for this study was identified by conducting a comprehensive search in electronic databases, including Web of Science, PubMed/Medline, and ProQuest. The search period ranged from January 2000 to December 2020. The keywords used in our searches were exercise, aerobic exercise, mental health, mental illness, and mental disorders. After removing duplicates, the titles and abstracts of the retrieved references were screened to exclude articles that did not meet the inclusion criteria (22). The full texts of the remaining articles were obtained and fully assessed by the authors (LY and JL). The reference lists of the final included articles were also screened to identify additional studies. The decision to include disputed articles was made jointly with the corresponding author (JC).
Selection Criteria
Studies were considered for inclusion if they met the following criteria (23): (1) the article was written in English; (2) a randomized controlled trial design was used to compare the aerobic exercise intervention group with a control group (either daily life or other forms of exercise); (3) the research question involved cognitive or mental health; (4) the study subjects were 60 years of age or older; and (5) the effect of aerobic exercise on the subjects' mental health was assessed. Studies were excluded if (1) the study subject was completely unable to care for himself/herself (had a severe physical disability); (2) the study design included other types of interventions (e.g., intervention diets); or (3) the study results did not include a cognitive or mental health component.
Risk-of-BIAS Assessment
A risk-of-bias assessment was performed to ensure the rigor of the sources of evidence. According to the PRISMA-Scr guidelines, we conducted a partial risk-of-bias assessment based on the Cochrane Guidelines (21). The Cochrane Risk of Bias Tool was used on Review Manager 5.4 (https://community.cochrane.org). Two reviewers independently assessed the sequence generation, allocation concealment, blinding of participants, blinding of assessors, incomplete outcome data, and selective outcome reporting for the included studies (21).
Data Extraction and Analysis
Data were extracted from each article using a pre-designed template according to the study design, sample characteristics, measures, intervention duration, intervention design, and intervention effects (22). The randomized controlled trials (RCTs) had to distinguish between two and three groups in their designs. The specific headings of the summary table included the author (as well as the year of publication and country where the study was conducted), subjects' health characteristics, sample size, mean or age range of the sample, measure/intervention involving aerobic exercise, and intervention effect size (ES). If the study provided values for the intervention effect sizes, the data were extracted directly. If the study did not directly provide values for the effect size, conversion was performed using means, standard deviations (standard errors) and sample sizes; F-values and sample sizes; or t-values, p-values and sample sizes. Specific conversions were performed using an online program developed by Wilson (24). Additionally, Cohen's d shows a large bias when the sample is small (<20 for the overall sample or <10 for each group). Therefore, Cohen's d calculated based on small samples needs to be corrected using a method proposed by Hedges and Olkin (25). Descriptive statistics and one-way ANOVA were performed on the extracted data using the SPSS 24.0 software.
Results
Selection of Sources of Evidence
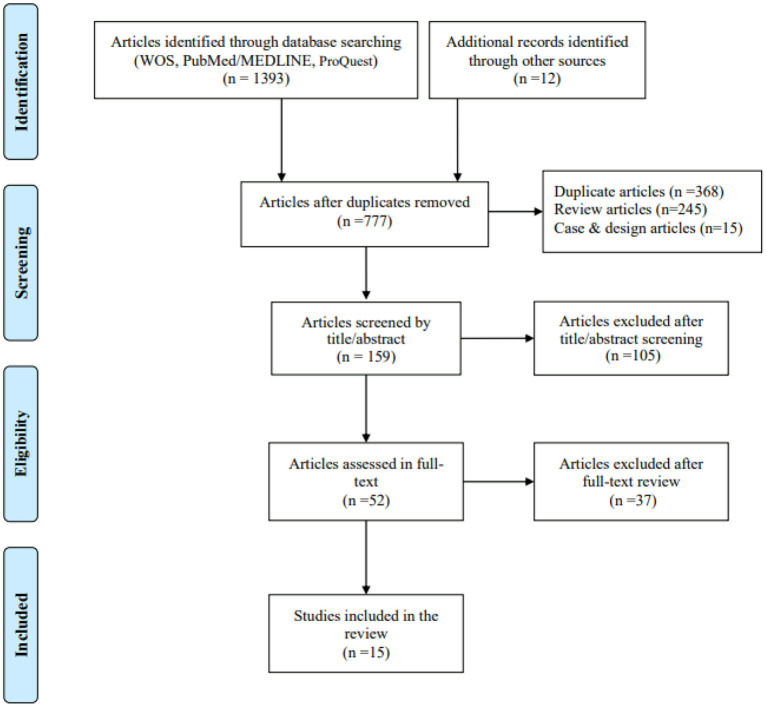
A total of 1,393 articles were identified using electronic databases such as Web of Science, PubMed/Medline and ProQuest, as were 12 articles from other systematic reviews. After removing duplicates and reviewing the titles, abstracts and full texts, 15 studies were finally included in the present study (Figure 1). Of these studies, two reported two and three measures of testing, respectively. Thus, a total of 18 intervention-effect-size results needed to be extracted.
Figure 1.
Flow diagram for evidence source search. From: Moher et al. (26).
Characteristics of Sources of Evidence
Data from 1,487 participants from 15 studies were included in the evidence analysis (see Table 1). Overall, the mean age of the participants was a minimum of 66.43 years and a maximum of 83.59 ± 7.05 years, with five studies (29, 31, 35, 37, 40) in which the subjects were over 65 years of age, the rest being over 60 years of age. The duration of the exercise interventions was at least 8 weeks (2 months) and at most 15 months. The frequency of the exercise interventions was 2–7 times per week, with the frequency of those in the majority of the studies being 3–5 times per week. In addition, five studies specified maximum loads for exercise, with the load controlled at 50–75% of the maximum heart rate (27, 33, 34, 39, 40); three studies also emphasized that subjects' attendance had to be no <70 or 80% (29, 37, 39).
Table 1.
The characteristics of sources of evidence.
| ID | Name, country | Time | N (f) | Subjects | Age | RCT | Measure | Exercise design | Exercise frequency |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Anderson-Hanley et al. (11) USA |
3 m | 111 (73) | MCI | All: 78.1 ± 9.9 EG1: 80.9 ± 12.3 EG2: 75.4 ± 9.83 |
RCT three groups | Stroop A/C | EG1: exer-tour, low cognitive load, virtual scenic bike tour EG2: exer-score, high cognitive demand, videogame CG: game-only |
EG1: 20–45 min, 3–5 tim/wk EG2: 20–45 min, 3–5 tim/wk CG: N/R |
| 2 | Awick et al. (27) USA |
12 m | 179 (N/R) | Low-active non-CI | All: 66.4 | RCT | SF12-MCS | EG: walking CG: strengthening and flexibility |
EG: first 7 weeks, 50–60% HR; next, 65–75% HR CG: 4 muscle resistance, 2 balance, 1 yoga, and 1 exercise of self-choice in each class |
| 3 | Bieler et al. (28) Denmark | 4 m | 152 (103) | OA | EG1: 69.6 ± 5.4 EG2: 70.0 ± 6.3 CG: 69.3 ± 6.4 |
RCT three groups observer-blinded | SF36-MCS | EG1: NW, Nordic Walking EG2: ST, strength training CG: HBE, home-based exercise |
EG1: NW, 1 h × 3 tim/wk, 12–14 on the Borg scale EG2: ST, 1 h × 3 tim/wk, 75% of 1RM CG: HBE, exercises recommended by the DAA |
| 4 | Cancela et al. (29) Spain | 15 m | 189 (126) | Dementia | EG: 80.63 ± 8.32 CG: 82.90 ± 7.42 |
RCT blinded | CSDD | EG: aerobic exercise CG: not exercise |
EG: daily cycling sessions; 15 min/s, >70% monthly attendance; CG: daily life |
| MEC | |||||||||
| NPI | |||||||||
| 5 | Cheung et al. (30) USA |
8 w | 73 (73) | OA | EG1: 68.9 ± 7.1 EG2: 74.4 ± 7.5 CG: 71.8 ± 8.0 |
RCT three arms blinded | SF12-MCS | EG1: HY, yoga EG2: ASE, aerobic and strengthening CG: education |
EG1: HY, (a) 45 min/wk × 8 wk, and (b) 30 min/day, 4 tim/wk of yoga EG2: ASE, (a) 8 tim/wk, (b) aerobic 15–30 min/day, 4 tim/week, and (c) strengthening 30 min/day, 2 tim/wk CG: education brochures, weekly telephone |
| 6 | Eggenberger et al. (31) Switzerland | 6 m | 89 (46) | Non-CI | EG1: 77.3 ± 6.3 EG2: 78.5 ± 5.1 CG: 80.8 ± 4.7 |
RCT three groups blinded | PACES | EG1: DANCE, virtual reality video game dancing EG2: MEMROY, treadmill walking with simultaneous verbal memory training CG: PHYS, treadmill walking |
Each group training: 2 tim/wk, 1 h/tim, vigorous intensity |
| 7 | Hall et al. (32) USA |
12 w | 54 (5) | PTSD | EG: 67.7 ± 3.2 CG: 66.9 ± 4.3 |
RCT-Pilot blinded | SF36-PCS | EG: exercise CG: wait-list usual care |
3 tim/wk, >150 min/wk, moderate intensity |
| 8 | Karssemeijer et al. (33) Netherlands | 12 w | 115 (54) | Dementia | EG1: 80.9 ± 6.1 EG2: 79.0 ± 6.9 CG: 79.8 ± 6.5 |
RCT: three arms | EFIP | EG1: stationary cycling training EG2: cognitive-aerobic bicycle training CG: relaxation and flexibility |
EG1: 30-50 min/tim, 3 tim/wk, 65–75% HR EG2: 30–50 min/tim, 3 tim/wk, 65–75% HR CG: 30 min/tim, 3 tim/wk |
| 9 | Langoni et al. (34) Brazil |
24 w | 52 (40) | MCI | EG: 72.6 ± 7.8 CG: 71.9 ± 7.9 |
RCT, single-blinded | MMSE | EG: group exercise (aerobic and strength)CG: no exercise | EG: 60 min/tim, 2 tim/wk, 60–75% HR CG: N/R |
| 10 | Middleton et al. (35) Canada | 12 w | 126 (82) | cognitive complaints | All: 73.0 ± 6.0 EG: 72.5 ± 5.9 CG: 74.3 ± 5.9 |
RCT: blinded 2 × 2 factorial design | SF12-MCS | EG: aerobic or stretching/toning,CG: mental activity, computer-based cognitive training or educational DVDs | EG: 3 × 60 min/wk CG: 3 ×60 min/wk |
| 11 | Parvin et al. (36) Iran |
12 w | 32 (N/R) | AD | All: 67.4 ± 8.8 | RCT, single-blinded | MoCA | EG: visual stimulation (muscle endurance, balance, flexibility, and aerobic exercises)CG: daily life | EG: 2 ×40–60 min/wk, warm-up 10 min, exercises 20–40 min, cool down 10 min. CG: daily life |
| 12 | Suzuki et al. (37) Japan |
12 m | 50 (27) | amnestic MCI | EG: 75.3 ± 7.5 CG: 76.8 ± 6.8 |
RCT | MMSE | EG: aerobic exercises, muscle strength, postural balance CG: education classes |
EG: 90 min/d, 2 d/wk CG: 3 d /12 mos |
| 13 | Varela et al. (38) Spain |
15 m | 39 (15) | Non-CI | EG: 83.59 ± 7.05 CG: 77.94 ± 8.79 |
RCT, single-blinded | MEC | EG: cycling CG: recreational activities |
EG: self-selected intensity 15 min/d, >70% completion rate CG: 3 d /12 mos |
| NPI | |||||||||
| 14 | Wanderley et al. (39) Portugal | 8 m | 105 (27) | Non-CI | EG1: 70.0 ± 5.7 EG2: 67.3 ± 4.9 CG: 67.8 ± 5.5 |
RCT three-groups blinded | SF36-MCS | EG1: aerobic training EG2: resistance training CG: daily lifestyle |
EG1: AT-−70–80% HR reserve, attendance rate >80%; 3 d/wk, 50 min/d; EG2: RT-−80% 1RM, attendance rate >80%; 3 d/wk, 50 min/d; CG: WL— not to change daily lifestyle |
| 15 | Zanetidou et al. (40) Italy | 24 w | 121 (86) | Late-life depression | EG: 74.9 ± 6.2 CG: 75.6 ± 5.6 |
RCT single-blinded | Anxiety | EG1: AD + NPE (low-intensity, non-progressive exercise), mat work and instrumental exercises EG2: AD + PAE (high-intensity, progressive aerobic exercise), cycling exercise CG: AD (sertraline) |
EG1: 3 tim/wk, <70% HR EG2: 3 tim/wk, <70% HR CG: daily lifestyle |
N/R, not reported; EG, experimental group; CG, control group; w, week; m, month; tim/wk, times/week; HR, heart rate. MCI, mild cognitive impairment; Non-CI, without cognitive impairment; PTSD, post-traumatic stress disorder; AD: Alzheimer's disease; OA: osteoarthritis. EFIP, Evaluative Frailty Index for Physical activity; SF12/36, SF-12/36 Health related Short Form 12/36; MCS, mental component summary; CSDD, Cornell Scale for Depression in Dementia; MEC, Mini-Examen Cognoscitive; NPI, Neuropsychiatric Inventory; PACES, Physical Activity enjoyment scale; MMSE, the mini-mental state examination; MoCA, Montreal cognitive assessment.
All the experimental designs included in this study were performed RCTs. Of all the included studies, eight used a three-group experimental design, with two groups for the exercise intervention and one non-exercise control group. For the other seven studies, participants were randomized into two groups for the exercise intervention and control group (41). In the three-group experimental design, except for the aerobic exercise, another exercise intervention group was studied, focusing on resistance training (27), stretching training (30), or a cognitive intervention plus aerobic training (33). The subjects in the study included three categories: no cognitive impairment (27, 31, 38, 39), cognitive impairment [mild (11, 34, 35, 37), dementia (29, 33), depression (32, 40), and Alzheimer's disease (36)] and physical impairment (osteoarthritis, etc.) (28, 30).
Risk-of-Bias Assessment for Sources of Evidence
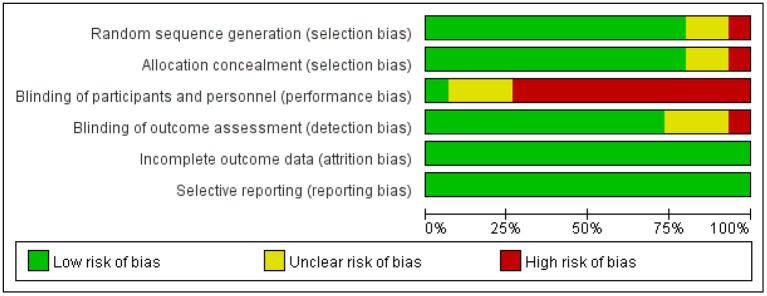
Figure 2 shows the assessment of the risk of bias for the sequence generation, allocation concealment, participant blinding, assessor blinding, incomplete outcome data, and selective outcome reporting (21). As shown in Figure 2, 3 of the 15 studies were unclear in the sequence generation (11, 33, 37), and four, in allocation concealment (32, 37) and the blinding of the assessor (11, 27, 37). Only one study reported blinding of the participants (31). Otherwise, all the studies had a low risk of bias in all domains (for details, see the online Supplementary Table 1).
Figure 2.
Risk-of-bias chart for studies included in the quantitative analysis.
ANOVA of the Intervention Effect Sizes
ANOVA was performed to facilitate the analysis of differences according to the various types of measures, durations, study subjects and exercise frequencies (42). We first categorized the data presented in Table 1. The measurements were coded as follows. (1) Measure category: 1 = SF12/SF36; 2 = NPI; 3 = Attitude (CSDD/EFIP/PACES/Anxiety); 4 = MMSE/MoCA/MEC. (2) Duration category: 1 = ≤3 months or 13 weeks; 2 = 3–6 months or 14–26 weeks; 3 = >6 months or 26 weeks. (3) Subject category: 1 = MCI/Dementia; 2 = Non-CI; 3 = OA. (4) Frequency category: 1 = <3 times/week; 2 = 3–5 times/week; 3 = >5 times/week.
Table 2 shows the effect sizes of the included studies and the recoded data. The mean effect size of the 15 included studies was 0.56 ± 0.39 (95%CI: 0.36–0.76). Table 3 shows the results of the descriptive statistics and one-way ANOVA for the effect sizes of the included studies. The results of the one-way ANOVA show that there were no significant differences in the intervention duration [F(2,15) = 0.919, p = 0.420], subject category [F(2,15) = 0.046, p = 0.955], or measure category [F(3,14) = 0.967, p = 0.436]. However, there was a significant difference in the exercise frequency factor [F(2,15) = 6.03, p = 0.012].
Table 2.
Inclusion of study effect sizes and classification of indicators.
| ID | Author, year | ES (Cohen's d) | ES level | Measure category | Duration category | Subject category | Frequency category |
|---|---|---|---|---|---|---|---|
| 1 | Anderson-Hanley (11) | 0.52 | M | 4 | 1 | 1 | 2 |
| 2 | Awick (27) | 0.44 | S | 4 | 3 | 2 | 2 |
| 3 | Bieler (28) | 1.13 | L | 2 | 2 | 3 | 2 |
| 4 | Cancela (29) | 0.35 | S | 4 | 3 | 1 | 3 |
| 5 | Cancela (29) | 0.48 | S | 4 | 3 | 1 | 3 |
| 6 | Cancela (29) | 0.42 | S | 2 | 3 | 1 | 3 |
| 7 | Cheung (30) | 0.16 | S | 4 | 1 | 3 | 2 |
| 8 | Eggenberger (31) | 1.06 | L | 2 | 2 | 2 | 1 |
| 9 | Hall (32) | 0.48 | S | 1 | 1 | 1 | 2 |
| 10 | Karssemeijer (33) | 0.35 | S | 1 | 1 | 1 | 2 |
| 11 | Langoni (34) | 0.65 | M | 1 | 2 | 1 | 1 |
| 12 | Middleton (35) | 0.08 | S | 3 | 1 | 1 | 2 |
| 13 | Parvin (36) | 1.70 | L | 3 | 1 | 1 | 1 |
| 14 | Suzuki (37) | 0.74 | M | 1 | 3 | 1 | 1 |
| 15 | Varela (38) | 0.54 | M | 1 | 3 | 2 | 3 |
| 16 | Varela (38) | 0.22 | S | 3 | 3 | 2 | 3 |
| 17 | Wanderley (39) | 0.47 | S | 1 | 3 | 2 | 2 |
| 18 | Zanetidou (40) | 0.30 | S | 4 | 2 | 1 | 2 |
ES Level: S, Small; M, Medium; L, Large.
Measure category: 1, SF12/SF36; 2, NPI; 3, Attitude (CSDD/EFIP/PACES/Anxiety); 4, MMSE/MoCA/MEC.
Duration category: 1, ≤3 months or 13 weeks; 2, 3–6 months or 14–26 weeks; 3, >6 months or 26 weeks.
Subject category: 1, MCI/Dementia; 2, Non-CI; 3, OA.
Frequency category: 1. <3 tim/wk; 2, 3–5 tim/wk; 3. >5 tim/wk.
Table 3.
Outcomes for descriptive statistics and ANOVA.
| N | M | SD | 95%CI | Min | Max | F | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Subjects | |||||||||
| MCI/dementia | 11 | 0.55 | 0.42 | 0.27 | 0.83 | 0.08 | 1.70 | 0.046 | 0.955 |
| Non-CI | 5 | 0.55 | 0.31 | 0.16 | 0.93 | 0.22 | 1.06 | ||
| OA | 2 | 0.65 | 0.69 | −5.52 | 6.81 | 0.16 | 1.13 | ||
| Duration (weeks/months) | |||||||||
| ≤13/3 | 6 | 0.55 | 0.59 | −0.07 | 1.17 | 0.08 | 1.70 | 0.919 | 0.420 |
| 14–26/3–6 | 4 | 0.79 | 0.39 | 0.17 | 1.40 | 0.30 | 1.13 | ||
| >26/6 | 8 | 0.46 | 0.15 | 0.33 | 0.58 | 0.22 | 0.74 | ||
| Measure | |||||||||
| SF12/36 | 6 | 0.46 | 0.37 | 0.07 | 0.85 | 0.08 | 1.13 | 0.967 | 0.436 |
| NPI | 2 | 0.32 | 0.14 | −0.95 | 1.59 | 0.22 | 0.42 | ||
| Attitude | 4 | 0.52 | 0.36 | −0.06 | 1.09 | 0.30 | 1.06 | ||
| MMSE/MoCA | 6 | 0.77 | 0.46 | 0.28 | 1.26 | 0.48 | 1.70 | ||
| Frequency (times/week) | |||||||||
| <3 | 4 | 1.04 | 0.48 | 0.28 | 1.79 | 0.65 | 1.70 | 6.030 | 0.012* |
| 3~5 | 9 | 0.44 | 0.30 | 0.21 | 0.67 | 0.08 | 1.13 | ||
| >5 | 5 | 0.40 | 0.12 | 0.25 | 0.56 | 0.22 | 0.54 | ||
p < 0.05.
Discussion
This study focused on the effects of aerobic exercise on the mental health of older adults (43). One-way ANOVA was used to examine four influencing factors across the study subjects, measures, intervention durations, and exercise frequency (44). The results show that only the ANOVA results were significantly different between different exercise frequencies. By contrast, there were no significant differences in the ANOVA results between the subjects, measurement indicators and intervention durations. This may not be in line with traditional studies. Therefore, we need to further analyze the possible reasons for this.
First, the quality of the included literature needs to be analyzed in terms of reliability. All the included studies were RCTs with the highest experimental grade, and all the studies were conducted in strict accordance with the established process for randomized controlled trials (45), except for four experimental designs with unclear random assignment methods and blinding points (11, 27, 33, 37). The included studies were reliable, with more than 70% to ensure a low risk of bias.
Second, was the coding of the impact factor classification scientific? The four impact factors selected for this study were reclassified and coded according to the needs of the study, and this classification was based on conventional experience (46). Therefore, the blind spots in the application of this method are currently unclear.
Finally, was the quality of the intervention effect size data extraction reliable? In addition to the categorical coding, the proposed intervention effect size is also an important factor influencing the results of the ANOVA in this study (47). Only one paper in this study provided effect size values directly (36), and the rest of the data were transformed using effect size calculation formula, which reduced the reliability of the data source. However, two people independently extracted and calculated the effect size separately, ensuring data integrity for the study. Despite all the three issues mentioned above, we followed strict scientific procedures to guarantee the quality of the included literature, coding classification and data extraction. However, the accuracy of the results provided by the original studies and the bias in the publication of the results could have affected the results of this study.
Comparison among the mean effect sizes of different exercise frequency groups (EFGs) showed that the lowest EFG obtained the largest effect size. The finding is similar to the results of a recent meta-analysis study of the cognitive function of older adults. That study suggested, in older adults, high-frequency exercise interventions did not affect cognitive function more than low-frequency ones (48). Similarly, another study of a 6-week exercise intervention showed no significant difference in effect size between the high-frequency and low-frequency groups (49). We reasoned that there might be methodological flaw in using only exercise frequency as an indicator of influencing factor. Yet another study revealed that exercise duration of more than 6 months was more effective than that of <6 months (50). In this study, the duration of the intervention was 6 months or more in 75% of the low-frequency group. Although the current evidence does not directly conclude that duration affects the effect of the intervention, regular and continuous exercise is undoubtedly beneficial for older adults. Thus, considering the benefits of low-frequency exercise with slightly higher or high-frequency exercise, older adults should primarily engage in low-frequency exercise.
In summary, there are several weaknesses in the present study. First, mixing different populations, outcome measures and exercise programs into the study may lead to high heterogeneity of fitting results. Second, one-way ANOVA only investigates the impact of a single factor on the observed variables, and cannot diagnose the interaction effects between factors (51). Third, selecting only effect size indicators ignores the value of sample size, which may produce uncontrollable errors (52). Therefore, future research should focus on seeking methodology breakthroughs while addressing the above issues.
Conclusions
This retrospective study confirmed the positive effect of aerobic exercise on the mental health of older adults with a moderate overall intervention effect (ES Cohen's d = 0.56). The results of the one-way ANOVA revealed that adults over 60 years of age, regardless of whether they have an intellectual disability or not, or are undergoing physical rehabilitation or not (mild motor impairment), can improve their mental health through aerobic exercise. We recommend low-frequency exercise for older adults when the exercise benefits of various modes are compared.
Author Contributions
LY, HF, WL, and JL: data collection. LY and JC: data analysis, conception, and design. LY, JL, and JC: research design, writing the manuscript, and revision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Education Science 13th Five-Year Plan Ministry of Education of the People's Republic of China Key Program (DLA190425).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely appreciate thank the reviewers for their comments and advices.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.748257/full#supplementary-material
References
- 1.Kessler RC, Haro JM, Heeringa SG, Pennell BE, Ustün TB. The world health organization world mental health survey initiative. Epidemiol Psichiatr Soc. (2006) 3:161–6. 10.1017/S1121189X00004395 [DOI] [PubMed] [Google Scholar]
- 2.Dunn AL, Jewell JS. The effect of exercise on mental health. Curr Sports Med Rep. (2010) 4:202–7. 10.1249/JSR.0b013e3181e7d9af [DOI] [PubMed] [Google Scholar]
- 3.Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, et al. Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J Affect Disord. (2007) 103:113–20. 10.1016/j.jad.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 4.Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the world health organization world mental health surveys. JAMA. (2004) 21:2581–90. 10.1001/jama.291.21.2581 [DOI] [PubMed] [Google Scholar]
- 5.Wang PS, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Borges G, Bromet EJ, et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet. (2007) 370:841–50. 10.1016/S0140-6736(07)61414-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aron L, Honberg R, Duckworth K, Kimball A, Edgar E, Carolla B, et al. Grading the States 2009: A Report on America's Health Care System for Adults with Serious Mental Illness. Arlington, VA: National Alliance on Mental Illness; (2009). [Google Scholar]
- 7.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. (2005) 58:175–89. 10.1016/j.biopsych.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Dubbert PM, White JD, Grothe KB, O'Jile J, Kirchner KA. Physical activity in patients who are severely mentally ill: feasibility of assessment for clinical and research applications. Arch Psychiatr Nurs. (2006) 5:205–9. 10.1016/j.apnu.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 9.Benson K. The effects of exercise on mental health: a research review. (Honors College Theses) (2017). p. 36. Available online at: https://digitalcommons.wayne.edu/honorstheses/3 (accessed July 07, 2021).
- 10.Veenhuis PE. Intellectual disability and ill health: a review of the evidence. JAMA. (2011) 7:770–1. 10.1001/jama.2011.1167 [DOI] [Google Scholar]
- 11.Anderson-Hanley C, Barcelos NM, Zimmerman EA, Gillen RW, Dunnam M, Cohen BD, et al. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neurosci. (2018) 10:76. 10.3389/fnagi.2018.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDowell CP, Dishman RK, Gordon BR, Herring MP. Physical activity and anxiety: a systematic review and meta-analysis of prospective cohort studies. Am J Prev Med. (2019) 4:545–56. 10.1016/j.amepre.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Kandola A, Ashdown-Franks G, Stubbs B, Osborn DPJ, Hayes JF. The association between cardiorespiratory fitness and the incidence of common mental health disorders: a systematic review and meta-analysis. J Affect Disord. (2019) 257:748–57. 10.1016/j.jad.2019.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennie JA, Teychenne MJ, De Cocker K. Biddle SJH. Associations between aerobic and muscle-strengthening exercise with depressive symptom severity among 17,839 US adults. Prev Med. (2019) 121:121–7. 10.1016/j.ypmed.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 15.Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. (2021) 72:45–62. 10.1146/annurev-med-060619-022943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Chen Y, Liu C, Yong L, Yang M, Zhu W, et al. Effect of square dance exercise on older women with mild mental disorders. Front Psychiatry. (2021) 12:699778. 10.3389/fpsyt.2021.699778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang J, Zhu W, Zhang J, Yong L, Yang M, Wang J, et al. The effect of Chinese square dance exercise on cognitive function in older women with mild cognitive impairment: the mediating effect of mood status and quality of life. Front Psychiatry. (2021) 12:711079. 10.3389/fpsyt.2021.711079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong L, Liu L, Ding T, Yang G, Su H, Wang J, et al. Evidence of effect of aerobic exercise on cognitive intervention in older adults with mild cognitive impairment. Front Psychiatry. (2021) 12:71361. 10.3389/fpsyt.2021.713671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. (2005) 1:1–8. 10.1016/j.amepre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 20.Stillman CM, Erickson KI. Physical activity as a model for health neuroscience. Ann N Y Acad Sci. (2018) 1:103–11. 10.1111/nyas.13669 [DOI] [PubMed] [Google Scholar]
- 21.Pascoe MC, Bailey AP, Craike M, Carter T, Patten R, Stepto NK, et al. Exercise interventions for mental disorders in young people: a scoping review. BMJ Open Sport Exerc Med. (2020) 6:e000678. 10.1136/bmjsem-2019-000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen W. A systematic review of measures for psychological well-being in physical activity studies and identification of critical issues. J Affect Disord. (2019) 256:473–85. 10.1016/j.jad.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 23.Aldridge AA, Roesch SC. Coping and adjustment in children with cancer: a meta-analytic study. J Behav Med. (2007) 2:115–29. 10.1007/s10865-006-9087-y [DOI] [PubMed] [Google Scholar]
- 24.Wilson DB. Practical Meta-Analysis Effect Size Calculator. George Mason University (2019). Available online at: https://wwwcampbellcollaborationorg/escalc/html/EffectSizeCalculator-Homephp (accessed June 20, 2021).
- 25.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic Press; (1985). [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta- analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awick EA, Wójcicki TR, Olson EA, Fanning J, Chung HD, Zuniga K, et al. Differential exercise effects on quality of life and health-related quality of life in older adults: a randomized controlled trial. Qual Life Res. (2015) 2:455–62. 10.1007/s11136-014-0762-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bieler T, Siersma V, Magnusson SP, Kjaer M, Christensen HE, Beyer N. In hip osteoarthritis, Nordic Walking is superior to strength training and home-based exercise for improving function. Scand J Med Sci Sports. (2017) 8:873–86. 10.1111/sms.12694 [DOI] [PubMed] [Google Scholar]
- 29.Cancela JM, Ayán C, Varela S, Seijo M. Effects of a long-term aerobic exercise intervention on institutionalized patients with dementia. J Sci Med Sport. (2016) 4:293–8. 10.1016/j.jsams.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Cheung C, Wyman JF, Bronas U, McCarthy T, Rudser K, Mathiason MA. Managing knee osteoarthritis with yoga or aerobic/strengthening exercise programs in older adults: a pilot randomized controlled trial. Rheumatol Int. (2017) 3:389–98. 10.1007/s00296-016-3620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin ED. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin Interv Aging. (2015) 10:1335–49. 10.2147/CIA.S87732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall KS, Morey MC, Beckham JC, Bosworth HB, Sloane R, Pieper CF, et al. Warrior wellness: a randomized controlled pilot trial of the effects of exercise on physical function and clinical health risk factors in older military veterans with PTSD. J Gerontol A Biol Sci Med Sci. (2020) 11:2130–8. 10.1093/gerona/glz255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karssemeijer EGA, Bossers WJR, Aaronson JA, Sanders LMJ, Kessels RPC, Olde Rikkert MGM. Exergaming as a physical exercise strategy reduces frailty in people with dementia: a randomized controlled trial. J Am Med Direct Assoc. (2019) 12:1502–8.e1. 10.1016/j.jamda.2019.06.026 [DOI] [PubMed] [Google Scholar]
- 34.Langoni CDS, Resende TL, Barcellos AB, Cecchele B, Knob MS, Silva TDN, et al. Effect of exercise on cognition, conditioning, muscle endurance, and balance in older adults with mild cognitive impairment: a randomized controlled trial. J Geriatr Phys Ther. (2019) 2:E15–22. 10.1519/JPT.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 35.Middleton LE, Ventura MI, Santos-Modesitt W, Poelke G, Yaffe K, Barnes DE. The mental activity and exercise (MAX) trial: effects on physical function and quality of life among older adults with cognitive complaints. Contemp Clin Trials. (2018) 64:161–6. 10.1016/j.cct.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parvin E, Mohammadian F, Amani-Shalamzari S, Bayati M, Tazesh B. Dual-task training affect cognitive and physical performances and brain oscillation ratio of patients with Alzheimer's disease: a randomized controlled trial. Front Aging Neurosci. (2020) 12:605317. 10.3389/fnagi.2020.605317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, et al. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. (2012) 12:128. 10.1186/1471-2377-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varela S, Cancela JM, Seijo-Martinez M, Ayán C. Self-paced cycling improves cognition on institutionalized older adults without known cognitive impairment: a 15-month randomized controlled trial. J Aging Phys Act. (2018) 4:614–23. 10.1123/japa.2017-0135 [DOI] [PubMed] [Google Scholar]
- 39.Wanderley FAC, Oliveira NL, Marques E, Moreira P, Oliveira J, Carvalho J. Aerobic versus resistance training effects on health-related quality of life, body composition, and function of older adults. J Appl Gerontol. (2015) 3:NP143–65. 10.1177/0733464812468502 [DOI] [PubMed] [Google Scholar]
- 40.Zanetidou S, Belvederi Murri M, Menchetti M, Toni G, Asioli F, Bagnoli L, et al. Safety efficacy of exercise for depression in seniors study group. Physical exercise for late-life depression: customizing an intervention for primary care. J Am Geriat Soc. (2017) 2:348–55. 10.1111/jgs.14525 [DOI] [PubMed] [Google Scholar]
- 41.Valiquette-Tessier SC, Vandette MP, Gosselin J. Is family structure a cue for stereotyping? A systematic review of stereotypes and parenthood. J Fam Stud. (2016) 2:162–81. 10.1080/13229400.2015.1049955 [DOI] [Google Scholar]
- 42.Elmussareh M, Traxer O, Somani BK, Biyani CS. Laser endopyelotomy in the management of pelviureteric junction obstruction in adults: a systematic review of the literature. Urology. (2017) 107:11–22. 10.1016/j.urology.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 43.Nuzum H, Stickel A, Corona M, Zeller M, Melrose RJ, Wilkins SS. Potential benefits of physical activity in MCI and dementia. Behav Neurol. (2020) 2020:7807856. 10.1155/2020/7807856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stillman CM, Esteban-Cornejo I, Brown B, Bender CM, Erickson KI. Effects of exercise on brain and cognition across age groups and health states. Trends Neurosci. (2020) 7:533–43. 10.1016/j.tins.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toohey K, McKune A, Nahon I, Kavanagh PS, Newton RU, Paterson C. Improving physical and mental health in patients with prostate cancer undergoing androgen deprivation therapy: strategies to promote and improve physical activity quality and quantity. Semin Oncol Nurs. (2020) 4:151051. 10.1016/j.soncn.2020.151051 [DOI] [PubMed] [Google Scholar]
- 46.Wolpert M, Dalzell K, Ullman R, Garland L, Cortina M, Hayes D, et al. Strategies not accompanied by a mental health professional to address anxiety and depression in children and young people: a scoping review of range and a systematic review of effectiveness. Lancet Psychiatry. (2019) 1:46–60. 10.1016/S2215-0366(18)30465-6 [DOI] [PubMed] [Google Scholar]
- 47.Ströhle A. Sports psychiatry: mental health and mental disorders in athletes and exercise treatment of mental disorders. Eur Arch Psychiatry Clin Neurosci. (2019) 5:485–98. 10.1007/s00406-018-0891-5 [DOI] [PubMed] [Google Scholar]
- 48.Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. (2019) 1:181. 10.1186/s12877-019-1175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berkhuysen MA, Nieuwland W, Buunk BP, Sanderman R, Viersma JW, Rispens P. Effect of high- versus low-frequency exercise training in multidisciplinary cardiac rehabilitation on health-related quality of life. J Cardiopulm Rehabil. (1999) 1:22–8. 10.1097/00008483-199901000-00003 [DOI] [PubMed] [Google Scholar]
- 50.Stubbs B, Koyanagi A, Hallgren M, Firth J, Richards J, Schuch F, et al. Physical activity and anxiety: a perspective from the World Health Survey. J Affect Disord. (2017) 208:545–52. 10.1016/j.jad.2016.10.028 [DOI] [PubMed] [Google Scholar]
- 51.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M, et al. comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. (2008) 5:56. 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matta Mello Portugal E, Cevada T, Sobral Monteiro-Junior R, Teixeira Guimarães T, da Cruz Rubini E, Lattari E, et al. Neuroscience of exercise: from neurobiology mechanisms to mental health. Neuropsychobiology. (2013) 1:1–14. 10.1159/000350946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.