Abstract
Background:
Short bowel syndrome is a potentially fatal condition with inadequate management options. Tissue-engineered small intestine (TESI) is a promising solution, but confirmation of TESI function will be crucial prior to human application. We sought to define intestinal epithelial barrier function in human intestinal organoid (HIO)-derived TESI.
Materials and Methods:
HIOs were generated in vitro from human embryonic stem cells (hESCs). After 1 month, HIOs were collected for analysis or transplanted into the kidney capsule of immunocompromised mice. Transplanted HIOs (tHIOs) were harvested for analysis at 4 or 8 weeks. RT-qPCR and immunofluorescent (IF) staining were performed for tight junction components: claudin 3 (CLDN3), claudin 15 (CLDN15), occludin (OCLN), and zonula occludens-1, or tight junction protein-1 (TJP1/ZO-1).
Results:
Four week old tHIOs demonstrated significantly (p<0.05) higher levels of CLDN15 (6x), OCLN (4x), and TJP1/ZO-1 (3x) normalized to GAPDH compared to in vitro HIOs. Eight week old tHIOs demonstrated significantly (p<0.05) higher expression levels of CLDN3 (26x), CLDN15 (29x), OCLN (4x), and TJP1/ZO-1 (5x) compared to in vitro HIOs. There was no significant difference in expression of these tight junction components between 4- and 8-week old tHIOs. IF staining revealed the presence of claudin 3, claudin 15, occludin, and zonula occludens-1 in both in vitro HIOs and tHIOs, however the morphology appeared more mature in tHIOs.
Conclusions:
In vitro HIOs have lower levels of tight junction mRNA and tight junction proteins appear morphologically immature. Transplantation facilitates maturation of the HIOs and enhances select tight junction gene expression.
Keywords: Human intestinal organoid, Short bowel syndrome, Intestine, Organoid, Tight junctions, Intestinal epithelial barrier function
Introduction
Short bowel syndrome (SBS) is clinically significant problem incurring over $500,000 in costs per patient in the first year alone1. Although parenteral nutrition has decreased morbidity and mortality, current therapies are associated with serious complications and are insufficient in some patients2. Tissue-engineered small intestine (TESI) may be a potential therapeutic solution3–9.
One of the challenges precluding human therapeutic use of TESI for SBS is the confirmation of appropriate function. The small intestine has three major functions: propulsive peristalsis, nutrient absorption, and barrier maintenance10. Intestinal epithelial tight junctions preserve the barrier against luminal pathogens while allowing selective absorption of nutrients. Three of the major tight junction proteins include claudins, occludin, and zonula occludens-1, also known as tight junction protein-111–14. The intestinal epithelial barrier has been previously studied in human intestinal organoid (HIO)-derived TESI6,15. HIO-derived TESI is generated from in vitro HIOs that are transplanted into non-obese diabetic, severe combined immunodeficiency, gamma chain deficient (NSG™) mice. This in vivo HIO is termed a transplanted HIO (tHIO). Watson et al demonstrated that tHIOs have a functional intestinal epithelial barrier as shown by a permeability assay with FITC-dextran6. Poling et al compared the intestinal epithelial barrier function between tHIOs, tHIOs with applied mechanical strain from an implanted nitinol spring (tHIO+S), and human adult jejunum. They showed that corrected short-circuit currents had a decreasing trend from tHIO to tHIO+S but the difference was not statistically significant. Interestingly, the corrected calculated FITC-dextran flux was significantly decreased in tHIO+S versus tHIO, and the tHIO+S trended toward the level of the human adult jejunum. The transepithelial resistance (TER) levels of tHIO, tHIO+S, and human adult jejunum were similar15. Poling et al additionally performed RNA-seq and evaluated normalized FPKMs for tHIO, tHIO+S, and human adult jejunum for tight junction components: tight junction protein 1 (TJP1)/zonula occludens-1 (ZO-1), junctional adhesion molecule 1 (F11R/JAM1), and metadherin (MTDH). The expressional levels for F11R/JAM-1 and MTDH were significantly increased in the tHIO+S versus the tHIOs, but TJP-1/ZO-1 gene expression was not significantly increased15. These results suggest that tHIOs have the presence of some tight junction components required to maintain intestinal barrier function, but the function is not yet equivalent to that of human adult jejunum. Furthermore, two major tight junction components: claudins and occludin, have not yet been investigated in HIOs or tHIOs. Therefore, we sought to further evaluate intestinal epithelial barrier function in HIO-derived TESI.
In this study, we hypothesized that tight junction components claudin 3, claudin 15, occludin, and zonula occludens-1 would be present in HIOs and tHIOs, but with enhanced production and morphology after in vivo transplantation.
Material and methods
Animal use
All animals were housed at The Center for Laboratory Animal Medicine and Care (CLAMC) at The University of Texas Health Science Center at Houston (UTHealth) and all animal procedures were performed with the approval of the Animal Welfare Committee (protocol #AWC-17–0017). The mouse colony was established from breeder non-obese diabetic, severe combined immunodeficiency, gamma chain deficient (NSG™) mice obtained from The Jackson Laboratory.
Generation and growth of human intestinal organoids
Human intestinal organoids (HIOs) were generated and grown in vitro using previously published protocols6,16. Human embryonic stem cells (hESCs) (H9, WiCell) were maintained in 6-well plates (Corning) coated with hESC-qualified Matrigel (Corning) in mTeSR™1 media (STEMCELL Technologies) changed daily. Induction of definitive endoderm (DE) was accomplished by passaging hESCs with ReLeSR™ (STEMCELL Technologies) and plated at a density of 75,000 cells per well in a hESC-qualified Matrigel (Corning) coated 24-well plate (Corning). When hESCs reached a 70–80% confluence, they were then treated with 100ng/mL of Activin A (Cell Guidance Systems) for three days. DE was then treated with hindgut induction medium (RPMI 1640, 2% defined FBS, NEAA 100x, 500ng/mL FGF4 (Peprotech), and 3μM Chiron 99021 (Tocris)) to induce mid-hindgut spheroid formation. Spheroids were collected at day 4 and suspended in GFR Basement Membrane phenol-free Matrigel and maintained using minigut medium (Advanced DMEM/F-12, N2, B27, 15 mM HEPES, 2 mM glutamax, penicillin-streptomycin) supplemented with RSpondin conditioned media (Texas Medical Center (TMC) Digestive Diseases Center (DDC) GEMS Core, Enteroid/Organoid Sub-Core) and 100 ng/ml EGF (ThermoFisher Scientific). Additionally, the gut spheroids were treated for the first 3 days with 10% Noggin conditioned media (TMC DDC GEMS Core, Enteroid/Organoid Sub-core) to generate HIOs. The media was changed at three days and then changed twice weekly thereafter. HIOs were grown in minigut medium for 25–40 days without passaging and then either collected for histology and RNA isolation or they were transplanted into NSG™ mice.
Transplantation of human intestinal organoids
Adult 9–18 week old male NSG™ mice served as hosts for HIO transplantation. Male mice were used because they tend to be larger than females of the same age, which makes transplantation easier. Additionally, some studies have suggested that sex hormones such as estrogen or progesterone may improve intestinal epithelial barrier function via the upregulation of tight junction proteins17. Therefore, in this preliminary study, we used only male mice to ensure that our results would not be confounded by potential sex-related differences. However, future studies are needed to compare HIO transplantation between male and female hosts to identify any sex-related distinctions. The mice were maintained in a temperature-regulated environment on a 12-hour light-dark cycle and given access to regular chow and water ad libitum pre- and post-operatively. A single HIO, grown in vitro for 25–40 days was removed from Matrigel with cold phosphate-buffered saline (Gibco by Life Technologies) and transplanted under the left kidney capsule similar to previously described18. We chose to transplant beneath the kidney capsule6,8 as opposed to the intestinal mesentery19 or the omentum7,9 because technical proficiency is more easily achieved and host mouse mortality is decreased20. The mice were anesthetized first using 4–5% inhaled isoflurane in an induction chambered, followed by 1–2% isoflurane titrated to effect via face mask with a precision vaporizer for maintenance. The fur on the left side of the mice was clipped and the left flank prepped in the usual sterile fashion with alternating chlorhexidine and 70% alcohol. A sterile draped was placed and then the mice were given bupivacaine 0.25% at <2.5mg/kg subcutaneously as well as ketofen 5mg/kg subcutaneously in the left thigh. A small left flank incision was made to expose the left kidney. A subcapsular pocket was created and the HIO was placed directly into the pocket. The kidney was returned to the retroperitoneum and the muscle and skin were closed in two layers with 4–0 or 5–0 Vicryl. Mice were checked daily for the first five postoperatively days and then daily Monday-Friday. Mice received carprofen 5 mg/kg PO for the first two postoperative days for analgesia. Mice received trimethoprim/sulfamethoxazole 40mg/200mg/5mL oral suspension at 1mL per 100mL of drinking water as antibiotic prophylaxis for seven postoperative days. Mice were humanely euthanized at either 4, 8, or 12 weeks after transplantation, and the transplanted HIO (tHIO) was harvested for histology and RNA isolation.
Human intestinal organoid tissue processing
HIOs were grown in vitro and then collected at 28 days and fixed in 4% paraformaldehyde (PFA) for 30 minutes on ice at 4°C. HIOs were then washed once with PBS (Gibco by Life Technologies) in a petri dish. HIOs were then placed in 50 μL of 0.02% methylene blue (Sigma-Aldrich) for 15 minutes at room temperature (RT). HIOs were then placed in 30% sucrose (Fisher Chemical) at 4°C until they sunk to the bottom of the Eppendorf. HIOs were then dab dried with a kim-wipe (Kimtech), and suspended in optimum cutting temperature (OCT) compound (Sakura). The OCT compound was fixed using a cooling block on dry ice. After fixation, the HIOs were cryosectioned at 10μm and placed on glass slides (Surgipath) at −20°C.
Transplanted human intestinal organoid tissue processing
tHIOs were grown in vivo and harvested at 4, 8, or 12 weeks from the NSG™ mouse host. tHIOs were placed in 4% PFA at RT for one hour. tHIOs were then washed in PBS three times for 5 minutes each. tHIOs were then either processed for cryosection or paraffin section. For cryosection, tHIOs were then placed in 30% sucrose at 4°C until they sunk to the bottom of the Eppendorf. tHIOs were then dab dried with a kim-wipe, and suspended in OCT. The OCT compound was fixed using a cooling block on dry ice. After fixation, tHIOs were cryosectioned at 5–7μm and placed on glass slides (Surgipath) at −20°C. For paraffin section, tHIOs were then placed into 70% ethanol overnight at RT. The tHIOs were then dehydrated from 70% to 95% to 100% ethanol for an hour each, followed by Xylene for an additional hour, and finally melted paraffin. tHIOs were then embedded in paraffin blocks. Paraffin blocked tHIOs were then sectioned at 5–7μm and placed on glass slides (Surgipath) at RT.
Immunofluorescence and microscopy
Paraffin sectioned tHIO slides were stained with hematoxylin and eosin. Cryosectioned HIOs and tHIO slides were thawed for 30 minutes at RT and stained with hematoxylin and eosin or subjected to immunohistochemistry. For immunohistochemistry, cryosections were subsequently washed in PBS three times for 5 minutes each. During the permeabilization step, cryosections were placed in 0.5% Triton TX-100 (Sigma) for 20 minutes and then washed in PBS three times for five minutes each. The slides were then wiped dry with a kim-wipe and a pap pen was used to encircle the cryosections. The cryosections were blocked with 100–200μL of 5% normal goat serum (Vector Laboratories) in PBS with 0.1% Tween 20 (Sigma) for one hour at RT. The serum was then aspirated from the slide. Cryosections were then incubated in primary antibody overnight at 4°C according to Table 1. Cryosections were washed in PBS three times for 5 minutes each, followed by incubation in secondary antibody for one hour at RT according to Table 2. The cryosections were then washed in PBS three times for 5 minutes each. Slides were then mounted with Vectashield with DAPI (Vector Laboratories) and coverslipped. Slides were imaged on a Leica upright DM4000 fluorescent microscope or EVOS FL auto 2 microscope.
Table 1.
Immunofluorescence primary antibodies.
| 1° Antibody | Animal | Dilution | Company | Catalog # |
|---|---|---|---|---|
| ZO-1 | rabbit | 1:100 | ThermoFisher | 61–7300 |
| OCLN | rabbit | 1:50 | ThermoFisher | 71–1500 |
| CLDN-3 | rabbit | 1:40 | abcam | ab15102 |
| CLDN-15 | rabbit | 1:20 | ThermoFisher | 38–9200 |
Table 2.
Immunofluorescence secondary antibodies.
| 2° Antibody | Animal | Dilution | Company | Catalog # |
|---|---|---|---|---|
| Alexa Fluor 488 | Anti-rabbit | 1:200 | Invitrogen | A-11008 |
| Alexa Fluor 647 | Anti-rabbit | 1:500 | Life Science Technologies | A-21244 |
RNA isolation and RT-qPCR
RNA was extracted from HIOs and tHIOs using the E.Z.N.A.® Total RNA Kit I (Omega Bio-Tek) according to the manufacturer’s protocol instructions. A nanodrop was used to measure the purity and concentration of the isolated RNA. An iScript™ reverse transcription kit (BIO-RAD) was used to synthesize cDNA with a Mastercycler (Eppendorf). RT-qPCR was then performed on duplicate samples using the Applied Biosystems PowerUp™ SYBR™ Green Master Mix protocol (ThermoFisher Scientific) and a QuantStudio 3 Real-Time PCR System (Applied Biosystems). See Table 3 for a list of primers (Integrated DNA Technologies) used.
Table 3.
RT-qPCR primers.
| Gene | Gene Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | GAA GTT GAA GGT CGG AGT CA | TTG AGG TCA ATG AAG GGG TC |
| CLDN3 | Claudin 3 | AAC ACC ATT ATC CGG GAC TTC T | GCG GAG TAG ACG ACC TTG G |
| CLDN15 | Claudin 15 | CTG CGC TGC ACC AAC ATT G | GGT ACA AGG GGT CGA AGA AGT |
| OCLN | Occludin | ACA AGC GGT TTT ATC CAG AGT C | GTC ATC CAC AGG CGA AGT TAA T |
| TJP1/ZO-1 | Tight Junction Protein 1/Zonula Occludens-1 | ACC AGT AAG TCG TCC TGA TCC | TCG GCC AAA TCT TCT CAC TCC |
Statistical analyses
All data are represented as the mean ± s.e.m. t-tests were completed using Prism v 7.0 (GraphPad). No statistical method was used to predetermine sample size.
Results
Transplantation of human intestinal organoids in vivo facilitates maturation and growth.
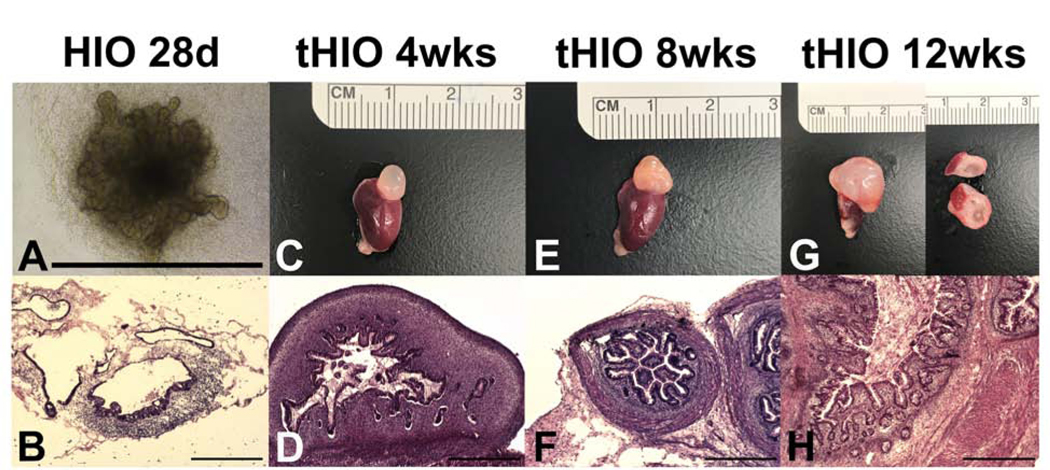
In order to demonstrate that sufficient intestinal epithelium would be present to identify, locate, and quantify tight junction genes and proteins, we grew HIOs in vitro for 28 days. HIOs exhibited a primitive epithelium lacking a crypt-villus axis with surrounding mesenchyme on brightfield microscopy (Figure 1A) as well as H&E staining (Figure 1B). To confirm that the tHIO epithelium would mature in vivo, we transplanted HIOs into NSG™ mice and harvested them after 4, 8, or 12 weeks of growth. tHIOs grew significantly in size from approximately 1000 μm on the day of transplantation to approximately 5–10mm after a period of in vivo growth. Figure 1C, 1E, and 1G are photos of tHIOs harvested at 4, 8, and 12 weeks, respectively. At 12 weeks, the tHIO measures approximately 10mm in maximum diameter, and transection of the tHIO reveals a visible lumen with a mucosal layer (Figure 1G). H&E staining revealed a rudimentary epithelium with surrounding mesenchyme in the 4 week old tHIO (Figure 1D), whereas 8 and 12 week old tHIOs demonstrated a mature intestinal epithelium with villi and crypts, distinct goblet cells, submucosa, and muscularis (Figure 1F and 1H). This data suggests that in vivo transplantation facilitates maturation of the intestinal tissue and an intestinal epithelium is present in order to assess the identity, location, and quantify tight junction genes and proteins.
Figure 1. Transplantation of human intestinal organoids in vivo facilitates maturation and growth of intestinal tissue.
A. Brightfield micrograph of an HIO after 28 days of in vitro growth. Scale bar 1000 μm.
B. H&E demonstrates a primitive epithelium lacking a crypt-villus axis with surrounding mesenchyme in a 28 day old HIO. Scale bar 500 μm. C,E,G. Harvested transplanted HIO (tHIO) adjacent to host mouse kidney at 4, 8, and 12 weeks, with the 12 week old tHIO demonstrating a visible mucosa and lumen after transection. D. H&E of a 4 week old tHIO revealed a rudimentary epithelium with surrounding mesenchyme. Scale bar 500 μm. F,H. H&E of 8 and 12 week old tHIOs exhibit a mature epithelium with villi and crypts as well as a robust mesenchyme with submucosa and muscular layers. Scale bar 500 μm.
Transplantation of human intestinal organoids in vivo promotes expression of select tight junction genes.
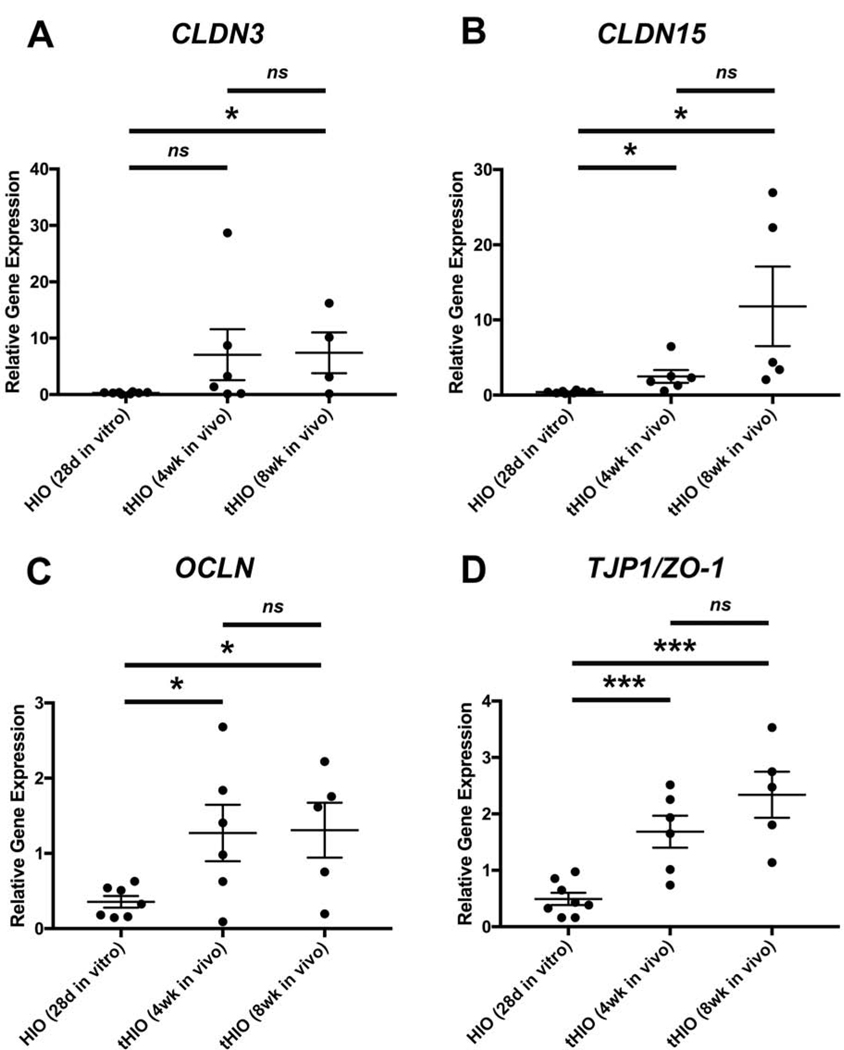
To determine if transplantation enhanced expression of select tight junction genes, we performed RT-qPCR for CLDN-3 (Figure 2A), CLDN-15 (Figure 2B), OCLN (Figure 2C), and TJP1/ZO-1 (Figure 2D) in HIOs grown in vitro for 28 days versus tHIOs after 4 or 8 weeks in vivo. The tight junction is complex and consists of numerous components11–14. For this preliminary study, we chose to focus on the components that would be most influential on the intestinal epithelial barrier function in the small intestine. Three of the major tight junction proteins include zonula occludens-1, also known as tight junction protein-1, occludin, and claudins11–14. Since there are 27 different claudin genes, we elected to focus on Claudins 3 and 15 as these are the most highly expressed throughout the small bowel with peak expression levels later in postnatal development21. We found that 4-week old tHIOs demonstrated significantly higher levels of CLDN15 (2.493 ± 0.8452, n=6 vs 0.4064 ± 0.0516, n=8, p=0.0138), OCLN (1.27 ± 0.3751, n=6 vs 0.3558 ± 0.07678, n=7, p=0.0256), and TJP1/ZO-1 (1.685 ± 0.2842, n=6 vs 0.4929 ± 0.1076, n=8, p=0.0009) normalized to GAPDH compared to in vitro HIOs. Eight week old tHIOs revealed significantly higher expression levels of CLND3 (7.406 ± 3.612, n=4 vs 0.2896 ± 0.05539, n=8, p=0.0149), CLDN15 (11.81 ± 5.292, n=5 vs 0.4064 ± 0.0516, n=8, p=0.0172), OCLN (1.308 ± 0.3656, n=5 vs 0.3558 ± 0.07678, n=7, p=0.0131), and TJP1/ZO-1 (2.339 ± 0.4081, n=5 vs 0.4929 ± 0.1076, n=8, p=0.0002) compared to in vitro HIOs, with the largest increase observed in CLDN15, 29-fold. There was no significant difference in expression of these tight junction genes between 4- and 8-week old tHIOs. This suggests that the exposure of tHIOs to biological factors in vivo for a minimum of 4 weeks augments the transcription of genes important for the function of the human intestinal barrier, but 8 weeks provides no additional benefit.
Figure 2. Transplantation of human intestinal organoids promotes expression of tight junction genes.
RT-qPCR demonstrates significant increases in relative gene expression of various tight junction components (A. CLDN3, B. CLDN15, C. OCLN, D. TJP1/ZO-1) in transplanted HIOs (tHIOs) after four (n=6) or eight (n-4–5) weeks in vivo compared to HIOs after 28 days in vitro (n=7–8). There was no significant difference in relative gene expression of tight junction genes between tHIOs at four and eight weeks in vivo. Values are expressed as the mean ± SEM. *p<0.05, ***p<0.001, ns denotes not significant (p≥0.05).
Transplanted human intestinal organoids demonstrate a more mature morphology of select tight junction proteins.
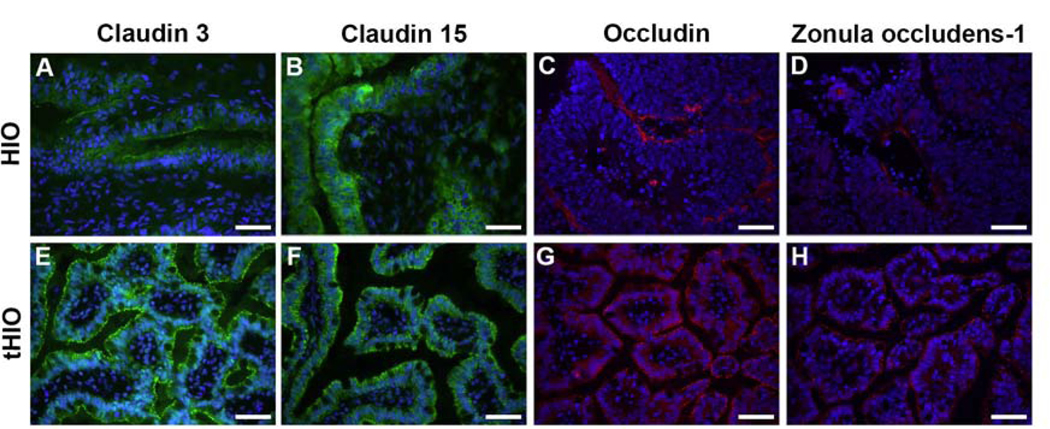
In order to ascertain if transplantation would facilitate appropriate production and morphology of select tight junction proteins, we performed immunofluorescence staining for CLDN3, CLDN15, OCLN, and TJP1/ZO-1 in HIOs (Figure 3A-D) and tHIOs (Figure 3E-H). These tight junction proteins were found in all HIOs and tHIOs, but more prevalent in tHIOs with an appropriate paracellular distribution. OCLN demonstrated the most disparate staining between tHIOs and HIOs, with OCLN protein in HIOs identified beneath the epithelial basement membrane. HIOs stained for all tight junction proteins, but did not have adequately mature morphology, to confirm proper location of tight junction proteins within the epithelium. Mature morphology is typically defined by the presence of villus-crypt units. These have previously been shown to be absent in 16 week old in vitro HIOs22, but present in 6–12 week old tHIOs6–9. Overall, a qualitatively more mature morphology and enhanced protein production was observed in tHIOs when compared to HIOs.
Figure 3. Transplanted HIOs (tHIOs) demonstrate a more mature morphology and paracellular tight junction protein distribution when compared to HIOs.
A-D HIOs after 28 days in vitro (n=4). E-H tHIOs after 8 weeks in vivo (n=5). A+E Claudin 3, B+F Claudin 15, C+G Occludin, D+H Zonula occludens-1. Scale bar 50 μm.
Discussion
In this paper, we demonstrated that transplantation facilitated maturation and growth, promoted select tight junction gene expression, and enabled proper tight junction protein production and morphology. Qualitative observations, with gross photomicrographs (Figure 1A,C,E,G) and H&E histologic examination (Figure 1B,D,F,H), showed that transplantation promotes intestinal tissue growth and architectural maturity. We additionally confirmed the production of select major tight junction mRNA (Figure 2) and proteins (Figure 3) required to maintain intestinal epithelial barrier function, which was enhanced after in vivo growth.
In vivo transplantation of HIOs has been previously shown to enable development of mature architecture and enhance cellular differentiation22. Watson et al previously observed expansion of a variety of cell lineages after transplantation in vivo including: enterocytes, Paneth cells, tuft cells, goblet cells, enteroendocrine cells, and smooth muscle cells6. Finkbeiner et al demonstrated mature intestinal histology in tHIOs with characteristic crypt-villus units found in adult small intestine as well as crypt-like domains containing proliferative Ki67 staining22. Figure 1 supports the previous finding that transplantation induces structural maturation of HIOs. We found that in vitro HIOs demonstrate a primitive, immature epithelium and mesenchyme while older tHIOs have an organized villus-crypt architecture similar to adult human small intestine with a discernible smooth muscle layer and goblet cell-containing epithelium. Thus, tHIOs are better able to recapitulate human small intestine histology.
Our study and other prior studies6,22, illustrate that biological factors in vivo are critical for maturation of the HIO into a TESI that more closely resembles functional human small intestine. Furthermore, these biological factors may include circulating humoral factors, acting in paracrine fashion as described by Watson et al6. In order to build a functional human TESI for patients suffering from SBS, it is critical to understand how transcriptionally similar HIOs and tHIOs are to each other as well as human small intestine. The global RNA expression data obtained by RNA-seq to characterize the transcriptomes of HIOs has been previously compared to the transcriptomes of tHIOs, fetal human intestine, and adult human intestine22. HIOs contained a transcriptome that most closely resembles fetal intestine. After in vivo transplantation, tHIOs demonstrated enhanced expression of brush border enzymes, revealed fully differentiated Paneth cells with increased expression of DEFA5 and REG3A (usually low expression in fetal intestine and increases as intestine matures), and finally, exhibited robust expression of well-characterized stem cell gene OLFM422. Finkbeiner et al showed that not only cellular differentiation and structural maturity are improved by in vivo transplantation, but that the entire global RNA expression of tHIOs is affected by elements that have yet to be simulated in vitro. However, this study did not look specifically at tight junction genes. In our RT-qPCR experiments, we were able to demonstrate that select major tight junction components (CLDN3, CLDN15, OCLN, and TJP1/ZO-1), were more highly expressed after in vivo transplantation. This appears fairly consistent with prior reports. Poling et al previously performed RNA-seq on tHIOs, tHIOs with applied mechanical strain from an implanted nitinol spring (tHIO+S), and human adult jejunum, including tight junction genes TJP1/ZO-1, F11R/JAM1, and MTDH. Interestingly, although F11R/JAM1 and MTDH expression were increased in tHIO+S versus tHIOs, TJP1/ZO-1 was not15. Furthermore, they did not compare tHIOs to HIOs in their study, so it is difficult to compare with our data. Finally, Poling et al did not investigate two of the main tight junction components: claudins and occludin, which we show here are also upregulated after in vivo transplantation.
In addition to mRNA expression, we felt it was imperative to evaluate tight junction proteins in our HIOs and tHIOs as intestinal epithelial barrier function depends heavily on protein presence, localization, and morphology. Tight junction proteins CLDN3, CLDN15, OCLN, and TJP1/ZO-1 were found to be present in both HIOs and tHIOs, but improperly localized with immature morphology in the in vitro HIOs. This suggests that tHIOs have the potential for tight junction function, and therefore an intact intestinal epithelial barrier. Prior studies have evaluated the intestinal epithelial barrier function in tHIOs with a FITC-dextran permeability assay6 as well as short-circuit currents, corrected calculated FITC-dextran flux, and TER in an Ussing chamber15. Although these studies confirmed some rudimentary intestinal epithelial barrier function, it did not recapitulate that of adult small intestine. Moreover, no prior studies have evaluated protein expression including localization and morphology in depth of these select major tight junction components such as claudins, occludin, and zonula occludens-1 in HIOs. Our study confirms the importance of in vivo transplantation on protein production and maturation in HIOs.
To our knowledge, tight junction genes and proteins, claudin 3, claudin 15, occludin, and zonula occludens-1 have not been previously evaluated in HIOs or tHIOs. The findings of this study have confirmed some previous limited tight junction data, while expanding on this topic. Specifically, the crucial role in vivo transplantation plays in enhancing HIO growth, architectural maturity, select tight junction gene expression, as well as select tight junction protein production, localization and morphology vital to the maintenance of the intestinal epithelial barrier.
Although in vivo transplantation appears to improve select tight junction gene expression in HIOs, it is critical to discuss the impact of transplant location. In our study, HIOs were transplanted beneath the kidney capsule rather than orthotopically. This retroperitoneal location may prevent normal levels of tight junction expression and epithelial barrier function seen in adult human intestine since the expression of tight junction genes and proteins is highly tissue and context dependent. There is only one publication directly comparing the effects of HIO transplantation sites (renal subcapsular space versus intestinal mesentery)20. Singh et al found that both transplantation sites are feasible and successful, and both demonstrated significant maturation of the tHIO after 10 weeks in vivo. Additionally, there was no difference in tHIO size, histologic heterogeneity, villus height, crypt depth, crypt fission, as well as gene and protein expression for epithelial development, proliferation, secretory lineages, epithelial stem cell markers, mesenchymal components, and markers of carbohydrate digestion20. However, they did not investigate tight junction gene and protein expression, nor intestinal epithelial barrier function. Thus, it is difficult to speculate whether these lack of differences between HIO transplant locations would extend to tight junction expression and function. It is also important to note that HIOs transplanted in the intestinal mesentery share a blood supply with the native host intestine and develop a dominant lumen more frequently enabling a luminal connection with the native host intestine (e.g. a side-to-side organoid to intestinal anastomosis)18–20. Thus, the intestinal mesentery location appears to be more clinically relevant, enabling functional in vivo studies and ultimately, translational applications. It will be imperative in future studies to evaluate tight junction expression and function in tHIOs at an orthotopic transplant site such as the intestinal mesentery, and ultimately, to establish a connection between the tHIO lumen and the native host intestinal lumen. This will be more physiologic as it would allow for the critical luminal contribution including nutrients, microbiota and their metabolites, which interact with the intestinal epithelium and can impact tight junction expression and function.
Finally, there are several important limitations of this study that deserve discussion. First, the composition of tight junctions is complex, including almost 40 different proteins, and we only evaluated four components in this preliminary study13. Future studies should investigate the gene expression and protein production of other essential tight junction components such as ZO-2, JAM, Cingulin, F-actin and other claudins, to see if our findings are corroborated. Second, the lack of a more relevant in vitro HIO control to compare to the tHIOs, e.g. in vitro HIOs grown for an additional 4 and 8 weeks in parallel with the in vivo tHIOs, makes it difficult to know if HIO transplantation alone is responsible for the observed tissue maturation as well as the increase in select tight junction gene expression and protein production within tHIOs, or if additional growth time additionally contributes. We did not utilize this type of control because Finkbeiner et al have previously demonstrated that in vitro HIOs remain immature and fetal-like after 16 weeks in vitro22. Specifically, they demonstrated that 16 week old in vitro HIOs lacked the characteristic crypt-villus units found in the in vivo tHIOs and adult human small intestine. These 16 week old in vitro HIOs additionally had decreased expression of brush border enzymes, DEFA5 (marker of Paneth cell differentiation), and OLFM4 (intestinal stem cell marker), although they did not evaluate tight junction gene expression. Interestingly, these differences were not due to variation in the tissue collected or the amount of epithelium present as the study also examined expression of multiple epithelial genes (EPCAM, CDH1, SOX9, KRT8, KLF5, CDX2) and found no consistent trends in epithelial-specific gene expression between any of the samples, thus providing confidence that gene expression differences were reflective of true biological difference22. Therefore, we felt that it would be unnecessary to grow the in vitro HIOs beyond 28 days as it would not result in intestinal maturation nor enhancement of tight junction gene expression and protein production. Third, we are unable to determine if tight junction gene expression correlates with differentiation marker expression and development as we did not measure differentiation markers. Although we were able to follow HIO growth and maturation histologically from 28 days in vitro to 4, 8, and 12 weeks in vivo, we did not assess markers of epithelial or mesenchymal differentiation in addition to our select tight junction genes and proteins. Thus, we cannot ascertain any developmental correlation between the two until further experiments are undertaken. Fourth, we cannot comment on potential sex-related differences in tight junctions as only male mice were used as hosts. Some studies have demonstrated that sex hormones such as estrogen and progesterone may improve intestinal epithelial barrier function via the upregulation of tight junction proteins17. In this preliminary study, we used only male mice hosts rather than both males and females to ensure that our results would not be confounded by potential sex-related differences. However, there may be important contributions to tight junction expression and epithelial barrier function that occur when HIOs are transplanted within female mice hosts. Unfortunately, no studies on tHIOs to date have investigated sex-related differences6–9,15,20. Thus, future studies directly comparing tHIOs grown in vivo within male versus female mice hosts will be required to identify sex-related distinctions and specifically, to determine their influence on tight junction development and epithelial barrier function. Fifth, it is well known that the expression and permeability properties of tight junctions are regulated by the immune system; however, immune signaling is absent in our HIO/tHIO model. HIOs lack an immune system of their own23 and the NSG™ mouse hosts are B and T cell deficient with an IL2rgnull mutation that prevents cytokine signaling through multiple receptors, leading to a functional natural killer cell deficiency. Thus, the NSG™ mouse host readily accepts engraftment of human cells such as HIOs, but there is likely very little immune signaling that could impact tight junction expression and function. The incorporation of immune cells into our HIO/tHIO model in the future will help address this limitation. Sixth, although protein presence, location and morphology were evaluated, we did not quantify protein levels. Finally, the additional comparison of human fetal and adult small intestine for the RT-qPCR and immunofluorescence staining studies would provide insight into the translational capabilities of the HIO-derived TESI we generate.
Conclusions
HIOs have lower levels of select tight junction gene expression and tight junction proteins appear morphologically immature. In vivo transplantation facilitates maturation of the HIO and enhances select tight junction gene and protein production as well as morphology. Our future lab studies plan to address the limitations above and also seek to confirm function of tight junctions in tHIOs, specifically in the presence of an enteric nervous system (ENS) as the ENS has been shown to play a role in intestinal barrier maintenance24–26.
Highlights.
In vivo transplantation of human intestinal organoids enhances select tight junction gene expression
Acknowledgments
Funding
This work was supported in part by NIH grant P30DK056338, which supports the Texas Medical Center Digestive Diseases Center, NIH grant U01DK103117, as well as the McGovern Medical School at UTHealth Department of Pediatric Surgery and Dean’s Office of Educational Programs.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, & Teitelbaum DH Pediatric short-bowel syndrome: The cost of comprehensive care. Am J Clin Nutr 2008;88(6):1552–1559. 10.3945/ajcn.2008.26007 [DOI] [PubMed] [Google Scholar]
- 2.Duggan CP and Jaksic T. Pediatric Intestinal Failure. N Engl J Med 2017;377 (7):666–75. 10.1056/NEJMra1602650 [DOI] [PubMed] [Google Scholar]
- 3.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, Vacanti JP. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004. November;240(5):748–54. 10.1097/01.sla.0000143246.07277.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. AMulticellular Approach Forms a Significant Amount of Tissue-Engineered Small Intestine in the Mouse. Tissue Eng Part A. 2011. July;17(13–14):1841–50. 10.1089/ten.tea.2010.0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Torashima Y, Grikscheit TC. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg. 2013. January;48(1):129–37. 10.1016/j.jpedsurg.2012.10.029 [DOI] [PubMed] [Google Scholar]
- 6.Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, & Helmrath MA An in vivo model of human small intestine using pluripotent stem cells. Nat Med 2014;20(11):1310–1314. 10.1038/nm.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkbeiner SR, Freeman JJ, Wieck MM, et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open 2015;4(11):1462–72. 10.1242/bio.013235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 2017;23(1):49–59. 10.1038/nm.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlieve CR, Fowler KL, Thornton M, et al. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue-engineered small intestine. Stem Cell Reports 2017;9(3):883–896. 10.1016/j.stemcr.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells JM and Spence JR. How to make an intestine. Development 2014;141:752–760. 10.1242/dev.097386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. intestinal research 2015;13(1):11. 10.5217/ir.2015.13.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins FL, Rios-Arce ND, Atkinson S, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. 2017;5(9):1–22. 10.14814/phy2.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Itallie CM and Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006;68:403–429. 10.1146/annurev.physiol.68.040104.131404 [DOI] [PubMed] [Google Scholar]
- 14.Buckley A,Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10(1):a029314. 10.1101/chperspect.a029314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poling HM, Wu D, Brown N, Baker M, Hausfeld TA, Huynh N, Chaffron S, Dunn JCY, Hogan SP, Wells JM, Helmrath MA, & Mahe MM Mechanically induced development and maturation of human intestinal organoids in vivo. Nat Biomed Eng 2018;2(6):429–442. 10.1038/s41551-018-0243-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, & Wells JM Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011;470(7332):105–110. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells. 2019; 19(8):261. 10.3390/cells8030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahe MM, Brown NE, Poling HM, Helmrath MA. In vivo model of small intestine. Methods Mol Biol 2017;1597:229–245. 10.1007/978-1-4939-6949-4_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez AR, Poling HM, Brown NE, Singh A, Mahe MM, Helmrath MA. Transplantation of human intestinal organoids into the mouse mesentery: A more physiologic and anatomic engraftment site. Surgery. 2018; 164(4):643–650. 10.1016/j.surg.2018.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Poling HM, Sundaram N, Brown N, Wells JM, Helmrath MA. Evaluation of transplantation sites for human intestinal organoids. PLoS ONE. 2020; 15(8):e0237885. 10.1371/journal.pone.0237885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expression Patterns. 2006;6:581–588. 10.1016/j.modgep.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 22.Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH, Chin AM, Mahe MM, Watson CL, Freeman JJ, Nattiv R, Thomson M, Klein OD, Shroyer NF, Helmrath MA, Teitelbaum DH, Dempsey PJ, & Spence JR Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Reports 2015;4(6):1140–1155. 10.1016/j.stemcr.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinagoga KL and Wells JM. Generating human intestinal tissues from pluripotent stem cells to study development and disease. The EMBO journal. 2015; 34:1149–1163. 10.15252/embj201490686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998; 93:189–201. 10.1016/S0092-8674(00)81571-8 [DOI] [PubMed] [Google Scholar]
- 25.Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. BJP. 2006; 149:463–479. 10.1038/sj.bjp.0706889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meir M, Flemming S, Burkard N, et al. The glial cell-line derived neurotrophic factor: a novel regulator of intestinal barrier function in health and disease. Am J Physiol Gastrointest Liver Physiol. 2016; 310: G1118–G1123. Published April 28, 2016. 10.1152/ajpgi.00125.2016 [DOI] [PubMed] [Google Scholar]