Abstract
Purpose:
To characterize long-term clinical outcomes of monotherapy selective laser trabeculoplasty (SLT) in Afro-Caribbean patients with open-angle glaucoma (OAG).
Design:
This was a post hoc analysis of nearly 8 years (median 3.2, interquartile range 2.1–7.1) of pooled data from the West Indies Glaucoma Laser Study (WIGLS) and its preliminary study.
Methods:
Setting:
Three eye care practices in Saint Lucia and Dominica.
Participants:
Afro-Caribbean adults with mild-moderate OAG treated with ≤2 medications (61 in preliminary study, 72 in WIGLS).
Intervention:
Participants underwent medication washout, baseline IOP determination, and bilateral 360° SLT. Participants were followed for up to 94 months. Repeat SLT was performed according to pre-specified criteria.
Main Outcome Measures:
The primary outcome was mean intraocular pressure (IOP) reduction from baseline. The secondary outcome was medication-free survival with SLT repeated as needed.
Results:
The pooled data set included 265 eyes of 133 Afro-Caribbean participants. Mean (standard deviation) baseline IOP was 21.2 (3.4) and 21.2 (3.9) mmHg in right and left eyes, respectively. Over 8 years, mean IOP ranged from 12.8–15.7 mmHg and from 13.1–15.8 mmHg, respectively (p<0.0001 for every comparison to baseline). Median medication-free survival time for initial SLT was 85.4 months in both eyes. The 94-month medication-free survival of SLT repeated as needed was 71.2% and 71.7%, respectively.
Conclusions:
Monotherapy SLT, repeated as needed, safely provides significant IOP reductions in most Afro-Caribbean adults with POAG through nearly 8 years of follow-up and has significant potential to delay or prevent glaucoma-related vision loss in Black patients in low-resource regions.
TOC Statement
In this analysis of pooled data from 2 prospective studies, monotherapy SLT (repeated as needed) safely provides significant IOP reductions in most Afro-Caribbean adults with POAG through up to 8 years of follow-up, with 94-month medication-free survival >70%, and has significant potential to delay or prevent glaucoma-related vision loss in Black patients in low-resource regions.
Introduction
Glaucoma is the leading cause of irreversible vision loss and blindness worldwide.1 Blacks are at the highest risk of developing glaucoma and glaucoma-related blindness.2–4 The burden of disease is particularly problematic in low-resource areas through sub-Saharan Africa and other regions populated with African descendants such as the Caribbean. The prevalence of glaucoma in the United States has been estimated at 1.9%;2 in contrast, in Blacks in sub-Saharan Africa the prevalence has been estimated at 4.2%,5 and in various Caribbean countries at 7–8%.6,7 In the United States, glaucoma accounts for 6.4% of blindness in Whites and 26.0% in Blacks;8 globally, glaucoma accounts for 8.5% of blindness while in sub-Saharan Africa 11.7–15.5% of blindness is from glaucoma.9
Glaucoma cannot be cured, but the reduction of intraocular pressure (IOP) can reduce the risk of progression and vision loss.10,11 IOP reduction can be achieved using medical therapy, laser procedures, or surgical interventions. In low-resource countries as are found throughout Sub-Saharan Africa and the Caribbean, barriers to care are significant and likely explain in part the higher rates of glaucoma blindness in these regions.12
Both the West Indies Glaucoma Laser Study (WIGLS)13–16 and its preliminary study in Saint Lucia17 prospectively explored the utility of selective laser trabeculoplasty (SLT) as sole therapy for open-angle glaucoma (OAG) among Afro-Caribbean adults. These studies, coupled with other reports from various African locations,18–20 demonstrated a meaningful reduction in IOP among Blacks with open-angle glaucoma. The longest follow-up in these studies is 12 months, limiting the characterization of SLT’s role in the long-term management of glaucoma in this population.
In this report, we describe the long-term (up to 7.8 years) outcomes of SLT in Afro-Caribbean patients with OAG in an analysis of the pooled data from WIGLS and its preliminary study.
Methods
This was a post hoc analysis of the pooled data from both WIGLS and its preliminary study. The preliminary study was conducted in Saint Lucia to establish proof of concept that SLT monotherapy was a viable therapeutic approach to OAG among Afro-Caribbeans and also to inform the design of WIGLS, which was conducted in Saint Lucia and Dominica. Given their relatedness, most design elements, including eligibility criteria and methods, were closely harmonized between studies. The preliminary study was a prospective interventional case series, and WIGLS was a prospective study utilizing a stepped-wedge design to randomize subjects to immediate versus delayed washout of IOP lowering medications followed by SLT. Both were reviewed and approved by all relevant ethics boards at West Virginia University as well as in the countries where they were conducted. All subjects provided written informed consent to participate. The preliminary study was launched in December 2010, at which time the study’s design did not require trial registration; WIGLS was launched in March 2015 and was registered at ClinicalTrials.gov (NCT 02375009) before enrollment began. Both studies were concluded in September 2018.
The eligibility criteria and methods have been previously reported for both the preliminary study17 and WIGLS.16 Briefly, subjects were recruited from two eye care practices in Saint Lucia (for both studies) and one in Dominica (for WIGLS); they were adults age 40 years or older (WIGLS) or 30 years or older (preliminary study) previously diagnosed with OAG and treated with up to 1 (preliminary study) or 2 (WIGLS) topical IOP-lowering medications. Qualifying eyes had best-corrected visual acuity (BCVA) of 20/400 or better, open anterior chamber angles, vertical cup-disc ratio of ≤0.8 (preliminary study) or ≤0.9 (WIGLS), and no visual field defects extending into the central 10 degrees on automated perimetry (Humphrey Matrix frequency-doubling perimeter in the preliminary study; Humphrey Field Analyzer II in WIGLS). Following washout of IOP-lowering therapy, a qualifying IOP ≤35 mmHg (preliminary study) or 18–32 mmHg inclusive (WIGLS) was required. If both eyes qualified, both could be enrolled.
Following a comprehensive eye examination to determine eligibility, qualifying subjects in the preliminary study underwent immediate 4–6 week washout of topical IOP-lowering medications, while in WIGLS qualifying subjects were randomized to immediate washout or to continue current IOP-lowering therapy for an additional 3 or 6 months before commencing washout (to quantify post-randomization regression to the mean). After washout, baseline IOP was established in two sessions 1–3 hours apart (preliminary study) or 1–3 days apart (WIGLS). In both studies at all visits, IOP was measured by a single examiner using the same Perkins tonometer at the same time of day (± 2 hours) for each subject following a modified OHTS protocol as follows: with the Perkins tonometer set to 10 mmHg, the examiner adjusted the dial without looking at it to achieve applanation; the Perkins tonometer was removed from the eye, the IOP read and recorded, and the dial set back to 10 mmHg. This process was repeated in the same eye, with a third reading taken only if the first two differed by 4 mmHg or more. These two (or three) values were averaged. The procedure was then repeated on the fellow eye. The average of all IOP measurements in both sessions was taken as the baseline IOP. Following the final baseline IOP assessment, SLT was performed as follows: after instillation of topical anesthetic and brimonidine 0.2%, approximately 100 treatment spots were applied to the full 360 degrees of the trabecular meshwork in each eye; the energy was adjusted throughout the procedure to produce tiny champagne bubbles with every second or third spot. All SLT treatments were delivered using the Lumenis Selecta II portable slit-lamp mounted platform and the Latina lens. Subjects were then reexamined 1 hour, 1 week, and 1 month post-SLT, and then every 3 months (through January 2017) or every 4 months (after January 2017) thereafter through September 2018. SLT retreatment was based on pre-specified retreatment criteria related to exceeding target IOP at 2 consecutive study visits.
The overall goal of this analysis of the pooled data set was to characterize the long-term efficacy of SLT as sole therapy for glaucoma in a population of Black patients with OAG. Right and left eyes were analyzed separately. Post-SLT mean IOP was calculated in one-year blocks of time, with mean IOP in each year representing the mean IOP at all study time points within each 12-month period. Each year’s mean IOP was compared to baseline using paired t-tests with alpha=0.05. Survival analysis was conducted using the Kaplan-Meier technique to assess long-term success following a single SLT, up to 2 SLTs, and up to 3 SLTs, to demonstrate the utility of a treatment plan consisting of SLT repeated as needed; failure was defined as IOP reduction <20% from baseline on 2 consecutive visits. Means are reported with standard deviations. The sample size was fixed and no hypotheses were tested, so a formal power analysis was not conducted.
Results
Overall, 265 eyes of 133 subjects were included in this analysis, including 122 eyes of 61 subjects from the preliminary study and 143 eyes of 72 subjects in WIGLS (one subject had BCVA < 20/400 in one eye which was not enrolled). Demographic and baseline glaucoma status data, as well as SLT laser data, are given in Table 1. Subjects were on average 60.9 years of age, approximately two-thirds (64.7%) were female, and all were self-identified Afro-Caribbean. Most had been prescribed 1 or 2 medications (4 subjects reported being prescribed 3 medications but reported taking none of them and were enrolled), of which beta-blockers and prostaglandin analogues were most common. The stage of glaucoma was generally mild, with mean cup-disc ratio of 0.63 and mean HFA visual field mean deviation of approximately −4.1 dB (in WIGLS subjects only). Eyes received an average of 103 SLT treatment spots totaling an average of 82–87 mJ of energy. There were no meaningful differences in demographics, glaucoma disease status, or SLT laser parameters between the preliminary study and WIGLS cohorts (data not shown). The median (interquartile range [IQR]) follow-up of the preliminary study cohort was 89.4 (45.7–93.6) months, of the WIGLS cohort was 35.5 (23.0–38.9) months, and of the pooled data set analyzed in this report was 38.8 (25.5–85.4) months.
Table 1.
Demographic and glaucoma status data of subjects in the study sample. Mean values are reported with standard deviations (SD).
| Characteristic | Value (N=133 subjects) | |
| Gender, n (%) | ||
| Male | 47 (35.3) | |
| Female | 86 (64.7) | |
| Age (yr), mean (SD) | 60.9 (10.51) | |
| Right Eye (N=133) | Left Eye (N=132) | |
| Number of glaucoma medications, n (%) | ||
| 0 | 8 (6.0) | 7 (5.3) |
| 1 | 102 (76.7) | 103 (78.0) |
| 2 | 19 (14.3) | 18 (13.7) |
| 3 | 4 (4.0) | 4 (3.0) |
| Glaucoma medication classes, n (%) | ||
| Beta-blockers | 79 (59.4) | 79 (59.9) |
| Prostaglandins | 51 (38.4) | 50 (37.9) |
| Carbonic anhydrase inhibitors | 9 (6.8) | 9 (6.8) |
| Adrenergic agonists | 10 (7.5) | 10 (7.6) |
| Central corneal thickness (μm), mean (SD) | 535.9 (35.9) | 537.5 (46.5) |
| Vertical cup-disc ratio, mean (SD) | 0.63 (0.14) | 0.63 (0.13) |
| Visual field mean deviation (dB), mean (SD) | −4.18 (5.14) | −4.07 (4.24) |
| Laser parameters, mean (SD) | ||
| Total energy (milllijoules) | 82.5 (18.8) | 87.0 (18.5) |
| Number of treatment spots | 103.3 (3.5) | 103.1 (3.1) |
All 265 enrolled eyes underwent washout and received initial SLT. Subsequently, 39 right eyes and 36 left eyes of 42 subjects (33 subjects bilaterally) underwent a second SLT; the median (IQR) time between first and second SLT in right and left eyes was 15.9 (9.1–64.2) months and 17.2 (10.7–70.7) month, respectively. Of these, 9 right eyes and 8 left eyes of 11 subjects (6 subjects bilaterally) underwent a third SLT; the median time between second and third SLT was 21.3 (12.7–25.1) months in right eyes and 23.8 (9.4–33.6) months in left eyes.
Mean IOP before medication washout, after medication washout (baseline), and in each post-enrollment year, as well as changes from baseline, through up to 7.8 years of follow-up for right and left eyes are given in Table 2 and Figure 1. Prior to SLT, mean medicated IOP was 16.2 (3.9) mmHg in right eyes and 16.3 (3.9) mmHg in left eyes and mean washout IOP was 21.2 (3.4) and 21.2 (3.9) mmHg, respectively. Over the following 8 years, under a treatment regimen of initial SLT repeated as needed (when IOP exceeded target IOP on 2 consecutive visits) with no medical therapy, mean IOP in right eyes ranged from 12.8–15.7 mmHg and in left eyes from 13.1–15.8 mmHg. These represent mean IOP reductions of 6.1–9.0 mmHg in right eyes and 6.5–9.2 mmHg in left eyes. At every annualized time point, mean IOP in each eye was significantly lower than baseline IOP (p<0.0001 for every comparison) and was arithmetically lower than pre-washout medicated IOP in each eye at every time point.
Table 2.
Mean (and standard deviation [SD]) IOP following initial SLT repeated as needed throughout follow-up.
| Time | IOP (mmHg) | IOP change from baseline (mmHg) | Significance (p) | |||||
|---|---|---|---|---|---|---|---|---|
| Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | |||
| N | Mean (SD) | N | Mean (SD) | |||||
| Pre-Washout | 133 | 15.5 (3.4) | 132 | 15.8 (3.6) | -- | -- | -- | -- |
| Baseline | 133 | 21.2 (3.4) | 132 | 21.2 (3.9) | -- | -- | -- | -- |
| Year 1 | 132 | 14.1 (3.1) | 132 | 13.5 (2.9) | −7.1 (3.3) | −7.6 (4.1) | <.0001 | <.0001 |
| Year 2 | 109 | 14.6 (3.2) | 113 | 14.3 (3.2) | −6.8 (3.8) | −7.1 (4.7) | <.0001 | <.0001 |
| Year 3 | 96 | 14.6 (2.8) | 99 | 13.9 (2.6) | −6.7 (3.3) | −7.5 (4.4) | <.0001 | <.0001 |
| Year 4 | 69 | 14.2 (2.6) | 73 | 13.7 (2.8) | −7.7 (2.9) | −8.4 (4.8) | <.0001 | <.0001 |
| Year 5 | 45 | 12.8 (2.7) | 44 | 13.1 (2.8) | −9.0 (3.1) | −9.2 (5.0) | <.0001 | <.0001 |
| Year 6 | 43 | 14.6 (3.2) | 42 | 14.2 (2.9) | −7.1 (2.8) | −7.9 (5.5) | <.0001 | <.0001 |
| Year 7 | 39 | 15.7 (3.3) | 39 | 15.8 (3.6) | −6.1 (2.8) | −6.5 (6.0) | <.0001 | <.0001 |
| Year 8 | 34 | 15.7 (2.6) | 34 | 14.7 (2.5) | −6.1 (2.8) | −7.4 (5.6) | <.0001 | <.0001 |
Figure 1.

Mean IOP at screening (on medications), after washout, and at each 1-year interval. Solid lines represent data from right eyes; dotted lines represent data from left eyes. Error bars represent standard deviation.
Survival analysis was conducted to evaluate the longevity of SLT’s effect on IOP. In these analyses, eyes were considered to have failed when IOP exceeded target IOP on 2 consecutive visits. In descending order of frequency, loss to follow-up, initiation of topical IOP-lowering therapy by non-study personnel, cataract surgery, repeat SLT while achieving (but very close to) target IOP at the time of fellow eye failure and retreatment, unrelated ocular events requiring topical IOP-lowering therapy (e.g., neovascular glaucoma, panuveitis), progression at target warranting the addition of topical therapy, and death were considered censoring events. Figure 2 gives the Kaplan-Meier survival curves for first SLT, SLT repeated as needed up to once, and SLT repeated as needed up to twice. The median survival time for initial SLT was 85.4 months in both right (Figure 2A) and left (Figure 2B) eyes, with 45.4% of right eyes and 44.2% of left eyes surviving through the end of follow-up after a single SLT. The median survival time of up to 2 or 3 SLT treatments remains unknown as fewer than half of eyes reached the failure endpoint in these analyses through up to 94 months of follow-up. As can be seen in Figure 2, SLT repeated as needed re-established IOP control and prolonged medication-free survival. At the end of follow-up—7.8 years after initial SLT—survival of SLT repeated up to once was 62.8% in right eyes and 63.1% in left eyes, and survival of SLT repeated up to twice was 71.2% in right eyes and 71.7% in left eyes.
Figure 2.
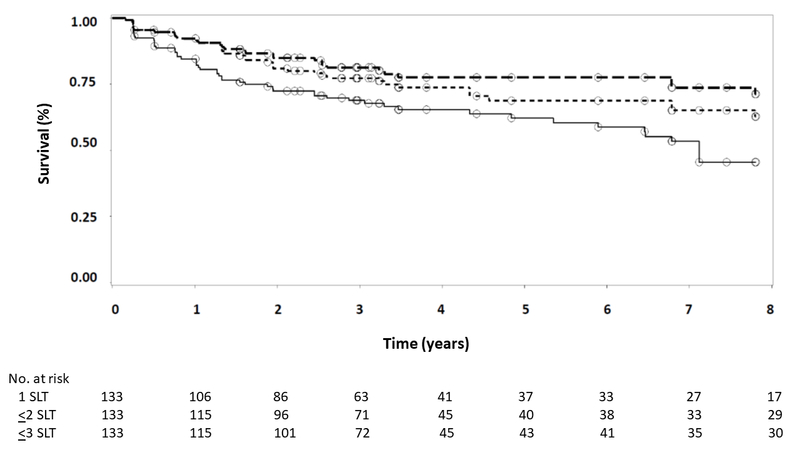
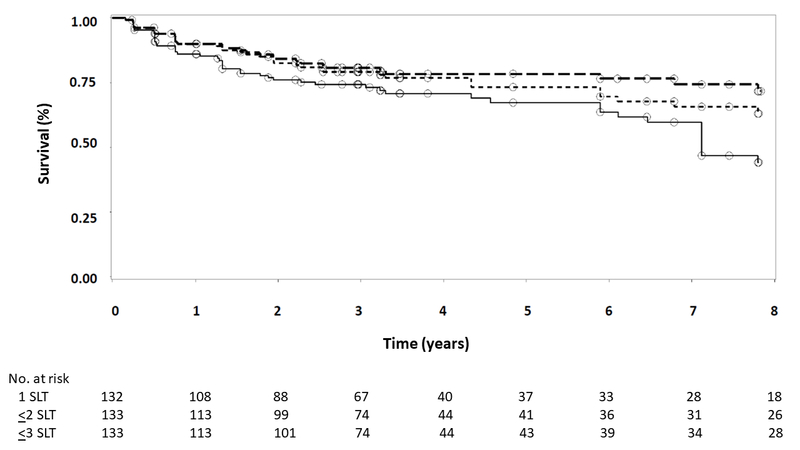
Kaplan-Meier survival analysis for right eyes (A) and left eyes (B). Failure was defined as IOP exceeding target IOP on 2 consecutive visits. Solid line represents initial SLT only; short dashed line represents up to 2 SLT treatments; long dashed line represents up to 3 SLT treatments. Circles indicate censored observations.
The 12-month safety profile of SLT in these Afro-Caribbean samples has been reported previously.16,17 Two treatment-related adverse events of note—one case of corneal edema and one case of symptomatic anterior iritis—have been reported previously16 and resolved without sequellae. No unexpected problems arising more than 1 week after the most recent SLT or related to repeat SLT were observed.
Discussion
In what we believe is the longest prospective study of SLT reported to date in any population, we have demonstrated that a regimen of monotherapy SLT repeated as needed can provide up to 8 years of medication-free glaucoma management in >70% of eyes of Afro-Caribbean adults with open-angle glaucoma. This was achieved with no sight-threatening complications.
The magnitude of IOP reduction observed in this study—mean IOP reductions of 6–9 mmHg from a mean baseline IOP of 21 mmHg—is consistent with other reports of SLT in Black patents18–20 and meets or exceeds the therapeutic goals recommended by both the American Academy of Ophthalmology21 and the European Glaucoma Society.22 The mean IOP levels achieved—13–16 mmHg—are equal to or lower than those demonstrated to prevent progressive visual field loss in the Collaborative Initial Glaucoma Treatment Study.23 Thus, while limitations in the availability of perimetry and optical coherence tomography in the locations where these studies were conducted precluded the assessment of structural and functional outcomes in this analysis, both the IOP reductions and the absolute IOP levels attained with SLT would be expected to delay or prevent progression of glaucoma in the majority of these eyes.
Medication-free survival through almost 8 years of follow-up exceeded 70% in both right and left eyes. This was accomplished with a single SLT treatment in roughly half (~45%) of all eyes. At all time points, mean IOP was significantly lower than pre-SLT baseline and was arithmetically lower than pre-washout medicated IOP, indicating that SLT is at least non-inferior to medical therapy in this population. The landmark Laser in Glaucoma and Hypertension (LiGHT) study also demonstrated at least similar outcomes with primary SLT versus medications in treatment-naïve patients with mild-moderate POAG or high-risk ocular hypertension through 3 years of follow-up.24 Collectively, these findings support the use of SLT as primary therapy for Black patients in both high- and low-resource settings. Particularly in the latter, where up to 80% of patients newly diagnosed with glaucoma never return for care,25 a single SLT treatment at the diagnostic visit could safely prevent or delay functional vision loss by many years in the majority of patients even if they never return for further care. Other features of SLT—it being office-based, not painful, requiring no postoperative care, its ease of performance, ease of skills transfer to providers, and ease of equipment portability—also favor its use in high-prevalence, low-resource areas where the potential for long-term follow-up is poor. A strategy for widespread utilization of SLT throughout sub-Saharan Africa, based on the L. V. Prasad Eye Institute model, has been described.26
Of note, the median survival time of SLT2 was longer than that of SLT 1 in both right eyes (by 5.4 months) and left eyes (6.6 months). This is consistent with prior reports of greater durability of repeat SLT compared to initial SLT.27,28 A biological basis for this observation is unknown. A selection bias is unlikely as patients would be more likely to be offered repeat SLT if initial SLT’s effect were long-lasting. Also, selection bias is less likely in the current study because it relied on protocol-specified retreatment criteria rather than investigator/patient discretion as was the case in the retrospective studies.27,28
Strengths of this study include its prospective design, the inclusion of an under-studied population, long-term follow-up, and inclusion of participants from multiple sites in the Caribbean. There are several important limitations of this study. Neither the preliminary study nor WIGLS incorporated a control group. To mitigate this, WIGLS utilized a stepped-wedge design in which some participants remained on pre-enrollment medications after randomization for 3 or 6 months before washout/SLT; this approach allowed us to rule out any effect of regression to the mean on our results.16 This was a long-term study, and two important attrition events reduced the sample size followed beyond 42 months. Eighty eyes (38 right, 42 left) were censored between months 23 and 42. In September 2017, 23–29 months after initial SLT in the WIGLS cohort, Hurricane Maria struck Dominica as a category 5 storm, devastating infrastructure and resulting in the emigration of 20–30% of the island’s population.29 In September 2017, 35–42 months after initial SLT in the WIGLS cohort, the study came to its end. All outcomes beyond the 42-month time point were derived solely from participants in the preliminary study. Finally, this was a study of SLT monotherapy but not primary therapy, as economic considerations required a convenience sample of previously diagnosed patients under treatment at enrollment.
In summary, this analysis of pooled data from both the West Indies Glaucoma Laser Study and its preliminary study demonstrates that monotherapy SLT, repeated as needed, provides statistically and clinically significant IOP reductions (~30–40%) in most eyes (~70%) of Afro-Caribbean adults with POAG through nearly 8 years of follow-up. SLT has significant potential to delay or prevent glaucoma-related vision loss in Black patients in low-resource regions.
Table 3.
Median (and interquartile range [IQR]) time to subsequent SLT treatments.
| Right Eyes | Left Eyes | |
|---|---|---|
| Number of eyes undergoing SLT2, n | 39 | 36 |
| Median (IQR) time from SLT1 to SLT2, months | 15.9 (9.1 – 64.2) | 17.2 (10.7 – 70.7) |
| Number of eyes undergoing SLT3, n | 9 | 8 |
| Median (IQR) time from SLT2 to SLT3, months | 21.3 (12.7 – 25.1) | 23.8 (9.4 – 33.6) |
Acknowledgements
A. Funding/Support: The preliminary study was funded in part by the American Glaucoma Society (2010 Mid-Career Physician Scientist Award to TR) and the West Indies Glaucoma Laser Study was funded by the National Eye Institute at the National Institutes of Health (R01 EY023620 to TR).
Footnotes
B. Financial disclosures (last 12 months):
TR (all as consultant):
• Aerie
• Asclepix
• IStar Med
• Ivantis
• New World Medical
• Nicox
• Notal
• Ocular Therapeutix
• Sight Sciences
GKB (all as consultant):
• New World Medical
• Ocular Therapeutix
• Sight Sciences
BD: None
HS-R: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5–ORSF13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 5.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology. 1989;96(9):1363–1368. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112(6):821–829. [DOI] [PubMed] [Google Scholar]
- 8.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 9.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 10.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. [DOI] [PubMed] [Google Scholar]
- 11.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. [DOI] [PubMed] [Google Scholar]
- 12.Kyari F, Adekoya B, Abdull MM, Mohammed AS, Garba F. The Current Status of Glaucoma and Glaucoma Care in Sub-Saharan Africa. Asia Pac J Ophthalmol (Phila). 2018;7(6):375–386. [DOI] [PubMed] [Google Scholar]
- 13.Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. West Indies Glaucoma Laser Study (WIGLS) 3. Anterior Chamber Inflammation Following Selective Laser Trabeculoplasty in Afro-Caribbeans with Open-Angle Glaucoma. J Glaucoma. 2019;28:622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. Crystalline lens changes after selective laser trabeculoplasty in Afro-Caribbean patients with open-angle glaucoma; report 4 of the West Indies Glaucoma Laser Study (WIGLS). J Cataract Refract Surg. 2019;45(10):1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. West Indies Glaucoma Laser Study (WIGLS)-2: Predictors of Selective Laser Trabeculoplasty Efficacy in Afro-Caribbeans With Glaucoma. J Glaucoma. 2018;27(10):845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. West Indies Glaucoma Laser Study (WIGLS): 1. 12-Month Efficacy of Selective Laser Trabeculoplasty in Afro-Caribbeans With Glaucoma. Am J Ophthalmol. 2017;184:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Realini T Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA Ophthalmol. 2013;131(3):321–327. [DOI] [PubMed] [Google Scholar]
- 18.Goosen E, Coleman K, Visser L, Sponsel WE. Racial Differences in Selective Laser Trabeculoplasty Efficacy. J Curr Glaucoma Pract. 2017;11(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seck SM, Agboton G, Dieng M, et al. [Selective laser trabeculoplasty (SLT): our experience in African blacks]. J Fr Ophtalmol. 2015;38(3):238–246. [DOI] [PubMed] [Google Scholar]
- 20.Ouattara OAS, Coulibaly F, Ouffoue YG, et al. [Selective laser trabeculoplasty in African blacks]. J Fr Ophtalmol. 2019;42(1):44–48. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Ophthalmology. Primary open-angle glaucoma: Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 2020. [Google Scholar]
- 22.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition. Chapter 3: Treatment Principles and Options. Br J Ophthalmol. 2017;101(6):130–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. [DOI] [PubMed] [Google Scholar]
- 24.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kizor-Akaraiwe NN. Follow-up and adherence to glaucoma care by newly diagnosed glaucoma patients in enugu, nigeria. Ophthalmic Epidemiol. 2019;26(2):140–146. [DOI] [PubMed] [Google Scholar]
- 26.Realini T, Olawoye O, Kizor-Akaraiwe N, Manji S, Sit A. The Rationale for Selective Laser Trabeculoplasty in Africa. Asia Pac J Ophthalmol (Phila). 2018;7(6):387–393. [DOI] [PubMed] [Google Scholar]
- 27.Polat J, Grantham L, Mitchell K, Realini T. Repeatability of selective laser trabeculoplasty. Br J Ophthalmol. 2016;100(10):1437–1441. [DOI] [PubMed] [Google Scholar]
- 28.Avery N, Ang GS, Nicholas S, Wells A. Repeatability of primary selective laser trabeculoplasty in patients with primary open-angle glaucoma. Int Ophthalmol. 2013;33(5):501–506. [DOI] [PubMed] [Google Scholar]
- 29.The New Humanitarian. Exodus from hurricane-hit Dominica as recovery remains elusive https://www.thenewhumanitarian.org/feature/2017/10/18/exodus-hurricane-hit-dominica-recovery-remains-elusive. Accessed January 5, 2021.


