Abstract
Plants, in contrast to animals, are unique in their capacity to postembryonically develop new organs due to the activity of stem cell populations, located in specialized tissues called meristems. Above ground, the shoot apical meristem generates aerial organs and tissues throughout plant life. It is well established that auxin plays a central role in the functioning of the shoot apical meristem. Auxin distribution in the meristem is not uniform and depends on the interplay between biosynthesis, transport, and degradation. Auxin maxima and minima are created, and result in transcriptional outputs that drive the development of new organs and contribute to meristem maintenance. To uncover and understand complex signaling networks such as the one regulating auxin responses in the shoot apical meristem remains a challenge. Here, we will discuss our current understanding and point to important research directions for the future.
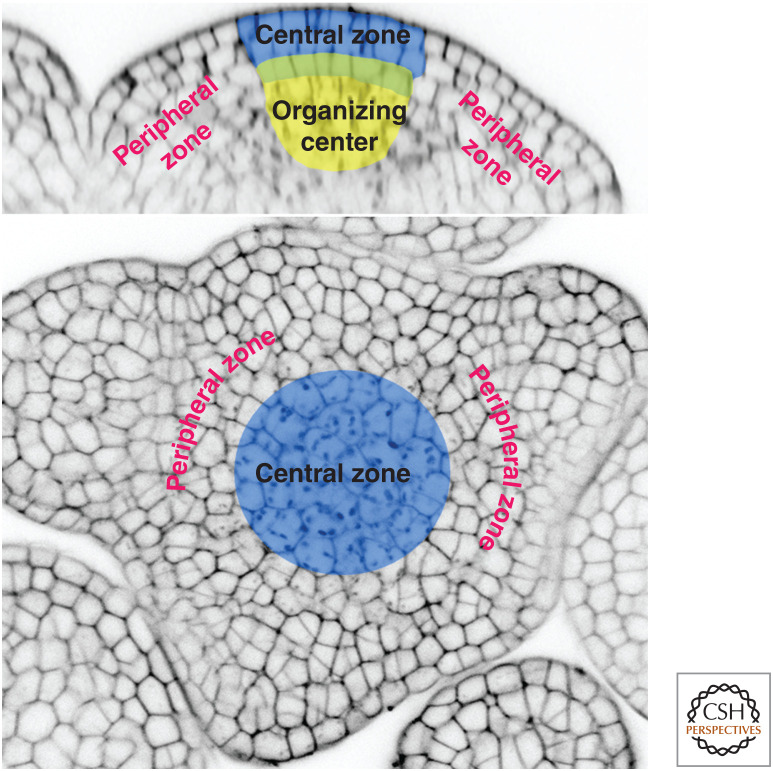
The establishment and maintenance of precise developmental patterns to construct a body architecture is a common characteristic of all multicellular living organisms. Understanding the spatiotemporal regulation of patterning is central to the comprehension of development processes. A unique characteristic of plants is their ability to continuously create structures postembryonically. The dynamic building of the architecture of the aerial part of the plant is controlled in the shoot apical meristem and regulated by a combination of both developmental and environmental factors. The shoot apical meristem is a highly organized structure containing a central zone, where the stem cells are located (Fig. 1). Below the central zone, the organizing center produces signals to regulate stem cells and is itself regulated by signals originating from the central zone as we will see below. New organs including leaves and flowers are initiated in the organogenic peripheral zone that surrounds the central zone.
Figure 1.
Shoot apical meristem structure in Arabidopsis.
Among the factors controlling shoot apical meristem function, phytohormones play a key role and compelling evidence show that auxin is one of the main regulators of both stem cell activity and organogenesis. Several recent reviews have discussed how auxin transport generates dynamic auxin distribution in the shoot apical meristem and the central role of polar auxin transport and auxin carriers in this regulation (Galvan-Ampudia et al. 2016; Kuhlemeier 2017; Bhatia and Heisler 2018; Shi and Vernoux 2019), and we will not cover those aspects here. We will focus on auxin signaling and on how it participates to regulate the function of the shoot apical meristem. In this introduction, we will first briefly review the different mechanisms mediating the perception and transduction of the auxin signal in plant cells.
Auxin acts through different signaling pathways, known as the canonical and noncanonical pathways. The canonical pathway also called the nuclear auxin pathway (NAP) involves three families of proteins (the numbers of members are given for Arabidopsis): 6 TRANSPORT INHIBITOR RESPONSE1 (TIR1)/AUXIN SIGNALING F-BOX (AFB) F-box proteins acting as auxin coreceptors, 29 AUXIN/INDOLE 3-ACETIC ACID INDUCIBLE (Aux/IAA) repressor proteins, and 23 AUXIN RESPONSE FACTOR (ARF) transcription factors. ARFs can be divided into three classes, A, B, and C, based on their evolutionary origin (Finet et al. 2013; Mutte et al. 2018). Class A ARFs are described to have a transcriptional activation activity and classes B and C as repressors (reviewed in Israeli et al. 2020). Individual class B ARFs likely compete with class A ARFs by binding to the same DNA sites (Boer et al. 2014; Kato et al. 2020). In the NAP, the presence of auxin is perceived by TIR1/AFB and triggers changes in the expression of target genes, which then induce tissue-specific biological responses. When auxin is low or absent, Aux/IAAs interact with ARF transcription factors through the shared PB1 domain and repress gene transcription and auxin responses. Auxin binding to TIR1/AFB coreceptors induces the proteasome-dependent degradation of Aux/IAA proteins by targeting them for ubiquitination, thus releasing the repression on ARFs and allowing transcriptional auxin responses. Several Aux/IAAs are themselves ARF targets thus establishing a negative feedback loop (reviewed in Gallei et al. 2020).
Differential expression of regulators of the NAP, as can be seen with the variety of expression patterns of particular ARFs in the shoot apical meristem of Arabidopsis (Fig. 2; Vernoux et al. 2011; Truskina et al. 2020), suggests different possible regulatory outputs arising from the auxin signaling cascade and an implication in all aspects of shoot apical meristem function. Many auxin targets, regulated by ARFs and involved in shoot apical meristem maintenance and activity, have been identified notably in Arabidopsis, especially targets regulated by ARF5/MONOPTEROS (ARF5/MP) (Hardtke and Berleth 1998): ARF5 itself (Lau et al. 2011), TARGET OF MONOPTEROSs (TMOs) (Schlereth et al. 2010), ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN 6 (AHP6) (Besnard et al. 2014), LEAFY (LFY) (Blázquez et al. 1997; Yamaguchi et al. 2013), AINTEGUMENTA (ANT) (Yamaguchi et al. 2013), ANT-LIKE6/PLETHORA3 (AIL6/PLT3) (Yamaguchi et al. 2013), ARABIDOPSIS RESPONSE REGULATOR 7 and ARR15 (Zhao et al. 2010), and DORNRÖSCHEN/ENHANCER OF SHOOT REGENERATION (DRN/ESR1) (Cole et al. 2009). We will discuss in this review the role of individual ARFs and their targets in the shoot apical meristem.
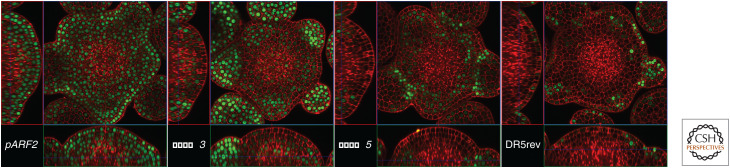
Figure 2.
ARFs are differentially expressed in the shoot apical meristem of Arabidopsis. proARF2, proARF3, proARF5 (Rademacher et al. 2011), and DR5rev (Wabnik et al. 2013) (from the left to the right) reporters show possible combinatorial actions of ARFs in the regulation of auxin transcriptional response, which might lead to different responses in the shoot apical meristem. Note that the expression of the synthetic auxin responsive promoter DR5rev (last image on the right) does not fully reflect the expression of ARFs.
There are currently two noncanonical auxin pathways that have been identified. The first noncanonical auxin response pathway involves the receptor-like kinase TRANSMEMBRANE KINASE 1 (TMK1) (Dai et al. 2013). TMK1 interacts with AUXIN-BINDING PROTEIN 1 (ABP1) and activates Rho-like guanosine triphosphatases (GTPase) from plants (ROP2/ROP6). The interaction between TMK1 and ABP1 was shown to occur in an auxin-dependent manner (Xu et al. 2014). Moreover, after auxin sensing, TMK1 phosphorylates and stabilizes IAA32 and IAA34, thus regulating ARF transcription factors (Cao et al. 2019). The role for ABP1 in this auxin signaling cascade is largely debated due to the absence of clear phenotypes in loss-of-function mutants (Gao et al. 2015; Gelová et al. 2021). An alternative noncanonical auxin pathway has been proposed and involves auxin binding to the atypical ARF3/ETTIN (ETT), resulting in modulation of chromatin and interaction with other transcriptional regulators (Simonini et al. 2016). Direct auxin binding to ARF3 leads to dissociation from corepressors of the TOPLESS/TOPLESS-RELATED family followed by histone acetylation and induction of gene expression and enables switching between repressive and derepressive chromatin states in a reversible manner (Kuhn et al. 2020).
In the next two sections, we will discuss how the canonical and noncanonical auxin signaling pathways act in the meristem to highlight the crucial role for auxin signaling not only in the dynamic initiation of organs but also in the maintenance of the shoot apical meristem.
AUXIN SIGNALING IN THE CENTRAL ZONE: A FUNCTION IN STEM CELL MAINTENANCE
Shoot apical meristem maintenance depends on the coordinated action of signals in the central zone and the organizing center, as well as on interactions with the peripheral zone. In Arabidopsis, the localization of the stem cell niche is primarily controlled by expression of the homeodomain transcription factor WUSCHEL (WUS) in the organizing center (Mayer et al. 1998; Schoof et al. 2000). WUS function is essential for proper shoot apical meristem development as wus mutants fail to organize a shoot apical meristem in the embryo. Postembryonically, an aberrant flattened shoot apical meristem is initiated and terminated prematurely (Laux et al. 1996). When produced, WUS protein migrates from the organizing center upward into the central zone and activates the synthesis of the secreted peptide CLAVATA3 (CLV3) (Fletcher et al. 1999; Yadav et al. 2011). CLV3 in turn negatively regulates WUS expression via the CLV1 receptor that delimits WUS expression to the organizing center (Yadav et al. 2011; Knauer et al. 2013; Daum et al. 2014). Thus, the WUS–CLV negative feedback loop serves as a self-controlling mechanism that maintains a stem cell niche of constant size (Brand 2000; Schoof et al. 2000; Somssich et al. 2016; Soyars et al. 2016). In addition, a plethora of other genes have been identified that are involved in stem cell maintenance alongside the WUS-CLV loop, likely contributing to provide the robust control mechanism that regulates the activity of the stem cell niche (for review, see, e.g., Kitagawa and Jackson 2019; Lee et al. 2019; Han et al. 2020; Jha et al. 2020).
The function of WUS in shoot apical meristem maintenance is intricately linked with cytokinin signaling. WUS represses the transcription of two ARR type-A genes, ARR7 and ARR15 (Leibfried et al. 2005; Zhao et al. 2010), which encode negative regulators of the cytokinin signaling pathway. WUS thus provides a positive input for cytokinin signaling in the center of the shoot apical meristem. Further, the cytokinin signaling pathway increases WUS levels and regulates the stem cell niche (Gordon et al. 2009). Moreover, WUS is stabilized by the transcription factor ARR1, a type-B response regulator that is part of the cytokinin pathway (Snipes et al. 2018), thus creating a positive feedback between WUS and cytokinins. Cytokinins produced in the L1 layer (epidermis) by the cytokinin biosynthetic enzyme LONELY GUY 4 (LOG4), along with CLV signaling, can act as positional cues for patterning the WUS domain within the stem cell niche (Chickarmane et al. 2012). These regulations are likely conserved in other plants, although with variations. For example, in maize, a mutation in ZmLOG7 causes meristem termination (Knauer et al. 2019), which is not the case in Arabidopsis where mutations in single LOG genes result in either a minor or no phenotype (Kuroha et al. 2009). Also, ZmWUS1 expression is not conserved within the vegetative shoot apical meristem (Nardmann and Werr 2006). The maize CLV1 ortholog, thick tassel dwarf1, is expressed in leaf primordia (Nardmann and Werr 2007). Moreover, it is ZmFCP1 and not the CLV3 ortholog that specifically signifies the shoot apical meristem in the corresponding organizing center region (Knauer et al. 2019). This indicates a conservation of the WUS-CLV signaling logic but also the existence of diversifications of the components of the circuit (Kellogg 2019; Rodriguez-Leal et al. 2019).
In maize, KNOTTED1 (KN1) (Vollbrecht et al. 1991) acts as a master regulator of meristem activity in the shoot apical meristem. Consistent with that, KN1 binds a substantial number of transcription factors acting in meristematic tissues. Moreover, genes acting in auxin signaling are overrepresented among KN1 targets (Bolduc et al. 2012; Knauer et al. 2019), providing a functional link between meristematic activity and the regulation of auxin signaling. More information on the function of auxin in meristem maintenance has come from extensive studies in Arabidopsis. Among the effectors involved in auxin signaling in Arabidopsis, nine ARFs and eight Aux/IAAs are expressed at relatively higher levels at the periphery and at comparatively lower levels in the center of the meristem (Vernoux et al. 2011; Truskina et al. 2020). The nine ARFs comprise four class A ARFs but also class B ARFs, indicating that ARFs acting as activators as well as repressors of auxin signaling show significant coexpression in the shoot apical meristem. ARF and Aux/IAA genes are expressed at low levels in the central zone and at relatively higher levels in the peripheral zone. Predictive mathematical modeling arising from a large-scale analysis of AUX/IAA-ARF interaction networks could correctly predict that the variation in expression levels between ARF activators and repressors results in different sensitivity to auxin between the central zone (low sensitivity) and the peripheral zone (high sensitivity). Furthermore, the relative expression ratios of ARF activators and repressors can buffer against input fluctuations, thus maintaining a stable regulatory response (Vernoux et al. 2011).
In a recent study, the auxin signaling input sensor R2D2, and the output signaling reporter DR5v2 (Liao et al. 2015) confirmed previous findings and identified WUS as a key regulator of the reduced sensitivity of the central zone cells to auxin (Ma et al. 2019). The auxin signaling minimum in the meristem center depends on stem cell fate. WUS binds to the promoter region of target genes and activates or represses their expression (Busch et al. 2010; Yadav et al. 2013). However, Ma et al. (2019) reported that WUS acts by triggering histone acetylation at target loci, including regulators of the NAP (e.g., TIR1, ARF5, IAA9) but also a large number of genes acting downstream of auxin (Ma et al. 2019). Histone acetylation is thought to lead to repression of gene expression, suggesting that auxin signaling and response capacity are rheostatically controlled by WUS, restricting auxin signaling to the stem cell population, while at the same time allowing instructive low levels of signaling output. Simultaneously, low levels of auxin signaling are required for stem cell maintenance (Ma et al. 2019). A yeast one-hybrid screen recently identified a network of transcription factors regulating the expression of Arabidopsis class A ARFs (Truskina et al. 2020). This work identified WUS as part of this network and further demonstrated that a vast majority of class A ARF regulators are transcriptional repressors. A mutation in one of these transcriptional repressors, WRKY38, leads to increased ARF7 expression in the center of the shoot apical meristem (Truskina et al. 2020). While a more global analysis of the function of these genes in the shoot apical meristem is required, this suggests that lower ARFs expression (and thus auxin sensitivity) in the central zone implicates transcriptional repressors acting in combination with WUS in regulating ARFs. This complex regulation of signaling output of auxin together with the regulation of cytokinin signaling results in the maintenance of a constant population of stem cells in the central zone, allowing for continuous replenishment of the peripheral zone. Recent evidence suggests that the regulation of stem cell activity by auxin might be an ancestral mechanism. Indeed, in moss, stem cell activity depends on the inability of stem cells to sense the auxin they produce while stem cell progeny responds to auxin (Landberg et al. 2021). Thus, a low level of auxin signaling appears to be required for the maintenance of shoot stem cells and suggests a requirement for certain ARFs to drive this process.
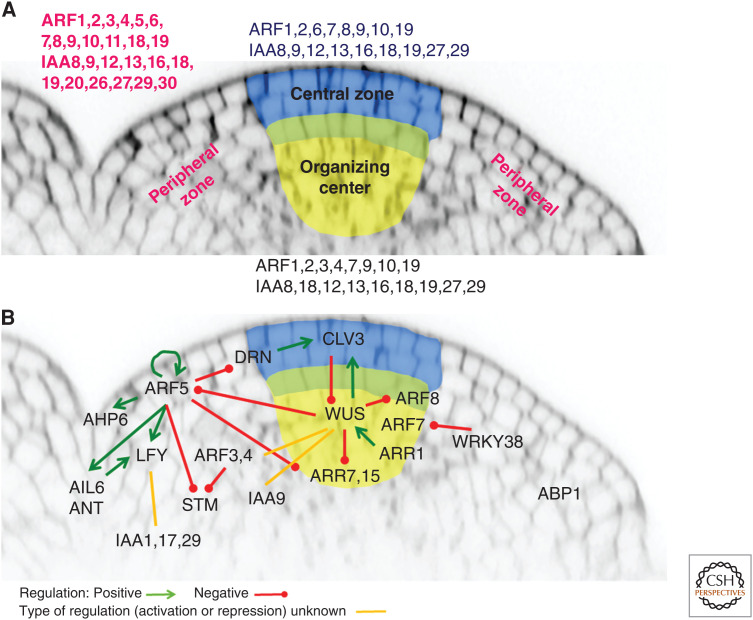
Expression patterns revealed by in situ hybridization or green fluorescent protein (GFP) fusion of single Arabidopsis ARFs pinpointed several candidates that might have a regulatory function in the central zone (Fig. 3A). ARF2, a possible repressor of auxin signaling, seems to be expressed throughout the shoot apical meristem, and seems to be one of the few ARFs present in the central zone (Fig. 2; Vernoux et al. 2011). arf2 mutants display a severe shoot phenotype with late flowering and a very long, thick, and wavy inflorescence stem, suggesting impaired timing of the vegetative-to-generative switch and growth determination. High numbers of undeveloped or retarded siliques point to pleiotropic defects in shoot apical meristem regulation (Okushima et al. 2005a). Weak homogenous expression in the shoot apical meristem was also detected for ARF9 and ARF10 (Vernoux et al. 2011) but their possible function in the central zone is still unknown. In the inner core of the meristem, in the organizing center, ARF4 (Vernoux et al. 2011) as well as ARF7 and ARF19 (Truskina et al. 2020) are expressed at relatively high levels. Mature arf7 mutant plants (nph4-1, arf7-1, and msg1-2/nph4-102) do not show any prominent developmental defects connected with the shoot apical meristem, except for slightly shorter inflorescence stems compared to wild-type plants. Finally, arf19 mutants are identical to wild-type, while an ARF19 overexpressor has severely impaired shoot development as well as apical dominance (Okushima et al. 2005b). So far, evidence for a role for ARFs in meristem maintenance in other species than Arabidopsis is missing (Matthes et al. 2019). Whether specific ARFs are regulating shoot apical meristem maintenance and stem cell niche activity is thus still largely unknown, which calls for further investigation.
Figure 3.
Auxin signaling in the shoot apical meristem of Arabidopsis. (A) ARFs and IAAs expression overview, and (B) ARF-dependent regulatory network.
INITIATION AND OUTGROWTH OF PRIMORDIA IN PERIPHERAL ZONE (PHYLLOTAXIS)—“ARF5 RULES THEM ALL”
Lateral organ primordia are initiated in the peripheral zone, following a regular spatiotemporal pattern called phyllotaxis. The most widely accepted model for phyllotaxis proposes that the spatiotemporal patterns of organ initiation emerge from a self-organizing process that involves morphogen-based inhibitory fields generated by preexisting organ primordia (Mitchison 1977; Douady and Couder 1992). While inhibitory fields block organ initiation in the vicinity of existing organs, new primordia are initiated in the position where the sum of inhibitory effects is the lowest outside of the center of the shoot apical meristem, where initiation is also inhibited. The spatiotemporal patterns of organ initiation then emerge thanks to these inhibitory fields as a result of growth. Over the last two decades, auxin has been demonstrated to be the central regulator of phyllotaxis (for review, see Shi and Vernoux 2019; Godin et al. 2020; Véron et al. 2020). Local auxin maxima trigger organ formation while auxin depletion around organs generates inhibitory fields. Auxin spatiotemporal distribution is generated by PIN1-dependent auxin transport in cooperation with auxin influx and efflux carriers and local auxin biosynthesis (Benková et al. 2003; Reinhardt et al. 2003; Heisler et al. 2005; Jonsson et al. 2006; Smith et al. 2006; Bainbridge et al. 2008; Stoma et al. 2008; Vernoux et al. 2011; Bhatia et al. 2016; Galvan-Ampudia et al. 2020).
As discussed previously, the role of the NAP (and also of the ARF3-mediated noncanonical pathway) in organ initiation is highlighted by the expression of ARF transcription factors and Aux/IAAs. In Arabidopsis, ARF1, 2, 5, 7, 8, 18 and IAA12, 13, 18, 27 are expressed homogeneously throughout the periphery of the shoot apical meristem and ARF3, 4, 6 and IAA8, 9 show a stronger expression in organ primordia (Fig. 3; Vernoux et al. 2011; Truskina et al. 2020). This suggests the involvement of a large network of ARFs and Aux/IAAs in controlling sensitivity to auxin in the peripheral zone. Among these, the class A ARF5/MP, an activator of transcription, seems to work as a central regulator. The ARF5 mutant mp exhibits a pin-like phenotype (Przemeck et al. 1996)—a naked inflorescence stem without lateral organs—similarly to the auxin transport mutant pin1 (Galweiler et al. 1998), even though leaves are still formed in both. But unlike the pin1 mutant, exogenous auxin application does not rescue the defect in flower primordia initiation of the arf5 mutant (Hardtke and Berleth 1998; Reinhardt et al. 2003; Zhao et al. 2010), demonstrating a critical role for auxin signaling in organ initiation in the inflorescence meristem. In the shoot apical meristem, ARF5 not only mediates gene activation in response to auxin as we will see below, but its activity is also determinant for regulating the dynamics of auxin distribution (Bhatia et al. 2016). ARF5 expression is required for regulating polarization of PIN1, with cell polarity patterns following ARF5 expression, which in turn follows auxin distribution. In the epidermal layer as well as in subepidermal tissues, high levels of ARF5-mediated auxin signaling are sufficient to instruct PIN1 polarity directions non-cell-autonomously. ARF5 in the shoot apical meristem has been shown to impact a chromatin-state switch by promoting histone acetylation (Wu et al. 2015). In the presence of auxin, ARF5 recruits SWI/SNF chromatin-remodeling ATPases and increases DNA accessibility through the regulation of histone acetylation in regions of chromatin that contain genes involved in flower formation, thus driving the flower primordium fate at the periphery of the shoot apical meristem. In the absence of auxin, Aux/IAA proteins bind to ARF5 preventing recruitment of SWI/SNF, in addition to recruiting a corepressor/histone deacetylase complex (Long 2006; Wu et al. 2015). This auxin-mediated chromatin state switch is key for iterative primordia initiation in the shoot apical meristem and could coordinate additional auxin-regulated cell fate transitions. Interestingly, the promotion of histone acetylation by ARF5 suggests that ARF5 might be able to counteract the histone deacetylation promoted by WUS, thus providing a possible mechanism by which auxin could contribute to the transition of stem cells to the peripheral zone.
Although playing a dominant role, ARF5 regulates organ initiation in the shoot apical meristem together with ARF3 and ARF4. A recent work demonstrates that these ARFs stimulate flower initiation by histone-deacetylation-mediated transcriptional silencing of the SHOOTMERISTEMLESS gene (Chung et al. 2019), a key meristem identity gene, and the ortholog of maize KN1, Oryza sativa homeobox15 (OSH15) or Tomato Kn2 (TKn2)/LeT6 (Hay and Tsiantis 2010). The arf3/ett loss-of function mutant displays perturbations in phyllotaxis, indicating that this noncanonical pathway is required for stable spatiotemporal patterns of organogenesis (Simonini et al. 2017; Galvan-Ampudia et al. 2020). A recent live imaging analysis has also provided important new insights on the properties of the NAP and of the ARF3-mediated noncanonical pathway in the shoot apical meristem. By comparing the dynamics of auxin accumulation and of transcription activation by auxin and exogenous auxin treatments, the authors could demonstrate that the NAP has time-integration properties, allowing to “count” cell exposure to auxin to trigger organ initiation (Galvan-Ampudia et al. 2020). The molecular mechanisms involved are still to be identified but ARF3 was shown to be involved, as the arf3 mutant loses the capacity to integrate the auxin signal. The temporal integration capacity could then rely on perception of auxin by ARF3 but possibly also by the NAP. Whether the ARF3-dependent noncanonical pathway is functionally conserved in species other than Arabidopsis is unknown (Matthes et al. 2019). Among the other ARFs, ARF6 and ARF8 mutants showed no other shoot phenotype except one in flower maturation (Nagpal 2005), although aerial tissues are dwarfed in the arf6 arf8 double mutant (Okushima et al. 2005b). Apart from ARF5, information about other ARFs and their function in organ initiation at the shoot apical meristem is fragmented and detailed genetic studies (on appropriate multiple mutants) are needed (Ori 2019).
In addition to ARFs, a role for several Aux/IAAs in the shoot apical meristem was uncovered in maize. BARREN INFLORESCENCE1 (BIF1/IAA27) and BARREN INFLORESCENCE4 (BIF4/IAA20) regulate the early steps required for inflorescence formation, particularly proper formation of new axillary meristems. The corresponding gain-of-function mutants produce fewer spikelets and flowers or even naked ears without any grains. These phenotypes are the result of defects in primordia initiation at the peripheral zone of the inflorescence meristem (Galli et al. 2015).
In Arabidopsis, a significant number of genes regulated by ARF5 have been identified, and some of them are crucial players in organ initiation and development (Fig. 3). The transcription factor LFY acts as a master coordinator of the floral network (Moyroud et al. 2010; Siriwardana and Lamb 2012), and is activated by auxin directly via ARF5 (Blázquez and Weigel 2000; Vernoux et al. 2000; Li et al. 2013; Yamaguchi et al. 2013). LFY specifies the floral fate of meristems and its loss-of-function mutation causes partial transformation of flowers into inflorescence shoots in Arabidopsis. Whereas auxin regulates LFY expression by directly binding to the ARF5 promoter region (Yamaguchi et al. 2013), LFY feeds back to the auxin pathway by modulating auxin transport, signaling, or biosynthesis. LFY targets a key regulator of auxin transport, PINOID (PID) (Friml et al. 2004) as seen in the pid mutant that produces a naked inflorescence like arf5/mp or pin1 (Yamaguchi et al. 2013). Moreover, LFY positively regulates auxin signaling by affecting IAA1, IAA17, and IAA29 expression and suppressing several auxin biosynthetic genes (Li et al. 2013). Therefore, floral meristem formation is under the feedback control of both auxin and the master transcription factor LFY. In addition to LFY, two other transcription factors play important roles in mediating auxin responses during floral meristem specification. ANT and AIL6/PLT3 are also direct targets of ARF5 (Yamaguchi et al. 2013), and they further activate LFY expression (Yamaguchi et al. 2016). Taken together, auxin (mainly through ARF5) activates LFY and other key regulators to define primordium founder cells. Last, but not least, ARF5 regulates its own expression by binding to its promoter region (Lau et al. 2011), which could potentiate its activation during organ initiation (Bhatia et al. 2016). In addition, ARF5 also directly represses the transcription of DRN/ESR1 in the shoot apical meristem, and DRN is involved in the activation of CLV3 in the stem cells (Luo et al. 2018). It seems that ARF5 restricts DRN expression in the meristem center thus aiding CLV3 activation. Although the drn mutant shows no obvious phenotype in shoot apical meristem development, overexpression of DRN results in specific defects in meristem maintenance and lateral organ formation (Kirch et al. 2003) and enhances shoot regeneration efficiency in tissue cultures (Banno et al. 2001).
Interaction of auxin with cytokinin was shown to be crucial for organ initiation in the peripheral zone as well. The cytokinin signaling inhibitor AHP6 as a direct target of ARF5 was shown to regulate the timing of organ initiation (Besnard et al. 2014). During flower initiation, AHP6 is a direct target of ARF5 and is activated one plastochrone (the time difference between two successive organ initiation, which is close to 12 h in Arabidopsis) (Besnard et al. 2014) after the induction of the auxin responsive reporter DR5. Sharp gradients of AHP6 centered on primordia and extending beyond its boundaries indicate that this protein creates fields around organs thus making additional, secondary inhibitory fields. Other than AHP6, ARF5 transcriptionally represses ARR7 and ARR15 (both negative regulators of cytokinin response) in the peripheral zone (Zhao et al. 2010). As ARR7 and ARR15 negatively regulate shoot apical meristem size, auxin signaling could thus promote shoot apical meristem activity. This demonstrates cooperation between both hormones in organ initiation and that cytokinin signaling acts in parallel or downstream of auxin to regulate new meristem initiation in the shoot.
CONCLUSION AND PERSPECTIVES
Insights about shoot apical meristem maintenance and patterning have increased at a steady pace over the last two decades. Although the role of auxin transport in the shoot apical meristem has been studied in great detail using mutants in auxin influx and efflux transporters (for review, see Bhatia and Heisler 2018), data regarding auxin action via transcriptional regulation (activation or repression) still remains fragmented, both in the central zone and in organ initiation. Moreover, the role for the noncanonical auxin pathway involving TMK1 in the shoot apical meristem remains unclear, as well as the function of ABP1 in auxin signaling. A recent paper uncovered a negative role for ABP1 in the initiation of leaf primordia using an ABP1 overexpressor line that was observed to produce a smaller number of rosette leaves (Gelová et al. 2021). While this raises the possibility that an ABP1-dependent mechanism acts in organ initiation in the shoot apical meristem, the absence of phenotypes in aerial organ initiation in abp1 mutants does not allow a conclusion at the moment. Large-scale gene-functional studies will likely be critical for shedding light on the role of the effectors of auxin signaling in the shoot apical meristem, allowing to decipher the role of individual transcriptional regulators (or groups of them) in response to auxin signaling. As functional redundancy between closely related genes complicates genetic studies of single members, the availability of CRISPR/Cas9 gene-editing tools should help generate higher order mutants and overcome this redundancy limitation or alternatively gain-of-function lines could be employed (Ori 2019; Israeli et al. 2020).
We foresee that quantitative imaging will be key to advancing our knowledge on auxin signaling function in the shoot apical meristem. More sensitive auxin signaling reporters could certainly help analyze auxin-dependent transcription and understanding the role of the low auxin signaling activity in the central zone and the organizing center of the shoot apical meristem. More generally, reporters and sensors with higher sensitivity (e.g., FRET-based biosensors for detecting auxin with subcellular resolution) (Herud-Sikimic et al. 2020), together with microscopes equipped with the latest-generation highly sensitive detectors, will be important to visualize and analyze auxin distribution and signaling activity in the internal layers of the shoot apical meristem. Most imaging studies we have discussed in this review focus on the epidermal layer, leaving many questions open on how auxin signaling contribute to shoot apical meristem activity in the rest of the meristem. Obtaining quantitative data on auxin distribution and auxin signaling activity in 3D over time in the entire shoot apical meristem will certainly be key to understanding how the auxin signal is processed and how it is translated into specific cellular responses such as fate changes, cell expansion, or cell division and ultimately in morphogenesis. A recent study demonstrated that cells integrate the auxin signal over time to trigger organ initiation in the shoot apical meristem, providing a striking example of how such approaches can expand our knowledge on the role of auxin signaling in the shoot apical meristem (Galvan-Ampudia et al. 2020).
Future approaches will also need to link genome-scale transcriptional changes to the activity of the auxin signaling pathways. Recent works have begun to use mathematical approaches to integrate large-scale data sets in predictive models (for review, see Mjolsness 2019). Such a cell-based model of the Arabidopsis shoot apical meristem was recently developed (Banwarth-Kuhn et al. 2019). The model incorporates individual cell behaviors such as cell–cell interactions, anisotropy in cell growth, cell division, differentiation, and biochemical signaling events to quantify the impact of individual cell processes on overall tissue shape, size, and function. Such approaches are flexible enough to model diverse biological processes such as intercellular chemical kinetics, intercellular signaling, cell differentiation, and motion of cells. More importantly, they can be used to perform simulations that can capture the underlying properties of the real biological system and help pinpoint other candidates as shoot apical meristem regulators mediating auxin signaling activity.
Finally, expanding the analysis of the role of auxin signaling in the shoot apical meristem to nonmodel plant meristems that develop different shoot architecture (Wang et al. 2018a,b; Kitagawa and Jackson 2019), as for example maize given the extensive amount of genetic and expression data available or tomato (Knauer et al. 2019; Matthes et al. 2019), will undoubtedly provide valuable complementary information as highlighted in this review for maize Aux/IAA mutants (Galli et al. 2015). It will also allow the identification of conserved function(s) for auxin signaling in shoot apical meristem function.
ACKNOWLEDGMENTS
We acknowledge funding sources, particularly the European Structural and Investment Funds, Operational Programme Research, Development and Education, Project MSCAfellow@MUNI (CZ.02.2.69/0.0/0.0/17_050/0008496) to M.P., and ANR-18-CE12-0014-02 (ChromAuxi) to T.V.
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C. 2008. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev 22: 810–823. 10.1101/gad.462608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno H, Ikeda Y, Niu QW, Chua NH. 2001. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13: 2609–2618. 10.1105/tpc.010234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwarth-Kuhn M, Nematbakhsh A, Rodriguez KW, Snipes S, Rasmussen CG, Reddy GV, Alber M. 2019. Cell-based model of the generation and maintenance of the shape and structure of the multilayered shoot apical meristem of Arabidopsis thaliana. Bull Mathematical Biol 81: 3245–3281. 10.1007/s11538-018-00547-z [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Lainé S, et al. 2014. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 505: 417–421. 10.1038/nature12791 [DOI] [PubMed] [Google Scholar]
- Bhatia N, Heisler MG. 2018. Self-organizing periodicity in development: organ positioning in plants. Development 145: dev149336. 10.1242/dev.149336 [DOI] [PubMed] [Google Scholar]
- Bhatia N, Bozorg B, Larsson A, Ohno C, Jönsson H, Heisler MG. 2016. Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Curr Biol 26: 3202–3208. 10.1016/j.cub.2016.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Weigel D. 2000. Integration of floral inductive signals in Arabidopsis. Nature 404: 889–892. 10.1038/35009125 [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. 1997. LEAFY expression and flower initiation in Arabidopsis. Development 124: 3835–3844. [DOI] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WAM, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. 2014. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589. 10.1016/j.cell.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Bolduc N, Yilmaz A, Mejia-Guerra MK, Morohashi K, O'Connor D, Grotewold E, Hake S. 2012. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev 26: 1685–1690. 10.1101/gad.193433.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand U. 2000. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619. 10.1126/science.289.5479.617 [DOI] [PubMed] [Google Scholar]
- Busch W, Miotk A, Ariel FD, Zhao Z, Forner J, Daum G, Suzaki T, Schuster C, Schultheiss SJ, Leibfried A, et al. 2010. Transcriptional control of a plant stem cell niche. Dev Cell 18: 841–853. 10.1016/j.devcel.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Cao M, Chen R, Li P, Yu Y, Zheng R, Ge D, Zheng W, Wang X, Gu Y, Gelová Z, et al. 2019. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568: 240–243. 10.1038/s41586-019-1069-7 [DOI] [PubMed] [Google Scholar]
- Chickarmane VS, Gordon SP, Tarr PT, Heisler MG, Meyerowitz EM. 2012. Cytokinin signaling as a positional cue for patterning the apical-basal axis of the growing Arabidopsis shoot meristem. Proc Natl Acad Sci 109: 4002–4007. 10.1073/pnas.1200636109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Zhu Y, Wu MF, Simonini S, Kuhn A, Armenta-Medina A, Jin R, Østergaard L, Gillmor CS, Wagner D. 2019. Auxin response factors promote organogenesis by chromatin-mediated repression of the pluripotency gene SHOOTMERISTEMLESS. Nat Commun 10: 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M, Chandler J, Weijers D, Jacobs B, Comelli P, Werr W. 2009. DORNROSCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development 136: 1643–1651. 10.1242/dev.032177 [DOI] [PubMed] [Google Scholar]
- Dai N, Wang W, Patterson SE, Bleecker AB. 2013. The TMK subfamily of receptor-like kinases in Arabidopsis display an essential role in growth and a reduced sensitivity to auxin. PLoS ONE 8: e60990. 10.1371/journal.pone.0060990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU. 2014. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc Natl Acad Sci 111: 14619–14624. 10.1073/pnas.1406446111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douady S, Couder Y. 1992. Phyllotaxis as a physical self-organized growth process. Phys Rev Lett 68: 2098–2101. 10.1103/PhysRevLett.68.2098 [DOI] [PubMed] [Google Scholar]
- Finet C, Berne-Dedieu A, Scutt CP, Marlétaz F. 2013. Evolution of the ARF gene family in land plants: old domains, new tricks. Mol Biol Evol 30: 45–56. 10.1093/molbev/mss220 [DOI] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914. 10.1126/science.283.5409.1911 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al. 2004. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865. 10.1126/science.1100618 [DOI] [PubMed] [Google Scholar]
- Gallei M, Luschnig C, Friml J. 2020. Auxin signalling in growth: Schrödinger's cat out of the bag. Curr Opin Plant Biol 53: 43–49. 10.1016/j.pbi.2019.10.003 [DOI] [PubMed] [Google Scholar]
- Galli M, Liu Q, Moss BL, Malcomber S, Li W, Gaines C, Federici S, Roshkovan J, Meeley R, Nemhauser JL, et al. 2015. Auxin signaling modules regulate maize inflorescence architecture. Proc Natl Acad Sci 112: 13372–13377. 10.1073/pnas.1516473112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Chaumeret AM, Godin C, Vernoux T. 2016. Phyllotaxis: from patterns of organogenesis at the meristem to shoot architecture. Wiley Interdiscip Rev Dev Biol 5: 460–473. 10.1002/wdev.231 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Cerutti G, Legrand J, Brunoud G, Martin-Arevalillo R, Azais R, Bayle V, Moussu S, Wenzl C, Jaillais Y, et al. 2020. Temporal integration of auxin information for the regulation of patterning. eLife 9: e55832. 10.7554/eLife.55832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230. 10.1126/science.282.5397.2226 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y. 2015. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci 112: 2275–2280. 10.1073/pnas.1500365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelová Z, Gallei M, Pernisová M, Brunoud G, Zhang X, Glanc M, Li L, Michalko J, Pavlovičová Z, Verstraeten I, et al. 2021. Developmental roles of auxin binding protein 1 in Arabidopsis thaliana. Plant Sci 303: 110750. 10.1016/j.plantsci.2020.110750 [DOI] [PubMed] [Google Scholar]
- Godin C, Golé C, Douady S. 2020. Phyllotaxis as geometric canalization during plant development. Development 147: dev165878. 10.1242/dev.165878 [DOI] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM. 2009. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci 106: 16529–16534. 10.1073/pnas.0908122106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Liu X, Zhou Y. 2020. Transcriptional circuits in control of shoot stem cell homeostasis. Curr Opin Plant Biol 53: 50–56. 10.1016/j.pbi.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411. 10.1093/emboj/17.5.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Tsiantis M. 2010. KNOX genes: versatile regulators of plant development and diversity. Development 137: 3153–3165. 10.1242/dev.030049 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911. 10.1016/j.cub.2005.09.052 [DOI] [PubMed] [Google Scholar]
- Herud-Sikimic O, Stiel AC, Ortega-Perez M, Shanmugaratnam S, Höcker B, Jürgens G. 2020. Design of a biosensor for direct visualisation of auxin. bioRxiv 10.1101/2020.01.19.911735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli A, Reed JW, Ori N. 2020. Genetic dissection of the auxin response network. Nat Plants 6: 1082–1090. 10.1038/s41477-020-0739-7 [DOI] [PubMed] [Google Scholar]
- Jha P, Ochatt SJ, Kumar V. 2020. WUSCHEL: a master regulator in plant growth signaling. Plant Cell Rep 39: 431–444. 10.1007/s00299-020-02511-5 [DOI] [PubMed] [Google Scholar]
- Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. 2006. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci 103: 1633–1638. 10.1073/pnas.0509839103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Mutte SK, Suzuki H, Crespo I, Das S, Radoeva T, Fontana M, Yoshitake Y, Hainiwa E, van den Berg W, et al. 2020. Design principles of a minimal auxin response system. Nat Plants 6: 473–482. 10.1038/s41477-020-0662-y [DOI] [PubMed] [Google Scholar]
- Kellogg EA. 2019. Different ways to be redundant. Nat Genet 51: 770–771. 10.1038/s41588-019-0406-y [DOI] [PubMed] [Google Scholar]
- Kirch T, Simon R, Grünewald M, Werr W. 2003. The DORNRÖSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell 15: 694–705. 10.1105/tpc.009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Jackson D. 2019. Control of meristem size. Ann Rev Plant Biol 70: 269–291. 10.1146/annurev-arplant-042817-040549 [DOI] [PubMed] [Google Scholar]
- Knauer S, Holt AL, Rubio-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker MR, Laux T. 2013. A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 24: 125–132. 10.1016/j.devcel.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Knauer S, Javelle M, Li L, Li X, Ma X, Wimalanathan K, Kumari S, Johnston R, Leiboff S, Meeley R, et al. 2019. A high-resolution gene expression atlas links dedicated meristem genes to key architectural traits. Genome Res 29: 1962–1973. 10.1101/gr.250878.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C. 2017. Phyllotaxis. Curr Biol 27: R882–R887. 10.1016/j.cub.2017.05.069 [DOI] [PubMed] [Google Scholar]
- Kuhn A, Ramans Harborough S, McLaughlin HM, Natarajan B, Verstraeten I, Friml J, Kepinski S, Østergaard L. 2020. Direct ETTIN-auxin interaction controls chromatin states in gynoecium development. eLife 9: e51787. 10.7554/eLife.51787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H. 2009. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21: 3152–3169. 10.1105/tpc.109.068676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K, Šimura J, Ljung K, Sundberg E, Thelander M. 2021. Studies of moss reproductive development indicate that auxin biosynthesis in apical stem cells may constitute an ancestral function for focal growth control. New Phytol 229: 845–860. 10.1111/nph.16914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Smet ID, Kolb M, Meinhardt H, Jürgens G. 2011. Auxin triggers a genetic switch. Nat Cell Biol 13: 611–615. 10.1038/ncb2212 [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jurgens G. 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Lee ZH, Hirakawa T, Yamaguchi N, Ito T. 2019. The roles of plant hormones and their interactions with regulatory genes in determining meristem activity. Int J Mol Sci 20: 4065. 10.3390/ijms20164065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175. 10.1038/nature04270 [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Liu X, Yu P, Cohen JD, Meyerowitz EM. 2013. LEAFY controls auxin response pathways in floral primordium formation. Sci Signal 6: ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Smet W, Brunoud G, Yoshida S, Vernoux T, Weijers D. 2015. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods 12: 207–210. 10.1038/nmeth.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA. 2006. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312: 1520–1523. 10.1126/science.1123841 [DOI] [PubMed] [Google Scholar]
- Luo L, Zeng J, Wu H, Tian Z, Zhao Z. 2018. A molecular framework for auxin-controlled homeostasis of shoot stem cells in Arabidopsis. Mol Plant 11: 899–913. 10.1016/j.molp.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Ma Y, Miotk A, Šutiković Z, Ermakova O, Wenzl C, Medzihradszky A, Gaillochet C, Forner J, Utan G, Brackmann K, et al. 2019. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat Commun 10: 5093. 10.1038/s41467-019-13074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes MS, Best NB, Robil JM, Malcomber S, Gallavotti A, McSteen P. 2019. Auxin EvoDevo: conservation and diversification of genes regulating auxin biosynthesis, transport, and signaling. Mol Plant 12: 298–320. 10.1016/j.molp.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. 10.1016/S0092-8674(00)81703-1 [DOI] [PubMed] [Google Scholar]
- Mitchison GJ. 1977. Phyllotaxis and the Fibonacci series. Science 196: 270–275. 10.1126/science.196.4287.270 [DOI] [PubMed] [Google Scholar]
- Mjolsness E. 2019. Prospects for declarative mathematical modeling of complex biological systems. Bull Math Biol 81: 3385–3420. 10.1007/s11538-019-00628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyroud E, Kusters E, Monniaux M, Koes R, Parcy F. 2010. LEAFY blossoms. Trends Plant Sci 15: 346–352. 10.1016/j.tplants.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GK-S, Weijers D. 2018. Origin and evolution of the nuclear auxin response system. eLife 7: e33399. 10.7554/eLife.33399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P. 2005. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132: 4107–4118. 10.1242/dev.01955 [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W. 2006. The shoot stem cell niche in angiosperms: expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Mol Biol Evol 23: 2492–2504. 10.1093/molbev/msl125 [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W. 2007. The evolution of plant regulatory networks: what Arabidopsis cannot say for itself. Curr Opin Plant Biol 10: 653–659. 10.1016/j.pbi.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Okushima Y, Mitina I, Quach HL, Theologis A. 2005a. AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator: ARF transcription factors. Plant J 43: 29–46. 10.1111/j.1365-313X.2005.02426.x [DOI] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. 2005b. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463. 10.1105/tpc.104.028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N. 2019. Dissecting the biological functions of ARF and Aux/IAA genes. Plant Cell 31: 1210–1211. 10.1105/tpc.19.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck GH, Mattsson J, Hardtke C, Sung ZR, Berleth T. 1996. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200: 229–237. 10.1007/BF00208313 [DOI] [PubMed] [Google Scholar]
- Rademacher EH, Möller B, Lokerse AS, Llavata-Peris CI, van den Berg W, Weijers D. 2011. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family: a cellular expression map of ARF gene expression. Plant J 68: 597–606. 10.1111/j.1365-313X.2011.04710.x [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260. 10.1038/nature02081 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Leal D, Xu C, Kwon C-T, Soyars C, Demesa-Arevalo E, Man J, Liu L, Lemmon ZH, Jones DS, Van Eck J, et al. 2019. Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat Genet 51: 786–792. 10.1038/s41588-019-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. 10.1038/nature08836 [DOI] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. 10.1016/S0092-8674(00)80700-X [DOI] [PubMed] [Google Scholar]
- Shi B, Vernoux T. 2019. Patterning at the shoot apical meristem and phyllotaxis. Curr Topic Dev Biol 131: 81–107. 10.1016/bs.ctdb.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Simonini S, Deb J, Moubayidin L, Stephenson P, Valluru M, Freire-Rios A, Sorefan K, Weijers D, Friml J, Østergaard L. 2016. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev 30: 2286–2296. 10.1101/gad.285361.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonini S, Bencivenga S, Trick M, Østergaard L. 2017. Auxin-induced modulation of ETTIN activity orchestrates gene expression in Arabidopsis. Plant Cell 29: 1864–1882. 10.1105/tpc.17.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwardana NS, Lamb RS. 2012. The poetry of reproduction: the role of LEAFY in Arabidopsis thaliana flower formation. Int J Dev Biol 56: 207–221. 10.1387/ijdb.113450ns [DOI] [PubMed] [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. 2006. A plausible model of phyllotaxis. Proc Natl Acad Sci 103: 1301–1306. 10.1073/pnas.0510457103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes SA, Rodriguez K, DeVries AE, Miyawaki KN, Perales M, Xie M, Reddy GV. 2018. Cytokinin stabilizes WUSCHEL by acting on the protein domains required for nuclear enrichment and transcription. PLoS Genet 14: e1007351. 10.1371/journal.pgen.1007351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich M, Je BI, Simon R, Jackson D. 2016. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143: 3238–3248. 10.1242/dev.133645 [DOI] [PubMed] [Google Scholar]
- Soyars CL, James SR, Nimchuk ZL. 2016. Ready, aim, shoot: stem cell regulation of the shoot apical meristem. Curr Opin Plant Biol 29: 163–168. 10.1016/j.pbi.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Stoma S, Lucas M, Chopard J, Schaedel M, Traas J, Godin C. 2008. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput Biol 4: e1000207. 10.1371/journal.pcbi.1000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truskina J, Han J, Chrysanthou E, Galvan-Ampudia CS, Lainé S, Brunoud G, Macé J, Bellows S, Legrand J, Bågman AM, et al. 2020. A network of transcriptional repressors modulates auxin responses. Nature 589: 116.– . [DOI] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. 2000. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127: 5157–5165. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. 2011. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7: 508. 10.1038/msb.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron E, Vernoux T, Coudert Y. 2020. Phyllotaxis from a single apical cell. Trends Plant Sci 26: 124–131. 10.1016/j.tplants.2020.09.014 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S. 1991. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243. 10.1038/350241a0 [DOI] [PubMed] [Google Scholar]
- Wabnik K, Robert HS, Smith RS, Friml J. 2013. Modeling framework for the establishment of the apical-basal embryonic axis in plants. Curr Biol 23: 2513–2518. 10.1016/j.cub.2013.10.038 [DOI] [PubMed] [Google Scholar]
- Wang B, Smith SM, Li J. 2018a. Genetic regulation of shoot architecture. Ann Rev Plant Biol 69: 437–468. 10.1146/annurev-arplant-042817-040422 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang T, Wang R, Zhao Y. 2018b. Recent advances in auxin research in rice and their implications for crop improvement. J Exp Botany 69: 255–263. 10.1093/jxb/erx228 [DOI] [PubMed] [Google Scholar]
- Wu M-F, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D. 2015. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. eLife 4: e09269. 10.7554/eLife.09269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusova H, et al. 2014. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028. 10.1126/science.1245125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Girke T, Jonsson H, Reddy GV. 2011. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev 25: 2025–2030. 10.1101/gad.17258511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Ohno C, Heisler M, Girke T, Jönsson H, Reddy GV. 2013. Plant stem cell maintenance involves direct transcriptional repression of differentiation program. Mol Syst Biol 9: 654. 10.1038/msb.2013.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Wu MF, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA, Wagner D. 2013. A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell 24: 271–282. 10.1016/j.devcel.2012.12.017 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Jeong CW, Nole-Wilson S, Krizek BA, Wagner D. 2016. AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 induce LEAFY expression in response to auxin to promote the onset of flower formation in Arabidopsis. Plant Physiol 170: 283–293. 10.1104/pp.15.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU. 2010. Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092. 10.1038/nature09126 [DOI] [PubMed] [Google Scholar]