Abstract
The auxin-binding protein 1 (ABP1) has endured a history of undulating prominence as a candidate receptor for this important phytohormone. Its capacity for binding auxin has not been in doubt, a feature adequately explained by its crystal structure, but any relevance of this to auxin signaling and plant development has been far more demanding to define. Over its research lifetime, it has been associated with many auxin-induced activities, including ion fluxes across the plasma membrane, rearrangement of the cytoskeleton and cell shape, and the abundance of PIN proteins at the plasma membrane via control of endocytosis, all of which required its presence in the apoplast. Yet, ABP1 has a KDEL sequence that targets it to the endoplasmic reticulum, where most of it remains. This mismatch has been more than adequately compensated for by the need for an auxin receptor to account for responses far too rapid to be executed through transcription and translation and the TIR1/AuxIAA coreceptor system. However, discoveries showing that abp1-null mutants are not compromised for auxin signaling or development, that TIR1 or AFB1 are necessarily involved with very rapid responses at the plasma membrane, and that these rapid responses are mediated with intracellular auxin all suggest that ABP1's auxin-binding capacity is not physiologically relevant. Nevertheless, ABP1 is ubiquitous in higher plants and throughout plant tissues. We need to complete its history by defining its function inside plant cells.
The auxin-binding protein 1 (ABP1) has had a colorful and vexatious history, being referred to as both a red herring (Hertel 1995; Habets and Offringa 2015) and an outsider (Sauer and Kleine-Vehn 2011), and with reviews ending with lists of questions rather than conclusions (Sauer and Kleine-Vehn 2011; Feng and Kim 2015). Throughout its history, its fortunes have ebbed and flowed. First discovered in 1972 using radiolabeled assays for auxin binding with preparations of Zea coleoptile membranes (Hertel et al. 1972), there was a rush to refine and describe its properties (for review, see Napier 1995 and Napier et al. 2002). It was then all but discounted as a receptor because it was shown to be associated with the endoplasmic reticulum (ER), not the plasma membrane (Ray 1977). Purification, using its property of binding auxin as an assay through chromatography steps (Löbler and Klämbt 1985), led to antibodies, peptide sequences, and the full primary sequence was published in 1989 (Inohara et al. 1989) starting another surge in research on ABP1.
Antibodies and sequence information offered tools with which the signaling pathway of ABP1 might be discovered. The first report claiming that an auxin-specific response was dependent on ABP1 and, hence, that it was an auxin receptor was also in 1989 (Barbier-Brygoo et al. 1989), and a series of reports extended these observations in the 1990s. However, these experiments opened again the questions about how a protein targeted to the ER might be functional at the cell surface. As details were added to the picture of receptor-like activity at the plasma membrane, the lack of genetic tools and genetic evidence to support its role in auxin signaling became ever more conspicuous.
In 2001, an abp1-null mutant was shown to be embryo lethal, which did support the hypothesis that ABP1 was vital to plants (Chen et al. 2001b). It also slowed further enquiries using genetics at a time when the power of genetics was starting to reveal another auxin receptor, leading to the description of the TIR1-Aux/IAA coreceptor complex in 2005 (for review, see Leyser 2018). However, a further resurgence of ABP1 research was initiated by reports that extracellular ABP1 could promote clathrin-mediated endocytosis with associated effects on the population of PIN-formed (PIN) proteins on the plasma membrane (Robert et al. 2010; Čovanová et al. 2013). As with the earlier physiological responses at the plasma membrane, this occurred rapidly, too soon after an auxin stimulus to pass through a loop of transcription and translation, and treatments to exclude transcription and translation confirmed the effects to be independent of TIR1. Once again, this resurgence grew and linked ABP1 to rearrangements of the cytoskeleton and membrane-bound kinases (Feng and Kim 2015), but all these observations were rapidly overshadowed by the description of two new abp1-null alleles, which were shown to have phenotypes indifferent from wild-type (Gao et al. 2015). In other words, plants were shown not to need ABP1 to interpret auxin signals, leaving all the ABP1 literature in need of reinterpretation, again.
Given its undulating history, it is no wonder that plant science is confused about ABP1. In spite of this, there are definite knowns as well as the many unknowns in its biography. In this review, I will attempt to summarize what we can be confident about (certainties) and what is less certain (uncertainties) and attempt to move our understanding toward the former. There have been thorough reviews written about ABP1 throughout its history (Napier 1995; Napier et al. 2002; Tromas et al. 2010; Sauer and Kleine-Vehn 2011; Feng and Kim 2015; Grones and Friml 2015) and I will use these where appropriate rather than list extensive original literature.
CERTAINTIES—WHAT WE KNOW ABOUT ABP1
The Biochemistry and Structure of ABP1
One aspect of ABP1 that has stood the test of time has been its name—it does bind auxin. It was identified using an auxin-binding assay using radiolabeled 1-naphthylacetic acid (1-NAA), it has been purified using auxin affinity chromatography, it has been photo-affinity labeled with an auxin derivative, and, perhaps most convincingly, it has been crystallized with auxin bound (Napier et al. 2002; Woo et al. 2002). In almost all these cases, the auxin in question was 1-NAA, or derivatives of it, although phenylacetic acid is a good affinity matrix for ABP1 purification. Kinetic measurements on the purified protein have shown that ABP1 from maize (Zea mays L.) binds 1-NAA with affinities varying from 6 × 10−8 M (Shimomura et al. 1986) to 2.4 × 10−7 M (Hesse et al. 1989), and from Nicotiana between 5 and 7 × 10−7 M (Shimomura et al. 1999). These are good affinities and are consistent with concentrations of auxins necessary for physiological activity when applied in vivo. Whether or not this binding is physiologically relevant will be discussed below.
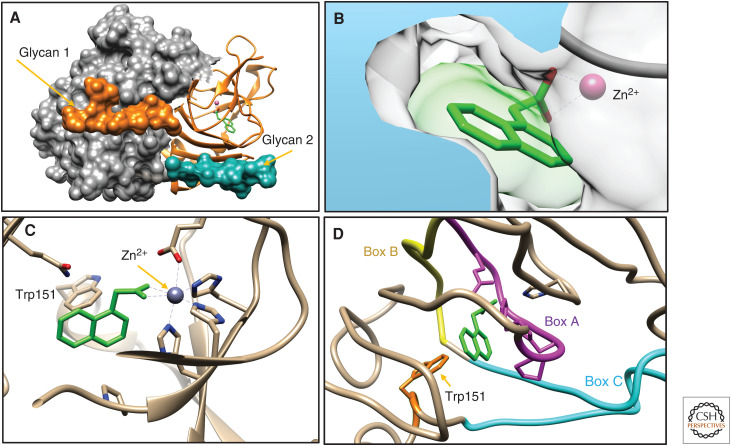
The structure of ABP1 was solved at a resolution of 1.9 Å and with an R-free value of 0.24, suggesting a robust model for the protein (PDB 1LRH) (Woo et al. 2002). The model shows ABP1 as a dimer and with the glycan of each monomer hugging its partner monomer (Fig. 1). The structure was solved in the absence and presence of bound 1-NAA with the auxin shielded inside a tight pocket, which is the cavity of the β-barrel fold, also known as the RmlC-like jelly roll fold (Giraud et al. 2000). This barrel structure is a conserved feature for the family of proteins known as the cupins, which are numerous in plants and have diverse functions (Dunwell et al. 2008). A key feature is a sequence motif for coordinating a divalent metal ion, which is zinc in the case of ZmABP1 (Fig. 1B,C). This zinc ion hydrogen bonds to the acetic acid group of 1-NAA, positioning it in the binding site. The aromatic ring of the auxin is held in an edge-to-face pi stack with an adjacent tryptophan residue (Trp151).
Figure 1.
Structural features of auxin-binding protein 1 (ABP1). In all panels, 1-NAA is shown in green and Zn2+ in purple. (A) The dimer of ABP1 with one monomer shown as a molecular surface (gray), the other as a ribbon (orange). The glycans are both shown as surfaces, with the glycan of the orange monomer (glycan 1) folded across the gray monomer, and vice versa. (B) The aperture to the auxin-binding site with the surface of 1-NAA also shaded in transparent green. (C) The Zn2+ ion is coordinated by a cluster of histidine residues and an aspartate, and when 1-NAA binds a water molecule is displaced (not shown) to allow the carboxylic acid group to hydrogen bond to the Zn2+ (dashed lines). Tryptophan151 is seen behind the auxin at the end of an α helix (receding into the background). (D) The conserved sequence boxes of ABP1: box A (magenta), box B (yellow), and box C (cyan), plus the conserved Trp151 (orange). PDB 1LRH visualized using Chimera (Pettersen et al. 2004).
There is a small aperture to the binding site (Fig. 1B) and no significant change in backbone carbons was reported between unbound and bound structures, which implies no auxin-induced conformational change (Woo et al. 2002). A rationale was put forward for crystal constraints promoting crystallization only of the bound structure, alongside a hypothetical model for conformational movement of the carboxy-terminal α helix upon auxin binding. At one end of this helix lies Trp151 (Fig. 1C), and the activation model suggested that as auxin binds, this Trp is displaced, pushing away the helix like a piston. This model remains unproven, although it is consistent with evidence of an auxin-induced conformational change detected using antibodies mapped to the carboxyl-terminus of the protein (Napier and Venis 1990).
Molecular dynamics simulations of ABP1 have variously supported changes in the arrangement of the carboxyl-terminus induced by auxin with general agreement that it unfolds in the absence of auxin (Bertoša et al. 2008; Grandits and Oostenbrink 2014; Tesser da Costa et al. 2017). Simulations of the glycosylated protein for Arabidopsis as well as maize also suggested small differences between dicot and monocot proteins, which may be linked to different auxin-binding specificities (Tesser da Costa et al. 2017).
The Sequence of ABP1
At the protein level, ABP1 is highly conserved throughout the angiosperms, and residue identity remains high with possible homologs in the lycophytes and mosses (Tromas et al. 2010). A series of very highly conserved motifs were defined as boxes A–C (Napier et al. 2002), with the cluster of zinc-binding residues being box A (Fig. 1) and the other boxes forming the walls of the barrel around the auxin-binding pocket in ABP1 (Fig. 1D).
Two additional key motifs that are conserved across higher plants are the carboxy-terminal KDEL ER retention sequence and two cysteine residues, one close to the amino-terminus of the mature ABP1 and the second just carboxy-terminal to Trp151. These cysteines are disulfide bonded, locking the two ends of the protein together, an important structural constraint (Woo et al. 2002). Both of these features are missing in lycophyte and moss sequences. The absence of these two structural features raises doubts about whether lower plant sequences are really of ABP1 homologs. The analysis by Tromas and colleagues also discussed possible homologs further back in plant ancestry, and it is certainly true that there are sequences from some algae with high similarity across the boxes but, again, they lack other key features.
Expression Profiles of ABP1
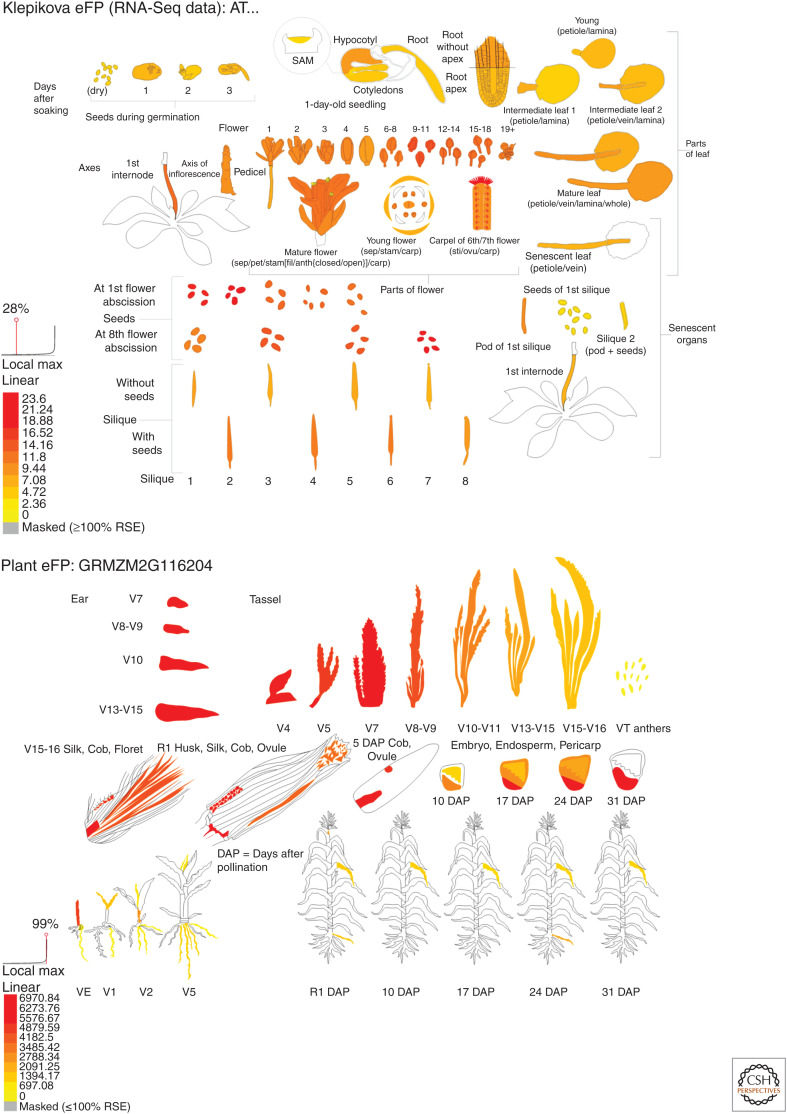
The early work of Hertel and Ray screened plant tissues for auxin-binding activity, which resulted in maize seedlings becoming the material of choice for early work. They remain the tissue with highest levels of extractable ABP1. In most plants and most tissues, ABP1 expression is modest. In Arabidopsis, expression appears highest in stylar tissue and seeds (Fig. 2, eFP browser for AT4G02980; Winter et al. 2007; Waese et al. 2017). Expression is elevated during early- to mid-seed maturation and in germinating endosperm, as well as in the root meristem and stele in trichomes and transiently in response to heat (38°C). Its transcript levels do not respond to hormone treatments (including auxin); there is some increase in response to some pathogen challenges although nothing consistent across different biotic stresses. Transcript levels change little with photoperiod and not at all to photoreceptor-linked light treatments. The eFP browser summaries agree in general with results from pABP1:GUS analyses, which suggested elevated expression in young growing leaves, the root meristem, vascular tissue, and hydathodes (Klode et al. 2011).
Figure 2.
Expression maps for auxin-binding protein 1 (ABP1) in Arabidopsis (top, AT4G02980) and maize (bottom, GRMZM2FG116204). The Arabidopsis image was generated from data from Klepikova et al. (2016). The maize image is based on data from Downs et al. (2013). Both images were prepared by bar.utoronto.ca/eplant (Waese et al. 2017). (RSE) Relative standard error.
Expression maps for maize suggest somewhat more variation over tissues and lifetime (Fig. 2), with the highest expression in floral and embryo tissues, which is consistent with the picture from Arabidopsis, plus the young seedling as used for biochemical extractions.
The expression data from Arabidopsis are also consistent with earlier results from Zea, which showed that both ABP1 protein and its mRNA were remarkably stable with long half-lives, which did not respond to hormone or stress treatments, except heat, which led to rapid loss of ABP1 protein (Oliver et al. 1995). There is also an interesting report of ABP1 expression being highly enhanced in active cambium of the tree Eucommia, and poorly expressed in dormant cambium (Hou et al. 2006). However, if we summarize all these observations on expression, we learn little that helps explain or define the role of ABP1. Its expression profiles are not distinctive, although the highlights like trichomes and hydathodes, stele and cambium, and stylar tissues should provide clues if only we could decipher them.
Genetic Analyses of ABP1 Function
Genes for ABP1 are ubiquitous across the higher plants, with ABP1-like sequences in lower plants, as noted above (Tromas et al. 2010). In many plant species, there is a small family of ABP1-like genes, although there is a single-copy ABP1 gene in Arabidopsis. The observation that ABP1 pervades higher plant genomes suggests that ABP1 is essential, and this presumption was kindled when a reverse-engineered Arabidopsis abp1-1 knockout line was reported to be embryo lethal (Chen et al. 2001b). This awkward genetic system slowed follow-up experimentation for over 10 years. Then, when attempts to complement the abp1-1 mutation by expressing wild-type ABP1 failed (Gao et al. 2015), additional work revealed that the embryo lethality in abp1-1 was caused by disruption of an adjacent gene (Dai et al. 2015; Michalko et al. 2015). Further, two new verified abp1 knockout lines showed that plant development and auxin signaling were not dependent on ABP1 (Gao et al. 2015). Thus, genetic analysis of ABP1 function was both freed up and appeared to separate ABP1 from auxin action.
A number of alternative genetic tools had been developed to help validate proposed roles of ABP1 in auxin signaling and these were rapidly reevaluated after the paper by Gao et al. (2015). A partial loss of function line, abp1-5, was shown to be unreliable after second site mutations were recorded (Enders et al. 2015) and strong phenotypes were observed in the absence of ABP1 in a set of conditional ABP1 knockdown lines (Michalko et al. 2016). Taken together, it became clear that another certainty about ABP1 was that it was not involved in the bulk of developmental and signaling roles driven by auxin. Indeed, it had long been accepted that ABP1 was not involved with the TIR1-Aux/IAA coreceptor complex nor in the genetic control of auxin action through this gene derepression pathway (Leyser 2018).
Antisense and Overexpression of ABP1
The observations on abp1 mutant lines do not necessarily disqualify data generated using antisense and overexpression genetic strategies. Antisense data have been reported little, probably due to a lack of marked phenotypes consistent with data from the knockout lines noted above (Gao et al. 2015). Overexpression has been reported a number of times and in most cases has resulted in subtle developmental phenotypes such as increased leaf epidermal cell size and interdigitation of epidermal cell lobes (Jones et al. 1998; Grones et al. 2015), increased sensitivity of K+ influx to applied auxin (Bauly et al. 2000), and positive regulation of endocytosis of PIN efflux carriers (Čovanová et al. 2013; Grones et al. 2015), probably through promotion of clathrin recruitment to the plasma membrane (Robert et al. 2010; Chen et al. 2014; Grones et al. 2015). Thus, there remain a cluster of phenotypes associated with overexpression of ABP1, which are linked to activities at the plasma membrane.
UNCERTAINTIES—REPORTS AND ASSOCIATIONS OF ABP1 WITH ACTIVITIES THAT NEED MORE EXPLANATION
ABP1 and Responses at the Plasma Membrane
Once antibodies and the sequence of ABP1 were available, there was a series of reports that suggested that ABP1 was active at the outer face of the plasma membrane and that this was the site of perception for auxin. The first was the demonstration that anti-ABP1 antibodies could selectively repress plasma membrane depolarization in response to added auxin in tobacco protoplasts (Barbier-Brygoo et al. 1989). There followed many reports using antibodies, purified ABP1 protein, and peptides based on parts of the ABP1 protein-associating ABP1 with the cell surface (Thiel et al. 1993; Napier et al. 2002; Scherer 2011). In addition to linking ABP1 with auxin perception on the plasma membrane, all these responses were rapid with response times of seconds or minutes, linked to ion flux across the membrane and, crucially, faster than gene-activation responses engaged by the TIR-Aux/IAA coreceptor complex (Badescu and Napier 2006). All these reports have been brought into question by subsequent findings.
ABP1 and Ion Transfer across the Plasma Membrane
One of the key drivers for seeking a TIR1-independent site for auxin perception has been the need to explain rapid responses. Many of these were recorded in ex vivo systems, but two very rapid responses to auxin have been reported in intact Arabidopsis tissues, the inhibition of primary root growth (Fendrych et al. 2018), and plasma membrane depolarization in root hairs (Dindas et al. 2018). In both cases, the response was maximal within ∼30 sec, and in both cases the use of mutants showed that the auxin effect depended on both the auxin uptake carrier AUX1 and the receptor TIR1, although later work showed that auxin F-Box1 (AFB1) from the TIR1 family dominated in these rapid responses (Prigge et al. 2020). How AFB1 transduces the auxin signal remains to be determined but the work shows that ABP1 is not necessary for rapid, plasma-membrane-associated auxin signaling. Indeed, the abp1-null lines (Gao et al. 2015) have been used to show that membrane depolarization and inhibition of endocytosis are independent of ABP1 (Paponov et al. 2019).
How then may we explain the many publications implicating ABP1 in auxin perception at the plasma membrane? It seems likely that the responses were associated with off-site activity of antibodies and peptides, perhaps because of features shared between ABP1 and other cupins. The conserved box sequences of ABP1 (Fig. 1) are accessible on the surface of what is a cupin fold (Fig. 1), making it difficult to discount antibody cross-reactivity. Such speculation is easy and not easy to prove, but these observations might also spur on some to explore the wider roles of cupins in cell surface signaling.
ABP1 as an Apoplastic Protein
All reports associating ABP1 with a receptor-like function rely on it being active at the outer face of the plasma membrane. Other cupins are certainly apoplastic (Dunwell et al. 2008), including Gossipium ABP19 (e.g., Pei et al. 2019) and Arabidopsis germin-like protein 4 (GLP4) (Yin et al. 2009). Peach ABP19 and AtGLP4 have also been shown to bind auxin (Ohmiya et al. 1998; Yin et al. 2009), albeit at pH 7, and so the claims for auxin binding by extracellular proteins are not exclusive. However, ABP1 (and not these other proteins) displays an ER retention sequence, KDEL at its carboxyl-terminus, and all studies of its subcellular localization place the majority of ABP1 in the ER (Tian et al. 1995; Napier et al. 2002; Xu et al. 2014).
Studies that have not relied on antibodies (which could cross-react with other apoplastic cupins, as noted above) to establish the subcellular localization of ABP1 are few. Quantification of glycans characteristic of post-Golgi compartments estimated that <15% of ABP1 ever reaches the Golgi apparatus and <2% passes further down the secretory pathway in maize (Henderson et al. 1997). Immunoelectron microscopy of Arabidopsis root cells measured 22% at the plasma membrane (Xu et al. 2014). Evidence of antibody specificity was given using western blotting but given sequence and structural similarities to other cupins, it is challenging to confirm that this holds for non-denatured proteins in microscopy, and rigorous evidence for a physical presence of ABP1 at the cell surface is lacking.
Auxin Binding and pH
One argument that has often been used to vindicate APB1's role as a receptor for auxin at the cell surface is its pH optimum for binding auxin. Many studies showed this to be between pH 5 and 5.5 (Napier 1995), which is within the pH range of the apoplast, whereas the pH in the ER is considered to be similar to that of the cytoplasm, between pH 7 and 7.5. Hence, ABP1 could work well as an ABP in the apoplast, not in the ER, and a set of careful studies confirmed that ABP1 does not bind auxin in the ER (Tian et al. 1995). Yet, most ABP1 is in the ER. Thus, one of our certainties, that ABP1 binds auxin, needs qualification: ABP1 binds auxin in vitro. In living cells, most of it does not. Of course, if any does reach the apoplast, binding conditions are favorable.
Auxin-Binding Selectivity
A further question that has often arisen over the activity of ABP1 as a receptor candidate is over its higher affinity for binding the synthetic auxin 1-NAA than for IAA. Where comparative measurements have been made, the affinity is poorer for IAA than for 1-NAA by one or two orders of magnitude (Shimomura et al. 1986; Hesse et al. 1989). This preference was also seen with unpurified ABP1 in Zea microsomes (Ray et al. 1977) and in an antibody-based assay for a binding-induced conformational change in purified ABP1 (Napier and Venis 1990). Molecular dynamic simulations of ABP1 have consistently found 1-NAA to bind with higher affinity than IAA, and better able to stabilize both Arabidopsis and maize ABP1s (Grandits and Oostenbrink 2014; Tesser da Costa et al. 2017). Yet in vivo, IAA is among the most active of auxins and this lack of correspondence of binding affinity with physiological activity formed part of the red herring argument that questioned the validity of the hypothesis that ABP1 is a receptor (Hertel 1995). Synthetic ligands often outperform natural ligands. This lack of correspondence does not undermine arguments for or against the functionality of ABP1.
The primary location of ABP1 in the ER corresponds to the growing awareness of the ER as an important contributor to auxin homeostasis (Skalický et al. 2018). It is a compartment where IAA conjugates accumulate and where there are auxin amidohydrolases to release IAA from such conjugates, for AUX1 (as well as the plasma membrane) (Dharmasiri et al. 2006) and PIN5 and PIN8 (Mravec et al. 2009; Ding et al. 2012). However, there is no evidence of ABP1 having a function related to auxin signaling in the ER.
A Binding Partner for ABP1 at the Plasma Membrane, the Docking Protein
As soon as there was evidence for ABP1 being a receptor candidate at the cell surface, it was recognized that this soluble glycoprotein would need help to transduce a signal back across the plasma membrane and a docking protein was posited (Barbier-Brygoo et al. 1991). This docking protein would act as an anchor in the membrane and help transfer the auxin signal into the cell. A glycosylphosphatidylinositol (GPI)-anchored protein known as carboxy-terminal peptide-binding protein 1 (CBP1) was shown to interact with the carboxyl-terminus of ABP1 (Shimomura 2006), but no further work has linked ABP1 with CBP1 or the related SKU5-like GPI-anchored proteins (Zhou 2019). Likewise, yeast two-hybrid work identified an Arabidopsis ortholog of human RING membrane-anchor E3 Ub ligase (AtRMA2) as an interactor for ABP1 (Son et al. 2010), but there has been no subsequent confirmation.
ABP1 Overexpression, Auxin-Mediated Control of Endocytosis, and TMKs
Intracellular cycling of the Rho Of Plants (ROPs) small GTPases has been linked to auxin control of cytoskeletal rearrangements and cell shape, all under the control of extracellular ABP1 (Xu et al. 2010). As noted above, some of these findings are uncertain because of the use of the abp1-5 mutant line, but the results were supported by antisense experiments, which gave phenotypes consistent with those of the mutant. A development in the story came with the discovery that a transmembrane receptor-like kinase (TMK1) formed a complex with ABP1 at the plasma membrane and that this was the auxin-sensing unit for activating the intracellular ROPs (Xu et al. 2014). The association was demonstrated by a series of coimmunoprecipitation experiments, notably with the extracellular domain of TMK1, which showed an auxin-driven dose-dependent interaction with ABP1 (for review, see Feng and Kim 2015).
The story of the ABP1/TMK complex became uncertain when the abp1 null lines showed no measurable changes in plant phenotype (Gao et al. 2015). More recent work links auxin activation of TMK1 (and TMK4) with downstream intracellular kinase cascades without the mention of what protein binds and perceives the IAA (Huang et al. 2019). In separate work on auxin-induced rapid cytoskeletal rearrangements, it was shown that the response required the uptake carrier AUX1, hence, intracellular auxin (Arieti and Staiger 2020), reducing the need for ABP1 as an auxin sensor in the apoplast. Further, calcium influx has been linked to rapid auxin-induced plasma membrane depolarization (Dindas et al. 2018), and calcium influx plus auxin-induced calcium-binding interactors of ROPs have been shown to be involved in root growth (Hazak et al. 2019). The need for an extracellular auxin sensor is diminishing, making it less certain that ABP1 is such a protein.
SYNTHESIS
The discussion above has served to highlight that there remain more uncertainties about ABP1 than certainties when considering auxin perception. There is little doubt that it lives up to its name in vitro, but when this is put into the context of its primary cellular destination (another certainty), the ER, the certainty is dissipated. The greatest challenge remaining is to assign a role for ABP1 in the ER—where it does not bind auxin.
ABP1 is certainly a cupin, and this has also brought uncertainty to our analysis of many of the reports on ABP1 because it is not possible to be certain the tools used were sufficiently specific. The cupin fold and key sequence motifs, most of which are accessible on the surface of the protein (Fig. 1), are shared, and many cupins are apoplastic. On these grounds, it is reasonable to question the interpretation of past research undertaken with antibodies and peptides, although this is not meant to question the validity of the work at the time. This leads us to question many of the reports linking ABP1 at the cell surface with signaling. Such uncertainty has been compounded by research suggesting that very rapid responses in intact tissues are dependent on the uptake carrier AUX1 and the intracellular receptors TIR1 and AFB1, albeit in a nontranscriptional mode of action. Hence, there is no longer a receptor missing for rapid auxin-mediated events at the plasma membrane and, hence, no longer a need for an extracellular auxin sensor.
An intriguing part of the story is the consistency of some phenotypes reported from use of techniques, such as antisense and overexpression, and the relationship of many such phenotypes to elements of auxin physiology (Jones et al. 1998; Bauly et al. 2000; Chen et al. 2001a; Čovanová et al. 2013). Another is the fact that the ABP1 gene family is ubiquitous in higher plants. There is much to be learned about cupins and their functions in subcellular compartments.
CONCLUDING REMARKS
We have learned a lot about ABP1 and understand very little. Its expression patterns are starting to tell a story, perhaps about sugars and secretion, but the plot remains obscure. We have probably now learned enough to concur with the statement that, “Auxin-binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development” (Gao et al. 2015). Consequently, we should separate ABP1 from auxin physiology. Auxin signaling probably no longer needs an extracellular receptor, nor a second class of receptor for rapid responses. However, until we understand what function ABP1 does play in plant biology, its storybook remains open.
ACKNOWLEDGMENTS
The author thanks members of the ABP1 community for enthusiasm and support over many years.
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Arieti RS, Staiger CJ. 2020. Auxin-induced actin cytoskeleton rearrangements require AUX1. New Phyt 226: 441–459. 10.1111/nph.16382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badescu GO, Napier RM. 2006. Receptors for auxin: will it all end in TIRs? Trends Plant Sci 11: 217–223. 10.1016/j.tplants.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Ghislain M, Guern J. 1989. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci 86: 891–895. 10.1073/pnas.86.3.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Maurel C, Palme K, Schell J, Guern J. 1991. Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J 1: 83–93. 10.1111/j.1365-313X.1991.00083.x [DOI] [PubMed] [Google Scholar]
- Bauly JM, Sealy IM, Macdonald H, Brearley J, Dröge S, Hillmer S, Robinson DG, Venis MA, Blatt MR, Lazarus CM, et al. 2000. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol 12: 1299–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoša B, Kojić-Prodić B, Wade RC, Tomić S. 2008. Mechanism of auxin interaction with auxin binding protein (ABP1): a molecular dynamics simulation study. Biophys J 94: 27–37. 10.1529/biophysj.107.109025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Shimomura S, Sitbon F, Sandberg G, Jones AM. 2001a. The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J 28: 607–617. 10.1046/j.1365-313x.2001.01152.x [DOI] [PubMed] [Google Scholar]
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. 2001b. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15: 902–911. 10.1101/gad.866201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benkova E, Perrot-Rechenmann C, Friml J. 2014. Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93. 10.1038/nature13889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čovanová M, Sauer M, Rychtář J, Friml J, Petrášek J, Zažímalová E. 2013. Overexpression of the auxin binding protein1 modulates PIN-dependent auxin transport in tobacco cells. PLoS ONE 8: e70050. 10.1371/journal.pone.0070050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhang Y, Zhang D, Chen J, Gao X, Estelle M, Zhao Y. 2015. Embryonic lethality of Arabidopsis abp1-1 is caused by deletion of the adjacent BSM gene. Nat Plants 1: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S, Swarup R, Mockaitis K, Dharmasiri N, Singh SK, Kowalchyk M, Marchant A, Mills S, Sandberg G, Bennett MJ, et al. 2006. AXR4 is required for localization of the auxin influx facilitator AUX1. Science 312: 1218–1220. 10.1126/science.1122847 [DOI] [PubMed] [Google Scholar]
- Dindas J, Scherzer S, Roelfsema MRG, von Meyer K, Müller HM, Al-Rasheid KAS, Palme K, Dietrich P, Becker D, Bennett MJ, et al. 2018. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat Commun 9: 1174. 10.1038/s41467-018-03582-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Wang B, Moreno I, Dupláková N, Simon S, Carraro N, Reemmer J, Pěnčík A, Chen X, Tejos R, et al. 2012. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun 3: 941. 10.1038/ncomms1941 [DOI] [PubMed] [Google Scholar]
- Downs GS, Bi YM, Colasanti J, Wu W, Chen X, Zhu T, Rothstein SJ, Lukens LN. 2013. A developmental transcriptional network for maize defines coexpression modules. Plant Physiol 161: 1830–1843. 10.1104/pp.112.213231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwell JM, Gibbings JG, Mahmood T, Naqvi SMS. 2008. Germin and germin-like proteins: evolution, structure, and function. Crit Rev Plant Sci 27: 342–375. 10.1080/07352680802333938 [DOI] [Google Scholar]
- Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC. 2015. Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes. Plant Cell 27: 1820–1826. 10.1105/tpc.15.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Akhmanova M, Merrin J, Glanc M, Hagihara S, Takahashi K, Uchida N, Torii KU, Friml J. 2018. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat Plants 4: 453–459. 10.1038/s41477-018-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Kim JY. 2015. Revisiting apoplastic auxin signaling mediated by AUXIN BINDING PROTEIN 1. Mol Cells 38: 829–835. 10.14348/molcells.2015.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y. 2015. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Nat Acad Sci 112: 2275–2280. 10.1073/pnas.1500365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M-F, Leonard GA, Field RA, Berlind C, Naismith JH. 2000. RmlC, the third enzyme of dTDP-L-rhamnose pathway, is a new class of epimerase. Nat Struct Biol 7: 398–402. 10.1038/75178 [DOI] [PubMed] [Google Scholar]
- Grandits M, Oostenbrink C. 2014. Molecular dynamics simulations of the auxin-binding protein 1 in complex with indole-3-acetic acid and naphthalen-1-acetic acid. Proteins 82: 2744–2755. 10.1002/prot.24639 [DOI] [PubMed] [Google Scholar]
- Grones P, Friml J. 2015. Auxin transporters and binding proteins at a glance. J Cell Sci 128: 1–7. 10.1242/jcs.159418 [DOI] [PubMed] [Google Scholar]
- Grones P, Chen X, Simon S, Kaufmann WA, De Rycke R, Nodzyński T, Zažímalová E, Friml J. 2015. Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J Exp Bot 66: 5055–5065. 10.1093/jxb/erv177 [DOI] [PubMed] [Google Scholar]
- Habets MEJ, Offringa R. 2015. Auxin binding protein 1: a red herring after all? Mol Plant 8: 1131–1134. 10.1016/j.molp.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Hazak O, Mamon E, Lavy M, Sternberg H, Behera S, Schmitz-Thom I, Bloch D, Dementiev O, Gutman I, Danziger T, et al. 2019. A novel Ca2+-binding protein that can rapidly transduce auxin responses during root growth. PLoS Biol 17: e3000085. 10.1371/journal.pbio.3000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Bauly JM, Ashford DA, Oliver SC, Hawes CR, Lazarus CM, Venis MA, Napier RM. 1997. Retention of maize auxin-binding protein in the endoplasmic reticulum: quantifying escape and the role of auxin. Planta 202: 313–323. 10.1007/s004250050133 [DOI] [PubMed] [Google Scholar]
- Hertel R. 1995. Auxin binding protein 1 is a red herring. J Exp Bot 46: 461–462. 10.1093/jxb/46.5.461-a [DOI] [Google Scholar]
- Hertel R, Thomson KS, Russo VE. 1972. In-vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta 107: 325–340. 10.1007/BF00386394 [DOI] [PubMed] [Google Scholar]
- Hesse T, Feldwisch J, Balshusemann D, Bauw G, Puype M, Vandekerckhove J, Lobler M, Klambt D, Schell J, Palme K. 1989. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J 8: 2453–2461. 10.1002/j.1460-2075.1989.tb08380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HW, Zhou YT, Mwange KN, Li WF, He XQ, Cui KM. 2006. ABP1 expression regulated by IAA and ABA is associated with the cambium periodicity in Eucommia ulmoides Oliv. J Exp Bot 57: 3857–3867. 10.1093/jxb/erl150 [DOI] [PubMed] [Google Scholar]
- Huang R, Zheng R, He J, Zhou Z, Wang J, Xiong Y, Xu T. 2019. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc Natl Acad Sci 116: 21285–21290. 10.1073/pnas.1910916116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Shimomura S, Fukui T, Futai M. 1989. Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure. Proc Natl Acad Sci 86: 3564–3568. 10.1073/pnas.86.10.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN. 1998. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114–1117. 10.1126/science.282.5391.1114 [DOI] [PubMed] [Google Scholar]
- Klepikova AV, Kasianov AS, Gerasimov ES, Logacheva MD, Penin AA. 2016. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J 88: 1058–1070. 10.1111/tpj.13312 [DOI] [PubMed] [Google Scholar]
- Klode M, Dahlke RI, Sauter M, Steffens B. 2011. Expression and subcellular localization of Arabidopsis thaliana auxin-binding protein 1 (ABP1). J Plant Growth Reg 30: 416–424. 10.1007/s00344-011-9203-2 [DOI] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiol 176: 465–479. 10.1104/pp.17.00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M, Klämbt D. 1985. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I: Purification by immunological methods and characterization . J Biol Chem 260: 9848–9853. 10.1016/S0021-9258(17)39314-6 [DOI] [PubMed] [Google Scholar]
- Michalko J, Dravecká M, Bollenbach T, Friml J. 2015. Embryo-lethal phenotypes in early abp1 mutants are due to disruption of the neighboring BSM gene. F1000 Res 4: 1104. 10.12688/f1000research.7143.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalko J, Glanc M, Perrot-Rechenmann C, Friml J. 2016. Strong morphological defects in conditional Arabidopsis abp1 knock-down mutants generated in absence of functional ABP1 protein. F1000Res 5: 86. 10.12688/f1000research.7654.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Krecek P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD, et al. 2009. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459: 1136–1140. 10.1038/nature08066 [DOI] [PubMed] [Google Scholar]
- Napier RM. 1995. Towards an understanding of ABP1. J Exp Bot 46: 1787–1795. 10.1093/jxb/46.12.1787 [DOI] [Google Scholar]
- Napier RM, Venis MA. 1990. Monoclonal antibodies detect an auxin-induced conformational change in the maize auxin-binding protein. Planta 182: 313–318. 10.1007/BF00197128 [DOI] [PubMed] [Google Scholar]
- Napier RM, David KM, Perrot-Rechenmann C. 2002. A short history of auxin-binding proteins. Plant Mol Biol 49: 339–348. 10.1023/A:1015259130955 [DOI] [PubMed] [Google Scholar]
- Ohmiya A, Tanaka Y, Kadowaki KI, Hayashi T. 1998. Cloning of genes encoding auxin-binding proteins (ABP19/20) from peach: significant peptide sequence similarity with germin-like proteins. Plant Cell Physiol 39: 492–499. 10.1093/oxfordjournals.pcp.a029396 [DOI] [PubMed] [Google Scholar]
- Oliver SC, Venis MA, Freedman RB, Napier RM. 1995. Regulation of synthesis and turnover of maize auxin-binding protein and observations on its passage to the plasma membrane: comparisons to maize immunoglobulin-binding protein cognate. Planta 197: 465–474. 10.1007/BF00196668 [DOI] [PubMed] [Google Scholar]
- Paponov IA, Dindas J, Król E, Friz T, Budnyk V, Teale W, Paponov M, Hedrich R, Palme K. 2019. Auxin-induced plasma membrane depolarization is regulated by auxin transport and not by auxin binding protein1. Front Plant Sci 9: 1953. 10.3389/fpls.2018.01953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Li X, Zhu Y, Ge X, Sun Y, Liu N, Jia Y, Li F, Hou Y. 2019. GhABP19, a novel germin-like protein from Gossypium hirsutum plays an important role in the regulation of resistance to verticillium and fusarium wilt pathogens. Front Plant Sci 10: 583. 10.3389/fpls.2019.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Platre M, Kadakia N, Zhang Y, Greenham K, Szutu W, Kumar Pandey B, Bhosale RA, Bennett MJ, Busch W, et al. 2020. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife 9: e54740. 10.7554/eLife.54740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM. 1977. Auxin-binding sites of maize coleoptiles are localized on membranes of the endoplasmic reticulum. Plant Physiol 59: 594–599. 10.1104/pp.59.4.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM, Dohrmann U, Hertel R. 1977. Specificity of auxin-binding sites on maize coleoptile membranes as possible receptor sites for auxin action. Plant Physiol 60: 585–591. 10.1104/pp.60.4.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Čovanová M, et al. 2010. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121. 10.1016/j.cell.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Kleine-Vehn J. 2011. Auxin binding protein1: the outsider. Plant Cell 23: 2033–2043. 10.1105/tpc.111.087064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GFE. 2011. AUXIN-BINDING-PROTEIN1, the second auxin receptor: what is the significance of a two-receptor concept in plant signal transduction? J Exp Bot 62: 3339–3357. 10.1093/jxb/err033 [DOI] [PubMed] [Google Scholar]
- Shimomura S. 2006. Identification of a glycosylphosphatidylinositol-anchored plasma membrane protein interacting with the C-terminus of auxin-binding protein 1: a photoaffinity crosslinking study. Plant Mol Biol 60: 663–677. 10.1007/s11103-005-5471-1 [DOI] [PubMed] [Google Scholar]
- Shimomura S, Sotobayashi T, Futai M, Fukui T. 1986. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem 99: 1513–1524. 10.1093/oxfordjournals.jbchem.a135621 [DOI] [PubMed] [Google Scholar]
- Shimomura S, Watanabe S, Ichikawa H. 1999. Characterization of auxin-binding protein 1 from tobacco: content, localization and auxin-binding activity. Planta 209: 118–125. 10.1007/s004250050613 [DOI] [PubMed] [Google Scholar]
- Skalický V, Kubeš M, Napier R, Novák O. 2018. Auxins and cytokinins—the role of subcellular organization on homeostasis. Int J Mol Sci 19: 3115. 10.3390/ijms19103115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son O, Cho SK, Kim SJ, Kim WT. 2010. In vitro and in vivo interaction of AtRma2 E3 ubiquitin ligase and auxin binding protein 1. Biochem Biophys Res Commun 393: 492–497. 10.1016/j.bbrc.2010.02.032 [DOI] [PubMed] [Google Scholar]
- Tesser da Costa C, Pedebos C, Verli H, Fett-Neto AG. 2017. The role of Zn2+, dimerization and N-glycosylation in the interaction of auxin-binding protein 1 (ABP1) with different auxins. Glycobiology 27: 1109–1119. 10.1093/glycob/cwx080 [DOI] [PubMed] [Google Scholar]
- Thiel G, Blatt MR, Fricker MD, White IR, Millner P. 1993. Modulation of K+ channels in Vicia stomatal guard cells by peptide homologs to the auxin-binding protein C terminus. Proc Nat Acad Sci 90: 11493–11497. 10.1073/pnas.90.24.11493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Klämbt D, Jones AM. 1995. Auxin-binding protein 1 does not bind auxin within the endoplasmic reticulum despite this being the predominant subcellular location for this hormone receptor. J Biol Chem 270: 26962–26969. 10.1074/jbc.270.45.26962 [DOI] [PubMed] [Google Scholar]
- Tromas A, Paponov I, Perrot-Rechenmann C. 2010. Auxin binding protein 1: functional and evolutionary aspects. Trends Plant Sci 15: 436–446. 10.1016/j.tplants.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Waese J, Fan J, Pasha A, Yu H, Fucile G, Shi R, Cumming M, Kelley LA, Sternberg MJ, Krishnakumar V, et al. 2017. ePlant: visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell 29: 1806–1821. 10.1105/tpc.17.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. 2007. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. 10.1371/journal.pone.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo EJ, Marshall J, Bauly J, Chen JG, Venis M, Napier RM, Pickersgill RW. 2002. Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J 21: 2877–2885. 10.1093/emboj/cdf291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z. 2010. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110. 10.1016/j.cell.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, et al. 2014. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028. 10.1126/science.1245125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Han X, Xu Z, Xue H. 2009. Arabidopsis GLP4 is localized to the Golgi and binds auxin in vitro. Acta Biochim Biophys Sin 41: 478–487. 10.1093/abbs/gmp036 [DOI] [PubMed] [Google Scholar]
- Zhou K. 2019. GPI-anchored SKS proteins regulate root development through controlling cell polar expansion and cell wall synthesis. Biochem Biophys Res Comm 509: 119–124. 10.1016/j.bbrc.2018.12.081 [DOI] [PubMed] [Google Scholar]