Abstract
Objective:
To determine the utility of the HAS-BLED and CHA2DS2-VASc scores among patients on anticoagulation (AC) for atrial fibrillation (AF) who have evidence of cerebral amyloid angiopathy (CAA).
Patients/Methods:
Patients >55 years with a diagnosis of AF who had a non-traumatic ICH while on AC between 1995–2016 were identified using the Rochester Epidemiology Project Database. Medical records were reviewed, including brain imaging, to identify baseline characteristics, AC use, and outcomes.
Results:
65 patients were identified (mean age 81.3); 35 (53.8%) had evidence of possible/probable CAA. Mean HAS-BLED scores in the CAA group were significantly lower (2.1) than that of the non-CAA group (2.9) (p<0.001). Mortality following ICH, adjusted for HAS-BLED scores, was not significantly different among patients with CAA and those without. 16 patients restarted on AC following ICH, CHA2DS2-VASc scores were no different between this group and those who weren’t restarted. Among CAA patients, the overall rate of ICH recurrence was 8.6% over 93.5 person-years of follow up. Among CAA patients, the rate of ICH recurrence was 3.2/100 patient years, higher than their HAS-BLED scores would predict (1.9 bleeds/100 patient years).
Conclusions:
HAS-BLED scores were lower in patients who had evidence of CAA compared to those without, suggesting underestimation of ICH risk in patients with CAA. CHA2DS2-VASc scores did not impact resumption of AC. ICH recurrence was higher in patients with CAA than their HAS-BLED scores predicted. Future scoring systems that account for the presence of CAA may improve the prediction of ICH for AF patients on AC.
Introduction:
Atrial fibrillation (AF) is a common arrhythmia which predisposes patients to stroke1–3. Oral anticoagulants are used to reduce the risk of ischemic stroke, but increase the risk of intracerebral hemorrhage (ICH)4, 5. Clinicians are often presented with the challenge of balancing cardioembolic stroke prevention with the risk of ICH. Scoring schemata have been developed to help guide these decisions in clinical practice. Two in particular are heavily relied upon in the United States. The HAS-BLED score was developed to predict the one year risk of a major bleed in patients with AF on oral anticoagulation (AC)6, 7. The CHA2DS2-VASc score was developed to predict the one year risk of stroke in patients with AF who are not on AC8–11.
Cerebral Amyloid Angiopathy (CAA) is a common cause of lobar ICH in the elderly, particularly among those on AC12. ICH on AC has a high mortality rate13, 14. CAA related ICH has a higher risk of recurrence compared to hypertensive related ICH15. Due to the higher risk of recurrence, some studies have recommended discontinuing AC after lobar ICH16, leading to complicated decision making scenarios17, 18. Concern about the risk of ICH may lead to underutilization of AC in a subset of patients19, 20. There is wide variety in the practice of resuming AC in patients with AF after an ICH among practitioners21, and a lack of well supported recommendations in current guidelines. Clinicians often rely on the HAS-BLED and CHA2DS2-VASc scores to make individualized decisions based on presumed risk of repeat ICH and ischemic stroke.
It is currently unknown how a diagnosis of CAA may impact the risks associated with oral AC therapy in patients with AF. The objectives of our population-based study were to 1: describe the HAS-BLED and CHA2DS2-VASc scores in relation to ICH occurrence and underlying CAA findings and 2: to determine the outcomes (mortality and ICH recurrence) among patients with AF on oral AC who suffered a non-traumatic ICH, comparing those with possible/probable CAA versus those without CAA. Our hypothesis was that the scores would have a worse predictive performance in patients with CAA.
Methods:
A retrospective review of data from all patients who presented to medical facilities within Olmsted County, MN between January 1, 1995 and December 31, 2016 was conducted. The study was approved by the Mayo Clinic Rochester and Olmsted Medical Center IRB’s; Mayo Clinic IRB #: 17–07542 & Olmsted Medical Center IRB #: 056-OMC-17. Given the nature of the study, informed consent was waived.
Data was extracted from the Rochester Epidemiology Project (REP)22. The REP database contains medical records for all patients within Olmsted County and codifies their diagnoses using ICD codes and a unique internal research code23–25. A reliable record of prescribed medications for each patient is also maintained since 2003.
All patients older than 55 presenting between 1995 and 2016 with diagnoses of non-traumatic ICH, AF and concomitant AC use were identified. Patients with hemorrhage secondary to trauma, hemorrhagic conversion of an ischemic stroke, and those with a known intracranial vascular lesion or tumor were excluded. Traumatic vs non-traumatic etiology of the hemorrhage was evaluated through chart and imaging review. Further, patients who were not on AC at the time of their ICH were excluded.
Medical records were reviewed to gather the etiology of ICH, baseline characteristics, data on restarting of AC, and mortality and ICH recurrence outcomes. Computerized Tomography (CT) and magnetic resonance imaging (when available) were reviewed by a neurologist (S.E.) using the Modified Boston Criteria to determine characteristics consistent with possible or probable CAA26, 27. Patients with CT evidence of lobar hemorrhage without MRI were included as possible CAA if no other cause could be identified. Similarly, patients who underwent MRI without hemosiderin-sensitive sequences, patients with isolated cerebellar ICH, or patients with convexal subarachnoid hemorrhage were included as possible CAA if no other etiologies were identified. Hemosiderin sensitive sequences include gradient recalled-echo and susceptibility weighted imaging sequences.
Baseline characteristics were used to calculate HAS-BLED and CHA2DS2-VASc Scores. HAS-BLED and CHA2DS2-VASc Scores were then used to estimate the predicted risk of major hemorrhage and ischemic stroke following the incident ICH, based on their respected validation cohorts. Predicted risk, based on these scores, was compared to actual outcomes in our cohort.
Outcomes and endpoints included recurrent ICH, ischemic stroke, and death. These were adjudicated by chart and imaging review. Recurrent ICH was defined as a new ICH outside of the expected evolution of the initial ICH based on clinical context and imaging. Ischemic stroke was recorded when chart review was consistent with a new neurological deficit in the setting of imaging evidence of new infarction. Death included all-cause mortality.
Statistics:
Continuous variables are presented as mean ± standard deviation and categorical variables as frequency (percentage). Comparisons between dichotomous subgroups were carried out using the Pearson χ2 test for categorical variables and the Student t-test for continuous variables. 7 and 30 day mortality was analyzed using logistic regression. Cox proportional hazard models were used to model long term outcomes. The Kaplan-Meier method was used to evaluate survival. Probability (p) values of <0.05 were considered statistically significant. Statistical analyses were completed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results:
6,045 patients over the age of 55 with AF on AC were identified. Among those patients, 262 (4.3%) were identified with any type of intra-cranial hemorrhage. 65 patients had a non-traumatic ICH while on AC. 61 patients were taking warfarin, 3 were taking direct oral anticoagulants, and 1 was on heparin. Among those 65 cases, 44 had MR imaging available while 21 only had CT imaging available for review. Of the 44 patients with MR imaging, 34 had hemosiderin-sensitive sequences to detect microbleeds and superficial siderosis.
The mean age was 81.3 years; 36 were female (55%). 35 (54%) were identified as having evidence of possible or probable CAA based on Revised Boston Criteria. [Table 1]. Mortality following ICH was 26.2% at 7 days, 36.9% at 30 days, 40.0% at 90 days, 41.5% at 182 days, 44.6% at 1 year, 47.9% at 2 years, and 63.1% at 5 years [Figure 1] [Figure 2]. Baseline HAS-BLED scores ranged from 1 to 5 (Score=1, 19%; 2, 35%; 3, 32%; 4, 12%; 5, 2%). Mean baseline HAS-BLED scores among patients with evidence of possible/probable CAA was significantly lower (2.1) than mean scores among patients without evidence of CAA (2.9) (p<0.001). Logistic regression showed that HAS-BLED scores was not a significant predictor of mortality at 7 and 30 days after ICH (p= .29 & .22 respectively). Cox proportional hazard models adjusted for possible/probable CAA showed that HAS-BLED score was not a significant predictor of mortality (p=.06).
Table 1:
Baseline Patient Characteristics
| Patient Characteristics | ||||
|---|---|---|---|---|
| Variable | Overall (N=65) | No Evidence of CAAa (N=30) | Possible/Probable CAA (N=35) | P-value |
| Age (years) | 81.3 (7.2) | 80.9 (8.4) | 81.8 (6.0) | 0.63 |
| Gender, n (%) | 0.76 | |||
| Male | 29 (45%) | 14 (47%) | 15 (43%) | |
| Female | 36 (55%) | 16 (53%) | 20 (57%) | |
| Race, n (%) | 0.18 | |||
| Caucasian | 63 (97%) | 30 (100%) | 33 (94%) | |
| Asian | 2 (3%) | 0 (0%) | 2 (6%) | |
| Obesity, n (%) | 24 (37%) | 12 (40%) | 12 (34%) | 0.63 |
| Tobacco Use, n (%) | 0.68 | |||
| No Use | 31 (48%) | 16 (53%) | 15 (43%) | |
| Past Use | 32 (49%) | 13 (43%) | 19 (54%) | |
| Current | 2 (3%) | 1 (3%) | 1 (3%) | |
| Alcohol Use, n (%) | 0.81 | |||
| No Use | 41 (63%) | 20 (67%) | 21 (60%) | |
| Past Use | 11 (17%) | 5 (17%) | 6 (17%) | |
| Current | 13 (20%) | 5 (17%) | 8 (23%) | |
| Diabetes Mellitus, n (%) | 16 (25%) | 9 (30%) | 7 (21%) | 0.45 |
| Hyperlipidemia, n (%) | 40 (61%) | 19 (64%) | 21 (60%) | 0.30 |
| Hypertension, n (%) | 53 (82%) | 23 (77%) | 30 (86%) | 0.08 |
| Coronary Artery Disease, n (%) | 22 (34%) | 8 (28%) | 14 (40%) | 0.30 |
| Chronic Kidney Disease, n (%) | 6 (9%) | 3 (10%) | 3 (9%) | 0.84 |
| Peripheral Vascular Disease, n (%) | 6 (9%) | 2 (7%) | 4 (11%) | 0.51 |
| Stroke/TIA, n (%) | 21 (32%) | 10 (33%) | 11 (31%) | 0.87 |
| Obstructive Sleep Apnea, n (%) | 11 (17%) | 5 (17%) | 6 (17%) | 0.99 |
| Rate Control, n (%) | 55 (85%) | 22 (73%) | 33 (94%) | 0.02 |
| Rhythm Control, n (%) | 5 (8%) | 5 (17%) | 0 (0%) | 0.01 |
| Prior Ablation, n (%) | 4 (6%) | 2 (7%) | 2 (6%) | 0.87 |
| HAS-BLED Score | 2.4 (1.0) | 2.9 (0.9) | 2.1 (0.9) | <.001 |
| HAS-BLED Score > 2, n (%) | 30 (46%) | 18 (60%) | 12 (34%) | 0.04 |
| CHA2DS2-VASc Score | 4.3 (1.3) | 4.2 (1.3) | 4.5 (1.4) | 0.33 |
| Hemorrhage Type, n (%) | ||||
| Clinical Hemorrhage | 65 (100%) | 30 (100%) | 35 (100%) | |
| Hemorrhage Location, n (%) | <.001 | |||
| Deep | 27 (42%) | 26 (87%) | 0 (0%) | |
| Lobar | 30 (46%) | 1 (3%) | 30 (86%) | |
| Cerebellar | 5 (8%) | 0 (0%) | 5 (14%) | |
| Primary IVH | 3 (5%) | 3 (10%) | 0 (0%) | |
CAA: Cerebral Amyloid Angiopathy
Figure 1.
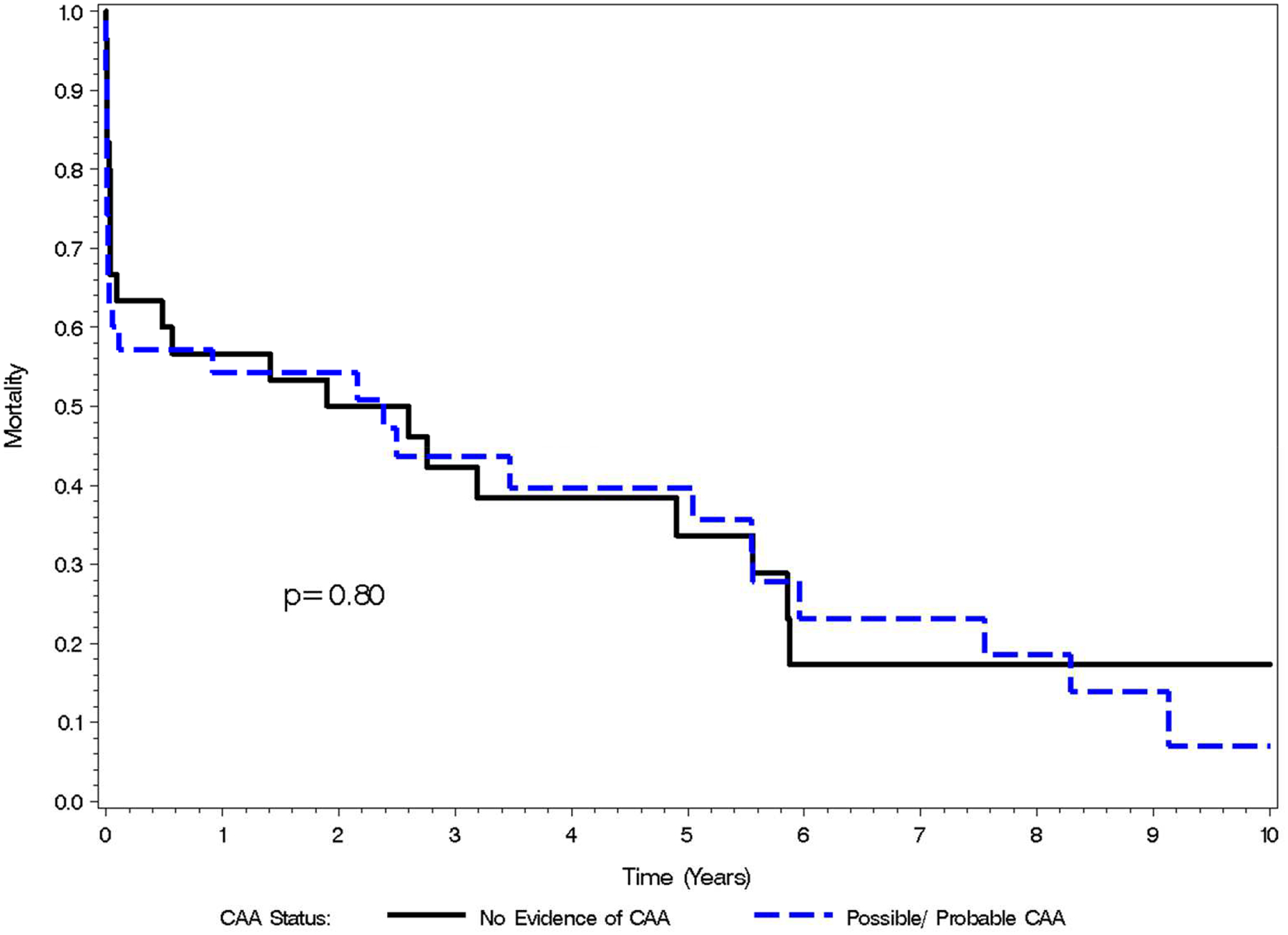
Kaplan Meier Mortality Curve
Figure 2.
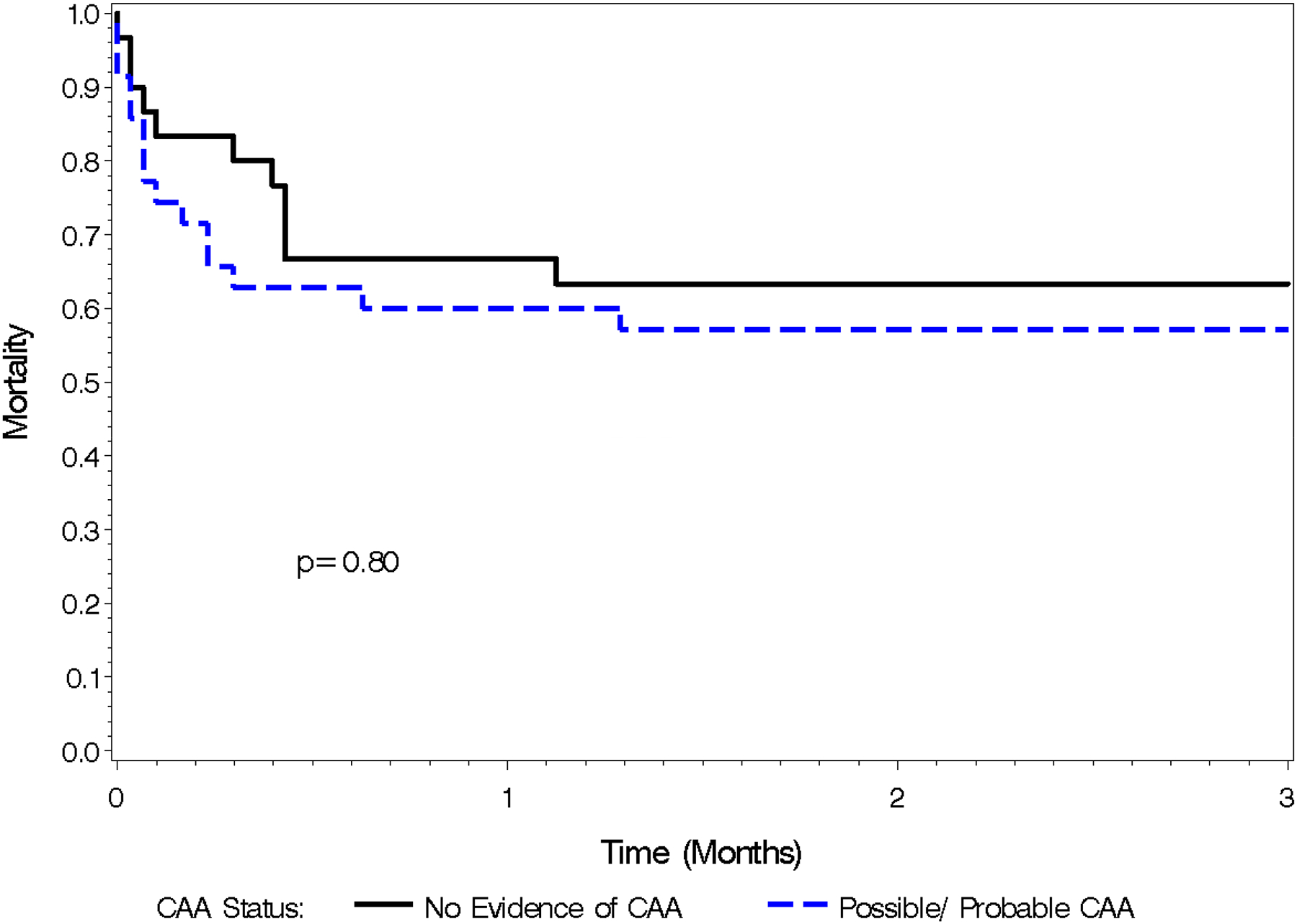
Kaplan Meier Mortality Curve, 0–3 months
Baseline CHA2DS2-VASc scores ranged from 2–8 (Score=2, 8%; 3, 15%; 4, 37%; 5, 25%; 6, 8%; 4, 6%; 8, 1%). Mean scores were not significantly different between patients with evidence of CAA (4.5) and those without (4.2) (p=.33). Logistic regression models showed that CHA2DS2-VASc score was not a significant predictor of mortality at 7 and 30 days (p=.18 & p=.18 respectively). Cox proportional hazards models adjusted for possible/probable CAA showed that CHA2DS2-VASc score was not a significant predictor of mortality (p=.09).
Mean HAS-BLED scores for patients with CAA (2.1) correlated to an estimated future hemorrhage risk of 3.6% overall and 1.9 bleeds per 100 patient years, while mean HAS-BLED scores for patients without CAA (2.9) correlated to an estimated future hemorrhage risk of 5.8% overall and 3.7 bleeds per 100 patient years6, 7. A CHA2DS2-VASc score of 4 correlated to an estimated future annual risk of stroke of 4.8% and a score of 5 correlated to an estimated annual risk of 7.2%, among patients not on AC8–11.
Of the 65 patients, 16 were re-initiated on AC following their ICH (39% of patients who were alive 30 days after the index ICH). Baseline CHA2DS2-VASc scores were not different between patients who were re-started on AC (4.2) compared to those who were not (4.4) (p=0.06). 10 (63%) of the patients restarted on AC had possible/probable CAA. Among the 16 re-initiated on AC, 1 (6%) suffered a second ICH and 1 (6%) suffered an ischemic stroke. Among the 49 who did not restart AC, 2 (4%) suffered a second ICH (3,335 and 2,176 days after the index ICH) [Figure 3] and 1 (2%) suffered an ischemic stroke. Kaplan Meier curve for all outcomes is shown in Figure 4. All 3 of the patients who suffered a second ICH had HAS-BLED scores <3 and all 3 had imaging suggestive of possible/probable CAA. Among patients with possible/probable CAA the rate of ICH recurrence was 8.6% overall, over a mean of 2.7 years and 93.45 total person-years of follow up, with 3.2 bleeds per 100 patient years (Table 2).
Figure 3.
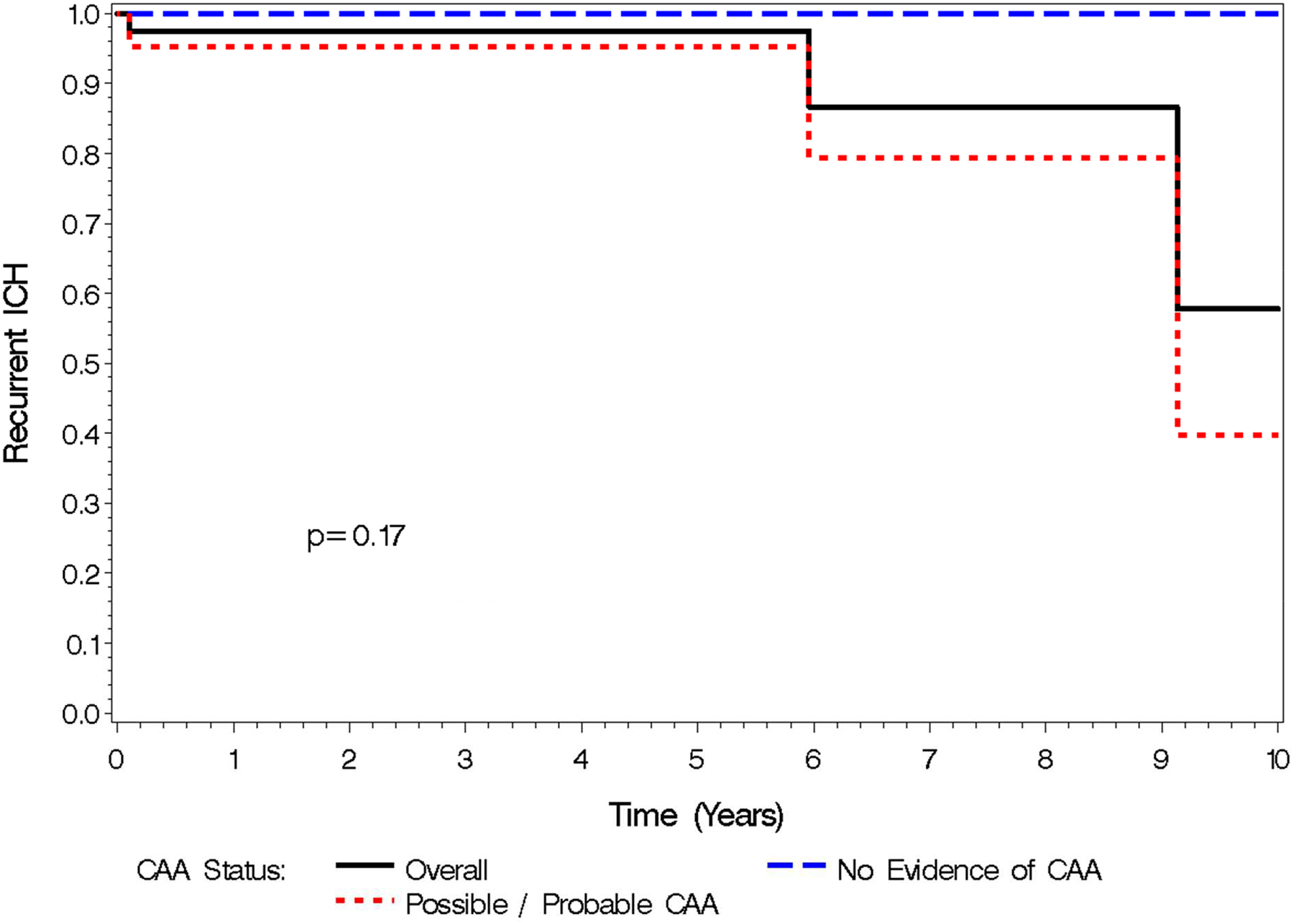
Kaplan Meier Curve: Recurrent ICH
Figure 4.
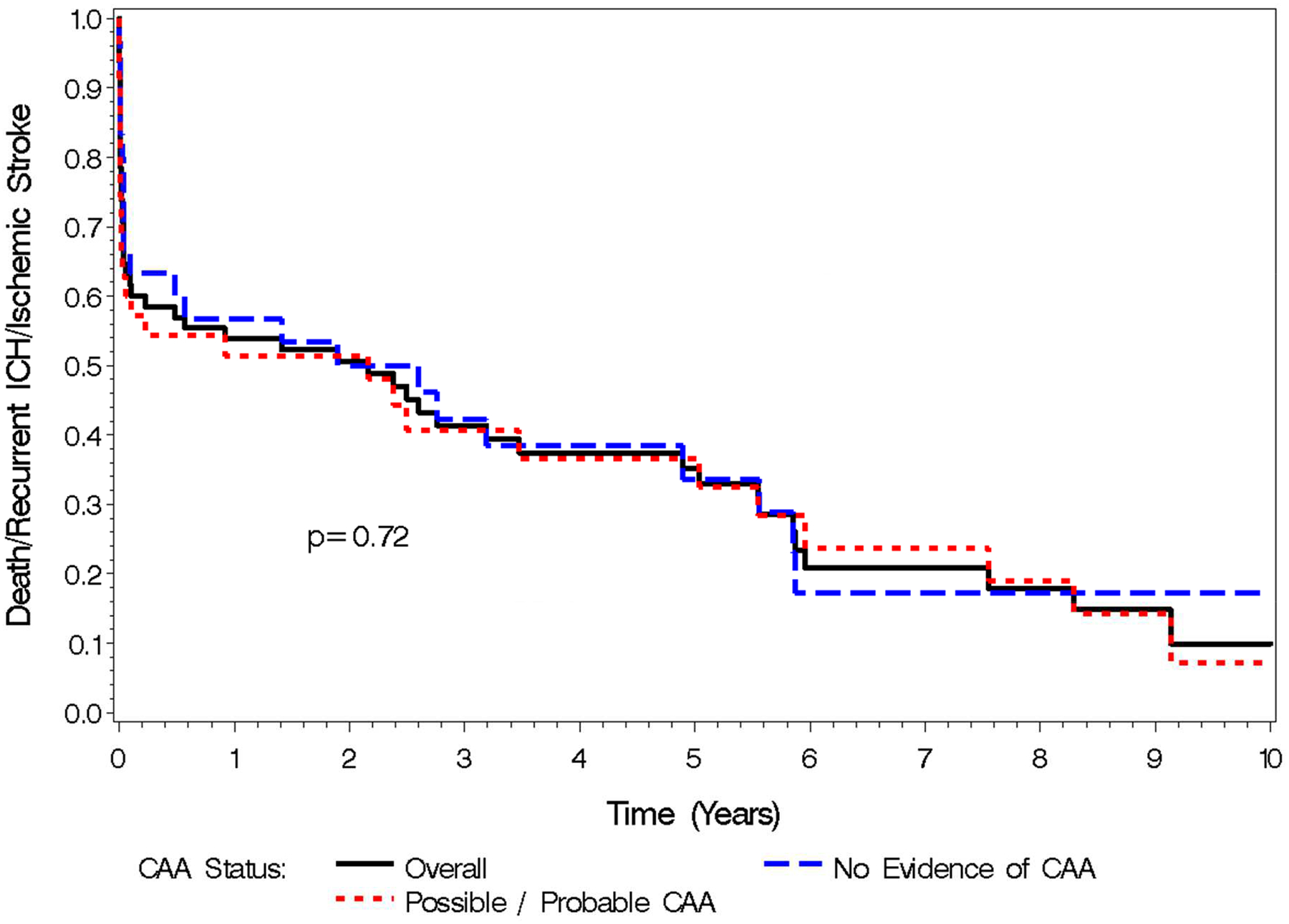
Kaplan Meier Curve: Death, Recurrent ICH, Ischemic Stroke
Table 2:
Predicted risk of Hemorrhage and Actual Recurrence of ICH by CAA Status
| Evidence of CAAa | No CAA | |
|---|---|---|
| Average HAS-BLED Score | 2.1 | 2.9 |
| Predicted risk of Hemorrhage | 1.9/100 patient years | 3.7/100 patient years |
| Actual recurrence of ICHb | 3.2/100 patient years | 0 |
CAA: Cerebral Amyloid Angiopathy
ICH: Intracerebral Hemorrhage
Discussion:
The main findings of our population based study are as follows: 1) Among patients with AF on AC who suffered ICH, baseline HAS-BLED scores were significantly lower in patients who also had evidence of CAA compared to those without. 2) Baseline HAS-BLED scores were not predictive of mortality following ICH. 3) Baseline CHA2DS2-VASc scores were not significantly different between patients who had evidence of CAA compared to those who did not, nor were they different between patients who were restarted on AC and those who were not. 4) The recurrence of ICH in patients with evidence of CAA was higher than their HAS-BLED scores would have predicted. 5) All patients who suffered recurrent ICH had evidence of CAA.
The HAS-BLED score was developed to give clinicians an estimate of the risk of a major bleed in patients with AF who were initiated on AC6, 7. CAA increases the risk of ICH12, 15 and yet it is largely unaccounted for in the HAS-BLED scoring system. This is despite series showing the prevalence of pathology proven CAA in patients with ICH as high as 25–83%28–30. The HAS-BLED score is derived from multiple factors that have little to no bearing on a diagnosis of CAA. Specific features of CAA not accounted for by the HAS-BLED score include lobar versus deep location of an ICH, cortical superficial siderosis and lobar cerebral microbleeds. Cerebral microbleeds alone have been associated with increased risk of ICH in patients with AF on AC31. Our study demonstrates that among a cohort of patients with AF on AC who suffered an ICH, those with evidence of CAA had statistically significant lower HAS-BLED scores compared to those without CAA. It is possible that in patients with CAA, the HAS-BLED score underestimates their risk of an ICH. Patients with CAA might be prone to suffer an ICH at a higher rate than what their HAS-BLED score might otherwise predict, and therefore, in the-post ICH group the clinical utility is questionable. While the majority of AF patients over 55 will not have a brain MRI evaluating for CAA before starting AC, consideration of CAA status with a brain MRI following an index ICH may prevent further morbidity associated with recurrent ICH.
The mean HAS-BLED score for patients in our study with possible or probable CAA was 2.1. That would correspond to an estimated future hemorrhage risk of 3.6% overall and 1.9 bleeds per 100 patient years6, 7. In contrast, we know that the risk of recurrent ICH after CAA related lobar hemorrhage is approximately 10% per year32, 33. Of the 16 patients in our study who were resumed on AC, 1 (6%) suffered a recurrent ICH, and 8.6% of possible/probable CAA patients overall suffered a recurrent ICH over a mean of 2.7 years of follow up. Patients with evidence of CAA in our cohort had rate of ICH recurrence of 3.2 bleeds per 100 patient years. The risk of a recurrent ICH within our subgroup was much higher than predicted by the mean HAS-BLED scores, albeit with very low overall numbers. This may further suggest the discordance between the predicted risk based on the HAS-BLED scores versus risk of a recurrent ICH related to CAA and the need for better risk predictors.
Baseline HAS-BLED scores were not predictive of mortality following ICH at neither 7 nor 30 days. The mortality related to ICH at 7 days and 30 days is known to be high8, 9, 11, and this was demonstrated again in our study with 7 and 30 day mortality at 26% and 37% respectively. While the HAS-BLED score was not intended to predict mortality, it has been validated to estimate the risk of a major hemorrhage, defined as an ICH, a fatal hemorrhage, a hemorrhage requiring a transfusion, or a >2 g/L drop in hemoglobin6, 7. Therefore, clinicians should not use HAS-BLED to estimate mortality risk when discussing with patients with AF whether or not to use AC.
In our cohort, there was no difference in baseline CHA2DS2-VASc scores between patients with possible/probable CAA versus those without. This would be expected, as CAA should not significantly impact the CHA2DS2-VASc scores. Our study showed no difference in baseline scores between patients who were restarted on AC following their ICH compared to those who were not restarted, highlighting the lack of consensus on the correct score threshold for restarting AC, especially in the setting of CAA.
Baseline CHA2DS2-VASc scores were not predictive of overall mortality following ICH in our study. The average baseline CHA2DS2-VASc score among patients who were not restarted on AC was high at 4.4. Despite this, there was no difference in recurrent ischemic stroke between patients who were restarted on AC compared to those who were not, albeit with low numbers in both categories and high mortality rate limiting length of follow up. There was only one instance of ischemic stroke in the group who were not restarted on AC, despite their average CHA2DS2-VASc score of 4.4, which would correlate to an estimated annual risk of ischemic stroke between 4.8% and 7.2%8.
Overall, our study suggests that more investigation is needed to better estimate the risks and benefits of AC in patients with CAA. The current most commonly used risk predictors do not take into account CAA status and may be inappropriate for use in patients with CAA. Given the wide prevalence AF and increasing findings of CAA3, the clinical scenario of a patient with both is going to become more common. Clinicians will need better tools to estimate the potential downside of AC in order to make informed decisions.
The main strength of our study is its population-based design and the completeness of follow-up data, which offers a unique cohort. Yet, our study does have limitations, chief among them being the relatively small numbers of events and particularly recurrent events which compromise the power of our statistical analysis. Not all patients with ICH had brain MRI for review, which may have led to underdiagnosis of CAA. The prescription data contained in the REP database is not complete prior to 2003 because of limitations in the electronic record keeping before this date, and therefore our study may have underestimated the number of patients on AC between 1995 and 2003.
Conclusion:
The main clinical message of this study is that the HAS-BLED score may be insufficient to estimate the risk of ICH in patients with AF who are >55 years. When these patients have underlying CAA, they may have a higher risk of ICH than predicted by the HAS-BLED score. Further research is needed to optimize ICH risk prediction in older patients.
Acknowledgements:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AF
Atrial Fibrillation
- AC
Anticoagulation
- CAA
Cerebral Amyloid Angiopathy
- CHA2DS2-VASc
Clinical score used to estimate the risk of an ischemic stroke
- ICD
International Statistical Classification of Diseases and Related Health Problems
- ICH
Intracerebral Hemorrhage
- INR
International Normalized Ratio
- IRB
Institutional Review Board
- HAS-BLED
Clinical score used to estimate the risk of a major hemorrhage
- MRI
Magnetic Resonance Imaging
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. [DOI] [PubMed] [Google Scholar]
- 2.Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NA 3rd. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clinic proceedings. 2016;91(12):1778–1810. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes RD, Guimaraes PO, Kolls BJ, et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood. 2017;129(22):2980–2987. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of internal medicine. 2007;146(12):857–867. [DOI] [PubMed] [Google Scholar]
- 6.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. Journal of the American College of Cardiology. 2011;57(2):173–180. [DOI] [PubMed] [Google Scholar]
- 8.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. European heart journal. 2012;33(12):1500–1510. [DOI] [PubMed] [Google Scholar]
- 9.Okumura K, Inoue H, Atarashi H, Yamashita T, Tomita H, Origasa H. Validation of CHA(2)DS(2)-VASc and HAS-BLED scores in Japanese patients with nonvalvular atrial fibrillation: an analysis of the J-RHYTHM Registry. Circulation journal : official journal of the Japanese Circulation Society. 2014;78(7):1593–1599. [DOI] [PubMed] [Google Scholar]
- 10.Dzeshka MS, Lane DA, Lip GY. Stroke and bleeding risk in atrial fibrillation: navigating the alphabet soup of risk-score acronyms (CHADS2, CHA2 DS2 -VASc, R2 CHADS2, HAS-BLED, ATRIA, and more). Clinical cardiology. 2014;37(10):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 12.Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2012;83(3):275–281. [DOI] [PubMed] [Google Scholar]
- 13.Cervera A, Amaro S, Chamorro A. Oral anticoagulant-associated intracerebral hemorrhage. Journal of neurology. 2012;259(2):212–224. [DOI] [PubMed] [Google Scholar]
- 14.Grysiewicz R, Gorelick PB. Incidence, mortality, and risk factors for oral anticoagulant-associated intracranial hemorrhage in patients with atrial fibrillation. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23(10):2479–2488. [DOI] [PubMed] [Google Scholar]
- 15.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75(8):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34(7):1710–1716. [DOI] [PubMed] [Google Scholar]
- 17.Murthy SB, Gupta A, Merkler AE, et al. Restarting Anticoagulant Therapy After Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Stroke. 2017;48(6):1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkes MA, Rabinstein AA. Anticoagulation for atrial fibrillation after intracranial hemorrhage: A systematic review. Neurology. Clinical practice 2018;8(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasishta S, Toor F, Johansen A, Hasan M. Stroke prevention in atrial fibrillation: physicians’ attitudes to anticoagulation in older people. Archives of gerontology and geriatrics. 2001;33(3):219–226. [DOI] [PubMed] [Google Scholar]
- 20.Mas Dalmau G, Sant Arderiu E, Enfedaque Montes MB, Sola I, Pequeno Saco S, Alonso Coello P. Patients’ and physicians’ perceptions and attitudes about oral anticoagulation and atrial fibrillation: a qualitative systematic review. BMC family practice. 2017;18(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Shoamanesh A, Schulman S, et al. Oral anticoagulant re-initiation following intracerebral hemorrhage in non-valvular atrial fibrillation: Global survey of the practices of neurologists, neurosurgeons and thrombosis experts. PloS one. 2018;13(1):e0191137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American journal of epidemiology. 2011;173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–539. [DOI] [PubMed] [Google Scholar]
- 27.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidoux C, Hauw JJ, Klein IF, et al. Amyloid Angiopathy in Brain Hemorrhage: A Postmortem Neuropathological-Magnetic Resonance Imaging Study. Cerebrovascular diseases (Basel, Switzerland). 2018;45(3–4):124–131. [DOI] [PubMed] [Google Scholar]
- 29.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55(7):947–951. [DOI] [PubMed] [Google Scholar]
- 30.Lin CM, Arishima H, Kikuta KI, et al. Pathological examination of cerebral amyloid angiopathy in patients who underwent removal of lobar hemorrhages. Journal of neurology. 2018;265(3):567–577. [DOI] [PubMed] [Google Scholar]
- 31.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. The Lancet. Neurology 2018;17(6):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology. 2017;89(8):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charidimou A, Boulouis G, Gurol ME, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain : a journal of neurology. 2017;140(7):1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]


