Abstract
A significant number of chronic venous ulcers (CVUs) fail to heal despite guideline-conform standards of care. Skin-derived ABCB5+ mesenchymal stem cells can dampen the sustained IL-1β‒driven inflammation present in chronic wounds. On the basis of their wound healing‒facilitating effects in a mouse CVU model and an autologous first-in-human study, ABCB5+ mesenchymal stem cells have emerged as a potential candidate for cell-based advanced therapy of nonhealing CVUs. In this interventional, multicenter, single-arm, phase I/IIa clinical trial, subjects whose CVUs had emerged as standard therapy resistant received one or two topical applications of 1 × 106 allogeneic ABCB5+ mesenchymal stem cells per cm2 wound area, in addition to standard treatment. Of 83 treatment-emergent adverse events, only three were judged related to the cell product; they were mild or moderate and recovered without sequelae. Wound size markedly decreased from baseline to week 12, resulting in a median wound size reduction of 76% (full analysis set, n = 31), 78% (per-protocol set, n = 27), and 87% (subset of responders, n = 21). In conclusion, the study treatment was well-tolerated and safe. The treatment elicited a profound wound size reduction within 12 weeks, identifying ABCB5+ mesenchymal stem cells as a potential candidate for adjunctive therapy of otherwise incurable CVUs. These results justify the conduct of a larger, randomized, controlled trial to confirm clinical efficacy.
Abbreviations: CVU, chronic venous ulcer; FAS, full analysis set; IQR, interquartile range; MSC, mesenchymal stem cell; PP, per-protocol set; TEAE, treatment-emergent adverse event
Introduction
Although venous leg ulcers can often be successfully treated, a significant number of ulcers become chronic (Chaby et al., 2013; Fife et al., 2018; Parker et al., 2015) (Table 1). Even after 5 years of repeated conservative therapy, 8% of chronic venous ulcers (CVUs) were still remaining unresolved (Callam et al., 1987). From a pathophysiologic perspective, CVUs are unable to progress through the normal wound repair pattern (Gurtner et al., 2008; Raffetto, 2016; Zhao et al., 2016). Instead, they remain stuck in an inflammatory state characterized by defective transition of proinflammatory M1 macrophages to granulation-promoting M2 macrophages, which is accompanied by an excess release of ROS and proinflammatory cytokines, including IL-1β and TNF-α. The sustained oxidative attack induces a senescence program in wound fibroblasts associated with the release of proinflammatory cytokines, chemokines, and matrix-degrading metalloproteinases, whereas IL-1β and TNF-α trigger a self-perpetuating cycle of autocrine recruitment and activation of M1 macrophages. Ultimately, the wound is arrested in a nonhealing state (Jiang and Scharffetter-Kochanek, 2020; Krzyszczyk et al., 2018; Sindrilaru et al., 2011).
Table 1.
Reported Healing Failure Rates of Venous Leg Ulcers Under Standard Therapy
| Treatment Duration | Failure Rate | Source | n |
|---|---|---|---|
| 4 weeks | 89% | Control group from an RCT (Cullen et al., 2017) | 27 patients |
| 12 weeks | 38% | Retrospective cohort study (Margolis et al., 1999) | 260 patients |
| 41% | Control group from an RCT (Cullen et al., 2017) | 27 patients | |
| 55% | US Wound Registry data (Fife et al., 2018) | 97,420 ulcers | |
| 55% | Retrospective cohort study (Gelfand et al., 2002) | 29,189 patients (56,488 ulcers) | |
| 57% | Control groups from 20 RCTs (Fife et al., 2018) | 1,372 patients | |
| 65% | Control group from an RCT (Bianchi et al., 2018) | 57 patients | |
| 85% | Control group from an RCT (Hayes et al., 2020) Failure rate dependent on basal wound size: ≤10 cm: 75% >10 cm: 93% |
26 patients | |
| 16 weeks | 56% | Control group from an RCT (Bianchi et al., 2018) | 57 patients |
| 20 weeks | 55% | Control group of an RCT (Kelechi et al., 2012) | 20 patients |
| 24 weeks | 14‒23% | RCT on compression therapy (Finlayson et al., 2014) | 103 patients |
| 24% | Prospective study (Gohel et al., 2005) | 1,186 patients (1,324 legs) | |
| 25% | Prospective study (Guest et al., 1999) | 198 legs | |
| 26‒34% | RCT on compression therapy (Polignano et al., 2004) | 68 patients | |
| 35% | RCT comparing surgery and compression with compression alone (Barwell et al., 2004) | 500 patients | |
| 35–44% | Retrospective cohort study (Margolis et al., 1999, 2000) Failure rate dependent on basal wound size and duration before treatment: ≤5 cm and ≤6 months: 5–7% >5 cm and >6 months: 63–87% |
260 patients | |
| 38% | Retrospective cohort study (Margolis et al., 2004) Failure rate dependent on basal wound size and duration before treatment: ≤10 cm and ≤12 months: 29% >10 cm and >12 months: 78% |
20,793 patients | |
| 45% | Retrospective cohort study (Gelfand et al., 2002) | 29,189 patients (56,488 ulcers) | |
| 51% | Control group of an RCT (Falanga et al., 1998) | 129 patients | |
| 56% | Control group of an RCT (Jull et al., 2020) | 72 patients | |
| 30 weeks | 29% | Retrospective cohort study (Margolis et al., 1999) | 260 patients |
| 1 year | 13% | Prospective study on compression therapy (Milic et al., 2009) | 189 patients |
| 30‒31% | Prospective study on compression therapy (Ashby et al., 2014) | 453 patients | |
| 40% | Control group of an RCT (Klonizakis et al., 2018) | 21 patients | |
| 2 years | 20% | Survey (Callam et al., 1987) | 600 patients |
| 5 years | 8% | Survey (Callam et al., 1987) | 600 patients |
Abbreviations: RCT, Randomized controlled trial; US, United States.
Among proinflammatory mediators, IL-1β plays a predominant role in chronic wound development (Harrell et al., 2020). Its counterpart, the naturally occurring receptor antagonist IL-1RA, is crucial for the alleviation of IL-1‒driven inflammation (Gabay et al., 2010). In a mouse chronic wound model mimicking the healing-impairing iron overload occurring in CVU tissue (Sindrilaru et al., 2011), adaptive IL-1RA release by locally injected mesenchymal stem cells (MSCs) expressing ABCB5 (Frank et al., 2003) dampened the IL-1β‒driven unrestrained M1 activation and promoted a shift toward M2 macrophages (Vander Beken et al., 2019).
ABCB5+ MSCs can be retrieved from skin tissue, expanded to a clinical scale, isolated as a highly pure, vital and viable cell population, and manufactured as a readily available advanced-therapy medicinal product (Ballikaya et al., 2020) with a favorable safety and tolerability profile (Tappenbeck et al., 2019). In mice, systemically applied ABCB5+ MSCs survived in the skin across a fully allogeneic barrier for at least 17 days (Schatton et al., 2015). In a first-in-human trial, topically applied patient-derived (autologous) ABCB5+ MSCs facilitated the healing of standard therapy‒resistant CVUs (Kerstan et al., 2021). In this study, we report on a phase I/IIa clinical trial (Figure 1a) evaluating the safety and efficacy of donor-derived, allogeneic ABCB5+ MSCs for topical treatment of CVUs in a high-medical-need population.
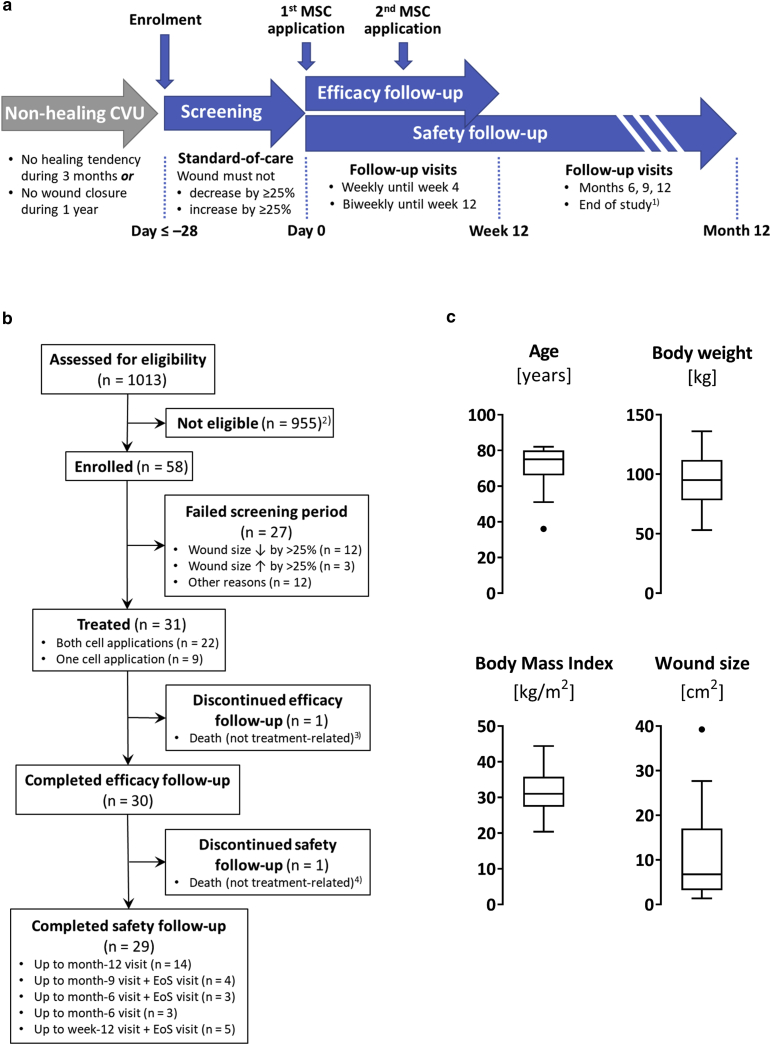
Figure 1.
Trial design, flow diagram, and patient characteristics. (a) Scheme of the trial design. 1Only subjects who did not reach month-12 visit before June 30, 2020 and were not scheduled for a planned safety follow-up visit in June 2020 were subjected to an end-of-study visit. (b) Study participants flow chart. For EoS visit, see a. 2Most frequent reasons for ineligibility were ulcer <1.5 cm2 or >100 cm2 (n = 268), ulcer not matching the CVU definition specified in the protocol (n = 207), patient aged >85 years (n = 110), BMI <20 or >45 (n = 101), and infected ulcer (n = 94). 3Owing to cardiac failure. 4Owing to pulmonary embolism. (c) Tukey’s boxplots of patient characteristics at screening and baseline wound size of all treated subjects (n = 31). BMI, body mass index; CVU, chronic venous ulcer; EoS, end of study; MSC, mesenchymal stem cell.
Results
Progress of the study
Patients were enrolled between November 2017 and January 2020. Forced by the COVID-19 pandemic, which was associated with critical issues, including staffing shortages, impairments of supply chains, and increased infection risk for the elderly and/or comorbid study participants, recruitment and treatment were discontinued as of April 2020. After consultation with the ethics committee and the regulatory authority, the trial was prematurely completed as of the end of June 2020. At that time, all treated subjects had completed the efficacy follow-up. Participants who had already entered the safety follow-up period but were not scheduled for a safety visit in June 2020 were subjected to a supplementary end-of-study visit (Figure 1a and b).
Participants
In total, 1,013 patients with CVU were assessed for eligibility, 58 of whom could be enrolled. Of these, 27 failed the screening period, so 31 subjects (16 male, 15 female) were treated (Figure 1b). In these subjects, only slight intraindividual wound sizes changes had occurred during the screening period (median duration of 34 days, interquartile range [IQR] = 28–41 days), which resulted in a median wound size reduction of ‒1% (IQR = ‒10 to 17%) (Figure 2a, run in). At baseline, the median age of the subjects was 75 (IQR = 66‒80) years, median bodyweight was 95 (IQR = 78‒112) kg, median body mass index was 31 (IQR = 27.4‒35.8) kg/m2, and median wound size was 6.79 (IQR = 3.21‒17.05) cm2 (Figure 1c).
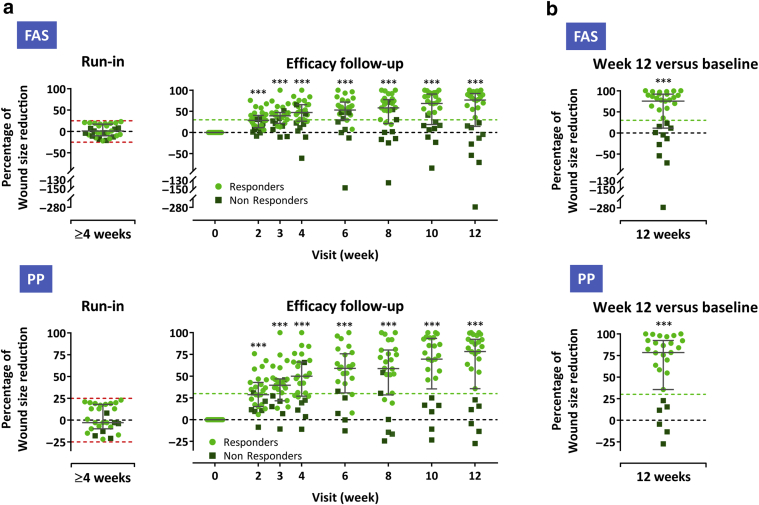
Figure 2.
Wound healing progress during the run-in and the treatment and efficacy follow-up period. (a) Percent wound size reduction during ≥4-week screening (run-in) period and during 12-week treatment/efficacy follow-up (presented as a reduction from baseline) in the FAS (n = 31) (upper panel) and PP (n = 27) (lower panel). (b) Percent wound size reduction from baseline at week 12 (last observation carried forward) in the FAS (upper panel) and PP (lower panel). Subjects whose wounds diminished or enlarged by >25% (dashed red lines) during the screening period did not qualify for study treatment. Subjects who had wound size reductions of at least 30% from baseline (indicated by light green dashed lines) at week 12 were considered responders. Error bars indicate median and interquartile range; ∗∗∗P < 0.001 versus baseline, two-sided Wilcoxon signed-rank test. FAS, full analysis set; PP, per-protocol set.
Of the 31 treated participants, 22 subjects received both, and nine subjects received only one topical cell application. Of the nine subjects who received only one, three subjects did because they had been enrolled under previous protocol versions before the second application was amended to the protocol, one subject did because of death (see details in the following paragraph), two subjects did because their wounds were already closed at the week-6 visit, and three subjects did because of the COVID-19 pandemic‒related treatment discontinuation. Two subjects discontinued study participation because of death (cardiac failure, pulmonary embolism; not related to study treatment): one was during efficacy, and the other was during safety follow-up (Figure 1b).
Four subjects had major protocol deviations: only week-2 efficacy assessment available owing to premature discontinuation, per-protocol wound size assessment at baseline missing, use of a wound irrigation solution, and exclusion criterion (potentially wound healing‒affecting condition) not met. These subjects were analyzed in the full analysis set (FAS) but were excluded from the per-protocol set (PP) (for a definition of the analysis sets, see Materials and Methods and statistical analysis.)
Safety outcomes
During the whole-study period, 83 treatment-emergent adverse events (TEAEs) were reported by 27 of 31 subjects (Table 2). Most TEAEs were mild or moderate, singular events; nine TEAEs reported by four subjects were severe. Nine TEAEs reported by seven subjects were serious (Table 3); two of them (cardiac failure and pulmonary embolism) had a fatal outcome. Only three TEAEs (increased wound exudation, mild; erythema, moderate; venous ulcer pain, moderate) were judged related to the cell product. These events recovered without sequelae.
Table 2.
Adverse Events
| Event | Number of Events | Number (%) of Subjects |
|---|---|---|
| Any adverse event1 | 96 | 28 (90) |
| Any TEAE | 83 | 27 (87) |
| Any serious TEAE2 | 9 | 7 (23) |
| Any TEAE | 3 | 3 (10) |
| Frequent TEAEs by MedDRA system organ class3 | ||
| General disorders and administration site conditions | 6 (19) | |
| Condition aggravated | 3 (10) | |
| Oedema peripheral | 2 (6) | |
| Infections and infestations | 9 (29) | |
| Nasopharyngitis | 3 (10) | |
| Wound infection | 2 (6) | |
| Skin and subcutaneous tissue disorders | 22 (71) | |
| Allergic contact dermatitis | 2 (6) | |
| Irritant contact dermatitis | 4 (13) | |
| Eczema, other | 2 (6) | |
| Pruritus | 2 (6) | |
| Skin ulcer | 12 (39) | |
| Stasis dermatitis | 2 (6) | |
| Venous ulcer pain | 5 (16) | |
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
Adverse events are reported for the safety analysis set (n = 31).
Includes pretreatment-emergent (occurring between giving written consent and first cell application) and treatment-emergent (occurring between first cell application and the end of safety follow-up) adverse events.
None of the serious TEAEs was related to study treatment.
Only TEAEs that were reported by at least two subjects.
Table 3.
Serious Treatment-Emergent Adverse Events
| MedDRA System Organ Class Preferred Term |
Number (%) of Subjects |
|---|---|
| Cardiac disorders | 1 (3) |
| Cardiac failure1 | 1 (3) |
| General disorders and administration site conditions | 1 (3) |
| Malaise | 1 (3) |
| Infections and infestations | 2 (6) |
| Cellulitis2 | 1 (3) |
| Wound infection3 | 1 (3) |
| Musculoskeletal and connective tissue disorders | 1 (3) |
| Foot deformity | 1 (3) |
| Renal and urinary disorders | 1 (3) |
| Renal amyloidosis | 1 (3) |
| Respiratory, thoracic, and mediastinal disorders | 1 (3) |
| Pulmonary embolism1 | 1 (3) |
| Skin and subcutaneous tissue disorders | 2 (6) |
| Skin ulcer4,5 | 2 (6) |
| Any event | 7 (23) |
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
Serious treatment-emergent adverse events are reported for the safety analysis set (n = 31). None of these events was related to the study treatment.
Event had a fatal outcome.
Moderate cellulitis originating from a superinfected nontarget (not cell-treated) ulcer, starting 9 months after the first and only cell application.
Severe postoperative wound infection after toe amputation due to foot deformation caused by rheumatoid arthritis, starting 11 months after the first and only cell application.
Worsening of the CVU, starting 3 months after the second cell application, required hospitalization owing to poor social situation of the subject.
Worsening of a skin ulcer at the contralateral foot, starting 4 weeks after the first cell application.
During efficacy follow-up, no clinically relevant changes in vital signs occurred (Table 4). A total of 14 new (not present at screening) physical examination findings were documented in 11 subjects (Table 5). Five findings (36%) represented improvements.
Table 4.
Vital Signs
| Parameter | Baseline (Day 0) (n = 31) | Change at Week 12 (n = 28) |
|---|---|---|
| Body temperature (°C) | 36.7 (0.5) | ‒0.1 (0.5) |
| Blood pressure (mm Hg) | ||
| Systolic | 135 (21) | ‒4 (16) |
| Diastolic | 76 (11) | 1 (10) |
| Heart rate (bpm) | 73 (9) | 1 (13) |
Abbreviation: bpm, beats per minute.
Vital signs are reported for the safety analysis set (n = 31). Data are presented as mean (SD).
Table 5.
Changes in Physical Examination Findings from Screening Visit
| Subject1 | Organ System | Visit at | Specification |
|---|---|---|---|
| 1 | Extremities | Week 12 | Oedema lower legs significantly reduced |
| Skin | Week 12 | Target ulcer almost closed, newly occurred nontarget ulcer | |
| 2 | Extremities | Week 12 | Ulcer left leg closed |
| Head | Week 12 | Scar occipital | |
| 3 | Skin | Week 6.12 | Skin irritation left lower leg |
| 4 | Skin | Week 6.12 | Plaster allergy left lower leg3 |
| 5 | Skin | Week 6.12 | Scar from carpal tunnel surgery |
| Skin | Week 12 | Pruritus4 | |
| 6 | Skin | Week 12 | Target ulcer and nontarget ulcers smaller |
| 7 | Skin | Week 12 | Clavus left hallux5 |
| 8 | Skin | Week 12 | Target ulcer closed |
| 9 | Skin | Week 6.12 | Intertrigo at the right mamma6 |
| 10 | Skin | Week 12 | Stasis dermatitis at the right foot/ankle7 |
| 11 | Ears | Week 6.12 | Disorder of the tuba auditiva |
Abbreviation: TEAE, treatment-emergent adverse event.
These changes are reported for the safety analysis set (n = 31).
Subjects presenting with changes in physical examinations; they are numbered consecutively.
Week 6.1: this visit was intended for the second cell application, scheduled 1–5 days after the week-6 efficacy follow-up visit.
Documented as TEAE (allergic contact dermatitis) not related to study treatment.
Documented as TEAE (pruritus) not related to study treatment.
Documented as TEAE (hyperkeratosis) not related to study treatment.
Documented as TEAE (allergic contact dermatitis) not related to study treatment.
Documented as TEAE (stasis dermatitis) not related to study treatment.
Efficacy outcomes
Efficacy assessments were performed on the FAS (n = 31) and the PP (n = 27) as specified in the Materials and Methods (see Statistical analysis). The primary efficacy outcome, median wound size reduction from baseline to week 12, was 76% (FAS) and 78% (PP) (Figure 2b). A summary of the secondary efficacy outcomes (see Material and Methods) is given in Table 6.
Table 6.
Summary of the Secondary Efficacy Outcomes
| Parameter | FAS (n = 31) | PP (n = 27) | Details |
|---|---|---|---|
| Absolute wound size reduction, (cm2)1 | |||
| Change from baseline at week 12 | 2.4 (14.0)2 | 5.2 (6.6) | Table 7 |
| Complete wound closure | |||
| Subjects with complete closure at week 12, n (%) | 6 (20)2 | 6 (22) | Table 9 |
| Subjects with complete closure at any time up to week 12, n (%) | 7 (23) | 7 (26) | Table 9 |
| Time to complete closure, days3 | Not reached | Not reached | Figure 3a |
| ≥30% wound size reduction | |||
| Subjects with ≥30% reduction at week 12 (responders), n (%) | 21 (70)2 | 21 (78) | Table 9 |
| Subjects with ≥30% reduction at any time up to week 12, n (%) | 26 (84) | 24 (89) | Table 9 |
| Time to ≥30% reduction, days3 | 21 (12; 27) | 15 (9; 27) | Figure 3b |
| Reopening after complete wound closure | |||
| Subjects with wounds reopened at week 12, n (%) | 1 (3.2) | 1 (3.7) | n.a. |
| Granulation, % of wound area | |||
| Day 0 | not evaluable | not evaluable | n.a. |
| Week 12 | not evaluable | not evaluable | n.a. |
| Epithelialization, % of wound area | |||
| Day 0 | not evaluable | not evaluable | n.a. |
| Week 12 | not evaluable | not evaluable | n.a. |
| Exudation | |||
| Wounds with low exudation, n (%) | |||
| Day 0 | 14 (45) | 11 (41) | Table 10 |
| Week 12 | 18 (62)4 | 16 (62)5 | Table 10 |
| Wounds with moderate exudation, n (%) | |||
| Day 0 | 15 (48) | 14 (52) | Table 10 |
| Week 12 | 10 (34)4 | 10 (38)5 | Table 10 |
| Pain score1 | |||
| Day 0 | 3.6 (3.2) | n.a. | Table 11 |
| Week 12 | 2.5 (2.2)2 | n.a. | Table 11 |
| QOL6 | |||
| Dermatology Life Quality Index7 | |||
| Day 0 | 9.5 (4–16)2 | n.a. | Table 12 |
| Week 12 | 6.0 (3–12)2 | n.a. | Table 12 |
Abbreviations: CI, confidence interval; FAS, full analysis set; IQR, interquartile range; n.a., not applicable; PP, per-protocol set.
Mean (SD).
n = 30.
Median (95% CI).
n = 29.
n = 26.
Owing to space limitations, the Short Form (36) Health Survey subscale scores (which remained virtually unchanged during the efficacy follow-up) are not shown in this table but are given in Table 12.
Median (IQR).
In more detail, wound size reduction was most pronounced during the first 2 weeks after the first MSC application. Thereafter, the median wound size continued to decrease until the end of efficacy follow-up at week 12 (Figure 2a and Table 7; for absolute wound size measurements by subject and visit, see Table 8).
Table 7.
Absolute Wound Size Reduction from Baseline by Visit
| Visit at | FAS (n = 31) |
PP (n = 27) |
||
|---|---|---|---|---|
| n | Difference from Baseline (cm2)1 | n | Difference from Baseline (cm2)1 | |
| Week 2 | 31 | 2.6 (3.6) | 27 | 2.8 (3.8) |
| Week 3 | 29 | 3.3 (4.2) | 26 | 3.2 (4.1) |
| Week 4 | 29 | 3.6 (4.4) | 27 | 3.6 (4.2) |
| Week 6 | 28 | 4.1 (5.8) | 25 | 4.4 (5.4) |
| Week 8 | 29 | 4.1 (6.6) | 26 | 4.2 (6.1) |
| Week 10 | 28 | 4.6 (6.2) | 25 | 5.0 (6.1) |
| Week 12 | 30 | 2.4 (14.0) | 27 | 5.2 (6.6) |
Abbreviations: FAS, full analysis set; PP, per-protocol set.
Mean (SD).
Table 8.
Absolute Wound Size Measurements by Visit
| Subject No. | Wound Size (cm2) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | |
| 1 | 24.6 | 20.4 | 19.2 | 18.1 | 12.9 | 10.0 | 10.8 | 11.1 |
| 2 | 27.7 | 24.8 | 25.8 | 26.7 | 25.7 | 27.6 | 23.1 | 24.4 |
| 3 | 2.4 | 1.4 | 0.8 | 0.6 | 0.4 | 0.6 | 0.1 | 0.3 |
| 4 | 3.2 | 2.1 | 1.7 | 1.0 | 0.8 | 0.3 | 0.2 | 0.0 |
| 5 | 9.6 | 8.5 | 8.1 | 8.5 | 9.6 | 11.2 | 10.6 | 10.9 |
| 6 | 2.3 | 1.7 | 1.6 | 1.3 | 1.3 | 1.1 | 0.7 | 0.5 |
| 7 | 15.6 | 10.2 | 9.9 | 10.8 | 9.1 | 12.6 | 6.6 | 4.7 |
| 8 | 9.8 | 5.6 | 5.3 | 3.8 | 4.0 | 2.8 | 2.6 | 1.3 |
| 9 | 5.5 | 5.6 | 6.0 | 8.9 | 13.6 | 13.0 | 10.2 | 9.4 |
| 10 | 4.6 | 4.5 | — | — | — | — | — | — |
| 11 | 3.2 | 2.0 | 1.9 | 1.6 | 1.5 | 1.5 | 1.0 | 1.3 |
| 12 | 3.2 | 1.2 | 1.3 | 0.5 | 1.3 | 0.3 | — | 0.3 |
| 13 | 3.9 | 2.7 | 2.1 | 1.3 | — | 4.4 | 2.9 | 4.0 |
| 14 | 17.6 | 5.6 | 4.5 | 4.2 | 1.2 | 0.3 | 0.2 | 0.3 |
| 15 | 22.5 | 20.9 | 10.9 | 12.6 | 11.9 | 6.5 | 13.3 | 85.3 |
| 16 | 18.9 | 10.1 | 9.8 | 8.8 | 7.6 | 7.9 | 5.8 | 3.3 |
| 17 | 25.9 | 10.8 | 9.9 | 8.9 | 5.9 | 6.3 | 6.0 | 4.1 |
| 18 | 8.2 | 7.4 | 6.0 | 6.6 | 6.2 | 3.8 | 6.9 | 6.3 |
| 19 | 14.7 | 11.6 | 11.6 | 11.4 | 9.9 | 10.3 | 13.4 | 12.4 |
| 20 | 17.0 | 12.2 | 14.8 | 11.7 | 12.1 | 8.2 | 8.9 | 6.2 |
| 21 | 10.0 | 8.5 | 6.3 | 7.2 | 9.2 | 6.9 | 5.4 | 2.4 |
| 22 | 39.3 | 42.6 | 43.5 | 43.6 | 44.3 | 48.9 | 48.5 | 50.0 |
| 23 | 8.9 | 6.3 | — | 5.1 | 3.2 | 3.0 | 1.2 | 0.7 |
| 24 | 1.7 | 1.1 | 1.0 | 0.9 | 0.7 | 0.3 | 0.1 | 0.1 |
| 25 | 3.8 | 2.6 | 2.5 | — | 2.0 | 3.7 | 3.6 | 5.8 |
| 26 | 4.8 | 4.5 | 2.7 | 1.6 | 1.7 | 3.7 | 0.3 | 0.1 |
| 27 | 4.0 | 2.8 | 2.6 | 2.1 | 1.8 | 1.2 | 0.6 | 0.4 |
| 28 | 1.9 | 1.6 | 1.0 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 |
| 29 | 4.8 | 2.6 | 2.9 | 2.4 | 0.9 | 0.9 | 0.7 | 1.0 |
| 30 | 6.8 | 5.9 | 5.8 | 5.0 | — | — | — | 4.4 |
| 31 | 1.4 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Abbreviation: No., number.
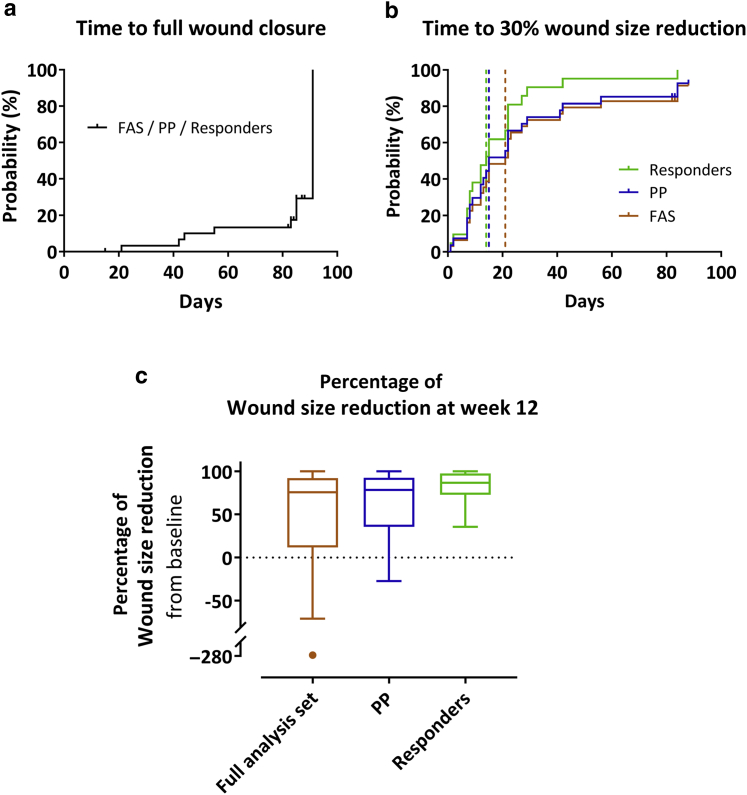
In six subjects (20% for FAS and 22% for PP), the wound was completely closed at week 12 (Table 9). A further subject presented with complete wound closure at the visit intended for the second cell application; however, the wound had enlarged at the subsequent visits (89% wound size reduction at week 12 from baseline). The median time to complete wound closure was not reached (Figure 3a).
Table 9.
Subjects with Complete Wound Closure and with ≥30% Wound Size Reduction by Visit
| Visit at | Complete Wound Closure |
≥30% Wound Size Reduction |
||||||
|---|---|---|---|---|---|---|---|---|
| FAS (n = 31) |
PP (n = 27) |
FAS (n = 31) |
PP (n = 27) |
|||||
| n | Subjects with Complete Wound Closure, n (%) | n | Subjects with Complete Wound Closure, n (%) | n | Subjects with 30% Wound Size Reduction, n (%) | n | Subjects with 30% Wound Size Reduction, n (%) | |
| Week 2 | 31 | 0 (0) | 27 | 0 (0) | 31 | 14 (45) | 27 | 13 (48) |
| Week 3 | 29 | 1 (3) | 26 | 1 (4) | 29 | 20 (69) | 26 | 18 (69) |
| Week 4 | 29 | 1 (3) | 27 | 1 (4) | 29 | 20 (69) | 27 | 19 (70) |
| Week 6 | 28 | 2 (7) | 25 | 2 (8) | 28 | 21 (75) | 25 | 19 (76) |
| Week 8 | 29 | 3 (10) | 26 | 3 (12) | 29 | 21 (72) | 26 | 20 (77) |
| Week 10 | 28 | 3 (11) | 25 | 3 (12) | 28 | 20 (71) | 25 | 19 (76) |
| Week 12 | 30 | 6 (20) | 27 | 6 (22) | 30 | 21 (70) | 27 | 21 (78) |
| Any time | 31 | 7 (23) | 27 | 7 (26) | 31 | 26 (84) | 27 | 24 (89) |
Abbreviations: FAS, full analysis set; PP, per-protocol set.
Figure 3.
Time-to-event and subgroup analyses. (a, b) Kaplan–Meier plots for the time to (a) full wound closure and (b) first 30% wound size reduction, expressed as the probability of the first occurrence of the event at a respective day during the efficacy follow-up period in the FAS (n = 31), PP (n = 27), and the subgroup of responders (n = 21). Subjects without event were censored at the date of the last available wound size assessment (indicated by small vertical ticks). Vertical dashed lines indicate the median time to event (not reached for full wound closure). Note that nearly all (except two) responders had reached 30% wound closure already by week 4 (day 28). (c) Tukey’s boxplots of the primary efficacy outcome parameter (percent wound size reduction from baseline at week 12) in the FAS (last observation carried forward), PP, and the subgroup of responders. FAS, full analysis set; PP, per-protocol set.
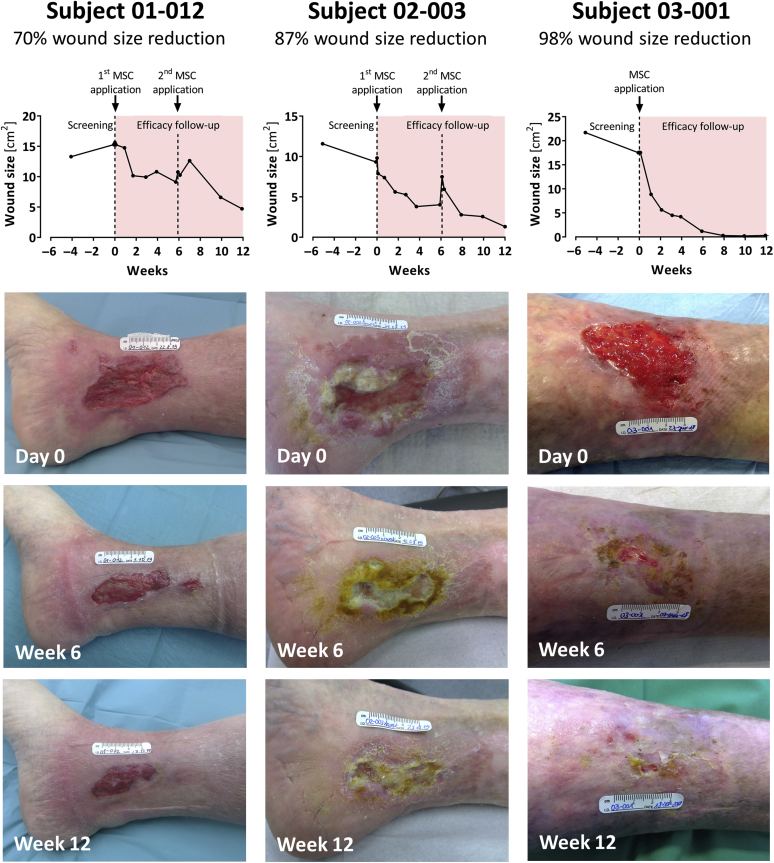
Wound size reduction by at least 30% at week 12 was observed in 70% (21 of 30; FAS) and 78% (21 of 27; PP) of subjects (Table 9). These subjects were considered responders. The median time to first 30% wound size reduction was 21 days (95% confidence interval = 12‒27; FAS) and 15 days (95% confidence interval = 9‒27; PP) (Figure 3b). The wound healing progress of three representative responders is illustrated in Figure 4.
Figure 4.
Wound healing progress during the treatment and efficacy follow-up period in three representative subjects in the subgroup of responders. All subjects had consented to publication. MSC, mesenchymal stem cell.
Most subjects showed low or moderate wound exudation. The group of subjects with low wound exudation increased from 45 to 62% (FAS) and from 41 to 62% (PP), whereas the percentage of subjects with moderate wound exudation decreased from 48 to 34% (FAS) and from 52 to 38% (PP) (Table 10). Owing to data inconsistencies resulting from measurement difficulties, formation of granulation and epithelial tissue was not evaluable.
Table 10.
Wound Exudation by Visit
| Visit at | FAS (n = 31) | PP (n = 27) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of Subjects | No. (%) of Subjects | |||||||
| n | Low | Moderate | High | n | Low | Moderate | High | |
| Day 0 | 31 | 14 (45) | 15 (48) | 2 (7) | 27 | 11 (41) | 14 (52) | 2 (7) |
| Day 1–3 | 31 | 9 (29) | 18 (58) | 4 (13) | 27 | 8 (30) | 15 (56) | 4 (15) |
| Week 1 | 31 | 12 (39) | 16 (52) | 3 (10) | 27 | 11 (41) | 14 (52) | 2 (7) |
| Week 2 | 31 | 13 (42) | 18 (58) | 0 (0) | 27 | 13 (48) | 14 (52) | 0 (0) |
| Week 3 | 29 | 14 (48) | 14 (48) | 1 (3) | 26 | 11 (42) | 14 (54) | 1 (4) |
| Week 4 | 28 | 13 (46) | 14 (50) | 1 (4) | 26 | 12 (46) | 14 (54) | 0 (0) |
| Week 6 | 28 | 18 (64) | 10 (36) | 0 (0) | 25 | 16 (64) | 9 (36) | 0 (0) |
| Week 8 | 29 | 16 (55) | 12 (41) | 1 (3) | 26 | 15 (58) | 10 (39) | 1 (4) |
| Week 10 | 28 | 16 (57) | 12 (43) | 0 (0) | 25 | 14 (56) | 11 (44) | 0 (0) |
| Week 12 | 29 | 18 (62) | 10 (34) | 1 (3) | 26 | 16 (62) | 10 (38) | 0 (0) |
Abbreviations: FAS, full analysis set; No., number; PP, per-protocol set.
Wound exudation was rated as low, moderate, or high according to Romanelli et al. (2010).
The mean pain score decreased slightly from 3.6 ± 3.2 at baseline to 2.5 ± 2.2 at week 12 (Table 11). The Short Form (36) Health Survey subscale scores remained virtually unchanged, whereas the median Dermatology Life Quality Index dropped from 9.5 (IQR = 4–16) at baseline to 6.0 (IQR = 3–12) at week 12 (Table 12).
Table 11.
Pain Score by Visit
| Visit at | n | Score1,2 |
|---|---|---|
| Day 0 | 31 | 3.6 (3.2) |
| Day 1–3 | 31 | 2.8 (2.7) |
| Week 1 | 31 | 3.3 (2.9) |
| Week 2 | 31 | 2.9 (2.7) |
| Week 3 | 29 | 2.4 (2.4) |
| Week 4 | 29 | 2.8 (2.4) |
| Week 6 | 28 | 2.6 (2.2) |
| Week 8 | 29 | 2.6 (2.0) |
| Week 10 | 28 | 2.5 (2.0) |
| Week 12 | 30 | 2.5 (2.2) |
The scores are reported for the full analysis set (n = 31).
Pain was rated using a 0–10 point numerical rating scale with 0 = no pain and 10 = worst pain imaginable.
Mean (SD).
Table 12.
QOL Scores by Visit
| Scale | Day 0 (n = 31) | Week 4 (n = 29) | Week 8 (n = 29) | Week 12 (n = 30) |
|---|---|---|---|---|
| Short Form (36) Health Survey subscale scores | ||||
| Subscales | ||||
| Physical functioning1 | 45 (30–65) | 45 (25–75) | 40 (25–70) | 45 (25–70) |
| Role functioning (physical)1 | 25 (0–100) | 75 (0–100) | 50 (0–75) | 38 (0–75) |
| Role functioning (emotional)1 | 100 (0–100) | 100 (33–100) | 67 (0–100) | 67 (0–100) |
| Social functioning1 | 75 (50–100) | 88 (63–100) | 88 (63–100) | 88 (63–100) |
| Mental health1 | 64 (52–80) | 68 (60–80) | 68 (56–76) | 60 (48–80) |
| Bodily pain1 | 51 (22–74) | 62 (41–74) | 52 (41–74) | 53 (41–74) |
| Vitality1 | 45 (35–60) | 50 (45–65) | 50 (35–65) | 45 (35–65) |
| General health perceptions1 | 52 (37–65) | 52 (40–65) | 50 (40–70) | 52 (35–62) |
| Health transition2 | 3 (3–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) |
| Dermatology Life Quality Index | ||||
| Summary score | 9.5 (4–16)3 | 6.5 (2–11)4 | 6.0 (2–8) | 6.0 (3–12) |
Abbreviation: IQR, interquartile range.
Scores are reported for the full analysis set (n = 31). Data are presented as median (IQR).
Transformed scale (0–100).
Raw scale.
n = 30.
n = 28.
Posthoc analyses
Posthoc analyses were performed on the subgroup of responders. In this group, median wound size reduction from baseline at week 12 was 87% (IQR = 73–97%) (Figure 3c), and 29% (6 of 21) of responders had complete wound closure at week 12. All except two responders had achieved the first 30% wound size reduction by week 4; the median time to first 30% wound size reduction was 14 days (95% confidence interval = 8‒22) (Figure 3b).
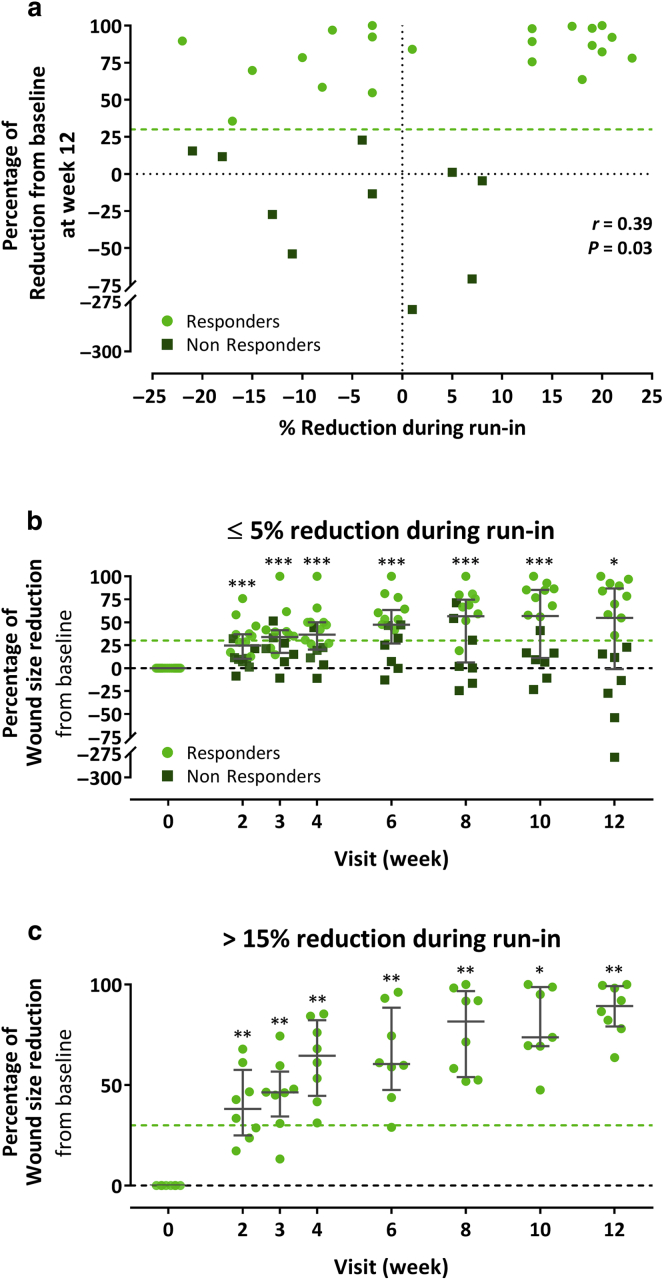
Another posthoc analysis was conducted to ascertain a possible relationship between the wound size change during the screening period and the wound healing progress after MSC treatment. A Spearman’s rank correlation test revealed a weak association (r = 0.39, P = 0.03) between percent wound size reduction during screening and percent wound size reduction from baseline at week 12 (Figure 5a). Whereas most (8 of 10) of the nonresponders had ≤5% wound size reduction during screening, almost half (10 of 21) of the responders fell also in this category (Figure 5a). A separate analysis of the subjects with low (≤5%) wound size reduction or wound enlargement during screening showed median postbaseline reductions that were still highly significant at each postbaseline visit, although they were numerically smaller than in the FAS (Figure 5b). On the other end, in the subjects with high (>15%) wound size reduction during screening, the wounds also improved further during the treatment/efficacy period because it became obvious, for example, from wound size reductions of 31 to 85% (median = 65%) 4 weeks after the first cell application compared with 17‒23% (median = 20%) during the ≥4-week screening period (Figure 5c).
Figure 5.
Assessment of association between wound size reduction during run-in and following MSC treatment. (a) Spearman’s rank correlation analysis between percent wound size reduction during screening and percent wound size reduction from baseline at week 12 in the full analysis set. (b, c) Percent wound size reduction from baseline during the treatment and efficacy period in subjects with (b) low (‒25 to 5%) and (c) high (15–25%) wound size reduction during screening. Error bars indicate median and interquartile range; ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.001 versus baseline, two-sided Wilcoxon signed-rank test. MSC, mesenchymal stem cell.
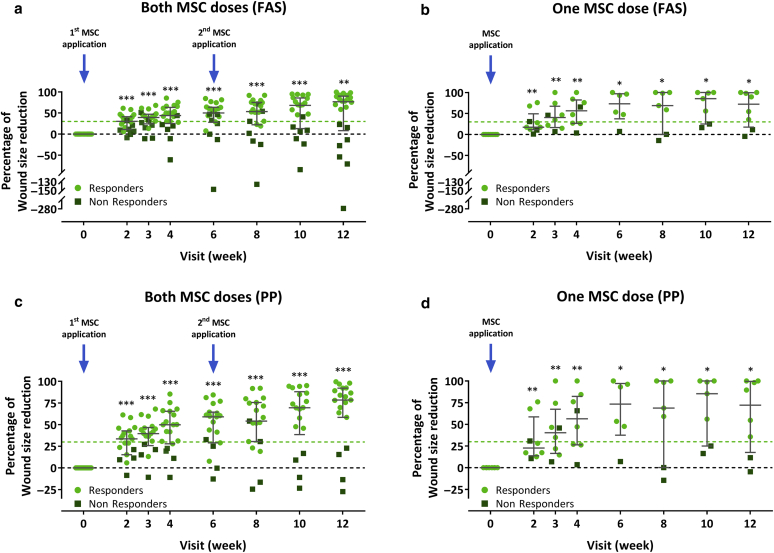
A further posthoc analysis was conducted to compare the wound healing progress between the subjects who had received both versus those who had received only one cell application. In both groups, the median percent wound size reduction from baseline was significant at all postbaseline visits. There was also no obvious difference in the median percent wound size reduction from baseline at week 12 between the two-dose and the one-dose groups (77% vs. 72% for the FAS and 78% vs. 72% for the PP). The percentage of responders did also not differ between the two-dose and the one-dose groups (15 of 22 [68%] vs. 6 of 9 [67%] for the FAS and 15 of 19 [79%] vs. 6 of 8 [75%] for the PP]) (Figure 6).
Figure 6.
Comparison of the wound healing progress during the treatment and efficacy follow-up period in the subjects receiving both versus those receiving only one-cell dose. (a, b) Percent wound size reduction from baseline in subjects receiving (a) subjects receiving both versus (b) those receiving one-cell dose in the FAS. (c, d) Percent wound size reduction from baseline in (c) subjects receiving both versus (d) those receiving one-cell dose in the PP. Subjects who had wound size reductions of at least 30% from baseline (indicated by light green dashed lines) at week 12 were considered responders. Error bars indicate median and interquartile range; ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.001 versus baseline, two-sided Wilcoxon signed-rank test. FAS, full analysis set; PP, per-protocol set; MSC, mesenchymal stem cell.
Discussion
On the basis of evidence that locally applied ABCB5+ MSCs can dampen IL-1β‒driven M1 macrophage overactivation present in nonhealing wounds (Vander Beken et al., 2019), ABCB5+ MSCs have been considered a potential treatment option for patients suffering from noncurable CVUs (Kerstan et al., 2021). To identify patients who were indeed in high need of an advanced wound closure strategy, all subjects underwent a two-step selection procedure. Basically, only such patients whose target CVUs qualified as therapy resistant, that is, having failed to improve within 3 months or to heal within 12 months of optimal phlebological treatment before enrolment (European Dermatology Forum, 2016), were enrolled. Furthermore, according to the anticipated mode of action of MSC therapy, that is, to advance wounds that are stalled in the inflammation phase of wound healing into the next stage of wound healing, we needed to verify that at the time of the first MSC application, the wound was indeed stalled. To this end, all enrolled subjects underwent at least 4-week screening period with standard-of-care treatment, during which the ulcer size was required to be static, defined as not changing by ≥25%. This rigorous selection process markedly outreached the approach advocated by the United States Food and Drug Administration, that is, to exclude subjects whose ulcer decreases by ≥30–50% during an initial 1–2 week standard-of-care period (U.S. Department of Health and Human Services and Food and Drug Administration, 2006). As a result, 21% of the enrolled therapy-resistant subjects were subsequently excluded again because their wounds appeared not clearly static. Finally, only 3.1% of the overall 1,013 prescreened patients with CVU could participate in this study (Figure 1b). In this way, we enrolled a study population for which a particularly high likelihood of healing failure at standard-of-care conditions can be presumed.
In this highly therapy-refractory population, topically applied ABCB5+ MSCs elicited a median wound size reduction of 76% (FAS) and 78% (PP) after 12 weeks (Figure 2b). Similar results (63% reduction) were observed in a previous first-in-human pilot trial with patient-derived (autologous) ABCB5+ MSCs (Kerstan et al., 2021). In this study, using donor-derived cells, we show that ABCB5+ MSCs display their wound healing-promoting capacity also in an allogeneic therapy setting. Because donor-derived ABCB5+ MSCs can be expanded to a clinical scale and manufactured as an off-the-shelf available, standardized advanced-therapy medicinal product of proven biological activity (Ballikaya et al., 2020), the hurdles associated with autologous therapy strategies, mainly potential interdonor variations and a long waiting time until treatment initiation owing to the time-consuming cell expansion process, can be overcome. Whether the greater effect on wound size reduction observed in this study than the autologous pilot trial may be attributed to the allogeneic approach and/or to the higher cells dose (1 × 106 vs. 5 × 105 cells/cm2 wound area) remains to be elucidated.
When viewed over time (Figure 2a), the effect of the applied cells appears most pronounced during the first weeks of treatment. This was expected considering that the action of ABCB5+ MSCs on wound healing relies on rather transient immunomodulatory activity that re-establishes a regenerative local environment, which enables the wound to resume physiologic healing. In line with this concept, subjects whose ulcers had not achieved a roughly 30% decrease at 4 weeks of study treatment had a high likelihood of emerging as nonresponders, staying below 30% wound size reduction until week 12 (Figures 2 and 3b). Similar observations were reported from other studies on CVU healing (Cardinal et al., 2008; Chaby et al., 2013), supporting a 30% wound size decrease as a suitable discriminator between potential responders and nonresponders. Moreover, in this study, the second cell dose at 6 weeks did neither enhance the percent wound size reduction achieved at week 12 nor increase the percentage of responders over the subjects who received only the first cell dose at day 0 (Figure 6). It may therefore be speculated that patients who will not respond to ABCB5+ MSC therapy can be detected already at around 4 weeks, which would help to adjust therapy decisions early as advocated by current treatment guidelines (Marston et al., 2016; O’Donnell et al., 2014).
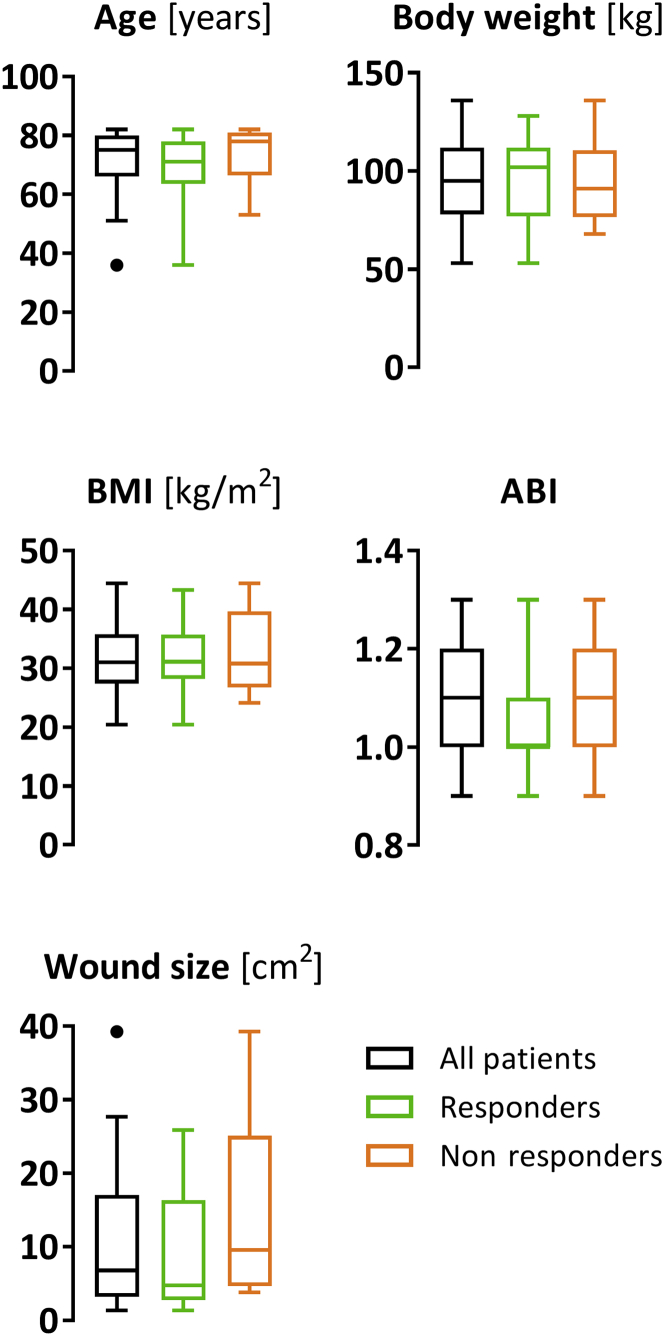
The phenomenon that some patients (30% of FAS and 22% of PP in this study) emerge as nonresponders is widely known in MSC therapy approaches (Caplan, 2018; Levy et al., 2020). In this trial, the strongly standardized quality of the cell product rules out potential differences in product quality as a cause of variation in the treatment responses (Supplementary Table S1). In addition, as shown in a posthoc subgroup analysis, achievement of responder status did not appear to be impacted by the wound size changes that had occurred during the run-in period (Figure 5). When comparing the characteristics that have been considered as unfavorable predictors for CVU healing, including higher patient age, higher body mass index, larger baseline wound size, and lower ankle‒brachial index (Gohel et al., 2005; Kulkarni et al., 2007; Labropoulos et al., 2012; Margolis et al., 1999, 2004; Marston et al., 2017; Meaume et al., 2005; Milic et al., 2009; Parker et al., 2015; Phillips et al., 2000; Taylor et al., 2002; Weller et al., 2020), there were no obvious differences between the responders and the nonresponders that could be called responsible for failure of treatment response (Figure 7). The absence of a clear association between treatment success and independent risk factors is not surprising, given the multifactorial etiology of impaired CVU healing, which involves more numerous factors such as nutritional status, mobility, and comorbidities (Gohel et al., 2005) or, on a cellular level, the differential expression or polymorphisms of genes associated with tissue inflammation, regeneration, or iron homeostasis (Charles et al., 2008; Gemmati et al., 2009; Tognazzo et al., 2006; Zamboni and Gemmati, 2007). To further investigate and understand what segregates responders from nonresponders could help detect the predictors of response to ABCB5+ MSC therapy, which might enable to identify patients with a high likelihood of nonresponsiveness already before treatment initiation.
Figure 7.
Comparison of patient characteristics at screening and baseline wound size between all treated subjects (n = 31) and subgroups of responders (n = 21) and nonresponders (n = 9). Depicted are Tukey’s boxplots. ABI, ankle‒brachial index; BMI, body mass index.
Nevertheless, the majority of subjects (70 and 78% for FAS and PP, respectively) responded to the study treatment, and these responders achieved a substantial median wound size reduction from baseline at week 12 of 87% (Figure 3c). Even though partial wound closure is a clinically less meaningful outcome than full wound closure, it is considered valid to “indicate relevant biological activity and help guide subsequent trials design” (U.S. Department of Health and Human Services and Food and Drug Administration, 2006). After all, in the present poor-prognosis population, 20% (FAS), 22% (PP), and 29% (responders) of subjects achieved full-wound closure. Moreover, it seems reasonable to expect that this rate would increase if the follow-up period was extended, given that at 12 weeks, the median time to full wound closure was not reached (Figure 3a), whereas the median wound size reduction was still increasing (Figure 2a). Another intriguing question to be studied in a subsequent trial is whether a higher cell dose would increase the healing effect, considering that in a preclinical dose selection study, a dose-dependent effect of ABCB5+ MSCs on wound size reduction was seen (Kerstan et al., 2021).
Naturally, the present conclusions are limited by factors typically associated with early-phase trials, mainly a relatively small sample size and an open, noncomparative design. Even though all wounds had been judged refractory to standard treatment, we cannot rule out that part of the observed improvements can be attributed to additional attention and care—for example, an optimized wound dressing strategy—the subjects may have received during the trial. Furthermore, wound healing can be affected by various patient-individual factors that were not controlled for. In addition, not all patients received a second cell dose. Nevertheless, we conclude that donor-derived ABCB5+ MSCs emerge as a promising candidate for adjunctive therapy of otherwise incurable CVUs. The very low rate of treatment-related adverse events verified good tolerability and overall safety of the cell product. Together, the results justify the conduct of a subsequent larger study with a randomized controlled, dose-ranging design, an extended efficacy follow-up period, and enhanced outcome parameters to validate the potential benefit and optimize the treatment strategy.
Materials and Methods
Participants
Adults (aged 35–85 years) were eligible if they had a lower leg CVU (sized 1.5‒100 cm2) confirmed by Doppler ultrasonography and unremarkable ankle‒brachial index (0.9–1.3) and were judged as therapy resistant according to the European Dermatology Forum S3 guideline (European Dermatology Forum, 2016), that is, having shown no healing tendency within 3 months or having not healed within 1 year of optimum phlebological therapy.
The main exclusion criteria were involvement of underlying muscle, tendon, or bone; diabetes; treatment-requiring peripheral artery disease; acute or untreated deep vein thrombosis; ulcer infection; adjacent skin disorders; other potentially wound healing‒affecting conditions; surgical procedures within 2 months before treatment; use of active wound care agents within 2 weeks before treatment; and current use of systemic glucocorticoids, immunosuppressants, or cytotoxic drugs.
Trial design
The study was a national, multicentric (nine sites in Germany), single-arm, phase I/IIa trial comprising screening (≥4 weeks), treatment and efficacy follow-up (weeks 1–12), and safety follow-up (until the end of month 12) periods (Figure 1a). During screening, the wound was required to be static, that is, subjects whose ulcers enlarged or diminished by >25% under standard of care were excluded.
The trial complied with the principles of the Helsinki Declaration and Good Clinical Practice. The study protocol and all other relevant documents had been approved by the local independent ethics committees (lead: Ethics Committee at the University of Würzburg, Germany; reference number 63/17_ff-sc) and the competent regulatory authority (Paul Ehrlich Institute, Langen, Germany). Before any trial-related activities/procedures, all participants gave written informed consent. The trial was registered with EudraCT (2017-000233-31) and ClinicalTrials.gov (NCT03257098).
Interventions
Treatment consisted of up to two topical applications of 1 × 106 allogeneic ABCB5+ MSCs (suspended in Ringer’s lactate solution containing 2.5% human serum albumin and 0.4% glucose at a target concentration of 1 × 107 cells/ml) per cm2 wound area at 6 weeks apart. The cells were delivered as Good Manufacturing Practice‒conforming standardized advanced-therapy medicinal products of proven vitality, viability, and biological activity (potency) (for product release data, see Supplementary Table S1). Originally, only one cell application was planned. The second application was amended to the protocol only after first-in-human data (Kerstan et al., 2021) suggested that a second application at 6 weeks after the first cell dose might provide additional benefit. For cell application, the wound was debrided, and after the bleeding had entirely stopped, the cell suspension was carefully dropped and evenly distributed on the wound surface. Thereafter, cells were allowed to settle for 15–30 minutes, and then the wound was covered with a waterproof film dressing (Tegaderm; 3M, Neuss, Germany) to hold the cell suspension in place. After 1–3 days, the film dressing was replaced by a foam (Mepilex; Mölnlycke, Düsseldorf, Germany or Biatain; Coloplast, Hamburg, Germany) or microbe-binding (Cutimed Sorbact; BSN Medical GmbH, Hamburg, Germany) dressing. In addition, participants received standard compression dressings. Dressings were changed during follow-up at the discretion of the investigator, depending on the subject’s individual wound situation.
Outcome measures
Safety outcome measures included adverse events (during efficacy and safety follow-up) and vital signs and changes in physical examination findings (during efficacy follow-up). The primary efficacy endpoint was percent wound size reduction at week 12 or the last available post-baseline measurement. Secondary efficacy endpoints were percent and absolute wound size reduction at predefined visits, the proportion of subjects achieving complete and 30% wound closure, time to complete and time to 30% wound closure, reopening after complete wound closure, granulation, epithelialization, wound exudation, pain, and QOL.
Outcome determination
Wound healing was documented by standardized digital photographs, and wound sizes were measured by the investigator using PictZar (BioVisual, Elmwood Park, NJ) planimetry software (98% accuracy, 94% inter-rater reliability, 98% intra-rater reliability according to Wendelken et al. [2011]). Formation of granulation and epithelial tissue was estimated by the investigator in the percentage of wound area from standardized wound photographs. Wound exudation was rated by the investigator as low (dry), moderate (moist), and high (wet), according to the criteria defined by the World Union of Wound Healing Societies (Romanelli et al., 2010). Pain was rated by the participant using a 0‒10‒point numerical rating scale with 0 meaning no pain and 10 meaning worst imaginable pain. QOL was assessed using the participant-reported Short Form (36) Health Survey and Dermatology Life Quality Index questionnaires.
Sample size
Enrolment followed a Simon optimal two-stage design, with responders defined as subjects showing at least 30% wound size reduction at week 12. The sample size required to achieve 80% power at a 5% significance level was calculated to be 37 subjects using PASS 13 software (NCSS, East Kaysville, UT). This enabled the option to terminate the trial if ≤6 or ≥14 of the first 18 treated subjects were responders. Because 13 of 18 subjects emerged as responders in an interim analysis, recruitment was continued. However, forced by the emerging COVID-19 pandemic in early 2020, the trial was prematurely completed. At that time, 31 subjects had been treated.
Statistical analysis
Safety assessments were performed on the safety analysis set (n = 31), which included all subjects who received at least one cell dose. All efficacy assessments were performed on the FAS, which included all subjects of the safety analysis set who underwent wound size assessments at baseline and at least one post-baseline visit (n = 31). In addition, the wound assessment parameters were also analyzed on the PP, which is a subset of the FAS, including all subjects of the FAS who had no major protocol deviations (n = 27).
Normally (D'Agostino–Pearson normality test) distributed parameters are presented as mean ± SD, and non-normally distributed parameters are presented as median and IQR. Time to complete wound closure and to attain 30% wound size reduction was analyzed using the Kaplan–Meier method. Statistical significance of percent wound size changes from baseline was tested against the null hypothesis (median change = 0) using a two-sided Wilcoxon signed-rank test. Spearman’s rank correlation analyses were performed to test for associations between variables.
Data availability statement
The individual participant data related to this article cannot be made publicly available for ethical/privacy reasons. The datasets are available from the corresponding author on reasonable request.
Conflict of Interest
NYF and MHF are inventors or coinventors of United States and international patents assigned to Brigham and Women’s Hospital (Boston, MA) and/or Boston Children’s Hospital (Boston, MA), licensed to TICEBA (Heidelberg, Germany) and RHEACELL (Heidelberg, Germany). MHF and KSK serve as scientific advisors to TICEBA and RHEACELL and participate in corporate-sponsored research collaborations with RHEACELL. KD, AKD, KK, and HS are employees of RHEACELL. ENR, JE, SB, and SS are employees of TICEBA. CG and MAK are Chief Executive Officer and Chief Scientific Officer, respectively, of RHEACELL and TICEBA. The remaining authors state no conflict of interest.
Acknowledgments
The authors thankfully acknowledge FGK Clinical Research GmbH (Munich, Germany) for expert support in project management, monitoring, and data analysis and the Coordination Centre for Clinical Trials (KKS) at the University of Heidelberg (Heidelberg, Germany) for advice on statistical analysis. We also gratefully thank the tissue donors and the tissue retrieval facilities: Aesthetic Quartier Heidelberg GmbH (Germany) (Director: Joachim Beck) and Cologne Dermatology (Germany) (Director: Wolfgang G. Philipp-Dormston). The trial was sponsored by RHEACELL (Heidelberg, Germany). The contributions by NYF and MHF to this work were supported by the National Institutes of Health/National Eye Institute grants RO1EY025794 and R24EY028767. DPO declared a grant from TICEBA to Brigham and Women's Hospital (Boston, MA).
ORCIDs
Andreas Kerstan: http://orcid.org/0000-0001-6483-0191
Kathrin Dieter: http://orcid.org/0000-0001-6341-4161
Elke Niebergall-Roth: http://orcid.org/0000-0001-7846-3388
Ann-Kathrin Dachtler: http://orcid.org/0000-0003-3744-1937
Korinna Kraft: http://orcid.org/0000-0001-9650-3988
Markus Stücker: http://orcid.org/0000-0001-5564-5285
Georg Daeschlein: http://orcid.org/0000-0002-4650-199X
Michael Juenger: http://orcid.org/0000-0002-7574-6646
Tobias Görge: http://orcid.org/0000-0002-3631-8841
Ulrich Meyer-Pannwitt: http://orcid.org/0000-0001-9181-6555
Cornelia Erfurt-Berge: http://orcid.org/0000-0002-3722-6383
Charlotte von Engelhardt: http://orcid.org/0000-0003-1888-9015
Andreas Klare: http://orcid.org/0000-0002-8887-126X
Christiane Pfeiffer: http://orcid.org/0000-0001-7770-168X
Jasmina Esterlechner: http://orcid.org/0000-0002-9840-9431
Hannes Matthias Schröder: http://orcid.org/0000-0002-0427-5867
Martin Gasser: http://orcid.org/0000-0002-4987-8389
Ana M. Waaga-Gasser: http://orcid.org/0000-0002-5587-5256
Matthias Goebeler: http://orcid.org/0000-0001-7095-9848
Seda Ballikaya: http://orcid.org/0000-0001-9746-0422
Samar Sadeghi: http://orcid.org/0000-0001-6846-4264
George F. Murphy: http://orcid.org/0000-0001-9362-5146
Dennis P. Orgill: http://orcid.org/0000-0002-8279-7310
Natasha Y. Frank: http://orcid.org/0000-0001-8196-0449
Christoph Ganss: http://orcid.org/0000-0003-0396-6591
Karin Scharffetter-Kochanek: http://orcid.org/0000-0002-9655-685X
Markus H. Frank: http://orcid.org/0000-0002-1312-0488
Mark A. Kluth: http://orcid.org/0000-0003-0764-4645
Author Contributions
Conceptualization: AKe, KD, MAK, GFM, DPO, NYF, KSK, MHF, MGa; Data Curation: KD, ENR, AKD, KK, MAK, HMS; Formal Analysis: ENR, AKe, KD, MAK; Investigation: AKe, MS, GD, MJ, TG, UMP, CEB, CVE, AKl, CP, MGa, AMWG, MGo; Methodology: AKe, KD, MAK, GFM, DPO, NYF, KSK, MHF, MGa; Project Administration: KD ENR AKD KK MAK; Resources: JE, HMS, SB, SS, CG; Validation: JE, HMS, SB, SS, CG, AKe; Visualization: ENR, KD, AKD, KK, MAK, HMS; Writing - Original Draft Preparation: ENR; Writing - Review and Editing: AKe, MS, GD, MJ, TG, UMP, CEB, CVE, AKl, CP, MGa, AMWG, MGo, KD, ENR, AKD, KK, MAK, JE, HMS, SB, SS, CG, GFM, DPO, NYF, KSK, MHF
accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2021;X:100067
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.xjidi.2021.100067.
Supplementary Material
References
- Ashby R.L., Gabe R., Ali S., Adderley U., Bland J.M., Cullum N.A., et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet. 2014;383:871–879. doi: 10.1016/S0140-6736(13)62368-5. [DOI] [PubMed] [Google Scholar]
- Ballikaya S., Sadeghi S., Niebergall-Roth E., Nimtz L., Frindert J., Norrick A., et al. Process data of allogeneic ex vivo-expanded ABCB5+ mesenchymal stromal cells for human use: off-the-shelf GMP-manufactured donor-independent ATMP. Stem Cell Res Ther. 2020;11:482. doi: 10.1186/s13287-020-01987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwell J.R., Davies C.E., Deacon J., Harvey K., Minor J., Sassano A., et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363:1854–1859. doi: 10.1016/S0140-6736(04)16353-8. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Cazzell S., Vayser D., Reyzelman A.M., Dosluoglu H., Tovmassian G., et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix®) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15:114–122. doi: 10.1111/iwj.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callam M.J., Harper D.R., Dale J.J., Ruckley C.V. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389–1391. doi: 10.1136/bmj.294.6584.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A.I. Cell-based therapies: the nonresponder. Stem Cells Transl Med. 2018;7:762–766. doi: 10.1002/sctm.18-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal M., Eisenbud D.E., Phillips T., Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008;16:19–22. doi: 10.1111/j.1524-475X.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Chaby G., Senet P., Ganry O., Caudron A., Thuillier D., Debure C., et al. Prognostic factors associated with healing of venous leg ulcers: a multicentre, prospective, cohort study. Br J Dermatol. 2013;169:1106–1113. doi: 10.1111/bjd.12570. [DOI] [PubMed] [Google Scholar]
- Charles C.A., Tomic-Canic M., Vincek V., Nassiri M., Stojadinovic O., Eaglstein W.H., et al. A gene signature of nonhealing venous ulcers: potential diagnostic markers. J Am Acad Dermatol. 2008;59:758–771. doi: 10.1016/j.jaad.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B.M., Serena T.E., Gibson M.C., Snyder R.J., Hanft J.R., Yaakov R.A. Randomized controlled trial comparing collagen/oxidized regenerated cellulose/silver to standard of care in the management of venous leg ulcers. Adv Skin Wound Care. 2017;30:464–468. doi: 10.1097/01.ASW.0000524452.80170.d8. [DOI] [PubMed] [Google Scholar]
- European Dermatology Forum Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol. 2016;30:1843–1875. doi: 10.1111/jdv.13848. [DOI] [PubMed] [Google Scholar]
- Falanga V., Margolis D., Alvarez O., Auletta M., Maggiacomo F., Altman M., et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- Fife C.E., Eckert K.A., Carter M.J. Publicly reported wound healing rates: the fantasy and the reality. Adv Wound Care (New Rochelle) 2018;7:77–94. doi: 10.1089/wound.2017.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson K.J., Courtney M.D., Gibb M.A., O'Brien J.A., Parker C.N., Edwards H.E. The effectiveness of a four-layer compression bandage system in comparison with Class 3 compression hosiery on healing and quality of life in patients with venous leg ulcers: a randomised controlled trial. Int Wound J. 2014;11:21–27. doi: 10.1111/j.1742-481X.2012.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank N.Y., Pendse S.S., Lapchak P.H., Margaryan A., Shlain D., Doeing C., et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- Gabay C., Lamacchia C., Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gelfand J.M., Hoffstad O., Margolis D.J. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol. 2002;119:1420–1425. doi: 10.1046/j.1523-1747.2002.19629.x. [DOI] [PubMed] [Google Scholar]
- Gemmati D., Federici F., Catozzi L., Gianesini S., Tacconi G., Scapoli G.L., et al. DNA-array of gene variants in venous leg ulcers: detection of prognostic indicators. J Vasc Surg. 2009;50:1444–1451. doi: 10.1016/j.jvs.2009.07.103. [DOI] [PubMed] [Google Scholar]
- Gohel M.S., Taylor M., Earnshaw J.J., Heather B.P., Poskitt K.R., Whyman M.R. Risk factors for delayed healing and recurrence of chronic venous leg ulcers--an analysis of 1324 legs. Eur J Vasc Endovasc Surg. 2005;29:74–77. doi: 10.1016/j.ejvs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Guest M., Smith J.J., Sira M.S., Madden P., Greenhalgh R.M., Davies A.H. Venous ulcer healing by four-layer compression bandaging is not influenced by the pattern of venous incompetence. Br J Surg. 1999;86:1437–1440. doi: 10.1046/j.1365-2168.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Harrell C.R., Markovic B.S., Fellabaum C., Arsenijevic N., Djonov V., Volarevic V. The role of interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. BioFactors. 2020;46:263–275. doi: 10.1002/biof.1587. [DOI] [PubMed] [Google Scholar]
- Hayes P.D., Harding K.G., Johnson S.M., McCollum C., Téot L., Mercer K., et al. A pilot multi-centre prospective randomised controlled trial of RECELL for the treatment of venous leg ulcers. Int Wound J. 2020;17:742–752. doi: 10.1111/iwj.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Scharffetter-Kochanek K. Mesenchymal stem cells adaptively respond to environmental cues thereby improving granulation tissue formation and wound healing. Front Cell Dev Biol. 2020;8:697. doi: 10.3389/fcell.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull A., Wadham A., Bullen C., Parag V., Weller C., Waters J. Wool-derived keratin dressings versus usual care dressings for treatment of slow healing venous leg ulceration: a randomised controlled trial (Keratin4VLU) BMJ Open. 2020;10:e036476. doi: 10.1136/bmjopen-2019-036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelechi T.J., Mueller M., Hankin C.S., Bronstone A., Samies J., Bonham P.A. A randomized, investigator-blinded, controlled pilot study to evaluate the safety and efficacy of a poly-N-acetyl glucosamine-derived membrane material in patients with venous leg ulcers. J Am Acad Dermatol. 2012;66:e209–e215. doi: 10.1016/j.jaad.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Kerstan A., Niebergall-Roth E., Esterlechner J., Schröder H.M., Gasser M., Waaga-Gasser A.M., et al. Ex vivo-expanded highly pure ABCB5+ mesenchymal stromal cells as good manufacturing practice-compliant autologous advanced therapy medicinal product for clinical use: process validation and first in-human data. Cytotherapy. 2021;23:165–175. doi: 10.1016/j.jcyt.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonizakis M., Tew G.A., Gumber A., Crank H., King B., Middleton G., et al. Supervised exercise training as an adjunct therapy for venous leg ulcers: a randomized controlled feasibility trial. Br J Dermatol. 2018;178:1072–1082. doi: 10.1111/bjd.16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.R., Gohel M.S., Wakely C., Minor J., Poskitt K.R., Whyman M.R. The Ulcerated Leg Severity Assessment score for prediction of venous leg ulcer healing. Br J Surg. 2007;94:189–193. doi: 10.1002/bjs.5597. [DOI] [PubMed] [Google Scholar]
- Labropoulos N., Wang E.D., Lanier S.T., Khan S.U. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2012;129:179–186. doi: 10.1097/PRS.0b013e3182362a53. [DOI] [PubMed] [Google Scholar]
- Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D.J., Allen-Taylor L., Hoffstad O., Berlin J.A. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12:163–168. doi: 10.1111/j.1067-1927.2004.012207.x. [DOI] [PubMed] [Google Scholar]
- Margolis D.J., Berlin J.A., Strom B.L. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol. 1999;135:920–926. doi: 10.1001/archderm.135.8.920. [DOI] [PubMed] [Google Scholar]
- Margolis D.J., Berlin J.A., Strom B.L. Which venous leg ulcers will heal with limb compression bandages? Am J Med. 2000;109:15–19. doi: 10.1016/s0002-9343(00)00379-x. [DOI] [PubMed] [Google Scholar]
- Marston W., Tang J., Kirsner R.S., Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen. 2016;24:136–144. doi: 10.1111/wrr.12394. [DOI] [PubMed] [Google Scholar]
- Marston W.A., Ennis W.J., Lantis J.C., 2nd, Kirsner R.S., Galiano R.D., Vanscheidt W., et al. Baseline factors affecting closure of venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2017;5:829–835.e1. doi: 10.1016/j.jvsv.2017.06.017. [DOI] [PubMed] [Google Scholar]
- Meaume S., Couilliet D., Vin F. Prognostic factors for venous ulcer healing in a non-selected population of ambulatory patients. J Wound Care. 2005;14:31–34. doi: 10.12968/jowc.2005.14.1.26723. [DOI] [PubMed] [Google Scholar]
- Milic D.J., Zivic S.S., Bogdanovic D.C., Karanovic N.D., Golubovic Z.V. Risk factors related to the failure of venous leg ulcers to heal with compression treatment. J Vasc Surg. 2009;49:1242–1247. doi: 10.1016/j.jvs.2008.11.069. [DOI] [PubMed] [Google Scholar]
- O’Donnell T.F., Jr., Passman M.A., Marston W.A., Ennis W.J., Dalsing M., Kistner R.L., et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg. 2014;60(Suppl. 2):3s–59s. doi: 10.1016/j.jvs.2014.04.049. [DOI] [PubMed] [Google Scholar]
- Parker C.N., Finlayson K.J., Shuter P., Edwards H.E. Risk factors for delayed healing in venous leg ulcers: a review of the literature. Int J Clin Pract. 2015;69:1029–1030. doi: 10.1111/ijcp.12677. [DOI] [PubMed] [Google Scholar]
- Phillips T.J., Machado F., Trout R., Porter J., Olin J., Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol. 2000;43:627–630. doi: 10.1067/mjd.2000.107496. [DOI] [PubMed] [Google Scholar]
- Polignano R., Bonadeo P., Gasbarro S., Allegra C. A randomised controlled study of four-layer compression versus Unna's Boot for venous ulcers. J Wound Care. 2004;13:21–24. doi: 10.12968/jowc.2004.13.1.26563. [DOI] [PubMed] [Google Scholar]
- Raffetto J.D. Pathophysiology of wound healing and alterations in venous leg ulcers-review. Phlebology. 2016;31(Suppl. 1):56–62. doi: 10.1177/0268355516632998. [DOI] [PubMed] [Google Scholar]
- Romanelli M., Vowden K., Weir D. Exudate management made easy. Wounds International. 2010. https://www.woundsinternational.com/resources/details/exudate-management-made-easy
- Schatton T., Yang J., Kleffel S., Uehara M., Barthel S.R., Schlapbach C., et al. ABCB5 identifies immunoregulatory dermal cells. Cell Rep. 2015;12:1564–1574. doi: 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenbeck N., Schröder H.M., Niebergall-Roth E., Hassinger F., Dehio U., Dieter K., et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy. 2019;21:546–560. doi: 10.1016/j.jcyt.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.J., Taylor A.D., Smyth J.V. Using an artificial neural network to predict healing times and risk factors for venous leg ulcers. J Wound Care. 2002;11:101–105. doi: 10.12968/jowc.2002.11.3.26381. [DOI] [PubMed] [Google Scholar]
- Tognazzo S., Gemmati D., Palazzo A., Catozzi L., Carandina S., Legnaro A., et al. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J Vasc Surg. 2006;44:815–819. doi: 10.1016/j.jvs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, Food and Drug Administration Guidance for industry: chronic cutaneous ulcer and burn wounds – developing products for treatment. 2006. https://www.fda.gov/media/71278/download
- Vander Beken S., de Vries J.C., Meier-Schiesser B., Meyer P., Jiang D., Sindrilaru A., et al. Newly defined ATP-binding cassette subfamily B Member 5 positive dermal mesenchymal stem cells promote healing of chronic iron-overload wounds via secretion of interleukin-1 receptor antagonist. Stem Cells. 2019;37:1057–1074. doi: 10.1002/stem.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller C.D., Bouguettaya A., Team V., Flegg J., Kasza J., Jayathilake C. Associations between patient, treatment, or wound-level factors and venous leg ulcer healing: wound characteristics are the key factors in determining healing outcomes. Wound Repair Reg. 2020;28:211–218. doi: 10.1111/wrr.12773. [DOI] [PubMed] [Google Scholar]
- Wendelken M.E., Berg W.T., Lichtenstein P., Markowitz L., Comfort C., Alvarez O.M. Wounds measured from digital photographs using photodigital planimetry software: validation and rater reliability. Wounds. 2011;23:267–275. [PubMed] [Google Scholar]
- Zamboni P., Gemmati D. Clinical implications of gene polymorphisms in venous leg ulcer: a model in tissue injury and reparative process. Thromb Haemost. 2007;98:131–137. [PubMed] [Google Scholar]
- Zhao R., Liang H., Clarke E., Jackson C., Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant data related to this article cannot be made publicly available for ethical/privacy reasons. The datasets are available from the corresponding author on reasonable request.