Abstract
Salinity is abiotic stress that inhibits seed germination and suppresses plant growth and root development in a dose-dependent manner. Fusarium pseudograminearum (Fg) is a plant pathogen that causes wheat crown rot. Chemical control methods against Fg are toxic to the environment and resistance has been observed in wheat crops. Therefore, an alternative approach is needed to manage this devastating disease and the effects of salinity. Our research focused on the mycoparasitic mechanisms of Trichoderma longibrachiatum (TG1) on Fg and the induction of defenses in wheat seedlings under salt and Fg stress at physiological, biochemical and molecular levels. The average inhibition rate of TG1 against Fg was 33.86%, 36.32%, 44.59%, and 46.62%, respectively, in the four NaCl treatments (0, 50, 100, and 150 mM). The mycoparasitic mechanisms of TG1 against Fg were coiling, penetration, and wrapping of Fg hyphae. In response to inoculation of TG1 with Fg, significant upregulation of cell wall degrading enzymes (CWDEs) was observed. The expression of β-1, 6-glucan synthase (PP4), endochitinase precursor (PH-1), and chitinase (chi18-15) increased by 1. 6, 1. 9, and 1.3-fold on day 14 compared with day 3. Wheat seedlings with combined TG1 + Fg treatments under different NaCl stress levels decreased disease index by an average of 51.89%; increased the superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activity by an average of 38%, 61%, and 24.96%, respectively; and decreased malondialdehyde (MDA) and hydrogen peroxide (H2O2) content by an average of 44.07% and 41.75% respectively, compared with Fg treated seedlings. The combined TG1 + Fg treatment induced the transcription level of plant defense-related genes resulting in an increase in tyrosin-protein kinase (PR2), chitinase class I (CHIA1), and pathogenesis-related protein (PR1-2) by an average of 1.15, 1.35, and 1.37-fold, respectively compared to Fg treatment. However, the expression levels of phenylalanine ammonia-lyase (PAL) increased 3.40-fold under various NaCl stresses. Our results suggest that TG1 enhances wheat seedling growth and controls wheat crown rot disease by strengthening the plant defense system and upregulating the expression of pathogenesis-related genes under both Fg and salt stress.
Keywords: biocontrol microbes, salinity stress, Fusarium, Trichoderma, mycoparasitism, antioxidative defense system
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereals grown in arid and semi-arid regions, providing 20% of the total dietary calorie and proteins intake that promotes human nutrition and healthy living (Shiferaw et al., 2013). Drought, soil nutrient deficiency, salinity stress, and disease-causing phytopathogens are all factors that limit wheat development. Fusarium pseudograminearum is one of the wheat pathogens that cause catastrophic yield losses by causing crown and root rots in wheat and barley crops (Pasquali et al., 2016). Infected seedlings can experience pre-emergence and post-emergence damping-off as a result of this pathogen (Kazan and Gardiner, 2018). Plants that survive damping-off may exhibit stunted growth and unfilled kernels due to blockage of water and nutrient transfer in the plants. Due to the rot, infected seedlings have dark brown to black crowns. Fusarium infections, including Fusarium foot rot (FFR) and Fusarium root rot (FRR), are responsible for outbreaks of seedling blight, epidemic scab, and Fusarium head blight (FHB) in the United States (Subedi et al., 2007). They directly or indirectly cause millions of dollars of losses in wheat and barley production (El-Allaf et al., 2001). The effects of F. pseudograminearum on most cereals are often due to the accumulation of deoxynivalenol (DON), an internationally regulated mycotoxin (Bolanos-Carriel et al., 2020). One of the most important methods for controlling plant diseases is chemical control. However, fungicide control of F. pseudograminearum in wheat is minimal and does not provide year-round protection against the pathogen (Moya-Elizondo and Jacobsen, 2016; Alahmad et al., 2018) and may pose a risk to human health. Therefore, an alternative for the use of environmentally-friendly and plant-growth-promoting fungi is urgently needed in recent years. Plant-growth-promoting microbes (PGPM) have several potentials that enable them to enhance plant growth. These include ability to fix atmospheric nitrogen and dissolve phosphate in soil, production of siderophores for iron extraction, ACC deaminase that contributes to stress tolerance, and production of indole-3-acetic acid (IAA). They also indirectly promote plant growth by the combating pathogens through nutrient and space competition, secretion of antibiotic substances and induction of defense systems in the plant.
Soil salinization is considered one of the major threats to agricultural production (Isayenkov and Maathuis, 2019), and affects over 1 billion hectares of arable land worldwide (Ivushkin et al., 2019). Increasing salinization is expected to affect 50% of arable land by 2050 (Wang et al., 2003). Salt stress suppresses plant growth, impedes germination and root development in a dose-dependent manner by blocking auxin signaling (Contreras-Cornejo et al., 2014) and triggering dehydration, nutrient deficiency, membrane dysfunction, and oxidative stress, leading to tissue damage or early senescence (Wu and Wang, 2012; Hossain et al., 2017). The accumulation of reactive oxygen species (ROS) is a well-known consequence of stress (Saghafi et al., 2018). Plants evolve scavenging mechanisms that include both enzymatic and non-enzymatic antioxidants to effectively mitigate ROS damage. The major enzymatic systems for ROS scavenging mechanisms, such as SOD, POD, and CAT are important parameters for assessing plant stress resistance. These ROS scavenging mechanisms mediated by antioxidant enzymes are the first line of defense against stress and directly reflect the effects of stress on plants. To maintain the balance between ROS development and interception and to mitigate the negative effects of stress on physiological metabolism and growth of plants, effective antioxidant capacity is essential (Saghafi et al., 2018). Plant responses to combined biotic and abiotic stresses are complex due to the multiple interactions between plants, pathogens and abiotic stresses. It is well known that the physiological and molecular responses of plants to combined abiotic-pathogen stresses differ significantly from their responses to individual stresses (Lin et al., 2015; Ramegowda and Senthil-Kumar, 2015).
Salicylic acid (SA) plays an important role in regulating plant growth, production, maturation, and defense responses (Miura and Tada, 2014). For instance, SA significantly increased seedling size and mass compared to the untreated (control) when applied exogenously to wheat seedlings (Purcǎrea et al., 2010), and high salt tolerance of wheat seedlings was observed when treated with the SA solution (Cornelia et al., 2011). The application of SA as a soil drench stimulates antioxidant protective responses that may lead to systemic acquired resistance (SAR) resulting in F. pseudograminearum (Sorahinobar et al., 2016). These findings shed light on the physiological and molecular function of SA in plant resistance to hemibiotrophic pathogens. Therefore, an alternative method of using plant-growth-promoting fungi that can increase SA in plants instead of exogenous SA application is more beneficial to the current agricultural system.
Trichoderma species are plant-growth-promoting fungi that play an important role in alleviating abiotic and biotic stresses due to their antimicrobial, mycoparasitic, competitive and secondary metabolic potentials, antioxidant enzyme activity and gene expression and synthesis of phytohormones such as IAA and ACC-deaminase (Qiu et al., 2012; Fu et al., 2017; Rubio et al., 2017). Previous studies reported by Pereira et al. (2014) showed that T. harzianum ALL-42 enhanced the response of field bean to Rhizoctonia solani by increasing the expression of β-1–3-glucanase and peroxidase genes compared to the host response when exposed to the pathogen alone. Similarly, cucumber roots colonized by T. harzianum T-203 showed increased activity of chitinase, β-1,3-glucanase, cellulase and peroxidase at 72 h after inoculation (Chatterton, 2010) which also supports the molecular mechanism of biocontrol- fungi in response to biotic stress.
However, there are fewer reports on the application of Trichoderma as biocontrol agent (BCA) in controlling wheat crown rot caused by F. pseudograminearum (Fg) and inducing salinity tolerance in wheat seedlings. Thus, the objectives of the present study were (i) to clarify the mycoparasitic mechanisms of TG1 against Fg by morphological and molecular techniques, (ii) to determine the mechanisms of TG1 in controlling wheat crown rot disease under salinity and Fg stress.
Materials and Methods
Fungal Inoculum Preparation
The salt tolerance strain of TG1 and Fg were obtained from the Laboratory of Plant Pathology, Gansu Agricultural University. TG1 and Fg were cultured on potato dextrose agar (PDA) in Petri dishes for 7 and 14 days at 25°C, respectively. The conidia suspensions of TG1 and Fg were prepared according to the method of Zhang et al. (2014). The conidial suspensions of TG1 (1.0 × 108 spores per ml) and Fg (5 × 108 spores per ml) were quantified and stored at 4°C.
Salt Concentration Preparation
Salt tolerance was tested according to Zhang S. et al. (2016) where one liter of liquid medium PDA was prepared and NaCl concentrations of 0, 50, 100, and 150 mM (0, 2.922, 5.844, and 8.766 g) were added and autoclaved at 121°C. The solutions were then poured into 8.5 cm Petri dishes.
Determination of the Activity of TG1 Against F. pseudograminearum Under Salinity Stress
The antagonistic activity of TG1 against F. pseudograminearum was tested in vitro with modified settings using a dual culture approach by Rahman et al. (2009). The salinity tolerance test was performed using a colony diameter growth approach in PDA media supplemented with 0–150 mM NaCl. In the dual culture approach, mycelial plugs (5 mm diameter) of Fg (7-day-old culture) and TG1 (3-day-old culture) were transferred in parallel to opposite sides of a PDA plate at a distance (1.5 cm) from the outer edge of the plate. The experimental design was completely randomized with two controls, positive control (medium inoculated with Fg and salt), negative control (medium inoculated with Fg without salt), and treatment (medium inoculated with TG1, Fg, and salt). Each of these groups consisted of six replicates. Plates were incubated after inoculation at 25 ± 1°C with intermittent light. Fg colony diameters and percentage of Fg colony growth inhibition (CGI) were determined from the first to the fourth day after incubation and calculated. CGI (%) = [(C − T)/(C − 5)] × 100, where C = growth of colony in control, T = growth of diameter in treatment and the 5 are the mycelial plugs.
Observation of the Mycoparasitic Effect of TG1 on F. pseudograminearum Using Microscope
The physical interactions between TG1 and F. pseudograminearum were monitored using a dual plate assay similar to that mentioned above at 150 mM salt stress. Cellophane fragments were removed from the interaction zone after contact (overgrowth), according to the protocols by Yassin et al. (2019). Using S-3400N Fully Automated VP Scanning Electron Microscope (Hitachi High Technologies America, Inc.) at Gansu Agricultural University, Lanzhou, China, imaging of the <2 mm of the interaction zone was achieved. Samples were dehydrated in 100% ethanol for 1 h, dried with carbon dioxide using a samdri®-790 critical point dryer (Tousimis, Rockville, MD, United States), and mounted in aluminum stubs with double-sided tape. The samples were then coated with a tiny layer of gold to make the surface electrically conductive using a Denton DESK II (JMB-3500VA). The electron scans showed the surface structure of the material. The beam settings for surface analysis were 5.0 kV and 1.5 nA, with a spot size of 150. For light microscopy, a wet-mounted glass slide was prepared and viewed under an inverted microscope (Accu Scope, Commack, NY) EXI-410.
Assessment of TG1 Mycoparasitism Genes
A dual plate assay was performed between TG1 and F. pseudograminearum. In a Petri plate containing PDA media with salt (150 mM salt stress) and covered with parafilm, 5 mm mycelial plugs of the pathogen and TG1 were equally spaced following the approach of Lopes et al. (2012). The plates were incubated in the dark at 25 ± 1°C. TG1 mycelia were harvested at three-time points: before contact with F. pseudograminearum mycelia (T1), on contact (T2), and after contact (overgrowth) (T3), as defined (Atanasova et al., 2013). The control consisted of TG1 facing itself in a salt medium. Following the method of Pimentel et al. (2020), the induction of mycoparasitism-related genes in TG1 was investigated by quantitative RT-PCR. The selected genes were β-1, 6-glucan synthase (PP4), endochitinase precursor (PH-1), and chitinase (chi18-15). The α-tubulin gene was used as a reference gene. The primers used in the experiments were designed according to the NCBI candidate protein sequences of Trichoderma EST. The primer sequences and NCBI genes ID are listed in the Supplementary Table 1. Total ribonucleic acid (RNA) was extracted from the collected samples according to the instructions of the manufacturer of the fungal RNA kit (OMEGA Bio-Tek). The quantity and purity of the isolated RNA were analyzed using a NanophotometerTM (IMPLEN, Schatzbogen, Germany). The A260/A280 ratio indicated that the RNA was free from protein contamination. First-strand cDNA (Tiangen Biotechnology, Beijing, China) was synthesized using Revert AidTM First Strand cDNA Synthesis Kit. qRT-PCR was performed using 2× M5 HiPer Real-time PCR Supermix with Low Rox (Mei5 Biotechnology, Co., Ltd., Beijing, China). According to the manufacturer’s instructions, the 20 μl reaction mixture contained 0.5 μl of each primer, 1.0 μl of cDNA, 10 μl of 2× M5 HiPer Realtime PCR Super mix with Low Rox and 8.0 μl of ddH2O qRT-PCR was performed using the following thermal profile: 95°C for 30 s, 40 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 30 s. Three technical replicates were used for each gene. The 2–ΔΔCt method (Livak and Schmittgen, 2001) was used to measure the relative expression of each target gene.
Plant Material and Treatment Conditions
The wheat (Triticum aestivum L.) cultivar ‘Yongliang 4’ provided by Gansu Academy of Agricultural Sciences (Lanzhou, China) was used in all the experiments. The cultivar has been well adapted to the major wheat-growing areas of northwest China. The biocontrol potential of TG1 against F. pseudograminearum inoculum was tested in artificial saline soil according to the method of Gond et al. (2015). Wheat seeds of equal sizes were surface sterilized with 1% (v/v) NaOCl for 5 min and were rinsed with sterile water six to ten times after disinfection. Thereafter, wheat seeds were soaked in (i) TG1 suspension (1.0 × 108 spores per ml) only, (ii) TG1 suspension plus Fg suspension (5 × 108 spores per ml), (iii) Fg suspension only, and (iv) sterile water only for 12 h. Seeds were air-dried overnight under aseptic conditions before sowing, according to Zhang et al. (2019b).
Effect of TG1 on Wheat Seeds Emergence Under F. pseudograminearum and Salt Stress
Fungi-treated wheat seeds and control seeds were exposed to artificial saline soil at 0, 50, 100, and 150 mM NaCl. The soil used for the study was collected from an agricultural crop field in Lanzhou, China (36.061°N, 103.834°E, and 1,518 m above sea level) had a sandy loam texture. Then completely randomized experimental design with two factorial arrangements having inoculation of TG1 and Fg (plus or minus) as the main variable and salinity as the second variable. For each procedure, 6 plastic pots were used (9 cm in diameter and 10.5 cm in depth), each containing 1 kg of air-dried sterile saline soil. Twenty uniform seeds were sown 1 cm deep in the soil and lightly covered. Plants were irrigated every 24 h with non-saline water and maintained at a constant temperature of 25°C ± 0.5, with supplemental day/night lighting of 16/8 h and relative humidity of 65%. Seedling emergence was counted according to the method Oluwaranti et al. (2015) and the percentage of seed emergence potential (EP%) was determined as follows:
Based on the formulation of Niu et al. (2013) with minor modification, emergence index [EI (%) = NESi/Ti × 100], where NESi is the number of emerged seeds in a given time and Ti is the incubation time, and emergence rate [ER (%) = (NSE/TNS) × 100], where NSE is the number of emerged seeds 5 days after sowing and TNS is the total number of seeds in each pot.
Growth Parameters
After 28 days of NaCl treatment, the wheat seedlings were harvested. The shoots and roots of wheat seedlings were removed, washed three times with distilled water, dried, and weighed. Root length and weight were measured with the meter rule and weighing balance. To determine the dry weight, all samples of wheat shoots and roots were oven-dried at 105°C for 30 min and then held at 80°C to maintain a constant weight before being weighed. Each preservation and control was performed six times. The relative water content (RWC) of the shoots and roots was measured according to Tian and Philpot (2015). RWC (%) = [(FW − DW)/FW] × 100, where RWC represents relative water content, FW represents fresh weight, and DW represents dry weight.
Disease Assessment
Wheat crown rot disease index was recorded 28 days after sowing. A disease index (DI) based on crown rot, yellowing, and chlorosis of cotyledons and leaves at 28 days was used to classify disease symptoms. The degree of the disease was graded using one of five scales adapted from Zhang et al. (2015) where; 0 indicates that there is no disease; 1 = trace to 10% discoloration of the first leaf sheath; 2 = 11 percent −25 percent discolored first leaf sheath; 3 = 26 percent −50 percent discolored first leaf sheath; 4 = 50 percent discolored first leaf sheath or necrotic second leaf sheath; 5 = third leaf sheath necrotic or entire plant badly to entirely rotten.
Chlorophyll Content Determination
Following the procedure of Miazek and Ledakowicz (2013), chlorophyll was extracted with methanol. A new 0.2 g wheat seedling leaves was homogenized with 10 ml of methanol. At 665 and 652 nm absorbance, the content of chlorophyll was evaluated in a dual-wavelength spectrophotometer (EPOCH2 Plate Reader, BioTek, United States). This was repeated six times.
Lipid Peroxidation and H2O2 Measurements
At 28 days after wheat seeds treatments, the root samples were used for oxidants investigations. Oxidants activity such as MDA and H2O2 were investigated according to the manufacturer’s protocol using the assay kits provided (Solarbio, China). The absorbance of the MDA sample was measured at three different wavelengths 450, 532, and 600 nm, and H2O2 at 415 nm using a spectrophotometer (EPOCH2 Plate Reader, BioTek, United States). The content of MDA and H2O2 were expressed as μmol g–1 FW. This was repeated six times.
Antioxidant Enzymes Activities
At 28 days after wheat seeds treatments, the root samples were used for antioxidants investigations. The antioxidants activity of SOD (EC 1.15.1.1), POD (EC 1.11.1.7), PAL (EC 4.3.1.5), and CAT (EC 1.11.1.6) were measured according to the manufacturer’s protocol using the assay kits provided (Solarbio, China). SOD was measured at 560 nm, POD at 470 nm, PAL at 290 nm, and CAT at 240 nm, respectively, using a spectrophotometer (EPOCH2 Plate Reader, BioTek, United States). This was repeated six times.
Determination of Endogenous SA in Wheat Seedlings
Free SA determination in wheat leaves was performed by high-pressure-liquid chromatography (HPLC) (Shimadzu Prominence LC System, France) following the method of Allasia et al. (2018) with slight modification where 0.1 g of the leaf sample was homogenized using liquid nitrogen. Separations by HPLC were performed on a C18 column (250 × 4.6 mm, 5 μm) using a linear aqueous MeOH gradient from 10 to 82% (v/v), at a flow rate of 1 ml min−1, over 30.4 min. Quantify with fluorimetric detection (measured at 305 nm; emission at 407 nm) and determine areas under the corresponding peaks of the standard [2-Methoxybenzoic acid (o-Anisic acid, OAA; internal standard)]. Briefly, stock solution of 152 mg OAA in 10 ml 70% aqueous EtOH (v/v) and diluted 1:1, 000 in ultra-pure water. Using the peak area, the standard curve and linear equation were used to determine the amount of free SA.
Extraction of Total RNA and Analysis of Gene Expression by Quantitative Real-Time Reverse Transcriptase (qRT-PCR)
Total RNA extraction and analysis of 100 mg wheat seedlings root exposed to different levels of NaCl stress and pathogen infection were performed using the E.Z.N.A.® plant RNA kit (OMEGA Bio-Tek, China) (White et al., 2008; Xie et al., 2013). The quantity and quality of isolated RNA and qRT-PCR were analyzed by the same procedure previously used in the analysis of mycoparasitic gene expression. Following the methodology of Zhang S. et al. (2016), unique primers for the bread wheat genes SOD, POD, and CAT and the internal control actin were used to amplify amplicons specific to wheat seedlings. The above genes were selected based on their role in wheat seedlings under stress in previous studies (Asthir et al., 2011). Primers used in the experiments were designed using Primer Express 3.0 software, which amplifies target genes according to the sequences of candidate proteins available in NCBI wheat EST (Wang et al., 2014). Using the previously described method for mycoparasitic gene expression, the relative expression of antioxidant genes (SOD, POD, and CAT) and the expression level of plant defense-related genes [tyrosine-protein kinase (PR2), PR1-2 pathogenesis-related protein, phenylalanine ammonia-lyase (PAL), and chitinase class I (CHIA1)] were assessed by quantitative qRT-PCR. Primer sequences and NCBI gene IDs for all genes can be found in the Supplementary Table 1.
Statistical Analysis
The data were tested in each experiment included TG1 strain on controlling F. pseudograminearum and inducing salinity tolerance in wheat seedlings. Data were analyzed using two-way ANOVA in SPSS Version 16.0 (SPSS Inc., Chicago, IL, United States), and mean comparisons were made using Duncan’s new multiple range test and the significance was considered at P < 0.05.
Results
Salinity Tolerance and in vitro Colony Growth Inhibition
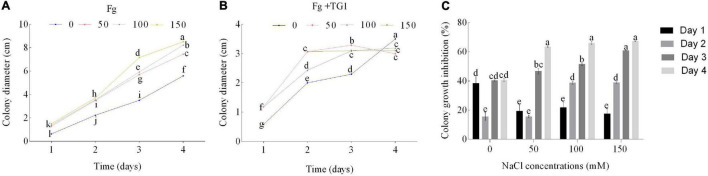
Salt stress and TG1 significantly (p < 0.05) affected the colony growth of Fg at different days after incubation (Figures 1A,B). The average inhibitory rate of TG1 against Fg was 33.86%, 36.32%, 44.59%, and 46.62%, respectively, for the four NaCl treatments (0, 50, 100, and 150 mM NaCl) (Figure 1C). Additionally, an increase in NaCl concentration increased the inhibitory rate of TG1 against the Fg colony growth at 4 days under different concentrations of salt stress (Figures 1C, 2A,B).
FIGURE 1.
Effect of T. longibrachiatum (TG1) and salt stress on F. pseudograminearum (Fg) growth. (A) colony diameter of Fg only, (B) colony diameter of Fg after inoculation with TG1, and (C) colony growth inhibition of TG1 against Fg under different salt stresses (0, 50, 100, and 150 mM NaCl) at different days. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA.
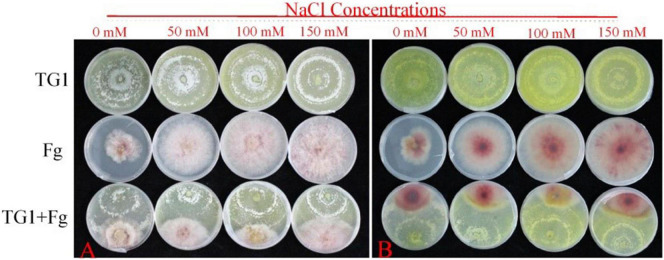
FIGURE 2.
Effect of different NaCl concentrations on colony growth and inhibition between T. longibrachiatum (TG1) and F. pseudograminearum (Fg) at 4 days after inoculation using dual plate assay (0–150 mM of NaCl concentrations). (A) front colony, and (B) reverse colony.
Microscopic Observations of Mycoparasitism
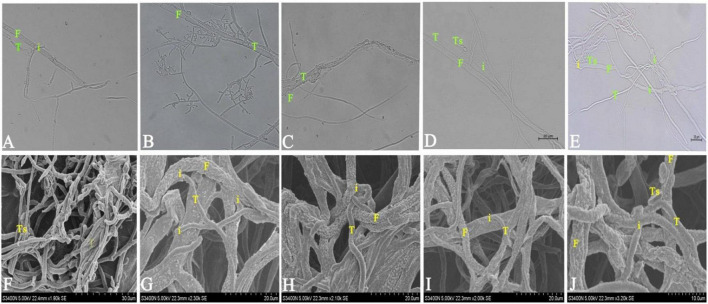
Light microscopy revealed that TG1 wrapped around the host hyphae of Fg and formed appressoria and hook-like structures that allowed firm attachment to the fungal host (Figure 3A). Growth of TG1 conidia was observed in the fungal host indicating the use of the fungal host as a nutrient source (Figures 3B,C). Visible disruption and decomposition of Fg hyphae by TG1 was observed coupled with a parallel growth in close hyphal association, also suggesting mycoparasitism. Germinating spore tubes of TG1 were found in the hyphae of Fg (Figures 3D,E). Again, the strain TG1 showed a mycoparasitic mechanism by invading the hyphae of Fg (Figures 3F–J).
FIGURE 3.
Mycoparasitism of T. longibrachiatum (TG1) (T) against F. pseudograminearum (Fg) (F) observed under light microscopy at 40× magnification and scanning electron microscopy (SEM) at 4 days after inoculation using dual plate assay at 150 mM NaCl concentration. (A) TG1wrapped around F. pseudograminearum hyphae with obvious degradation of F. pseudograminearum cell wall. (B) TG1 conidial growth internally and externally on F. pseudograminearum hyphae. (C) TG1 hyphal disruption, penetration and breakage of F. pseudograminearum hyphae. (D) TG1 spore germ tube (Ts) growing on F. pseudograminearum hyphae. (E) F. pseudograminearum hyphae looped by TG1 hyphae and TG1 hyphae extending parallel to F. pseudograminearum showing hyphal depression. (F–H) SEM images of TG1 (T) coiling the hyphae of F. pseudograminearum. (I,J) SEM images of TG1 coiling and invading the hyphae of F. pseudograminearum. Ts represent Trichoderma spore and (i) represent the point of interaction.
Mycoparasitism Genes
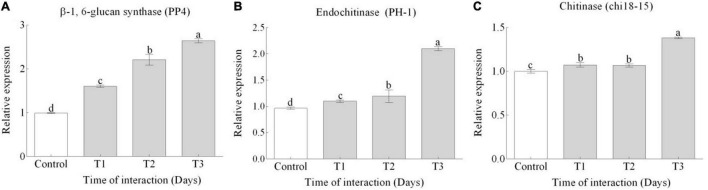
In response to inoculation of TG1 with and Fg, significant (p < 0.05) upregulation of CWDEs was observed in TG1 at 150 mM salt stress. The expression of β-1, 6-glucan synthase (PP4) (Figure 4A), endochitinase precursor (PH-1) (Figure 4B), and chitinase (chi18-15) (Figure 4C) genes increased by 1.6, 1.9, and 1.3-fold, respectively, at T3 compared to T1. In appressoria and fast-growing biotrophic hyphae, CWDEs are required for cell wall stiffness, and their rigorous upregulation during biocontrol activity represents a strategy to enhance immunity and mycoparasitism.
FIGURE 4.
Relative expression levels of (A) β-1, 6-glucan synthase (PP4), (B) endochitinase (PH-1), and (C) endochitinase (chi18-15) genes were measured by qRT-PCR during the interaction of T. longibrachiatum (TG1) and F. pseudograminearum (Fg) in a dual plate assay at 150 mM salt stress. Gene expression was evaluated at three different interaction time points at Days 3 (T1), 7 (T2), and 14 (T3) after the interaction. The 2–ΔΔct method was used for the relative expression of each target gene using the control and α-tubulin as the reference gene. Small bars represent the standard errors of the means. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA.
Effect of TG1 on Wheat Seed Growth Under F. pseudograminearum and Different Salt Stresses
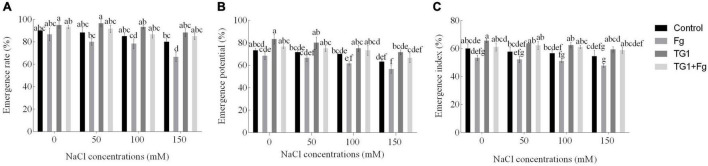
Compared to the treatments with sterile water (control), the seeds treated with TG1 increased emergence rate (ER) (Figure 5A) by an average of 8.71%, emergence potential (EP) (Figure 5B) by 11.39%, and emergence index (EI) (Figure 5C) by 13.19%, in the four NaCl treatments (0, 50, 100, and 150 mM). However, seeds treated with the combination TG1 + Fg increased ER by an average of 15.10%, EP by 15.32%, and EI by 19.15% in all four NaCl treatments compared to the Fg treatment alone. Without TG1 treatment, ER, EP, and EI of the control decreased significantly with increasing NaCl concentration.
FIGURE 5.
Effect of T. longibrachiatum (TG1) on wheat seeds (A) emergence rate, (B) emergence potential, and (C) emergence index under F. pseudograminearum (Fg) and salt stress. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA. Emergence rate, potential, and index were determined after treatment, respectively. Control treatments represent wheat seedlings pretreated with distilled water only; TG1 represents wheat seeds pretreated with the suspension of T. longibrachiatum (TG1); TG1 + Fg represents wheat seeds pretreated with T. longibrachiatum (TG1) and F. pseudograminearum (Fg); and Fg represents wheat seeds pretreated with F. pseudograminearum (Fg) for 12 h before planting with different NaCl treatments (0, 50, 100, and 150 mM).
Plant Biomass Accumulations and Relative Water Content
Salt and pathogenic stress significantly (p < 0.05) affected fresh weight (FW), dry weight (DW), and relative water content (RWC) of shoot and root of wheat seedlings (Table 1). Compared with the control, TG1-treated seedlings increased FW and DW of the shoot by an average of 36.53% and 20%; the root FW, DW, and RWC by 25.59%, 6.35%, and 11.16%, respectively, across the four NaCl treatments levels (from 0 to 50, 100, and 150 mM). However, TG1 + Fg-treated seedlings increased FW and DW of the shoot by an average of 63.37% and 43.47%; the root FW, DW, and RWC by 56.20%, 35.89%, and 12.05%, respectively, across the four NaCl treatment levels, compared with Fg treatment alone. Increasing NaCl concentration along with pathogen infection without treatment with strain TG1 decreased seedling FW, DW, and RWC.
TABLE 1.
Effect of T. longibrachiatum (TG1) on wheat seedling biomass and relative water content under F. pseudograminearum (Fg) and different salt stresses.
| NaCl (mM) | Treatment |
Wheat shoot
|
Wheat root
|
||||
| Fresh weight | Dry weight | Relative water | Fresh weight | Dry weight | Relative water | ||
| (g plant–1) | (g plant–1) | content (%) | (g plant–1) | (g plant–1) | content (%) | ||
| 0 | Control | 0.600.03bcd | 0.100.00c | 83.330.24abcd | 0.250.04c | 0.090.00b | 64.001.03abcd |
| Fg | 0.500.05fg | 0.090.00d | 82.001.52bcde | 0.210.00de | 0.080.00c | 61.900.05abcd | |
| TG1 | 0.760.03a | 0.120.01a | 84.210.032abcd | 0.300.00a | 0.100.01a | 66.670.03ab | |
| TG1 + Fg | 0.670.02b | 0.110.00b | 83.580.46abcd | 0.280.03b | 0.100.00a | 64.290.02abc | |
| 50 | Control | 0.580.02cdef | 0.100.00c | 82.760.44abcde | 0.220.06d | 0.090.03b | 59.091.01cde |
| Fg | 0.430.03h | 0.080.01e | 81.401.57cde | 0.180.00gh | 0.070.00d | 61.110.15bcd | |
| TG1 | 0.750.00a | 0.110.01b | 85.330.85a | 0.270.00b | 0.090.03b | 67.670.23a | |
| TG1 + Fg | 0.640.02bc | 0.100.01c | 84.380.90abc | 0.240.03c | 0.090.02b | 62.500.12abcd | |
| 100 | Control | 0.520.05efg | 0.090.00d | 82.690.78abcde | 0.190.04fg | 0.080.00c | 57.890.09de |
| Fg | 0.320.01i | 0.060.01f | 81.250.69de | 0.130.00j | 0.060.01e | 53.850.32e | |
| TG1 | 0.590.02cde | 0.090.00d | 84.750.89ab | 0.240.00c | 0.080.02c | 66.670.21ab | |
| TG1 + Fg | 0.560.03cdef | 0.100.01c | 82.141.13bcde | 0.220.03d | 0.090.01b | 59.090.03cde | |
| 150 | Control | 0.300.01i | 0.060.00f | 80.002.28ef | 0.150.00i | 0.070.01d | 53.330.15e |
| Fg | 0.230.02j | 0.050.00g | 78.261.90f | 0.090.00k | 0.050.00f | 44.440.05f | |
| TG1 | 0.530.06def | 0.090.00d | 83.021.38abcde | 0.200.00ef | 0.080.00c | 60.000.01cd | |
| TG1 + Fg | 0.450.01gh | 0.080.00e | 82.220.30bcde | 0.170.03h | 0.070.01d | 58.820.02cde | |
Data are mean ± standard error of replicates in a column followed by different letters are significantly different at P < 0.05 based on Duncan’s multiple range test using two-way ANOVA. The treatments are detailed in the footnote of Figure 5.
Effect of TG1 on Wheat Seedlings Growth, Chlorophyll Content and ROS Accumulation Under F. pseudograminearum (Fg) and Different Salt Stresses
Salt and pathogenic stress significantly (p < 0.05) affected shoot height and root length of wheat seedlings (Table 2). Increasing NaCl concentration along with pathogen stress decreased shoot height and root length. However, TG1 treatment increased shoot height and root length of wheat seedlings irrespectively of the presence of the pathogen or different NaCl concentrations (from 0 to 50, 100, and 150 mM). Compared with the control, shoot height and root length increased by an average of 22.76% and 21.35%, respectively, in the seedlings treated with the strain TG1 at the different NaCl concentrations. However, the seedlings treated with TG1 + Fg showed an average increase of 27.21% and 25.21% in shoot height and root length, respectively, at the different NaCl concentrations compared to the seedlings treated with Fg alone.
TABLE 2.
Effect of T. longibrachiatum (TG1) on wheat seedlings growth, chlorophyll content and ROS accumulation under F. pseudograminearum (Fg) and different salt stresses.
| NaCl concentration (mM) | Treatment | Shoot length | Root length | Chlorophyll content | MDA content in root | H2O2 content in root |
| (cm) | (cm) | (mg g–1) | (μmol g–1 FW) | (μmol g–1 FW) | ||
| 0 | Control | 27.13 ± 1.89bc | 14.87 ± 0.38bc | 1.37 ± 0.11bcd | 0.86 ± 0.02ghi | 0.99 ± 0.01fg |
| Fg | 26.10 ± 0.06cd | 13.27 ± 1.13def | 1.33 ± 0.10bcde | 1.01 ± 0.01fg | 1.16 ± 0.11f | |
| TG1 | 31.83 ± 0.77a | 18.03 ± 0.62a | 1.72 ± 0.13a | 0.70 ± 0.05i | 0.55 ± 0.01h | |
| TG1 + Fg | 29.77 ± 0.18ab | 15.27 ± 0.49bc | 1.63 ± 0.02ab | 0.74 ± 0.08hi | 0.63 ± 0.10h | |
| 50 | Control | 25.67 ± 0.64cd | 14.20 ± 0.06bcde | 1.32 ± 0.09bcd | 1.10 ± 0.05f | 1.17 ± 0.00f |
| Fg | 25.03 ± 0.33cd | 13.17 ± 0.07def | 1.26 ± 0.06cdef | 1.65 ± 0.05d | 1.58 ± 0.04e | |
| TG1 | 31.90 ± 1.47a | 17.57 ± 0.03a | 1.63 ± 0.05ab | 0.86 ± 0.01ghi | 0.75 ± 0.00gh | |
| TG1 + Fg | 30.87 ± 1.69b | 15.13 ± 0.03bc | 1.54 ± 0.13abc | 0.89 ± 0.01gh | 0.84 ± 0.03gh | |
| 100 | Control | 23.97 ± 1.05cde | 12.70 ± 0.45efg | 1.14 ± 0.07def | 1.67 ± 0.06d | 1.89 ± 0.04d |
| Fg | 23.20 ± 0.74de | 11.30 ± 0.06g | 0.99 ± 0.03f | 2.52 ± 0.03b | 2.35 ± 0.27c | |
| TG1 | 31.73 ± 1.13a | 15.57 ± 0.14b | 1.51 ± 0.03abc | 1.36 ± 0.11e | 1.25 ± 0.02f | |
| TG1 + Fg | 30.63 ± 1.62a | 13.97 ± 0.27cde | 1.36 ± 0.16bcd | 1.41 ± 0.08e | 1.28 ± 0.02f | |
| 150 | Control | 21.13 ± 1.25e | 12.17 ± 0.19fg | 1.06 ± 0.05ef | 2.21 ± 0.07c | 3.82 ± 0.07b |
| Fg | 16.87 ± 1.49f | 8.97 ± 1.02h | 0.68 ± 0.06g | 4.04 ± 0.02a | 5.22 ± 0.03a | |
| TG1 | 24.73 ± 0.28cd | 14.33 ± 0.22bcd | 1.43 ± 0.04abcd | 1.34 ± 0.02e | 2.11 ± 0.21cd | |
| TG1 + Fg | 23.03 ± 0.23de | 13.20 ± 0.25def | 1.25 ± 0.14cdef | 1.65 ± 0.03d | 3.71 ± 0.03b |
Data are mean ± standard error of replicates in a column followed by different letters are significantly different at P < 0.05, based on Duncan’s multiple range test using two-way ANOVA. The treatments are detailed in the footnote of Figure 5.
Compared to seedlings treated with Fg alone, TG1 treatment increased chlorophyll content by an average of 55.39% at the four NaCl stresses. However, the combined TG1 + Fg treatment increased the chlorophyll content of wheat seedlings by 37.14% and 85.38% at 100 and 150 mM NaCl stress, respectively (Table 2).
MDA and H2O2 content in wheat seedlings treated with control and Fg increased significantly with the increase in NaCl concentration. Averaged over the four NaCl concentration treatments (from 0 to 50, 100, and 150 mM), seedlings treated with strain TG1 decreased MDA and H2O2 content in roots by 24.28% and 39.79%, respectively, compared to the control; similarly, the combination of TG1 + Fg treated seedlings decreased MDA and H2O2 content by 44.07% and 41.75%, respectively, compared to seedlings treated with Fg alone. The extent of MDA and H2O2 content was greater in seedlings treated with Fg at 150 mM NaCl concentration than at 50 and 100 mM NaCl concentrations. Strain TG1 decreased both MDA and H2O2 content with or without the pathogen and NaCl stress (Table 2).
Crown Rot, Seedling Blight, and Seedling Death
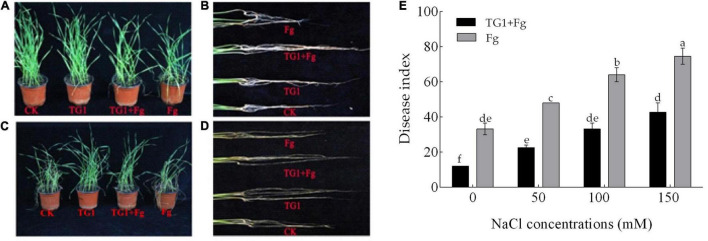
The positive effect of TG1 on reducing the disease index of crown rot was observed when the seeds were treated with BCA. However, the severity of F. pseudograminearum increased significantly (p < 0.05) in saline soils compared to non-saline soils (Figure 6E). The combined treatment with the antagonist and the pathogen (TG1 + Fg) reduced disease index in wheat seedlings by 64%, 52.78%, 47.92%, and 42.86%, respectively, compared to the pathogen alone across the four NaCl concentrations. The conidiospores of the pathogen germinated and invaded the xylem and pith of both the crown and root, developed a reddish-brown or white discoloration (Figure 6B). Salinity and pathogen stress caused the crown to turn from green to dull yellow, causing dysfunction, and reducing water and nutrient uptake (Figure 6D). Seedling death, root rot and crown rot were observed under pathogen and salinity stress at 150 mM NaCl compared to 0 mM NaCl concentration (Figures 6A,C).
FIGURE 6.
Effect of T. longibrachiatum (TG1) on wheat seedling disease index at 28 days after inoculation under F. pseudograminearum (Fg) and different NaCl stress. Panels (A,B) represent treatments without NaCl; (C,D) represent treatments with 150 mM of NaCl stress; and (E) represents the disease index across the four NaCl concentrations (0, 50, 100, and 150 mM). Small bars represent the standard errors of the means. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA. The treatments are detailed in the footnote of Figure 5.
Endogenous SA Content and PAL Activity
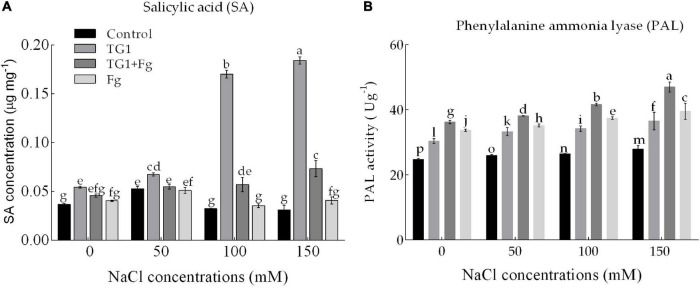
NaCl and Fg stress significantly (p < 0.05) affected SA content and PAL activity in wheat seedlings (Figures 7A,B). Compared to the control, seeds treated with strain TG1 increased SA by 47.96% at 0 mM NaCl. However, TG1 + Fg treated seedlings increased SA content by an average of 40.56% compared to Fg treated seedlings (Figure 7A).
FIGURE 7.
Effect of T. longibrachiatum (TG1) on (A) salicylic acid content and (B) phenylalanine ammonia-lyase (PAL) activity of wheat seedlings subjected to F. pseudograminearum (Fg) and different salinity stress at 28 days after inoculation. Small bars represent the standard errors of the means. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA. Treatments are detailed in the footnote of Figure 5.
Fg and NaCl stress significantly affected the activity of PAL. Fg-treated seedlings increased the activity of PAL compared with the control with or without NaCl stress. Averaged over the four levels of NaCl concentration treatment, seeds treated with TG1 + Fg increased the activity of PAL by 11% compared to seedlings treated with Fg alone. Similarly, an increase in NaCl concentration increased the PAL activity of TG1 compared with the control. Across the four NaCl concentration treatments, seedlings treated with TG1 increased the activity of PAL by an average of 28% compared with the control (Figure 7B).
Antioxidant Enzymes Activities and Gene Expression
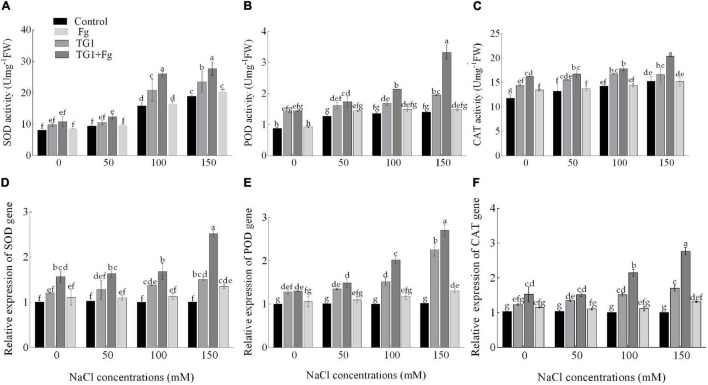
The combined biotic and abiotic stressors had effects on antioxidant enzyme activity. At all four NaCl concentrations (from 0 to 50, 100, and 150 mM), antioxidant enzyme activity increased with an increasing NaCl concentration. Compared with the control, wheat seedlings treated with TG1 increased SOD activity by 32% and 24% at 100 and 150 mM NaCl concentrations, respectively. However, wheat seedlings treated with TG1 increased POD and CAT at all four NaCl concentrations by an average of 38% and 17%, respectively, compared to the control. Similarly, wheat seedlings treated with the combination TG1 + Fg increased the activity of SOD, POD, and CAT by an average of 38%, 61%, and 24.96%, respectively, compared with Fg treatment alone at the four NaCl concentrations (Figures 8A–C).
FIGURE 8.
Effect of T. longibrachiatum (TG1) on SOD (A), POD (B), and CAT (C) activities and relative expressions of SOD (D), POD (E), and CAT (F) genes in the root of wheat seedlings under F. pseudograminearum (Fg) and different salt stress at 28 days after inoculation. Small bars represent the standard errors of the means. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA. The treatments are detailed in the footnote of Figure 5.
Fg and NaCl stress-induced higher transcript levels of the genes SOD, POD, and CAT compared with control. Significant upregulation of the genes SOD, POD, and CAT was observed in response to TG1 treatment (Figures 8D–F). An increase in NaCl concentration along with Fg increased the expression levels of the genes compared with the house-keeping gene. Wheat seedlings treated with TG1 increased the transcript levels of SOD by 1.11 and 1.22-fold at 100 and 150 mM NaCl concentration, respectively, compared with 0 mM NaCl concentration in TG1 treatment. Similarly, the transcript level of POD increased by 1.76-fold in TG1-treated seedlings at 150 mM NaCl concentration compared to 0 mM NaCl concentration. At NaCl concentrations of 50, 100, and 150 mM, TG1-treated seedlings increased the transcription level of the gene CAT by an average of 1.24-fold compared to 0 mM NaCl concentration in TG1 treatment. The combined TG1 + Fg-treated wheat seedlings with 150 mM NaCl-stress increased the expression levels of SOD, POD, and CAT by 1.61, 2.07, and 1.81-fold, respectively, compared to 0 mM NaCl concentration in TG1+Fg treatment.
Plant Defense-Related Genes Expression
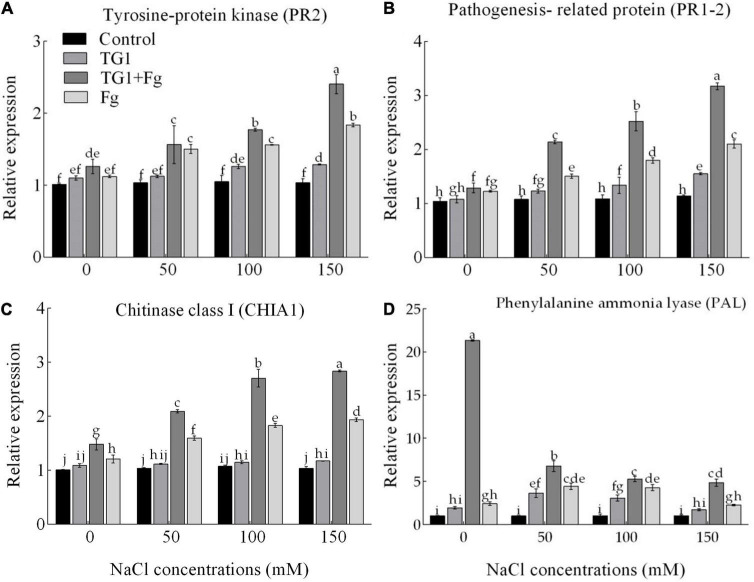
NaCl and Fg stress significantly (p < 0.05) affected the expression of the proteins associated with pathogenesis (Figures 9A–C). The wheat seedlings treated with strain TG1 increased the transcript levels of the tyrosine-protein kinase (PR2) by 1.15 and 1.17-fold at 100 and 150 mM NaCl concentration, respectively, compared to 0 mM NaCl concentration in TG1 treatment. Similarly, transcription of pathogenesis-related protein (PR1-2) increased by 1.24 and 1.44-fold at 100 and 150 mM of NaCl concentration, respectively, under TG1 treatment. Likewise, the transcript level of chitinase class I (CHIA1) in TG1-treated wheat seedlings increased by 1.08-fold at 150 mM NaCl concentration compared to 0 mM NaCl concentration in TG1 treatment. However, the PAL transcript level of TG1-treated wheat seedlings increased by 1.87 and 1.58-fold at 50 and 100 mM NaCl compared with 0 mM of NaCl concentration, respectively (Figure 9D). The combined TG1 + Fg treatment induced the transcription level of plant defense-related genes resulting in an increase in PR2, CHIA1, PR1-2, and PAL gene expression by an average of 1.15, 1.35, 1.37, and 3.40-fold, respectively, compared to Fg treatment.
FIGURE 9.
Effect of T. longibrachiatum (TG1) on relative expressions of tyrosine-protein kinase PR2 (A), pathogenesis-related protein PR 1-2 (B), chitinase class I CHIA1 (C), and phenylalanine ammonia-lyase (D) genes in the root of wheat seedlings under F. pseudograminearum (Fg) and different salinity stress at 28 days after inoculation. Small bars represent the standard errors of the means. Different lower case letters indicate significant differences at P < 0.05 in Duncan’s multiple range test using two-way ANOVA. Treatments are detailed in the footnote of Figure 5.
Discussion
High NaCl stress affected colony color and sporulation of both the antagonist and the pathogen. Previous studies found that salt stress decreased colony diameter growth, spore germination, morphology, conidial sporulation and environmental sensitivity of the fungus (Paulitz and Bélanger, 2001). Other studies revealed that F. solani was the most resistant among the Furarium to an increase in NaCl and temperature (Mandeel, 2006). The NaCl tolerance of the species was followed by the development of massive thick-walled, drought-resistant chlamydospores, indicating that the species is predominantly thermo-osmotolerant (Mandeel, 2006). Similarly, in this study, both the antagonist and the pathogen tolerated 150 mM NaCl stress. Furarium pseudograminearum tolerated high sodium chloride stress with slow growth compared to the antagonist. Both fungi developed salt-resistant hyphae, thick-walled conidia and chlamydospores. Interestingly, the antagonist T. longibrachiatum TG1 inhibited the growth of the pathogen under both normal and NaCl stress, which is helpful for its activity as a BCA. This result is in agreement with the findings of Narayan et al. (2017) whose study showed that T. viride and Trichoderma spp. significantly inhibited the mycelial growth of Fusarium.
In the present study, the microscopic analysis showed that TG1 wraps around the hyphae of F. pseudograminearum and grows parallel with the tight hyphae association, indicating mycoparasitism (Monteiro et al., 2010). Previous studies have shown that during mycoparasitism, Trichoderma wraps around the hyphae of the fungal host and forms appressoria and hook-like structures that allow firm attachment to the fungal host (Sachdev and Singh, 2020). Also in this study, during the interaction between T. longibrachiatum TG1 and F. pseudograminearum, coiling, disintegration of host hyphae, formation of appressoria and hook-like structures and penetration into the hyphae were observed. This suggests that the mechanisms of host recognition and lysis by Trichoderma may be different in different Trichoderma-host interactions. In addition, a possible CWDEs activity was detected with the degradation of F. pseudograminearum cell walls and hyphal deepening in the area infested by T. longibrachiatum TG1. These results were confirmed by the findings of Vinale et al. (2008) who reported that CWDEs can degrade or hydrolyze the cell wall of host pathogens and inhibit their growth.
Trichoderma spp. are well-known BCA with demonstrated activity against a range of pathogenic fungi (Harman and Uphoff, 2019). CWDEs, such as chitinases and glucanases, have been reported to be directly involved in the mycoparasitic activity of Trichoderma by facilitating lysis of the fungal host cell wall during parasitism, thus enabling invasion of the fungal host (Jaroszuk-Ściseł et al., 2019; Pimentel et al., 2020). Trichoderma longibrachiatum TG1 showed an increase in the expression level of CWDEs upon interaction with F. pseudograminearum. Significant expression of β-1, 6-glucan synthase (PP4), endochitinase (PH-1), and chitinase (chi 18-15) was conspicuous after contact between the two fungi, at a time when T. longibrachiatum TG1 overgrew F. pseudograminearum. The significant increase in expression of genes during the interaction of TG1 with F. pseudograminearum suggests that these enzymes are involved in the mycoparasitic activity of BCA against F. pseudograminearum. Previous studies have shown that genomic studies of mycoparasitic Trichoderma species have revealed higher numbers of chitinases and glucanases (Karlsson et al., 2017). In other studies, induction and secretion of β-1,3-glucanase and chitinase have been shown to play a role in the mycoparasitism of F. oxysporum by Trichoderma species (Rashad and Moussa, 2020) and mycoparasitism of Sclerotinia sclerotiorum by T. harzianum T-aloe (Zhang F. et al., 2016). Consistent with this study, other secondary metabolites produced by T. longibrachiatum TG1 species include these CWDEs (Lorito et al., 1994). In biocontrol, these enzymes are often assigned to both parasitism and antibiosis. CWDEs such as chitinase, β-1,3-glucanases, and cellulase, for example, were not only essential features of the mycoparasitism for colonizing the pathogen but also had a significant antifungal effect on the pathogen. This result is in agreement with that of Yuef et al. (2018) where the chitinase and glucanase activities of T. asperellum, T. virens, T. gamsii, and T. logibrachiatum inhibited 15% of mycelia growth of Phytophthora parasitica and 45% of F. oxysporum. The identification of biological control mechanisms that are highly effective against plant pathogens represents a tool that can be explored for plant disease management, especially in the case of important phytopathogens that are difficult to control, such as F. pseudograminearum.
In the pot experiment, the different NaCl concentrations and the pathogen decreased the emergence parameters of wheat seeds, which is clearly in accordance with the hypothesis of the study. Several previous studies have reported the adverse effects of salinity on germination and growth of plant seeds both in vitro and in the greenhouse (Azooz et al., 2013). Similarly, sodium chloride treatment was reported to inhibit root hair growth (Dey et al., 2004). Previous studies also showed that F. pseudograminearum infection increased from less than 20% 10 days after anthesis to more than 98% at maturity under significant environmental stress in both resistant and susceptible wheat cultivars, resulting in unacceptable standard germination and seed vigor (Argyris et al., 2003).
In the present study, the interaction of pathogen and salt stress resulted in poor seed germination and seed vigor. However, the combined treatment (TG1 + Fg) improved seedling emergence and vigor compared with the pathogen alone treatment. Moreover, the application of T. longibrachiatum TG1 reduced the deleterious effects of NaCl stress and the pathogen on wheat seedling growth, increasing shoot and root length and enhancing root hair formation, which is beneficial for stressed plants. These results were supported by Mastouri et al. (2012) who reported that application of T. harzianum T22 improved germination of tomato (Lycopersicum esculentum L.) seeds under abiotic and biotic stress conditions. Similarly, T. longibrachiatum T6 promoted the growth of wheat seedlings (T. aestivum L.) under NaCl stress by increasing shoot and root length (Zhang et al., 2019b). These results and the general observation show that Trichoderma treatments benefit plants most when they are stressed, support the theory that these beneficial fungi alleviate both biotic and abiotic plant stress.
In this study, T. longibrachiatum TG1 induced salt tolerance and reduced disease incidence (e.g., seedling blight, crown rot caused by F. pseudograminearum on wheat seedlings). In previous studies, Trichoderma was shown to promote plant root growth and nutrient solubilization (Contreras-Cornejo et al., 2009). The adverse effects of Fusarium wilt are caused by the blockage of xylem vessels leading to leaf senescence and reduced photosynthesis which in turn leads to lower crop yields as previously reported (Singh et al., 2017). The antagonist was an endophytic fungus that increased the relative water content and membrane protection. Infection with a pathogen usually triggers systemic acquired resistance (SAR), a general defense response, first in infected host cells, then in uninfected neighboring tissues. Similarly, the interaction of pathogen and salt stress increased the disease index of wheat seedlings due to the salt tolerance potential of the pathogen. The antagonistic effect of T. longibrachiatum TG1 against salinity and the pathogen seems to be related not only to direct competition and mycoparasitism but also to activation of plant defenses through the activation of SA, the synthesis of phytohormones and an increased number of ROS scavenging antioxidant enzymes and the maintenance of osmotic balance and metabolic homeostasis in wheat seedlings. This result was supported by Hasan et al. (2014) who found that the production of lytic enzymes by antagonists has been shown to control pathogenic fungi by competing for tissue nutrients, thus suppressing plant pathogens caused by Trichoderma spp.
The abiotic and biotic stresses in this study affected the chlorophyll of the seedlings and decreased the relative water content. Chlorophyll is an important molecule in photosynthesis, and plant bioregulators help to increase the quality of chlorophyll by maintaining cellular osmotic reactions (Burman et al., 2004; Seckin et al., 2009). The decrease in chlorophyll content in wheat under pathogen and salt stress may be due to increased chlorophyll degradation or decreased activity of chlorophyll biosynthesis enzymes (Singh et al., 2016; Mann et al., 2019). Larger decreases in chlorophyll concentration have also been associated with exacerbation of stress symptoms (Kumar et al., 2018). Previous studies have shown that salinity and F. pseudograminearum are known to affect photosynthetic processes in most plants by altering organelle ultrastructure, the concentration of various pigments, metabolites and enzymatic activities (Akram et al., 2018). However, under both stress conditions, the antagonist T. longibrachiatum TG1 maintained cells at an optimal hydration level by accumulating osmolytes, which maintained water uptake and increased tissue RWC (Yadav et al., 2020). Other studies have also shown that enhancement of gas exchange properties by endogenous SA through the application of T. longibrachiatum helps to improve photosynthetic efficiency and chlorophyll fluorescence, which enables plants to withstand environmental stress (Wang and Li, 2005). Osmolytes are stress tolerance systems that plants use to protect themselves from abiotic stress (Li and Dami, 2016). Similar to the current study, chlorophyll content increased when T. longibrachiatum TG1 and the pathogen were applied together, with the antagonist causing rapid root colonization due to its rapid growth and high salt tolerance. This was due to the delay of pathogenic stress and promotion of root development for water and nutrient uptake. In seedlings exposed to salt and pathogen stress, RWC in the plant decreased and photosynthetic structures were damaged due to reduced nutrient uptake.
Endogenous SA which enhances osmotic changes serves as a stress indicator that stabilizes macromolecules and protects against the effects of ROS (Ahmad et al., 2012; Lata et al., 2019). In both treatments, higher endogenous SA content correlated with higher stress intensity as shown by the results of this study (Khan et al., 2013). Previous studies have shown that abiotic and biotic stresses trigger the oxidation of acids in the lipid bilayer leading to a shift in the cell membrane (Carrasco-Ríos et al., 2013). In this study, MDA levels increased in wheat seedlings under saline conditions, while pathogen infection doubled the effects. Higher MDA levels were associated with increased electrolyte loss and H2O2 accumulation (Nandwal et al., 2007), which has long been used as a marker of stress tolerance due to lipid peroxidation. However, T. longibrachiatum TG1 induced SA to decrease fatty acid degradation, increase antioxidant enzyme activity and decrease MDA and H2O2 levels (Abdelkader et al., 2012). Moreover, wheat seedlings treated with the antagonist and pathogen with or without NaCl accumulated low H2O2 and MDA levels by increasing the activities of scavenging ROS antioxidant enzymes (SOD, POD, and CAT), compared to what was reported by Zhang et al. (2019a) where T. harzianum T-soybean increased scavenging antioxidant enzymes for ROS in cucumber under salt stress.
The expression levels of antioxidant genes were upregulated at all stress levels, which stimulated stress response signaling, increased shoot height and root length, and further increased biomass content in the seedlings treated with the antagonist and the pathogen (TG1 + Fg) compared seedlings treated with the pathogen alone and mock seedlings. This result is similar to that of Zhang et al. (2019c) where T. longibrachiatum T6 promoted the growth of wheat seedlings under salt stress through an antioxidant defense system. However, this study involves a different BCA strain that combats both salt and pathogen stress through enzymatic antioxidants, expression of protein enzymes associated with pathogenesis and induction of endogenous SA content of seedlings. Previous studies have shown that exogenous SA treatment increases the transcripts of genes encoding ascorbate and glutathione cycle enzymes (Chen et al., 2016), and overexpression of these genes conferred increased resistance to salt and chilling stress. SA, a phenolic phytohormone, regulates plant growth and production, photosynthesis, transpiration, ion absorption, and transport (Khan et al., 2014), and thus SA has shown positive responses in mitigating both pathogenic and osmotic stress (Chini et al., 2004). Similarly, the increase of endogenous SA by TG1 played an important role in the improvement of the root system, which allowed correction of salt-induced growth and improved biomass production by inducing the expression of enzymatic antioxidants and plant protective genes in plants. Our results are in agreement with those of Luan et al. (2020), who showed that Trichoderma isolates ThTrx5 confers salt tolerance to Arabidopsis by activating stress response signals and overexpressing of SOD, POD, and CAT, and increase root length and fresh weight of ThTrx5 transgenic plants.
Salicylic acid regulates the activities of various enzymes, such as enzymatic antioxidants and PAL, which are the main components of induced plant protection against biotic and abiotic stresses. In this study, the salicylic acid pathway was determined by the activity of PAL. Previous studies reported that PAL plays a central role in the hypersensitive response (HR) and SAR is associated with early signaling events in response to pathogens, such as activation of PR genes (Dong, 2001; Gruner et al., 2013). By catalyzing cell wall lignification, these enzymes play an important role in protecting plants from pathogens. Other studies have shown that rapid SAR-activation through the expression of various PR genes leads to a significant increase in resistance to pathogen infection (Qi et al., 2019). Pretreatment of wheat seedlings with T. longibrachiatum TG1 increased endogenous SA-content and stimulated cell wall degrading enzymes and plant cell wall lignification to reduce pathogen infection. Previous studies by Valifard et al. (2015) showed that salt stress could induce PAL expression (12 to 18 times) and subsequently increase PAL activity (42%–45%) and total phenolic accumulation (35%–43%) in the early hours after stress treatment. Again, Cuong et al. (2020) revealed that, treatment with various concentrations of NaCl (50, 100, and 200 mM) resulted in increased epicatechin levels but decreased accumulation of benzoic acid (TaPAL) in wheat sprouts compared with the control (0 mM). Upregulation of PAL and the enzymes PR1, PR1-2, and chitinase reduced the number of certain mycotoxin forms that accumulated in seedlings and induced salt tolerance. The current study results are in agreement with those of Pereira et al. (2014) who reported that T. harzianum ALL-42 appears to enhance the response of field beans to R. solani by increasing the expression of β-1–3-glucanase (glu1) and peroxidase (pod3) genes, compared to the host response when exposed to the pathogen alone. Similarly, cucumber roots colonized by T. harzianum T-203 showed increased activity of chitinase, β-1,3-glucanase, cellulase, and peroxidase 72 h after inoculation (Chatterton, 2010) which also supports the current result. Therefore, another mechanism by which T. longibrachiatum TG1 increases seedling salt tolerance and resistance to pathogen attack could be realized via the SAR pathway. These results are supported by those of Ali et al. (2018) who found that genes related to pathogenesis (PR) show basal expression under control conditions but increase dramatically after fungal infection both at the locally infected site and in uninfected parts of the host, triggering the SAR pathway.
Conclusion
Our results suggest that T. longibrachiatum TG1 is a plant growth-promoting fungus that can tolerate salt stress and control F. pseudograminearum in vitro and in vivo, and induce salinity tolerance of wheat seedlings by attenuating the negative effects of pathogen and salt stress. Rigorous physiological, biochemical, and molecular assays used in the study allowed us to explore the possible mechanisms and pathways in which TG1 attenuates the suppressive effects of pathogen and salt stress. The mechanism involves are; (i) mycoparasitism (ii) overgrowth and space competition (iii) antioxidant defense system and expression and increase of endogenous SA in stressed plants. The identification of biological control agents that are highly effective against salt stress and plant pathogens represents a tool that can be explored for plant disease management, especially in the case of important phytopathogens that are difficult to control, such as F. pseudograminearum. These results and the repeated observation show that the greatest benefit of Trichoderma treatments to plants occurs when they are under stress, lend credence to the concept that these beneficial fungi alleviate both biotic and abiotic plant stress. Our results provide a basis for future incorporation of biological control agents into strategies to control seedling blight, crown rot, and root rot of plants under salt stress.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
SB and TL performed the experiments, analyzed data, and wrote and edited the manuscript. SZ, BX, and AC-U conceived the idea and revised the manuscript. SZ designed the experiments, conceived the idea, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the College of Plant Protection research team, Gansu Agricultural University, China.
Abbreviations
- IAA
indole-3-acetic acid
- ROS
reactive oxygen species
- MDA
malondialdehyde
- H2O2
hydrogen peroxide
- PGPR
plant growth-promoting rhizobacteria
- RNA
ribonucleic acid
- ACC
1-aminocyclopropane-1-carboxylate
- SOD
superoxide dismutase
- POD
peroxidase
- CAT
catalase
- DNA
deoxyribonucleic acid
- ER
emergence rate
- EI
emergence index
- EP
emergence potential
- NaCl
sodium chloride
- Fg
Fusarium pseudograminearum
- SEM
scanning electron microscopy.
Funding
This study was funded by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University (project GSCS-2017-1); Fuxi Outstanding Talent Cultivation Program, Gansu Agricultural University (Project Gaufx-03J03); Scientific Research Start-up Funds for Openly recruited Doctors (project 2017RCZX-07); National Natural Science Foundation of China (project 31860526); and Gansu Provincial Science Fund for Distinguished Young Scholars (project 18JR3RA161).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.741231/full#supplementary-material
References
- Abdelkader A. F., Hassanein R. A., Ali H. (2012). Studies on effects of salicylic acid and thiourea on biochemical activities and yield production in wheat (Triticum aestivum var. Gimaza 9) plants grown under drought stress. Afri. J. Biotechnol. 11 12728–12739. [Google Scholar]
- Ahmad P., Kumar A., Ashraf M., Akram N. A. (2012). Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afri. J. Biotechnol. 11 2694–2703. [Google Scholar]
- Akram N. A., Iqbal M., Muhammad A., Ashraf M., Al-Qurainy F., Shafiq S. (2018). Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255 163–174. 10.1007/s00709-017-1140-x [DOI] [PubMed] [Google Scholar]
- Alahmad S., Simpfendorfer S., Bentley A., Hickey L. (2018). Crown rot of wheat in Australia: Fusarium pseudograminearum taxonomy, population biology and disease management. Australasian Plant Pathol. 47 285–299. 10.1007/s13313-018-0554-z [DOI] [Google Scholar]
- Ali S., Ganai B. A., Kamili A. N., Bhat A. A., Mir Z. A., Bhat J. A., et al. (2018). Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 212 29–37. 10.1016/j.micres.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Allasia V., Ponchet M., Quentin M., Favery B., Keller H. (2018). Quantification of salicylic acid (SA) and SA-glucosides in Arabidopsis thaliana. Bio-protocol 8:e2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris J., Van Sanford D., Tekrony D. (2003). Fusarium graminearum infection during wheat seed development and its effect on seed quality. Crop Sci. 43 1782–1788. 10.2135/cropsci2003.1782 [DOI] [Google Scholar]
- Asthir B., Koundal A., Bains N. (2011). Kinetic properties of cell wall bound superoxide dismutase in leaves of wheat (Triticum aestivum L.) following stripe rust (Puccinia striiformis) infection. Indian J. Biochem. Bio. 48 341–345. [PubMed] [Google Scholar]
- Atanasova L., Le Crom S., Gruber S., Coulpier F., Seidl-Seiboth V., Kubicek C. P., et al. (2013). Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics 14:121. 10.1186/1471-2164-14-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azooz M. M., Alzahrani A. M., Youssef M. M. (2013). The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (‘Vicia faba’L.). Australian J. Crop Sci. 7 2091–2100. [Google Scholar]
- Bolanos-Carriel C., Wegulo S. N., Baenziger P. S., Eskridge K. M., Funnell-Harris D., Mcmaster N., et al. (2020). Tri5 gene expression analysis during postharvest storage of wheat grain from field plots treated with a triazole and a strobilurin fungicide. Can. J. Plant Pathol. 42 547–559. 10.1080/07060661.2019.1700169 [DOI] [Google Scholar]
- Burman U., Garg B., Kathju S. (2004). Interactive effects of thiourea and phosphorus on clusterbean under water stress. Biol. Plant. 48 61–65. 10.1023/b:biop.0000024276.03834.8d [DOI] [Google Scholar]
- Carrasco-Ríos L., Rojas C., Pinto M. (2013). Contrasting physiological responses to high salinity between two varieties of corn ‘Lluteño’(salt tolerant) and ‘Jubilee’(salt sensitive). Chilean J. Agric. Res. 73 205–212. 10.4067/s0718-58392013000300001 27315006 [DOI] [Google Scholar]
- Chatterton S. G. D. (2010). Mechanisms of biological control of Fusarium root and stem rot of greenhouse cucumber by Gliocladium catenulatum. Ph.D. Thesis. Burnaby, BC: Biological Sciences Department-Simon Fraser University. [Google Scholar]
- Chen S. C., Zhao H. J., Wang Z. H., Zheng C. X., Zhao P. Y., Guan Z. H., et al. (2016). Trichoderma harzianum-induced resistance against Fusarium oxysporum involves regulation of nuclear DNA content, cell viability and cell cycle-related genes expression in cucumber roots. Eur. J. Plant Pathol. 147 43–53. 10.1007/s10658-016-0978-7 [DOI] [Google Scholar]
- Chini A., Grant J. J., Seki M., Shinozaki K., Loake G. J. (2004). Drought tolerance established by enhanced expression of the CC–NBS–LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. The Plant J. 38 810–822. 10.1111/j.1365-313x.2004.02086.x [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo H. A., Macías-Rodríguez L., Alfaro-Cuevas R., López-Bucio J. (2014). Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe Interact. 27 503–514. [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo H. A., Macías-Rodríguez L., Cortes-Penagos C., López-Bucio J. (2009). Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 149 1579–1592. 10.1104/pp.108.130369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelia P., Bogdan A. T., Ipate L., Chis A., Borbely M. (2011). Exogenous salicylic acid involvement in ameliorating the negative effect of salt stress in wheat (Triticum aestivum cv. crisana) plants in vegetative stage. J. Anal. Univ. din Oradea Fascicula Protecñ. Med. 7 137–146. [Google Scholar]
- Cuong D. M., Kwon S. J., Nguyen B. V., Chun S. W., Kim J. K., Park S. U. (2020). Effect of salinity stress on phenylpropanoid genes expression and related gene expression in wheat sprout. Agronomy 10:390. 10.3390/agronomy10030390 [DOI] [Google Scholar]
- Dey R., Pal K., Bhatt D., Chauhan S. (2004). Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 159 371–394. 10.1016/j.micres.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Dong X. (2001). Genetic dissection of systemic acquired resistance. Curr. Opin. Plant Biol. 4 309–314. 10.1016/s1369-5266(00)00178-3 [DOI] [PubMed] [Google Scholar]
- El-Allaf S., Lipps P., Madden L., Johnston A. (2001). “Effect of foliar fungicides and biocontrol agents on Fusarium head blight development and control in Ohio,” in Proceedings of the 2001 National Fusarium Head Blight Forum: Citeseer, Michigan State University, United States (US), (Kinkos, MI: Okemos Press; ), 49–53. [Google Scholar]
- Fu J., Liu Z., Li Z., Wang Y., Yang K. (2017). Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS One 12:e0179617. 10.1371/journal.pone.0179617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gond S. K., Bergen M. S., Torres M. S., White Jr, J. F. (2015). Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 172 79–87. 10.1016/j.micres.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Gruner K., Griebel T., Návarová H., Attaran E., Zeier J. (2013). Reprogramming of plants during systemic acquired resistance. Front. Plant Sci. 4:252. 10.3389/fpls.2013.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman G. E., Uphoff N. (2019). Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019:9106395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S., Gupta G., Anand S. (2014). Lytic enzymes of Trichoderma: their role in plant defense. Int. J. App. Res. Stud. 3 2–5. [Google Scholar]
- Hossain M. S., Elsayed A. I., Moore M., Dietz K. J. (2017). Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J. Exp. Bot. 68 1283–1298. 10.1093/jxb/erx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov S. V., Maathuis F. J. (2019). Plant salinity stress: many unanswered questions remain. Front. Plant Sci. 10:80. 10.3389/fpls.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivushkin K., Bartholomeus H., Bregt A. K., Pulatov A., Kempen B., De Sousa L. (2019). Global mapping of soil salinity change. Remote Sens. Environ. 231:111260. 10.1016/j.rse.2019.111260 [DOI] [Google Scholar]
- Jaroszuk-Ściseł J., Tyśkiewicz R., Nowak A., Ozimek E., Majewska M., Hanaka A., et al. (2019). Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 20:4923. 10.3390/ijms20194923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Atanasova L., Jensen D. F., Zeilinger S. (2017). “Necrotrophic mycoparasites and their genomes,” in The Fungal Kingdom, eds Heitman J., Howlett B. J., Crous P. W., Stukenbrock E. H., James T. Y., Gow N. A. R. (Washington, DC: ASM Press; ), 1005–1026. 10.1128/9781555819583.ch50 [DOI] [Google Scholar]
- Kazan K., Gardiner D. M. (2018). Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects. Mol. Plant Pathol. 19 1547–1562. 10.1111/mpp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. I. R., Asgher M., Khan N. A. (2014). Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 80 67–74. 10.1016/j.plaphy.2014.03.026 [DOI] [PubMed] [Google Scholar]
- Khan M. I. R., Iqbal N., Masood A., Per T. S., Khan N. A. (2013). Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 8:e26374. 10.4161/psb.26374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Sharma S. K., Lata C., Devi R., Kulshrestha N., Krishnamurthy S., et al. (2018). Impact of water deficit (salt and drought) stress on physiological, biochemical and yield attributes on wheat (Triticum aestivum) varieties. Indian J. Agric. Sci. 88 1624–1632. [Google Scholar]
- Lata C., Soni S., Kumar N., Kumar A., Pooja, Mann A., et al. (2019). Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Indian J. Agric. Sci. 89 1050–1053. [Google Scholar]
- Li S., Dami I. E. (2016). Responses of Vitis vinifera ‘Pinot gris’ grapevines to exogenous abscisic acid (ABA): I. Yield, fruit quality, dormancy, and freezing tolerance. J. Plant Growth Regul. 35 245–255. 10.1007/s00344-015-9529-2 [DOI] [Google Scholar]
- Lin Y., Liu T., Liu J., Liu X., Ou Y., Zhang H., et al. (2015). Subtle regulation of potato acid invertase activity by a protein complex of invertase, invertase inhibitor, and sucrose nonfermenting1-related protein kinase. Plant Physiol. 168 1807–1819. 10.1104/pp.15.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lopes F. A. C., Steindorff A. S., Geraldine A. M., Brandão R. S., Monteiro V. N., Júnior M. L., et al. (2012). Biochemical and metabolic profiles of Trichoderma strains isolated from common bean crops in the Brazilian Cerrado, and potential antagonism against Sclerotinia sclerotiorum. Fungal Biol. 116 815–824. 10.1016/j.funbio.2012.04.015 [DOI] [PubMed] [Google Scholar]
- Lorito M., Hayes C., Di Pietro A., Woo S., Harman G. (1994). Purification, characterization, and synergistic activity of a glucan 1, 3-beta-glucosidase and an N-acetyl-beta-glucosaminidase from Trichoderma harzianum. Phytopathology 84 398–405. 10.1094/phyto-84-398 [DOI] [Google Scholar]
- Luan J., Dong J., Song X., Jiang J., Li H. (2020). Overexpression of Tamarix hispida ThTrx5 confers salt tolerance to Arabidopsis by activating stress response signals. Int. J. Mol. Sci. 21:1165. 10.3390/ijms21031165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeel Q. A. (2006). Biodiversity of the genus Fusarium in saline soil habitats. Basic J. Microbiol. 46 480–494. 10.1002/jobm.200510128 [DOI] [PubMed] [Google Scholar]
- Mann A., Kaur G., Kumar A., Sanwal S. K., Singh J., Sharma P. (2019). Physiological response of chickpea (Cicer arietinum L.) at early seedling stage under salt stress conditions. Leg. Res. 42 625–632. [Google Scholar]
- Mastouri F., Björkman T., Harman G. E. (2012). Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interact. 25 1264–1271. 10.1094/mpmi-09-11-0240 [DOI] [PubMed] [Google Scholar]
- Miazek K., Ledakowicz S. (2013). Chlorophyll extraction from leaves, needles and microalgae: a kinetic approach. Int. J. Agric. Biol. Eng. 6 107–115. [Google Scholar]
- Miura K., Tada Y. (2014). Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 5:4. 10.3389/fpls.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro V. N., Do Nascimento Silva R., Steindorff A. S., Costa F. T., Noronha E. F., Ricart C. A. O., et al. (2010). New insights in Trichoderma harzianum antagonism of fungal plant pathogens by secreted protein analysis. Curr. Microbiol. 61 298–305. [DOI] [PubMed] [Google Scholar]
- Moya-Elizondo E. A., Jacobsen B. J. (2016). Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control 92 153–163. 10.1016/j.biocontrol.2015.10.006 [DOI] [Google Scholar]
- Nandwal A. S., Kukreja S., Kumar N., Sharma P. K., Jain M., Mann A., et al. (2007). Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J. Plant Physiol. 164 1161–1169. 10.1016/j.jplph.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Narayan O. P., Verma N., Singh A. K., Oelmüller R., Kumar M., Prasad D., et al. (2017). Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci. Reports 7 1– 11. 10.1038/s41598-017-12944-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Mi L., Li Y., Wei A., Yang Z., Wu J., et al. (2013). Physiological and biochemical responses of rice seeds to phosphine exposure during germination. Chemosphere 93 2239–2244. 10.1016/j.chemosphere.2013.07.074 [DOI] [PubMed] [Google Scholar]
- Oluwaranti A., Fakorede M., Adeboye F. (2015). Maturity groups and phenology of maize in a rainforest location. Int. Agric. Innov. Res. 4 124–127. [Google Scholar]
- Pasquali M., Beyer M., Logrieco A., Audenaert K., Balmas V., Basler R., et al. (2016). A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 7:406. 10.3389/fmicb.2016.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulitz T. C., Bélanger R. R. (2001). Biological control in greenhouse systems. Annu. Rev. Phytopathol. 39 103–133. [DOI] [PubMed] [Google Scholar]
- Pereira J. L., Queiroz R. M., Charneau S. O., Felix C. R., Ricart C. A., Da Silva F. L., et al. (2014). Analysis of Phaseolus vulgaris response to its association with Trichoderma harzianum (ALL-42) in the presence or absence of the phytopathogenic fungi Rhizoctonia solani and Fusarium solani. PLoS One 9:e98234. 10.1371/journal.pone.0098234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel M. F., Arnão E., Warner A. J., Subedi A., Rocha L. F., Srour A., et al. (2020). Trichoderma isolates inhibit Fusarium virguliforme growth, reduce root rot, and induce defense-related genes on soybean seedlings. Plant Dis. 104 1949–1959. 10.1094/pdis-08-19-1676-re [DOI] [PubMed] [Google Scholar]
- Purcǎrea C., Cachitǎ D., Petrus A., Pop L., Chis A. (2010). Exogenous salicylic acid treatments enhance tolerance to salinity of wheat (Triticum aestivum) plantlets. J. Acta Agraria Debreceniensis 5 34–38. 10.34101/actaagrar/i/8371 [DOI] [Google Scholar]
- Qi P.-F., Zhang Y. Z., Liu C. H., Chen Q., Guo Z. R., Wang Y., et al. (2019). Functional analysis of FgNahG clarifies the contribution of salicylic acid to wheat (Triticum aestivum) resistance against Fusarium head blight. Toxins 11:59. 10.3390/toxins11020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Zhang R., Xue C., Zhang S., Li S., Zhang N., et al. (2012). Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol. Fert. Soils 48 807–816. 10.1007/s00374-012-0675-4 [DOI] [Google Scholar]
- Rahman M., Begum M., Alam M. (2009). Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Microbiology 37 277–285. 10.4489/myco.2009.37.4.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramegowda V., Senthil-Kumar M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 176 47–54. 10.1016/j.jplph.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Rashad Y. M., Moussa T. A. (2020). “Biocontrol agents for fungal plant diseases management,” in Cottage Industry of Biocontrol Agents and Their Applications, eds Saleh M., Abu-hashim M., El-Wakeil N. (Cham: Springer; ), 337–363. 10.1007/978-3-030-33161-0_11 [DOI] [Google Scholar]
- Rubio M. B., Hermosa R., Vicente R., Gómez-Acosta F. A., Morcuende R., Monte E., et al. (2017). The combination of Trichoderma harzianum and chemical fertilization leads to the deregulation of phytohormone networking, preventing the adaptive responses of tomato plants to salt stress. Front. Plant Sci. 8:294. 10.3389/fpls.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev S., Singh R. P. (2020). “Trichoderma: a multifaceted fungus for sustainable agriculture,” in Ecological and Practical Applications for Sustainable Agriculture, eds Korstad J., Bauddh K., Singh R. P., Kumar S. (Singapore: Springer; ), 261–304. 10.1007/978-981-15-3372-3_13 [DOI] [Google Scholar]
- Saghafi D., Ghorbanpour M., Lajayer B. A. (2018). Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J. Soil Sci. Plant Nutrit. 18 253–268. [Google Scholar]
- Seckin B., Sekmen A. H., Türkan I. (2009). An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 28 12–20. 10.1007/s00344-008-9068-1 [DOI] [Google Scholar]
- Shiferaw B., Smale M., Braun H. J., Duveiller E., Reynolds M., Muricho G. (2013). Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 5 291–317. 10.1007/s12571-013-0263-y [DOI] [Google Scholar]
- Singh A., Sharma P., Meena M., Kumar A., Mishra A., Kumar P., et al. (2016). Effect of salinity on gas exchange parameters and ionic relations in bael (Aegle marmelos Correa). Indian J. Hort. 73 48–53. [Google Scholar]
- Singh R., Mahmoudpour A., Rajkumar M., Narayana R. (2017). A review on stripe rust of wheat, its spread, identification and management at field level. Res. Crops 18 528–533. 10.5958/2348-7542.2017.00091.2 [DOI] [Google Scholar]
- Sorahinobar M., Niknam V., Ebrahimzadeh H., Soltanloo H., Behmanesh M., Enferadi S. T. (2016). Central role of salicylic acid in resistance of wheat against Fusarium graminearum. J. Plant Growth Regul. 35 477–491. 10.1007/s00344-015-9554-1 [DOI] [Google Scholar]
- Subedi K., Ma B., Xue A. (2007). Planting date and nitrogen effects on Fusarium head blight and leaf spotting diseases in spring wheat. Agron. J. 99 113–121. 10.2134/agronj2006.0171 [DOI] [Google Scholar]
- Tian J., Philpot W. D. (2015). Relationship between surface soil water content, evaporation rate, and water absorption band depths in SWIR reflectance spectra. Remote Sens. Environ. 169 280–289. 10.1016/j.rse.2015.08.007 [DOI] [Google Scholar]
- Valifard M., Mohsenzadeh S., Niazi A., Moghadam A. (2015). Phenylalanine ammonia lyase isolation and functional analysis of phenylpropanoid pathway under salinity stress in Salvia species. Aust. J. Crop Sci. 9 656–665. [Google Scholar]
- Vinale F., Sivasithamparam K., Ghisalberti E. L., Marra R., Woo S. L., Lorito M. (2008). Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 40 1–10. 10.1079/9780851995120.0001 [DOI] [Google Scholar]
- Wang L., Li S. (2005). “The effects of salicylic acid on distribution of 14C-assimilation and photosynthesis in young grape plants under heat stress,” in Proceedings of the International Symposium on Biotechnology of Temperate Fruit Crops and Tropical Species, Vol. 738 Daytona Beach, FL, 779–785. 10.17660/actahortic.2007.738.104 [DOI] [Google Scholar]
- Wang L., Shi Z. M., Jiang C. F., Liu X., Chen Q. D., Qian X., et al. (2014). MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 5 5416–5427. 10.18632/oncotarget.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Altman A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218 1–14. 10.1007/s00425-003-1105-5 [DOI] [PubMed] [Google Scholar]
- White E. J., Venter M., Hiten N. F., Burger J. T. (2008). Modified Cetyltrimethylammonium bromide method improves robustness and versatility: the benchmark for plant RNA extraction. Biotechnol. J. 3 1424–1428. 10.1002/biot.200800207 [DOI] [PubMed] [Google Scholar]
- Wu G., Wang S. (2012). Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 58 121–127. 10.17221/374/2011-pse [DOI] [Google Scholar]
- Xie C., Wang C., Wang X., Yang X. (2013). Two modified RNA extraction methods compatible with transcript profiling and gene expression analysis for cotton roots. Prep. Biochem. Biotechnol. 43 500–511. 10.1080/10826068.2012.759967 [DOI] [PubMed] [Google Scholar]
- Yadav T., Kumar A., Yadav R., Yadav G., Kumar R., Kushwaha M. (2020). Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J. Biol. Sci. 27 2010–2017. 10.1016/j.sjbs.2020.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin S. M., Aly A., Abdel-Kader D. A., Morsy K., Atallah O. J. Z. (2019). Antagonistic potential of rhizospheric biocontrol agents against soybean root rot-wilt disease complex syndrome. Agric. Res. 46 1395–1418. 10.21608/zjar.2019.48159 [DOI] [Google Scholar]
- Yuef M. I., Ariel T. J., ul R. I., Alberto L., Benigno E., Eduardo O. A. (2018). Identification and evaluation of secondary metabolites by gas chromatography-mass spectrometry (GC-MS) in native strains of Trichoderma species. Afri. J. Biotechnol. 17 1162–1171. 10.5897/ajb2018.16546 [DOI] [Google Scholar]
- Zhang F., Ge H., Zhang F., Guo N., Wang Y., Chen L., et al. (2016). Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 100 64–74. [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang Y., Liu C., Chen F., Ge H., Tian F., et al. (2019a). Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 170 436–445. 10.1016/j.ecoenv.2018.11.084 [DOI] [PubMed] [Google Scholar]
- Zhang S., Gan Y., Xu B. (2016). Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front Plant Sci. 7:1405. 10.3389/fpls.2016.01405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Gan Y., Xu B. (2019b). Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 19:22. 10.1186/s12870-018-1618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Gan Y., Xu B., Xue Y. (2014). The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control 72 1–8. 10.1016/j.biocontrol.2014.01.009 [DOI] [Google Scholar]
- Zhang S., Xu B., Gan Y. (2019c). Seed treatment with Trichoderma longibrachiatum T6 promotes wheat seedling growth under NaCl stress through activating the enzymatic and nonenzymatic antioxidant defense systems. Int. J. Mol. Sci. 20:3729. 10.3390/ijms20153729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-X., Sun H. Y., Shen C. M., Li W., Yu H. S., Chen H. G. (2015). Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 99 1610–1615. 10.1094/pdis-04-14-0422-re [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.