Abstract
Background:
Manganese neurotoxicity is associated with parkinsonism; the associated motor deficits can affect individuals’ quality of life (QoL). We investigated associations between manganese (Mn) exposure, parkinsonian signs, and QoL in Mn mine workers.
Methods:
We assessed parkinsonian signs and QoL in 187 black South African Mn mine workers, using the Unified Parkinson Disease Rating Scale motor subsection 3 (UPDRS3), and the Parkinson Disease Questionnaire (PDQ-39), respectively. We estimated cumulative Mn exposure in mg Mn/m3-years using complete occupational histories and a job exposure matrix. We investigated the cross-sectional association between cumulative Mn exposure and UPDRS3 score, and the UPDRS3 score and PDQ-39, adjusting for age, using linear regression.
Results:
Participants mean age was 41.8 years (range 21–67 years); 97.3% were male. Estimated mean cumulative Mn exposure at the time of examination was 5.4 mg Mn/m3-years, with a mean of 14.0 years working in a Mn mine. The mean UPDRS3 score was 10.1, and 25.7% of the workers had a UPDRS3 score ≥ 15. There was a U-shaped dose-response relation between cumulative Mn exposure and UPDRS3 score, with a positive association up to 15 mg Mn/m3-years of exposure and an inverse association thereafter. Greater UPDRS3 scores were associated with poorer self-reported QoL.
Conclusion:
In this cohort of employed Mn mine workers, parkinsonian signs were common, and were associated with both estimated cumulative Mn exposure and poorer quality of life.
Keywords: Parkinsonian Disorders, Manganese, Miners
INTRODUCTION
Manganese (Mn) is an essential micronutrient but, at high doses, it is neurotoxic, especially when inhaled.1 Occupational exposures to Mn-containing welding fume and dust in Mn mines, in particular, have been associated with Mn neurotoxicity.2,3 Common adverse health outcomes associated with excess levels of airborne Mn exposure are motor dysfunction, cognitive dysfunction, and mood changes.3–5 More recently, there has been renewed interest in occupational Mn neurotoxicity, given evidence of adverse health effects occurring at levels below regulatory thresholds.6
Classically, Mn neurotoxicity, especially in occupational settings, has been linked to parkinsonism – a movement disorder characterized by the presence of two or more of the cardinal clinical signs of Parkinson Disease (PD): rigidity, bradykinesia, rest tremor, and postural instability.7,8 Occupational Mn-induced parkinsonism sometimes manifests as severe and rapidly progressive degenerative neurologic syndrome, manganism, typically associated with extremely high Mn exposures.9 These clinical signs can worsen progressively, even after exposure to Mn has ceased.9–11 More recently, we described Mn dose-dependent progressive parkinsonian in Mn-exposed welders with contemporary occupational exposures.12 Parkinsonism and the associated motor deficits can affect an individual’s daily activities and, hence, quality of life (QoL),13 including limitations in performing self-care activities, depression, and social seclusion. In the workplace, poor health related to Mn exposure may affect job performance and worker safety.13
South Africa contains more than 80% of the world’s known Mn resources, primarily in the Northern Cape province.14,15 Currently, the Mn mining workforce comprises approximately 7,000 workers.16 The current regulated, time-weighted average (TWA) occupational exposure limit (OEL) for Mn dust and compounds in South Africa is 5 mg/m3.17 This OEL has not been updated since 2008, and some speculate that the exposure limit should be lowered.18 Given the potentially serious consequences of high or prolonged exposure to Mn, including chronic neurologic disorders, and the large number of workers exposed, it is important to understand better the relationships between Mn exposure, parkinsonian signs, and QoL in these workers. Epidemiological examination of these associations is limited in the African context.19 Therefore, we investigated these associations in a cohort of Mn mine workers in South Africa. The a priori hypothesis was that Mn exposure in this cohort of miners would be associated with parkinsonism, and the associated motor deficits would affect individuals’ QoL (Figure 1).
Figure 1: Directed acyclic graph (DAG) for manganese, parkinsonism, quality of life, and potential confounders.
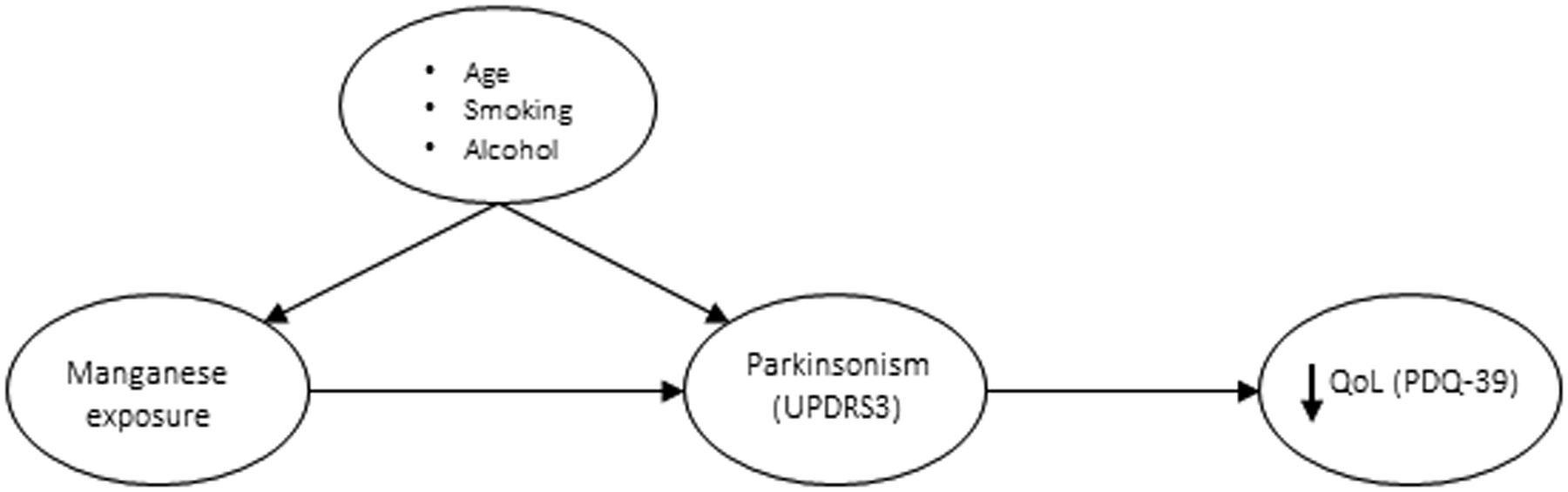
The DAG illustrates the hypothesised causal relationships between manganese exposure, parkinsonism, and QoL, and the associations between potential confounders (age, smoking, and alcohol) and both manganese exposure and parkinsonism. (Note: Age is also a potential confounder in the association between parkinsonism and QoL.)
Abbreviations: UPDRS3 = Unified Parkinson’s Disease Rating Scale motor subsection part 3; PDQ-39 = Parkinson’s Disease Questionnaire-39.20
METHODS
Protection of human subjects
This study was approved by the appropriate Ethics Committee and Human Research Protection Office for the institutions involved. All participants provided written informed consent.
Study participants and assessment of signs of parkinsonism, quality of life, and work histories
Participants were employed black mine workers recruited in the period 2010–2014 from five Mn mines located in Hotazel, a mining town the Northern Cape province of South Africa. The five mines, owned by two large, multi-national mining companies, are in relative close proximity to each other and draw workers from the same surrounding communities. All active workers from the five Mn mines were eligible to participate in the study. We focused on active workers because the town is located in a remote region and is predominantly a labor force town. Retired workers usually return to their distant home towns, thereby limiting access to them for examination. In addition, an actively working cohort without a diagnosis of a parkinsonian condition better characterizes neurological effects and QoL in a relatively healthy group of Mn mine workers. Mine workers who had been employed at the mine for more than one year were invited to participate. Posters about the study were displayed at strategic locations in the mining town, e.g. outside the only supermarket in the town. Due to the nature of our recruitment method, we do not have information on those who opted not to participate. All participants were given R50 for participation.
All mine workers (N = 418) who attended the first visit were enrolled into the cohort and examined using the UPDRS3. They also completed demographics, work history, and PDQ-39 questionnaires on the day of that examination. We only retained the 187 participants (Figure 2) who had complete data available. We excluded 231 participants from our final analysis due to missing information: UPDRS3 (n = 54), Mn exposure data (n = 56), and PDQ-39 (n = 121). Missing values of the PDQ-39 might have resulted from respondents not completing the self-administered, health status questionnaire, whereas missing UPDRS3 was a result of some missing subscores, which can occur when participants are unable to perform the specified neurologist-directed movements for various non-neurological reasons, e.g. fracture.
Figure 2: Participating South African Mn mine workers (N = 187).
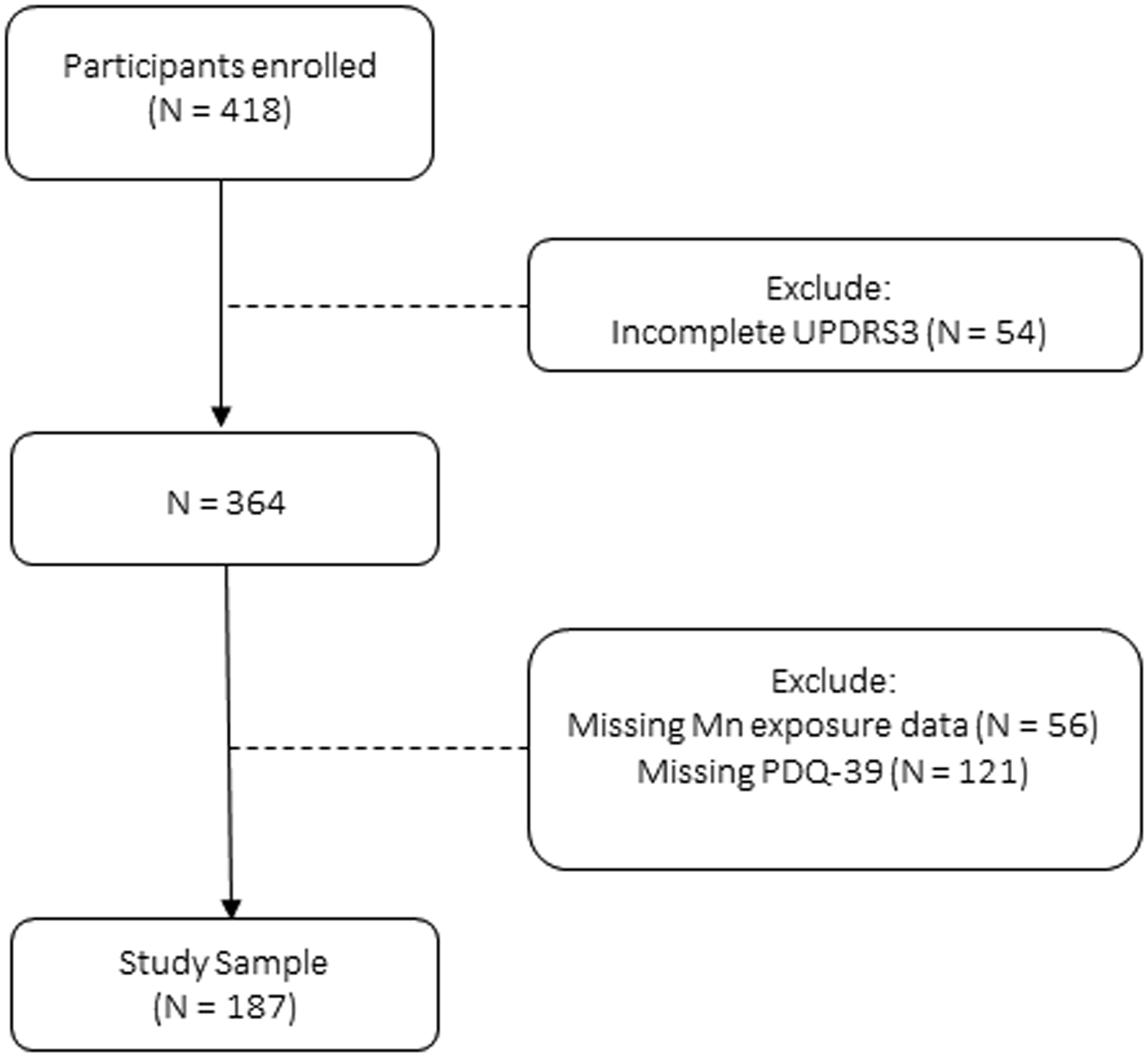
We included all Mn mine workers who had a complete UPDRS3 score (examination), who were not missing Mn exposure data (work history data as of the date of the UPDRS3 exam), and who were not missing the PDQ-39 questionnaire data.
Abbreviations: Mn = manganese; UPDRS3 = Unified Parkinson’s Disease Rating Scale motor subsection part 3; PDQ-39 = Parkinson’s Disease Questionnaire-39.20
The participant-completed questionnaires included a demographic questionnaire which inquired about current cigarette smoking status (never, former, current) and ever alcohol consumption, a structured work history questionnaire which we used to assess cumulative Mn exposure, and the 39-item Parkinson Disease Questionnaire (PDQ-39),20 which we used to characterize quality of life (QoL). The PDQ-39 is a self-administered questionnaire commonly used in PD to measure QoL and health status (i.e. to assess the impact of PD on well-being). The overall PDQ-39 score can range from 0 to 156, with a score of 0 to 4 on each of 39 questions; higher scores indicate poorer QoL.20 This instrument covers eight QoL dimensions (mobility, activities of daily living [ADL], bodily discomfort, emotional well-being, social support, stigma, communication, and cognition). From these eight PDQ-39 subscores and the PDQ-39 total score, we excluded scores from three questions that were only applicable to individuals who either had a spouse or who had PD, because these workers did not have a diagnosis of PD and were not all married. At the time they completed these questionnaires, participants also were examined by one American Board of Psychiatry and Neurology board-certified neurologist/movement disorders specialist. Severity of parkinsonism signs was assessed using the Unified Parkinson’s Disease Rating Scale motor subsection 3 (UPDRS3)21 while blinded to responses on the PDQ-39 and exposure questionnaire. The UPDRS is a widely used rating scale for assessing severity of parkinsonism.21,22 The motor subsection, part 3 (UPDRS3) used in this study assesses motor signs specific to parkinsonism, including bradykinesia, rigidity, tremor, postural instability, and gait impairment, with higher scores indicating greater severity of parkinsonian symptoms. The scale is typically analysed as a continuous variable and there are no absolute thresholds for clinically relevant parkinsonism. Most patients with newly diagnosed PD have UPDRS3 scores >15.23
Estimation of lifetime occupational exposure to Mn
For each worker, we estimated the cumulative airborne Mn exposure in mg Mn/m3-years, using a previously published job exposure matrix,24 and participants’ complete job histories. The estimated cumulative Mn exposure metric accounts for both intensity (mg Mn/m3) and duration of mining job (years) exposure components. Exposure intensity was initially calculated as a product of Mn total dust concentration and estimated Mn percentage content for each job.25 Specifically, for each Mn mining job, we multiplied the number of years in that job by the estimated mg Mn/m3 (exposure intensity) for that job, and then summed these products across the entire work history. In total, 55 job titles for jobs held within the mines were reported (Supplemental Table I).
Statistical analysis
We conducted all analysis in Stata version MC 14.2.26 We compared mine workers characteristics using the Mann-Whitney test for continuous variables and the Pearson chi-square (or Fisher’s exact where appropriate) test for categorical variables. We used multivariable linear regression to estimate, in the form of β estimates, the cross-sectional association between cumulative Mn exposure and the UPDRS3 score, as well as between the UPDRS3 score and the PDQ-39 score and subscores. We retained all of these variables as continuous measures, but transformed the PDQ-39 score and subscores to z-scores in order to present results in terms of standard deviations from the mean, i.e. to make the results (β estimates) comparable across all subscores. For all models, we adjusted a priori for age (as a continuous variable) because of its strong association with cumulative Mn exposure, the UPDRS3 score, and the PDQ-39 score. Secondarily, we further adjusted for other potentially confounding variables, smoking and consumption of alcohol, in order to examine their effects on the associations of interest. Previous reports have consistently demonstrated an inverse association between cigarette smoking and PD.27,28 Although there is mixed evidence for associations of PD with alcohol, some studies have noted reduced risk of PD associated with alcohol consumption.29,30 There is also some evidence that there may be an interaction between Mn exposure, alcohol consumption, and neurobehavioral outcomes.31 We did not adjust for sex due to the small number of women in this miner cohort.
In all linear regression models, we initially modeled cumulative Mn exposure linearly but then examined the appropriateness of linearity by using locally weighted scatterplot smoothing (LOWESS) graphs.32 The purpose of LOWESS was to support our primary analysis method, i.e. to develop appropriate regression models, by indicating whether the relationship between our outcome and exposure variables was non-linear. LOWESS suggested two alternative models, a linear spline model and a quadratic model. We also modeled cumulative Mn exposure categorically (4 categories based on quartiles of the exposure distribution) to further evaluate potential non-linear relationships. We compared all models to the linear model, using the Akaike information criterion (AIC) for each model. A model with a lower AIC value indicates a superior model. In addition, we used the variance inflation factor (VIF) to assess multi-collinearity because of the potential collinearity between age and cumulative Mn exposure. A VIF value not larger than 10 is preferred and, prior to reporting any models, we conservatively verified that the VIF was < 10. We used distribution plots (e.g. histogram, kernel density estimation) to examine each model’s residuals prior to reporting the model. As noted, LOWESS suggested two alternative models, viz. a linear spline model (comprising two linear splines with a knot at mg Mn/m3-years) and a quadratic model (a square term in addition to a linear term). For mg Mn/m3-years age-adjusted analysis, the quadratic and spline models had lower AICs than the linear model (AIC = 1230.9), indicating that estimated cumulative Mn exposure was better modelled as a non-linear term. For all analyses, a two-sided p-value of 0.05 defined significance.
RESULTS
Characteristics of participants
All participants were black and most were male (97.3%) (Table I). They were 21–67 years old with a mean age of 41.8 years (standard deviation [SD] 11.4). Nearly a quarter (23.7%) currently smoked cigarettes, and 49.7% reported ever consuming alcohol. Estimated mean cumulative Mn exposure was 5.4 mg Mn/m3-years (SD 7.4). Mean time-weighted concentration of airborne occupational exposure to Mn was 0.6 mg Mn/m3 (SD 0.5). The mean total lifetime duration of exposure to work in a Mn mine was 14.0 years (SD 11.4). UPDRS3 scores ranged from 0 to 35.5 with a mean of 10.1 (SD 6.6). Forty-eight participants (25.7%) had a UPDRS3 score ≥ 15. The mean PDQ-39 score was 11.7 (SD 13.4), and ranged from 0 to 83. Participants’ characteristics, stratified by the two managing companies, did not differ by sex (p = 0.32), age (p = 0.51), alcohol consumption (p = 0.34), cigarette smoking (p = 0.90), and UPDRS3 (p = 0.20).
Table I:
Participant characteristics
| Characteristic | N = 187 |
|---|---|
| n (%) | |
| Male | 182 (97.3) |
| Black | 187 (100.0) |
| Cigarette smoking status a | |
| Never | 128 (68.8) |
| Former | 14 (7.5) |
| Current | 44 (23.7) |
| Ever consume alcohol a | 92 (49.7) |
| Mean (SD) | |
| Age, years | 41.8 (11.4) |
| Minimum | 21 |
| Median | 42 |
| Maximum | 67 |
| Total duration of Mn exposure, years | 14.0 (11.4) |
| Minimum | 0.4 |
| Median | 9.8 |
| Maximum | 41.5 |
| Mean Mn concentration b , mg Mn/m 3 | 0.6 (0.5) |
| Minimum | 0.1 |
| Median | 0.4 |
| Maximum | 2.5 |
| Cumulative Mn exposure, mg Mn/m 3 -years | 5.4 (7.4) |
| Minimum | 0.2 |
| Median | 2.1 |
| Maximum | 36.8 |
| UPDRS3 | 10.1 (6.6) |
| Minimum | 0 |
| Median | 9 |
| Maximum | 35.5 |
| Mean (SD) | |
| PDQ-39 score | 11.7 (13.4) |
| Minimum | 0 |
| Median | 8 |
| Maximum | 83 |
Abbreviations: Mn = manganese; SD = standard deviation; UPDRS3 = Unified Parkinson’s Disease
Rating Scale motor subsection part 3; PDQ-39 = Parkinson’s Disease Questionnaire-39.20
Percent excludes participants with missing data on smoking (n = 1) or alcohol (n = 2).
Time-weighted mean airborne Mn concentration while working in a job with Mn exposure, based upon the mg Mn/m3 estimated from a job exposure matrix for each job, while taking into account the duration in each job.24
Association between estimated cumulative Mn exposure and UPDRS3
The LOWESS presented a U-shape association between estimated cumulative Mn exposure and UPDRS3, with a decreasing trend at exposures beyond 15 mg Mn/m3-years (Figure 3; Table II), which suggested two alternative models, a linear spline model and a quadratic model. The two models provided the best fit [i.e. had lower AICs than the linear model (AIC = 1230.9)]. Whether we adjusted for age only or for age, alcohol and smoking, the models consistently yielded a similar conclusion - the spline and the quadratic models were better than the linear term model; however, the spline model was superior to both. With adjustment for age, alcohol, and smoking, each mg Mn/m3-year of exposure, when exposure was < 15 mg Mn/m3-years, was associated with a 0.25 (95% CI −0.04, 0.55) point difference on the UPDRS3 score (p = 0.10). In contrast, when estimated cumulative Mn exposure was > 15 mg Mn/m3-years (N = 18 workers), there was no positive association between exposure and UPDRS3 score. In fact, the association in this small group with the highest cumulative Mn exposure was inverse (p = 0.02). We verified that results were unchanged by modelling cumulative Mn exposure as a categorical term. The categorical exposure model had higher AICs (AIC = 1234.1: age-adjusted model; AIC = 1209.6: age, alcohol, smoking-adjusted model) than the other models, indicating that a continuous exposure variable was most appropriate.
Figure 3: Association between cumulative Mn exposure (mg Mn/m3-years) and UPDRS3 score (N = 187).
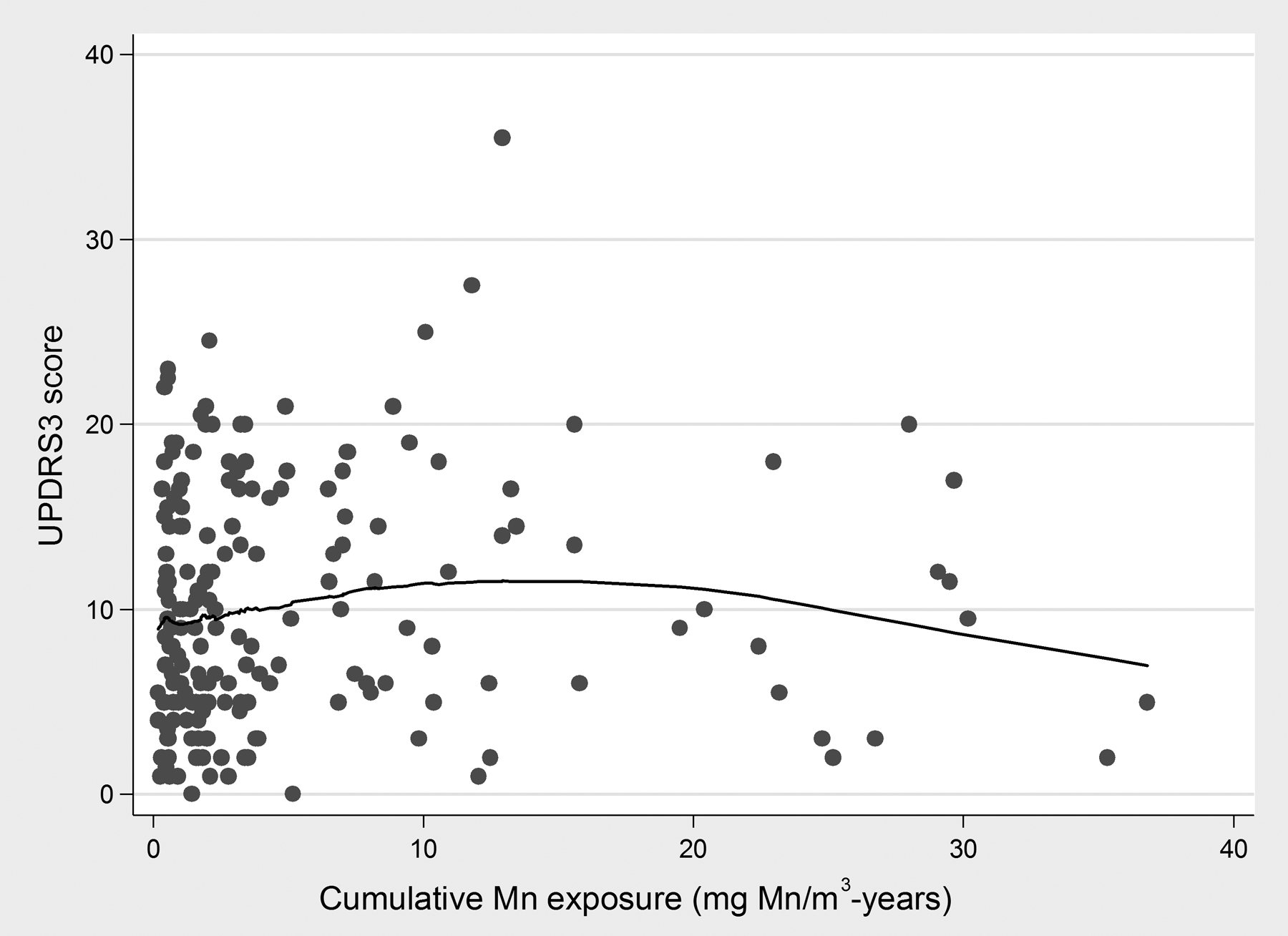
LOWESS demonstrated a U-shaped association between cumulative Mn exposure (mg Mn/m3-years) and the UPDRS3 score, with a positive association in approximately the lowest 15 mg Mn/m3-years of exposure and an inverse association when cumulative Mn exposure was > 15 mg Mn/m3-years of exposure.
Abbreviations: LOWESS = Locally weighted scatterplot smoothing graph; UPDRS3 = Unified Parkinson’s Disease Rating Scale motor subsection 3.
Table II:
Association between cumulative Mn exposure (mg Mn/m3-years) and UPDRS3 score
| Age-adjusteda | Age, alcohol, smoking-adjusteda,b | |||||||
|---|---|---|---|---|---|---|---|---|
| n | β (95% CI)c | p-value | AICd | n | β (95% CI)c | p-value | AICd | |
| Linear spline model f | ||||||||
| < 15 mg Mn/m3-years | 169 | 0.22 (−0.09, 0.52) | 0.16 | 1229.2 | 166e | 0.25 (−0.04, 0.55) | 0.10 | 1203.5 |
| ≥ 15 mg Mn/m3-years | 18 | −0.35 (−0.69, −0.004) | 0.05 | 18 | −0.39 (−0.73, −0.06) | 0.02 | ||
| Quadratic model | 187 | 184e | ||||||
| Linear main effect term | 0.34 (−0.110, 0.79) | 0.14 | 1229.7 | 0.40 (−0.05, 0.85) | 0.08 | 1204.2 | ||
| Square term | −0.01 (−0.03, 0.001) | 0.08 | −0.01 (−0.03, −0.001) | 0.04 | ||||
Abbreviations: AIC = Akaike Information Criterion; CI = confidence interval; Mn = manganese; UPDRS3 = Unified
Parkinson’s Disease Rating Scale motor subsection part 3.
Adjusted for age as a continuous variable.
Also adjusted for ever/never alcohol consumption and never/former/current cigarette smoking. Not adjusted for sex due to small numbers.
Change in UPDRS3 score (95% CI) per mg Mn/m3-year.
A smaller relative AIC suggests a better model.
Excludes participants with missing data on smoking (n = 1) or alcohol (n = 2).
Two linear splines with a knot at 15 mg Mn/m3-years. We determined the position of the knot from the LOWESS (Figure 3). Eighteen participants had ≥ 15 mg Mn/m3-years of exposure
Association between UPDRS3 and PDQ-39 score and subscores
We observed a strong, positive association between UPDRS3 and PDQ-39 scores (p = 0.001) (Table III). Specifically, participants had a PDQ-39 score that was 0.04 (95% CI 0.02, 0.06) standard deviations greater than the mean for each unit increase in the UPDRS3 score. This worsening of self-reported QoL with worsening parkinsonian signs appeared to be driven primarily by mobility, ADL, emotional, and bodily discomfort dimensions of the PDQ-39 (all p ≤ 0.01 in the fully adjusted model). Although non-significant, the cognitive dimension also was positively associated with the UPDRS3 score (p = 0.09). For all regression models (UPDRS3 and PDQ-39), the mean VIF for age, as a measure of multi-collinearity was stable and remained below 2.
Table III:
Association between UPDRS3 score and PDQ-39 score and subscores
| Age-adjusteda N = 187 |
Age, alcohol, smoking-adjusteda,b N = 184c |
|||
|---|---|---|---|---|
| Z-scored | β (95% CI)e | p-value | β (95% CI)e | p-value |
| PDQ-39 score | 0.04 (0.02, 0.06) | 0.001 | 0.04 (0.02, 0.06) | 0.001 |
| PDQ-39 subscore | ||||
| Mobility | 0.04 (0.01, 0.06) | 0.001 | 0.04 (0.01, 0.06) | 0.002 |
| ADL | 0.03 (0.01, 0.05) | 0.01 | 0.03 (0.01, 0.05) | 0.01 |
| Emotional | 0.04 (0.02, 0.06) | 0.001 | 0.04 (0.01, 0.06) | 0.001 |
| Stigma | 0.03 (0.01, 0.05) | 0.01 | 0.02 (−0.005, 0.04) | 0.13 |
| Social support | 0.01 (−0.01, 0.03) | 0.30 | 0.01 (−0.01, 0.04) | 0.22 |
| Cognition | 0.02 (−0.01, 0.04) | 0.17 | 0.02 (−0.003, 0.04) | 0.09 |
| Communication | 0.01 (−0.01, 0.03) | 0.31 | 0.02 (−0.01, 0.04) | 0.19 |
| Bodily discomfort | 0.02 (−0.001, 0.04) | 0.06 | 0.03 (0.01, 0.05) | 0.01 |
Abbreviations: ADL = activities of daily living; CI = confidence interval; SD = standard deviation;
UPDRS3 = Unified Parkinson’s Disease Rating Scale motor subsection part 3.
Adjusted for age as a continuous variable.
Also adjusted for ever/never alcohol consumption and never/former/current cigarette smoking. Not adjusted for sex due to small numbers.
Excludes participants with missing data on smoking (n = 1) or alcohol (n = 2).
Higher score indicates poor QoL. We transformed the PDQ-39 to z-scores so that the magnitude of the β estimates could be compared across subscores.
Standard deviation difference in PDQ-39 score (95% CI) per one point increase in the UPDRS3 score.
DISCUSSION
There are several important findings from this study. First, a relatively high proportion (25.7%) of Mn mine workers had a UPDRS3 score ≥ 15, which is somewhat higher than that found in U.S. workers exposed to Mn-containing welding fume.12,33 Second, parkinsonian signs were associated with greater impairment in several dimensions assessed by the PDQ-39, indicating that parkinsonism in these workers likely resulted in noticeable impairments in daily function. Finally, we observed a U-shaped dose-response association between cumulative Mn exposure and the UPDRS3, which we also observed between a similar intensity-weighted cumulative Mn exposure metric and UPDRS3 in a U.S. welder cohort.33 Our study had important strengths that lend weight to these findings. Notably, we used one physician expert for clinical examination, and participants were assessed with clinically relevant, validated, and reliable data collection instruments: the UPDRS321 and the PDQ-39.20
The U-shaped association that we observed between estimated cumulative Mn exposure and the UPDRS3 score is highly consistent with the healthy worker survivor effect34 given the cross-sectional nature of the present study and the earlier welder study33 in which a U-shaped association was also observed. The healthy worker survivor effect is a well-established phenomenon in which workers who develop health effects that limit their ability to work, leave the work force and therefore are no longer observable in a cross-sectional sample. The healthy worker survivor effect typically blunts the dose-response relationship at the higher levels of exposure, leading to an apparent lack of association, or even a reversal of the association. In the present study, the severity and prevalence of parkinsonism might be underestimated if those cohort members with more severe parkinsonism left the workforce due to physical limitations. The finding that workers with more severe parkinsonism had greater impairments in QoL is consistent with this hypothesis.
The association between estimated cumulative Mn and UPDRS3 appeared to be most usefully represented by the spline model. While the quadratic model formally confirmed that the association between Mn exposure and parkinsonism was U-shaped, the spline model nicely complemented those results. The beta coefficient for the first linear spline, which represents the Mn-UPDRS3 association up to 15 mg Mn/m3-years of cumulative exposure, has a more useful interpretation than the coefficients from the quadratic model. However, the two linear spline model assumed a linear association in the lowest 15 mg Mn/m3-years of cumulative Mn exposure. Although this spline model fit the data slightly better than the quadratic model, according to the AIC, the U-shaped association between Mn and UPDRS3 remained consistent with the healthy worker survivor effect. Alternatively, these data are also consistent with a non-linear association in which the association between Mn exposure and UPDRS3 plateaus after the initial Mn exposure.
If the association between Mn exposure and UPDRS3 is linear, then the beta coefficient for the first linear spline might be a reasonable estimate of the linear association in the absence of the healthy worker survivor effect. The beta coefficient from the first spline in the spline model indicated that, for each mg Mn/m3-year of exposure, the UPDRS3 score was 0.25 points higher. For example, a worker exposed to a Mn air concentration of 0.5 mg Mn/m3 which is close to the median for our cohort, is estimated to have a UPDRS3 one point higher for every eight years of occupational Mn exposure. However, even this effect estimate might underestimate the true impact of cumulative occupational Mn mining exposure on parkinsonism in the workers in our study, given the strong evidence of a healthy worker survivor effect.
We have previously observed, in our welder cohort, an apparent critical Mn exposure window. There is a strong association between progression of clinical parkinsonism as measured by the UPDRS3 and Mn-containing welding fume exposure in the early years of exposure.12 There was also a suggestion of a similar non-linear upregulation of dopamine type 2 receptors (D2R) in the substantia nigra with positron emission tomography in relation to cumulative Mn exposure,32 which is interesting, given that we also observed a clear relation between D2R upregulation and UPDRS3 in the welders study. The Mn-UPDRS3 relationship in the present study is consistent with these reports and is, therefore, clinically meaningful. Furthermore, our analysis suggests that Mn exposure at levels below the current South African OEL and U.S. permissible exposure limit are associated with neurologic dysfunction. The estimated mean TWA exposure in our cohort was 0.6 mg Mn/m3 which is substantially lower than the South African TWA-OEL of 5 mg Mn/m3 for Mn dust.17 The estimated TWA Mn exposure for these workers is also below the U.S. Occupational Safety and Health Administration (OSHA) Permissible Exposure Limit (PEL) ceiling for Mn compounds of 5 mg Mn/m3.35
Another important finding of this study was that UPDRS3 was positively associated with poor QoL. Poorer QoL, as assessed by the PDQ-39 score, was driven by mobility, ADL, and emotional and bodily discomfort subscores. Mobility, ADL, and bodily discomfort are areas that are most plausibly affected by worsening signs of parkinsonism. These PDQ-39 subscores probably reflect motor deficits or functional impairments well-described in the Mn neurotoxicity literature.3–5 The association between the UPDRS3 score and the emotional subscore of the PDQ-39 is most likely a non-causal association, but is plausible, given that Mn exposure has been associated with emotional lability in some worker cohorts.36 Mobility issues likely contribute to impaired performance of workers’ ADL, while emotional instability can result in depression and other psychological and/or psychiatric disorders.13,37 These findings, in addition to the high prevalence of parkinsonism in our study, suggest that occupational health evaluations should include an assessment for signs of parkinsonism and related QoL in Mn-exposed workers. Identifying workers with parkinsonism and reduced QoL may provide an opportunity to intervene and reduce associated morbidity. Whether these impairments in QoL impact job performance, absenteeism, productivity, or risk of injury in the workplace is unclear, but should be investigated in future studies.
As with all studies, there are several potential limitations. Most notably, the cross-sectional nature of the study means that we cannot infer causality because exposure and outcome were simultaneously assessed, i.e. we cannot establish the temporal relationship between exposure and outcome. We also relied on a work history-based exposure metric to estimate airborne Mn levels, which might have introduced exposure measurement error, even though the job exposure matrix was derived from data obtained from the same mines in which our participants worked.24 Unfortunately, there are no biomarkers of cumulative Mn exposure, aside from brain MRI which was not available in this study. This is a single study in a cohort of workers exposed to Mn, and may not be representative of other Mn-exposed workforces. Finally, the PDQ-39 has not been validated in the context of low and middle income countries, although we still found a strong association between the UPDRS3 and the PDQ-39. Nevertheless, the results should be taken into consideration for future research and regulatory thresholds for airborne Mn.
CONCLUSION
In this cohort of employed Mn mine workers, parkinsonian signs were common, and were associated with both estimated cumulative Mn exposure and poorer quality of life.
Supplementary Material
Acknowledgements:
We thank the Hotazel Manganese Mine and clinic staff for allowing us to use their premises and providing access to medical information, as well as allowing the mine workers time off work to participate in this study. Sr. Marina Steenkamp was instrumental in organizing visits to the Northern Cape Province and collecting the data. We also thank the Hotazel Recreation Club, for use of its premises. The study could not have been conducted without the participating mine workers.
Funding:
Funding of this research was supported by the National Institute of Environmental Health Sciences (grants: R01ES026891, K24ES017765, K01ES028295, and R21ES017504); American Parkinson Disease Association; and the Association of Commonwealth Universities. The funding agencies had no role in the analysis of the data, interpretation of the results, or the decision to publish these results.
Footnotes
Institution and ethics approval and informed consent: This work was performed at the Department of Neurology, Washington University School of Medicine, St. Louis, United States. This study was approved by the appropriate Ethics Committee and Human Research Protection Office for the institutions involved. All participants provided written informed consent.
Disclosure (Authors): The authors declare no conflicts of interest.
Disclaimer: None.
Institution at which the work was performed: Department of Neurology, Washington University School of Medicine, St. Louis, United States
REFERENCES
- 1.Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27(3):315–326. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J Searching for a Relationship between Manganese and Welding and Parkinson’s Disease. Neurology. 2005;64(12):2021–2028. [DOI] [PubMed] [Google Scholar]
- 3.Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environmental research. 1997;73(1–2):92–100. [DOI] [PubMed] [Google Scholar]
- 4.Hudnell HK. Effects from environmental Mn exposures: a review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20(2–3):379–397. [PubMed] [Google Scholar]
- 5.Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368(2–3):542–556. [DOI] [PubMed] [Google Scholar]
- 6.Levy BS, Nassetta WJ. Neurologic effects of manganese in humans: a review. Int J Occup Environ Health. 2003;9(2):153–163. [DOI] [PubMed] [Google Scholar]
- 7.Femi OL, Ibrahim A, Aliyu S. Clinical Profile of Parkinsonian Disorders in the Tropics: Experience at Kano, Northwestern Nigeria. Journal of Neurosciences in Rural Practice. 2012;3(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Abbas AK, Fausto N. Degenerative Diseases of Basal Ganglia and Brainstem. In: Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, Pennsylvania: Saunders Elsevier; 2005:1391–1393. [Google Scholar]
- 9.Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann NYAcadSci. 2004;1012:209–223. [DOI] [PubMed] [Google Scholar]
- 10.Rodier J Manganese poisoning in Moroccan miners. Br J Ind Med. 1955;12(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JD, Huang CC, Hwang YH, Chiang JR, Lin JM, Chen JS. Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. British journal of industrial medicine. 1989;46(12):856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racette BA, Searles Nielsen S, Criswell SR, et al. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017;88(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RC, Lundin JI, Criswell SR, et al. Effects of parkinsonism on health status in welding exposed workers. Parkinsonism Relat Disord. 2011;17(9):672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonga MW. An Overview of the South African Iron, Manganese and Steel Industry During the Period 1984–2003. South Africa. 2005. R45/2005. [Google Scholar]
- 15.Blaurock-Busch E Environmental Exposure and the Toxicity of Metals, The South African vs Global Problems. North-West University;2010. [Google Scholar]
- 16.Minerals Council South Africa. Facts and Figures 2017. Johannesburg, South Africa. 2017. [Google Scholar]
- 17.Department of Labour South Africa. Regulation: OHS - Hazardous Chemical Substances. Pretoria, South Africa. 1995. R1179. [Google Scholar]
- 18.Mallon W Occupational Exposure Limits. Occupational Health Southern Africa. 2016;22(5):21. [Google Scholar]
- 19.Abd-Allah F, Kissani N, William A, et al. Neuroscience Research in Africa: Current Status. eNeurologicalSci. 2016;3:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–248. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9(1):76–83. [DOI] [PubMed] [Google Scholar]
- 22.Criswell SR, Searles Nielsen S, Warden M, et al. MRI Signal Intensity and Parkinsonism in Manganese-Exposed Workers. J Occ Env Med. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penney JB, Oakes D, Shoulson I, et al. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Annals of Neurology. 1996;39(1):37–45. [DOI] [PubMed] [Google Scholar]
- 24.Myers JE, Thompson ML, Ramushu S, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24(6):885–894. [DOI] [PubMed] [Google Scholar]
- 25.Myers JE, teWaterNaude J, Fourie M, et al. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology. 2003;24(4–5):649–656. [DOI] [PubMed] [Google Scholar]
- 26.Stata Statistical Software [computer program]. Version MC 14.2. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 27.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. [DOI] [PubMed] [Google Scholar]
- 28.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT Jr., Swanson PD. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. American journal of epidemiology. 2002;155(8):732–738. [DOI] [PubMed] [Google Scholar]
- 29.Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14(1):28–37. [DOI] [PubMed] [Google Scholar]
- 30.Godwin-Austen RB, Lee PN, Marmot MG, Stern GM. Smoking and Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1982;45:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingsen DG, Kusraeva Z, Bast-Pettersen R, et al. The interaction between manganese exposure and alcohol on neurobehavioral outcomes in welders. Neurotoxicology and teratology. 2014;41:8–15. [DOI] [PubMed] [Google Scholar]
- 32.Criswell SR, Warden MN, Searles Nielsen S, et al. Selective D2 Receptor PET in Manganese-Exposed Workers. Neurology. 2018;91(11):e1022–e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racette BA, Criswell SR, Lundin JI, et al. Increased Risk of Parkinsonism Associated with Welding Exposure. Neurotoxicology. 2012;33(5):1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occupational and environmental medicine. 2007;64(8):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Occupational Safety and Health Administration (OSHA). Permissible Exposure Limits, OSHA Annotated Table Z-1. 2018; https://www.osha.gov/dsg/annotated-pels/tablez-1.html. Accessed 11/19/2018.
- 36.Racette BA. Manganism in the 21st century: the Hanninen lecture. Neurotoxicology. 2014;45:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damiano AM, Snyder C, Strausser B, Willian MK. A review of health-related quality-of-life concepts and measures for Parkinson’s disease. Quality of Life Research. 1999;8(3):235–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


