Abstract
Background: Viscoelastic coagulation testing has been suggested to help manage coagulopathy in critically ill patients with COVID-19. However, results from different viscoelastic devices are not readily comparable. ClotPro® is a novel thromboelastometry analyzer offering a wider range of commercially available assays.
Methods: We compared the results from ClotPro with results from the well-established ROTEM® Delta device and conventional coagulation tests in critically ill patients with COVID-19.
Results: Viscoelastic parameters indicated the presence of a potentially hypercoagulable state in the majority of patients. In up to 95 paired measurements, we found strong correlations between several parameters routinely used in clinical practice: (i) EX test vs. EXTEM CT, A5, A10, MCF, (ii) IN test vs. INTEM A5, A10, MCF, and (iii) FIB test vs. FIBTEM A5, A10, MCF (all R > 0.7 and p < 0.001). In contrast, IN test CT vs. INTEM CT showed only a moderate correlation (R = 0.53 and p < 0.001). Clot strength parameters of both devices exhibited strong correlations with platelet counts and fibrinogen levels (all R > 0.7 and p < 0.001). Divergent correlations of intrinsically activated assays with aPTT and anti-factor Xa activity were visible. Regarding absolute differences of test results, considerable delta occurred in CT, CFT, and clot strength parameters (all p < 0.001) between both devices.
Conclusions: Several parameters obtained by ClotPro show strong correlations with ROTEM Delta. Due to weak correlations of intrinsically activated clotting times and considerable absolute differences in a number of parameters, our findings underline the need for device-specific algorithms in this patient cohort.
Keywords: ClotPro®, coagulation, comparison, COVID-19, critically ill, point-of-care, ROTEM Delta®, viscoelastic test
Introduction
Critically ill patients with COVID-19 might exhibit a number of complex coagulation abnormalities that have been consistently associated with a hypercoagulable state (1). Characteristic findings include the occurrence of endothelial injury (2), increase in factor VIII, von Willebrand factor, and fibrinogen (3), the release of cytokines (4), increased plasma viscosity (5), complement activation (6), and impaired fibrinolysis (7). Conventional coagulation tests such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and antithrombin activity cannot sufficiently depict these changes (8, 9). Recent evidence suggests that viscoelastic testing might be more sensitive to COVID-19-associated coagulopathy in comparison with conventional coagulation tests (10–12). In line with this, a number of recent publications have reported results from viscoelastic tests in patients with COVID-19, including ROTEM (10, 12–16), TEG (17), and ClotPro (1, 7, 18, 19). Additionally, recently published diagnostic pathways for predicting thromboembolic complications and guiding therapeutic interventions in patients with severe COVID-19 suggest that viscoelastic tests be used (11, 12, 15, 20, 21). Moreover, further prospective clinical trials assessing the occurrence of coagulopathy in patients with COVID-19 using viscoelastic tests are underway. However, due to methodological differences, the results of different viscoelastic devices are not interchangeable (22, 23). Studies evaluating the correlations between different viscoelastic coagulation analyzers—such as TEG 5000 and TEG 6s (24, 25), ROTEM Delta and ROTEM Sigma (22, 26), TEG 6s and ROTEM Sigma (27), TEG 5000 and ROTEM Delta (23, 28)—have come up with heterogeneous results, whereas no head-to-head comparison of ClotPro with other viscoelastic point-of-care devices has been published to date.
ClotPro® (enicor GmbH, Munich, Germany) is a novel Conformitè Europëenne (CE) marked viscoelastic whole blood coagulation analyzer that has recently been investigated in patients undergoing orthopedic surgery (29), under treatment with direct oral anticoagulants (30), or with COVID-19 disease (1, 7, 18, 19). In contrast to other viscoelastic devices, ClotPro offers standardized pipette tips prefilled with distinct reagents that allow an ample range of specific assays. Although ClotPro measurements result in thromboelastometry curves similar to those obtained by ROTEM, a direct comparison is pending. Thus, the aim of the present retrospective analysis was to compare simultaneously obtained viscoelastic test results between the novel ClotPro analyzer and the well-established ROTEM® Delta (Tem Innovations GmbH, Munich, Germany) device in critically ill patients with COVID-19.
Materials and Methods
This retrospective analysis was approved by the Ethics Committee of the Medical University of Vienna (EK 2269/2020) and was performed in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. We conducted a review of data from all critically ill patients with COVID-19 disease (SARS-CoV2 detected by polymerase chain reaction) admitted to a single tertiary center, the Medical University of Vienna, between 1 April 2020 and 15 December 2020. Two different viscoelastic test systems with a range of diverse assays are regularly used at our institution: ClotPro (enicor GmbH, Munich, Germany, Software Version 1.45a) and ROTEM Delta (Tem Innovations GmbH, Munich, Germany, Software Version 2.8.1). Due to the simultaneous availability of these test systems, viscoelastic tests were run in parallel on both devices in a considerable number of critically ill patients with COVID-19 throughout their ICU stay. Furthermore, all critically ill patients with COVID-19 underwent daily evaluation with conventional coagulation tests at the same timepoint as viscoelastic testing. Blood samples were routinely obtained at the same time every morning by staff of the Intensive Care Units. We thus designed this study to compare results from the novel ClotPro device with (i) results obtained by ROTEM Delta and (ii) conventional coagulation tests. Electronic medical records were reviewed to identify all patients with (i) laboratory-confirmed COVID-19 disease, (ii) admission to an intensive care unit (ICU) and (iii) the presence of simultaneous viscoelastic test results from both devices (i.e., ClotPro and ROTEM) performed from an identical blood sample. Exclusion criteria were (i) pre-existing coagulation disorder, (ii) liver cirrhosis, and (iii) intake of oral anticoagulants within 48 h before admission to the ICU. Patient characteristics, results from viscoelastic tests and laboratory values—of samples drawn at the same time as viscoelastic tests—were extracted into a spreadsheet. Every blood sample with an available viscoelastic test, simultaneously performed on both devices (ClotPro and ROTEM Delta), was included.
ClotPro tests that were routinely performed included (i) EX test [tissue factor (TF)-activated assay), (ii) IN test (ellagic acid-activated assay), (iii) FIB test (TF-activated assay using cytochalasin D and a glycoprotein IIb/IIIa inhibitor for platelet inhibition), and (iv) AP test (TF-activated assay using aprotinin to inhibit fibrinolysis). Corresponding ROTEM Delta tests were (i) EXTEM (TF-activated assay), (ii) INTEM (ellagic acid-activated assay), (iii) FIBTEM (TF-activated assay using cytochalasin D for platelet inhibition), and (iv) APTEM (TF-activated assay using aprotinin to inhibit fibrinolysis). Except for the IN test and INTEM, all tests contain polybrene to neutralize heparin. Measurements and regular quality controls for both viscoelastic devices were run by experienced and trained personnel in accordance with the manufacturers' instructions. We assessed the results of ClotPro and ROTEM Delta and performed a descriptive analysis of the following parameters: coagulation time (CT; time until clot reaches a firmness of 2 mm, measured in s), clot amplitude 5 min after CT (A5; measured in mm), clot amplitude 10 min after CT (A10; measured in mm), clot amplitude 20 min after CT (A20; measured in mm), clot formation time (CFT; time between 2 and 20 mm of clot firmness, measured in s), maximum clot firmness (MCF; maximum amplitude of the clot during measurement, measured in mm), and alpha angle (alpha; angle between baseline and the tangent to the clotting curve through the 2-mm point, measured in degrees). Furthermore, the parameter maximum lysis (ML; measured in percentage of MCF during measurement) was analyzed for tests with an equal runtime of 60 min on both devices. In accordance with clinical routine, blood samples for conventional coagulation tests, platelet count, and viscoelastic testing were drawn from an arterial line at the same time. As CFT of fibrinogen assays is rarely reached in healthy subjects, CFT of FIB test/FIBTEM assays is not used in clinical practice. Furthermore, ClotPro—as set in the device settings by default—did not record results of FIB test CFT, alpha and ML in the database during the study period. Therefore, CFT, alpha, and ML of fibrinogen assays were not included in our analysis.
We analyzed the following conventional coagulation tests: PT (Owren, reference range: 24.6–32.7 s), aPTT (reference range: 27–41 s), fibrinogen (Clauss method; reference range: 2–4 g.l−1), and anti-factor Xa activity (STA-Liquid AntiXa, REF 00691 and REF 00322, reference range: <0.1 IU.ml−1) via the STA R Max 2 coagulometer (Diagnostica Stago SAS, Asnières-sur-Seine, France). Furthermore, platelet count (reference range: 150–350 109.l−1) was determined via the Sysmex XE-2100 cell counter (Sysmex, Kobe, Japan). Normality for continuous data was assessed using the Shapiro–Wilk test. Normally distributed data are presented as mean (SD), whereas non-normally distributed data are presented as median (25–75th percentile). Non-normally distributed variables were compared using the Wilcoxon Signed Rank test. We used Spearman rank correlation to evaluate correlations between the various tests performed on the ClotPro and the ROTEM Delta and standard laboratory parameters. Bland-Altman plots were used to describe agreement between the ClotPro and ROTEM Delta tests. Correlations were considered as very strong correlation (R 0.9–1.0), strong correlation (R 0.7–0.89), moderate correlation (R 0.5–0.69), and weak correlation (R 0.3–0.49). We considered two-sided p-values ≤ 0.05 to be statistically significant and performed statistical analyses and graphical representations using RStudio (RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, Vers. 4.0.3).
Results
Between 1 April 2020 and 15 December 2020, 86 critically ill patients with COVID-19 were admitted to an ICU of the Medical University of Vienna. We identified 27 patients (17 men and 10 women) who met the inclusion criteria, resulting in a range of 31 (AP test/APTEM) to 95 (EX test/EXTEM) paired measurements simultaneously performed on ClotPro and ROTEM Delta. Baseline patient characteristics upon ICU admission are depicted in Table 1. Median length of ICU stay was 14 (8–26) days, and throughout their ICU stay, nine (33%) patients received venovenous extracorporeal membrane oxygenation for treatment of acute respiratory distress syndrome. EX test/EXTEM were measured simultaneously at 95 different timepoints in 27 patients, IN test/INTEM at 64 different timepoints in 24 patients, FIB test/FIBTEM at 94 different timepoints in 27 patients, and AP test/APTEM at 31 different timepoints in 16 patients. The median runtime of viscoelastic tests was 60 (60–60) min for ClotPro and 60 (60–60) min for ROTEM Delta. All patients received heparin for pharmacological thromboprophylaxis or anticoagulation. At the time of IN Test/INTEM analysis, 22 (92%) patients received subcutaneous LMWH (Enoxaparin) at a median dose of 80 (40–120) mg.day−1, whereas two (8%) patients received continuous intravenous UFH at a mean rate of 1,117 (324) IU.h−1. Median time between the last subcutaneous application of LMWH and conduction of IN test/INTEM was 11 (10–14) h. The results of conventional coagulation tests and anti-factor Xa activity at the time of corresponding viscoelastic measurements are depicted in Table 2.
Table 1.
Patient baseline characteristics upon ICU admission (n = 27).
| Age (years)tb | 58 (15) |
| Height (cm) | 173.8 (7.9) |
| Weight (kg) | 88.8 (16.14) |
| Male | 17 (63%) |
| BMI (kg.m−2) | 29.5 (5.8) |
| SOFA score | 11 (10–12) |
| PaO2/FiO2 ratio | 104 (80–145) |
| Invasive ventilation | 20 (74%) |
Values are mean (SD), median (25–75th percentile), or number (proportion).
Table 2.
Platelet count and conventional coagulation tests at the timepoint of ROTEM/ClotPro measurements.
| EX test/EXTEM | IN test/INTEM | FIB test/FIBTEM | |
|---|---|---|---|
| Platelet count (109.l−1) | 243 (155–353) | 235 (154–339) | 243 (156–351) |
| Fibrinogen (g.l−1) | 6.1 (2) | 6.1 (2.2) | 6.1 (5–7.1) |
| PT (s) | 30.6 (4.2) | 31.0 (4.2) | 30.6 (4.2) |
| INR | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) |
| aPTT (s) | 39 (35–44) | 40 (35–45) | 39 (35–44) |
| anti-Xa (IU.ml−1) | 0.3 (0.2–0.4) | 0.2 (0.2–0.3) | 0.3 (0.2–0.4) |
Values are mean (SD) and median (25–75th percentile).
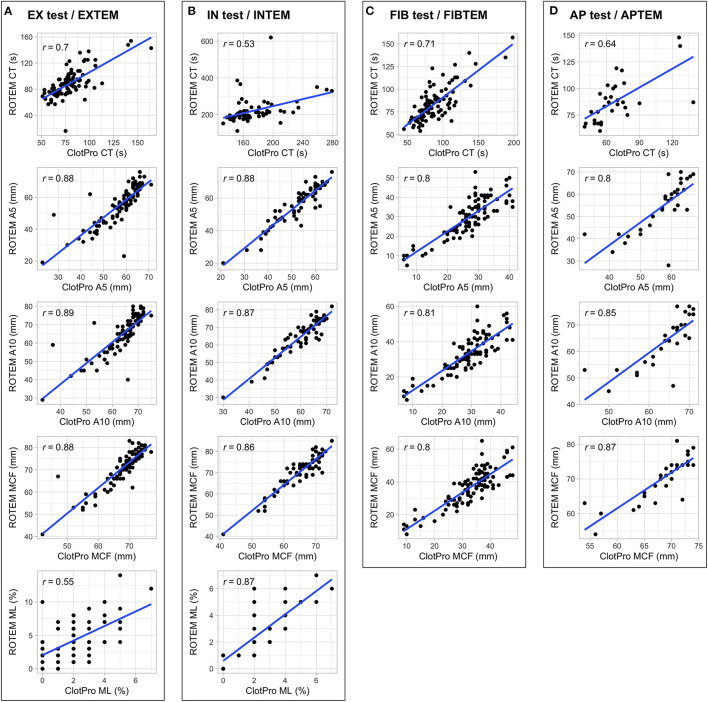
Figure 1 shows scatterplots and the correlation coefficients for the parameters CT, A5, A10, MCF, and ML for all four distinct assays performed on both devices. We found strong correlations between several parameters routinely used in clinical practice: (i) EX test vs. EXTEM CT, A5, A10, MCF, (ii) IN test vs. INTEM A5, A10, MCF, and (iii) FIB test vs. FIBTEM A5, A10, MCF. In contrast, IN test CT showed only moderate correlations with INTEM CT. Separate analysis for patients receiving LMWH and UFH revealed a similar grade of correlation between IN test CT and INTEM CT (LMWH: 55 timepoints, r = 0.45, p < 0.001; UFH: 9 timepoints, r = 0.47, p < 0.001). Scatterplots for further parameters (CFT, alpha, and A20) are shown in Supplementary Figure 1.
Figure 1.
Scatterplots and correlation coefficients for the parameters CT, A5, A10, MCF, and ML for EX test/EXTEM (A), IN test/INTEM (B), FIB test/FIBTEM (C), AP test/APTEM (D) performed on both devices.
Table 3 shows absolute differences (delta) and correlations between ClotPro and ROTEM for EX test/EXTEM, IN test/INTEM, and FIB test/FIBTEM measurements. In decreasing order, considerable absolute differences between the two devices occurred for IN test vs. INTEM CT, EX test vs. EXTEM CT, and EX test vs. EXTEM CFT. Furthermore, clot strength parameters (i.e., A5, A10, A20, and MCF) showed differences, with mainly lower values obtained by ClotPro in extrinsically activated, intrinsically activated, and functional fibrinogen assays. In comparison with ROTEM, ClotPro measurements resulted in smaller interquartile ranges in all assays. Bland-Altmann plots (Supplementary Figure 2) suggested a positive relationship between inter-device differences and the mean of paired measurements for clot strength parameters (i.e., A5, A10, A20, and MCF) throughout all assays. Furthermore, we observed the same pattern for EX test vs. EXTEM ML and IN test vs. INTEM CT. Differences and correlations between AP test/APTEM can be found in Supplementary Table 1.
Table 3.
Differences and correlations between ROTEM Delta and ClotPro.
| n | Patients | ClotPro | ROTEM | Δ* | p-value† | r ‡ | p-value‡ | |
|---|---|---|---|---|---|---|---|---|
| EX test/EXTEM | ||||||||
| CT (s) | 95 | 27 | 77 (68–85) | 86 (75–95) | 11 (3–18) | <0.001 | 0.70 | <0.001 |
| CFT (s) | 95 | 27 | 52 (45–76) | 60 (49–89) | 9 (−4–23) | <0.001 | 0.60 | <0.001 |
| alpha | 95 | 27 | 78 (75–80) | 78 (73–80) | −1 (−3–1) | 0.062 | 0.66 | <0.001 |
| A5 (mm) | 95 | 27 | 60 (54–63) | 58 (47–65) | −2 (−6–1) | 0.004 | 0.88 | <0.001 |
| A10 (mm) | 95 | 27 | 66 (62–69) | 67 (59–74) | 1 (−2–4) | 0.028 | 0.89 | <0.001 |
| A20 (mm) | 95 | 27 | 69 (65–72) | 72 (65–77) | 3 (0–5) | <0.001 | 0.89 | <0.001 |
| MCF (mm) | 95 | 27 | 70 (66–72) | 73 (68–77) | 3 (1–5) | <0.001 | 0.88 | <0.001 |
| ML (%)§ | 69 | 24 | 2 (1–3) | 4 (2–6) | 2 (0–4) | <0.001 | 0.55 | <0.001 |
| IN test/INTEM | ||||||||
| CT (s) | 64 | 24 | 165 (155–189) | 206 (188–225) | 34 (19–54) | <0.001 | 0.53 | <0.001 |
| CFT (s) | 64 | 24 | 57 (50–80) | 62 (50–84) | 2 (−4–9) | 0.074 | 0.78 | <0.001 |
| alpha | 64 | 24 | 78 (74–80) | 78 (73–80) | 0 (−2–1) | 0.211 | 0.79 | <0.001 |
| A5 (mm) | 64 | 24 | 54 (46–60) | 58 (48–65) | 3 (5) | <0.001 | 0.88 | <0.001 |
| A10 (mm) | 64 | 24 | 62 (56–66) | 67 (59–73) | 5 (4) | <0.001 | 0.87 | <0.001 |
| A20 (mm) | 64 | 24 | 66 (60–69) | 72 (66–76) | 6 (4) | <0.001 | 0.86 | <0.001 |
| MCF (mm) | 64 | 24 | 67 (63–70) | 72 (68–76) | 6 (3) | <0.001 | 0.86 | <0.001 |
| ML (%)§ | 44 | 21 | 2 (0–3) | 2 (1–4) | 0 (0–1) | 0.078 | 0.87 | <0.001 |
| FIB test/FIBTEM | ||||||||
| CT s | 95 | 27 | 84 (71–100) | 81 (70–91) | −1 (−12–4) | 0.018 | 0.71 | <0.001 |
| A5 mm | 95 | 27 | 28 (24–32) | 30 (10) | 2 (−2–6) | <0.001 | 0.80 | <0.001 |
| A10 mm | 95 | 27 | 30 (26–34) | 34 (11) | 4 (0–9) | <0.001 | 0.81 | <0.001 |
| A20 mm | 95 | 27 | 32 (28–36) | 36 (11) | 4 (0–10) | <0.001 | 0.79 | <0.001 |
| MCF mm | 95 | 27 | 35 (29–38) | 37 (12) | 3 (−1–8) | <0.001 | 0.80 | <0.001 |
Values are mean (SD) and median (25–75th percentile). CT, coagulation time; CFT, clot formation time; alpha, alpha angle; A5, clot amplitude 5 min after CT; A10, clot amplitude 10 min after CT; A20, clot amplitude 20 min after CT; MCF, maximum clot firmness; ML, maximum lysis.
Delta.
Wilcoxon signed-rank test.
Spearman correlation coefficient.
Only for tests with a runtime of 60 min.
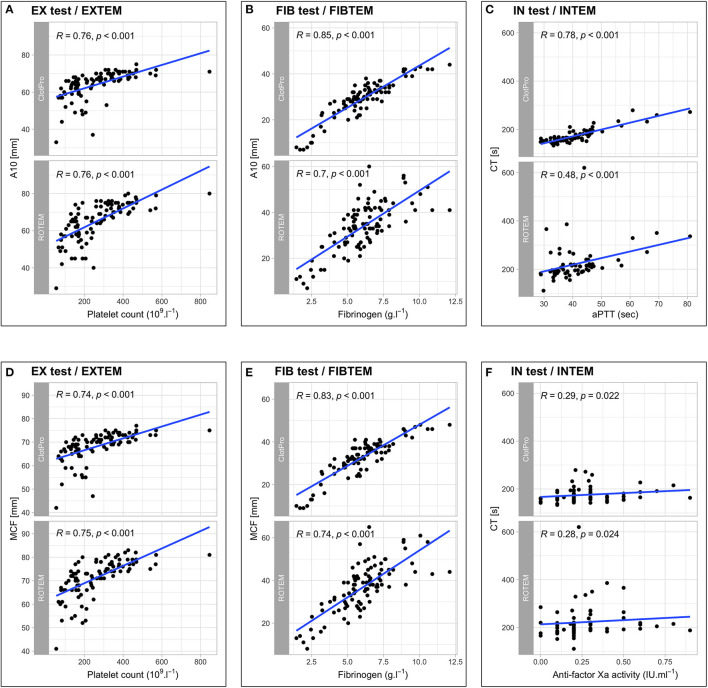
With regard to conventional coagulation tests, we found no correlation between EX test or EXTEM CT and PT (r = 0.15, p = 0.161 and r = 0.16, p = 0.132), whereas clot firmness parameters in the extrinsically activated assays (A5, A10, MCF) showed strong correlations with platelet counts. Furthermore, fibrinogen levels correlated strongly with both the FIB test and FIBTEM A10 and MCF. In contrast, intrinsically activated assays revealed differences between the two devices regarding correlations with the aPTT and anti-factor Xa activity. IN test CT showed a strong correlation with aPTT, whereas the correlation between INTEM CT and aPTT was weak. This difference in correlation remained when analyzing for subgroups of pharmacological thromboprophylaxis: LMWH r = 0.70, p < 0.001 (IN test CT) vs. r = 0.40, p < 0.001 (INTEM CT); UFH r = 0.85, p = 0.004 (IN test CT) vs. r = 0.38, p = 0.308 (INTEM CT). Regarding the correlation between IN test/INTEM CT and anti-factor Xa activity, ClotPro, and ROTEM Delta showed no relevant differences in patients receiving LMWH (r = 0.34, p = 0.012 vs. r = 0.36, p = 0.007, respectively), whereas different correlations occurred in patients receiving UFH (r = 0.75, p = 0.020 vs. r = 0.55, p = 0.125, respectively). Scatterplots and correlation coefficients between ClotPro/ROTEM measurements and conventional coagulation tests are depicted in Figure 2.
Figure 2.
Scatterplots and correlation coefficients between ClotPro/ROTEM measurements and conventional coagulation tests for EX test/EXTEM A10 and platelet count (A), FIB test/FIBTEM A10 and fibrinogen (B), IN test/INTEM CT and aPTT (C), EX test/EXTEM MCF and platelet count (D), FIB test/FIBTEM MCF and fibrinogen (E), IN test/INTEM CT and anti-factor Xa activity (F).
Discussion
To the best of our knowledge, we are the first to present a direct head-to-head comparison between the novel ClotPro analyzer and the well-established ROTEM Delta device in a clinical setting, i.e., a cohort of critically ill patients with COVID-19. Critically ill patients with COVID-19 regularly exhibit hemostatic alterations associated with an increased risk for thromboembolic complications (31), and conventional coagulation tests fail to adequately depict these complex changes (9). Hence, a multimodal diagnostic approach, including the use of viscoelastic testing, may confer benefits in the management of this specific patient cohort. However, the comparability of results obtained by different viscoelastic devices has repeatedly been questioned due to methodological discrepancies (22, 23, 28). Therefore, the assessment of correlation between results from distinct viscoelastic devices in defined patient populations is crucial.
Generally, the characteristics of patients included in the present study were similar in comparison to current literature describing the use of viscoelastic tests in critically ill patients with COVID-19. Our patients showed a higher sequential organ failure assessment (SOFA) score, a lower Horowitz index and younger age (1, 7, 10, 12, 13, 15, 32). This can be explained by the fact that the majority of patients were transferred from other hospitals to our ICUs for extracorporeal life support evaluation. Regarding conventional coagulation tests, our results corroborate those found in current literature, largely reporting high levels of fibrinogen, normal to increased platelet counts, and normal or slightly prolonged PT and aPTT (1, 7, 14, 15, 32, 33). Moreover, our viscoelastic test results are comparable with those reported by other study groups (1, 7, 11, 12, 32, 34). In line with a possible shift of haemostatic balance toward a prothrombotic state in critically ill patients with COVID-19, the median results of most viscoelastic clot strength parameters exceeded both manufacturer reference ranges and values of healthy controls (1, 7, 35).
Critical illness due to COVID-19 has been shown to result in similarly high rates of venous thromboembolism compared to critically ill patients with sepsis (36). Both patient cohorts share a number of similar pathophysiologic features, such as the excessive release of cytokines. In this regard, impaired fibrinolysis has been previously described as a hallmark of both sepsis-induced and COVID-19-associated coagulopathy (7, 10, 37). Of particular interest, impaired fibrinolysis cannot be depicted by conventional coagulation tests. In our study, fibrinolytic shutdown, previously defined as an ML <3.5% in patients with sepsis and severe trauma (37, 38), was present in 26 (96%) of the included patients at some timepoint throughout their ICU stay (extrinsic TF-activated assay; ClotPro or ROTEM Delta). Notably, however, ClotPro might provide a higher sensitivity with regard to detecting impaired fibrinolysis: 57 (82%) of ClotPro EX test results fulfilled the criteria for fibrinolytic shutdown, whereas only 32 (46%) of ROTEM Delta EXTEM results did so. These differences might be of importance when designing viscoelastically guided algorithms for the treatment of this specific patient cohort.
Patients included in the present study received heparin for pharmacological thromboprophylaxis or therapeutic anticoagulation. Despite the use of mainly intermediate doses of LMWH and UFH, we found both IN test and INTEM CT values to remain within normal values. The manufacturers of ClotPro and ROTEM provide divergent reference ranges: IN test CT 100–240 s and INTEM CT 136–187 s, which is in line with available published results of healthy controls (Bachler et al.: IN test CT 153–166 s; Lang et al.: INTEM CT 137–246 s) (7, 35). These differences might be attributable to distinct reagent compositions and might explain the poor correlation between the IN test and INTEM CT found in our study. Up to date, no evidence-based viscoelastically guided algorithms for patients with COVID-19 have been established. But literature suggesting the use of viscoelastic testing to guide prevention and treatment of thromboembolic complications in this patient cohort has emerged (39, 40). Against this background, our findings represent a clinically relevant discovery. In clinical practice, the moderate correlation of IN test/INTEM CT implies that algorithms based on results from one device cannot readily be used with results obtained from the other device. On the other hand, some statistically significant absolute differences between these two devices might not translate into clinical relevance. For example, a difference between FIB test and FIBTEM CT would almost certainly not result in changes in patient management, whereas different results of clot strength parameters (A5, A10, MCF) might lead to alterations in medical interventions in bleeding patients (41, 42).
Regarding conventional coagulation tests, we found correlations between (i) EX test/EXTEM MCF and platelet count and (ii) FIB test/FIBTEM MCF and fibrinogen that were stronger than previously reported (19). Furthermore, we found strong correlations between IN test CT and aPTT as well as anti-factor Xa activity, except for anti-Xa levels obtained in patients receiving LMWH. In contrast, INTEM CT showed moderate to no correlations with aPTT and anti-Xa levels. A previous study found moderate (r = 0.69, p < 0.001) correlations between ROTEM INTEM CT and aPTT in patients not receiving heparin and healthy volunteers, whereas in our study population ROTEM INTEM CT only showed poor correlations in comparison with aPTT (38). Our findings suggest that the novel ClotPro might show higher sensitivity for UFH than ROTEM Delta.
The limitations of the present study need to be recognized. First, the retrospective design of our study carries an inherent risk of bias. Second, baseline characteristics indicate a selection of patients that were most severely affected by COVID-19, which can be explained by the status of our institution as an ECMO referral center. Finally, our study reports results obtained from a rather small sample size. Taken together with the fact that we conducted a single-center study, this might limit the generalizability and transferability of our results. Although we are convinced that our findings add important knowledge to the field of viscoelastic testing in critically ill patients, they remain to be confirmed by future prospective multi-center trials.
In conclusion, we found several parameters obtained by the novel viscoelastic coagulation analyzer ClotPro to show strong correlations with the well-established ROTEM Delta device in critically ill patients with COVID-19. In contrast, intrinsically activated clotting times showed only weak correlations. Moreover, in a large number of parameters, considerable differences occurred between the results from the two devices. Although these results remain to be confirmed by prospective trials, our findings underline the need for the development of device-specific algorithms in defined patient cohorts.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical University of Vienna. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LI and JG: study conception and design and first draft of the manuscript. LI, CD, ES, SU, MW, CL, and JG: data collection and interpretation. LI and CD: statistical analysis. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by departmental funds of the Department of Anaesthesia, Critical Care and Pain Medicine, Division of General Anaesthesia and Intensive Care Medicine, Medical University of Vienna.
Conflict of Interest
MW received speaker's fees from Tem Innovations GmbH and Enicor GmbH. JG has received honoraria, research funding, and travel reimbursement from Alexion, Boehringer Ingelheim, CSL Behring, Johnson & Johnson, Instrumentation Laboratory, Mitsubishi Tanabe Pharma, Octapharma, and Portola. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the biomedical scientists of the ICU 9D at the University Hospital Vienna for their help and expertise and Andreas Baierl, Ph.D. (University of Vienna, Department of Statistics and Operations Research) for his support regarding statistical analyses.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.777145/full#supplementary-material
References
- 1.Heinz C, Miesbach W, Herrmann E, Sonntagbauer M, Raimann FJ, Zacharowski K, et al. Greater fibrinolysis resistance but no greater platelet aggregation in critically ill COVID-19 patients. Anesthesiology. (2021) 134:457–67. 10.1097/ALN.0000000000003685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation. (2020) 142:1609–11. 10.1161/CIRCULATIONAHA.120.050354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. (2020) 18:1738–42. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begbie M, Notley C, Tinlin S, Sawyer L, Lillicrap D. The factor VIII acute phase response requires the participation of NFkappaB and C/EBP. Thromb Haemost. (2000) 84:216–22. 10.1055/s-0037-1613999 [DOI] [PubMed] [Google Scholar]
- 5.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley C-L, Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. (2020) 395:1758–9. 10.1016/S0140-6736(20)31209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter JN, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol. (2021) 6:eabh2259. 10.1126/sciimmunol.abh2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachler M, Bosch J, Sturzel DP, Hell T, Giebl A, Strohle M, et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br J Anaesth. (2021) 126:590–8. 10.1016/j.bja.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. (2020) 46:1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. (2020) 3:e2011192. 10.1001/jamanetworkopen.2020.11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creel-Bulos C, Auld SC, Caridi-Scheible M, Barker NA, Friend S, Gaddh M, et al. Fibrinolysis shutdown and thrombosis in a COVID-19 ICU. Shock (Augusta, Ga). (2021) 55:316–20. 10.1097/SHK.0000000000001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. (2020) 231:193.e1–203.e1. 10.1016/j.jamcollsurg.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse JM, Magomedov A, Kurreck A, Munch FH, Koerner R, Kamhieh-Milz J, et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. (2020) 24:676. 10.1186/s13054-020-03401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. (2020) 18:2215–9. 10.1111/jth.15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Veenendaal N, Scheeren TWL, Meijer K, van der Voort PHJ. Rotational thromboelastometry to assess hypercoagulability in COVID-19 patients. Thromb Res. (2020) 196:379–81. 10.1016/j.thromres.2020.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almskog LM, Wikman A, Svensson J, Wanecek M, Bottai M, van der Linden J, et al. Rotational thromboelastometry results are associated with care level in COVID-19. J Thromb Thrombolysis. (2021) 51:437–45. 10.1007/s11239-020-02312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss E, Roux O, Moyer JD, Paugam-Burtz C, Boudaoud L, Ajzenberg N, et al. Fibrinolysis resistance: a potential mechanism underlying COVID-19 coagulopathy. Thromb Haemost. (2020) 120:1343–5. 10.1055/s-0040-1713637 [DOI] [PubMed] [Google Scholar]
- 17.Maatman TK, Jalali F, Feizpour C, Douglas A, II, McGuire SP, Kinnaman G, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. (2020) 48:e783–90. 10.1097/CCM.0000000000004466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duque P, Chasco-Ganuza M, Burgos-Santamaria A, Terradillos E. Increased resistance to fibrinolysis activation in patients with coronavirus disease 2019: a case series. Blood Coagul Fibrinolysis. (2021) 32:298–301. 10.1097/MBC.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 19.Mitrovic M, Sabljic N, Cvetkovic Z, Pantic N, Zivkovic Dakic A, Bukumiric Z, et al. Rotational thromboelastometry (ROTEM) profiling of COVID-19 patients. Platelets. (2021) 32:690–6. 10.1080/09537104.2021.1881949 [DOI] [PubMed] [Google Scholar]
- 20.Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J, et al. COVID-19 and hypercoagulability: a review. Clin Appl Thromb Hemost. (2020) 26:1076029620962853. 10.1177/1076029620962853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. (2020) 18:1752–5. 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillissen A, van den Akker T, Caram-Deelder C, Henriquez D, Bloemenkamp KWM, Eikenboom J, et al. Comparison of thromboelastometry by ROTEM((R)) delta and ROTEM((R)) sigma in women with postpartum haemorrhage. Scand J Clin Lab Invest. (2019) 79:32–8. 10.1080/00365513.2019.1571220 [DOI] [PubMed] [Google Scholar]
- 23.Hagemo JS, Naess PA, Johansson P, Windelov NA, Cohen MJ, Roislien J, et al. Evaluation of TEG((R)) and RoTEM((R)) inter-changeability in trauma patients. Injury. (2013) 44:600–5. 10.1016/j.injury.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 24.Gurbel PA, Bliden KP, Tantry US, Monroe AL, Muresan AA, Brunner NE, et al. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets. (2016) 27:642–9. 10.3109/09537104.2016.1153617 [DOI] [PubMed] [Google Scholar]
- 25.Neal MD, Moore EE, Walsh M, Thomas S, Callcut RA, Kornblith LZ, et al. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J Trauma Acute Care Surg. (2020) 88:279–85. 10.1097/TA.0000000000002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenk B, Gorlinger K, Treml B, Tauber H, Fries D, Niederwanger C, et al. A comparison of the new ROTEM((R)) sigma with its predecessor, the ROTEMdelta. Anaesthesia. (2019) 74:348–56. 10.1111/anae.14542 [DOI] [PubMed] [Google Scholar]
- 27.Ziegler B, Voelckel W, Zipperle J, Grottke O, Schochl H. Comparison between the new fully automated viscoelastic coagulation analysers TEG 6s and ROTEM Sigma in trauma patients: a prospective observational study. Eur J Anaesthesiol. (2019) 36:834–42. 10.1097/EJA.0000000000001032 [DOI] [PubMed] [Google Scholar]
- 28.Solomon C, Sorensen B, Hochleitner G, Kashuk J, Ranucci M, Schochl H. Comparison of whole blood fibrin-based clot tests in thrombelastography and thromboelastometry. Anesth Analg. (2012) 114:721–30. 10.1213/ANE.0b013e31824724c8 [DOI] [PubMed] [Google Scholar]
- 29.Groene P, Sappel SR, Saller T, Nitschke T, Sa PA, Paulus A, et al. Functional testing of tranexamic acid effects in patients undergoing elective orthopaedic surgery. J Thromb Thrombolysis. (2020) 51:989–96. 10.1007/s11239-020-02272-8 [DOI] [PubMed] [Google Scholar]
- 30.Oberladstatter D, Voelckel W, Schlimp C, Zipperle J, Ziegler B, Grottke O, et al. A prospective observational study of the rapid detection of clinically-relevant plasma direct oral anticoagulant levels following acute traumatic injury. Anaesthesia. (2020) 76:373–80. 10.1111/anae.15254 [DOI] [PubMed] [Google Scholar]
- 31.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. (2020) 324:799–801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsantes AE, Frantzeskaki F, Tsantes AG, Rapti E, Rizos M, Kokoris SI, et al. The haemostatic profile in critically ill COVID-19 patients receiving therapeutic anticoagulant therapy: an observational study. Medicine (Baltimore). (2020) 99:e23365. 10.1097/MD.0000000000023365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. (2020) 18:1747–51. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa TD, Cordioli RL, Campos Guerra JC, Caldin da. Silva B, Dos Reis Rodrigues R, de Souza GM, et al. Coagulation profile of COVID-19 patients admitted to the ICU: an exploratory study. PLoS ONE. (2020) 15:e0243604. 10.1371/journal.pone.0243604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang T, Bauters A, Braun SL, Potzsch B, von Pape KW, Kolde HJ, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. (2005) 16:301–10. 10.1097/01.mbc.0000169225.31173.19 [DOI] [PubMed] [Google Scholar]
- 36.Gratz J, Wiegele M, Maleczek M, Herkner H, Schochl H, Chwala E, et al. Risk of clinically relevant venous thromboembolism in critically ill patients with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne). (2021) 8:647917. 10.3389/fmed.2021.647917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamzik M, Eggmann M, Frey UH, Gorlinger K, Brocker-Preuss M, Marggraf G, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. (2010) 14:R178. 10.1186/cc9284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scala E, Coutaz C, Gomez F, Alberio L, Marcucci C. Comparison of ROTEM sigma to standard laboratory tests and development of an algorithm for the management of coagulopathic bleeding in a tertiary center. J Cardiothorac Vasc Anesth. (2020) 34:640–9. 10.1053/j.jvca.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 39.Bunch CM, Thomas AV, Stillson JE, Gillespie L, Khan RZ, Zackariya N, et al. Preventing thrombohemorrhagic complications of heparinized COVID-19 patients using adjunctive thromboelastography: a retrospective study. J Clin Med. (2021) 10:3097. 10.3390/jcm10143097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seelhammer TG, Plack D, Lal A, Nabzdyk CGS. COVID-19 and ECMO: an unhappy marriage of endothelial dysfunction and hemostatic derangements. J Cardiothorac Vasc Anesth. (2020) 34:3193–6. 10.1053/j.jvca.2020.09.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. (2017) 34:332–95. 10.1097/EJA.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 42.Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol. (2018) 182:789–806. 10.1111/bjh.15524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.