Abstract
Purpose:
To develop a reliable and reproducible statistical model to predict the survival outcome of patients with localized melanoma.
Methods:
A total of 25,734 patients with localized melanoma from the 2008 AJCC Melanoma Database were used for the model development and validation. The predictive model was developed from the model development dataset (n=14,760) contributed by 9 major institutions and study groups and was validated on an independent model validation dataset (n=10,974) consisting of patients from a separate melanoma center. Multivariate analyses based on the Cox model were performed for the model development, and the concordance correlation coefficients calculated to assess the adequacy of the predictive model.
Results:
Patient characteristics in both datasets were virtually identical, and tumor thickness was the single most important prognostic factor. Other key prognostic factors identified by stratified analyses included ulceration, lesion site and age. Direct comparisons of the predicted 5- and 10-year survival rates calculated from the predictive model and the observed Kaplan-Meier 5- and 10-year survival rates estimated from the validation dataset yielded high concordance correlation coefficients of 0.90 and 0.93 respectively. A web-based electronic prediction tool was also developed (www.melanomaprognosis.org).
Conclusions:
This is the first predictive model for localized melanoma that was developed based on a very large dataset and was successfully validated on an independent dataset. The high concordance correlation coefficients demonstrated the accuracy of the predicted model. This predictive model provides a clinically useful tool for treatment decisions, patient risk assessment, and for planning and analysis of clinical trials.
Keywords: localized melanoma, predictive model, model validation, electronic prediction tool
INTRODUCTION
Myriad clinical and pathologic features affecting melanoma patient survival have been studied extensively for more than three decades at major melanoma centers around the world. With the aid of powerful statistical techniques, remarkable progress has been made in the identification of dominant prognostic factors that characterize the natural history and outcome of melanoma.1–13 Most of the large melanoma series were analyzed with multivariate regression analysis methods so that the relative importance of the prognostic factors considered could be accurately assessed. With an application of the Cox regression model, almost all major multivariate prognostic factor studies have identified a remarkably consistent set of independent prognostic factors for melanoma patients treated worldwide.14 These advances, in turn, have facilitated a fundamental revamping in the staging of melanoma and criteria used for interpreting results of prospective clinical trials in melanoma with dominant prognostic factors identified by Cox regression analyses.
Although a great deal of research attention has been focused on identification of dominant prognostic factors, few researchers have developed practical and reliable models for predicting patient survival and disease recurrence in melanoma. The first statistical model and scoring system for predicting outcome in patients with localized melanoma was developed in 1985.15 Various predictive models of survival for localized melanoma have been subsequently developed by investigators in the United States and other countries.16–21 Most of these models were developed based on a relatively small number of patients and were not validated. In 1992 a generalized multivariable prognostic model was developed for localized melanoma to address both survival following diagnosis and outcome after a disease-free interval.22 The model was developed on the basis of a combined database of 4,568 patients from the Sydney Melanoma Unit (SMU) and the University of Alabama at Birmingham (UAB).
Since 2000, we have collaborated with melanoma clinical investigators worldwide to create a unique melanoma staging and prognosis database under the auspices of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer. The first version of this Melanoma Staging Database incorporated the clinical and pathologic results of more than 17,600 prospectively observed melanoma patients treated on three continents. The results using the Cox regression methodology led to a major revision of the melanoma staging criteria and stage grouping. These results were first published in the Journal of Clinical Oncology in 2001.12, 23 A methodologic study of a parametric model (the generalized gamma regression model) as a potential alternative to the Cox regression model for melanoma prognosis and modeling was also done using this database.24 Since 2007, an updated AJCC Melanoma Staging Database has been created that contains pathology and treatment outcome data on more than 50,000 prospectively observed melanoma patients treated in the United States, Australia, and Europe. The database was finalized in 2008, and a series of statistical analyses for staging and prognostic modeling were subsequently performed. The revised AJCC melanoma staging system based on the analyses of this database has recently been published.25
This paper summarizes the results of developing and validating a reliable and reproducible statistical model to predict survival outcome of patients with localized melanoma. Development of a web-based electronic prediction tool based on this predictive model is also described. The predictive model contains several key prognostic factors that were not included in the AJCC melanoma staging system and thus provides more accurate survival estimation for patients with localized melanoma compared to the AJCC melanoma staging system.
METHODS
AJCC Melanoma Database
The model predicting outcome from initial diagnosis for patients with localized melanoma was developed using the data from the current 2008 AJCC melanoma database. The cohort of data with localized melanoma (Stage I and II) consists of 31,337 patients, 25,734 (82%) of whom had information available for all of the factors required for the model development and validation. Of the 25,734 patients included in this analysis, 18,965 (73.7%) were diagnosed prior to 2002. Ten institutions and cooperative study groups contributed patients to this study. The following prognostic factors were included in the multivariate analysis of localized melanomas: age, gender, primary melanoma site, primary tumor thickness, level of invasion, and primary tumor ulceration. Axial lesion site includes trunk, head and neck. Survival times were calculated from onset of primary melanoma diagnosis and considered censored for patients who were alive at the last follow-up or who died without evidence of melanoma. Staging evaluation, including indication for sentinel node biopsy and recurrence monitoring, were performed based on respective institutional guidelines, but were generally consistent with those promulgated by the National Comprehensive Cancer Network (NCCN) and other guideline-setting organizations.
Description of the Statistical Model
The derived statistical model for predicting survival rates in patients with localized or regional melanoma was based on the well-known proportional hazard model proposed by Cox.26 The introduction of the Cox model represents the most important methodological development in the area of survival data analysis for more than three decades. It permits nonparametric assessment of survival data and allows the statistical inference to be restricted to the effect of concomitant information (e.g., prognostic factors) without knowledge of the form of survival distribution. The Cox model has been shown to be well suited to serve as a basis for evaluating prognostic factors and for developing predictive models for melanoma.1,3,7,8,15,21,22 The survival function of the Cox model can be described as follows:
where X1, X2, …, Xp are the values for p measured patient characteristics (or prognostic factors), are the mean values of those variables; β1, β2, …, βp are regression coefficients to be estimated from the data; S0 (t) is a baseline survival function to be estimated from data; and S(t) is the probability that a patient with given set of characteristics X1, X2, …,Xp survives to at least time t.
Multivariate Analyses and Modeling
The predictive model for localized melanoma was developed from the model development (training) dataset (n=14,760) consisting of data from nine major institutions and cooperative study groups from the United States and was validated on the model validation (testing) dataset (n=10,974) comprising patients treated at the Sydney Melanoma Unit (SMU), Australia. The list of these institutions and groups is shown in Table 1. Key patient characteristics for both datasets were compared and a multivariate analysis based on the Cox regression model performed for each dataset. The results of multivariate analyses from both datasets were also compared. It has been well established in virtually all melanoma studies that tumor thickness is the single most important prognostic factor in localized melanoma. We postulated that other key factors may have varying degrees of prognostic value among patients subgrouped by tumor thickness. To develop a more accurate predictive model, stratified multivariate survival analyses based on the Cox model were performed for the six subgroups of patients divided according to tumor thickness (0.01–0.50mm, 0.51–1.00mm, 1.01–2.00mm, 2.01–3.00mm, 3.01–6.00mm and >6.00mm) using the model development dataset. A submodel was developed for each of the six subgroups of patients, resulting in six submodels for the overall predictive model. The overall validity of the six predictive submodels developed was tested on the independent model validation dataset. The predicted 5- and 10-year survival rates were calculated for various combinations of significant factors within each thickness subgroup based on the model developed from the model development dataset. The Kaplan-Meier 5- and 10-year survival rates were also estimated for the corresponding combination of factors for each subgroup in the model validation dataset. Concordance correlation coefficients were calculated based on the direct comparisons of the predicted 5- and 10-year survival rates and the observed 5- and 10-year survival rates estimated from the validation dataset.
Table 1.
Institutions/Study Groups contributing to the model development and validation datasets for localized melanoma.
| 1. Model Development (Training) Dataset (n=14,760) |
| This data set consists of data from the following 9 institutions and study groups: |
| 1) Memorial Sloan Kettering Cancer Center |
| 2) The University of Texas M. D. Anderson Cancer Center |
| 3) University of Pennsylvania |
| 4) Sunbelt Melanoma Group |
| 5) Sentinel Lymph Node Working Group |
| 6) University of Michigan |
| 7) Moffitt Cancer Center |
| 8) University of Alabama at Birmingham |
| 9) Intergroup Melanoma Clinical Trial Group |
| 2. Model Validation (Testing) Dataset (n=10,974) |
| This dataset consists of patients treated at Sydney Melanoma Unit (SMU) |
Web-based Electronic Prediction Tool
To facilitate access and usefulness of the predictive model by clinicians, patients and clinical researchers, an electronic prediction tool was developed based on the data generated from the predictive model and implemented on the worldwide web for public usage. The web-based electronic prediction tool page contains: introduction of the tool, usage disclaimer, option selection for survival prediction for localized melanoma or regional melanoma (description of the model development for regional melanoma is not included in this paper and will be published separately), user input of key prognostic features of an individual patient, and an output of estimated 1-, 2-, 5- and 10-year survival rates (with 95% confidence intervals) for this patient based on the input data.
RESULTS
Patient populations from the model development dataset and the model validation datasets were very similar. As shown in Table 2, distributions of key patient characteristics in these two datasets were virtually identical with the exception that there appeared to be a higher percentage of axial melanoma in the model validation dataset as compared to that in the model testing dataset (60.8% vs. 48.5%, respectively, p<0.001).
Table 2.
Comparisons of patient characteristics in the model development and validation datasets for localized melanoma
| Variable | Model Development (Training) Dataset (n=14,760) % | Model Validation (Testing) Dataset (n=10,974) % |
|---|---|---|
| Age | ||
| <50 | 44.9 | 45.0 |
| 50–59 | 20.7 | 20.4 |
| 60–69 | 19.1 | 18.0 |
| 70–79 | 11.8 | 12.6 |
| ≥80 | 3.6 | 4.0 |
| Gender | ||
| Male | 54.2 | 52.9 |
| Female | 45.8 | 47.1 |
| Lesion Site | ||
| Axial (trunk, head, neck) | 60.8 | 48.3 |
| Extremity | 39.2 | 51.7 |
| Tumor Thickness (mm) | ||
| 0–0.50 | 22.3 | 18.1 |
| 0.51–1.00 | 23.3 | 28.1 |
| 1.01–2.00 | 29.6 | 26.4 |
| 2.01–3.00 | 11.9 | 12.5 |
| 3.01–6.00 | 9.9 | 11.7 |
| >6.00 | 3.2 | 3.1 |
| Ulceration | ||
| No | 80.8 | 80.3 |
| Yes | 19.2 | 19.7 |
| Level of Invasion | ||
| II | 22.0 | 20.7 |
| III | 28.8 | 33.6 |
| IV | 44.8 | 40.7 |
| V | 4.3 | 5.0 |
| Stage | ||
| IA | 36.0 | 37.4 |
| IB | 33.4 | 30.1 |
| IIA | 15.7 | 16.3 |
| IIB | 10.3 | 11.1 |
| IIC | 4.6 | 5.1 |
The results of multivariate analyses for both datasets were also very similar. Using the Cox regression model to analyze the model development dataset of 14,760 patients with localized melanoma, the following six factors were identified that significantly affected survival: tumor thickness (χ2=232.9; p<0.000001), ulceration (χ2=126.2; p<0.000001), age (χ2=91.9; p<0.000001), lesion site (χ2=45.5; p<0.00001), gender (χ2=23.9; p<0.00001), and level of invasion (χ2=21.2; p<0.00001). Similar procedures were used to analyze the model validation dataset of 10,974 patients. There were five significant factors from this dataset: tumor thickness (χ2=229.4, p<0.000001), ulceration (χ2=95.0, p<0.000001), lesion site (χ2=41.0, p<0.00001), age (χ2=27.9, p<0.0001) and gender (χ2=19.8, p<0.0001). The ranking of the top four most significant factors identified from this dataset were very similar to those from the model development dataset. For both datasets, tumor thickness was the most significant prognostic factor followed by ulceration; Clark’s level was the least significant. The results of these multivariate analyses with variable codings of the Cox model are given in Table 3. The estimated survival curves subgrouped by tumor thickness (0.01–0.50mm, 0.51–1.00mm, 1.01–2.00mm, 2.01–3.00mm, 3.01–6.00mm, and >6.00mm) for both the model development dataset and the model validation dataset indicated an extremely high correlation between tumor thickness and patient survival (p<0.000001) (Figure 1). The clear separations of survival curves by these six tumor thickness subgroups were overwhelmingly significant for both datasets.
Table 3.
Multivariate Cox regression analyses for localized melanoma by dataset
| Chi-square values (Wald) | |||
|---|---|---|---|
| Variable* | Degree of Freedom (DF) | Model Development (Training) Dataset (n=14,760) % | Model Validation (Testing) Dataset (n=10,974) % |
| Tumor Thickness | 5 | 232.9 | 229.4 |
| Ulceration | 1 | 126.2 | 95.0 |
| Age | 4 | 91.9 | 27.9 |
| Lesion Site | 1 | 45.5 | 41.0 |
| Gender | 1 | 23.9 | 19.8 |
| Level of Invasion | 3 | 21.2 | 5.5 |
Variable coding in the Cox regression model: Tumor thickness (1=0–0.50mm; 2=0.51–1.00mm; 3=1.01–2.00mm; 4= 2.01–3.00mm; 5=3.00–6.00mm; 6=>6.00mm); Ulceration (0=no; 1=yes); Age (1=<50; 2=50–59; 3=60–69; 4=70–79; 5=≥80); Lesion site (0=extremity; 1=axial); Gender (0=female; 1=male); and Level of invasion (1=level II; 2=level III; 3=level IV; 4=level V)
Figure 1.
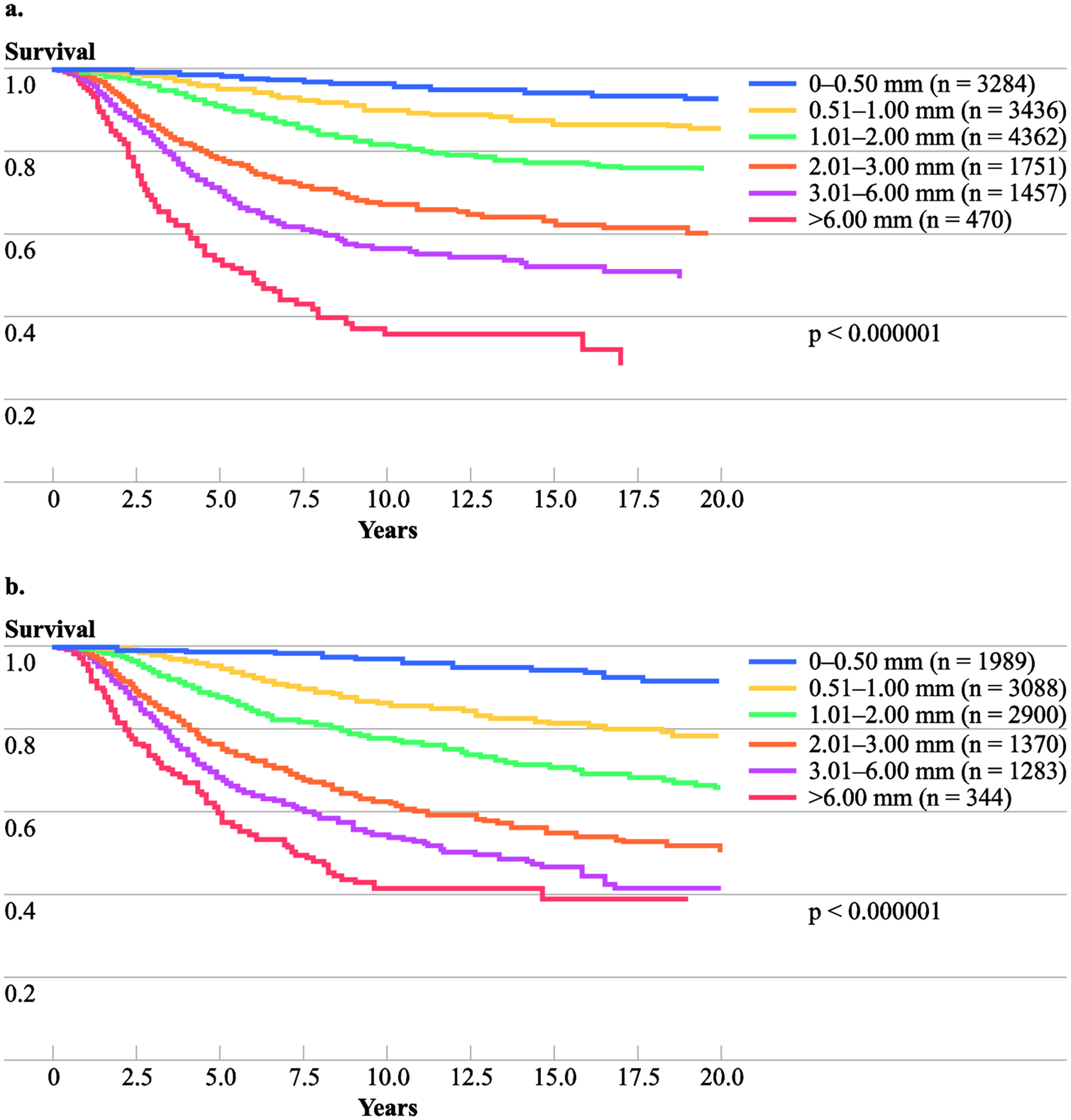
Kaplan-Meier survival curves by tumor thickness group; (A) Model development dataset; (B) Model validation dataset
To increase the accuracy of the overall predictive model, multivariate analyses based on the Cox regression model were performed for each of the above six tumor thickness subgroups, and the results of the six submodels developed are shown in Table 4. Ulceration was significant in all of the thickness subgroups. Lesion site remained significant in all subgroups, with the exception of patients with tumor thickness less than or equal than 0.50mm and those with tumor thickness greater than 6.00mm. Age was a significant factor in all tumors less than 6.00mm. Ulceration, lesion site and age were significant in all four subgroups of patients with tumor thickness greater than 0.50mm and less than 6.00mm. Thus, the overall predictive model developed for localized melanoma consisted of six submodels stratified according to tumor thickness. The significant factors (covariates) with their codings and estimated regression coefficients for each submodel are given in Table 4.
Table 4.
Prognostic submodels for patients with localized melanoma by tumor thickness subgroup based on the Cox regression model.
| Thickness subgroup (mm) | Covariate (Xi) and coding | Regression coefficient (95% CI*) | Hazard Ratio (95% CI) |
|---|---|---|---|
| ≤0.50 (n=3284) |
Ulceration (0: No; 1:Yes) | 1.24 (0.08–2.41) | 3.46 (1.08–11.10) |
| Age (0: <60; 1: ≥60) | 1.41 (0.90–1.93) | 4.11 (2.46–6.86) | |
| 0.51–1.00 (n=3436) |
Ulceration (0: No; 1:Yes) | 1.11 (0.65–1.56) | 3.02 (1.92–4.75) |
| Lesion site (0: Extremity; 1: Axial) |
0.92 (0.55–1.29) | 2.51 (1.73–3.65) | |
| Age (0: <60; 1: ≥60) | 0.62 (0.28–0.96) | 1.85 (1.32–2.60) | |
| 1.01–2.00 (n=4362) |
Ulceration (0: No; 1:Yes) | 0.97 (0.76–1.17) | 2.63 (2.13–3.24) |
| Lesion site (0: Extremity; 1: Axial) |
0.71 (0.49–0.94) | 2.04 (1.63–2.57) | |
| Age (0: <70; 1: ≥70) | 0.76 (0.49–1.03) | 2.14 (1.63–2.81) | |
| 2.01–3.00 (n=1751) |
Ulceration (0: No; 1:Yes) | 0.54 (0.32–0.75) | 1.71 (1.37–2.13) |
| Lesion site (0: Extremity; 1: Axial) |
0.20 (−0.04–0.44) | 1.22 (0.97–1.55) | |
| Age (0: <70; 1: ≥70) | 0.57 (0.30–0.85) | 1.77 (1.35–2.33) | |
| 3.01–6.00 (n=1457) |
Ulceration (0: No; 1:Yes) | 0.56 (0.33–0.79) | 1.75 (1.391–2.21) |
| Lesion site (0: Extremity; 1: Axial) |
0.28 (0.05–0.52) | 1.33 (1.06–1.68) | |
| Age (0: <70; 1: ≥70) | 0.48 (0.21–0.75) | 1.61 (1.23–2.11) | |
| >6.00 (n=470) |
Ulceration (0: No; 1:Yes) | 0.56 (0.21–0.91) | 1.75 (1.23–2.48) |
CI: confidence interval
The direct comparisons of the predicted 5- and 10-year survival rates using the predictive model and the observed 5- and 10-year survival rates estimated from the validation dataset yielded concordance correlation coefficients (CCC) of 0.90 and 0.93 for 5-` and 10-year survival rates, respectively. These high concordance correlation coefficients indicated high accuracy and precision of the predictive model developed for the localized melanoma; graphical illustration of the excellent concordance correlation for the 10-year survival rate is shown in Figure 2.
Figure 2.
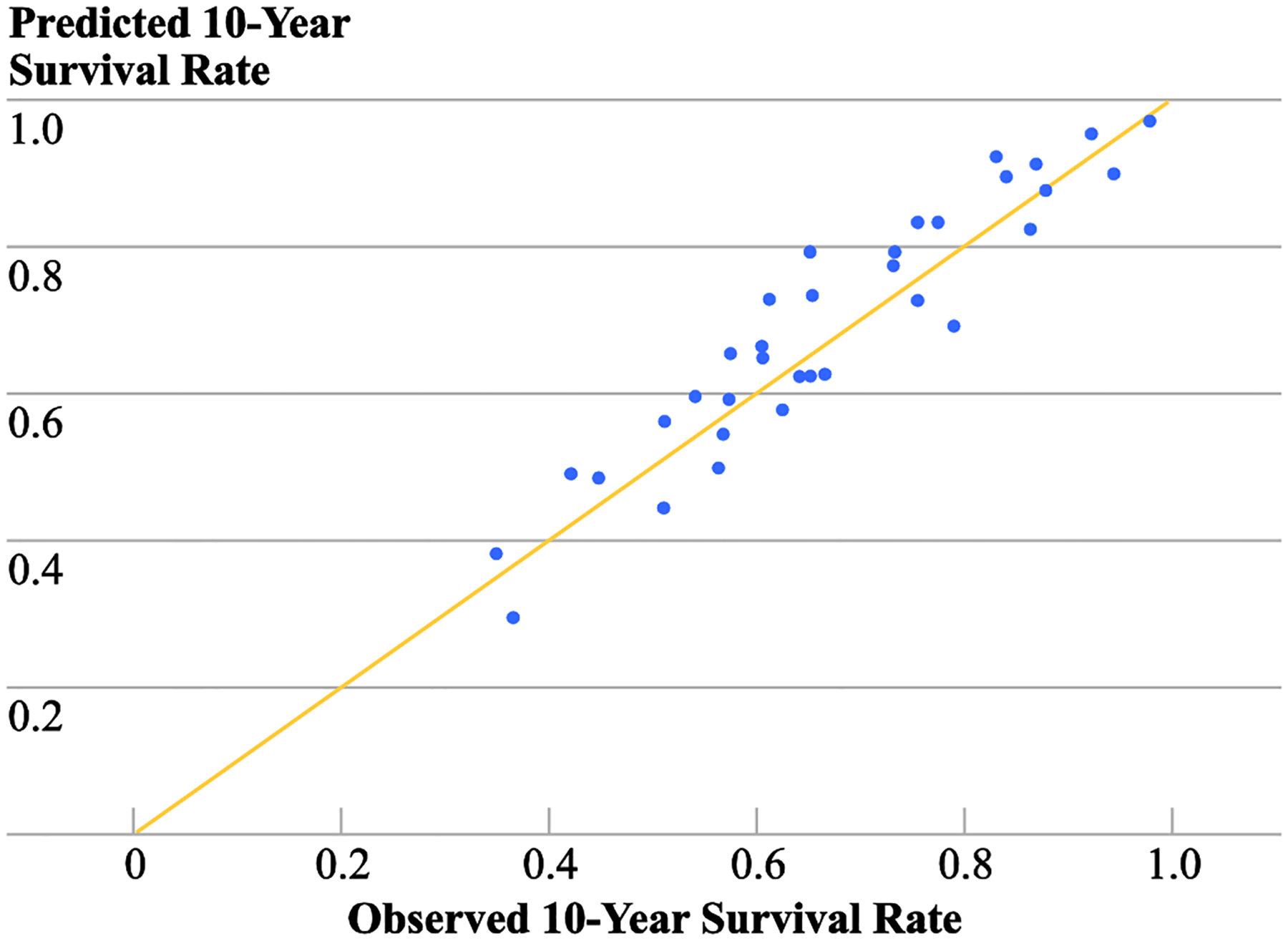
Concordance correlation plot of the predicted and observed 10-year survival rates by combination of prognostic factors within thickness groups
An electronic prediction tool was programmed based on the predictive model developed for localized melanoma. A website (www.melanomaprognosis.org) was created for easy access to this prediction tool. Users simply enter the individual patient’s key prognostic features, and the tool will instantaneously provide the output of the patient’s estimated 5- and 10-year survival rates with 95% confidence intervals (CI). Examples of the estimated 1-, 2-, 5- and 10-year survival rates and the associated confidence intervals for patients with various characteristics are given in Table 5. For instance, for a 54 year-old patient with a 0.8 mm, non-ulcerated, extremity melanoma, the projected 1-,−2-, 5- and 10-year survival rates are 99.8% (CI: 99.7%−99.9%), 99.6% (CI: 99.4%−99.8%), 98% (95% CI: 97%−99%) and 96% (95% CI: 95%−97%), respectively. In contrast, for a 60 year-old patient presenting with 7.0mm, ulcerated, melanoma of the extremity, the predicted 1-, 2-, 5- and 10-year survival rates are 88% (CI: 84%−92%), 75% (CI: 70%−80%), 47% (95% CI: 40%−55%), and 30% (95% CI: 22%−40%), respectively. Development of the application of this prediction tool for an electronic handheld device is currently underway.
Table 5.
Examples of predicted 1, 2, 5 and 10-year survival rates for individual patients with localized melanoma.
| Patient | Tumor Thickness | Ulceration | Lesion Site | Age | Predicted Survival Rate (%) (95% CI*) | |||
|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year 5-Year | 10-Year | ||||||
| 1 | 0.8 | No | Extremity | 54 | 99.8 (99.7–99.9) | 99.6 (99.4–99.8) | 98.1 (97.4–98.8) | 95.9 (94.4–97.4) |
| 2 | 1.5 | Yes | Extremity | 45 | 98.7 (98.3–99.2) | 96.9 (96.0–97.8) | 89.2 (86.6–91.7) | 79.7 (75.3–84.3) |
| 3 | 2.2 | No | Axial | 75 | 95.7 (94.2–97.3) | 87.5 (84.0–91.2) | 71.7 (65.2–78.7) | 59.6 (51.4–69.1) |
| 4 | 3.7 | Yes | Axial | 39 | 93.0 (91.3–94.8) | 85.3 (82.5–88.1) | 62.5 (57.8–67.6) | 49.6 (43.9–55.9) |
| 5 | 7.0 | Yes | Extremity | 60 | 88.1 (84.6–91.8) | 75.1 (70.0–80.7) | 47.2 (40.4–55.1) | 30.0 (22.4–40.2) |
CI: confidence interval
DISCUSSION
This is the first predictive model for localized melanoma that was developed based on a very large dataset and was successfully validated on an equally large independent dataset. The high concordance correlation coefficients demonstrated high accuracy of the predicted model we developed.
The 2008 AJCC Melanoma Database that we created and used for defining an evidence-based staging system and for predictive model development within different stages of melanoma is the world’s largest melanoma research database with combined data on over 50,000 melanoma patients treated at major melanoma centers on three continents.25 Relatively few melanoma predictive models have been published. Most such models were developed with a small number of patients (n<1,000); importantly, some did not include ulceration, an important independent prognostic factor for localized melanoma. Our study of predictive model development and validation for localized melanoma was based on a substantially large number of patients (n>25,000) with complete information on key prognostic features and patient follow-up derived from the 2008 AJCC Melanoma Database.
An important feature of our predictive model was that it was successfully validated on an independent dataset. Although there are several other validation methods based on division of the existing dataset (e.g., split-group method, jackknife method, and bootstrap method), the best method is one that tests the performance of the model with an independent dataset from a different institution.
The current AJCC melanoma staging system provides important initial risk classification of patients (e.g., stages IA, IB, IIA, IIB, and IIC for localized melanoma) based on anatomic factors. Our predictive model for localized melanoma includes not only patients’ key clinical features but also significant pathologic factors and thus provides much more accurate survival estimation that is customized for an individual patient compared to the AJCC staging system.
Our predictive model was developed based on the well-known Cox regression model. The Cox model has been used extensively in melanoma research over the past three decades and has been widely accepted as an excellent model to study multivariate melanoma prognosis and modeling. We recently investigated a class of parametric models as a potential alternative to the Cox model for melanoma prognosis and modeling. The comparative study revealed that the Cox model performed equally well compared to the best parametric model (the generalized gamma model) we identified for localized melanoma prognosis and modeling.24
One of the most important facets of the predictive model is its usefulness in a clinical setting. Prediction of the clinical course of disease and treatment outcome is an essential part of medical practice. Physicians face daily decisions as to the selection of an appropriate treatment and follow-up strategy for a particular patient. The predictive model generates a prognostic summary analysis for an individual patient based on that patient’s presenting characteristics. For example, a physician may choose to use these projections of survival in conjunction with other factors, including the morbidity planned stage-specific treatment, to guide the selection of an appropriate treatment for a given patient.
On the basis of this predictive model, a clinical scoring system representing an individual patient’s prognosis with localized melanoma can be generated based on the 10-year survival rate predicted by the model for that patient. For example, a patient is assigned a score of 80 if this patient’s predicted 10-year survival rate is 80%. The projected 10-year survival rate was proposed as a clinical score because in localized melanoma 10 years of follow-up is generally considered sufficient for adequate patient evaluation. Thus, the proposed clinical score could be considered a composite prognostic indicator of several dominant prognostic factors in localized melanoma, and it represents the probability of a patient’s long-term survival. Because the predictive model that generated the score is highly predictable, the scoring system itself is highly reproducible. It is also easy to remember because the score is simply the value of the patient’s predicted 10-year survival rate.
There are several important applications of the predictive model and this clinical scoring system. A practical patient risk classification system can be generated based on this clinical scoring system. For example, based on clinical scores, five patient risk groups can be defined as follows: patients with a clinical score of 80 to 100 are assigned to Risk Group I, 60–79 as Risk Group II, 40–69 as Risk Group III, 20–39 as Risk Group IV, and 0–19 as Risk Group IV. This patient risk classification system is obviously more accurate and useful compared to the traditional AJCC staging system because it contains additional information on other significant prognostic factors that cannot be used within the current constraints of the overall AJCC staging system criteria. In addition, one can create as many risk groups using the clinical score as one desires depending on proposed applications. For example, the risk classification can be used by clinicians to devise an appropriate treatment and follow-up plan, to select an appropriate patient population for a clinical trial, or to define a simple stratification criteria for patient randomization in clinical trials. Furthermore, it can be used to define patient subgroups for comparing the effectiveness of treatments within each subgroup in a clinical trial. In the multivariate analysis of clinical trial data, one can incorporate the patient’s actual clinical score in the regression model and compare the effectiveness of treatments adjusting for clinical score and other additional prognostic factors in the specific clinical trial patient population.
Another important application of this model is its potential use for life and health insurance purposes. Many studies show that a diagnosis of localized melanoma is not necessarily fatal and disease-free survival for many years is highly probable. For example, a significant portion of the patient population belongs to the Risk Group I as defined above. These patients have an average 5-year survival rate of at least 95% and a 10-year survival rate of at least 87%. Thus, this model can be used to generate actuarial life tables that more accurately reflects patient risk and may facilitate issuance and maintenance of a reasonable insurance policy by a patient with a diagnosis of melanoma.
In summary, the predictive model described in this paper represents the most comprehensive survival model developed for localized melanoma to date. The model was developed based on a very large dataset and was successfully validated on an equally large independent dataset. It provides accurate survival estimation for patients with localized melanoma. The electronic prediction tool developed based on this model is publically accessible through a user-friendly website. This prediction tool, coupled with the predictive model for predicting disease recurrence and survival following a specific period of disease-free survival (currently under development), will provide a comprehensive projection of an individual patient’s clinical outcomes based upon actuarial survival rates. The individualized information generated for a specific patient will significantly enhance the clinicians’ abilities to plan an optimum treatment and follow-up schedule for that patient. It can also provide crucial information for clinical investigators in the design and interpretation of melanoma clinical trials.
Synopsis.
A predictive survival model for localized melanoma was developed and validated based on the large AJCC Melanoma Database. High concordance correlation coefficients demonstrated high accuracy of this predictive model. A web-based electronic prediction tool is available on www.melanomaprognosis.org.
ACKNOWLEDGEMENTS
1. The following institutions and study groups generously contributed their patients to the 2008 AJCC Melanoma Database, and the localized melanoma data included in this study were contributed by the institutions and study groups with * indicated:
*Sydney Melanoma Unit, Sydney, Australia, (John F. Thompson, MD)
Istituto Nazionale Tumori, Milan Italy, (Natale Cascinelli, MD)
San Pio X Hospital, Milan Italy (Natale Cascinelli, MD)
*Memorial Sloan-Kettering Cancer Center, New York, NY, Daniel G. Coit, MD)
*The University of Texas M. D. Anderson Cancer Center, Houston, TX (Jeffrey E. Gershenwald, MD, Merrick I. Ross, MD, and Marcella Johnson)
John Wayne Cancer Institute, Santa Monica, CA (Donald L. Morton, MD)
Netherlands Cancer Institute, Amsterdam, The Netherlands (Omgo Niewig, MD)
*University of Pennsylvania Hospital, Philadelphia, PA (Keith Flaherty, MD, and Phyllis A. Gimotty, PhD)
*University of Michigan, Ann Arbor, MI (Timothy Johnson, MD)
*H. Lee Moffitt Cancer Center, Tampa, FL (Vernon K. Sondak, MD, and Douglas S. Reintgen, MD)
*University of Alabama at Birmingham, Birmingham, AL (Charles M. Balch, MD, Seng-jaw Soong, PhD, and Marshall Urist, MD)
Eastern Cooperative Oncology Group (John M. Kirkwood, MD, and Michael B. Atkins, MD).
*Sunbelt Melanoma Trial Group (Kelly M. McMasters, MD)
*Sentinel Lymph Node Working Group (Stanley Leong, MD)
*Intergroup Melanoma Surgical Trial Group (Charles M. Balch, MD, and Seng-jaw Soong, PhD)
National Cancer Institute, Naples, Italy (Corrado Caraco, PhD, MD)
2. The planning and development of the AJCC Staging System and predictive model has been guided by the AJCC Melanoma Task Force Committee consisting of the following members: Charles M. Balch, MD (Chair), Jeffrey E. Gershenwald, MD (Vice-chair), Seng-jaw Soong, PhD (Vice-chair), Michael B. Atkins, MD, David R. Byrd, MD, Antonio C. Buzaid, MD, Natale Cascinelli, MD, Alistair J. Cochran, MD, Daniel G. Coit, MD, Alexander M. M. Eggermont, MD, David Frishberg, MD, Keith T. Flaherty, MD, Phyllis A. Gimotty, PhD, Allan C. Halpern, MD, Alan N. Houghton, Jr, MD, Marcella M. Johnson, MS, John M. Kirkwood, MD, Kelly M. McMasters, MD, Martin F. Mihm, Jr. MD, Donald L. Morton, MD, Merrick I. Ross, MD, Arthur J. Sober, MD, Vernon K. Sondak, MD, Kristen Stephens, CTR, John F. Thompson, MD.
3. We thank Troy Bland, computer specialist, for programming the electronic prediction tool and for maintaining the website, and Connie Pitts for her assistance in manuscript preparation.
4. Supported in part by a grant from the AJCC, by unrestricted grants from Schering Plough to the AJCC, and by research funds from the Comprehensive Cancer Center of the University of Alabama at Birmingham.
REFERENCES
- 1.Balch CM, Murad TM, Soong SJ, Ingalls AL, Halpern NB, Maddox WA. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark’s and Breslow’s staging methods. Ann Surg 1978; 88(6):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eldh J, Boeryd B, Peterson LE. Prognostic factors in cutaneous malignant melanoma in stage I. A clinical, morphological and multivariate analysis. Scand J Plast Reconstr Surgery 1978;12(3):243–255. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery 1980;86(2):343–351. [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA. A multifactorial analysis of melanoma. III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg 1981;193(3):377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Der Esch EP, Cascinelli N, Preda F, Morabito A, Bufalina R. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer 1981;48(7):1668–1673. [DOI] [PubMed] [Google Scholar]
- 6.Drzewiecki KT, Andersen PK. Survival with malignant melanoma: a regression analysis of prognostic factors. Cancer 1982;49(11):2414–2419. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Soong SJ, Milton GW, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg 1982;196(6):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch CM, Soong SJ, Murad TM, Smith JW, Maddox WA, Durant JR. A multifactorial analysis of melanoma. IV. Prognostic factors in 200 melanoma patients with distant metastases (stage III). J Clin Oncol 1983;1(2):126–134. [DOI] [PubMed] [Google Scholar]
- 9.Coit DG, Rogatko A, Brennan MF. Prognostic factors in patients with melanoma metastatic to axillary or inguinal lymph nodes. A multivariate analysis. Ann Surg 1991;214(5):627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton DL, Davtyan DG, Wanek LA, et al. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer 71:3737, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Buttner P, Garbe C, Bertz J, et al. Primary cutaneous melanoma. Optimized cutoff points of tumor thickness and importance of Clark’s level for prognostic classification. Cancer 1995;75:2499. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19(16):3622–3634. [DOI] [PubMed] [Google Scholar]
- 13.Gershenwald JE, Balch CM, Soong SJ, Thompson JF. Chapter 3: Prognostic factors and natural history. In: Balch CM, Houghton AN, Sober AJ, Soong SJ, Atkins MB, Thompson JF, eds. Cutaneous Melanoma (5th Edition). Missouri: Quality Medical Publishing, Inc., 2009: 35–64. [Google Scholar]
- 14.Balch CM, Milton GW, Shaw HM, Soong SJ, eds. Cutaneous Melanoma: Clinical Management and Treatment Results Worldwide. Pennsylvania: JB Lippincott Co, 1985. [Google Scholar]
- 15.Soong SJ. A computerized mathematical model and scoring system for predicting outcome in melanoma patients. In: Balch CM, Milton GW, eds. Cutaneous Melanoma: Clinical Management and Treatment Results Worldwide. Pennsylvania: JB Lippincott, 1985:353. [Google Scholar]
- 16.Clark WH Jr, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based upon tumor progression. J Natl Cancer Inst 1989; 81:1893–904. [DOI] [PubMed] [Google Scholar]
- 17.MacKie RM, Aitchison T, Sirel JM, McLaren K, Watt DC. Prognostic models for subgroups of melanoma patients from the Scottish Melanoma Group database 1979–86, and their subsequent validation. Br J Cancer 1995; 71:173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 1996; 78:427–32. [DOI] [PubMed] [Google Scholar]
- 19.Schuchter L, Schultz DJ, Synnestvedt M, Trock BJ, Guerry D, Elder DE, Elenitsas R, et al. A prognostic model for predicting 10-years survival in patients with primary melanoma. The Pigmented Lesion Group. Ann Intern Med 1996; 125:369–75. [DOI] [PubMed] [Google Scholar]
- 20.Sahin S, Rao B, Kopf AW, Lee E, Rigel DS, Nossa R, Rahman IJ, et al. Predicting ten-year survival of patients with primary cutaneous melanoma: a corroboration of a prognostic model. Cancer 1997; 80:1426–31. [PubMed] [Google Scholar]
- 21.Soong SJ, Zhang Y, Desmond R. Models for Predicting Outcome. In Balch CM, Houghton AN, Sober A, Soong SJ, eds. Cutaneous Melanoma, 4th Edition. St. Louis, MO: Quality Medical Publishing, Inc., 2003:77–90. [Google Scholar]
- 22.Soong SJ, Shaw HM, Balch CM, McCarthy WH, Urist MM, Lee JY. Predicting survival and recurrence in localized melanoma: A multivariate approach. World J Surg 1992;16:191–5. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19:3635–48. [DOI] [PubMed] [Google Scholar]
- 24.Ding S, Soong SJ, Lin HY, Desmond R, Balch CM. Parametric modeling of localized melanoma prognosis and outcome. J Biopharm Stat 2009; 19:732–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol In Press. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables (with discussion). J R Statist Soc 1972; B34:187. [Google Scholar]


