Objectives:
Single-injection erector spinae plane block (ESPB) provides good control of pain relief after open thoracotomy surgeries. However, the duration of pain relief does not last long. For this purpose, we hypothesized that adding α2-adrenoceptor agonist, dexmedetomidine, for interfascial nerve blockade may increase the duration of analgesia. There are only few studies using dexmedetomidine for interfasical nerve blocks in humans. In this study, our aim is to investigate whether addition of dexmedetomidine to ropivacaine for ESPB could effectively prolong the duration of postoperative analgesia and reduce opioid consumption after open thoracotomy.
Materials and Methods:
Sixty patients with esophageal cancer were randomized to receive ESPB using 28 mL of 0.5% ropivacaine, with 2 mL of normal saline (group R) or 0.5 µg/kg dexmedetomidine in 2 mL (group RD) administered interfascially. ESPB was performed at the fifth thoracic level under ultrasound guidance. The primary outcome was the duration of analgesia. The secondary outcomes were total postoperative sufentanil consumption, Numeric Rating Scale pain scores, Ramsay Sedation Scale scores and adverse effects.
Results:
The duration of analgesia in group RD (505.1±113.9) was longer than that in group R (323.2±75.4) (P<0.001). The total postoperative sufentanil consumption was lower in group RD (23.3±10.0) than in group R (33.8±13.8) (P=0.001). There was no significant difference in the incidence of adverse effects between the 2 groups.
Conclusion:
After open thoracotomy, addition of dexmedetomidine to ropivacaine for ESPB effectively prolonged the duration of postoperative analgesia and reduced opioid consumption without increasing additional incidence of adverse effects.
Key Words: dexmedetomidine, open thoracic surgery, pain, postoperative, ultrasound-guided erector spinae plane block
Open thoracotomy for the treatment of thoracic esophageal cancer is associated with intense acute postoperative pain.1 Single-injection erector spinae plane block (ESPB) has been reported to be successfully used for analgesia after thoracotomy.2 Although ESPB is effective for alleviating pain after thoracotomy, the duration of analgesia is not long enough.3 Because the moderate-severe acute postoperative pain associated with open thoracotomy frequently outlasts the duration of single-injection ESPB and there is muted enthusiasm regarding the use of continuous perineural catheters, the potential for prolonging the duration of analgesia after single-injection ESPB is especially important.4–6 Adding adjuvants (eg, clonidine) to local anesthetics is frequently used to prolong the duration of single-injection regional nerve block.6–9 The use of another α2-adrenoceptor agonist, dexmedetomidine, for interfasical nerve blockade is not as widespread. Dexmedetomidine is a 7 times more selective α2-adrenoceptor agonist in comparison to clonidine and has a similar mechanism of blocking hyperpolarisation activated cation channels.10 There have been multiple studies claiming increased effectiveness of use of dexmedetomidine and this has been consolidated in a meta-analysis examining the effectiveness of dexmedetomidine as a peripheral nerve block adjuvant.11,12 However, whether interfasical dexmedetomidine prolongs the duration of single-injection ESPB and reduces postoperative opioid consumption after curative-intent open thoracotomy remains uncertain. Therefore, this prospective, randomized, double-blinded, controlled clinical study was performed to investigate whether adding dexmedetomidine to ropivacaine could prolong the duration of single-injection ESPB and reduce postoperative opioid consumption for open thoracotomy.
MATERIALS AND METHODS
Trial Design and Participants
This prospective, randomized, double-blinded, controlled clinical study was conducted in accordance with the Helsinki Declaration. The study was approved by the Institutional Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (18-079/1657). And this trial was registered on: www.chictr.org.cn (ChiCTR1800016583) on June 10, 2018. The patients were enrolled on July 2, 2018. Written informed consent was obtained from every patient.
The major inclusion criteria were as follows: diagnosed as lower thoracic esophageal cancer, American Society of Anesthesiologists (ASA) physical status of I to III, aged 18 to 64 years old (ie, adult, but not elderly), selected for open thoracic surgery (Sweet procedure), and able to communicate well and understood how to evaluate their pain score at rest or during coughing. To eliminate potential confounders from the surgeons, all open thoracic surgeries were performed by only the same experienced surgical team. The major exclusion criteria were as follows: infections at the site of injection for ultrasound-guided ESPB; neuropathy; coagulation disorders, morbid obesity (body mass index >40 kg/m2); allergy to ropivacaine or dexmedetomidine; adrenoreceptor agonist or antagonist therapy; greater than first-degree heart block; bradycardia (heart rate [HR] < 50 bpm); pregnancy clinically significant cardiovascular, pulmonary, hepatic or renal diseases; psychiatric illnesses that would interfere with the assessment of pain scores; and those given a painkiller within 1 week before surgery. During the preoperative interview, patients were taught how to evaluate their pain score by the Numeric Rating Scale (NRS).
Randomization and Blinding
A computer-generated randomization list was used to randomize consented study participants on a 1:1 ratio to receive ultrasound-guided ESPB with the trial medication.
Group R (control): 28 mL of 0.5% ropivacaine+2 mL of normal saline.
Group RD: 28 mL of 0.5% ropivacaine+0.5 µg/kg in 2 mL of interfascial dexmedetomidine.
The volume of the study drug was 30 mL. None of the patients were administered intravenous dexmedetomidine perioperatively. The patient-controlled intravenous analgesia (PCIA) protocol was programmed with 200 µg sufentanil diluted to 100 mL (1.5 mL bolus, lockout time interval of 10 minutes and 1 hour limit of 9 mL without any baseline infusion). The randomization table was kept in our hospital pharmacy. The study medication and PCIA solutions were prepared by a nurse who did not know the purpose of the study. The patients, surgeons, and research staff who enrolled participants and collected study data were blinded to the group assignments.
Interventions
Before the study, every patient was trained on how to use the PCIA pump and instructed on using the NRS; with 0: no pain to 10: the worst imaginable pain.
Upon arrival in the operating room, routine monitoring for open thoracotomy, including 5-lead electrocardiography, invasive blood pressure, HR, respiratory rate (RR), and pulse oxygen saturation was applied.
Before general anesthesia induction, the patient was placed in a right lateral decubitus position. Then, the spinous processes were palpated and marked, and the fifth thoracic transverse process was identified using a high-frequency linear ultrasound transducer (GE LOGIQe, Wauwatosa, WI) counting up from the 12th rib and also down from the first rib. And the position of the T5 transverse process was confirmed under ultrasound guidance. Then, by moving the probe to the outside, the probe was placed in a longitudinal orientation 3 cm lateral to the T5 spinous process. Then, 3 muscles were identified superficial to the hyperechoic transverse process shadow as follows: trapezius, rhomboid major, and erector spinae. Under ultrasound guidance, an 8-cm 22-G block needle (Contiplex; B Braun, Melsungen, Germany) was inserted in-plane in a caudad-to-cephalad direction until the tip lay in the surface of the transverse process (Fig. 1A). Correct needle tip position was confirmed by visualizing linear fluid spread that separated the erector spinae muscle from the transverse process. Then, 30 mL of the study drug was injected deep into the erector spinae muscle (Fig. 1B). The assessment of cutaneous sensory block by pinprick was initiated 30 minutes later by a blind observer. After confirmation and assessment of the sensory block to pinprick, general anesthesia was induced with 0.05 mg/kg midazolam, 1.5 to 2.5 mg/kg propofol, 0.2 to 0.4 µg/kg sufentanil and 0.15 mg/kg cisatracurium. General anesthesia was maintained with sevoflurane, sufentanil and cisatracurium. And train-of-four stimulation of the ulnar nerve at the adductor pollicis was used to monitor intraoperative neuromuscular blockade.
FIGURE 1.
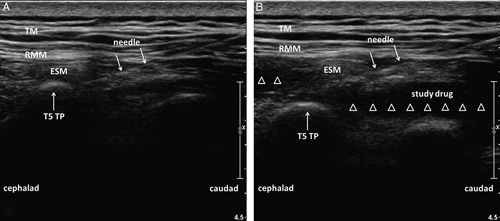
Ultrasound-guided erector spinae plane block performed deep to the ESM. A, Ultrasound imaging before injecting study drug. B, Injection of study drug into the interfascial plane deep to ESM produced a visible fluid spread (white hollow triangles) beneath the ESM. ESM indicates erector spinae muscle; RMM, rhomboid major; T5, the fifth thoracic vertebra; TM, trapezius; TP, transverse process.
The standardized anesthetic technique was used in the 2 groups. After anesthesia induction, a double-lumen endobronchial tube was placed under the guidance of fiberoptic bronchoscopy. Then, the patient was turned to a right lateral decubitus position from the supine position. The correct position of the double-lumen endobronchial tube was confirmed again by fiberoptic bronchoscopy. Before the skin incision, 1-lung ventilation was initiated. The volume-controlled ventilation mode was adopted, with the airway pressure and lung compliance monitored. General anesthesia was maintained with 2.5% to 3.0% sevoflurane (1.3 to 1.5 minimum alveolar concentration). The end-tidal carbon dioxide partial pressure was maintained between 35 and 45 mm Hg by mechanical ventilation.
After ipsilateral lung collapse, a posterolateral incision in the sixth intercostal space on the left side of the chest was made. At the conclusion of the surgical procedure, a chest drain was placed. At the end of the surgery, the intravenous analgesic pump was applied. PCIA was administered when NRS ≥4 or at the request of the patient.
Outcomes
The primary outcome was the duration of analgesia, which was defined as the time in minutes to the first request for postoperative analgesics. Total postoperative sufentanil consumption within the 48-hour period and total intraoperative sufentanil consumption were also recorded. Before surgery (baseline) and at 3, 6, 12, 24, 36, and 48 hours after surgery, the patient’s mean arterial pressure (MAP), HR, NRS scores at rest and during coughing, and Ramsay Sedation Scale (RSS) (1: anxious; 2: cooperative and tranquil; 3: responding to command; 4: brisk response to stimuli; 5: sluggish response to stimuli; and 6: no response to stimuli) were all assessed.
Adverse effects (AEs) such as hypotension (systolic blood pressure < 90 mm Hg or >20% decline from baseline), hypoxemia (pulse oxygen saturation<90%), bradycardia (HR<50 bpm), respiratory depression (RR<10 breaths/min lasting for >10 min), pruritus, neurotoxicity, headache, nausea and vomiting were recorded after surgery. Hypotension was treated with 6 mg ephedrine and 6 mL/kg normal saline; the same doses were repeated as required. Bradycardia was treated with intravenous boluses of 0.5 mg atropine, repeated as needed to a total of 3 mg. Hypoxemia was treated with inhalation of pure oxygen through a face mask. Respiratory depression was treated with naloxone and oxygen until the RR was >15 breaths/min.
Statistical Analysis
The primary outcome was the duration of analgesia by single-injection ESPB. We hypothesized that adding 0.5 µg/kg dexmedetomidine to 0.5% ropivacaine would prolong the duration of analgesia compared with only 0.5% ropivacaine after ESPB for open thoracotomy. In our preliminary study conducted in 10 adult patients (5 in each group), the mean duration of analgesia was 397±74 and 461±80 minutes in group R and group RD, respectively. PASS, version 11.0.7 (NCSS LLC) for Windows was used to calculate the sample size. The Student t test was selected, and the group allocation ratio was equal. The hypothesized means of the analgesia duration were 397 and 461, and the SDs were 74 and 80, respectively. Then, we calculated that a sample of 24 patients would provide 80% power at a 2-sided α level of 0.05. Ultimately, we recruited 30 patients in each group for a total of 60 patients considering possible dropouts and incomplete follow-up.
The continuous variables are presented as the mean±SDs or medians (25th to 75th percentiles), and categorical data are presented as numbers and percentages. Normality was tested by the Kolmogorov-Smirnov analysis. The Student t test was used for analysis of the duration of analgesia, total intraoperative and postoperative sufentanil consumptions, NRS score and RSS data. The Kaplan-Meier survival method was used for analysis of time-to-event outcomes, and the log-rank test was used to compare groups. For analysis of MAP and HR data, repeated measures analysis of variance with Bonferroni correction was used. To analyze the incidence of AEs, Fisher exact test was used. All data were processed by IBM SPSS Statistics 21.0 (IBM Inc., New York, NY). A 2-sided P-value < 0.05 was considered to be statistically significant.
RESULTS
A total of 113 patients were recruited to participate in this study from July 2018 to December 2019. In addition, 45 patients were ineligible because they did not meet the inclusion criteria or declined to participate. Eight patients were excluded from the trial because of nonadherence to the study protocol (5 patients were lost to follow-up; 1 patient underwent secondary emergency surgery in 48 hours; 1 patient refused to use PCIA after thoracotomy; and 1 patient developed atelectasis in 48 h). Ultimately, 60 patients were randomized and completed the study protocol (group R: n=30; group RD: n=30). The Consolidated Standards of Reporting Trials flow diagram depicts participants progressing through the study phases (Fig. 2).
FIGURE 2.
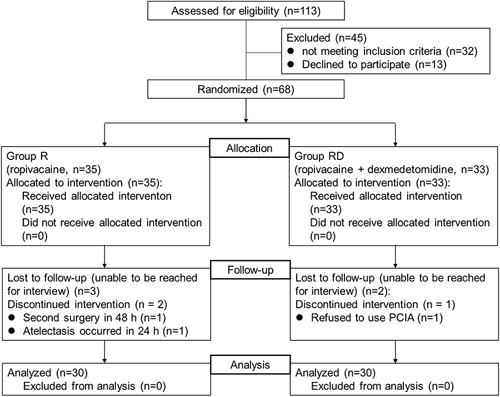
Patient enrollment and inclusion and exclusion process. PCIA indicates patient-controlled intravenous analgesia.
Ultimately, a total of 60 patients were included in the analysis in this study. There were no significant differences in the patient characteristics between the groups (Table 1).
TABLE 1.
Demographic Data and Surgical Characteristics
| Group R | Group RD | P | |
|---|---|---|---|
| Age (y) | 55.8±7.0 | 57.9±6.0 | 0.205 |
| BMI (kg/m2) | 22.5±2.0 | 22.8±2.2 | 0.614 |
| Sex (F/M) | 8/22 | 9/21 | 0.774 |
| Duration of surgery (min) | 141.8±32.9 | 148.1±35.4 | 0.473 |
| Extubation time (min) | 21.6±5.8 | 22.6±6.8 | 0.553 |
Data are expressed as the mean±SD for numbers. There were no significant differences between groups.
BMI indicates body mass index; F, female; M, male; R, ropivacaine; RD, ropivacaine+dexmedetomidine.
The duration of analgesia was significantly longer in group RD than in group R (P<0.001) (Table 2). Figure 3 represents the Kaplan-Meier survival analysis of the time to first analgesic request. The log-rank test revealed that the time to first analgesic request was prolonged in group RD compared with group R (P<0.001).
TABLE 2.
Duration of Analgesia and Sufentanil Consumption
| Variables | Group R | Group RD | P |
|---|---|---|---|
| Duration of analgesia (min) | 323.2±75.4 | 505.1±113.9 | <0.001 |
| Total postoperative sufentanil consumption (µg) | 33.8±13.8 | 23.3±10.0 | 0.001 |
| Total intraoperative sufentanil consumption (µg) | 44.3±7.2 | 38.3±6.1 | 0.01 |
The duration of analgesia and sufentanil consumption data are expressed as the mean±SD.
R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
FIGURE 3.
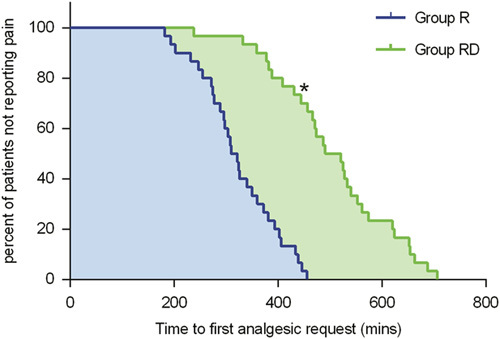
Kaplan-Meier survival plot representing the first analgesic request in the 2 groups. *P<0.001 between group RD and group R. R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
The total postoperative sufentanil consumption in group RD was significantly lower than that in group R (P=0.001) (Table 2). Also, the total intraoperative sufentanil consumption in group RD was significantly lower than that in group R (P=0.01) (Table 2).
At 12, 24, 36, and 48 hours postoperatively, the NRS score at rest in group R was higher than that in group RD (P<0.05) (Fig. 4A). At 12, 24, and 36 hours postoperatively, the NRS score during coughing in group R was higher than that in group RD (P<0.05) (Fig. 4B).
FIGURE 4.
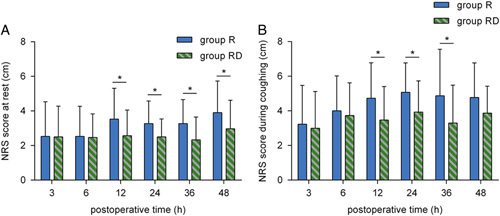
Postoperative pain severity NRS score at rest (A) and during coughing (B) at 3, 6, 12, 24, 36, and 48 hours postoperatively. *P<0.05. NRS indicates Numeric Rating Scale; R, ropivacaine; RD, ropivacaine+dexmedetomidine.
The MAPs at different time points were significantly different in each group (P<0.001) (Fig. 5). Similarly, the discrepancies in HR at different time points were statistically significant (P<0.01) (Fig. 6). However, no significant differences were observed in the MAP or HR between group R and group RD (P=0.250 and 0.099, respectively).
FIGURE 5.
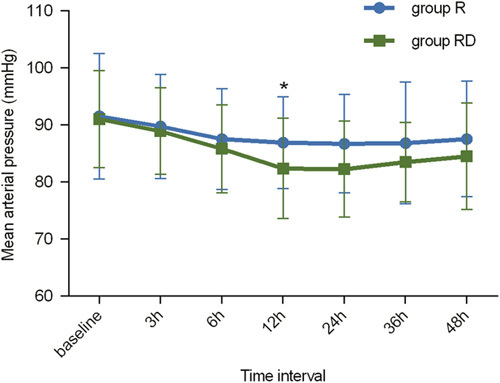
Mean arterial pressure (MAP) changes at different times in each group. In both groups, the changes in MAP showed a significant difference in relation to time, with *P<0.001. R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
FIGURE 6.
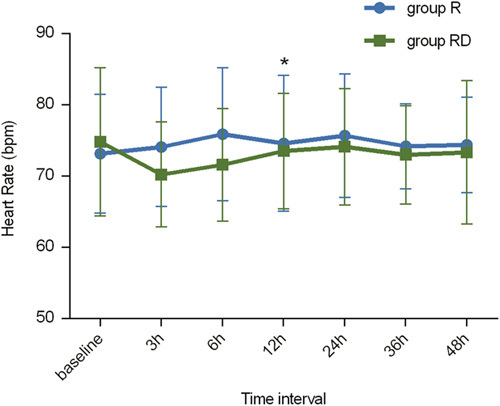
Heart rate changes at different times in each group. In both groups, the changes in heart rate showed a significant difference in relation to time with *P<0.01. R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
Table 3 shows the number of patients experiencing AEs. There were no significant differences in the incidences of bradycardia, hypotension, hypoxemia, respiratory depression, nausea, vomiting, pruritus, dizziness or neurotoxicity between group R and group RD. The RSS is shown in Figure 7. At 12, 24, 36, and 48 hours postoperatively, the RSS in group RD was higher than that in group R (P<0.05).
TABLE 3.
Incidence of Adverse Effects
| Adverse effects | Group R | Group RD | P |
|---|---|---|---|
| Bradycardia | 0 (0) | 0 (0) | — |
| Hypotension | 0 (0) | 0 (0) | — |
| Hypoxemia | 0 (0) | 0 (0) | — |
| Respiratory depression | 0 (0) | 0 (0) | — |
| Nausea | 5 (17) | 3 (10) | 0.706 |
| Vomiting | 3 (10) | 1 (3) | 0.306 |
| Pruritus | 0 (0) | 0 (0) | — |
| Dizziness | 3 (10) | 2 (7) | 1.000 |
| Neurotoxicity | 0 (0) | 0 (0) | — |
The incidence of adverse effects data are expressed as n (%).
R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
FIGURE 7.
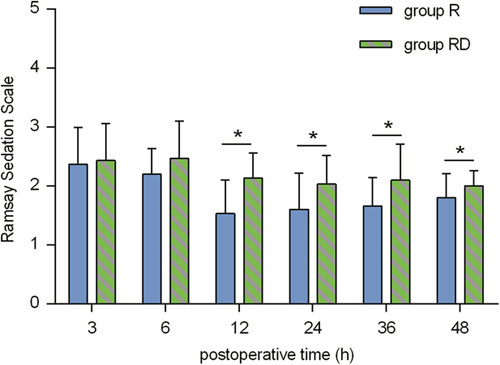
Ramsay Sedation Scale scores at 3, 6, 12, 24, 36, and 48 hours postoperatively. *P<0.05. R indicates ropivacaine; RD, ropivacaine+dexmedetomidine.
DISCUSSION
In this study, we demonstrated that adding dexmedetomidine to ropivacaine could prolong the analgesia duration and reduce postoperative sufentanil consumption compared with ropivacaine alone in patients undergoing open thoracotomy without additional AEs.
There are a few studies using dexmedetomidine for interfascial nerve blocks in humans. Although beneficial in acute and chronic pain management, the use of local anesthetics is limited by its duration of action and the dose dependent AEs on the cardiac and central nervous system. Adjuvants or additives are often used with local anesthetics for its synergistic effect by prolonging the duration of sensory-motor block and limiting the cumulative dose requirement of local anesthetics. Subsequently, there have been studies in supraclavicular, interscalene, cervical plexus, and ulnar nerve blocks where dexmedetomidine has been shown to increase quality and duration of analgesia of commonly used local anesthetics like ropivacaine and bupivacaine.13–15 An interesting study found that dexmedetomidine fared significantly better than clonidine when used as an adjuvant in supraclavicular blocks.16 Neurotoxicity of dexmedetomidine, especially when used in perineural spaces is a valid concern. Surprisingly, preliminary evidence seems to suggest that dexmedetomidine has potential for neuroprotection, especially when compared with lidocaine and bupivacaine.17,18
In a previous study by Xu et al,18 the authors found that adding 0.5 µg/kg dexmedetomidine to 0.25% ropivacaine for transversus abdominis plane block reduced the total amount of sufentanil in the first 24 hours after abdominal surgery. Similar to the results of their study, the total postoperative sufentanil consumption in this study was significantly lower in group RD than in group R in the first 48 hours after surgery, indicating that adding 0.5 µg/kg dexmedetomidine to 0.5% ropivacaine could provide a significant opioid-sparing effect after open thoracotomy. As Lee et al19 found, patients with esophageal cancer undergoing curative-intent surgeries were particularly vulnerable to opioid abuse, which was probably associated with intense postsurgical pain after thoracotomy. Regional block for effectively managing postoperative pain while simultaneously decreasing opioid consumption was found to decrease subsequent opioid misuse or dependence.20,21 On the basis of the currently available evidence, we speculated that adding dexmedetomidine to ropivacaine for ESPB probably contributed to minimizing opioid use and misuse in patients with esophageal cancer undergoing curative-intent thoracotomy.
As demonstrated in this study, the RSS scores at 12, 24, 36, and 48 hours postoperatively were higher in group RD than in group R. Furthermore, the RSS score in group RD was < 3 points. These results indicated that interfascial dexmedetomidine could provide a rational sedative effect in the first 48 hours after thoracotomy, including preventing patients from being restless, agitated, or anxious. Despite interfascial dexmedetomidine producing superior sedative effectiveness, no patients receiving dexmedetomidine experienced respiratory depression or desaturation in the first 48 hours after thoracotomy, which indicated that adding 0.5 µg/kg dexmedetomidine to ropivacaine for ESPB was safe. Although most available research has emphasized direct interfascial mechanisms,10,22,23 in a previous interesting study, Hong et al24 found that sedation with intravenous dexmedetomidine was able to prolong the analgesic effect of regional nerve block. However, the exact mechanism by which adding dexmedetomidine prolongs the analgesia time of ESPB remains speculative.
As demonstrated in this study, adding 0.5 µg/kg dexmedetomidine to ESPB did not cause significant fluctuations in MAP or HR. In addition, the incidence of other AEs, including nausea, vomiting and dizziness, was similar in the 2 groups. However, this result did not indicate that any dose of dexmedetomidine could be safely used for perineural injection. In a previous study by Esmaoglu et al,25 they found that adding 100 µg dexmedetomidine to the local anesthetic caused obvious declines in systolic blood pressure, diastolic blood pressure, and HR during the first 2 hours after surgery. In addition, a previous meta-analysis indicated that when the dose of dexmedetomidine for perineural injection was >50 µg, the possibility of intraoperative bradycardia significantly increased.26 As known, the common AEs of dexmedetomidine are bradycardia and hypotension, which are commonly seen in the elderly.27 The reasons for the absence of bradycardia and hypotension after interfascial use of dexmedetomidine in this study were probably as follows: first, none of the patients involved in the study were elderly. Second, the dose of dexmedetomidine adding for interfascial plane block in this study was not high. Therefore, these studies suggest that clinicians should select the dose of dexmedetomidine for interfascial injection with caution.
Similar to clonidine, dexmedetomidine pretreatment extends the time-to-cardiotoxicity from intravenous bupivacaine infusion in rats, and increases the total dose required to induce cardiac arrest.28,29 Whether this cardioprotective effect applies to coadministration with local anesthetics is not known. Whether coadministration of α2-adrenoceptor agonists and local anesthetics will have similar safety effects is not known and merits further study. While the data to date are supportive, the authors believe that further clinical studies are warranted to better establish the appropriate dosing, potential side effects and safety before widespread clinical use.
There were several limitations in this study. First, there was no intravenous dexmedetomidine group in this study. Without intravenous control, it was difficult to ascertain whether the analgesic prolongation of dexmedetomidine was mediated by systemic or local interfascial action. Second, the dose of dexmedetomidine selected in this study was based on our institution’s anecdotal experience. Further investigation is still needed to determine the dose-ranging data. Third, considering that repeatedly assessing the cutaneous sensory block area after surgery can significantly disturb patients’ rest, we used the time to first postoperative analgesic request instead of testing sensory block to ascertain the analgesia time of ESPB. However, the duration of postoperative pain relief might not be a reliable surrogate measure for the sensory blockade duration of ESPB. The former has been validated and extensively used to evaluate the effect of adjuncts on local anesthetic-based regional nerve block.30–34 Fourth, the patients might have fallen asleep when the investigators performed follow-up visits. This possibility might interfere with the measurements in this study. Fortunately, the randomized nature of this study probably balanced this situation. Finally, the use of dexmedetomidine in this study was off-label. Although this drug has been widely used internationally and studied in many clinical trials, the United States Food and Drug Administration has not approved dexmedetomidine for interfascial injection. Considering the inconclusive evidence on the safety of interfascial dexmedetomidine, we would like to suggest that clinic practitioners gain approval from local regulatory health institutions for the off-label use of this drug.
CONCLUSIONS
Our results suggest that in patients with esophageal cancer undergoing curative-intent open thoracotomy, adding 0.5 µg/kg dexmedetomidine to single-injection ESPB with 0.5% ropivacaine prolongs the duration of analgesia and reduces perioperative opioid consumption without producing additional evident AEs.
Footnotes
Q.W.: contributed to the formal analysis, investigation, writing of the original draft, and writing of the review and editing. H.L. and S.W.: contributed to the data curation and investigation. G.Z.: contributed to the formal analysis, investigation, and project administration. C.N.: contributed to the investigation, methodology, and project administration. L.S.: contributed to the formal analysis and methodology. H.Z.: contributed to the acquisition of funding, supervision, and writing of the review and editing.
Supported by the Beijing Hope Run Special Fund of Cancer Foundation of China (Beijing/China) (grant LC2017A09), the Management Research Project Special Fund of Cancer Hospital Chinese Academy of Medical Sciences (Beijing/China) (grant LC2018D01) and Sanming Project of Medicine in Shenzhen (Shenzhen/China) (grant SZSM201812069), Cancer Pain Treatment and Perioperative Medical Team of Professor Sun Li at Cancer Hospital, Chinese Academy of Medical Sciences (Beijing/China). The funding had no role in the study design, data collection or analysis, or in the decision to publish or prepare the manuscript. The authors declare no conflict of interest.
Contributor Information
Qiang Wang, Email: wqzjbd001@163.com.
Huixian Li, Email: wqzjbd002@163.com.
Shijing Wei, Email: wsjzlz@sina.com.
Guohua Zhang, Email: zhangguohua_zlyy@163.com.
Cheng Ni, Email: nicheng_zlyy@163.com.
Li Sun, Email: sunli_zlyy@163.com.
Hui Zheng, Email: zhenghui_zlyy@163.com.
REFERENCES
- 1.Miyata K, Fukaya M, Itatsu K, et al. Muscle sparing thoracotomy for esophageal cancer: a comparison with posterolateral thoracotomy. Surg Today. 2016;46:807–814. [DOI] [PubMed] [Google Scholar]
- 2.Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med. 2019;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tulgar S, Kapakli MS, Senturk O, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–106. [DOI] [PubMed] [Google Scholar]
- 4.Raft J, Chin KJ, Belanger ME, et al. Continuous erector spinae plane block for thoracotomy analgesia after epidural failure. J Clin Anesth. 2018;54:132–133. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Liu N, Zeng Q, et al. The efficacy of pregabalin for the management of acute and chronic postoperative pain in thoracotomy: a meta-analysis with trial sequential analysis of randomized-controlled trials. J Pain Res. 2018;12:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Liu S, Zhu J, et al. Dexamethasone and dexmedetomidine as adjuvants to local anesthetic mixture in intercostal nerve block for thoracoscopic pneumonectomy: a prospective randomized study. Reg Anesth Pain Med. 2019;8:rapm-2018-100221. [DOI] [PubMed] [Google Scholar]
- 7.Casati A, Magistris L, Fanelli G, et al. Small-dose clonidine prolongs postoperative analgesia after sciatic-femoral nerve block with 0.75% ropivacaine for foot surgery. Anesth Analg. 2000;91:388–392. [DOI] [PubMed] [Google Scholar]
- 8.Hong B, Lim C, Kang H, et al. Thoracic paravertebral block with adjuvant dexmedetomidine in video-assisted thoracoscopic surgery: a randomized, double-blind study. J Clin Med. 2019;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Yang JS, Dong BH, et al. The effect of dexmedetomidine added to preemptive ropivacaine infiltration on postoperative pain after lumbar fusion surgery: a randomized cntrolled trial. Spine (Phila Pa 1976). 2019;44:1333–1338. [DOI] [PubMed] [Google Scholar]
- 10.Brummett CM, Padda AK, Amodeo FS, et al. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39:37–47. [DOI] [PubMed] [Google Scholar]
- 12.Coviello A, Esposito D, Galletta R, et al. Opioid-free anesthesia-dexmedetomidine as adjuvant in erector spinae plane block: a case series. J Med Case Rep. 2021;15:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JH, Shim HY, Lee TJ, et al. Comparison of dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus block. Korean J Anesthesiol. 2014;66:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swami SS, Keniya VM, Ladi SD, et al. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: a randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Zhou F, Li C, et al. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitro experimental study. PLoS One. 2013;8:e55556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tüfek A, Kaya S, Tokgöz O, et al. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med. 2013;36:E95–E102. [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Hu Z, Shen J, et al. Efficacy of US-guided transversus abdominis plane block and rectus sheath block with ropivacaine and dexmedetomidine in elderly high-risk patients. Minerva Anestesiol. 2018;84:18–24. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trasolini NA, McKnight BM, Dorr LD. The opioid crisis and the orthopedic surgeon. J Arthroplasty. 2018;33:3379.e1–3382.e1. [DOI] [PubMed] [Google Scholar]
- 21.Soffin EM, Lee BH, Kumar KK, et al. The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. Br J Anaesth. 2019;122:e198–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brummett CM, Hong EK, Janda AM, et al. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummett CM, Amodeo FS, Janda AM, et al. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–431. [DOI] [PubMed] [Google Scholar]
- 24.Hong B, Jung C, Jo Y, et al. Sedation with dexmedetomidine prolongs the analgesic duration of brachial plexus block: a randomised controlled trial. Anaesth Crit Care Pain Med. 2019;38:231–236. [DOI] [PubMed] [Google Scholar]
- 25.Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–1551. [DOI] [PubMed] [Google Scholar]
- 26.Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42:184–196. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Kim WO, Kim HB, et al. Adequate sedation with single-dose dexmedetomidine in patients undergoing transurethral resection of the prostate with spinal anaesthesia: a dose-response study by age group. BMC Anesthesiol. 2015;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulec S, Aydin Y, Uzuner K, et al. Effects of clonidine pre-treatment on bupivacaine and ropivacaine cardiotoxicity in rats. Eur J Anaesthesiol. 2004;21:205–209. [DOI] [PubMed] [Google Scholar]
- 29.Hanci V, Karakaya K, Yurtlu S, et al. Effects of dexmedetomidine pretreatment on bupivacaine cardiotoxicity in rats. Reg Anesth Pain Med. 2009;34:565–568. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2015;40:125–132. [DOI] [PubMed] [Google Scholar]
- 31.Parrington SJ, O’Donnell D, Chan VWS, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35:422–426. [DOI] [PubMed] [Google Scholar]
- 32.Cummings KCR, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–453. [DOI] [PubMed] [Google Scholar]
- 33.Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111:445–452. [DOI] [PubMed] [Google Scholar]
- 34.Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2014;118:1113–1119. [DOI] [PubMed] [Google Scholar]


