Abstract
Objective:
The aim of this study was to appropriately plan for rollout and monitor impact of oral preexposure prophylaxis (PrEP). It is important to understand PrEP continuation and come to a consensus on how best to measure PrEP continuation. This study reviews data on PrEP continuation to document how it is reported, and to compare continuation over time and across populations.
Design:
A systematic review and meta-analysis.
Methods:
We searched MEDLINE, Embase and Global Health and reviewed abstracts from HIV conferences from 2017 to 2018 for studies reporting primary data on PrEP continuation. Findings were summarized along a PrEP cascade and continuation was presented by population at months 1, 6 and 12, with random-effects meta-analysis.
Results:
Of 2578 articles and 596 abstracts identified, 41 studies were eligible covering 22 034 individuals. Continuation data were measured and reported inconsistently. Results showed high discontinuation at month 1 and persistent discontinuation at later time points in many studies. Pooled continuation estimates were 66% at month 1 [n = 5348; 95% confidence interval (95% CI): 48–82], 63% at month 6 (n = 13 629; 95% CI: 48–77) and 71% at month 12 (n = 14 933; 95% CI: 60–81; higher estimate than previous timepoints due to inclusion of different studies). Adequate data were not available to reliably compare estimates across populations.
Conclusion:
This review found that discontinuation at one month was high, suggesting PrEP initiations may be a poor measure of effectiveness. Continuation declined further over time in many studies, indicating existing cross-sectional indicators may not be adequate to understand PrEP use patterns. Studies do not measure continuation consistently, and consensus is needed.
Keywords: HIV prevention, HIV/AIDS, oral preexposure prophylaxis, preexposure prophylaxis continuation
Introduction
The effectiveness of oral preexposure prophylaxis (PrEP) is contingent upon continued use during periods of risk for HIV, which evidence shows is difficult for many clients [1–3]. Continual engagement in care via follow-up visits is important not only for refills but also for ongoing continuation support, risk reduction counselling, screening for sexually transmitted infections (STIs) and management of side effects [4–6]. The WHO and national PrEP guidelines recommend that PrEP clients are tested for HIV at 1 and 3 months after initiation and every 3 months thereafter [7,8]. Despite increasing adoption of PrEP [9], there have been challenges in achieving continued engagement in follow-up visits.
As countries approve PrEP for HIV prevention and prepare for widespread provision, monitoring of continued follow-up and refill – or continuation – is essential to estimate the potential impact of the intervention and eventually measure the success of PrEP programs. Continuation data can inform costing of PrEP implementation by providing evidence of the likelihood that PrEP clients will return at each follow-up visit, helping to adequately project the cost of PrEP provision over time. Continuation data can also provide insight into whether some population groups are more or less likely to continue on PrEP and help ensure that strategies to promote PrEP continuation are designed to meet the requirements of specific populations.
Despite the importance of consistent measurement of PrEP continuation, there is little consensus on how to do so. Conversations about the appropriate indicators to measure PrEP programme success are ongoing, and approaches are rapidly evolving. Consensus is building that the word ‘retention’ is not appropriate for prevention, because unlike antiretroviral therapy (ART), PrEP is not taken for life; at times, clients may safely cycle on and off of PrEP, in consultation with their providers. However, in the literature, terms such as retention, adherence and continuation are often used interchangeably. Further complication arises with different dosing for different populations. WHO guidance supports intermittent, event-driven or ‘on demand’ dosing for MSM and provision of time-limited PrEP to HIV-negative people in serodiscordant relationships [7]. Existing PrEP indicators, including those endorsed by the WHO [7] and PEPFAR [10], do not account for differences in dosing schedules or well tolerated cycling.
This systematic review and meta-analysis will document reporting of continuation in published literature and compare continuation across diverse populations. The analysis will inform the ongoing conversation about how to measure PrEP programme success, support future modelling and costing studies, and highlight how PrEP continuation vary among target populations.
Materials and methods
Search strategy and selection criteria
The purpose of this systematic review and meta-analysis was to identify data from clinical trials, demonstration projects and real-world settings on participant continuation on oral PrEP. Due to the limited evidence base currently available on this topic, the search was intended to cover programmes run among any of the target groups considered to be at risk of HIV infection and in any setting. Only those studies reporting on primary data were deemed eligible, with modelling studies and simulations excluded. Studies on programmes utilizing methods other than oral PrEP delivery (e.g. topical gels, vaginal rings) were also excluded. Where trials or studies included two modes of delivery (i.e. one oral PrEP and one other, or one oral PrEP and one placebo), only data from the oral PrEP arm were included. Studies reporting on eligibility and enrolment figures only, and not continuation data, were excluded. Grey literature was not included.
A literature search was run on three databases (MEDLINE, Embase and Global Health) to identify articles written in English and published in 2010 or later. Search terms used were (’preexposure prophylaxis’ OR ‘PrEP’) AND (’HIV’ OR ‘HIV/AIDS’) AND (’implementation’ OR ‘demonstration’ OR ‘observation’ OR ‘trial’ OR ‘open label extension’). The search was completed on 6 November 2018. Titles and abstracts were screened initially, followed by review of full text articles deemed potentially eligible for inclusion. In addition to the database searches, abstracts from the Conference on Retroviruses and Opportunistic Infections (CROI) in 2017 and 2018, the 22nd International AIDS Conference (AIDS 2018) and the 2017 International AIDS Society (IAS) Conference were also screened on the basis of their titles, and full abstracts were reviewed when they were deemed potentially eligible for inclusion.
Study screening and extraction
The primary outcome for this review is continuation in PrEP services at various time points, which are represented along a simplified PrEP cascade in Fig. 1[11]. Continuation data were extracted from the literature and mapped to the timepoints in this cascade. It is important to note that studies reporting on continuation in PrEP services cover PrEP programmes of varying structures, with study visits occurring and continuation reported at different intervals. The cascade is not an accurate reflection of each study visit in every study included but affords the benefit of allowing for comparison between studies. When continuation was reported between time points, the continuation value was presented for the closest preceding timepoint. Note that many proposed PrEP services cascades also include adherence, or effective use [11–13]; however, this important indicator of PrEP programme success is beyond the scope of this review.
Fig. 1.
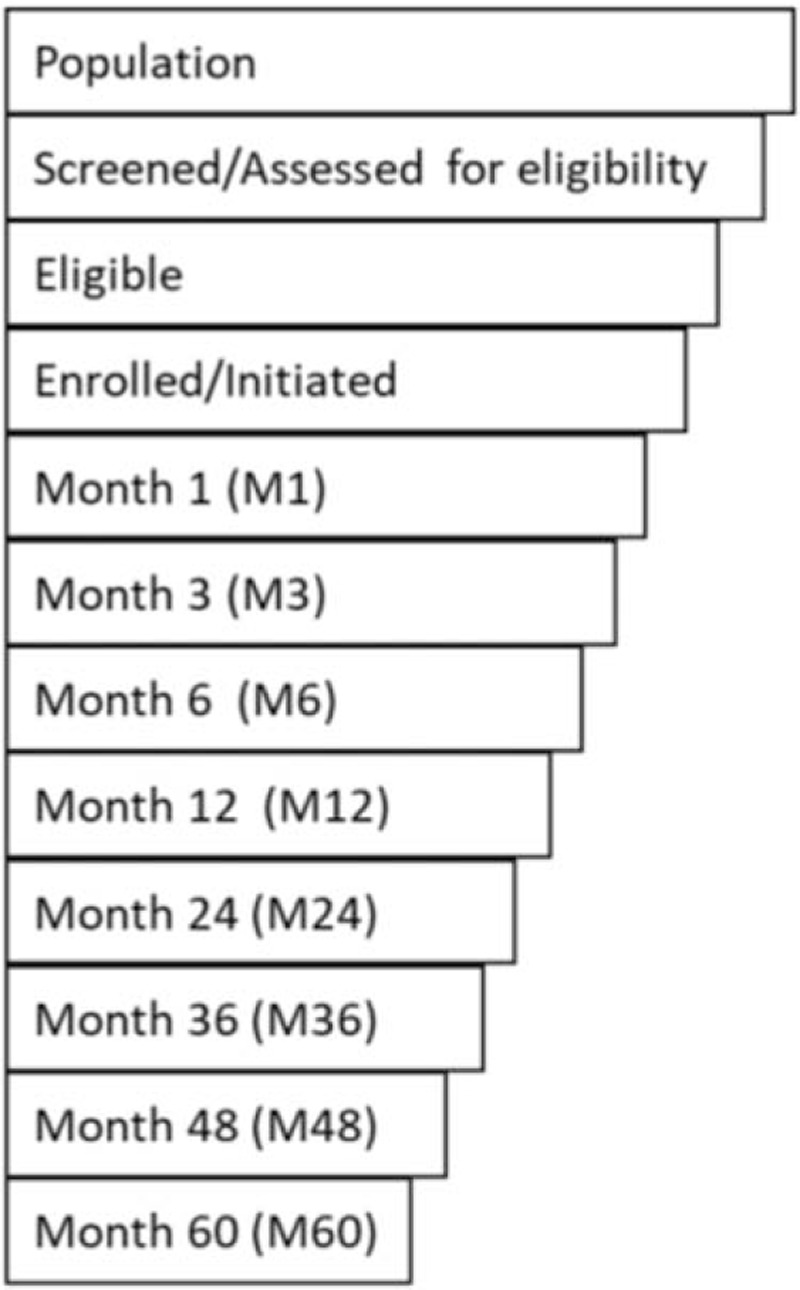
PrEP services cascade.
For the purpose of this analysis, continuation at each point in the PrEP cascade is defined as the number or proportion of enrolled or initiated study participants who returned for a follow-up visit at the relevant time point. This definition does not account for true duration of use among clients who discontinue as these data are not available; instead, clients who do not return to the study visit are considered to have discontinued use at the time of the visit. We also do not account for use of PrEP during periods of risk and discontinuation when no longer at risk, which is sometimes referred to as prevention-effective use or prevention-effective adherence [14–16]. Limiting our interpretation of effective continuation to only continual use over time could underestimate the impact of PrEP, ignoring the risk averted by those with other use patterns. However, none of the studies in this review tracked prevention-effective use, so we report on PrEP continuation only as continual use time.
Data were extracted and entered into Excel. Four weeks were considered 1 month for continuation reported at weekly intervals. If no specific population group was targeted in the study, the population was deemed ‘All at risk’. When continuation at different points in the cascade was reported separately by study population or study site, the continuation data were extracted separately for each population or site to allow for comparison.
Two independent reviewers (JL and KS) completed the full review process to determine studies for inclusion. Both reviewers then screened the full text of each study and extracted data, and discrepancies were discussed and resolved.
Data analysis
Study quality was assessed using the Joanna Briggs Institute Checklist for Prevalence Studies [17]. This tool allows assessment of studies based on study design, implementation and analysis. No papers were excluded, as all were deemed to be of sufficient quality (score of 5 or more).
All statistical and meta-analyses were completed using Stata 15 (StataCorp LLC, College Station, Texas, USA) [18]. We summarized reporting of continuation by calculating the percentage of studies reporting on each aspect of the cascade. We calculated percentage discontinuation between time periods by taking the difference in continuation between the time periods. PrEP continuation cascades were developed presenting continuation from studies reporting continuation from at least three of the four time points from month 1 to month 12. We created forest plots of continuation at month 1, month 6 and month 12, grouped by population, using random-effects meta-analysis with Freeman–Tukey double arcsine transformation. The analysis was done using Metaprop, a program specifically designed for binomial data that calculates confidence intervals (CIs) within the admissible values of 0–1 [19]. We estimated pooled continuation and 95% CIs overall, and not by population, because of high heterogeneity (I2 statistic) within groups.
Role of the funding source
This work was funded by the US Agency for International Development and led by the OPTIONS Consortium. The funders did not play any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Results
Search results
The search yielded 2578 articles and 596 abstracts, of which 249 were retained for full text review (As depicted in Appendix S1; see Supplemental Digital Content). Fifty records met the inclusion criteria for this review, covering 41 individual trials, demonstration projects or routine implementation/clinical programmes.
Study characteristics
Key features of the studies, including type of study, site and study population are included in Appendix 2 (see Supplemental Digital Content). Although some trials published multiple papers, they all contributed to a single record if they reported on the same data. On the contrary, a single paper is considered to contribute to multiple studies if it reported data along the cascade separately by population or location. The 41 programmes covered by the studies in this review included 24 open-label or demonstration projects, nine routine implementation/clinical programmes and eight randomized controlled trials (RCTs). Studies were most commonly conducted in Africa (16, 39%) and North America (12, 29%). Populations most commonly reported were MSM and transgender women (TGW) (18, 44%), all people at risk (9, 22%) and women (6, 15%).
Reporting of preexposure prophylaxis cascade components
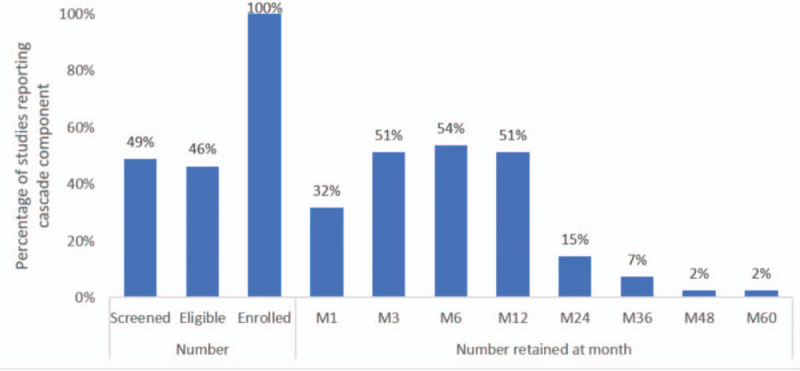
Table 1[20–56] presents the number of clients screened, eligible and enrolled/initiated for each study, along with the percentage of those enrolled/initiated still retained in care (continuation) at each time point of the PrEP services cascade. No studies reported prevention-effective use and none discussed a continuation definition that suggested continued need for PrEP was considered in the statistics presented. Reported components of the PrEP services cascade varied by study, as shown in Fig. 2.
Table 1.
Percentage of participants/clients who continued on PrEP over time.
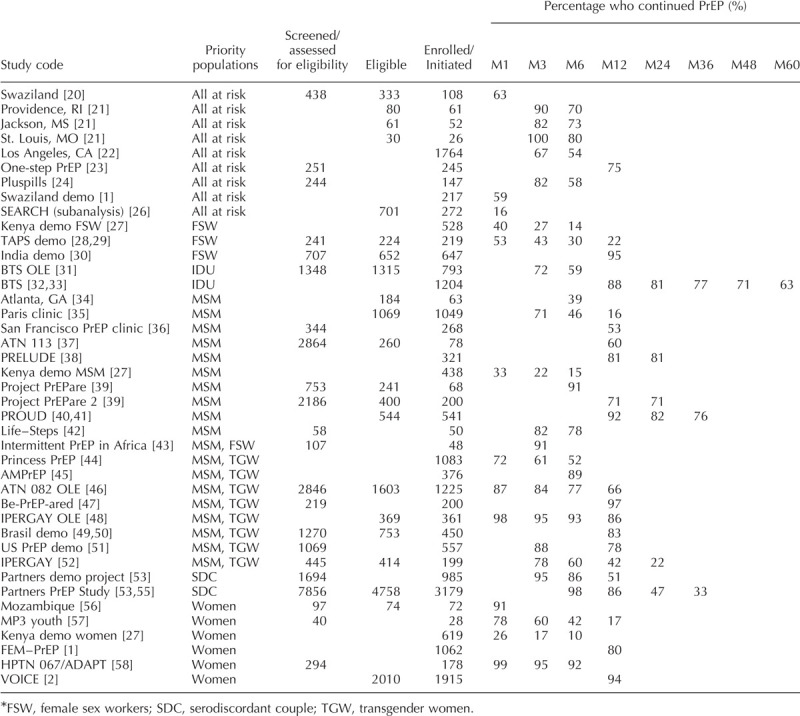
Fig. 2.
Components of the PrEP services cascade reported in literature review.
Just under half of the studies reported the numbers of clients screened and the number of clients determined eligible for PrEP. All studies reported number of clients enrolled/initiated (a requirement for inclusion) but reported this information in different ways. Some studies reported the number enrolled, while others also reported those who were prescribed PrEP or those who started taking PrEP, and sometimes these numbers differed from those enrolled [21]. We reported the number who started taking PrEP as enrolled/initiated when such information was provided.
Continuation was most commonly reported at month 6, followed by months 3 and 12, and then month 1. Continuation past one year was rarely reported and was not reported in any of the routine implementation studies included in this review. Other time points at which continuation was reported were months 4, 9, 15, 16, 18 and 20 [30,34,42], and weeks 6, 10, 14, 18, 22, 26, 30, 34 [53,56].
Some studies only reported continuation as total or average length of follow-up (in days, months, or years). As it was not possible to fit these data into the PrEP cascade, those studies were excluded. Other methods for reporting continuation included using the number who ‘opted out’ at certain time points [24] or the percentage retained among those still remaining in the study at the previous time point [47].
Continuation up to 1 year
All studies reported continuation at a minimum of one time point within 1 year of initiation. Continuation at each time point varied greatly across studies, with some studies maintaining relatively high continuation over time and others with an immediate drop-off. To compare continuation within studies over time, we looked at continuation among studies reporting three or more of the four timepoints up to month 12 in Fig. 3. Average continuation among these studies was: 65% (M1), 62% (M3), 51% (M6) and 43% (M12). Percentage discontinuation between time periods varied by study, with discontinuation ranging from 2 to 18% from months 1–3, from 1 to 25% from months 3–6, and from 7to 35% from months 6–12.
Fig. 3.
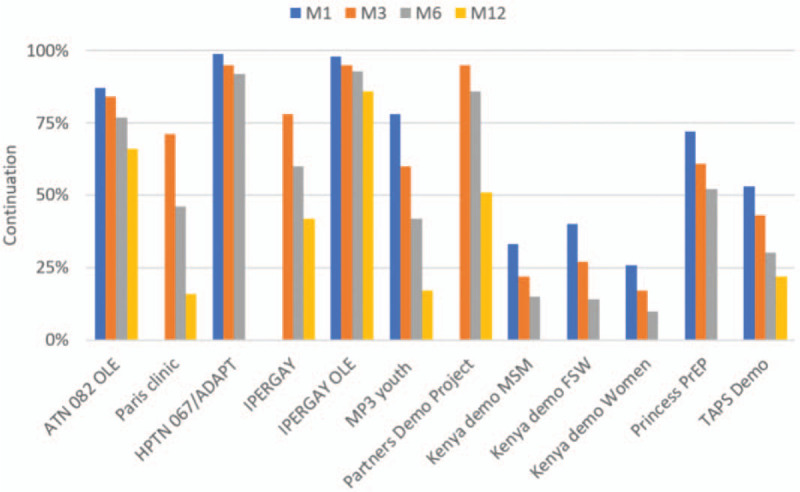
PrEP continuation cascade among studies reporting on three or more time periods up to month 12.
Continuation after 1 year
Few studies reported continuation after month 12. Six of the studies reported continuation at month 24, which ranged from 22 to 82%. At 36 months, continuation ranged from 33 to 77%, as reported by three studies. The only study providing continuation data past 36 months, the Bangkok Tenofovir Study (BTS), reported continuation on PrEP among IDU as 71% at 48 months and 63% at 60 months [31].
Continuation by population
We examined continuation by population visually over time using forest plots. Data from 3107 MSM, 897 women, 597 all at risk and 747 female sex workers (FSWs) were available for the meta-analysis of continuation at month 1 (Fig. 4a) [57,58].
Fig. 4.
(a) Forest plot of continuation by subpopulation at 1 month after initiation.
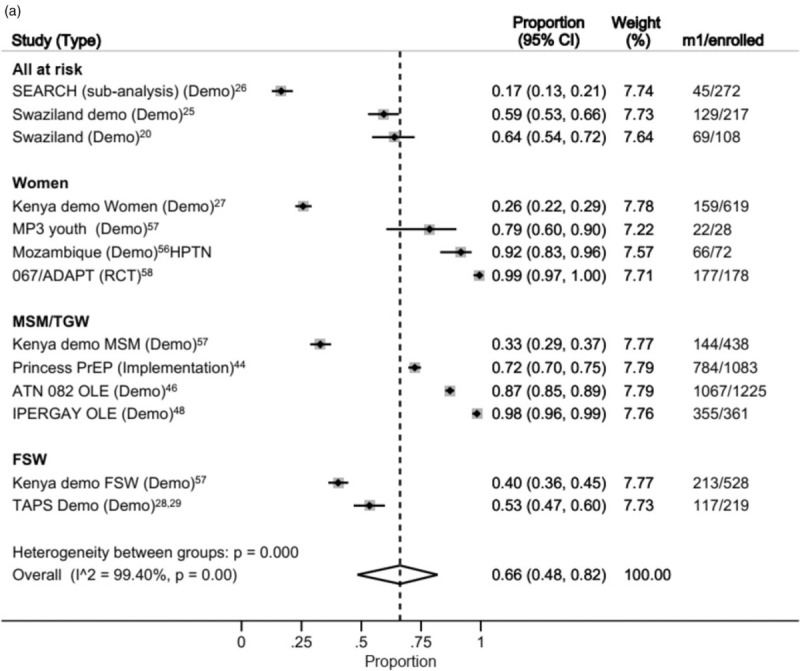
(b) Forest plot of continuation by subpopulation at 6 months after initiation. (c) Forest plot of continuation by subpopulation at 12 months after initiation.
Fig. 4 (Continued).
(a) Forest plot of continuation by subpopulation at 1 month after initiation.
(b) Forest plot of continuation by subpopulation at 6 months after initiation. (c) Forest plot of continuation by subpopulation at 12 months after initiation.
Fig. 4 (Continued).
(a) Forest plot of continuation by subpopulation at 1 month after initiation.
(b) Forest plot of continuation by subpopulation at 6 months after initiation. (c) Forest plot of continuation by subpopulation at 12 months after initiation.
Considerable variation was observed across studies and populations. Significant inter-group heterogeneity was observed (P < 0.001 and high I2 statistic in each group), so pooled estimates by population were omitted. Heterogeneity among groups was also significant, suggesting the pooling of all studies may not be appropriate.
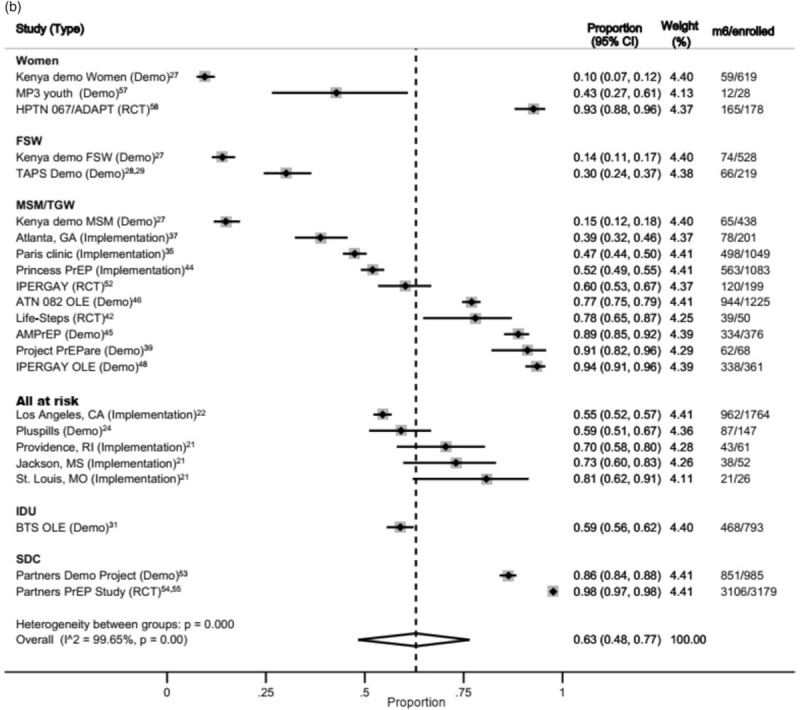
At 6 months, a total of 5050 MSM, 4164 serodiscordant couples (SDCs), 2050 all at risk, 825 women, 793 IDUs (from one study) and 747 FSW were available for the meta-analysis (Fig. 4b). Again, heterogeneity was high among studies and across population groups.
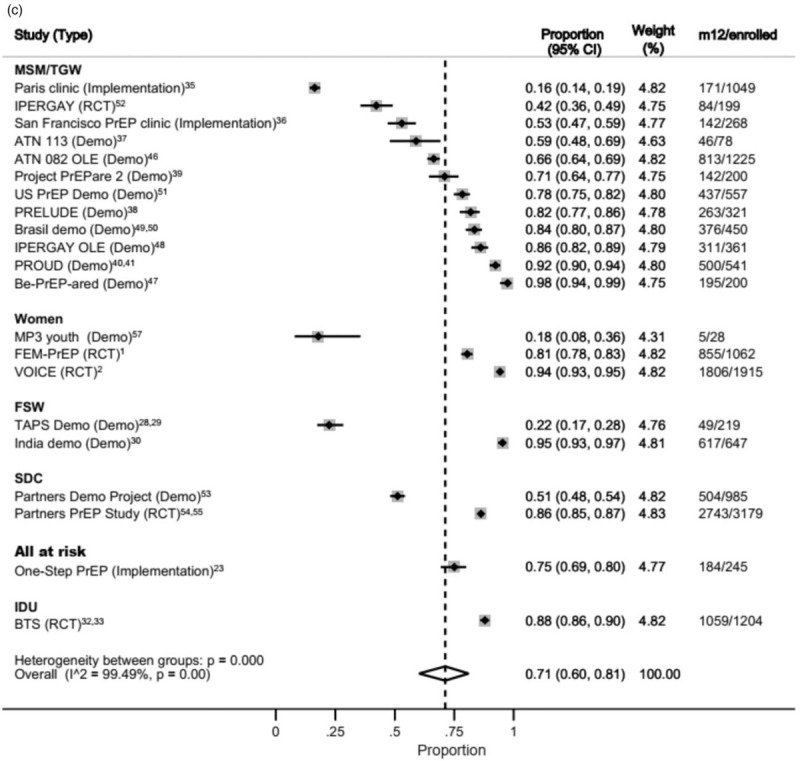
At month 12, data on 5449 MSM, 4164 SDC, 3005 women, 1204 IDU (from one study), 866 FSW and 245 members of the general population (from one study) were available for the meta-analysis (Fig. 4c). Again, inter-studies and intergroup heterogeneity were high.
Discussion
This systematic review synthesizes the growing body of literature on PrEP continuation. The results show that the metric by which oral PrEP continuation is measured and reported are not consistent. Continuation varies widely across studies and target populations, and continues to decline over time.
Collation of data along the PrEP cascade revealed that the time points at which continuation is reported vary widely. This is not surprising, given the abundance of proposed PrEP cascades in published literature and the lack of consensus on which components are most important to track [11–13]. In studies that reported multiple time points, we found that discontinuation often persisted over time, with discontinuation as high as 25 and 35% from months 3 to 6 and from months 6 to 12, respectively.
These results have implications for existing monitoring and evaluation guidelines, which focus heavily on cross-sectional indicators over client-level longitudinal indicators. The WHO PrEP M&E guidelines suggest a core indicator of ‘Continuation on PrEP’, defined as the ‘Percentage of PrEP users who continued on oral PrEP for three consecutive months after having initiated PrEP in the last 12 months [7]’. The decision to limit this indicator to 3 months was justified based on early data from demonstration projects suggesting that many users who discontinue oral PrEP do so during the first few months. This review contradicts those early results, given it has shown that discontinuation in the studies currently under review was common even after month 3.
The PEPFAR oral PrEP indicators also do not promote longitudinal monitoring, rather they parallel existing treatment indicators, which give a snapshot of changes in the total number in care over time, rather than allowing for an understanding of duration of continuation [10]. Although M&E indicators of client-level continuation may not be feasible, organizations should promote evaluation studies to understand this important dimension of PrEP rollout, without which impact and cost-effectiveness cannot be assessed.
Just under one-third of studies reported continuation at month 1; among those, discontinuation was high, averaging 37.3%. High discontinuation at month 1 indicates a large percentage of PrEP clients are not returning for the first follow-up visit and has important implications for PrEP effectiveness. Although discontinuation at subsequent time points could be due to periods of low risk, discontinuation at 1 month likely indicates other reasons for stopping. These findings suggest that when assessing whether a client should initiate PrEP, attention should be paid to not only PrEP eligibility, but also the client's readiness to take PrEP consistently over time. Initiations are costly [59], and no prevention impact can be assumed without at least one return visit. This finding suggests that the number of PrEP initiations may not be a very useful indicator in estimating PrEP effectiveness.
Recent studies show that side effects, stigma, influence of partners, difficulty accessing services and reduced HIV-risk perception have contributed to discontinuation in some PrEP users [26,60,61]. Discontinuation due to lack of risk is an important concept for continuation measurement, as discussed previously, which we were unable to account for in our analysis due to lack of data. More research is needed to determine the reasons for high early and ongoing discontinuation.
In designing this review, we felt it was important to make the distinction between continuation of all clients who initiated and continuation among just those still at risk or indicated for PrEP, known as prevention-effective use 16. No studies in this review reported prevention-effective use or stopping and restarting of clients on PrEP. Some studies reported planned cycling or dosing schedules, such as studies among serodiscordant couples that promoted PrEP as a bridge to ART and the Gaza miners study, which offered PrEP during periods of high risk [52,54,62]. Future research is needed to examine cycling among PrEP users and how to appropriately monitor prevention-effective use.
This review found that continuation varied by population and across time. Pooled estimates at 12 months were actually higher than previous timepoints. This is likely due to different studies reporting at the different timepoints, and some studies with particularly low continuation reporting at only months 1 and 6 [26]. Continuation also varied within populations. Some of this variation can likely be attributed to differences in study types, intervention models, and mechanisms for client support.
This systematic review has limitations. Studies had various designs, populations and geographic locations. Given the paucity of data on combinations of population, study type and geography, it is not currently possible to examine pooled continuation by just one population, study type and region. As PrEP delivery progresses, programmes should be encouraged to publish data on continuation across time so that these analyses can be completed and shed further light on this important topic. To better understand PrEP continuation, researchers should consider longitudinal studies that account for prevention effective adherence (time at-risk) and explore probabilities of continuation via survival analysis or other more robust methods.
Some studies had to be excluded because the reported continuation data did not align with the cascade used in the study design. We could not distinguish in this review participants who were lost to follow-up versus those who went off the product and stayed in the study. Finally, this study did not assess the influence of potential confounders. Further research is needed to examine the predictors of PrEP continuation and discontinuation to more fully understand this important component of PrEP programme effectiveness and efficiency.
Despite these limitations, the findings have implications for the evolving discussion on how to monitor PrEP programmes and provide valuable information for decision makers. Our analysis of continuation suggests that PrEP initiations may not be a good measure of effectiveness and that longitudinal monitoring of continuation may be important for understanding long-term use patterns. Research should examine methods of ensuring PrEP-readiness prior to initiation and reasons for early and later discontinuation. Guidance is needed on how best to measure prevention-effective use, which was not reported by any studies in this review.
Acknowledgements
This work was led by the OPTIONS Consortium, a programme made possible by the generous assistance from the American people through the U.S. Agency for International Development (USAID) and the U.S. President's Emergency Plan for AIDS Relief (PEPFAR). Financial assistance was provided by USAID to FHI 360 and the London School of Hygiene and Tropical Medicine under the terms of Cooperative Agreement No. AID-OAA-A-15-00035. The contents do not necessarily reflect the views of USAID or the United States Government. J.O. was supported by the Australian National Health and Medical Research Fund (APP1104781).
Conflicts of interest
K.S. conducted the systematic review, data validation, data analysis, data interpretation and led writing. H.G. contributed to study design, data interpretation and review. J.L. conducted the systematic review and supported study design, data analysis, data interpretation and writing. G.G. contributed to study design, data analysis, data interpretation and review. K.K. contributed to study design, data interpretation and review. K.T. contributed to study design, data interpretation and review. J.O. contributed to study design, data validation, data interpretation and review. F.T.P. contributed to study design, data interpretation and review.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV Infection among African women. N Engl J Med 2012; 367:411–422.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New Engl J Med 2015; 372:509–518.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rees H, Delany-Moretlwe SA, Baron D, Lombard C, Gray G, Myer L, et al.Facts 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women.Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA (abstr); 2015. [Google Scholar]
- 4.Mullins TLK, Lally M, Zimet G, Mullins TL, Lally M, Zimet G, Kahn JA. Clinician attitudes toward CDC interim preexposure prophylaxis (PrEP) guidance and operationalizing PrEP for adolescents. AIDS Patient Care ST 2015; 29:193–203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krakower DS, Mayer KH. The role of healthcare providers in the roll out of preexposure prophylaxis. Curr Opin HIV AIDS 2016; 11:41–48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong JJ, Baggaley RC, Wi TE, Tucker JD, Fu H, Smith MK, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open 2019; 2:e1917134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO WHO implementation tool for preexposure prophylaxis of HIV infection. Geneva, Switzerland:World Health Organization; 2017. [Google Scholar]
- 8.AVAC. National policies and guidelines for PrEP. https://www.prepwatch.org/prep-planning/national-policies-guidelines/ [Accessed 15 March 2019]. [Google Scholar]
- 9.AVAC. Global PrEP tracker. 2018. https://www.prepwatch.org/in-practice/global-prep-tracker/ [Accessed 6 October 2019]. [Google Scholar]
- 10. PEPFAR. Monitoring, evaluation, and reporting indicator reference guide, MER 2.0 (Version 2.4). Washington, DC: PEPFAR, 2019. [Google Scholar]
- 11.Hargreaves JR, Delany-Moretlwe S, Hallett TB, Johnson S, Kapiga S, Bhattacharjee P, et al. The HIV prevention cascade: integrating theories of epidemiological, behavioural, and social science into programme design and monitoring. Lancet HIV 2016; 3:e318–e322.. [DOI] [PubMed] [Google Scholar]
- 12.Dunbar MS, Kripke K, Haberer J, Castor D, Dalal S, Mukoma W, et al. Understanding and measuring uptake and coverage of oral preexposure prophylaxis delivery among adolescent girls and young women in sub-Saharan Africa. Sex Health 2018; 15:513–521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu A, Colfax G, Cohen S, Bacon O, Kolber M, Amico KR, et al.The spectrum of engagement in HIV prevention: proposal for a PrEP cascade. 7th International Conference on HIV Treatment and Prevention Adherence. Miami, FL, USA (abstr); 2012. [Google Scholar]
- 14.Gilbert HN, Wyatt MA, Pisarski EE, Muwonge TR, Heffron R, Katabira ET, et al. PrEP discontinuation and prevention-effective adherence: experiences of PrEP users in Ugandan HIV serodiscordant couples. J Acquir Immune Defic Syndr 2019; 82:265–274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution; from clinical trials to routine practice. Curr Opin HIV AIDS 2016; 11:10–17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, et al. Defining success with HIV preexposure prophylaxis: a prevention-effective adherence paradigm. AIDS 2015; 29:1277–1285.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Joanna Briggs Institute The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for prevalence studies. Adelaide, Australia:The Joanna Briggs Institute; 2017. [Google Scholar]
- 18.Stata statistical software: release 15. StataCorp LLC. 2017 [cited January 2019]. https://www.stata.com/stata15/. [Google Scholar]
- 19.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nesbitt R, Mpala Q, Mamba C, et al.PrEP for HIV prevention in Shiselweni, Swaziland: early uptake and month one retention.22nd International AIDS Conference. Amsterdam, The Netherlands (abstr); 2018. [Google Scholar]
- 21.Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, et al. Retention in care outcomes for HIV preexposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016; 19:20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shover CL, Javanbakht M, Shoptaw S, Bolan R, Gorbach P. High discontinuation of preexposure prophylaxis within six months of initiation.Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA (abstr); 2018. [Google Scholar]
- 23. Tung E, Thomas A, Eichner A, Shalit P. Feasibility of a pharmacist-run HIV PrEP clinic in a community pharmacy setting.Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA (abstr); 2017. [Google Scholar]
- 24. Gill K, Dietrich J, Gray G, Pidwell T, Kayamba F, Bennie T, et al.Pluspills: an open-label, safety and feasibility study of oral preexposure prophylaxis (PrEP) in 15-19-year-old adolescents in two sites in South Africa. 9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 25.Mayer CM, Owaraganise A, Kabami J, Kwarisiima D, Koss CA, Charlebois ED, et al. Distance to clinic is a barrier to PrEP uptake and visit attendance in a community in rural Uganda. J Int AIDS Soc 2019; 22:e25276–e25280.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kyongo JK, Kiragu M, Karugu F R, Ochieng C, Ngunjiri A, Wachihi C. How long will they take it? Oral preexposure prophylaxis (PrEP) retention for female sex workers, men who have sex with men and young women in a demonstration project in Kenya.22nd International AIDS Conference. Amsterdam, the Netherlands (abstr); 2018. [Google Scholar]
- 27.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV preexposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med 2017; 14:e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eakle R, Mutanha N, Mbogua J, Sibanyoni M, Bourne A, Gomez G, et al. Designing PrEP and early HIV treatment interventions for implementation among female sex workers in South Africa: developing and learning from a formative research process. BMJ Open 2018; 8:e019292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reza-Paul S, Lazarus L KN R, MS V, Ramaiah M, Becker M, et al.A PrEP demonstration project among female sex workers in India.22nd International AIDS Conference. Amsterdam, the Netherlands (abstr); 2018. [Google Scholar]
- 30.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV preexposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. Lancet HIV 2017; 4:e59–e66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–2090.. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS 2015; 29:819–824.. [DOI] [PubMed] [Google Scholar]
- 33. Rolle CP, Onwubiko U, Jo J, Sheth AN, Kelley CF, Holland DP. PrEP implementation and persistence in a county health department in Atlanta, GA.Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA (abstr); 2018. [Google Scholar]
- 34. Balavoine S, Noret M, Loze B, Pintado C, Leplatois A, Charbonneau P, et al.PrEP uptake, safety and efficacy in a hospital-based clinic in Paris.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 35.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV preexposure prophylaxis (PrEP) uptake and retention among men who have sex with men in a community-based sexual health clinic. AIDS Behav 2018; 22:1096–1099.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatrics 2017; 171:1063–1071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaccher SJ, Marzinke M, Grulich AE, Ooi C, Carr A, Haire BG, et al.Very high adherence to HIV PrEP over one year confirmed by four measures in an open-label demonstration project (PRELUDE) in NSW, Australia.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 38.Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr 2013; 62:447–456.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gafos M, White E, White D, Clarke A, Apea V, Brodnicki E. Adherence intentions, long-term adherence and HIV acquisition among PrEP users in the PROUD open-label randomised control trial of PrEP in England.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 40.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer KH, Safren SA, Elsesser SA, Psaros C, Tinsley J, Marzinke M, et al. Optimizing preexposure antiretroviral prophylaxis adherence in men who have sex with men: results of a pilot randomized controlled trial of ‘Life-Steps for PrEP’. AIDS Behav 2017; 21:1350–1360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutua G, Sanders E, Mugo P, Anzala O, Haberer J, Bangsberg D, et al. Safety and adherence to intermittent preexposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One 2012; 7:e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jantarapakde J, Pengnonyang S, Sungsing T Meekrua P, Brutrat P, Panpet P, et al.Retention and adherence to PrEP among MSM and transgender women in Thailand's ‘Princess PrEP program’: the key population-led PrEP program.22nd International AIDS Conference. Amsterdam, the Netherlands (abstr); 2018. [Google Scholar]
- 44.Hoornenborg E, Coyer L, van Laarhoven A, Achterbergh R, de Vries H, Prins M, van der Loeff MS. Change in sexual risk behaviour after 6 months of preexposure prophylaxis use. AIDS 2018; 32:1527–1532.. [DOI] [PubMed] [Google Scholar]
- 45.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of preexposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–829.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vuylsteke B, Reyniers T, De Baetselier I, Wouters K, Nöstlinger C, Laga M. Be-PrEP-ared’: a PrEP demonstration project among men who have sex with men in Belgium – preliminary results.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 47.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Chapitant C, et al. Efficacy, safety, and effect on sexual behaviour of on-demand preexposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4:e402–e410.. [DOI] [PubMed] [Google Scholar]
- 48. Grinsztejn B, Hoagland B, Moreira R, Kallas EG, Madruga JV, Goulart S, et al.High level of retention and adherence at Week 48 for MSM and TGW enrolled in the PrEP Brasil demonstration study.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 49.Grinsztejn B, Hoagland B, Moreira RI, Kallas EG, Madruga JV, Goulart S, et al. Retention, engagement, and adherence to preexposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5:e136–e145.. [DOI] [PubMed] [Google Scholar]
- 50.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–2246.. [DOI] [PubMed] [Google Scholar]
- 52.Baeten JM, Heffron R, Kidoguchi L, Mugo N, Katabira E, Bukusi E, et al. Integrated delivery of antiretroviral treatment and preexposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med 2016; 13:e1002099–e1002100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lahuerta M, Zerbe A, Baggaley R, Falcao J, Ahoua L, DiMattei P, et al. Feasibility, acceptability, and adherence with short-term HIV preexposure prophylaxis in female sexual partners of migrant miners in Mozambique. J Acquir Immune Defic Syndr 2017; 76:343–347.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Inwani I, Buttolph J, Kawango A, Cleland C, Kiarie J, Kinuthia J, et al.PrEP cohort results from MP3 youth combination HIV prevention pilot study.9th IAS Conference on HIV Science. Paris, France (abstr); 2017. [Google Scholar]
- 56.Bekker L-G, Roux S, Sebastien E, Yola N, Amico KR, Hughes JP, et al. Daily and nondaily preexposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV 2018; 5:e68–e78.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hughey A, Hettema A, Oldenburg C, Kohler S, McMahon S, Lejeune C, et al.Predictors of 1-month retention of PrEP for the general population in the public sector: a longitudinal study in routine care in Swaziland.22nd International AIDS Conference. Amsterdam, the Netherlands (abstr); 2018. [Google Scholar]
- 58.Baeten JM, Donnell D, Mugo NR, Ndase P, Thomas KK, Campbell JD, et al. Single-agent tenofovir versus combination emtricitabine plus tenofovir for preexposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis 2014; 14:1055–1064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mangenah C, Nhamo D, Terris-Prestholt F. The costs of PrEP implementation across high risk populations in Zimbabwe.20th International Conference on AIDS and STI's in Africa. Kigali, Rwangda (abstr); 2019. [Google Scholar]
- 60. McGrory E, Murungu J, Aguolu R, Alagi M, Alary M, Baeten J, et al.Delivering PrEP: lessons from early demonstration projects.HIV R4P. Madrid, Spain (poster); 2018. [Google Scholar]
- 61.Pillay D, Stankevitz K, Lanham M, Ridgeway K, Murire M, Briedenhann E, et al. Factors influencing uptake, continuation, and discontinuation of oral PrEP among clients at sex worker and MSM facilities in South Africa. PLoS One 2020; 15:e0228620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pyra M, Haberer JE, Heffron R, Kidoguchi L, Brown E, Bukusi E, et al. PrEP use during periods of HIV risk among East African women in serodiscordant relationships. J Acquir Immune Defic Syndr 2018; 77:41–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


