Précis:
Modeling of visual field and pharmacy data (Kaiser Permanente, 2001 to 2014) from open-angle/pseudoexfoliation glaucoma patients in clinical practice indicated a significant inverse association between the level of medication adherence and rate of visual field progression.
Purpose:
The aim was to quantify the effect of nonadherence to topical hypotensive medication on glaucomatous visual field progression in clinical practice.
Methods:
Retrospective analysis of combined visual field and pharmacy data from Kaiser Permanente Southern California’s HealthConnect electronic health record database. Patients with a diagnosis of primary open-angle glaucoma or pseudoexfoliation glaucoma (2001 to 2011) and ≥3 subsequent visual field tests of the same Swedish Interactive Threshold Algorithm type were followed up from first medication fill to final visual field test. Medication adherence (proportion of days covered) was estimated from pharmacy refill data. A conditional growth model was used to estimate the effect of adherence level in modifying the progression of mean deviation over time after adjusting for potential confounders, including age, sex, race/ethnicity, baseline glaucoma severity, and comorbidity.
Results:
In total, 6343 eligible patients were included in the study and followed for (mean) 5.8 years; average treatment adherence during follow-up was 73%. After controlling for confounders and the interaction between time and baseline disease severity, the model indicated that mean deviation progression was significantly (P=0.006) reduced by 0.006 dB per year for each 10% (absolute) increase in adherence. Model estimates of time to glaucoma progression (mean deviation change −3 dB from baseline) were 8.3 and 9.3 years for patients with adherence levels of 20% and 80%, respectively.
Conclusions:
Improving patient adherence to topical glaucoma medication may result in slower deterioration in visual function over time.
Key Words: glaucoma, medication adherence, visual field, disease progression
Open-angle glaucoma (OAG), the predominant form of glaucoma in the Western world,1 is a chronic, progressive optic neuropathy characterized by retinal ganglion cell loss with associated visual field defects.2,3 Because of its progressive and largely asymptomatic nature, it is crucial that OAG is diagnosed at an early stage and that its progression is monitored in a timely manner to prevent functional vision loss. Increased intraocular pressure (IOP) is an important prognostic risk factor for development of OAG and for progression of visual field loss in established OAG,4–6 and is the only modifiable etiologic factor.7–11 First-line treatment for OAG typically comprises topical IOP-lowering medication; however, patient adherence with treatment (consistent daily use of medication in accordance with dosage recommendations) and persistence (continued use of medication over time) is typically poor.12–17 Multiple factors can affect adherence, such as access to medication, remembering to use the medication every day, appropriately timing the doses, and instilling the medication accurately into the eye. Patients may have difficulty with administration—for example, administering the appropriate number of drops, missing their eye, or being unable to squeeze the bottle properly.18,19 In addition, tolerability issues and cost considerations may hinder patients’ ability to maintain treatment over the long term.18–21 Poor treatment adherence may contribute to suboptimal IOP control and hence to increased risk of glaucomatous damage, visual disability, and blindness.22–26 Strategies to improve treatment adherence in OAG may help to preserve visual function18,27–30; however, quantitative information on the relationship between treatment adherence and glaucomatous visual field progression is sparse.18,29,31,32 Analysis of data from the topical medication arm of the Collaborative Initial Glaucoma Treatment Study (CIGTS), one of the few studies to have captured longitudinal measures of both medication adherence and visual field, indicated a clinically significant association between patients’ reported levels of adherence and glaucomatous vision loss over 8 years of follow-up.32 However, the magnitude of the association is uncertain, as self-reported adherence to eyedrops is invariably an overestimate of true adherence.33
This retrospective cohort study was designed to assess the effect of adherence to topical glaucoma medication, as determined from pharmacy refill data, on long-term visual field progression among newly diagnosed OAG patients in a large integrated US health care system. The availability of pharmacy refill data in administrative claims databases provides a convenient and unobtrusive method for analyzing medication adherence patterns among patients in the real-world setting.34 This study is the first to quantify the impact of adherence on visual field progression in a real-world setting.
METHODS
This study was conducted at Kaiser Permanente Southern California (KPSC), a group model health maintenance organization, which provides health care to members living in Southern California. The membership is racially and ethnically diverse and representative of the population of Southern California.35
Database
Electronic health records from 2001 to 2014 were sourced from Kaiser Permanente HealthConnect, one of the world’s largest private electronic health record systems.36 This integrated, patient-level database contains records of all health care encounters, including office visits, outpatient clinic visits, and surgeries, with associated International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes and Current Procedural Terminology (CPT) procedural codes. Because of the closed pharmacy benefit of KPSC, prescriptions are dispensed through the KPSC pharmacy system, which enables verification that patients receive their prescribed medications. Pharmacy prescription fill data were obtained from Kaiser Permanente’s Pharmacy Information Management System database (a component of the HealthConnect database). The study protocol was reviewed and approved by the institutional review board of KPSC (IRB # 5818). All patient data were de-identified in compliance with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Study Population
The HealthConnect database was screened to identify patients who (i) received an initial diagnosis of primary OAG (ICD-9 diagnostic code 365.1x) or pseudoexfoliation glaucoma (ICD-9 code 365.52) in the outpatient setting between 2001 and 2011, (ii) filled a prescription (index) for topical ocular hypotensive medication within 90 days of their initial diagnosis, (iii) had drug benefit coverage, and (iv) had at least 1 year of continuous membership before diagnosis and at least 2 years of continuous membership after diagnosis (with a 45-day gap allowance for each period). For study inclusion, each patient was required to have at least 3 reliable visual field tests of the same Swedish Interactive Threshold Algorithm (SITA) type [either SITA-Standard (24 or 30) or SITA-Fast (24 or 30)] over the period of interest. Visual field tests with false-positive or false-negative rates >15% were removed from the analysis.
Patients were excluded from the study cohort if they were below 18 years of age at initial glaucoma diagnosis, had a glaucoma diagnosis before 2001, or had a condition that might affect the visual field of the eye, including a history of glaucoma because of other lens disorders (ICD-9 code 365.5x), low vision/blindness (ICD-9 code 369.xx), optic nerve/visual pathway disorder (ICD-9 code 377.xx), trabeculotomy ab externo (CPT code 65850), selective laser trabeculoplasty (CPT code 65855), trabeculectomy (CPT codes 66170, 66172), or aqueous shunt insertion (CPT codes 66179, 66180, 66183) before their initial glaucoma diagnosis (Fig. 1). Eligible patients were followed from their index date (ie, date of first prescription fill) to their last follow-up visual field assessment (the last visual field within the data set was performed on January 31, 2014) or earlier in the event of glaucoma-related surgery, death or disenrollment from the health plan.
FIGURE 1.
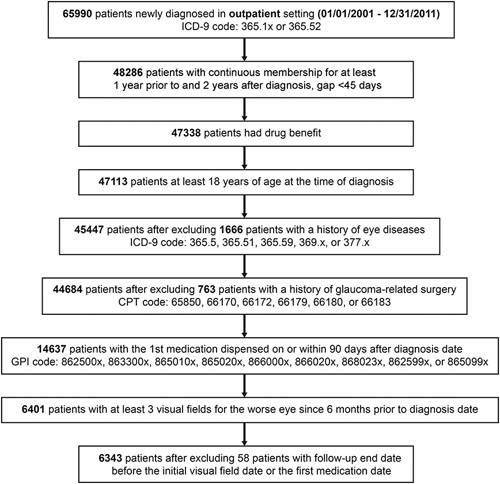
Flow chart for generation of the study cohort. CPT indicates Current Procedural Terminology; GPI, Generic Product Identifier; ICD-9, International Classification of Diseases, Ninth Revision.
Each patient contributed one eye to the study. If both eyes met the inclusion criteria, the eye with the worse mean deviation at the visual field test closest to the index date was designated as the study eye.
Visual Field Assessment
Visual field data from January 2001 to January 2014 were downloaded from Humphrey Field Analyzer II machines (Zeiss-Humphrey Systems, Dublin, CA). Where available, a visual field measurement performed within ±6 months of the index date was used to establish baseline disease severity. If a patient had more than one measurement within ±6 months of the index date, the visual field type (SITA-Standard vs. SITA-Fast) with the most measures and the measure closest to the index date served as the baseline measurement. Baseline disease severity was categorized according to mean deviation as mild (≥−2 dB), moderate (<−2.0 dB to ≥−6.0 dB), severe (<−6.0 dB to ≥−12.0 dB), or very severe (<−12.0 dB). Patients who did not have a visual field measurement within ±6 months of the index date were categorized as missing baseline disease severity. For this study the primary outcome of interest was the mean deviation in visual field over time. Disease progression in this study was defined as a loss of 3 dB in mean deviation; this threshold has previously been used to mark a significant change in visual field.37
Medication Adherence Assessment
Adherence to topical glaucoma medication was assessed from pharmacy refill data and expressed as the proportion of days covered (PDC), which is calculated as the number of days that the patient is supplied with medication divided by the number of days in the period of interest (Fig. 2).38 Patients who were prescribed multiple medication classes were assumed to be covered if they were supplied with at least 1 medication class. Treatment adherence was measured over the interval between medication initiation (index date) and the first visual field test, and over the individual intervals between consecutive visual field tests during the postindex period (Fig. 2). Adherence was variously expressed as (i) “first-year adherence” (covering the first 365 days from medication initiation), (ii) “total follow-up adherence” (from medication initiation to the final visual field test), and (iii) “average follow-up adherence” (mean of PDC estimates for the individual intervals between visual field tests).
FIGURE 2.
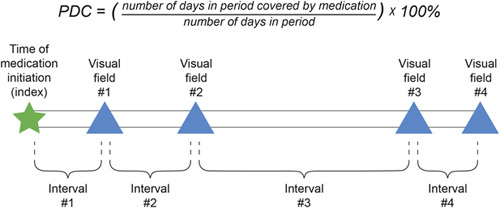
Formula for calculating proportion of days covered (PDC) with glaucoma medication, and time intervals used for calculation of medication adherence.
Conditional Growth Model Analysis
A conditional growth model (also known as a linear mixed effects model) was used to examine the impact of treatment adherence on the progression of mean deviation in visual field over the course of follow-up while controlling for potential confounders that might influence the change in mean deviation. In the model, the outcome was mean deviation at different time points during follow-up, while the predictors were time (years) from index date to mean deviation measurement, adherence level (proportion), and the interaction term between follow-up time and medication adherence. Potential confounders for adjustment included age (years) at first prescription fill, baseline glaucoma severity, sex, race/ethnicity, neighborhood household income, Charlson comorbidity index, history of diabetic retinopathy, history of stroke, and the interaction term between follow-up time and baseline glaucoma severity. Since mean deviation was a repeated measure for each patient, the intercept and coefficient of time were specified as random effects between patients, and within-patient correlations were specified using an autocorrelation structure. The coefficient of time indicated the mean deviation progression per year of follow-up, the coefficient of adherence indicated the mean deviation difference corresponding to a 100% (absolute) difference in adherence level (ie, 0% vs. 100% adherence) at a given time point, and the coefficient of the interaction between follow-up time and medication adherence indicated the effect of adherence in modifying the progression of mean deviation over time. Since mean deviation decreases (ie, deteriorates) over time in glaucoma, resulting in a negative coefficient of time, a positive coefficient of the interaction between time and adherence would suggest that higher adherence is associated with a slower decline in mean deviation over time.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.4.3 (R Core Team, Vienna, Austria). For all statistical tests, the significance level was set at 0.05.
RESULTS
Study Population
Of 65,990 patients identified with a diagnosis of primary OAG or pseudoexfoliation glaucoma between 2001 and 2011, 6343 patients (2936 men; 3407 women) met the study eligibility criteria (Fig. 1). Of this population (n=6343), 54% of patients were female and 46% were male. Within the study population, 4713 patients (74%) had a baseline visual field assessment, whereas 1630 (26%) did not. Among patients with a baseline visual field, 1096 (23.3%) had mild disease, 2055 (43.6%) had moderate disease, 1002 (21.3%) had severe disease, and 560 (11.9%) had very severe disease. Age at diagnosis differed significantly across the disease severity categories, ranging from mean (SD) 63.3 (10.2) years in patients with mild disease to 67.7 (10.6) years in those with severe disease, as did sex, race/ethnicity, neighborhood household income, Charlson comorbidity index, and history of diabetic retinopathy (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of the Study Population, Categorized by Baseline Glaucoma Severity*
| Mild (≥−2 dB), N=1096 (17.3%) | Moderate (<−2.0 dB to ≥−6.0 dB), N=2055 (33.4%) | Severe (<−6.0 dB to ≥−12.0 dB), N=1002 (15.8%) | Very Severe (<−12.0 dB), N=560 (8.8%) | Total, N=4713 | P † | VF Unavailable at Baseline, N=1630 (25.7%) | |
| Initial VF mean deviation | <0.001 | ||||||
| Mean (SD) | −0.8 (0.88) | −3.8 (1.10) | −8.3 (1.68) | −16.5 (3.77) | −5.6 (5.06) | −5.5 (5.26) | |
| Median | −1.0 | −3.7 | −8.0 | −15.4 | −4.1 | −3.9 | |
| Q1, Q3 | −1.6, −0.2 | −4.6, −2.8 | −9.6, −6.9 | −18.7, −13.5 | −7.5, −2.1 | −7.6, −1.8 | |
| Range | (−2.00 to 4.45) | (−6.00 to −2.01) | (−11.99 to −6.01) | (−31.94 to −12.02) | (−31.94 to 4.45) | (−29.26 to 2.95) | |
| Length of KPSC membership after index diagnosis, years | 0.417 | ||||||
| Mean (SD) | 7.72 (3.17) | 7.70 (3.29) | 7.82 (3.30) | 7.52 (3.15) | 7.71 (3.25) | 9.24 (3.09) | |
| Median | 7.46 | 7.44 | 7.55 | 7.31 | 7.46 | 9.36 | |
| Q1, Q3 | 5.24, 10.13 | 4.93, 10.22 | 5.25, 10.23 | 4.92, 9.98 | 5.05, 10.17 | 6.85, 11.66 | |
| Range | (2.06-17.42) | (2.03-17.72) | (2.02-17.11) | (2.04-15.82) | (2.02-17.72) | (2.10-19.26) | |
| Age at diagnosis with glaucoma, years | <0.001 | ||||||
| Mean (SD) | 63.3 (10.21) | 65.7 (10.76) | 67.7 (10.60) | 67.0 (11.30) | 65.7 (10.78) | 64.7 (10.75) | |
| Median | 64 | 67 | 69 | 68 | 67 | 66 | |
| Q1, Q3 | 56.0, 70.0 | 59.0, 73.0 | 61.0, 76.0 | 60.0, 75.0 | 59.0, 73.0 | 57.0, 73.0 | |
| Range | (21.0-88.0) | (19.0-95.0) | (26.0-94.0) | (29.0-96.0) | (19.0-96.0) | (18.0-89.0) | |
| Patient sex, N (%) | <0.001 | ||||||
| Female | 588 (53.6) | 1126 (54.8) | 525 (52.4) | 255 (45.5) | 2494 (52.9) | 913 (56.0) | |
| Male | 508 (46.4) | 929 (45.2) | 477 (47.6) | 305 (54.5) | 2219 (47.1) | 717 (44.0) | |
| Race/ethnicity, N (%) | <0.001 | ||||||
| White, non-Hispanic | 528 (48.2) | 798 (38.8) | 391 (39.0) | 183 (32.7) | 1900 (40.3) | 702 (43.1) | |
| Black, non-Hispanic | 204 (18.6) | 440 (21.4) | 197 (19.7) | 147 (26.3) | 988 (21.0) | 305 (18.7) | |
| Hispanic | 243 (22.2) | 499 (24.3) | 237 (23.7) | 144 (25.7) | 1123 (23.8) | 367 (22.5) | |
| Asian, non-Hispanic | 106 (9.7) | 286 (13.9) | 167 (16.7) | 78 (13.9) | 637 (13.5) | 235 (14.4) | |
| Other, non-Hispanic | 15 (1.4) | 32 (1.6) | 10 (1.0) | 8 (1.4) | 65 (1.4) | 21 (1.3) | |
| Neighborhood household income, $1000‡ | <0.001 | ||||||
| Mean (SD) | 69.4 (30.12) | 69.0 (31.86) | 65.8 (30.42) | 67.0 (32.74) | 68.2 (31.29) | 70.5 (30.06) | |
| Median | 65.4 | 63.6 | 60.9 | 60.5 | 62.8 | 66.3 | |
| Q1, Q3 | 47.3, 86.6 | 46.7, 85.9 | 44.3, 80.2 | 45.4, 81.0 | 46.1, 84.4 | 49.2, 87.0 | |
| Range | (16.3-318.8) | (10.0-375.0) | (10.0-274.7) | (13.0-239.9) | (10.0-375.0) | (12.1-383.9) | |
| Charlson comorbidity index§ | <0.001 | ||||||
| Mean (SD) | 0.5 (0.93) | 0.7 (1.05) | 0.8 (1.14) | 0.6 (1.00) | 0.7 (1.04) | 0.6 (0.95) | |
| N (%) | |||||||
| 0 | 716 (66.3) | 1207 (59.6) | 559 (57.0) | 329 (60.3) | 2811 (60.7) | 1025 (65.0) | |
| 1 to 2 | 311 (28.8) | 684 (33.8) | 337 (34.4) | 182 (33.3) | 1514 (32.7) | 483 (30.6) | |
| 3 or more | 53 (4.9) | 135 (6.7) | 84 (8.6) | 35 (6.4) | 307 (6.6) | 70 (4.4) | |
| History of stroke, N (%) | 242 (22.1) | 527 (25.6) | 269 (26.8) | 138 (24.6) | 1176 (25.0) | 0.063 | 336 (20.6) |
| History of diabetic retinopathy, N (%) | 46 (4.2) | 107 (5.2) | 79 (7.9) | 29 (5.2) | 261 (5.5) | 0.002 | 64 (3.9) |
Baseline is defined as the date of medication initiation (first prescription fill). Glaucoma severity is based on mean deviation from the visual field test conducted closest to, and within ±6 months of, initiation of medication.
Statistical testing was performed with the Kruskal-Wallis test (one-way analysis of variance on ranks) for continuous variables and the χ2 test for categorical variables.
Neighborhood household income is not reported income but is estimated on the basis of members’ addresses and neighborhood incomes from the US Census tract information.
Charlson comorbidity index (CCI) is assessed in the year before the initial diagnosis of glaucoma. CCI score (range: 0 to 35) is a weighted, age-adjusted estimate of mortality risk based on the presence and severity of 16 comorbid conditions; higher scores correlate with reduced 10-year survival.
dB indicates decibel; KPSC, Kaiser Permanente Southern California; Q, quartile; VF, visual field.
Visual Field and Treatment Adherence
The mean duration of patient follow-up after first prescription fill was 5.8 years (range: 0.1 to 12.9 y). Over this time period, the mean number of visual field tests performed was 0.83 per eye per year of follow-up. The average first-year medication adherence was 78%, total follow-up adherence was 72%, and average follow-up adherence was 73%. Patients with very severe disease at baseline consistently had the highest levels of medication adherence across all 3 measures (Table 2).
TABLE 2.
Medication Adherence and Length of Follow-up of the Study Population, Categorized by Baseline Glaucoma Severity*
| Mild (≥−2 dB), N=1096 (17.3%) | Moderate (<−2.0 dB to ≥−6.0 dB), N=2055 (33.4%) | Severe (<−6.0 dB to ≥−12.0 dB), N=1002 (15.8%) | Very Severe (<−12.0 dB), N=560 (8.8%) | Unavailable at Baseline, N=1630 (25.7%) | Total, N=6343 (100%) | P † | |
|---|---|---|---|---|---|---|---|
| First-year adherence‡ | <0.001 | ||||||
| Mean (SD) | 0.77 (0.24) | 0.76 (0.24) | 0.78 (0.23) | 0.81 (0.23) | 0.78 (0.24) | 0.78 (0.24) | |
| Median | 0.85 | 0.83 | 0.86 | 0.92 | 0.87 | 0.85 | |
| Q1, Q3 | 0.61, 0.99 | 0.60, 0.99 | 0.63, 1.00 | 0.66, 1.00 | 0.61, 0.99 | 0.61, 0.99 | |
| Range | (0.09-1.00) | (0.08-1.00) | (0.09-1.00) | (0.13-1.00) | (0.13-1.00) | (0.08-1.00) | |
| N (%) | |||||||
| 0% to <20% | 24 (2.2) | 58 (2.8) | 18 (1.8) | 8 (1.4) | 42 (2.6) | 150 (2.4) | |
| 20% to <80% | 458 (41.8) | 880 (42.8) | 405 (40.4) | 188 (33.6) | 639 (39.2) | 2570 (40.5) | |
| 80% to 100% | 614 (56.0) | 1117 (54.4) | 579 (57.8) | 364 (65.0) | 949 (58.2) | 3623 (57.1) | |
| Total follow-up adherence§ | <0.001 | ||||||
| Mean (SD) | 0.70 (0.26) | 0.70 (0.26) | 0.73 (0.25) | 0.78 (0.24) | 0.73 (0.25) | 0.72 (0.26) | |
| Median | 0.76 | 0.76 | 0.79 | 0.88 | 0.79 | 0.78 | |
| Q1, Q3 | 0.53, 0.92 | 0.53, 0.92 | 0.58, 0.95 | 0.63, 0.98 | 0.58, 0.94 | 0.56, 0.94 | |
| Range | (0.01-1.00) | (0.01-1.00) | (0.01-1.00) | (0.02-1.00) | (0.02-1.00) | (0.01-1.00) | |
| N (%) | |||||||
| 0% to <20% | 74 (6.8) | 126 (6.1) | 35 (3.5) | 20 (3.6) | 84 (5.2) | 339 (5.3) | |
| 20% to <80% | 543 (49.5) | 1001 (48.7) | 477 (47.6) | 208 (37.1) | 742 (45.5) | 2971 (46.8) | |
| 80% to 100% | 479 (43.7) | 928 (45.2) | 490 (48.9) | 332 (59.3) | 804 (49.3) | 3033 (47.8) | |
| Average follow-up adherence∥ | <0.001 | ||||||
| Mean (SD) | 0.71 (0.25) | 0.71 (0.25) | 0.75 (0.23) | 0.79 (0.23) | 0.74 (0.25) | 0.73 (0.25) | |
| Median | 0.77 | 0.78 | 0.81 | 0.89 | 0.81 | 0.8 | |
| Q1, Q3 | 0.55, 0.93 | 0.55, 0.93 | 0.60, 0.95 | 0.67, 0.98 | 0.60, 0.95 | 0.58, 0.94 | |
| Range | (0.01-1.00) | (0.01-1.00) | (0.01-1.00) | (0.03-1.00) | (0.01-1.00) | (0.01-1.00) | |
| N (%) | |||||||
| 0% to <20% | 55 (5.0) | 107 (5.2) | 27 (2.7) | 13 (2.3) | 78 (4.8) | 280 (4.4) | |
| 20% to <80% | 553 (50.5) | 986 (48.0) | 455 (45.4) | 196 (35.0) | 720 (44.2) | 2910 (45.9) | |
| 80% to 100% | 488 (44.5) | 962 (46.8) | 520 (51.9) | 351 (62.7) | 832 (51.0) | 3153 (49.7) | |
| Length of follow-up, years¶ | <0.001 | ||||||
| Mean (SD) | 5.71 (2.71) | 5.50 (2.77) | 5.35 (2.75) | 4.83 (2.66) | 6.99 (2.69) | 5.84 (2.82) | |
| Median | 5.45 | 5.1 | 4.95 | 4.4 | 6.8 | 5.52 | |
| Q1, Q3 | 3.51, 7.52 | 3.14, 7.42 | 3.05, 7.12 | 2.70, 6.59 | 4.81, 9.08 | 3.52, 7.81 | |
| Range | (0.40-12.60) | (0.08-12.94) | (0.48-12.45) | (0.11-12.44) | (0.75-12.80) | (0.08-12.94) | |
Baseline is defined as the date of medication initiation (first prescription fill). Glaucoma severity is based on mean deviation from the visual field test conducted closest to, and within ±6 months of, initiation of medication.
Statistical testing was performed with the Kruskal-Wallis test (1-way analysis of variance on ranks) for continuous variables and the χ2 test for categorical variables.
Defined as the proportion of days covered in the first year following the date of medication initiation.
Total follow-up adherence was defined as medication adherence from the date of medication initiation to the last visual field.
Average follow-up adherence was defined as the average of the interim follow-up measurement before each visual field.
Measured as the time between medication initiation and the last visual field test within the follow-up period.
dB indicates decibel; Q, quartile.
Conditional Growth Model Analysis
In the conditional growth model, the coefficient of time was found to have a significantly negative value (β=−0.218; P<0.001), signifying a deterioration in mean deviation of 0.218 dB per year of follow-up (Table 3). The coefficient of medication adherence also showed a significantly negative value (β=−0.358; P=0.002), equating to a 0.036 dB worsening in mean deviation at a given time point per 10% absolute increase in adherence. This counterintuitive finding reflects the influence of disease severity, since medication adherence is typically higher among glaucoma patients with more advanced disease.39 The primary parameter of interest—the coefficient of the interaction between follow-up time and medication adherence—was positively and significantly associated with mean deviation progression (β=0.061; P=0.006), indicating a reduction in mean deviation progression of 0.006 dB per year in response to a 10% absolute increase in medication adherence. To illustrate this effect, Figure 3 shows model-based predicted mean deviation over time at low (20%) and high (80%) levels of medication adherence among patients stratified by baseline disease severity. The model predicts that for each baseline severity group, patients with high adherence will experience slower mean deviation progression than those with low adherence. For patients with moderate baseline disease severity, glaucoma progression (signified by a threshold 3 dB deterioration in mean deviation) will occur on average after 8.7 years of treatment in those with constant 20% adherence, and 1 year later in those with constant 80% adherence. Model estimates of the average annual change in mean deviation at high (80%) and low (20%) adherence levels were −0.32 and −0.37 dB, respectively (P=0.006), suggesting that patients with higher adherence had more stable visual fields compared with patients with low adherence. Among patient subgroups defined by baseline glaucoma severity, the coefficient of the interaction between follow-up time and baseline glaucoma severity was found to be significantly negative (P<0.001) in the moderate, severe, and very severe disease subgroups compared with that in the mild disease subgroup (reference group), suggesting that early initiation of medication is associated with slower progression of mean deviation.
TABLE 3.
Conditional Growth Model With Random Intercept and Slope of Time
| Coefficient Estimate | Standard Error | t Statistic | P * | |
|---|---|---|---|---|
| (Intercept) | −0.024 | 0.446 | −0.053 | 0.958 |
| Time in years | −0.218 | 0.029 | −7.457 | <0.001 |
| Medication adherence (0% to 100% interval) | −0.358 | 0.113 | −3.160 | 0.002 |
| Disease severity at baseline† | ||||
| Mild (≥−2 dB) | Reference | |||
| Moderate (−2.0 dB through <−6.0 dB) | −2.124 | 0.147 | −14.424 | <0.001 |
| Severe (−6.0 dB through <−12.0 dB) | −5.577 | 0.172 | −32.363 | <0.001 |
| Very severe (<−12.0 dB) | −13.333 | 0.205 | −65.059 | <0.001 |
| Missing baseline disease severity | −3.782 | 0.154 | −24.508 | <0.001 |
| Age in years at initiation of medication | ||||
| Below 40 | Reference | |||
| 40 to below 60 | 0.168 | 0.426 | 0.394 | 0.694 |
| 60 to below 80 | −0.369 | 0.423 | −0.873 | 0.383 |
| 80 or above | −1.245 | 0.451 | −2.758 | 0.006 |
| Patient sex | ||||
| Female | Reference | |||
| Male | −0.427 | 0.092 | −4.632 | <0.001 |
| Race/ethnicity | ||||
| White, non-Hispanic | Reference | |||
| Black, non-Hispanic | −0.385 | 0.126 | −3.051 | 0.002 |
| Hispanic | −0.301 | 0.121 | −2.482 | 0.013 |
| Asian, non-Hispanic | −0.332 | 0.143 | −2.315 | 0.021 |
| Other, non-Hispanic | −0.273 | 0.398 | −0.686 | 0.493 |
| History of diabetic retinopathy | −0.303 | 0.210 | −1.447 | 0.148 |
| History of stroke | −0.209 | 0.110 | −1.903 | 0.057 |
| Interaction of follow-up time and medication adherence | 0.061 | 0.022 | 2.739 | 0.006 |
| Interaction of follow-up time and moderately severe disease at baseline | −0.140 | 0.030 | −4.613 | <0.001 |
| Interaction of follow-up time and severe disease at baseline | −0.315 | 0.036 | −8.847 | <0.001 |
| Interaction of follow-up time and very severe disease at baseline | −0.264 | 0.044 | −5.972 | <0.001 |
| Interaction of follow-up time and missing baseline disease severity | −0.054 | 0.030 | −1.789 | 0.074 |
Modeling was performed using a conditional growth model and the Wald test was used to test the regression coefficients.
Glaucoma severity is based on mean deviation in visual field closest to and within ±6 months of initiation of medication. Glaucoma severity is a clustering effect, not a covariate.
dB indicates decibel.
FIGURE 3.
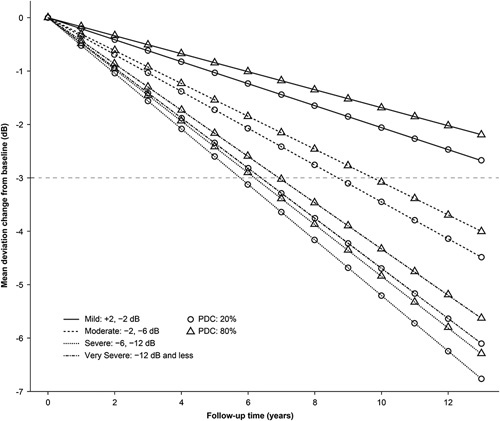
Model-based predicted growth curve for adherence levels of 20% and 80% stratified by baseline severity level. The y-axis represents the change in mean deviation (dB) from baseline and the x-axis represents years of follow-up. PDC indicates proportion of days covered.
DISCUSSION
To the best of our knowledge, this study is the first to quantify the effect of medication adherence on visual field progression over time in a real-world, medically treated glaucoma population. Previous studies that assessed the relationship between medication adherence and visual field progression were relatively small and of short duration, used data from a limited number of time points, and did not quantify progression rate over time.18,29,31 On the basis of electronic measures of adherence over a 3-month period, 1 study (n=102) found that glaucoma patients with <80% adherence were significantly more likely to have moderate or severe visual field defects at study end than those with ≥80% adherence.18 A small retrospective study (n=35) reported that stable visual field was strongly associated with medication adherence.29 A prospective Greek study conducted on an unselected cohort of patients with OAG or exfoliation glaucoma (n=100) reported significantly worse visual field loss among self-reported noncompliant patients (>2 doses of eyedrops missed per week) compared with compliant patients.31 A post hoc analysis of data (n=306) from the CIGTS demonstrated that even in a randomized controlled trial setting, worse self-reported medication adherence was significantly associated with greater glaucomatous visual field loss.32 However, our large study is the first to fully assess and quantify the impact of verified pharmacy refill nonadherence on visual field progression using repeated measures over time. The study findings complement those of the CIGTS analysis in demonstrating that the association between suboptimal medication adherence and glaucomatous progression also applies in clinical practice.
Categorization of patients by baseline glaucoma severity indicated that those with the most severe disease had the highest average level of adherence over the period of follow-up. There are several possible explanations for this finding. Patients presenting with more severe glaucoma have greater incentive to adhere to their medication as they are more likely to have experienced visual symptoms, and further disease progression will have noticeable detrimental impact on their visual function. In addition, previous studies have shown that patients presenting with severe glaucoma progress more rapidly.40,41 Severely affected patients are also likely to visit their physician more frequently, and this provides additional opportunity for patient education and discussion about adherence and options for future surgical interventions,42 which would be expected to encourage adherent behavior.
Since medication adherence is not only affected by disease severity but also likely to vary over time, we adopted a conditional growth model to assess the effect of the medication adherence-by-time interaction on visual field progression. This model relied on several strong assumptions. First, we assumed visual field progression to be linear as we observed linear patterns in the longitudinal data using a locally weighted scatterplot smoother. Second, we assumed that the interaction between medication adherence and time was constant across the different baseline severity groups, allowing for a single estimate of adherence on progression.
To illustrate the clinical relevance of these findings, we generated model-predicted growth curves assuming constant adherence levels. The curves illustrate distinct patterns of visual field progression at different adherence levels, with greater adherence being significantly associated with more stable mean deviation trajectories over time. Patients with higher medication adherence had a slower rate of visual field loss over time compared with patients with lower adherence. This preservation of vision would be anticipated to confer a benefit on patients’ quality of life, since it has been shown in previous studies (Los Angeles Latino Eye Study) that even marginal worsening of visual field impacts vision and quality of life.43 Higher adherence to medical therapy may also delay or reduce the need for more advanced treatments such as surgery.
In clinical practice, physicians often note that there are some patients with glaucoma that progresses more rapidly than in others. Although we did not analyze fast-progressers and slow-progressers separately, we would expect that differential effects would average out in a large study such as this, and that our findings are relevant to the entire population of treated patients with glaucoma. Even in an integrated health care environment such as KPSC, there are many factors that can lead to poor adherence, such as tolerability issues, lack of transportation to clinics and pharmacies, and poor patient education.20 Findings demonstrating a meaningful clinical impact at the population level can be helpful to clinicians in educating individual patients on the importance of treatment adherence in preserving vision. In addition, efforts to simplify treatment methods, such as the use of medications with longer durations of action that do not rely on daily patient compliance, should be explored to help improve medication adherence. In this regard, further research is needed to determine whether medication classes are associated with different adherence profiles and to identify facilitators for and barriers to long-term medication adherence.
Strengths of this study include its large patient population, extended duration of follow-up, and the ability to verify medication possession within a closed health care system. The changes in visual field caused by glaucoma can be slow and it is difficult for clinicians to distinguish noise from real change. Although in general treated glaucoma tends to progress slowly, KPSC members tend to be long-term members of the health plan, so the availability of long-term data allowed us to demonstrate an effect of delayed progression over time. In addition, this patient population was treated according to real-world paradigms by the KPSC health care team, allowing for extrapolation to other real-world clinical environments.
On account of its retrospective observational nature, this study is subject to several limitations. Although the analysis controlled for glaucoma severity at baseline, additional risk factors for glaucomatous visual field progression such as glaucoma type, baseline IOP,11 escalation of treatment,44,45 and separation of “fast-progressers” versus “slow-progressers” were not included as covariates. Patients who had multiple medication classes were considered covered if at least 1 medication class was supplied. This assumption leads to a conservative estimate of patient adherence. In our closed pharmacy system, where mostly generic medications are used and the plan covers a high proportion of the cost, allowing low patient copayments, most patients do not have a financial barrier to keeping their prescriptions filled as needed. Estimation of medication adherence was based on pharmacy refill using the PDC, a commonly used measure in other studies of adherence. Although there are various ways to measure adherence (self-report, physician report, direct observation, electronic medication monitors), pharmacy refill was selected because it is the measure most readily available for our large population and long duration of follow-up. However, pharmacy refill data cannot address whether the medication was dosed at the appropriate timings or instilled into the eye properly.
Our study used mean deviation as the main outcome measure. Mean deviation is a commonly accepted measure for performing retrospective analyses of visual field data, but is a weighted measure of the extent of damage across the entire visual field. Abnormalities in the central field are weighted higher than peripheral points.
In conclusion, using a conditional growth model, this study estimated the impact of medication nonadherence on visual field progression over time in OAG. It demonstrated that treated patients with glaucoma who were more adherent showed less deterioration in visual function over time and presumably had less need for additional interventions. On the basis of the variations in trajectory of disease progression, this study supports the clinical imperative of improving patient adherence to glaucoma medication. With higher adherence, it is more likely that glaucoma progression will be delayed, and that the disease will have a less detrimental impact on the patient’s quality of life.
ACKNOWLEDGMENTS
The authors thank Claire Mesirov of the Department of Regional Patient Care Services at the Kaiser Foundation Health Plan for her efforts in collecting visual field data for the study. Medical writing and editorial support was provided to the authors by Andrew Fitton, PhD, of Evidence Scientific Solutions (Horsham, UK) and was funded by Allergan, an AbbVie company, Irvine, CA, USA. All authors met the ICMJE authorship criteria. No honoraria or payments were made for authorship.
Footnotes
Research reported in this publication was supported by a grant from Allergan, an AbbVie company, Irvine, CA.
Disclosure: Y.-H.S., J.W., T.L., and D.S.F. have received grant funding from Allergan for the conduct of this study. T.L. and D.S.F. have received grant funding from Regeneron and Santen Inc., outside the conduct of this study. C.M. has received consultancy fees from Allergan. J.C., V.S., and C.Z. are employees of AbbVie Inc. The remaining authors declare no conflict of interest.
Contributor Information
Yu-Hsiang Shu, Email: Yu-Hsiang.Shu@kp.org.
Jun Wu, Email: Jun.X.Wu@kp.org.
Tiffany Luong, Email: Tiffany.Q.Luong@kp.org.
Cynthia Mattox, Email: cindiem@gmail.com.
Ervin N. Fang, Email: Ervin.N.Fang@kp.org.
Brian L. Lee, Email: Brian.L.Lee@kp.org.
Jason P. Jones, Email: jason.jones@healthcatalyst.com;Anne.Brown@envisionpharma.com.
Joanna Campbell, Email: joanna.campbell@abbvie.com.
Vanessa Shih, Email: vanessa.shih@abbvie.com.
Changgeng Zhao, Email: Changgeng.Zhao@allergan.com.
Donald S. Fong, Email: donald.s.fong@kp.org.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon YH, Fingert JH, Kuehn MH, et al. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon MO. The ocular hypertension treatment study. Arch Ophthalmol. 2002;120:714–720. [DOI] [PubMed] [Google Scholar]
- 5.Le A, Mukesh BN, McCarty CA, et al. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–3789. [DOI] [PubMed] [Google Scholar]
- 6.Nemesure B, Honkanen R, Hennis A, et al. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114:1810–1815. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 10.De Moraes CGV. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2010;129:562–568. [DOI] [PubMed] [Google Scholar]
- 11.Ernest PJ, Schouten JS, Beckers HJ, et al. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120:512–519. [DOI] [PubMed] [Google Scholar]
- 12.Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113:431–436. [DOI] [PubMed] [Google Scholar]
- 13.Olthoff C, Schouten J, Vandeborne B, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology. 2005;112:953–961.e957. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18:401–409. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53:S57–S68. [DOI] [PubMed] [Google Scholar]
- 16.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman-Casey PA, Blachley T, Lee PP, et al. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118:2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stryker JE, Beck AD, Primo SA, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010;19:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29:1515–1522. [DOI] [PubMed] [Google Scholar]
- 22.Stewart WC, Kolker AE, Sharpe ED, et al. Long-term progression at individual mean intraocular pressure levels in primary open-angle and exfoliative glaucoma. Eur J Ophthalmol. 2008;18:765–770. [DOI] [PubMed] [Google Scholar]
- 23.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635. [DOI] [PubMed] [Google Scholar]
- 24.Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study: the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooner KS, AlBdoor M, Cho BJ, et al. Risk factors for progression to blindness in high tension primary open angle glaucoma: comparison of blind and nonblind subjects. Clin Ophthalmol (Auckland, NZ). 2008;2:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pleet A, Sulewski M, Salowe RJ, et al. Risk factors associated with progression to blindness from primary open-angle glaucoma in an African-American population. Ophthalmic Epidemiol. 2016;23:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart WC, Chorak RP, Hunt HH, et al. Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol. 1993;116:176–181. [DOI] [PubMed] [Google Scholar]
- 28.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110:726–733. [DOI] [PubMed] [Google Scholar]
- 29.Rossi GCM, Pasinetti GM, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21:410–414. [DOI] [PubMed] [Google Scholar]
- 30.Paula JS, Furtado JM, Santos AS, et al. Risk factors for blindness in patients with open-angle glaucoma followed-up for at least 15 years. Arq Bras Oftalmol. 2012;75:243–246. [DOI] [PubMed] [Google Scholar]
- 31.Konstas AGP, Maskaleris G, Gratsonidis S, et al. Compliance and viewpoint of glaucoma patients in Greece. Eye. 2000;14:752–756. [DOI] [PubMed] [Google Scholar]
- 32.Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kass MA, Meltzer DW, Gordon M, et al. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101:515–523. [DOI] [PubMed] [Google Scholar]
- 34.Wilensky J, Fiscella RG, Carlson AM, et al. Management of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. Am J Ophthalmol. 2006;141:28–33. [DOI] [PubMed] [Google Scholar]
- 35.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser Permanente. HealthConnect enables care improvement and transformation. 2013. Available at: https://www.webwire.com/ViewPressRel.asp?ald=171227. Accessed October 3, 2021.
- 37.Musch DC, Gillespie BW, Niziol LM, et al. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. [DOI] [PubMed] [Google Scholar]
- 39.Ung C, Zhang E, Alfaro T, et al. Glaucoma severity and medication adherence in a county hospital population. Ophthalmology. 2013;120:1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan TCW, Bala C, Siu A, et al. Risk factors for rapid glaucoma disease progression. Am J Ophthalmol. 2017;180:151–157. [DOI] [PubMed] [Google Scholar]
- 41.Lee JM, Caprioli J, Nouri-Mahdavi K, et al. Baseline prognostic factors predict rapid visual field deterioration in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2228–2236. [DOI] [PubMed] [Google Scholar]
- 42.Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123:P41–P111. [DOI] [PubMed] [Google Scholar]
- 43.Hoki SL, Varma R, Lai MY, et al. Prevalence and associations of asymptomatic retinal emboli in Latinos: the Los Angeles Latino Eye Study (LALES). Am J Ophthalmol. 2008;145:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folgar FA, de Moraes CGV, Prata TS, et al. Glaucoma surgery decreases the rates of localized and global visual field progression. Am J Ophthalmol. 2010;149:258–264.e252. [DOI] [PubMed] [Google Scholar]
- 45.Bertrand V, Fieuws S, Stalmans I, et al. Rates of visual field loss before and after trabeculectomy. Acta Ophthalmol. 2013;92:116–120. [DOI] [PubMed] [Google Scholar]


