SUMMARY
Klebsiella oxytoca is actually a complex of nine species—Klebsiella grimontii, Klebsiella huaxiensis, Klebsiella michiganensis, K. oxytoca, Klebsiella pasteurii, Klebsiella spallanzanii, and three unnamed novel species. Phenotypic tests can assign isolates to the complex, but precise species identification requires genome-based analysis. The K. oxytoca complex is a human commensal but also an opportunistic pathogen causing various infections, such as antibiotic-associated hemorrhagic colitis (AAHC), urinary tract infection, and bacteremia, and has caused outbreaks. Production of the cytotoxins tilivalline and tilimycin lead to AAHC, while many virulence factors seen in Klebsiella pneumoniae, such as capsular polysaccharides and fimbriae, have been found in the complex; however, their association with pathogenicity remains unclear. Among the 5,724 K. oxytoca clinical isolates in the SENTRY surveillance system, the rates of nonsusceptibility to carbapenems, ceftriaxone, ciprofloxacin, colistin, and tigecycline were 1.8%, 12.5%, 7.1%, 0.8%, and 0.1%, respectively. Resistance to carbapenems is increasing alarmingly. In addition to the intrinsic blaOXY, many genes encoding β-lactamases with varying spectra of hydrolysis, including extended-spectrum β-lactamases, such as a few CTX-M variants and several TEM and SHV variants, have been found. blaKPC-2 is the most common carbapenemase gene found in the complex and is mainly seen on IncN or IncF plasmids. Due to the ability to acquire antimicrobial resistance and the carriage of multiple virulence genes, the K. oxytoca complex has the potential to become a major threat to human health.
KEYWORDS: β-lactamases, carbapenemases, resistance, Klebsiella oxytoca, virulence, taxonomy, Klebsiella, antimicrobial resistance
INTRODUCTION
Klebsiella oxytoca is a Gram-negative bacterium of the genus Klebsiella within the family Enterobacteriaceae and is widely distributed in nature (1–3). In humans, K. oxytoca is a member of the normal gut microflora and has been detected in the stool of 8% to 10% of healthy adults by culture-based methods (4). It is also found on the skin and in the oropharynx (5). In addition to being a member of the commensal microflora, K. oxytoca is an important human pathogen causing a large variety of infections ranging from mild diarrhea to life-threatening bacteremia and meningitis (5–7) and also causing outbreaks of health care-associated infections. Despite its importance, K. oxytoca is relatively under the radar and is largely masked by its notorious relative, Klebsiella pneumoniae (8). However, K. oxytoca is quite different from K. pneumoniae in many respects, such as antimicrobial resistance, virulence, and disease spectrum. Recently, new findings have significantly advanced our knowledge of this important pathogen. For example, genome-based taxonomic studies have shown that K. oxytoca is not a single species but in fact a complex comprising at least six species, i.e., Klebsiella grimontii, Klebsiella huaxiensis, Klebsiella michiganensis, K. oxytoca, Klebsiella pasteurii, and Klebsiella spallanzanii. In this review, we provide updates on the taxonomy, antimicrobial resistance, and virulence of the K. oxytoca complex and also summarize studies on its epidemiology and infections.
TAXONOMY
In 1886, an organism called “Bacillus oxytocus perniciosus” was recovered from old milk by Flugge and then renamed “Aerobacter oxytocum” by Bergey in 1923 and Klebsiella oxytoca by Lautrop in 1956 (9). K. oxytoca is indole positive and was considered a subgroup of K. pneumoniae for many years, but the clear distinction between the two species was finally revealed by DNA relatedness studies (9, 10). Through genome sequencing technologies and bioinformatics, K. oxytoca has been found to be a heterogeneous complex comprising multiple species (9). Sequence variations of the chromosomally encoded β-lactamase gene blaOXY can assign the K. oxytoca complex into phylogroups (9). Currently, nine phylogroups, Ko1 to Ko9, are assigned to reflect the blaOXY variant (blaOXY-1 to blaOXY-9) that they carry (Table 1). However, Ko5 is now known to be a sub-phylogroup of Ko1 (11), and Ko9 is a sub-phylogroup of Ko3 (12), while the taxonomic status of Ko7 needs to be determined, as no genome sequence of the strain carrying blaOXY-7 is available for analysis (13). K. oxytoca sensu stricto belongs to Ko2, as it carries blaOXY-2, and the type strain is ATCC 13182 (= NCTC13727 = CIP103434). The taxonomic determination of the Ko1, Ko3, Ko4, Ko6, and Ko8 phylogroups is summarized below according to the timeline of their species designations.
TABLE 1.
Species of the K. oxytoca complex
| Speciesa | Phylogroup | OXY variant(s) | Type or reference strain | Genome accession no. | Reference |
|---|---|---|---|---|---|
| K. michiganensis | Ko1 | OXY-1, OXY-5 | W14T | GCA_901556995 | 14 |
| K. oxytoca | Ko2 | OXY-2 | ATCC 13182T | GCA_900977765 | 9 |
| K. spallanzanii | Ko3 | OXY-3, OXY-9 | SPARK_775_C1T | ERS3550824 | 12 |
| K. pasteurii | Ko4 | OXY-4 | SPARK_836_C1T | ERS3550825 | 12 |
| K. grimontii | Ko6 | OXY-6 | 06D021T | GCA_900200035 | 17 |
| K. huaxiensis | Ko8 | OXY-8 | WCHKl090001T | GCA_003261575 | 20 |
| Taxon 1 | OXY-10 | 67 | QJJG00000000 | ||
| Taxon 2 | OXY-11 | P620 | CP046115 | ||
| Taxon 3 | OXY-12 | RHBSTW-00484 | CP055481 |
Taxa 1, 2, and 3 were identified here.
K. michiganensis represents the phylogroup Ko1, which also comprises Ko5 (11). In 2012, strain W14T of the phylogroup Ko1 was recovered from a toothbrush holder and shared the consistent biochemical profile of the genus Klebsiella (14). Analysis based on housekeeping rpoB, gyrB, and gyrA gene sequences showed its close relatedness with K. oxytoca. However, the strain was negative in the pectate degradation test and negative by PCR for the polygalacturonase gene pehX (involved in pectin degradation), which has been used to differentiate K. oxytoca from other Klebsiella species (15, 16). The DNA-DNA hybridization (DDH) value between W14T and the K. oxytoca type strain was 55.7% ± 6.2% (14), below the ≥70% cutoff for defining a bacterial species. Isolate W14T was therefore thought deserving of the status of new species and was named K. michiganensis to reflect the state of Michigan in the United States, where the type strain was isolated (14). The type strain is W14 (also designated ATCC BAA-2403 and DSM 25444) (14).
K. grimontii represents the phylogroup Ko6. Six Ko6 strains were found, forming a well-defined sequence cluster based on rpoB and gyrA sequencing and separate from K. michiganensis and K. oxytoca (17, 18). The average nucleotide identity (ANI) value of Ko6 was 91.2% with K. oxytoca and 93.47% with K. michiganensis, both of which were well below the ≥95% to 96% ANI cutoff for bacterial species distinction (19). The name Klebsiella grimontii, referring to Patrick A. D. Grimont (a French microbiologist), was proposed for the phylogroup Ko6 (19). The type strain is 06D021 (also designated CIP111401 and DSM 105630) (19).
K. huaxiensis represents the phylogroup Ko8. Strain WCHKl090001T was isolated from human urine in China in 2017 (20). WCHKl090001T had up to 87.18% ANI and an in silico DNA-DNA hybridization (isDDH) value of up to 35.2% with type strains of other Klebsiella species (20). Strain WCHKl090001T therefore belongs to a novel species of the genus Klebsiella, named K. huaxiensis (Ko8) to refer to West China (Huaxi in Chinese) Hospital, where the strain was isolated (20). The type strain is WCHKl090001 (also designated GDMCC 1.1379 and CNCTC 7650) (20).
K. spallanzanii represents the phylogroup Ko3. Strain SPARK_775_C1T, a representative Ko3 strain, had the highest ANI value, 90.7%, with K. huaxiensis WCHKl090001T compared with other members of the genus Klebsiella. The name K. spallanzanii, referring to Lazzaro Spallanzani (an Italian biologist), was proposed for the phylogroup Ko3 (12). The type strain is SPARK_775_C1 (also designated CIP 111695 and DSM 109531) (12).
K. pasteurii represents the phylogroup Ko4. Strain SPARK_836_C1T, a representative Ko4 strain, had the highest ANI value, 95.5%, with K. grimontii 06D021T, which falls into the 95% to 96% inconclusive zone of defining a bacterial species (21, 22). Nonetheless, the name K. pasteurii, commemorating Louis Pasteur, the well-known French microbiologist, was proposed for the Ko4 phylogroup. The type strain is SPARK_836_C1 (also designated CIP 111696 and DSM 109530) (12). We performed an analysis and found that the isDDH between K. pasteurii SPARK_836_C1T and K. grimontii 06D021T was 67.8%, below the 70% cutoff (23). The species status of K. pasteurii is therefore confirmed.
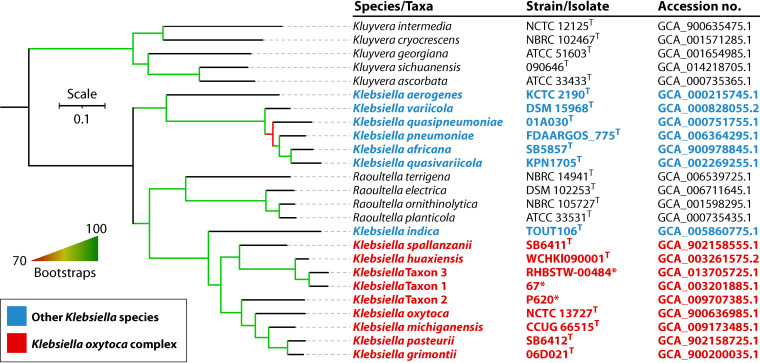
In addition to the blaOXY variants blaOXY-1 to blaOXY-9 reported in the literature (12), blaOXY-10, blaOXY-11, and blaOXY-12 have been assigned in the β-lactamase database curated by the Institute Pasteur (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef&page=alleleQuery&locus=blaOXY). These three blaOXY genes reflect three new phylogroups and may represent three novel species of the K. oxytoca complex. The corresponding genome sequences of strains harboring blaOXY-10, blaOXY-11, and blaOXY-12 were examined for precise species identification as described previously (23, 24). The strains harboring blaOXY-10, blaOXY-11, and blaOXY-12 indeed represent three novel species, which are designated taxa 1 to 3 here (Table 1 and Fig. 1), as the assignation of proper species names needs detailed phenotype characterization (25). Taxa 1 and 3 are most closely related to K. huaxiensis, with a 95.62 or 95.18% ANI and a 62.4% or 60.6% isDDH value, while taxon 2 is most closely related to K. grimontii, with a 90.42% ANI and a 40.8% isDDH value. The three novel species are therefore members of the K. oxytoca complex, which extends the complex to nine species (Table 1 and Fig. 1). Of note, blaOXY has not been found in species other than those of the K. oxytoca complex at present; in the chromosomal location corresponding to blaOXY in K. oxytoca, there is a gene encoding a myoinosose 2 dehydratase in K. pneumoniae, and the genetic context of blaOXY in the K. oxytoca complex has no similarities with that of blaSHV, which is intrinsic to K. pneumoniae.
FIG 1.
Phylogenomic tree based on the concatenated nucleotide sequence of core genes of Klebsiella species. Strains and their nucleotide accession numbers are listed alongside the species names. Species belonging to the K. oxytoca complex are in red, while other Klebsiella species are in blue. Raoultella species that were separated from Klebsiella 20 years ago (399) are included, while the genus Kluyvera, which is closely related to Klebsiella and Raoultella (400), is used as an outgroup. The tree was inferred by the core genome identification using PIRATE v1.0.4 (401) and subsequent phylogenetic inference using IQ-TREE v2.1.4 (402) using the GTR+G+ASC model with 10,000 rapid bootstraps. Branches with support over 70% are indicated by gradients. The bar shows nucleotide substitutions per site.
Recently, a novel species named Klebsiella indica was reported and is most closely related to species of the K. oxytoca complex (26). In the phylogenomic tree, K. indica is clustered with species of the K. oxytoca complex and is phylogenetically separated from other Klebsiella species and Raoultella species (Fig. 1). However, we found that K. indica contains no blaOXY gene, and instead, there is a 1,224-bp gene encoding a putative transporter of the major facilitator superfamily (MFS) in the location of blaOXY. In the phylogenomic tree, K. indica is also placed outside species within the K. oxytoca complex. The above findings suggest that K. indica should not be considered a member of the K. oxytoca complex at present.
SPECIES IDENTIFICATION
Phenotypic Tests
Strains of the K. oxytoca complex are non-spore forming and nonmotile and form smooth, circular, dome-shaped, glistening colonies on agar plates (12). The classical phenotypic tests for identification of K. oxytoca include indole, lactose, mannitol, malonate, lysine decarboxylase, ornithine decarboxylase, Voges-Proskauer, and O-nitrophenyl-β-d-galactopyranoside (ONPG) tests and the test for reduction of nitrate to nitrite (12). Strains of all six named species of the K. oxytoca complex are positive for indole, lactose, lysine decarboxylase, mannitol, ONPG, and reduction of nitrate to nitrite but are negative for ornithine decarboxylase (9, 12). The positive indole test could differentiate species of the K. oxytoca complex from K. pneumoniae, while the positive ONPG test or the negative ornithine decarboxylase test could differentiate the complex from Raoultella ornithinolytica (9, 27–29). A combination of Simmons’ citrate agar supplemented with bile salts, inositol, and tryptophan (SCITB agar) and a spot indole test for screening the K. oxytoca complex has shown a superior sensitivity (93.8% versus 63.3%) and specificity (99.9% versus 60.4%) and contributed to a reduction in workload and cost compared to the use of MacConkey agar for isolation (30). Conventional phenotypic identification kits such as API 20E and API 50CH and automated phenotypic identification systems such as Vitek II and VITK-JR30 systems are widely used in clinical and scientific laboratories, but they exhibit limited performance for differentiating members of the K. oxytoca complex at the species level (17, 31–35).
Based on currently available literature (9, 12), each of the six species of the K. oxytoca complex has unique phenotypic features (Table 2), which could help to design phenotypic tests to differentiate these closely related species. For instance, the combination of the Voges-Proskauer, urease, and α-ketoglutaric acid tests could correctly differentiate the six species based on results reported in the literature (Table 2). However, these phenotypic characterizations have been performed for only a very limited number of strains or even on just a single strain (9, 12), and therefore, these discriminatory features are prone to be changed as additional strains of each species are tested. More studies are warranted to investigate the phenotypic differences among species within the K. oxytoca complex. At present, it appears that phenotypic tests are more appropriate for screening purposes and preliminary identification to the K. oxytoca complex level rather than the individual species level.
TABLE 2.
Phenotypic characteristics of species of the K. oxytoca complexa
| Phenotypic characteristic test | Result for phylogroup (species) |
|||||
|---|---|---|---|---|---|---|
| Ko1 (K. michiganensis) | Ko2 (K. oxytoca) | Ko3 (K. spallanzanii) | Ko4 (K. pasteurii) | Ko6 (K. grimontii) | Ko8 (K. huaxiensis) | |
| n = 1 | NA | n = 3 | n = 13 | n = 6 | n = 1 | |
| Motility | − | − | − | − | − | − |
| Indole | + | + | + | + | + | + |
| Lysine decarboxylase | + | + | + | + | + | + |
| Lactose | + | + | + | + | + | + |
| Mannitol | + | + | + | + | + | + |
| ONPG | + | + | + | + | + | + |
| Reduction of nitrate to nitrite | + | + | + | + | + | + |
| Voges-Proskauer | + | + | − | + | + | − |
| Malonate | + | + | + | + | + | − |
| Urease | − | + | + | − | − | − |
| Ornithine decarboxylase | − | − | − | − | − | − |
| n = 7 | n = 5 | n = 4 | n = 5 | n = 6 | n = 3 | |
| l-Proline | + | + | − | + | + | − |
| d,l-α-Glycerol-phosphate | + | + | v | + | v | − |
| α-Ketoglutaric acid | − | − | − | − | + | − |
| Glyoxylic acid | − | − | − | v | − | − |
| Melezitose | + | + | + | + | − | v |
| Tricarballylic acid | + | + | − | + | + | − |
| Acetyl-β-d-mannosamine | v | + | v | + | + | + |
| 3-O-Methyl-glucose | − | − | − | − | − | + |
| γ-Amino-butyric acid | + | + | − | v | v | − |
| l-Tartaric acid | v | v | v | + | + | − |
| Reference(s) | 12, 14 | 9, 12 | 12 | 12 | 12, 17 | 12, 20 |
ONPG, O-nitrophenyl-β-d-galactopyranoside; +, positive; −, negative; v, between 20 and 80% positive strains; NA, not available.
MALDI-TOF MS
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been increasingly used in clinical microbiology laboratories for identifying microorganisms to the species level (36). It has been attempted for identification of the K. oxytoca complex, but misidentification occurs (12). For instance, some Raoultella strains have been incorrectly assigned to K. oxytoca by MALDI-TOF MS, although this accuracy of identification could be improved by applying a more stringent 10% differential score (37). It is more difficult to differentiate each species within the K. oxytoca complex, as most of them were identified very recently, and therefore, most laboratories may not have reference spectra of these new species in their databases (14, 38–40). MALDI-TOF MS patterns of all six species within the K. oxytoca complex were analyzed in a study (12) which also updated the data set to improve species identification by MALDI-TOF MS. With the updated data set, the specificity and sensitivity of correct identification for the six named species within the K. oxytoca complex by MALDI-TOF MS range from 60 to 100% and from 80 to 100%, respectively (12).
16S rRNA Gene Sequence Analysis
16S rRNA gene sequence analysis has been widely used for bacterial species identification. Typically, the nearly complete 16S rRNA gene sequences of bacterial strains are amplified using PCR with the universal primers 27F and 1492R (41), and the generated amplicons are then subjected to Sanger sequencing (42). Comparison of the 16S rRNA gene sequences can be performed using curated data sets such as EzBioCloud (43). In 1994, a <97% similarity was proposed as the cutoff to delineate species (44), and in 2008, it was proposed to update the cutoff to 99% (45). However, as shown in Table 3, type strains of species within the K. oxytoca complex have up to 99.9% similarity of the 16S rRNA gene sequences. This suggests that the current scheme of 16S rRNA gene sequence analysis does not have adequate resolution for correct species identifications in the K. oxytoca complex as previously demonstrated (12, 14, 17, 20).
TABLE 3.
16S rRNA gene sequence identity, ANI, and isDDH values between type strains of each species belonging to the K. oxytoca complex
| Organism | Identity, ANI, and isDDH (%) for: |
|||||||
|---|---|---|---|---|---|---|---|---|
| K. grimontii 06D021T | K. huaxiensis WCHKl090001T | K. michiganensis CCUG 66515T | K. oxytoca NCTC3727T | K. pasteurii SB6412T | K. spallanzanii SB6411T | Taxon 1 | Taxon 2 | |
| K. grimontii 06D021T | ||||||||
| K. huaxiensis WCHKl090001T | 99.8, 88.0, 35.2 | |||||||
| K. michiganensis CCUG 66515T | 99.7, 93.6, 53.8 | 99.5, 88.0, 35.5 | ||||||
| K. oxytoca NCTC3727T | 97.9, 91.5, 44.7 | 98.1, 87.6, 34.0 | 97.9, 92.3, 48.2 | |||||
| K. pasteurii SB6412T | 99.3, 96.0, 67.8 | 99.5, 87.7, 34.4 | 99.4, 93.7, 54.3 | 98.1, 91.2, 43.9 | ||||
| K. spallanzanii SB6411T | 98.4, 89.0, 37.7 | 98.6, 91.2, 44.2 | 98.5, 89.1, 37.9 | 98.5, 88.6, 36.3 | 98.6, 88.7, 36.9 | |||
| Taxon 1 | 98.5, 87.7, 34.2 | 98.8, 95.1, 62.4 | 98.6, 87.6, 34.3 | 99.4, 87.1, 32.8 | 98.6, 87.4, 33.6 | 98.9, 89.7, 39.4 | ||
| Taxon 2 | 98.4, 90.2, 40.8 | 98.2, 87.5, 34.0 | 98.5, 90.1, 40.9 | 97.6, 89.6, 39.4 | 98.7, 89.9, 39.8 | 97.9, 88.6, 36.4 | 98.1, 87.0, 32.7 | |
| Taxon 3 | 98.5, 88.0, 35.1 | 98.3, 94.8, 60.6 | 98.5, 88.0, 35.2 | 98.5, 87.2, 33.5 | 98.4, 87.5, 34.1 | 97.9, 89.9, 40.1 | 98.7, 95.4, 64.0 | 97.9, 87.1, 33.3 |
Single Gene Markers
All species of the K. oxytoca complex carry blaOXY, a β-lactamase-encoding gene intrinsic to the complex, which has not been reported in other species in the literature. In the β-lactamase database curated by the Institute Pasteur (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef&page=alleleQuery&locus=blaOXY; accessed 1 August 2021), there are blaOXY genes encoding 86 distinct OXY enzymes, including 38 that have not been reported in the literature. The pairwise amino acid identity between 12 OXY groups ranges from 82.82% (between OXY-9-1 and OXY-11-1) to 98.97% (between OXY-1-1 and OXY-6-1) (Table 4). Each of the blaOXY variants matches a species within the complex (Table 1), and therefore, amplification and sequencing of blaOXY genes may be used for species identification within the K. oxytoca complex.
TABLE 4.
Pairwise amino acid sequence identity between OXY groups
| Group | % identity with: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OXY-1-1 | OXY-2-1 | OXY-3-1 | OXY-4-1 | OXY-5-1 | OXY-6-1 | OXY-7-1 | OXY-8-1 | OXY-9-1 | OXY-10-1 | OXY-11-1 | |
| OXY-2-1 | 88.66 | ||||||||||
| OXY-3-1 | 89.35 | 87.59 | |||||||||
| OXY-4-1 | 96.56 | 88.28 | 89.66 | ||||||||
| OXY-5-1 | 97.94 | 90.00 | 89.66 | 97.24 | |||||||
| OXY-6-1 | 98.97 | 88.66 | 89.69 | 97.60 | 96.91 | ||||||
| OXY-7-1 | 97.25 | 88.28 | 90.69 | 97.24 | 96.21 | 98.28 | |||||
| OXY-8-1 | 88.32 | 86.90 | 93.10 | 87.93 | 88.62 | 88.32 | 88.97 | ||||
| OXY-9-1 | 86.94 | 84.83 | 94.10 | 87.24 | 87.24 | 87.29 | 87.93 | 92.41 | |||
| OXY-10-1 | 89.00 | 85.86 | 93.10 | 87.59 | 88.62 | 88.66 | 88.62 | 94.48 | 93.45 | ||
| OXY-11-1 | 86.25 | 86.60 | 86.25 | 86.25 | 86.25 | 86.25 | 86.94 | 85.57 | 82.82 | 84.88 | |
| OXY-12-1 | 90.03 | 87.93 | 94.14 | 89.31 | 90.35 | 89.69 | 90.35 | 97.59 | 93.10 | 96.55 | 85.57 |
The polygalacturonase-encoding gene pehX was found to be unique to K. oxytoca (15, 16). PCR for pehX alone (15, 16, 46–49) or in combination with other housekeeping genes, such as infB (50), has been widely used to differentiate K. oxytoca from K. pneumoniae and Raoultella spp. However, it has been reported that K. michiganensis is negative by PCR for pehX (14). In addition, we found by BLAST analysis that pehX was truncated between nucleotides 1,977 and 1,983 in a number of K. oxytoca complex genomes, such as K. michiganensis strains A10 (342 bp left; accession no. PIDR01001036.1) and A11 (531 bp; accession no. PIDS01000708.1), K. oxytoca strain 112_KOXY 226_19650_207590 (288 bp; accession no. WCM01000066.1), and K. pasteurii strain FDAARGOS_511 (247 bp; accession no. CP033824.1). These strains may be missed by the currently reported PCR for pehX.
The efflux pump-encoding genes oqxA and oqxB and the fosfomycin resistance gene fosA are intrinsic to K. pneumoniae (51). oqxA and oqxB are also intrinsic to some species of the K. oxytoca complex (52) but are absent from strains of K. huaxiensis, K. spallanzanii, taxon 1, and taxon 3 as identified by BLAST. fosA is seen in almost all isolates of the K. oxytoca complex as identified by BLAST. We found that the nucleotide identities of fosA between species of the K. oxytoca complex and K. pneumoniae are 76.13 to 85.19%. However, the maximum nucleotide sequence identity of fosA between different Klebsiella species and the minimum nucleotide identity within the same species overlap. For instance, the minimum nucleotide identity within K. huaxiensis is 94.93%, while the maximum nucleotide identity between K. huaxiensis and K. spallanzanii is 99.77%. The presence of oqxA and oqxB in only some species of the complex and the absence of clear, unified cutoffs of the nucleotide sequence identity for fosA suggest that these three genes are not suitable for species identification for the K. oxytoca complex.
Whole-Genome Sequencing and Analysis
Whole-genome sequencing provides a maximal level of resolution for precise bacterial species identification (53). Along with the rapidly increased use of whole-genome sequencing and a deluge of bacterial genomes, genome-based species identification has gained in popularity, at least in the research domain, and is usually used as the gold standard for precise species designations and evaluation of other methods, such as phenotypic tests and MALDI-TOF MS (53–55). ANI and isDDH are the two most commonly used algorithms for genome-based species identification (22, 23, 56). The K. oxytoca complex has been found to comprise multiple species, and each species has been assigned based on ANI and isDDH (12, 14, 19, 20) with the values shown in Table 3. As currently available phenotypic tests and MALDI-TOF MS are unable to correctly identify species in all cases, genome-based species identification using ANI and isDDH is usually required to determine the precise species for strains of the K. oxytoca complex.
EPIDEMIOLOGY, INFECTION, AND ANTIMICROBIAL TREATMENT
As K. oxytoca has only recently been identified as an actual complex comprising multiple species, strains called K. oxytoca in most studies have not been subjected to precise species identification, and the prevalence of each species of the complex in human colonization and infection remains largely unknown. Due to this absence of precise species identification, organisms referred to as K. oxytoca in the literature could actually be any species within the K. oxytoca complex. Therefore, “K. oxytoca” in this section represents the K. oxytoca complex as a whole unless otherwise specified. K. oxytoca colonizes the skin, oral cavity, and intestinal and respiratory tracts of both healthy and sick people (5, 57, 58). K. oxytoca is also an opportunistic pathogen able to cause a variety of infections, in particular antibiotic-associated hemorrhagic colitis (AAHC) after administration of antimicrobial agents and other health care-associated infections in patients with underlying diseases or immunocompromised conditions (49, 59–61). Of note, studies of antimicrobial susceptibility surveillance or virulence assays that contain information about sample types of the isolates but no clinical information are not included in the analysis here. In general, compared to K. pneumoniae, infection due to K. oxytoca is much less common, which may be partially due to its relatively low colonization rate, but is associated with relatively better prognoses, with a mortality rate of 7.14 to 23.58% (62–64) versus the 13.52 to 54.30% seen with K. pneumoniae (65). In addition, AAHC is commonly caused by K. oxytoca but not by K. pneumoniae.
Colonization
K. oxytoca is detected from the feces of 1.6 to 9% of healthy subjects, suggesting a relatively low colonization rate (6, 66), which is lower than the 3.9% to 87.7% colonization rate of K. pneumoniae (67–69). Nonetheless, the colonization rate is much higher in patients, as K. oxytoca was detected in 4.7% of those with inflammatory bowel diseases (IBD) (70), 14% of those with influenza A (71), and 25.5% of infants and neonates in neonatal intensive care units (NICUs) and intermediate care units (72). In addition, the intestinal colonization of K. oxytoca producing extended-spectrum β-lactamases (ESBL) has been found in 2.24% of NICU patients and 3.3% of pregnant women (73, 74), while another study reported that 1.4% of adult ICU patients are colonized with carbapenem-resistant K. oxytoca (75).
Geological Distribution of Infections
According to published clinical reports (Table 5), infections caused by the K. oxytoca complex have been reported mainly in the Asia-Western Pacific region, North America, and Western Europe, with few reports in Africa and South America. All types of infections caused by the K. oxytoca complex have been seen in the Asia-Western Pacific region, Europe, or North America, while AAHC appears to be more common in the Asia-Western Pacific region, in particular Iran and Japan (49, 76–79). In contrast, there are no reports of AAHC due to the complex in Africa and South America. However, as reports of infections due to the K. oxytoca complex are still limited in the literature, the exact geographical distribution of K. oxytoca infections, specifically regarding infection types, incidences, and prognoses, is yet to be elucidated.
TABLE 5.
Infections due to the K. oxytoca complexa
| Yr | Country | Underlying diseases | Ward or department | Age group | Infection type | No. of cases | Reference |
|---|---|---|---|---|---|---|---|
| 2011–2016 | Iran | — | ICU, infectious and internal medicine | 1–87 yr | AAHC | 50 | 49 |
| 2011–2013 | Iran | — | — | Children, adults | AAHC | 40 | 76 |
| — | Austria | — | — | — | AAHC | 13 | 94 |
| 2009–2011 | China | Chronic cardiopulmonary conditions, malignancy, diabetes | Medical, surgical | 1–100 yr | AAHC | 12 | 30 |
| 2001–2006 | Turkey | Sinusitis, tonsillitis | — | Adults | AAHC | 11 | 93 |
| 2007–2009 | Nigeria | Pregnancy | Antenatal clinics | Adults | UTI | 45 | 100 |
| 2011 | Japan | Neurogenic bladder, benign prostatic hyperplasia | Urology | Adults | UTI | 42 | 99 |
| 2009–2010 | Cameroon | — | Outpatients | 2–80 yr | UTI | 35 | 134 |
| 2012–2014 | India | Pregnant | — | Adults | UTI | 35 | 135 |
| 2016–2017 | Spain | — | — | Children, adults | UTI | 28 | 96 |
| 2001–2010 | Australia | Meningitis | — | 1 day–16 yr | UTI | 26 | 101 |
| 1994–1998 | Italy | — | Pediatrics | 0–17 yr | UTI | 23 | 102 |
| 2009–2010 | Bosnia and Herzegovina | — | Inpatients, outpatients | — | UTI | 15 | 103 |
| 2014–2016 | Tunisia | — | Medical, surgical | Neonates, adults | UTI | 10 | 104 |
| 1991–2000 | South Korea | Various medical and surgical diseases | — | Adults | BSI | 125 | 62 |
| 2003–2008 | USA | Various medical and surgical diseases | Medical, surgical | Adults | BSI | 68 | 142 |
| 2010–2015 | Afghanistan | — | Inpatients | 1 day–18 yr | BSI | 44 | 143 |
| 1980–1996 | China | Hepatobiliary disease, malignancy, diabetes | Medical, surgical and pediatric | 5–93 yr | BSI | 43 | 63 |
| 1986–1987 | USA | COPD, malignancy, diabetes | — | 1 day–95 yr | BSI | 34 | 144 |
| 1996 | Germany | — | PICU | Infants | BSI | 28 | 64 |
| 1985 | USA | — | ICU | Adults | BSI | 15 | 145 |
| 1981–1983 | UK | — | NICU | Neonates | BSI | 12 | 146 |
| 2016–2017 | Spain | — | — | Adults | BSI | 11 | 96 |
| 1983 | USA | Malignancy, heart disease, COPD, diabetes, alcoholism | Medical, surgical | Adults | Pneumonia | 14 | 108 |
| 1979–1981 | UK | Chronic bronchitis, malignancy, cryptogenic fibrosing alveolitis | Respiratory, chest | Adults | Pneumonia | 11 | 176 |
| 2016–2017 | Spain | — | — | Adults | Pneumonia | 10 | 96 |
| 2005–2006 | Spain | — | — | — | Peritonitis | 14 | 188 |
| 2006–2007 | Poland | — | Inpatients | — | SSTI | 44 | 210 |
| 2001–2011 | Australia | Malignancy, postsurgery status | ICU, other wards | Adults | Pleural empyema | 19 | 242 |
Reports with ≥10 cases are included. —, not available. AAHC, antibiotic-associated hemorrhagic colitis; BSI, bloodstream infection, also containing bacteremia; UTI, urinary tract infection; SSTI, skin and soft tissue infection. COPD, chronic obstructive pulmonary disease. NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
AAHC
K. oxytoca can cause various gastrointestinal infections in both children and adults, among which AAHC is particularly common (49). In fact, K. oxytoca is recognized and known to clinicians largely due to AAHC, which was first described in 1978 (80). K. oxytoca and Salmonella are the two pathogens causing AAHC (66), while K. pneumoniae has not been reported to cause this disease. In a recent study involving three major hospitals in Iran between 2011 and 2016, K. oxytoca was recovered from 50 (9.2%) of 545 patients with AAHC, while no pathogens were reported for the remaining patients (49). There are 22 published studies reporting a total of 161 cases of AAHC due to K. oxytoca, but large-scale surveys are lacking, and most of the studies are case reports (6, 30, 49, 66, 76–79, 81–94) (Table 5). The majority of these AAHC cases occurred after the patients received various antimicrobial agents, including β-lactams, fluoroquinolones, clarithromycin, clindamycin, and metronidazole, for 1 to 7 days with sudden onset of bloody diarrhea (6, 30, 66, 76, 93). AAHC due to K. oxytoca may develop in critically ill patients who received antimicrobial agents for more than 2 weeks (49). The abdominal cramps and diarrhea symptoms are not mild in AAHC patients but are usually alleviated within 24 to 48 h and resolve within 1 week after withdrawal of antimicrobial agents in nearly all cases (6, 81, 84, 93). This is different from non-AAHC infectious diarrhea caused by other species of the Enterobacterales, which often requires antimicrobial treatment to resolve (95).
Urinary Tract Infection
K. oxytoca is a relatively common pathogen of urinary tract infections (UTI) in both children and adults, primarily in pregnant women, immunocompromised patients, or those with genitourinary diseases (20, 96–136) (Table 5). K. oxytoca accounted for 1.3% (16/1,235), 0.7% (24/3,103), 1.9% (18/937), and 3.6% (109/3,038) of all UTI isolates in Mexico, China, Spain, and the United States, respectively, between 2009 and 2018 according to the Study for Monitoring Antimicrobial Resistance Trends (SMART) program (137–140). In hospitalized patients, the proportion of K. oxytoca in all bacterial uropathogens ranges from 2.5% to 3.5% (59, 101). For pregnant women, K. oxytoca appears to be more common in UTI and accounted for 19.4% and 38.1% of bacterial uropathogens, second only to Escherichia coli, in two studies (100, 135). Several studies have also reported UTI due to K. oxytoca in many patients with immunocompromised conditions, critical illness, or malignancies (97, 98, 115, 136). Most of these patients have a favorable outcome of UTI except for those with critical illness who always have infections at other sites (106, 114, 124, 127). In addition, UTI due to K. oxytoca is also common in patients with underlying genitourinary diseases or conditions, such as neurogenic bladder, renal lithiasis, urinary tract surgery, prostatic hyperplasia, and testicular infarction (99, 113, 125). In most of such cases, UTI can be resolved but are prone to recur, as the underlying diseases often continue to exist, and may then lead to long-term colonization with K. oxytoca (113, 119, 132).
Bloodstream Infection
Bacteremia refers to viable bacteria in the blood, which can evolve into a bloodstream infection (BSI) when the immune response mechanisms fail or become overwhelmed (141). K. oxytoca has not been reported as a common bacteremia pathogen in the past (62), but recently, a number of studies and cases have reported bacteremia or BSI due to K. oxytoca in patients across all age groups (62–64, 96, 98, 104, 105, 111, 114, 115, 127, 128, 142–173) (Table 5). In particular, there are three large-scale retrospective studies reporting the proportion of K. oxytoca in pathogens causing bacteremia or BSI (60, 62). K. oxytoca accounted for 0.57% of all bacteremia cases in South Korea between 1991 and 2001 (62), 3.7% (261/6,754) in Toronto, Canada, between 2006 and 2016 (60), and 4.2% (44/1,040) in Kabul, Afghanistan, between 2010 and 2015 (143).
Most K. oxytoca bacteremia or BSI cases are secondary to infections at other sites, such as UTI, skin and soft tissue infections, and pneumonia, and are associated with certain underlying diseases, including diabetes, malignancies, chemotherapy, radiation therapy, hepatobiliary diseases, cerebrovascular accidents, chronic obstructive pulmonary disease, chronic renal insufficiency, congestive heart failure, and various surgeries (63, 115, 144, 148–151, 153, 154). Septic shock, which is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with increased risks of mortality (174), was developed in many BSI cases (152, 156, 159, 160, 173, 175). The mortality of patients with K. oxytoca BSI varies significantly in different studies (62, 63, 98, 146), and only one large-scale study reported the mortality rate, which was 23.2% in South Korea (62). More large-scale studies are warranted to investigate the actual mortality of patients with K. oxytoca BSI.
Pneumonia
A mortality rate as high as 50% has been seen in pneumonia caused by Klebsiella spp. (71). K. pneumoniae is the most common Klebsiella species causing both community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP). K. oxytoca is also able to cause pneumonia, especially HAP (34, 96, 98, 104, 105, 108, 111, 114–116, 122, 127, 128, 147, 175–186), although much less commonly than K. pneumoniae. One large-scale study in China has reported that K. oxytoca constituted 3.6% (70/1,920) of bacterial isolates recovered from patients with pneumonia (138). Another retrospective study has found that K. oxytoca accounted for 10% of Klebsiella species causing acute respiratory tract infections in the United Kingdom between 1979 and 1981 (176). In Nepal in 2018 and 2019, K. oxytoca accounted for 2.86% of all Gram-negative bacteria causing lower respiratory tract infections (177). K. oxytoca pneumonia appears to be more commonly seen in patients with underlying respiratory diseases, including chronic bronchitis, small-cell carcinoma, chronic obstructive pulmonary disease (COPD), endobronchial tuberculosis, asthma, and obstructive sleep apnea (98, 108, 115, 128, 176, 186) (Table 5). Several studies have also shown that K. oxytoca is a relatively common pathogen causing ventilator-associated pneumonia (VAP) in critically ill patients with mechanical ventilation (116, 127, 186). A possible mechanism of VAP due to K. oxytoca is respiratory colonization acquired from hospital staff or equipment surfaces and then subsequent introduction into the lung via the tracheal tube. The prognosis of pneumonia due to K. oxytoca appears to be poor, as 12 of the 25 patients with such infection died (108, 176).
Intra-abdominal Infections
Intra-abdominal infections (IAI) are usually caused by E. coli and enterococci (187) but can also be due to K. oxytoca in some cases (98, 104, 127, 181, 188–209). The proportion of K. oxytoca in all pathogens isolated from IAI in the SMART program was 2.0% (54/2,682) in Mexico, 1.7% (65/3,758) in China, 4.8% (69/1,429) in Spain, and 5.9% (209/3,633) in the United States between 2009 and 2018 (137–140). In most cases, peritonitis and liver or spleen abscess due to K. oxytoca are secondary to abdominal surgeries or are seen in patients with malignancies, end-stage liver or renal diseases, or immunocompromised conditions, such as diabetes and renal transplantation (189, 194, 208). However, pancreatic abscess due to K. oxytoca is seen only in patients with pancreatitis (181, 200). Most patients with IAI due to K. oxytoca recovered after a combination of antimicrobial agents and surgeries (194, 199, 206, 207).
Skin and Soft Tissue Infections
Skin and soft tissue infections (SSTIs) due to K. oxytoca can be classified into three major types, i.e., wound infection, necrotizing fasciitis, and abscess (16, 96, 105, 108, 118, 128, 186, 210–220) (Table 5). Wound infection is usually secondary to surgeries (16, 96, 186), while necrotizing fasciitis is seen in patients with malignancies or receiving organ transplantations (118, 214, 215). Abscess mainly occurs in diabetic patients and may be due to skin damage (212, 217, 220). Patients usually recover from SSTIs due to K. oxytoca, except for those with other severe diseases (212, 214, 217, 219).
Other Infections
K. oxytoca has been found in central nervous system (CNS) infection (221–226), endocarditis (133, 173, 227–231), endophthalmitis (179, 232–237), septic arthritis (238–241), and many other types of infections, such as plueral empyema (242), prostatic infection (243), acute epididymitis (125), nonhemorrhagic diarrhea (244) or colitis (245), and malignant external otitis (246). CNS infections due to K. oxytoca, including meningitis, ventriculitis, and brain abscess, have been reported (221, 223–226), some of which are secondary to chronic otitis media (223, 225, 226). Most cases of CNS infections have a good prognosis after antimicrobial therapy and surgical procedures (aspiration or excision) (224–226). In the literature, there are seven cases of adults with endocarditis caused by K. oxytoca, six of whom recovered after antimicrobial treatment (133, 173, 227–231). Endophthalmitis, including keratitis, corneal ulcer, and suture abscess, has been reported in six adults and two neonates, and almost all of them recovered after topical antimicrobial drops (179, 232–237). Septic arthritis, an inflammation of the joints secondary to an infectious etiology, is usually caused by Staphylococcus aureus or Kingella kingae in children and S. aureus and Streptococcus pneumoniae in adults (247). Four cases of K. oxytoca septic arthritis, in two infants and two adults, have been reported (238–241), all of whom recovered after antimicrobial treatment. K. oxytoca was also found to be associated with hydropneumothorax in a case report (248).
Antimicrobial Treatment of Infections Due to the K. oxytoca Complex
Only few studies have specifically addressed the antimicrobial treatment in patients with infections due to the K. oxytoca complex. AAHC due to the K. oxytoca complex usually resolves spontaneously after withdrawal of antimicrobial agents that cause AAHC, and there is no need for antimicrobial treatment for AAHC (84, 90). Otherwise, as it is a member of the order Enterobacterales, antimicrobial treatment for infections due to the K. oxytoca complex is essentially the same as that for infections due to other Enterobacterales species, such as K. pneumoniae and E. coli. It is notable that rates of nonsusceptibility of the K. oxytoca complex to commonly used antimicrobial agents such as ceftazidime, carbapenems, amikacin, and levofloxacin are relatively low in surveillance programs such as SENTRY (see “Antimicrobial Resistance and Determinants” for details) and published reports (137, 139, 249). Therefore, many commonly used antimicrobial agents, including β-lactams (e.g., cephalosporins, carbapenems, and piperacillin-tazobactam) and non-β-lactam agents (e.g., amikacin, colistin, quinolones, tigecycline, and trimethoprim-sulfamethoxazole) could be therapeutic options for infections due to the K. oxytoca complex according to patient factors such as the disease severity, the immunity status, and the infection site (250). Strains of the complex share carbapenem resistance mechanisms with other Enterobacterales species, in particular K. pneumoniae. Antimicrobial treatment for carbapenem-resistant strains of the complex is the same as that for carbapenem-resistant Enterobacterales (CRE) (250–252). The antimicrobial options against CRE are usually stratified by the infection site (UTI or infections outside the urinary tract), the resistance profile (the susceptibility to meropenem in addition to resistance to ertapenem), and the types of carbapenemases, i.e., serine β-lactamases (e.g., KPC or OXA-48) or metallo-β-lactamases (MBLs, e.g., NDM). For infections outside the urinary tract, combinations containing new non-β-lactam β-lactamase inhibitors, such as ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam, are usually recommended against KPC-producing CRE, while cefiderocol (a novel siderophore cephalosporin) or ceftazidime-avibactam plus aztreonam are the preferred choice against NDM-producing CRE (250).
For cases with endophthalmitis caused by the K. oxytoca complex, symptoms disappeared after topical use of antimicrobial agents, including cefazolin, tobramycin, or fluoroquinolones, in combination with dexamethasone when necessary (232, 235). In addition to antimicrobial treatment, most patients with IAI, SSTI, or CNS infection caused by the K. oxytoca complex recovered after undergoing surgeries (118, 194, 225).
Outbreaks of Health Care-Associated Infections
To date, 15 outbreaks of health care-associated infections due to the K. oxytoca complex have been reported (Table 6), ranging in severity from conjunctivitis to sepsis (50, 175), with the number of cases ranging from 5 to 66 (105, 149). Most of these outbreaks occurred in hospitalized patients mainly in NICUs and several other types of wards, including hematology, neurology, and renal transplantation (40, 50, 97, 114, 115, 175). Microbiological source tracing was performed in most studies; handwashing sinks, drainage systems, humidifiers, blood gas analyzers, enteral feeding, and infusion preparation have been identified as likely sources of the outbreaks (115, 127, 149, 179, 253). This suggests that the K. oxytoca complex has environmental sources and may be well adapted to health care environments containing water, but studies examining the ability of the complex to survive and persist in relevant environments are largely lacking. The likely source of some of the outbreaks could not be identified, even though epidemiological and microbiological molecular screening methods were employed (50). Fortunately, all outbreaks with outcomes being reported were controlled by source control, such as sink modifications, and implementing bundles of infection control measures. These measures usually include strengthening hand hygiene, enhancing environment cleaning (particularly sinks and equipment), isolating infected patients, contact precautions, antimicrobial stewardship programs, and performance improvement of standard procedures (38, 50, 97, 115, 175, 253). In addition, there are two studies that reported clusters of NICU or pediatric ICU (PICU) patients with intestinal colonization of the K. oxytoca complex but without developing infection (38, 40). The two clusters were controlled after implementing bundles of infection control measures (38, 40).
TABLE 6.
Outbreaks due to the K. oxytoca complexa
| Yr | Country | Ward(s) | Age group | Infection type or site | No. of cases (infection/colonization) | Resistance | Source(s) | Control measures | Mortality | Clonality (ST) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016–2017 | Norway | NICU | Neonates | IC, pneumonia, necrotizing enterocolitis, conjunctivitis | 22 (5/17) | AMP only | — | Bundle: cleaning and disinfection, patient cohorting, hand hygiene, etc. | 1/22 | M (ST179) | 50 |
| 2014 | Spain | NICU | Neonates | IC, pneumonia, conjunctivitis | 20 (4/16) | Carbapenem | Other patients | Bundle: early detection, contact precautions, cohorting patients and HCWs, restricting β-lactams, etc. | 0/20 | P | 175 |
| 2013–2016 | Tunisia | Hospital-wide | Neonates, adults | UTI, hepatic abscess, pneumonia, sepsis | 19 (19/0) | PIP-TAZ, TIC, AMP-SUL | — | — | — | M (ST220) | 104 |
| 2011–2013 | Austria | Hematology | Adults | IC, pneumonia, BSI, abdominal abscess | 10 (6/4) | Carbapenem | Handwashing sinks | Isolating colonized patients; enforcing hand hygiene; cleaning the ward, particularly the sinks and equipment | 5/10 | M (ST4) | 115 |
| 2009–2011 | Spain | ICU | Adults | IC, VAP, BSI, UTI, peritonitis | 42 (14/28) | Multidrug resistant | Drainage system | Eliminating the horizontal drainage system | 18/42 | M | 127 |
| 2009–2010 | Japan | Neurosurgery | 17–75 yr | IC, postoperative SSTI, pneumonia | 8 (4/4) | PIP-TAZ | — | Enforcing hand hygiene, contact precaution, promoting AMS | 0/8 | M (ST9) | 114 |
| 2016–2017 | Greece | Oncology chemotherapy outpatient unit | — | CRBSI | 7 (7/0) | — | Chemotherapeutical prepn | — | 0/7 | M | 148 |
| 2006–2011 | Canada | ICU | — | IC, UTI, BSI, SSTI, pneumonia | 66 (24/42) | ESBLs | Handwashing sinks | Sink cleaning 3×/day, sink drain modifications, AMS | — | P | 105 |
| 2005 | Argentina | Renal transplant | — | UTI | 7 (7/0) | AMX-CLA, FUR, PIP-TAZ, ATM | — | Enhancing standard precautions (i.e., hand hygiene) via education and contact precaution | — | M | 97 |
| 2003 | Turkey | Neurology, ICU | 3–65 yr | BSI | 5 (5/0) | — | Saline solution during MRA | Changing and discarding saline solution | 0/5 | M | 149 |
| 2000 | Turkey | NICU | Premature neonates | BSI, pneumonia | 10 (10/0) | CAZ, CRO | — | — | — | P | 147 |
| 1997 | South Korea | NICU | Neonates | Endophthalmitis, pneumonia, UTI | 6 (6/0) | ATM, CRO | Humidifiers | Humidifiers were thoroughly washed and disinfected | — | M | 179 |
| 1996–1997 | France | Premature baby unit, NICU | Premature neonates | IC, BSI | 25 (1/24) | — | Enteral feeding procedures | Using gloves during enteral feeding, hand washing, isolation and cohorting | — | M | 253 |
| 1996 | Germany | NICU, PICU | Infants | BSI | 28 (28/0) | — | 0.25% disinfectant contaminated | Increasing the concn of disinfectant to 0.5%, replacing plastic pails. | 2/28 | M | 64 |
| 1981–1983 | UK | NICU | Neonates | BSI | 12 (12/0) | GEN | Blood gas analyzer | Disposing wastes from the blood gas analyzer in the lab rather than in the unit. | 8/12 | — | 146 |
| 2017–2018 | Australia | Neonatal | Neonates | IC | 10 (0/10) | ESBLs | Contaminated detergents | Isolating cases, destroying reusable detergent bottles, using prefilled single-use detergent bottles. | — | M | 40 |
| 2012–2013 | Germany | Pediatric center, ICU | Newborns, children | IC | 14 (0/14) | ESBLs | Washing machine | Replacing washing machine and sinks, and bundle: environmental monitoring; active patient screening, training HCWs, hand hygiene. | — | M | 38 |
AMS, antimicrobial stewardship; CRBSI, catheter associated bloodstream infection; ESBL, extended-spectrum β-lactamase; HCWs, health care workers; IC, intestinal colonization; MRA, magnetic resonance angiography; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; VAP, ventilator-associated pneumonia; AMP, ampicillin; AMX, amoxicillin; ATM, aztreonam; CAZ, ceftazidime; CLA, clavulanate; CRO, ceftriaxone; FUR, cefuroxime; GEN, gentamicin; PIP, piperacillin; SUL, sulbactam; TAZ, tazobactam; TIC, ticarcillin; M, monoclonal; P, polyclonal; —, not available.
Strain Clonal Background
Very few studies have addressed the clonal background of K. oxytoca clinical isolates. A multilocus sequencing typing (https://pubmlst.org/organisms/klebsiella-oxytoca) scheme has been developed (254). Using this scheme, 74 isolates from clinical samples or asymptomatic carriers were assigned to 60 sequence types (STs) (254), and in another study (13), 68 isolates (from rectal swabs in hospitals across Europe and Israel) that were not susceptible to expanded-spectrum cephalosporins were assigned to 34 STs. The studies suggest a hugely diverse clonal background within the K. oxytoca complex. Among the 359 STs of the K. oxytoca complex (https://pubmlst.org/organisms/klebsiella-oxytoca; accessed 1 April 2021), clonal complex 2, comprising ST2, -9, -18, -19, -57, -58, -61, -63, -141, -154, -155, and -176 (13, 254, 255), was the most common type, accounting for 32.4% of the 68 cephalosporin-nonsusceptible carriage isolates (13), and was also the most prevalent type in infants (255). Isolates of clonal complex 2 have been found in many countries in Europe and Israel (8, 13, 254) as well as Australia, China (254), and Japan (114). Clonal complex 2 has also been associated with carbapenem resistance and has caused outbreaks of health care-associated infections. In a multicenter study in Spain, eight of the 12 representative strains of carbapenem-resistant the K. oxytoca complex belonged to clonal complex 2 (96). In Japan, isolates of clonal complex 2 (ST9) caused an outbreak of various health care-associated infections in a university hospital (114). In the United Kingdom and Ireland, the rapid dissemination of isolates belonging to clonal complex 2 (ST2) has been identified due to clonal expansion (8). In light of the relatively high prevalence, the wide geographical distribution, and the association of carbapenem resistance and outbreaks, clonal complex 2 may have the potential to become a high-risk lineage for mediating the dissemination of antimicrobial resistance, and further studies are warranted.
ANTIMICROBIAL RESISTANCE AND DETERMINANTS
In Vitro Antimicrobial Susceptibility of the K. oxytoca Complex
SENTRY (https://www.jmilabs.com/sentry-surveillance-program/) is a worldwide antimicrobial surveillance program and has in vitro susceptibility data for 5,724 clinical isolates of the K. oxytoca complex from 2013 to 2019 (Table 7). In contrast, the K. oxytoca complex is not included in other large-scale international or regional surveillance programs (e.g., European Antimicrobial Resistance Surveillance System [EARSS]) or its overall in vitro susceptibility data of all participated regions are not available (e.g., the Assessing Worldwide Antimicrobial Resistance Evaluation [AWARE] global surveillance program and SMART). According to SENTRY data, almost all isolates of the K. oxytoca complex are susceptible to tigecycline and colistin, with a <1.0% nonsusceptibility rate, and the vast majority are also susceptible to aminoglycosides, with nonsusceptibility rates of 0.2 to 3.7%. The rates of nonsusceptibility to third-generation cephalosporins ranged from 4.1% (to ceftazidime) to 14.6% (to cefoperazone), while the carbapenem-nonsusceptible rate was 1.8% (to ertapenem) (Table 7). However, the rates of nonsusceptibility of the K. oxytoca complex to carbapenems and cephalosporins have been increasing during the past 7 years, although the rates of nonsusceptibility to aminoglycosides and piperacillin-tazobactam have remained stable. The rate of nonsusceptibility of the K. oxytoca complex to carbapenems varies across regions. The rate is higher in the Asia-West Pacific region and Europe than in North America, while since 2018, Latin America has shown a faster increase trend and a higher rate. For fluoroquinolones, the rates of nonsusceptibility to levofloxacin, ciprofloxacin, and moxifloxacin were 4%, 7.1%, and 10.7%, respectively, and have remained stable in the past 7 years.
TABLE 7.
In vitro susceptibility of antimicrobial agents against isolates of the K. oxytoca complex in the SENTRY program and other available large-scale (≥100 isolates) national or regional surveillance data in the Asia-West Pacific region and Africa
| Antimicrobial agent | % nonsusceptible (no. tested)a |
|||||
|---|---|---|---|---|---|---|
| SENTRY,b 2013–2019 (n = 5,724) | Japan,c 2019 (n = 10,551) | China,d 2019 (n = 30,781) | Thailand,e 2019 (n = 1,368) | Australia,f 2019 (n = 239) | Middle East and Africa,g 2015–2018 (n = 103) | |
| Amikacin | 0.2 (5,717) | 0.1 | 2.1 (29,638) | 2.4 (1,122) | 0 (239) | — |
| Gentamicin | 3.5 (5,723) | 1.2 | 11.9 (26,509) | 13.5 (966) | 0.4 (239) | — |
| Tobramycin | 3.7 (5,178) | — | — | — | 0.4 (239) | — |
| Amoxicillin-clavulanate | 7.5 (3,728) | 11 | 24.9 (11,487) | 17.7 (988) | 9.3 (215) | — |
| Ampicillin-sulbactam | 51.2 (5,724) | 29.1 | 43.5 (21,537) | 33.9 (115) | — | — |
| Cefoperazone-sulbactam | 12.1 (4,714) | — | 14.6 (14,697) | 17.1 (615) | — | — |
| Ceftazidime-avibactam | 1 (896) | — | — | — | — | — |
| Ceftolozane-tazobactam | 3.9 (894) | — | — | — | — | — |
| Piperacillin-tazobactam | 11 (5,716) | 9.1 | 12.6 (29,731) | 11.9 (986) | 9.2 (239) | 0 (103) |
| Doripenem | 0.8 (4,827) | — | — | — | — | — |
| Ertapenem | 1.8 (2,107) | — | — | 4.1 (917) | — | — |
| Imipenem | 1.1 (5,723) | — | 6.4 (30,057) | 6.1 (917) | — | — |
| Meropenem | 0.9 (5,723) | 0.2 | 5.5 (18,969) | 4.5 (1,050) | 0 (239) | — |
| Cefepime | 5.3 (5,723) | 1.5 | 11.9 (29,433) | 17.9 (380) | 0.4 (239) | 0 (103) |
| Cefoperazone | 14.6 (1,368) | — | — | — | — | — |
| Cefoxitin | 5.1 (790) | — | 15.2 (12,083) | 10.1 (513) | — | — |
| Ceftaroline | 17.9 (5,667) | — | — | — | — | 3.9 (103) |
| Ceftazidime | 4.1 (5,724) | 2.4 | 12.3 (27,814) | 21.2 (1,132) | 1.3 (239) | — |
| Ceftriaxone | 12.5 (5,724) | 9 | 20.8 (24,645) | 23.2 (858) | 7.6 (239) | — |
| Cefuroxime | 28.9 (1,539) | — | 27.1 (17,327) | — | — | — |
| Aztreonam | 11.9 (5,723) | 8.5 | 15.8 (22,996) | — | — | 0 (103) |
| Trimethoprim-sulfamethoxazole | 7.2 (5,717) | 6.9 | 20.7 (28,690) | — | — | — |
| Tigecycline | 0.1 (5,724) | — | 3.3 (8,257) | — | — | 0 (103) |
| Colistin | 0.8 (5,656) | — | — | — | — | — |
| Ciprofloxacin | 7.1 (5,710) | — | 16.1 (25,604) | 30.6 (1,032) | 1.7 (239) | — |
| Levofloxacin | 5.4 (5,715) | 5.3 | 12.9 (29,565) | 23.4 (444) | — | 3.9 (103) |
| Moxifloxacin | 10.7 (4,205) | — | — | — | — | — |
| Doxycycline | 7.6 (4,929) | — | — | — | — | — |
| Minocycline | 5.9 (4,971) | — | — | — | — | — |
| Tetracycline | 7.9 (4,976) | — | — | — | — | — |
Nonsusceptible, including intermediate and resistant, is defined using criteria of the Clinical and Laboratory Standards Institute (CLSI) (2019), except for colistin and moxifloxacin, for which the term is defined using criteria of EUCAST (https://www.eucast.org) (2019). For each agent, the number of isolates tested varies and therefore is shown in parentheses except the data from Japan, for which the number is not available. —, not available.
The SENTRY surveillance data are available at https://www.jmilabs.com/sentry-surveillance-program/.
Source: Japan Surveillance for Infection Prevention and Healthcare Epidemiology (J-SIPHE) program, comprising 2,223 hospitals (https://j-siphe.ncgm.go.jp/en).
Source: China Antimicrobial Resistance Surveillance System (CARSS), comprising 1,375 hospitals (http://www.carss.cn/).
Source: National Antimicrobial Resistant Surveillance Center, comprising 92 hospitals (http://narst.dmsc.moph.go.th/).
Source: Antimicrobial Use and Resistance in Australia (AURA) Surveillance System (https://www.safetyandquality.gov.au/). These isolates were collected from sepsis patients only.
Source: reference 256. These isolates were collected from patients with SSTI and respiratory tract infections.
The vast majority of isolates (92.3%) of the K. oxytoca complex in the SENTRY program are from North America (61.2%, n = 3,501) and Europe (31.1%, n = 1,783), while isolates from the Asia-West Pacific region and Latin America accounted for only 4.4% (n = 257) and 3.2% (n = 183), respectively, and no isolates were from Africa. In the Middle East and Africa, 103 isolates of the K. oxytoca complex from patients with SSTI and respiratory tract infections between 2015 and 2018 were reported in the AWARE global surveillance program (256). All of the 103 isolates were susceptible to aztreonam, cefepime, piperacillin-tazobactam, and tigecycline, while the rate of nonsusceptibility to ceftaroline and levofloxacin was 3.9% for both (Table 7) (256).
There are national or large-scale (with 100 or more isolates) surveillance systems, which have reported susceptibility data for clinical isolates of the K. oxytoca complex, in several countries (Australia, China, Japan, and Thailand) in the Asia-West Pacific region. In Japan, the rates of nonsusceptibility of the 10,551 clinical isolates of the K. oxytoca complex to all tested antimicrobial agents but amoxicillin-clavulanate (https://j-siphe.ncgm.go.jp/en) were lower than those in SENTRY (Table 7). In contrast, clinical isolates of the K. oxytoca complex in China (n = 30,781, from 1,375 hospitals in 2019; http://www.carss.cn/) and Thailand (n = 1,368, from 92 hospitals in 2019; http://narst.dmsc.moph.go.th/) had higher rates of nonsusceptibility to most antimicrobial agents than those in SENTRY and Japan, in particular to carbapenems, ceftazidime, cefepime, and fluoroquinolones (Table 7). For instance, the rates of nonsusceptibility to carbapenems were 6.4% in China and 6.1% in Thailand, which are higher than the 0.9% in SENTRY and the 0.2% in Japan (Table 7). In Australia, 239 clinical isolates of the K. oxytoca complex collected from sepsis patients in 2019 had lower rates of nonsusceptibility to almost all tested agents than those in SENTRY and Japan (Table 7), and no carbapenem-nonsusceptible isolates were identified (https://www.safetyandquality.gov.au/).
Antimicrobial Resistance Determinants
The K. oxytoca complex carries several intrinsic antimicrobial resistance genes, including the β-lactamase-encoding blaOXY and efflux pump-encoding oqxA-oqxB, to mediate low-level resistance to quinolones (52). However, we found that oqxA-oqxB was absent from strains of K. huaxiensis, K. spallanzanii, taxon 1, and taxon 3. In addition, we also found that the fosfomycin resistance gene fosA (257) is intrinsic to the K. oxytoca complex. Many isolates of the complex have also acquired genes mediating resistance to a variety of antimicrobial agents, including β-lactams (e.g., penicillins, cephalosporins, and carbapenems), aminoglycosides, quinolones, and colistin (258). These antimicrobial resistance genes are listed in Table 8.
TABLE 8.
Antimicrobial resistance genes
| Antimicrobial | Antimicrobial resistance gene(s)a | References |
|---|---|---|
| Carbapenems | blaGES-5, blaGIM-1, blaIMP-1, -4, -6, -8, -28, -29, -34, blaKPC-2, -3, blaNDM-1, -4, -5, blaOXA-48, -181, blaVIM-1, -2, -4 | 32, 34, 96, 104, 106, 119–121, 124, 150, 156, 158, 166, 183, 190, 213, 241, 244, 281, 285–287, 296, 299–303, 305–332, 393–398, 403–416 |
| Other β-lactams | blaACC-1, blaACT-36, blaBEL-1, blaCARB-2, blaCMY-2, -4, -6, -31, blaCTX-M-2, -3, -8, -9, -15, blaDHA-1, blaFOX-3, -5, blaGES-1, blaLAP-2, blaOXA-1, -2, -4, -9, -10, blaSHV-2, -5, -11, -12, -46, blaTEM-1, -15, -30, blaVEB | 32, 34, 40, 96, 106, 119–121, 124, 150, 156, 166, 183, 281, 285–289, 292, 296–303, 309, 310, 316, 319, 322, 323, 337, 338, 354, 393, 397, 406, 411, 414, 415, 417–424 |
| Colistin | mcr-1, -9 | 335 – 338 |
| Aminoglycosides | aac(3)-I, -Ia, -Ib, -II, -IIa, -IId, -IIg, -IV, -IVa, aac(6′)-30, -Ib, -Ib3, -Ib-cr, -Ib-cr5, -II, -IIa, -IIc, aacA44, aadA1, aadA2, aadA5, aadA13, aadB, ant(2′′)-Ia, ant(3′′)-Ia, aph(3′)-Ia, -Ib, -Ic, -VI, -XV, aph(3′′)-Ib, aph(6)-Id, armA, rmtC, sat2, strA, strB | 31, 32, 40, 96, 119, 150, 166, 169, 213, 244, 286, 287, 292, 296–300, 318–320, 323, 337, 338, 354, 393, 397, 406, 407, 418–420, 425 |
| Quinolones | oqxA, oqxB, qnrA1, qnrB1, qnrB2, qnrB4, qnrB6, qnrB19, qnrB32, qnrS1, qnrS2 | 31, 32, 34, 96, 119, 166, 169, 286, 287, 292, 296–298, 300, 306, 319, 323, 336–338, 388, 393, 397, 406, 413, 418, 425–430 |
| Fosfomycin | fosA3 | 34, 323, 337, 388, 393, 427 |
| Sulfonamides | sul1, sul2, sul3 | 32, 40, 96, 119, 166, 244, 286, 287, 292, 296–298, 300, 323, 337, 393, 397, 418–420 |
| Trimethoprim | dfrA1, dfrA12, dfrA14, dfrA16, dfrA17, dfrA19, dfrB1, dfrII, dfrIIIc | 32, 96, 119, 156, 166, 169, 286, 292, 296, 297, 300, 319, 320, 323, 338, 393, 397, 418, 420 |
| Chloramphenicol | catA1, catA2, catB2, catB3, catB11, cmlA1 | 32, 34, 96, 119, 287, 292, 296–298, 300, 320, 323, 337, 338, 393, 397 |
| Rifampin | arr-3, arr-8 | 32, 156, 297, 298, 323, 337, 393 |
| Tetracyclines | tet(A), tet(B), tet(D) | 34, 96, 166, 287, 296–298, 323, 338, 393, 418 |
| Macrolides | ere(A), mph(A), mph(E), msr(E) | 96, 166, 292, 323, 337, 393, 397 |
All strains of the K. oxytoca complex also have intrinsic blaOXY genes.
Resistance to β-lactams in the Enterobacteriaceae is mainly due to the production of β-lactamases. A large number of β-lactamases have been reported and can be divided into four classes, i.e., class A, B, C, and D, according to the molecular structure (259). Narrow-spectrum β-lactamases are able to hydrolyze commonly prescribed penicillins, while broad-spectrum β-lactamases are also capable of hydrolyzing first- and second-generation cephalosporins (260–262). However, it is worth noting that the boundary between narrow- and broad-spectrum β-lactamases is often blurred in the literature and the same β-lactamase may be referred to as either type in different publications. ESBLs have the ability to hydrolyze monobactams (e.g., aztreonam) and the oxyiminocephalosporins (e.g., cefotaxime, ceftazidime, and cefepime) (263). Compared to ESBLs, AmpC-type cephalosporinases are also able to hydrolyze cephamycins (e.g., cefoxitin) but not cefepime, and their hydrolysis mechanism is typically resistance to the inhibition by β-lactam-type β-lactamase inhibitors (clavulanate, sulbactam, and tazobactam) (264). Carbapenemases further extend the hydrolysis spectrum to carbapenems while typically retaining the activities of ESBLs and AmpC. ESBLs are of either class A or D, and AmpC belongs to class C, while carbapenemases can belong to class A, B, or D. In addition, some class A β-lactamases are also resistant to the inhibition of β-lactam-type β-lactamase inhibitors and are called inhibitor-resistant β-lactamases (261).
blaOXY.
The K. oxytoca complex has an intrinsic blaOXY gene encoding the chromosomal class A β-lactamase OXY, which is typically produced at a low level to confer resistance to aminopenicillins (ampicillin and amoxicillin), carboxypenicillins (carbenicillin and ticarcillin), and other penicillins (265, 266). Mutations in the promoter sequences of blaOXY have been observed in 10% to 20% of clinical isolates (266–269) and can lead to overexpression of this gene by 73- to 223-fold (268). Five point mutations in the promoter have been mentioned in the literature: four in the −10 consensus sequence (G to T at the first base, G to A at the fifth base, G to A at the eighth base, and G to T at the twelfth base) and a T-to-A mutation at the fourth base of the −35 sequence (267, 270). Compared to the G-to-A mutation at the fifth base (the −10 consensus sequence) and the T-to-A mutation at the fourth base (the −35 sequence), the G-to-T mutation at the first base (the −10 sequence) leads to a stronger promoter (268). blaOXY overexpression confers resistance to penicillins and some extended-spectrum β-lactams, especially aztreonam (265, 269–271), and leads to hydrolysis of ceftriaxone to a greater extent than cefotaxime but typically does not confer resistance to ceftazidime (269–272). Mutations in blaOXY may also extend the resistance spectrum to aztreonam and oxyimino-cephalosporins. The proline-to-serine substitution at Ambler position 167 enhances the ability to hydrolyze ceftazidime (243). At Ambler position 237, the alanine-to-threonine substitution confers resistance to cefotaxime (273), while the alanine-to-glycine substitution increases the hydrolysis of aztreonam and ceftriaxone and increases resistance to the inhibition of clavulanate but decreases the ability to hydrolyze benzylpenicillin, cephaloridine, and cefamandole (274). Amino acid substitutions at Ambler position 237 also reduce susceptibility to ceftazidime (275). Compared with OXY-2-2, OXY-2-15 has a deletion of two amino acids at Ambler positions 168 and 169 and acquires the ability to hydrolyze ceftazidime (201). Some mutations in blaOXY, such as the mutation resulting in a serine-to-glycine substitution at Ambler position 130 of OXY-2, generate an inhibitor-resistant β-lactamase (276). Typically, blaOXY is located on the chromosome of the K. oxytoca complex. However, plasmid-borne blaOXY has also been found in certain strains of the complex with the potential to be further transferred to other species, such as K. pneumoniae (136).
Class A noncarbapenemase β-lactamase-encoding genes.
blaTEM-1 appears to be the most common blaTEM variant in the K. oxytoca complex and encodes TEM-1, a broad-spectrum β-lactamase. Several other blaTEM variants have also been found in the K. oxytoca complex (Table 8). These variants encode either ESBLs, including TEM-3, TEM-15, TEM-26, and TEM-116 (263, 277), or the inhibitor-resistant β-lactamase TEM-30 (278). blaSHV is intrinsic to K. pneumoniae but not K. oxytoca (279). Nonetheless, blaSHV variants encoding SHV-2, −5, −7, −11, −12, −14, −30, and −46 have been found in the K. oxytoca complex (Table 8). Among these SHV enzymes, all but SHV-11 are ESBLs (263, 280, 281), while SHV-11 is a broad-spectrum β-lactamase (282). CTX-M enzymes are almost always ESBLs (283), and a few blaCTX-M variants have been found in the K. oxytoca complex (Table 8). Of note, the presence of OXY β-lactamases may cause false-positive detection of CTX-M by immunological panels (284). Genes encoding other class A β-lactamases, including GES-1 and VEB (the exact variant was not specified), have also been sporadically reported (285).
ampC genes.
Unlike many other Enterobacteriaceae (such as Citrobacter spp., Enterobacter spp., and E. coli), the K. oxytoca complex has no chromosomal ampC genes encoding AmpC β-lactamases. Nonetheless, plasmid-borne ampC genes, including blaACC (286), blaACT (287), blaCMY (106, 121, 288), blaDHA (289, 290), and blaFOX (291, 292), have been found in the K. oxytoca complex (Table 8).
Class D noncarbapenemase blaOXA genes.
To date, five blaOXA genes encoding OXA-1, OXA-2, OXA-4, OXA-9, and OXA-10, all of which are narrow-spectrum β-lactamases (293–295), have been sporadically found in the K. oxytoca complex (96, 119, 150, 286, 296–303). The five OXA β-lactamases can be assigned to four subfamilies, i.e., OXA-1 (OXA-1 and OXA-4), OXA-2, OXA-9, and OXA-10 (295). Of note, OXA-2 and OXA-10 have weak activity against carbapenems (304).
Carbapenemase-encoding genes.
Carbapenem-resistant K. oxytoca (CRKO) was first reported in 2003, with the strain being isolated from human urine in New York, USA, in 1998 (281). Since then, CRKO carrying a variety of carbapenemase genes has been identified all around the world (Table 8 and Fig. 2). It is worth noting that CRKO strains in most studies were not identified to the precise species level and that “K. oxytoca” in these studies could refer to any species of the complex. Therefore, in the following text, CRKO refers to all species within the K. oxytoca complex unless otherwise indicated.
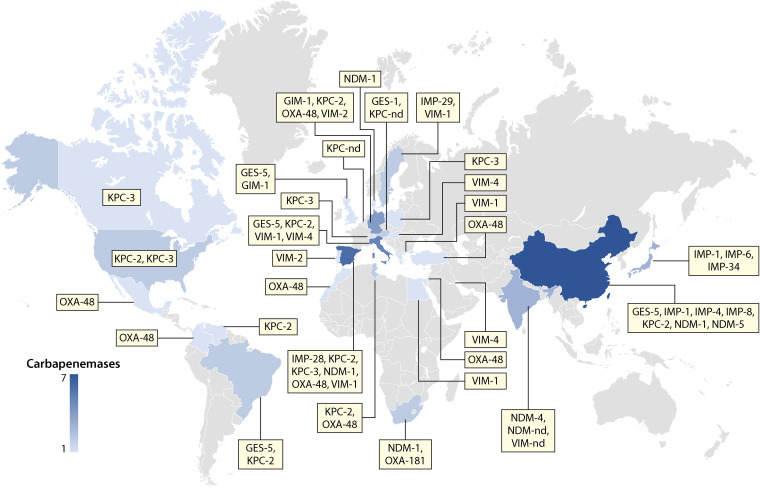
FIG 2.
Worldwide distribution of CRKO strains and their carbapenemase types. nd, not determined. The number of carbapenemase variants that have been reported in a given country is indicated by color gradients.
Class A carbapenemases KPC-2, KPC-3, and GES-5 have been found in CRKO, including K. grimontii (34), K. michiganensis (166, 190), and K. oxytoca (96, 119, 156, 183, 244, 281, 287, 299, 300, 302, 305–315). KPC-2 appears to be the most common carbapenemase in CRKO strains, and KPC-2-positive CRKO has been identified from patients and clinical environment settings in Brazil (156), China (34, 119, 244, 305, 306, 310), Germany (302), Spain (96), the United States (190, 281, 312), and Venezuela (183), from rivers in Spain (299) and Italy (300), and from a wild bird in Tunisia (287). KPC-3-positive CRKO is usually isolated from patients (166, 190, 314), while GES-5-positive CRKO has been found only in water samples so far (300, 316, 317).
Currently, genes encoding four types of class B MBLs, i.e., GIM, IMP, NDM, and VIM, have been found in CRKO including K. grimontii, K. michiganensis, and K. oxytoca. The first MBL gene reported in CRKO was blaVIM-2, which was found in four isolates from blood cultures of neonates in Portugal in 2005 (150). However, blaVIM-1 is the most common blaVIM variant in CRKO, and blaVIM-1-positive CRKO has been widely reported across Europe (96, 286, 299, 318–320) and in Egypt (321). blaIMP is another relatively common MBL gene in CRKO. Seven IMP enzymes encoded by blaIMP genes have been found in CRKO (Table 8), and IMP-4 is the most common one. Three blaNDM variants encoding NDM-1, NDM-4, and NDM-5 have been found in CRKO, including K. michiganensis and K. oxytoca. In particular, blaNDM-1-positive CRKO has been reported multiple times and all isolates were from patients or hospital environments (32, 96, 244, 296, 305, 322–326). In contrast, blaNDM-4 (301) and blaNDM-5 (244) have been found only in single isolates. blaGIM-1 was originally found in Pseudomonas aeruginosa in Germany in 2002 and has been found in one CRKO strain in the United Kingdom recovered in 2010 (327).
blaOXA-48 and the closely related blaOXA-181 are the two class D carbapenemase-encoding genes that have been found in CRKO, including K. michiganensis and K. oxytoca. blaOXA-48-positive CRKO has been found in hospital environments in Israel (328), Mexico (329), Morocco (120), Spain (96), and Turkey (285), from patients in Colombia (330) and Tunisia (104), and from companion animals in Germany (331). blaOXA-181 has been reported only in K. michiganensis in South Africa from urban hospital effluent (332) and a cancer patient (32).
The coexistence of two or three carbapenemase genes, in particular blaNDM-1 plus either blaKPC-2 (119, 244, 305), blaIMP-4 (119), blaNDM-5 (244), or blaOXA-181 (32), in the same CRKO strain has also been reported. Other coexistences in the same CRKO strain are blaKPC-2 plus either blaIMP-4 (119, 305) or blaIMP-8 (310).
Plasmid-borne colistin resistance genes.
Colistin resistance in the K. oxytoca complex is commonly due to the interruption of mgrB (158, 333), a negative regulator of the PhoP-PhoQ two-component system (334), or altered expression of this gene (211). Nonetheless, plasmid-borne colistin resistance genes, including mcr-1 (335, 336) and mcr-9 (337, 338), have also been seen in the complex. Plasmid-borne mcr-1 has been found in K. oxytoca from the superficial skin swab of a patient in South Africa (335) and from a lake in China (336). Plasmid-borne mcr-9 has been reported in K. oxytoca strains which were recovered from a rectal swab of a patient from Qatar (337) and from horses in Sweden (338).
Other resistance genes.
Genes mediating resistance to aminoglycosides, chloramphenicol, fosfomycin, macrolides, quinolones, rifampin, sulfonamides, tetracyclines, and trimethoprim are listed in Table 8. Aminoglycoside resistance in the K. oxytoca complex is mainly due to modifications, including acetylation (aac and sat genes) (339), adenylylation (ant and aad genes) (339), and phosphorylation (aph and str genes) (339). Genes (armA and rmtC) encoding 16S rRNA methylases that confer high-level resistance to all aminoglycosides that are commonly used in clinical settings, including amikacin, gentamicin, and tobramycin, have also been found in the K. oxytoca complex (340, 341). Acquired quinolone resistance in the K. oxytoca complex is due to plasmid-borne qnr genes (qnrA, qnrB, and qnrS). qnr genes encode pentapeptide repeat proteins to protect bacterial DNA gyrase and topoisomerase IV from inhibition by quinolones and result in low-level quinolone resistance (342). The sulfonamide resistance genes sul1, sul2, and sul3, encoding dihydropteroate synthases, which are able to catalyze the condensation of para-aminobenzoate with 6-hydroxymethyl-7,8-dihydropterin diphosphate (343), are seen in many isolates of the K. oxytoca complex. A number of variants of dfr trimethoprim resistance genes, which encode dihydrofolate reductases (343), are found in the K. oxytoca complex (Table 8). Chloramphenicol resistance in the K. oxytoca complex is mainly caused by acetylation of the drug via different types of cat-encoding chloramphenicol acetyltransferases (344). In addition, a specific exporter encoded by cmlA1 also confers chloramphenicol resistance (344) and has been found in the K. oxytoca complex. The rifampin resistance gene arr encodes ADP-ribosyl transferases able to inactivate rifampin (345) and is also found in the K. oxytoca complex. tet genes encode energy-dependent membrane-associated proteins to export tetracycline out of bacterial cells (346), and several tet genes have been identified in the complex. Macrolide resistance genes seen in the K. oxytoca complex include ere(A), mph(A), mph(E), and msr(E). ere(A) encodes a macrolide esterase, and mph genes encode macrolide phosphotransferases, while msr encodes an efflux pump able to reduce the intracellular concentration of macrolides (347).
Chromosomal Point Mutation-Associated Antimicrobial Resistance
In addition to intrinsic and acquired antimicrobial genes, nonsynonymous mutations in some chromosomal genes, including gyrA, mgrB, and parC, can also mediate resistance to quinolones (gyrA and parC) or colistin (mgrB).
gyrA and parC encode DNA topoisomerase II (gyrase) subunit A (GyrA) and DNA topoisomerase IV subunit A, respectively. Amino acid substitutions in GyrA at positions 83 and 87 and in ParC at position 80 are associated with reduced binding of quinolones to the topoisomerase-DNA complex and lead to quinolone resistance in the Enterobacteriaceae (348–350). In the K. oxytoca complex, amino acid substitutions of GyrA at position 83 (T83I) or 87 (D87G) and those of ParC at position 80 (S80R or S80I) have been reported to confer quinolone resistance (18, 107, 351–353). In addition, the D87N substitution in GyrA (354) and M157L in ParC (298, 351) have also been found in quinolone-resistant isolates of the K. oxytoca complex, but whether these substitutions confer quinolone resistance has yet to be verified.
mgrB is a negative regulator of the PhoP-PhoQ two-component system (334). It has been found that interruption of mgrB by insertion sequences (158, 333), altered expression of mgrB (211), or the C28Y amino acid substitution in MgrB (333, 355) is able to mediate colistin resistance in the K. oxytoca complex.
VIRULENCE FACTORS
Cytotoxins Causing AAHC
K. oxytoca is a well-characterized causative agent of AAHC, caused by the production of cytotoxins. K. oxytoca-specific cytotoxin was initially described in 1989 and was thought to be present only in clinical isolates (356). An early study demonstrated that the cytotoxin produced by K. oxytoca from an AAHC patient was able to cause fluid accumulation in the ileal and colonic loops and severe ileal mucosal hemorrhage with erosion in rabbits (357). The right-side colon was found to be the main target of K. oxytoca using a rat model involving inoculation with K. oxytoca or the administration of amoxicillin-clavulanate (6). This finding is consistent with a case report regarding infection sites (86). AAHC associated with K. oxytoca happens as a result of the administration of antimicrobial agents, especially penicillins (6, 81, 86), which disturb the normal intestinal microflora, contributing to favorable conditions for the overgrowth of K. oxytoca (94). Although K. oxytoca also exists in other body sites, such as skin, mouth, upper respiratory tract, and urinary tract, the cytotoxin-producing isolates are more prevalent in the intestinal tract (16, 94). One study reported that 46% (6/13) of isolates from the stool of the asymptomatic carriers exhibited cytotoxicity, while none from the urinary tract (n = 10) or respiratory tract (n = 16) displayed cytotoxicity (94).
There are two distinct cytotoxins produced by K. oxytoca, tilimycin (also known as kleboxymycin or carbinolamine) and tilivalline (generated by nucleophilic attack of free indole on tilimycin [358, 359]), which lead to the pathological changes seen in AAHC (359, 360). Both tilivalline and tilimycin are pyrrolobenzodiazepine (PBD) metabolites and are generated from a bimodular nonribosomal peptide synthetase (NRPS) pathway (359, 360). The kleboxymycin-biosynthetic gene cluster for tilimycin and tilivalline contains the regulators npsC and marR, an NRPS operon, an aroX operon, mfsX (encoding a multidrug efflux MFS transporter), and uvrX (encoding the excinuclease ABC subunit UvrA) (358, 361, 362). The NRPS operon consists of npsA (encoding an amino acid adenylation domain-containing protein), thdA (encoding an acyl carrier protein), and npsB (encoding a nonribosomal peptide synthetase). The aroX operon comprises five genes, aroX, dhbX, icmX, adsX, and hmoX, encoding a 3-deoxy-7-phosphoheptulonate synthase, a 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase, an isochorismatase, a 2-amino-2-deoxy-isochorismate synthase, and a 4-hydroxyphenyl acetate-3-monooxygenase, respectively (358, 361, 362).
PBDs are commonly produced by the actinobacteria, and therefore, it is suspected that the gene cluster encoding both toxins in K. oxytoca was acquired through horizontal gene transfer (359, 363). In a recent study of 7,170 Klebsiella genomes, including 178 belonging to the K. oxytoca complex (76 K. michiganensis, 66 K. oxytoca, 24 K. grimontii, 6 K. pasteurii, 5 K. huaxiensis, and 1 K. spallanzanii genome), the complete kleboxymycin gene cluster was found only in K. grimontii, K. michiganensis, K. oxytoca, and K. pasteurii (364). As K. grimontii, K. michiganensis, K. oxytoca, and K. pasteurii phylogenetically cluster as a lineage separated from other species of the K. oxytoca complex (Fig. 1), it is likely that the kleboxymycin gene cluster was acquired in this lineage before species divergence. However, the gene cluster has not been found to be plasmid borne (254).
Tilivalline was the first cytotoxin characterized in detail (360). It has been demonstrated that tilivalline suppresses microtubule-dependent processes in A549 lung carcinoma cells and HT-29 colon cancer cells by binding tubulin directly, making the microtubules stable and resulting in mitotic arrest (361). Tilimycin, also called kleboxymycin, exhibits at least 9-fold-higher toxicity than tilivalline in a cell culture assay based on MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole] and leads to increased virulence in the presence of glucose and lactose (359). Tilimycin is a genotoxin interacting with double-stranded DNA, inducing cellular DNA damage in host cells in vitro and in vivo and causing a more serious lesion in cecal enterocytes of colonized mice than in healthy controls (361). Both tilimycin and tilivalline were found to be related to perturbations of the intestinal barrier by decreasing the expression of claudin-1, a barrier-forming tight junction protein, in T84 monolayers (365). However, in the absence of the two toxins, the intestinal barrier damage can also be triggered by reducing the expression of claudin-5 and −8 in K. oxytoca in T84 monolayers (365).
CPS and LPS
Capsular polysaccharide (CPS; K antigen) and LPS (containing O antigen) are common virulence factors of K. pneumoniae (366–369). Compared to K. pneumoniae, K and O antigens of the K. oxytoca complex are less studied. Nonetheless, a few K types, including K6, K9, K15, K21, K23, K26, K29, K31, K41, K43, K47, K55, K61, K66, K68, K70, K74, and K79, have been identified in the K. oxytoca complex (370–376). In a study containing 150 strains of the K. oxytoca complex, K68 was the most prevalent type in human stool samples (371). Currently, no published reports of the O antigen in the K. oxytoca complex are available.
Other Virulence Factors in the Literature
Other than AAHC-associated cytotoxins, there are only a limited number of studies addressing virulence factors of the K. oxytoca complex, partially due to the fact that most members of the K. oxytoca complex were identified in recent years. Nonetheless, a number of K. pneumoniae virulence factors have also been reported in the K. oxytoca complex, including genes encoding capsules (matB) (48, 377, 378), adhesins or biofilm formation (cf29a, fimA, fimH, mrkABCDF, and pilQ) (48, 377–382), iron uptake (kfuBC) (379), and urease (ureA) (379, 383). Among these genes, very few have been experimentally tested to explore their association with virulence in the K. oxytoca complex. The mrk gene cluster (mrkABCDF) encodes the mannose-resistant Klebsiella-like hemagglutinins (the type 3 fimbriae), allowing attachment to surfaces and thus formation of biofilms in Klebsiella spp. (381, 382, 384–386). This operon has been detected in two of 100 K. oxytoca isolates (380). The two mrk-positive K. oxytoca isolates were able to cause type 3 fimbria-specific agglutination in testing on tannic acid-treated red blood cells, but attempts to prove that the expression of type 3 fimbriae leads to the colonization of the mouse urinary tract failed (380). In a study, 70% (35/50) of K. oxytoca isolates from AAHC patients produced moderate levels of biofilm with higher expression of fimA (encoding a type 1 fimbrial protein facilitating colonization of the epithelium), pilQ (encoding type IV pilus biogenesis and competence protein) and mrkA than the biofilm-free strains (49). In another study, 78% (156/200) of K. oxytoca isolates from patients with colorectal cancer generated moderate levels of biofilm production (377). Virulence genes found in other enteric pathogens, such as Citrobacter freundii, E. coli, and Vibrio cholerae (genes encoding type IV and type VI secretion systems and proteins with partial homology to the cholera toxin), have also been reported in K. oxytoca, but their association with virulence in the complex has not been determined (8, 360).
PLASMIDS FOUND IN THE COMPLEX
Replicon Types of Plasmids in the K. oxytoca Complex
Plasmids are mobile genetic elements which can replicate independently of the chromosome (387), and many carry genes encoding beneficial phenotypes for the survival of host strains, such as antimicrobial resistance and virulence. A variety of plasmids have been reported in the K. oxytoca complex (32, 35, 96, 156, 244, 296, 388, 389). Plasmids seen in the complex belong to various replicon types, but none is specific to the complex.
Plasmids Carrying Carbapenemase-Encoding Genes
No known carbapenemase-encoding genes have originated from the K. oxytoca complex, and therefore, plasmids play a pivotal role in introducing carbapenem resistance into the complex. Forty-three plasmids carrying genes encoding class A carbapenemases have been reported in the literature or have been deposited in NCBI (Table 9), most of which carry blaKPC-2. These blaKPC-2-carrying plasmids belong to various replicon types, including IncF, IncHI, IncN, IncP, IncW, and IncX. Among the plasmids, IncN appears to be particularly common and could be further assigned to plasmid sequence types using the plasmid multilocus sequence typing (pMLST) scheme (https://cge.cbs.dtu.dk/services/pMLST/). In particular, 9 ST15 IncN blaKPC-2-carrying plasmids were identified in Germany in 2014 or 2017, but unfortunately, no epidemiological information is available for these plasmids to determine whether there was an outbreak due to plasmid-mediated carbapenem resistance. Four ST6 IncN blaKPC-2-carrying plasmids were found in the United States in 1997 and 2015 (Table 9), while the remaining four IncN plasmids were reported in the United States in 2015 (190), Germany in 2016 (302), or Spain in 2013 (390), but their sequences are not available to determine the STs. A variety of IncF blaKPC-2-carrying plasmids have also been seen in the complex (34, 244, 313), but it appears that no particular IncF plasmids dominate (Table 9). Other blaKPC-2-carrying plasmids belong to IncHI1, IncP6 (96, 119, 390), IncW (156), or IncX3 (390). There were four blaKPC-3-carrying plasmids of either IncF or IncN in the United States in 2009 (190), Spain in 2016 to 2017 (96), and Switzerland in 2017 (166). blaGES-5 was found on two IncQ plasmids, one of which (pJF707; accession no. KX946994) has also been found to be widespread across other species and genera of the Enterobacteriaceae in multiple hospitals in the United Kingdom (391).
TABLE 9.
Plasmids carrying carbapenemase genes in the K. oxytoca complexa
| Plasmidb | Gene | Replicon typec | Country | Yr | Host speciesd | Accession no. and/or reference |
|---|---|---|---|---|---|---|
| pJF-789 | bla GES-5 | Q | NA | NA | Ko | KX912254 |
| pJF-707 | bla GES-5 | Q | UK | 2014–2016 | Ko | KX946994 (391) |
| pKPC-4b66 | bla KPC-2 | FIA(HI1), N (ST6) | USA | 2015 | Km | CP026274 |
| pK516_KPC | bla KPC-2 | FIA, FII(p14) | China | 2016 | Km | CP022349 (244) |
| pK518_KPC | bla KPC-2 | FIA, FII(p14) | China | 2017 | Km | CP023186 |
| pKPC2_020121 | bla KPC-2 | FII(p14), FII(Yp) | China | 2017 | Kg | MH192342 (34) |
| pKPC-f607 | bla KPC-2 | FII(pKP91) | USA | 2015 | Km | CP026272 |
| pKPC-55bf | bla KPC-2 | FII(pKP91) | USA | 2015 | Km | CP026280 |
| pKPC-727 | bla KPC-2 | FII(pKP91), FII(Yp) | USA | 2012 | Km | CP008791 |
| pKPC_CAV1099 | bla KPC-2 | FII(S), FII(SARC14) | USA | 2009 | Ko | CP011595 |
| pKPC_CAV1335 | bla KPC-2 | FII(S), FII(SARC14) | USA | 2010 | Ko | CP011615 |
| pKPC_UVA02 | bla KPC-2 | FII(S), FII(SARC14) | USA | 2007 | Ko | CP017929 (313) |
| — | bla KPC-2 | FIIK/FIB | Poland | 2008–2009 | Ko | 311 |
| pKPC_CAV1374 | bla KPC-2 | HI1A(NDM-CIT), HI1B(pNDM-CIT) | USA | 2010 | Km | CP011635 |
| pKO13459-1_KPC2 | bla KPC-2 | N (ST15) | Germany | 2017 | Km | VKMF01000347 |
| pKO17045_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNC01000072 |
| pKO16290_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKND01000090 |
| pKO16162_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNM01000153 |
| pKO14657_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNN01000158 |
| pKO14641_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNO01000140 |
| pKO13137_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNP01000116 |
| pKO13048_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNQ01000128 |
| pKO13047_KPC2 | bla KPC-2 | N (ST15) | Germany | 2014 | Km | VKNR01000115 |
| pKPC-8bc0 | bla KPC-2 | N (ST6) | USA | 2015 | Km | CP026277 |
| pKm38_N | bla KPC-2 | N (ST6) | USA | 1997 | Ko | KY128483 |
| pKm58_N | bla KPC-2 | N (ST6) | USA | 1997 | Km | KY128484 |
| — | bla KPC-2 | N | Germany | 2016 | Ko | 302 |
| (RK 171170-1) | bla KPC-2 | N | Germany | 2016 | Ko | 302 |
| (131SC04) | bla KPC-2 | N | Spain | 2013 | Ko | 390 |
| (YDC736-2) | bla KPC-2 | N | USA | 2015 | Km | 190 |
| (121SC72) | bla KPC-2 | P6 | Spain | 2012 | Ko | 390 |
| (121SC85) | bla KPC-2 | P6 | Spain | 2012 | Ko | 390 |
| (121SC07) | bla KPC-2 | P6 | Spain | 2012 | Ko | 390 |
| p5-KPC | bla KPC-2 | P6 | China | 2013 | Ko | KY913901 (119) |
| pKPC-cd17 | bla KPC-2 | P6 | Spain | 2016–2017 | Ko | CP026224 (96) |
| (KPN106) | bla KPC-2 | W | Brazil | 2008 | Ko | 156 |
| (131SC24) | bla KPC-2 | X3 | Spain | 2013 | Ko | 390 |
| pLSCH-KOX18040 | bla KPC-2 | NA | NA | NA | Ko | MN401418 |
| (8-CAR) | blaKPC-2, blaVIM-1 | N | Spain | 2014 | Ko | 299 |
| pCR14_3 | bla KPC-3 | FIB | Spain | 2016–2017 | Ko | CP015395 (96) |
| pKMISG1 | bla KPC-3 | FIB(pQil), FII(pKP91) | Switzerland | 2017 | Km | CM011641 (166) |
| unitig_2 | bla KPC-3 | FII(p14) | NA | NA | Km | CP020359 |
| (YD358) | bla KPC-3 | N | USA | 2009 | Km | 190 |
| p7121-IMP | bla IMP-1 | N (ST ua) | China | 2014 | Ko | KX784502 (395) |
| pKOI-34 | bla IMP-34 | M | Japan | NA | Ko | AB715422 (394) |
| p4-IPM | bla IMP-4 | N (ST20) | China | 2013 | Ko | KY913900 (119) |
| pC52_003 | bla IMP-4 | N (ST ua) | Australia | 2014 | Km | CP042548 |
| pKOX7525_1 | blaIMP-4, blaNDM-1 | FIA(HI1), R | China | 2020 | Km | CP065475 |
| (THC5) | bla IMP-6 | N | Japan | 2011 | Ko | 124 |
| pMRY14-187KOX_2 | bla IMP-6 | N (ST5), R | Japan | 2013 | Ko | AP019199–AP019209 (396) |
| pMRY14-192KOX_2 | bla IMP-6 | N (ST5), R | Japan | 2013 | Ko | AP019216–AP019229 (396) |
| pMRY14-247KOX_2 | bla IMP-6 | N (ST5), R | Japan | 2014 | Ko | AP019274–AP019290 (396) |
| (8-CAR) | blaKPC-2, blaVIM-1 | N | Spain | 2014 | Ko | 299 |
| (K310) | bla NDM | X3 | India | 2011–2013 | Ko | 303 |
| (K3682) | bla NDM | X3 | India | 2011–2013 | Ko | 303 |
| (K3739) | blaNDM, blaVIM | X3 | India | 2011–2013 | Ko | 303 |
| (IR5344) | bla NDM-1 | A/C | China | 2014 | Ko | 326 |
| unnamed5 (K9455) | bla NDM-1 | A/C2 | Spain | 2016–2017 | Ko | CP029118 (96) |
| (AMA942) | bla NDM-1 | A/C2 | Denmark | 2015 | Ko | 296 |
| pK516_NDM1 | bla NDM-1 | FIB(pB171), FII(Yp) | China | 2016 | Ko | CP022350 (244) |
| pK518_NDM1 | bla NDM-1 | FIB(pB171), FII(Yp) | China | 2017 | Km | CP023187 |
| pKOX_NDM1 | bla NDM-1 | FIB(pB171), FII(Yp) | China: Taiwan | 2010 | Ko | JQ314407 (323, 393) |
| pKOX_NDM | bla NDM-1 | FIB(pB171), FII(Yp) | Romania | 2013 | Km | MF042355 |
| pNDM-TJ11 | bla NDM-1 | FIB(pNDM-Mar), HI1B(pNDM-MAR) | NA | NA | Ko | MG845200 |
| (A105R1B5) | bla NDM-1 | X3 | South Africa | NA | Km | 32 |
| p3-NDM | bla NDM-1 | X3 | China | 2013 | Ko | KY913899 (119) |
| pK516_NDM5 | bla NDM-5 | X3 | China | 2016 | Ko | CP022351 (244) |
| pK518_NDM5 | bla NDM-5 | X3 | China | 2017 | Km | CP023188 |
| pKlox-45574cz | bla NDM-5 | X3 | NA | NA | Ko | MG833406 |
| pFDL-VIM | bla VIM-1 | A/C | Italy | 2019 | Ko | MN783744 (397) |
| pSE15-SA01028 | bla VIM-1 | HI2 | Spain | 2016–2017 | Ko | CP026661 (96) |
| pKp1050-3 | bla VIM-1 | L | Spain | 2016–2017 | Ko | CP023419 (96) |
| pKOX105(K9534) | bla VIM-1 | N (ST7) | Spain | 2016–2017 | Ko | NC_014208 (96) |
| pKOX105 | bla VIM-1 | N (ST7) | Italy | 2008 | Ko | HM126016 (319) |
| (E912) | bla VIM-1 | W | Greece | 2007 | Ko | 398 |
| — | bla VIM-4 | A/C | Italy | 2013 | Ko | 121 |
| — | bla OXA-48 | L | Tunisia | 2013–2016 | Ko | 104 |
| — | bla OXA-48 | L | Morocco | 2010 | Ko | 120 |
| p211DT2019_2 | bla OXA-48 | L | NA | 2019 | Km | JACYGO010000003 |
| pA6sk3_4 | bla OXA-48 | L | NA | 2019 | Km | JACYGP010000006 |
| — | bla OXA-48 | L | NA | NA | Ko | MK249860 |
| pKp_Goe_414-5 | bla OXA-48 | L | Spain | 2016–2017 | Ko | CP018342 (96) |
| pKp_Goe_588-2 | bla OXA-48 | L | Spain | 2016–2017 | Ko | CP018694 (96) |
| pKPoxa-48N1 | bla OXA-48 | L | Germany | 2012 | Ko | NC_021488 (331) |
NA, not available.
Names in parentheses are host strains of unnamed plasmids. —, unnamed.
The sequence type of IncN plasmids is shown in parentheses. ua, novel, unassigned ST in the pMLST scheme (https://cge.cbs.dtu.dk/services/pMLST/) for IncN plasmids.
Kg, K. grimontii; Km, K. michiganensis; Ko, K. oxytoca.
Thirty-three plasmids carrying class B MBL genes in the K. oxytoca complex have been reported in the literature or have been deposited in NCBI (Table 9). In the complex, most blaNDM-carrying plasmids belong to IncX3 (32, 119, 244, 303), which is well known to mediate the inter- and intraspecies transfer of blaNDM genes in the Enterobacteriaceae (392). blaNDM-carrying plasmids of IncA/C (96, 296, 326), IncF (244, 323, 393), or IncHI have also been reported in the complex. blaIMP-carrying plasmids in the complex belong to IncF, IncHI, IncL/M (394), IncN (119, 124, 395, 396), and IncR (124, 396), with IncN plasmids being relatively common and generally also containing an additional IncR replicon. blaVIM-carrying plasmids belong to IncA/C (121, 397), IncHI (96), IncL (96), IncN (96, 319), and IncW (398).
A total of 8 blaOXA-48-carrying plasmids have been found in the complex (Table 9), all of which belong to IncL/M and were isolated in Europe (Germany and Spain) (96, 331) and North Africa (Morocco and Tunisia) (104, 120). No plasmid MLST scheme is available for IncL/M plasmids at present, and further analysis of these IncL/M blaOXA-48-carrying plasmids is warranted to investigate whether there is one or several common plasmids mediating the dissemination of blaOXA-48 across different geographic locations.
Plasmids Carrying mcr or Virulence Genes in the Complex
Among mcr genes identified in the complex, six plasmids, all of which carried mcr-9, have been found in the K. oxytoca complex and belong to either IncFIB(K) or IncHI (337, 338) (GenBank accession no. CP011596, CP011617, and CP017930), while an mcr-1-carrying plasmid has been identified in the complex but its plasmid replicon type is unknown (336). As for virulence, genes encoding tilivalline and tilimycin associated with AAHC are chromosomally located in the K. oxytoca complex.
CONCLUDING REMARKS
The K. oxytoca complex comprises 6 known species—K. oxytoca, K. michiganensis, K. grimontii, K. huaxiensis, K. pasteurii, and K. spallanzanii—and three new unnamed species. These species are closely related and are difficult, if not impossible, to differentiate on the basis of phenotypic characteristics. Precise species identification relies on genome sequencing and analysis. blaOXY is characteristic of and omnipresent in the K. oxytoca complex. The gene can be assigned to 12 genotypes, i.e., blaOXY-1 to blaOXY-12, the carriage of which corresponds to species designation within the complex. The K. oxytoca complex is part of the human commensal microflora in the skin, mouth, gut, and respiratory system and is also an important pathogen causing AAHC and a number of other infections, but it is much less prevalent than K. pneumoniae. Two cytotoxins, tilivalline and tilimycin, cause the pathological changes of AAHC. The K. oxytoca complex has also been responsible for many outbreaks of health care-associated infections worldwide, many of which likely stem from water sources, such as sinks and humidifiers. The clonal background of K. oxytoca clinical isolates remains poorly understood, but isolates of clonal complex 2 appear to be widely distributed and have been associated with carbapenem resistance and outbreaks. In the worldwide bacterial collection of SENTRY, the rates of nonsusceptibility of the 5,724 clinical isolates of the K. oxytoca complex to carbapenems, ceftriaxone, ciprofloxacin, colistin, and tigecycline were 1.8%, 12.5%, 7.1%, 0.8%, and 0.1%, respectively. The rates of nonsusceptibility to carbapenems and cephalosporins have increased during the past 7 years. In addition to the intrinsic blaOXY, a number of genes encoding β-lactamases with various hydrolysis spectra, including the carbapenemases GES-5, GIM, IMP, KPC, NDM, OXA-48, and VIM and ESBLs such as a few CTX-M variants and several TEM and SHV variants, have been found in the complex. blaKPC-2 appears to be the most common carbapenemase gene and is mainly seen on IncN or IncF plasmids. The likelihood of being well adapted to health care environments, the flexibility to acquire antimicrobial resistance, and the presence of diverse virulence genes may help the K. oxytoca complex to become a major threat to human health. If not carefully monitored, it could easily go on to impose much greater challenges for therapy and infection control in the future, akin to those currently presented by K. pneumoniae.
There are a number of notable research gaps in our knowledge of the K. oxytoca complex. First, the three novel species of the K. oxytoca complex defined here by genome-based analysis warrant further investigation using phenotypic methods to establish their species status and to propose appropriate species names under the current code for prokaryotes (25). Second, the clinical significance of each species of the K. oxytoca complex, including the colonization incidence in patients, their prevalence as pathogens in various infections, and the disease spectrum, manifestation, severity, and prognosis, remains largely unknown. In other words, whether precise species identification within the K. oxytoca complex has implications for patient treatment and prognosis prediction as well as epidemiological surveillance and infection control is yet to be elucidated. Unless such clinical significance of each species has been demonstrated, we believe that the precise species identification within the K. oxytoca complex is required for research purposes but may not be necessary for routine clinical practice at present. Third, virulence factors crucial to the K. oxytoca complex causing infections other than AAHC are largely not understood. Fourth, the ability of the K. oxytoca complex to survive and persist in health care environments, in particular water-containing ones such as sinks, needs to be fully characterized. Fifth, more surveillance of the antimicrobial susceptibility of the K. oxytoca complex clinical isolates in international or regional collections is required, in particular isolates from Africa, Asia, and South America, to provide a comprehensive view of the current status and changing trend of antimicrobial resistance. Sixth, the population structure and global epidemiology of the K. oxytoca complex isolates are understudied. Whether there are certain high-risk clones of the K. oxytoca complex mediating resistance to key antimicrobial agents, particularly carbapenems, across different geographic locations remains to be determined. Seventh, more studies of plasmids in the complex are needed to explore whether there are particular plasmids mediating the wide dissemination of important antimicrobial resistance genes, such as blaKPC-2.
ACKNOWLEDGMENTS
Our relevant work was supported by the DETECTIVE project funded by the National Natural Science Foundation of China (81861138055) and the Medical Research Council (MR/S013660/1) and by a grant from the West China Hospital of Sichuan University (1.3.5 project for disciplines of excellence, grant ZYYC08006).
We declare no conflict of interest.
Biographies

Jing Yang is a specialist in critical care medicine. She obtained her M.B.B.S. and Ph.D. from West China School of Medicine, Sichuan University (Chengdu, China). She received research training as a visiting fellow in the Department of Genetic Medicine, Weill Cornell Medical College (New York, USA) from 2013 to 2015. She completed her residency in critical care medicine and has been an intensivist at West China Hospital. Her research interests have been focused on the management of critically ill patients with serious infections, especially those caused by multidrug-resistant microorganisms. She started her postdoctoral training in Center of Infectious Disease in 2019 and is currently working on understanding the relationship between human gut microbiota and colonization/infection of carbapenem-resistant Gram-negative bacteria.

Haiyan Long is a physician and a postdoctoral fellow at West China Hospital, Sichuan University. She obtained her doctoral and master degrees from Sichuan University and her M.B.B.S. from Hebei Medical University, China. She received a Chinese government scholarship from the China Scholarship Council to study at the University of Birmingham, UK, as a joint Ph.D. student for one year. Her Ph.D. thesis was about the molecular epidemiology and dissemination mechanisms of carbapenem-resistant Escherichia coli, and she is currently interested in antiplasmid treatment and plasmid curing.

Ya Hu obtained her medical doctor’s degree from Sichuan University in 2020 and her M.B.B.S. from Sichuan University in 2016. She was funded by China Scholarship Council in 2018 and proceeded with one year of training in the field of bioinformatics from the University of Birmingham. She has been working at the Center of Infectious Diseases, West China Hospital, Sichuan University, as a physician since September 2020. She has also been working as a postdoctoral fellow since November 2020, and her research interests are antimicrobial resistance mechanisms and drug resistance plasmids in Enterobacteriaceae.

Yu Feng has been working at Center of Infectious Diseases, West China Hospital of Sichuan University, as a research assistant since 2014, received four-year training in the fields of microbiology, genetics, and biochemistry from the University of Adelaide, Australia, and was awarded the Honours Degree of Bachelor of Science with First Class Honours at the end of 2013. As a researcher in the hospital, he is interested in dealing with clinical isolates, especially multidrug-resistant strains of the family Enterobacteriaceae, focusing on epidemiology status and resistance mechanisms. In addition, he has gained valuable and practical knowledge of bioinformatics from a variety of training programs as well as long-term working experience and is currently responsible for in silico works of the laboratory.

Alan McNally is Director of the Institute of Microbiology and Infection in the College of Medical and Dental Sciences at University of Birmingham. Dr. McNally studied for a B.Sc. in molecular microbiology at University of Glasgow before moving to University of Edinburgh to undertake a Ph.D. in virulence gene regulation. Postdoctoral positions at University of Bristol and then the Veterinary Laboratories Agency in Surrey, UK, followed, working on subjects including bacterial pathogenesis and avian influenza virus molecular epidemiology. This led to an academic position at Nottingham Trent University, first as a senior lecturer and then reader, where he developed his research expertise in microbial genomics and evolution. Dr. McNally moved to University of Birmingham in 2016 and was promoted to Director of the Institute of Microbiology in 2018. He now combines his background in classical microbiology with his expertise in genomics gained over the last 12 years to study the evolution of antimicrobial resistance.

Zhiyong Zong is a specialist in infectious diseases. He is Head of the Infection Control Department, Director of Center for Pathogen Research, and a professor at West China Hospital, Sichuan University (Chengdu, China). He obtained his medical M.B.B.S. degree from West China University for Medical Sciences (Chengdu, China) and completed his medical training at West China Hospital. He obtained his Ph.D. from the University of Sydney (Sydney, Australia). He received the Newtown Advanced Fellowship from the Royal Society (UK) and was an International Ambassador of the Society for Hospital Epidemiology of America (SHEA). His main research interests are the epidemiology and mechanisms of antimicrobial resistance in the Enterobacteriaceae and the prevention and control of multidrug-resistant organisms.
REFERENCES
- 1.Evans HJ, Campbell NE, Hill S. 1972. Asymbiotic nitrogen-fixing bacteria from the surfaces of nodules and roots of legumes. Can J Microbiol 18:13–21. 10.1139/m72-003. [DOI] [PubMed] [Google Scholar]
- 2.Podschun R, Pietsch S, Holler C, Ullmann U. 2001. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl Environ Microbiol 67:3325–3327. 10.1128/AEM.67.7.3325-3327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley ST. 1985. Habitat association of Klebsiella species. Infect Control 6:52–58. 10.1017/s0195941700062603. [DOI] [PubMed] [Google Scholar]
- 4.Savino F, Cordisco L, Tarasco V, Calabrese R, Palumeri E, Matteuzzi D. 2009. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr 98:1582–1588. 10.1111/j.1651-2227.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- 5.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogenauer C, Langner C, Beubler E, Lippe IT, Schicho R, Gorkiewicz G, Krause R, Gerstgrasser N, Krejs GJ, Hinterleitner TA. 2006. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 355:2418–2426. 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 7.Neog N, Phukan U, Puzari M, Sharma M, Chetia P. 2021. Klebsiella oxytoca and emerging nosocomial infections. Curr Microbiol 78:1115–1123. 10.1007/s00284-021-02402-2. [DOI] [PubMed] [Google Scholar]
- 8.Moradigaravand D, Martin V, Peacock SJ, Parkhill J. 2017. Population structure of multidrug resistant Klebsiella oxytoca within hospitals across the UK and Ireland identifies sharing of virulence and resistance genes with K. pneumoniae. Genome Biol Evol 9:574–587. 10.1093/gbe/evx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisse S, Grimont F, Grimont PAD. 2006. The Genus Klebsiella, p 159–196. In Martin D, Stanley F, Eugene R, Karl-Heinz S, Erko S (ed), The prokaryotes: a handbook on the biology of bacteria, vol 6. Proteobacteria: Gamma subclass. Springer, New York, NY. [Google Scholar]
- 10.Jain K, Radsak K, Mannheim W. 1974. Differentiation of the oxytocum group from Klebsiella by deoxyribonucleic acid-deoxyribonucleic acid hybridization. Int J Syst Bacteriol 24:402–407. 10.1099/00207713-24-4-402. [DOI] [Google Scholar]
- 11.Fevre C, Jbel M, Passet V, Weill FX, Grimont PA, Brisse S. 2005. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob Agents Chemother 49:3453–3462. 10.1128/AAC.49.8.3453-3462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merla C, Rodrigues C, Passet V, Corbella M, Thorpe HA, Kallonen TVS, Zong Z, Marone P, Bandi C, Sassera D, Corander J, Feil EJ, Brisse S. 2019. Description of Klebsiella spallanzanii sp. nov. and of Klebsiella pasteurii sp. nov. Front Microbiol 10:2360. 10.3389/fmicb.2019.02360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izdebski R, Fiett J, Urbanowicz P, Baraniak A, Derde LP, Bonten MJ, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Brisse S, Gniadkowski M, MOSAR WP2 WP3 and WP5 Study Groups . 2015. Phylogenetic lineages, clones and β-lactamases in an international collection of Klebsiella oxytoca isolates non-susceptible to expanded-spectrum cephalosporins. J Antimicrob Chemother 70:3230–3237. [DOI] [PubMed] [Google Scholar]
- 14.Saha R, Farrance CE, Verghese B, Hong S, Donofrio RS. 2013. Klebsiella michiganensis sp. nov., a new bacterium isolated from a tooth brush holder. Curr Microbiol 66:72–78. 10.1007/s00284-012-0245-x. [DOI] [PubMed] [Google Scholar]
- 15.Kovtunovych G, Lytvynenko T, Negrutska V, Lar O, Brisse S, Kozyrovska N. 2003. Identification of Klebsiella oxytoca using a specific PCR assay targeting the polygalacturonase pehX gene. Res Microbiol 154:587–592. 10.1016/S0923-2508(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 16.Validi M, Soltan-Dallal MM, Douraghi M, Fallah-Mehrabadi J, Rahimi-Foroushani A, Frohesh-Tehrani H. 2017. Identification of cytotoxin-producing Klebsiella oxytoca strains isolated from clinical samples with cell culture assays. Microb Pathog 113:1–4. 10.1016/j.micpath.2017.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Passet V, Brisse S. 2018. Description of Klebsiella grimontii sp. nov. Int J Syst Evol Microbiol 68:377–381. 10.1099/ijsem.0.002517. [DOI] [PubMed] [Google Scholar]
- 18.Brisse S, Verhoef J. 2001. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol 51:915–924. 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- 19.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Wei L, Feng Y, Xie Y, Zong Z. 2019. Klebsiella huaxiensis sp. nov., recovered from human urine. Int J Syst Evol Microbiol 69:333–336. 10.1099/ijsem.0.003102. [DOI] [PubMed] [Google Scholar]
- 21.Richter M, Rossello-Mora R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106:19126–19131. 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosselló-Móra R, Amann R. 2015. Past and future species definitions for Bacteria and Archaea. Syst Appl Microbiol 38:209–216. 10.1016/j.syapm.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain C, Rodriguez RL, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker CT, Tindall TJ, Garrity GM. 2019. International code of nomenclature of prokaryotes. prokaryotic code (2008 revision). Int J Syst Evol Microbiol 69:S1–S111. [DOI] [PubMed] [Google Scholar]
- 26.Gujarati S, Chaudhari D, Hagir A, Khairnar M, Shouche Y, Rahi P. 2020. Klebsiella indica sp. nov., isolated from the surface of a tomato. Int J Syst Evol Microbiol 70:3278–3286. 10.1099/ijsem.0.004168. [DOI] [PubMed] [Google Scholar]
- 27.Alves MS, Dias RC, de Castro AC, Riley LW, Moreira BM. 2006. Identification of clinical isolates of indole-positive and indole-negative Klebsiella spp. J Clin Microbiol 44:3640–3646. 10.1128/JCM.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maslow JN, Brecher SM, Adams KS, Durbin A, Loring S, Arbeit RD. 1993. Relationship between indole production and differentiation of Klebsiella species: indole-positive and -negative isolates of Klebsiella determined to be clonal. J Clin Microbiol 31:2000–2003. 10.1128/jcm.31.8.2000-2003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakazaki R, Tamura K, Kosako Y, Yoshizaki E. 1989. Klebsiella ornithinolytica sp. nov., formerly known as ornithine-positive Klebsiella oxytoca. Curr Microbiol 18:201–206. 10.1007/BF01570291. [DOI] [Google Scholar]
- 30.Cheng VC, Yam WC, Tsang LL, Yau MC, Siu GK, Wong SC, Chan JF, To KK, Tse H, Hung IF, Tai JW, Ho PL, Yuen KY. 2012. Epidemiology of Klebsiella oxytoca-associated diarrhea detected by Simmons citrate agar supplemented with inositol, tryptophan, and bile salts. J Clin Microbiol 50:1571–1579. 10.1128/JCM.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang J, Tun HM, Mauroo NF, Ma AP, Chan SY, Leung FC. 2014. Complete genome sequence and comparative genome analysis of Klebsiella oxytoca HKOPL1 isolated from giant panda feces. BMC Res Notes 7:827. 10.1186/1756-0500-7-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Founou RC, Founou LL, Allam M, Ismail A, Essack SY. 2018. Genomic characterisation of Klebsiella michiganensis co-producing OXA-181 and NDM-1 carbapenemases isolated from a cancer patient in uMgungundlovu district, KwaZulu-Natal province, South Africa. S Afr Med J 109:7–8. 10.7196/SAMJ.2018.v109i1.13696. [DOI] [PubMed] [Google Scholar]
- 33.Minnan L, Jinli H, Xiaobin W, Huijuan X, Jinzao C, Chuannan L, Fengzhang Z, Liangshu X. 2005. Isolation and characterization of a high H2-producing strain Klebsiella oxytoca HP1 from a hot spring. Res Microbiol 156:76–81. 10.1016/j.resmic.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Feng Y, Hu Y, Kang M, Xie Y, Zong Z. 2018. Klebsiella grimontii, a new species acquired carbapenem resistance. Front Microbiol 9:2170. 10.3389/fmicb.2018.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izdebski R, Baraniak A, Zabicka D, Sekowska A, Gospodarek-Komkowska E, Hryniewicz W, Gniadkowski M. 2018. VIM/IMP carbapenemase-producing Enterobacteriaceae in Poland: epidemic Enterobacter hormaechei and Klebsiella oxytoca lineages. J Antimicrob Chemother 73:2675–2681. 10.1093/jac/dky257. [DOI] [PubMed] [Google Scholar]
- 36.Clark AE, Kaleta EJ, Arora A, Wolk DM. 2013. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26:547–603. 10.1128/CMR.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong E, de Jong AS, Smidts-van den Berg N, Rentenaar RJ. 2013. Differentiation of Raoultella ornithinolytica/planticola and Klebsiella oxytoca clinical isolates by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Diagn Microbiol Infect Dis 75:431–433. 10.1016/j.diagmicrobio.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Schmithausen RM, Sib E, Exner M, Hack S, Rosing C, Ciorba P, Bierbaum G, Savin M, Bloomfield SF, Kaase M, Jacobshagen A, Gemein S, Gebel J, Engelhart S, Exner D. 2019. The washing machine as a reservoir for transmission of extended-spectrum-β-lactamase (CTX-M-15)-producing Klebsiella oxytoca ST201 to newborns. Appl Environ Microbiol 85:e01435-19. 10.1128/AEM.01435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang KF, Wang WJ, Ji XJ. 2020. Draft genome sequence of tetracycline-resistant Klebsiella oxytoca CCTCC M207023 producing 2,3-butanediol isolated from China. J Glob Antimicrob Resist 20:160–162. 10.1016/j.jgar.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Chapman P, Forde BM, Roberts LW, Bergh H, Vesey D, Jennison AV, Moss S, Paterson DL, Beatson SA, Harris PNA. 2020. Genomic investigation reveals contaminated detergent as the source of an extended-spectrum-β-lactamase-producing Klebsiella michiganensis outbreak in a neonatal unit. J Clin Microbiol 58:e01980-19. 10.1128/JCM.01980-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane D. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, UK. [Google Scholar]
- 42.Hu Y, Feng Y, Zhang X, Zong Z. 2017. Acinetobacter defluvii sp. nov., recovered from hospital sewage. Int J Syst Evol Microbiol 67:1709–1713. 10.1099/ijsem.0.001847. [DOI] [PubMed] [Google Scholar]
- 43.Lee I, Chalita M, Ha SM, Na SI, Yoon SH, Chun J. 2017. ContEst16S: an algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int J Syst Evol Microbiol 67:2053–2057. 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 44.Stackebrandt E, Goebel BM. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Evol Microbiol 44:846–849. 10.1099/00207713-44-4-846. [DOI] [Google Scholar]
- 45.Edgar RC. 2018. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics 34:2371–2375. 10.1093/bioinformatics/bty113. [DOI] [PubMed] [Google Scholar]
- 46.Stojowska-Swędrzyńska K, Krawczyk B. 2016. A new assay for the simultaneous identification and differentiation of Klebsiella oxytoca strains. Appl Microbiol Biotechnol 100:10115–10123. 10.1007/s00253-016-7881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Validi M, Soltan Dallal MM, Douraghi M, Fallah Mehrabadi J, Rahimi Foroushani A. 2016. Identification of Klebsiella pneumoniae carbapenemase-producing Klebsiella oxytoca in clinical isolates in Tehran hospitals, Iran by chromogenic medium and molecular methods. Osong Public Health Res Perspect 7:301–306. 10.1016/j.phrp.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghasemian A, Mobarez AM, Peerayeh SN, Bezmin Abadi AT. 2019. The association of surface adhesin genes and the biofilm formation among Klebsiella oxytoca clinical isolates. New Microbes New Infect 27:36–39. 10.1016/j.nmni.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghasemian A, Mohabati Mobarez A, Najar Peerayeh S, Talebi Bezmin Abadi A, Khodaparast S, Mahmood SS. 2019. Expression of adhesin genes and biofilm formation among Klebsiella oxytoca clinical isolates from patients with antibiotic-associated haemorrhagic colitis. J Med Microbiol 68:978–985. 10.1099/jmm.0.000965. [DOI] [PubMed] [Google Scholar]
- 50.Ronning TG, Aas CG, Stoen R, Bergh K, Afset JE, Holte MS, Radtke A. 2019. Investigation of an outbreak caused by antibiotic-susceptible Klebsiella oxytoca in a neonatal intensive care unit in Norway. Acta Paediatr 108:76–82. 10.1111/apa.14584. [DOI] [PubMed] [Google Scholar]
- 51.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, Brisse S, Cao H, Wilksch J, Gorrie C, Schultz MB, Edwards DJ, Nguyen KV, Nguyen TV, Dao TT, Mensink M, Minh VL, Nhu NT, Schultsz C, Kuntaman K, Newton PN, Moore CE, Strugnell RA, Thomson NR. 2015. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA 112:E3574-81. 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong MH, Chan EW, Chen S. 2015. Evolution and dissemination of OqxAB-like efflux pumps, an emerging quinolone resistance determinant among members of Enterobacteriaceae. Antimicrob Agents Chemother 59:3290–3297. 10.1128/AAC.00310-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konstantinidis KT, Tiedje JM. 2005. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci USA 102:2567–2572. 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zong Z. 2020. Genome-based taxonomy for bacteria: a recent advance. Trends Microbiol 28:871–874. 10.1016/j.tim.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Parks DH, Chuvochina M, Chaumeil PA, Rinke C, Mussig AJ, Hugenholtz P. 2020. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat Biotechnol 38:1079–1086. 10.1038/s41587-020-0501-8. [DOI] [PubMed] [Google Scholar]
- 56.Ciufo S, Kannan S, Sharma S, Badretdin A, Clark K, Turner S, Brover S, Schoch CL, Kimchi A, DiCuccio M. 2018. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int J Syst Evol Microbiol 68:2386–2392. 10.1099/ijsem.0.002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker JL, Hendrickson EL, Tang X, Lux R, He X, Edlund A, McLean JS, Shi W. 2019. Klebsiella and Providencia emerge as lone survivors following long-term starvation of oral microbiota. Proc Natl Acad Sci USA 116:8499–8504. 10.1073/pnas.1820594116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netsvyetayeva I, Marusza W, Olszanski R, Szyller K, Krolak-Ulinska A, Swoboda-Kopec E, Sierdzinski J, Szymonski Z, Mlynarczyk G. 2018. Skin bacterial flora as a potential risk factor predisposing to late bacterial infection after cross-linked hyaluronic acid gel augmentation. Infect Drug Resist 11:213–222. 10.2147/IDR.S154328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouchillon SK, Badal RE, Hoban DJ, Hawser SP. 2013. Antimicrobial susceptibility of inpatient urinary tract isolates of Gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009–2011. Clin Ther 35:872–877. 10.1016/j.clinthera.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Mineau S, Kozak R, Kissoon M, Paterson A, Oppedisano A, Douri F, Gogan K, Willey BM, McGeer A, Poutanen SM. 2018. Emerging antimicrobial resistance among Escherichia coli strains in bloodstream infections in Toronto, 2006–2016: a retrospective cohort study. CMAJ Open 6:E580–E586. 10.9778/cmajo.20180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoban DJ, Badal R, Bouchillon S, Hackel M, Kazmierczak K, Lascols C, Hawser S. 2014. In vitro susceptibility and distribution of β-lactamases in Enterobacteriaceae causing intra-abdominal infections in North America 2010–2011. Diagn Microbiol Infect Dis 79:367–372. 10.1016/j.diagmicrobio.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 62.Kim BN, Ryu J, Kim YS, Woo JH. 2002. Retrospective analysis of clinical and microbiological aspects of Klebsiella oxytoca bacteremia over a 10-year period. Eur J Clin Microbiol Infect Dis 21:419–426. 10.1007/s10096-002-0738-9. [DOI] [PubMed] [Google Scholar]
- 63.Lin RD, Hsueh PR, Chang SC, Chen YC, Hsieh WC, Luh KT. 1997. Bacteremia due to Klebsiella oxytoca: clinical features of patients and antimicrobial susceptibilities of the isolates. Clin Infect Dis 24:1217–1222. 10.1086/513637. [DOI] [PubMed] [Google Scholar]
- 64.Reiss I, Borkhardt A, Fussle R, Sziegoleit A, Gortner L. 2000. Disinfectant contaminated with Klebsiella oxytoca as a source of sepsis in babies. Lancet 356:310. 10.1016/S0140-6736(00)02509-5. [DOI] [PubMed] [Google Scholar]
- 65.Xu L, Sun X, Ma X. 2017. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 16:18. 10.1186/s12941-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beaugerie L, Metz M, Barbut F, Bellaiche G, Bouhnik Y, Raskine L, Nicolas JC, Chatelet FP, Lehn N, Petit JC, Infectious Colitis Study Group . 2003. Klebsiella oxytoca as an agent of antibiotic-associated hemorrhagic colitis. Clin Gastroenterol Hepatol 1:370–376. 10.1053/S1542-3565(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 67.Conlan S, Kong HH, Segre JA. 2012. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS One 7:e47075. 10.1371/journal.pone.0047075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin RM, Cao J, Brisse S, Passet V, Wu W, Zhao L, Malani PN, Rao K, Bachman MA. 2016. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1:e00261-16. 10.1128/mSphere.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin YT, Siu LK, Lin JC, Chen TL, Tseng CP, Yeh KM, Chang FY, Fung CP. 2012. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 12:13. 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zollner-Schwetz I, Herzog KA, Feierl G, Leitner E, Schneditz G, Sprenger H, Prattes J, Petritsch W, Wenzl H, Kump P, Gorkiewicz G, Zechner E, Hogenauer C. 2015. The toxin-producing pathobiont Klebsiella oxytoca is not associated with flares of inflammatory bowel diseases. Dig Dis Sci 60:3393–3398. 10.1007/s10620-015-3765-y. [DOI] [PubMed] [Google Scholar]
- 71.Lee KM, Morris-Love J, Cabral DJ, Belenky P, Opal SM, Jamieson AM. 2018. Coinfection with influenza A virus and Klebsiella oxytoca: an underrecognized impact on host resistance and tolerance to pulmonary infections. Front Immunol 9:2377. 10.3389/fimmu.2018.02377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baier C, Pirr S, Ziesing S, Ebadi E, Hansen G, Bohnhorst B, Bange FC. 2019. Prospective surveillance of bacterial colonization and primary sepsis: findings of a tertiary neonatal intensive and intermediate care unit. J Hosp Infect 102:325–331. 10.1016/j.jhin.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 73.Strenger V, Feierl G, Resch B, Zarfel G, Grisold A, Masoud-Landgraf L, Dosch V, Riedl R, Zenz W, Muller W, Urlesberger B. 2013. Fecal carriage and intrafamilial spread of extended-spectrum β-lactamase-producing Enterobacteriaceae following colonization at the neonatal ICU. Pediatr Crit Care Med 14:157–163. 10.1097/PCC.0b013e31824ea2a2. [DOI] [PubMed] [Google Scholar]
- 74.Mwandigha AM, Kamori D, Kibwana UO, Masoud S, Manyahi J, Majigo M. 2020. Fecal carriage and factors associated with extended-spectrum β-lactamase-producing Enterobacteriaceae among pregnant women at the tertiary referral hospital, Tanzania. Trop Med Health 48:84. 10.1186/s41182-020-00271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eser F, Yılmaz GR, Güner R, Hasanoğlu İ, Ürkmez Korkmaz FY, Açıkgöz ZC, Taşyaran MA. 2019. Risk factors for rectal colonization of carbapenem-resistant Enterobacteriaceae in a tertiary care hospital: a case-control study from Turkey. Turk J Med Sci 49:341–346. 10.3906/sag-1810-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alikhani MY, Shahcheraghi F, Khodaparast S, Mozaffari Nejad AS, Moghadam MK, Mousavi SF. 2016. Molecular characterisation of Klebsiella oxytoca strains isolated from patients with antibiotic-associated diarrhoea. Arab J Gastroenterol 17:95–101. 10.1016/j.ajg.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Koga H, Aoyagi K, Yoshimura R, Kimura Y, Iida M, Fujishima M. 1999. Can quinolones cause hemorrhagic colitis of late onset? Report of three cases. Dis Colon Rectum 42:1502–1504. 10.1007/BF02235056. [DOI] [PubMed] [Google Scholar]
- 78.Sakurai Y, Tsuchiya H, Ikegami F, Funatomi T, Takasu S, Uchikoshi T. 1979. Acute right-sided hemorrhagic colitis associated with oral administration of ampicillin. Dig Dis Sci 24:910–915. 10.1007/BF01311944. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka K, Fujiya M, Sakatani A, Fujibayashi S, Nomura Y, Ueno N, Kashima S, Goto T, Sasajima J, Moriichi K, Okumura T. 2017. Second-line therapy for Helicobacter pylori eradication causing antibiotic-associated hemorrhagic colitis. Ann Clin Microbiol Antimicrob 16:54. 10.1186/s12941-017-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toffler RB, Pingoud EG, Burrell MI. 1978. Acute colitis related to penicillin and penicillin derivatives. Lancet ii:707–709. 10.1016/s0140-6736(78)92704-6. [DOI] [PubMed] [Google Scholar]
- 81.Zollner-Schwetz I, Hogenauer C, Joainig M, Weberhofer P, Gorkiewicz G, Valentin T, Hinterleitner TA, Krause R. 2008. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis 47:e74–e78. 10.1086/592074. [DOI] [PubMed] [Google Scholar]
- 82.Kato S, Ebina K, Ozawa A, Naganuma H, Nakagawa H. 1995. Antibiotic-associated hemorrhagic colitis without Clostridium difficile toxin in children. J Pediatr 126:1008–1010. 10.1016/S0022-3476(95)70235-0. [DOI] [PubMed] [Google Scholar]
- 83.Stampfer L, Deutschmann A, Dür E, Eitelberger FG, Fürpass T, Gorkiewicz G, Heinz-Erian P, Heller I, Herzog K, Hopfer B, Kerbl R, Klug E, Krause R, Leitner E, Mache C, Müller T, Pansy J, Pocivalnik M, Scheuba E, Schneditz G, Schweintzger G, Sterniczky E, Zechner E, Hauer AC, Högenauer C, Hoffmann KM. 2017. Causes of hematochezia and hemorrhagic antibiotic-associated colitis in children and adolescents. Medicine (Baltimore) 96:e7793. 10.1097/MD.0000000000007793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shinjoh M, Iwata S, Takahashi T. 2010. Klebsiella oxytoca-positive, penicillin-associated hemorrhagic enterocolitis in children. Pediatr Int 52:132–133. 10.1111/j.1442-200X.2009.02949.x. [DOI] [PubMed] [Google Scholar]
- 85.Youn Y, Lee SW, Cho HH, Park S, Chung HS, Seo JW. 2018. Antibiotics-associated hemorrhagic colitis caused by Klebsiella oxytoca: two case reports. Pediatr Gastroenterol Hepatol Nutr 21:141–146. 10.5223/pghn.2018.21.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann KM, Deutschmann A, Weitzer C, Joainig M, Zechner E, Hogenauer C, Hauer AC. 2010. Antibiotic-associated hemorrhagic colitis caused by cytotoxin-producing Klebsiella oxytoca. Pediatrics 125:e960–e963. 10.1542/peds.2009-1751. [DOI] [PubMed] [Google Scholar]
- 87.Miyauchi R, Kinoshita K, Tokuda Y. 2013. Clarithromycin-induced haemorrhagic colitis. BMJ Case Rep 2013:bcr2013009984. 10.1136/bcr-2013-009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Philbrick AM, Ernst ME. 2007. Amoxicillin-associated hemorrhagic colitis in the presence of Klebsiella oxytoca. Pharmacotherapy 27:1603–1607. 10.1592/phco.27.11.1603. [DOI] [PubMed] [Google Scholar]
- 89.Yamada M, Yamazawa K, Sekiguchi S, Shinjoh M, Tomita K, Takenouchi T, Takahashi T. 2014. A pediatric case of antibiotic-associated hemorrhagic colitis caused by Klebsiella oxytoca. Glob Pediatr Health 1:2333794x14550525. 10.1177/2333794X14550525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akanbi O, Saleem N, Soliman M, Pannu BS. 2017. Antibiotic-associated haemorrhagic colitis: not always Clostridium difficile. BMJ Case Rep 2017:bcr2017219915. 10.1136/bcr-2017-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gandhi S, Sohal S, Patel D. 2019. A case of recurring diarrhea in a young woman. Gastroenterology 156:883–884. 10.1053/j.gastro.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 92.Fisher A, Halalau A. 2018. A case report and literature review of Clostridium difficile negative antibiotic associated hemorrhagic colitis caused by Klebsiella oxytoca. Case Rep Gastrointest Med 2018:7264613. 10.1155/2018/7264613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yilmaz M, Bilir YA, Aygun G, Erzin Y, Ozturk R, Celik AF. 2012. Prospective observational study on antibiotic-associated bloody diarrhea: report of 21 cases with a long-term follow-up from Turkey. Eur J Gastroenterol Hepatol 24:688–694. 10.1097/MEG.0b013e328352721a. [DOI] [PubMed] [Google Scholar]
- 94.Joainig MM, Gorkiewicz G, Leitner E, Weberhofer P, Zollner-Schwetz I, Lippe I, Feierl G, Krause R, Hinterleitner T, Zechner EL, Hogenauer C. 2010. Cytotoxic effects of Klebsiella oxytoca strains isolated from patients with antibiotic-associated hemorrhagic colitis or other diseases caused by infections and from healthy subjects. J Clin Microbiol 48:817–824. 10.1128/JCM.01741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azer SA, Tuma F. 2021. Infectious colitis. StatPearls Publishing LLC., Treasure Island, FL, USA. [PubMed] [Google Scholar]
- 96.Perez-Vazquez M, Oteo-Iglesias J, Sola-Campoy PJ, Carrizo-Manzoni H, Bautista V, Lara N, Aracil B, Alhambra A, Martinez-Martinez L, Campos J, Spanish Antibiotic Resistance Surveillance Program Collaborating Group . 2019. Characterization of carbapenemase-producing Klebsiella oxytoca in Spain, 2016–2017. Antimicrob Agents Chemother 63:e02529-18. 10.1128/AAC.02529-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zarate MS, Gales AC, Picao RC, Pujol GS, Lanza A, Smayevsky J. 2008. Outbreak of OXY-2-producing Klebsiella oxytoca in a renal transplant unit. J Clin Microbiol 46:2099–2101. 10.1128/JCM.00194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vergara-Lopez S, Dominguez MC, Conejo MC, Pascual A, Rodriguez-Bano J. 2015. Lessons from an outbreak of metallo-β-lactamase-producing Klebsiella oxytoca in an intensive care unit: the importance of time at risk and combination therapy. J Hosp Infect 89:123–131. 10.1016/j.jhin.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Ishikawa K, Hamasuna R, Uehara S, Yasuda M, Yamamoto S, Hayami H, Takahashi S, Matsumoto T, Minamitani S, Kadota J-i, Iwata S, Kaku M, Watanabe A, Sunakawa K, Sato J, Hanaki H, Tsukamoto T, Kiyota H, Egawa S, Deguchi T, Matsumoto M, Tanaka K, Arakawa S, Fujisawa M, Kumon H, Kobayashi K, Matsubara A, Wakeda H, Amemoto Y, Onodera S, Goto H, Komeda H, Yamashita M, Takenaka T, Fujimoto Y, Tsugawa M, Takahashi Y, Maeda H, Onishi H, Ishitoya S, Nishimura K, Mitsumori K, Ito T, Togo Y, Nakamura I, Ito N, Kanamaru S, Hirose T, Muranaka T, Yamada D, et al. 2015. Japanese nationwide surveillance in 2011 of antibacterial susceptibility patterns of clinical isolates from complicated urinary tract infection cases. J Infect Chemother 21:623–633. 10.1016/j.jiac.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 100.Kehinde A, Adedapo K, Aimakhu C, Odukogbe AT, Olayemi O, Salako B. 2012. Urinary pathogens and drug susceptibility patterns of urinary tract infections among antenatal clinic attendees in Ibadan, Nigeria. J Obstet Gynaecol Res 38:280–284. 10.1111/j.1447-0756.2011.01635.x. [DOI] [PubMed] [Google Scholar]
- 101.Tebruegge M, Pantazidou A, Clifford V, Gonis G, Ritz N, Connell T, Curtis N. 2011. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One 6:e26576. 10.1371/journal.pone.0026576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghiro L, Cracco AT, Sartor M, Comacchio S, Zacchello G, Dall’Amico R, Veneto Urinary Tract Infection Study Group . 2002. Retrospective study of children with acute pyelonephritis. evaluation of bacterial etiology, antimicrobial susceptibility, drug management and imaging studies. Nephron 90:8–15. 10.1159/000046308. [DOI] [PubMed] [Google Scholar]
- 103.Ibrahimagic A, Bedenic B, Kamberovic F, Uzunovic S. 2015. High prevalence of CTX-M-15 and first report of CTX-M-3, CTX-M-22, CTX-M-28 and plasmid-mediated AmpC β-lactamase producing Enterobacteriaceae causing urinary tract infections in Bosnia and Herzegovina in hospital and community settings. J Infect Chemother 21:363–369. 10.1016/j.jiac.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Guzman-Puche J, Jenayeh R, Perez-Vazquez M, Manuel C, Asma F, Jalel B, Oteo-Iglesias J, Martinez-Martinez L. 2021. Characterization of OXA-48-producing Klebsiella oxytoca isolates from a hospital outbreak in Tunisia. J Glob Antimicrob Resist 24:306–310. 10.1016/j.jgar.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 105.Lowe C, Willey B, O'Shaughnessy A, Lee W, Lum M, Pike K, Larocque C, Dedier H, Dales L, Moore C, McGeer A, Mount Sinai Hospital Infection Control Team . 2012. Outbreak of extended-spectrum β-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis 18:1242–1247. 10.3201/eid1808.111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsakris A, Poulou A, Markou F, Pitiriga V, Piperaki ET, Kristo I, Pournaras S. 2011. Dissemination of clinical isolates of Klebsiella oxytoca harboring CMY-31, VIM-1, and a new OXY-2-type variant in the community. Antimicrob Agents Chemother 55:3164–3168. 10.1128/AAC.00102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horii T, Osaki M, Muramatsu H. 2008. Fluoroquinolone resistance in clinical isolates of Klebsiella oxytoca. Chemotherapy 54:323–327. 10.1159/000151266. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez S, Stinnett JA, Shell CG, Berk SL. 1985. Klebsiella oxytoca isolates in a general hospital. Infect Control 6:310–313. 10.1017/s0195941700063165. [DOI] [PubMed] [Google Scholar]
- 109.Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C, Agwu E, Bazira J. 2019. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi District, Uganda. Int J Microbiol 2019:4246780. 10.1155/2019/4246780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharara SL, Amoah J, Pana ZD, Simner PJ, Cosgrove SE, Tamma PD. 2020. Is piperacillin-tazobactam effective for the treatment of pyelonephritis caused by extended-spectrum β-lactamase-producing organisms? Clin Infect Dis 71:e331–e337. 10.1093/cid/ciz1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Subha A, Ananthan S, Alavandi SV. 2001. Extended-spectrum β-lactamase production and multidrug resistance in Klebsiella species isolated from children under five with intestinal and extraintestinal infections. Indian J Med Res 113:181–185. [PubMed] [Google Scholar]
- 112.Mohammed MA, Alnour TM, Shakurfo OM, Aburass MM. 2016. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac J Trop Med 9:771–776. 10.1016/j.apjtm.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 113.Shigemura K, Kitagawa K, Nomi M, Yanagiuchi A, Sengoku A, Fujisawa M. 2020. Risk factors for febrile genito-urinary infection in the catheterized patients by with spinal cord injury-associated chronic neurogenic lower urinary tract dysfunction evaluated by urodynamic study and cystography: a retrospective study. World J Urol 38:733–740. 10.1007/s00345-019-02743-5. [DOI] [PubMed] [Google Scholar]
- 114.Fujita A, Kimura K, Yokoyama S, Jin W, Wachino J, Yamada K, Suematsu H, Yamagishi Y, Mikamo H, Arakawa Y. 2015. Characterization of piperacillin/tazobactam-resistant Klebsiella oxytoca recovered from a nosocomial outbreak. PLoS One 10:e0142366. 10.1371/journal.pone.0142366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Hogenauer C, Sill H, Krause R, Zollner-Schwetz I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoenigl M, Valentin T, Zarfel G, Wuerstl B, Leitner E, Salzer HJ, Posch J, Krause R, Grisold AJ. 2012. Nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Klebsiella oxytoca in Austria. Antimicrob Agents Chemother 56:2158–2161. 10.1128/AAC.05440-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGee M, Brown A, Whelan C, Nicholson T. 2017. Unilateral hydronephrosis and urosepsis secondary to vaginal ring pessary. ANZ J Surg 87:E46–E47. 10.1111/ans.12960. [DOI] [PubMed] [Google Scholar]
- 118.Greer-Bayramoglu R, Matic DB, Kiaii B, Fortin AJ. 2008. Klebsiella oxytoca necrotizing fasciitis after orthotopic heart transplant. J Heart Lung Transplant 27:1265–1267. 10.1016/j.healun.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 119.Wang J, Yuan M, Chen H, Chen X, Jia Y, Zhu X, Bai L, Bai X, Fanning S, Lu J, Li J. 2017. First report of Klebsiella oxytoca strain simultaneously producing NDM-1, IMP-4, and KPC-2 carbapenemases. Antimicrob Agents Chemother 61:e00877017. 10.1128/AAC.00877-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hays C, Benouda A, Poirel L, Elouennass M, Nordmann P. 2012. Nosocomial occurrence of OXA-48-producing enterobacterial isolates in a Moroccan hospital. Int J Antimicrob Agents 39:545–547. 10.1016/j.ijantimicag.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 121.Caltagirone M, Bitar I, Piazza A, Spalla M, Nucleo E, Navarra A, Migliavacca R. 2015. Detection of an IncA/C plasmid encoding VIM-4 and CMY-4 β-lactamases in Klebsiella oxytoca and Citrobacter koseri from an inpatient in a cardiac rehabilitation unit. New Microbiol 38:387–392. [PubMed] [Google Scholar]
- 122.Tateno H, Yasuhara T, Sugano E, Tahara S, Ugajin K, Fukuchi K. 2014. Detection of metallo-β-lactamase genes in clinically isolated Klebsiella pneumoniae and Klebsiella oxytoca. Rinsho Byori 62:1191–1196. [PubMed] [Google Scholar]
- 123.Hagiya H, Ogawa H, Takahashi Y, Yamamoto A, Otsuka F. 2015. Klebsiella oxytoca-producing IMP-1 detected as the first strain of carbapenem-resistant Enterobacteriaceae in our hospital. Intern Med 54:2939–2941. 10.2169/internalmedicine.54.4965. [DOI] [PubMed] [Google Scholar]
- 124.Ohno Y, Nakamura A, Hashimoto E, Matsutani H, Abe N, Fukuda S, Hisashi K, Komatsu M, Nakamura F. 2017. Molecular epidemiology of carbapenemase-producing Enterobacteriaceae in a primary care hospital in Japan, 2010–2013. J Infect Chemother 23:224–229. 10.1016/j.jiac.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 125.Lee W, Park H, Lee G. 2016. A case of testicular infarction from the complications of Klebsiella oxytoca induced acute epididymitis. J Infect Chemother 22:254–256. 10.1016/j.jiac.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Osei Sekyere J, Amoako DG. 2017. Genomic and phenotypic characterisation of fluoroquinolone resistance mechanisms in Enterobacteriaceae in Durban, South Africa. PLoS One 12:e0178888. 10.1371/journal.pone.0178888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vergara-Lopez S, Dominguez MC, Conejo MC, Pascual A, Rodriguez-Bano J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–E498. 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 128.Aronsson B, Eriksson M, Herin P, Rylander M. 1991. Gentamicin-resistant Klebsiella spp. and Escherichia coli in a neonatal intensive care unit. Scand J Infect Dis 23:195–199. 10.3109/00365549109023400. [DOI] [PubMed] [Google Scholar]
- 129.Pai B, Shaw N, Högler W. 2012. Salt-losing crisis in infants-not always of adrenal origin. Eur J Pediatr 171:317–321. 10.1007/s00431-011-1541-3. [DOI] [PubMed] [Google Scholar]
- 130.Cheang HK, Rangecroft L, Plant ND, Morris AA. 1998. Hyperammonaemia due to Klebsiella infection in a neuropathic bladder. Pediatr Nephrol 12:658–659. 10.1007/s004670050523. [DOI] [PubMed] [Google Scholar]
- 131.Vaidyanathan S, Mansour P, Soni BM, Hughes PL, Singh G, Oo T. 2009. Marked hydronephrosis and hydroureter after distigmine therapy in an adult male patient with paraplegia due to spinal cord injury: a case report. Cases J 2:7333. 10.4076/1757-1626-2-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abo-Salem E, Phy M. 2011. Air in the urinary system. Am J Med Sci 341:67. 10.1097/MAJ.0b013e3181cc7634. [DOI] [PubMed] [Google Scholar]
- 133.Watanakunakorn C. 1985. Klebsiella oxytoca endocarditis after transurethral resection of the prostate gland. South Med J 78:356–357. 10.1097/00007611-198503000-00032. [DOI] [PubMed] [Google Scholar]
- 134.Akoachere JF, Yvonne S, Akum NH, Seraphine EN. 2012. Etiologic profile and antimicrobial susceptibility of community-acquired urinary tract infection in two Cameroonian towns. BMC Res Notes 5:219. 10.1186/1756-0500-5-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sibi G, Kumari P, Kabungulundabungi N. 2014. Antibiotic sensitivity pattern from pregnant women with urinary tract infection in Bangalore, India. Asian Pac J Trop Med 7S1:S116–S120. 10.1016/S1995-7645(14)60216-9. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Lopez JJ, Coelho A, Larrosa MN, Lavilla S, Bartolome R, Prats G. 2009. First detection of plasmid-encoded blaOXY β-lactamase. Antimicrob Agents Chemother 53:3143–3146. 10.1128/AAC.01473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ponce-de-Leon A, Rodríguez-Noriega E, Morfín-Otero R, Cornejo-Juárez DP, Tinoco JC, Martínez-Gamboa A, Gaona-Tapia CJ, Guerrero-Almeida ML, Martin-Onraët A, Vallejo Cervantes JL, Sifuentes-Osornio J. 2018. Antimicrobial susceptibility of Gram-negative bacilli isolated from intra-abdominal and urinary-tract infections in Mexico from 2009 to 2015: results from the study for monitoring antimicrobial resistance trends (SMART). PLoS One 13:e0198621. 10.1371/journal.pone.0198621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Q, Zhang H, Yu Y, Kong H, Duan Q, Wang Y, Zhang S, Sun Z, Liao K, Gu L, Jiang X, Wu A, Huang W, Shan B, Kang M, Hu F, Yu H, Zhang W, Xu Y. 2020. In vitro activity of imipenem/relebactam against Enterobacteriaceae isolates obtained from intra-abdominal, respiratory tract, and urinary tract infections in China: study for monitoring antimicrobial resistance trends (SMART), 2015–2018. Clin Infect Dis 71:s427–s435. 10.1093/cid/ciaa1519. [DOI] [PubMed] [Google Scholar]
- 139.Cantón R, Loza E, Aznar J, Castillo FJ, Cercenado E, Fraile-Ribot PA, González-Romo F, López-Hontangas JL, Rodríguez-Lozano J, Suárez-Barrenechea AI, Tubau F, Díaz-Regañón J, López-Mendoza D, SMART-Spain Working Group . 2019. Monitoring the antimicrobial susceptibility of Gram-negative organisms involved in intraabdominal and urinary tract infections recovered during the SMART study (Spain, 2016 and 2017). Rev Esp Quimioter 32:145–155. [PMC free article] [PubMed] [Google Scholar]
- 140.Karlowsky JA, Lob SH, Kazmierczak KM, Young K, Motyl MR, Sahm DF. 2020. In vitro activity of imipenem/relebactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples: SMART surveillance United States 2015–2017. J Glob Antimicrob Resist 21:223–228. 10.1016/j.jgar.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 141.Smith DA, Nehring SM. 2021. Bacteremia, StatPearls Publishing LLC, Treasure Island, FL, USA. [PubMed] [Google Scholar]
- 142.Cox ER, Nayak SU, Kuruppu JC. 2013. Klebsiella oxytoca bacteremia-causal relationship to symptomatic colitis? Int J Infect Dis 17:e472-3–e473. 10.1016/j.ijid.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 143.Tariq TM, Rasool E. 2016. Emerging trends of bloodstream infections: a six-year study at a paediatric tertiary care hospital in Kabul. J Coll Physicians Surg Pak 26:887–891. [PubMed] [Google Scholar]
- 144.Korvick JA, Bryan CS, Farber B, Beam TR, Jr, Schenfeld L, Muder RR, Weinbaum D, Lumish R, Gerding DN, Wagener MM. 1992. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 36:2639–2644. 10.1128/AAC.36.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beck-Sague CM, Jarvis WR. 1989. Epidemic bloodstream infections associated with pressure transducers: a persistent problem. Infect Control Hosp Epidemiol 10:54–59. 10.1086/645961. [DOI] [PubMed] [Google Scholar]
- 146.Smith MJ, Hart CA, Cooke KW. 1984. Gentamicin-resistant Klebsiella oxytoca on a special care baby unit. Lancet ii:586–587. 10.1016/s0140-6736(84)90812-2. [DOI] [PubMed] [Google Scholar]
- 147.Ayan M, Kuzucu C, Durmaz R, Aktas E, Cizmeci Z. 2003. Analysis of three outbreaks due to Klebsiella species in a neonatal intensive care unit. Infect Control Hosp Epidemiol 24:495–500. 10.1086/502245. [DOI] [PubMed] [Google Scholar]
- 148.Mauri D, Roumbkou S, Michalopoulou S, Tsali L, Spiliopoulou A, Panou C, Valachis A, Panagopoulos A, Polyzos NP. 2010. Port central venous catheters-associated bloodstream infection during outpatient-based chemotherapy. Med Oncol 27:1309–1313. 10.1007/s12032-009-9380-z. [DOI] [PubMed] [Google Scholar]
- 149.Sardan YC, Zarakolu P, Altun B, Yildirim A, Yildirim G, Hascelik G, Uzun O. 2004. A cluster of nosocomial Klebsiella oxytoca bloodstream infections in a university hospital. Infect Control Hosp Epidemiol 25:878–882. 10.1086/502313. [DOI] [PubMed] [Google Scholar]
- 150.Conceicao T, Brizio A, Duarte A, Barros R. 2005. First isolation of blaVIM-2 in Klebsiella oxytoca clinical isolates from Portugal. Antimicrob Agents Chemother 49:476. 10.1128/AAC.49.1.476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tohamy ST, Aboshanab KM, El-Mahallawy HA, El-Ansary MR, Afifi SS. 2018. Prevalence of multidrug-resistant Gram-negative pathogens isolated from febrile neutropenic cancer patients with bloodstream infections in Egypt and new synergistic antibiotic combinations. Infect Drug Resist 11:791–803. 10.2147/IDR.S163293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hagiwara S, Murata M, Aoki M, Kaneko M, Oshima K. 2013. Septic shock caused by Klebsiella oxytoca: an autopsy case and a survival case with driving extracorporeal membrane oxygenation. Hippokratia 17:171–173. [PMC free article] [PubMed] [Google Scholar]
- 153.Watson JT, Jones RC, Siston AM, Fernandez JR, Martin K, Beck E, Sokalski S, Jensen BJ, Arduino MJ, Srinivasan A, Gerber SI. 2005. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch Intern Med 165:2639–2643. 10.1001/archinte.165.22.2639. [DOI] [PubMed] [Google Scholar]
- 154.Al-Anazi KA, Al-Jasser AM, Al-Zahrani HA, Chaudhri N, Al-Mohareb FI. 2008. Klebsiella oxytoca bacteremia causing septic shock in recipients of hematopoietic stem cell transplant: two case reports. Cases J 1:160. 10.1186/1757-1626-1-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egger M, Kurath S, Strenger V, Grisold A, Schlenke P, Rosskopf K, Krakowitzky P, Lackner H, Schwinger W, Urban C. 2017. Klebsiella oxytoca bacteremia induced septic shock following platelet transfusion. Klin Padiatr 229:304–305. 10.1055/s-0043-116850. [DOI] [PubMed] [Google Scholar]
- 156.Almeida AC, Cavalcanti FL, Martins WM, Vilela MA, Gales AC, Morais Junior MA, Morais MM. 2013. First description of KPC-2-producing Klebsiella oxytoca in Brazil. Antimicrob Agents Chemother 57:4077–4078. 10.1128/AAC.02376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eshetu B, Gashaw M, Berhane M, Abdissa A, McClure EM, Goldenberg RL, Muhe LM. 2019. Intravenous fluid contaminated with Klebsiella oxytoca as a source of sepsis in a preterm newborn: case report. Am J Infect Control 47:840–842. 10.1016/j.ajic.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 158.Simon M, Melzl H, Hiergeist A, Richert K, Falgenhauer L, Pfeifer Y, Gerlach RG, Fuchs K, Reischl U, Gessner A, Jantsch J. 2017. Colistin- and carbapenem-resistant Klebsiella oxytoca harboring blaVIM-2 and an insertion in the mgrB gene isolated from blood culture. Int J Med Microbiol 307:113–115. 10.1016/j.ijmm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 159.Disse SC, Meyer S, Baghai-Arassi A. 2018. Sepsis-associated purpura fulminans due to Klebsiella oxytoca. Dtsch Arztebl Int 115:784. 10.3238/arztebl.2018.0784a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gortani G, Gregori M, Giannotta A, Barbi E. 2013. A “shocking” appendicitis. Pediatr Emerg Care 29:233–234. 10.1097/PEC.0b013e318280d80c. [DOI] [PubMed] [Google Scholar]
- 161.Umenai T, Saitoh Y, Chiba M. 1978. Septicemia caused by Klebsiella oxytoca. Tohoku J Exp Med 124:393–394. 10.1620/tjem.124.393. [DOI] [PubMed] [Google Scholar]
- 162.Tsubouchi N, Tsurukiri J, Numata J, Sano H. 2019. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med 58:1801–1802. 10.2169/internalmedicine.2350-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adachi I, Matsumiya G, Sakaguchi T, Kuratani T, Miyagawa S, Yamauchi T, Saito S, Sawa Y. 2009. Ventricular assist device infection necessitating device exchange following extensive myocardial resection. J Artif Organs 12:271–273. 10.1007/s10047-009-0478-z. [DOI] [PubMed] [Google Scholar]
- 164.Venkatanarasimha N, Yong YR, Gogna A, Tan BS. 2019. Case 265: Lemmel syndrome or biliary obstruction due to a periampullary duodenal diverticulum. Radiology 291:542–545. 10.1148/radiol.2019162375. [DOI] [PubMed] [Google Scholar]
- 165.Vergara-López S, Domínguez MC, Conejo MC, Pascual Á, Rodríguez-Baño J. 2015. Prolonged treatment with large doses of fosfomycin plus vancomycin and amikacin in a case of bacteraemia due to methicillin-resistant Staphylococcus epidermidis and IMP-8 metallo-β-lactamase-producing Klebsiella oxytoca. J Antimicrob Chemother 70:313–315. 10.1093/jac/dku341. [DOI] [PubMed] [Google Scholar]
- 166.Seiffert SN, Wuthrich D, Gerth Y, Egli A, Kohler P, Nolte O. 2019. First clinical case of KPC-3-producing Klebsiella michiganensis in Europe. New Microbes New Infect 29:100516. 10.1016/j.nmni.2019.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Uz E, Özlem A, Şahin Balçık Ö, Kanbay M, Işık A, Uz B, Kaya A, Koşar A. 2010. Portal vein thrombosis secondary to Klebsiella oxytoca bacteriemia. Turk J Haematol 27:213–215. 10.5152/tjh.2010.33. [DOI] [PubMed] [Google Scholar]
- 168.Burket JS, Chenoweth CE, Meyer TL, Barg NL. 1999. Donor-to-recipient transmission of bacteria as an unusual cause of mediastinitis in a heart transplant recipient. Infect Control Hosp Epidemiol 20:132–133. 10.1086/501603. [DOI] [PubMed] [Google Scholar]
- 169.Yang S, Hemarajata P, Shevy L, Maciariello M, Culbreath K, Bush K, Humphries R. 2018. Unusual carbapenem resistant but ceftriaxone and cefepime susceptible Klebsiella oxytoca isolated from a blood culture: case report and whole-genome sequencing investigation. IDCases 11:9–11. 10.1016/j.idcr.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heda RP, Savage Y, Nair SP, Verma R. 2019. Enterococcus casseliflavus, Streptococcus equinus and Klebsiella oxytoca septicaemia associated with perinephric haematoma in a post-liver transplant patient with allograft cirrhosis. BMJ Case Rep 12:e230096. 10.1136/bcr-2019-230096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maalouf M, Moon W, Leers S, Papasavas PK, Birdas T, Caushaj PF. 2007. Mycotic aneurysm of the infrarenal aorta after drainage of an infected chronic pancreatic pseudocyst: case report and review of the literature. Am Surg 73:1266–1268. 10.1177/000313480707301216. [DOI] [PubMed] [Google Scholar]
- 172.Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A, Long SS. 2000. Predictors of infectious complications after burn injuries in children. Pediatr Infect Dis J 19:990–995. 10.1097/00006454-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 173.Ullah S, Elbita O, Abdelghany M, Tahir H, Tuli P, Alkilani WZ, Suri J. 2016. Klebsiella oxytoca endocarditis with complete heartblock. J Invest Med High Impact Case Rep 4:2324709616663232. 10.1177/2324709616663232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 175.Herruzo R, Ruiz G, Gallego S, Diez J, Sarria A, Omenaca F. 2017. VIM-Klebsiella oxytoca outbreak in a neonatal intensive care unit. This time it wasn't the drain. J Prev Med Hyg 58:E302–E307. 10.15167/2421-4248/jpmh2017.58.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Power JT, Calder MA. 1983. Pathogenic significance of Klebsiella oxytoca in acute respiratory tract infection. Thorax 38:205–208. 10.1136/thx.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sherchan JB, Humagain S. 2021. Antimicrobial susceptibility pattern of Gram-negative bacteria causing lower respiratory tract infections in Kathmandu university hospital. J Nepal Health Res Counc 18:661–666. 10.33314/jnhrc.v18i4.2566. [DOI] [PubMed] [Google Scholar]
- 178.De Simone C, Chiodo F, Delia S, Pastore G, Scalise G, Sorice F, Tonietti G, Zanussi C, Paoloni M, Gargiulo M. 1991. Clinical results of a multicenter study with sulbactam/ampicillin for the treatment of patients with lower respiratory and urinary tract infections. J Chemother 3:321–327. 10.1080/1120009x.1991.11739113. [DOI] [PubMed] [Google Scholar]
- 179.Jeong SH, Kim WM, Chang CL, Kim JM, Lee K, Chong Y, Hwang HY, Baek YW, Chung HK, Woo IG, Ku JY. 2001. Neonatal intensive care unit outbreak caused by a strain of Klebsiella oxytoca resistant to aztreonam due to overproduction of chromosomal β-lactamase. J Hosp Infect 48:281–288. 10.1053/jhin.2001.1018. [DOI] [PubMed] [Google Scholar]
- 180.Kim SB, Lee WY, Lee JH, Lee SJ, Lee MK, Kim SH, Uh Y, Jung SH, Shin B. 2020. A variety of bacterial aetiologies in the lower respiratory tract at patients with endobronchial tuberculosis. PLoS One 15:e0234558. 10.1371/journal.pone.0234558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Umgelter A, Prinz C, Gaa J, Huber W. 2007. Ascending pneumonia complicating endoscopic therapy of a pancreatic abscess. Endoscopy 39(Suppl 1):e267. 10.1055/s-2007-966606. [DOI] [PubMed] [Google Scholar]
- 182.Al-Moamary MS, Copland GM. 1998. Nonresolving pneumonia due to Klebsiella oxytoca: an unusual presentation. Clin Infect Dis 26:765–766. 10.1086/517122. [DOI] [PubMed] [Google Scholar]
- 183.Labrador I, Araque M. 2014. First description of KPC-2-producing Klebsiella oxytoca isolated from a pediatric patient with nosocomial pneumonia in Venezuela. Case Rep Infect Dis 2014:434987. 10.1155/2014/434987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gera K, Roshan R, Varma-Basil M, Shah A. 2015. Chronic pneumonia due to Klebsiella oxytoca mimicking pulmonary tuberculosis. Pneumonol Alergol Pol 83:383–386. 10.5603/PiAP.2015.0061. [DOI] [PubMed] [Google Scholar]
- 185.Conejo MC, Domínguez MC, López-Cerero L, Serrano L, Rodríguez-Baño J, Pascual A. 2010. Isolation of multidrug-resistant Klebsiella oxytoca carrying blaIMP-8, associated with OXY hyperproduction, in the intensive care unit of a community hospital in Spain. J Antimicrob Chemother 65:1071–1073. 10.1093/jac/dkq063. [DOI] [PubMed] [Google Scholar]
- 186.Deutsch RF, Ross JW, Nailor MD. 2015. Carbapenem-resistant Enterobacteriaceae: a case series of infections at Hartford hospital. Conn Med 79:269–275. [PubMed] [Google Scholar]
- 187.Mazuski JE, Solomkin JS. 2009. Intra-abdominal infections. Surg Clin North Am 89:421–437. 10.1016/j.suc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 188.Tubau F, Linares J, Rodriguez MD, Cercenado E, Aldea MJ, Gonzalez-Romo F, Torroba L, Berdonces P, Plazas J, Aguilar L, Delgado A, Garcia-Escribano N, Study Group . 2010. Susceptibility to tigecycline of isolates from samples collected in hospitalized patients with secondary peritonitis undergoing surgery. Diagn Microbiol Infect Dis 66:308–313. 10.1016/j.diagmicrobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 189.Fan S, Wang J, Li Y, Li J. 2017. Bacteriology and antimicrobial susceptibility of ESBLs producers from pus in patients with abdominal trauma associated intra-abdominal infections. Eur J Trauma Emerg Surg 43:65–71. 10.1007/s00068-016-0650-0. [DOI] [PubMed] [Google Scholar]
- 190.Hazen TH, Mettus R, McElheny CL, Bowler SL, Nagaraj S, Doi Y, Rasko DA. 2018. Diversity among blaKPC-containing plasmids in Escherichia coli and other bacterial species isolated from the same patients. Sci Rep 8:10291. 10.1038/s41598-018-28085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang SB, Yang WC, Chen TW, Lin CC. 2004. Klebsiella oxytoca and polymicrobial infection in peritoneal dialysis-related peritonitis. Perit Dial Int 24:196–197. 10.1177/089686080402400215. [DOI] [PubMed] [Google Scholar]
- 192.Lai CC, Lin TL, Tseng SP, Huang YT, Wang JT, Chang SC, Teng LJ, Wang JT, Hsueh PR. 2011. Pelvic abscess caused by New Delhi metallo-β-lactamase-1-producing Klebsiella oxytoca in Taiwan in a patient who underwent renal transplantation in China. Diagn Microbiol Infect Dis 71:474–475. 10.1016/j.diagmicrobio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 193.Alvarez F, Hadchouel M, Bernard O. 1982. Latent chronic cholangitis in congenital hepatic fibrosis. Eur J Pediatr 139:203–205. 10.1007/BF01377359. [DOI] [PubMed] [Google Scholar]
- 194.Paasch C, Wilczek S, Strik MW. 2017. Liver abscess and sepsis caused by Clostridium perfringens and Klebsiella oxytoca. Int J Surg Case Rep 41:180–183. 10.1016/j.ijscr.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Borbone S, Cascone C, Santagati M, Mezzatesta ML, Stefani S. 2006. Bactericidal activity of ertapenem against major intra-abdominal pathogens. Int J Antimicrob Agents 28:396–401. 10.1016/j.ijantimicag.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 196.Shima H, Mizoguchi S, Morine Y, Tashiro M, Okada K, Minakuchi J, Kawashima S. 2018. Intestinal perforation by a peritoneal dialysis catheter in which fungal peritonitis led to diagnosis: a rare case report. CEN Case Rep 7:208–210. 10.1007/s13730-018-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nagamura T, Tanaka Y, Terayama T, Higashiyama D, Seno S, Isoi N, Katsurada Y, Matsubara A, Yoshimura Y, Sekine Y, Akitomi S, Sato K, Tsuda H, Saitoh D, Ikeuchi H. 2019. Fulminant pseudomembranous enterocolitis caused by Klebsiella oxytoca: an autopsy case report. Acute Med Surg 6:78–82. 10.1002/ams2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. 2019. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: a case report. Medicine (Baltimore) 98:e14079. 10.1097/MD.0000000000014079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fujinaga T, Nishida T, Miyazaki M, Shigekawa M, Ikezawa K, Iwahashi K, Inoue T, Yamada T, Ezaki H, Shinzaki S, Yakushijin T, Iijima H, Tsujii M, Takehara T. 2013. Acute suppurative pancreatic ductitis associated with pancreatic duct obstruction. Endoscopy 45(Suppl 2 UCTN):E135. 10.1055/s-0032-1326450. [DOI] [PubMed] [Google Scholar]
- 200.Suzuki A, Matsunaga T, Aoki S, Hirayama T, Nakagawa N, Shibata K, Yabana T, Kawasaki H, Takasaka H, Sasaki K, Katsuramaki T, Mukaiya M, Hirata K, Imai K. 2002. A pancreatic abscess 7 years after a pancreatojejunostomy for calcifying chronic pancreatitis. J Gastroenterol 37:1062–1067. 10.1007/s005350200179. [DOI] [PubMed] [Google Scholar]
- 201.Nijhuis RH, Oueslati S, Zhou K, Bosboom RW, Rossen JW, Naas T. 2015. OXY-2–15, a novel variant showing increased ceftazidime hydrolytic activity. J Antimicrob Chemother 70:1429–1433. 10.1093/jac/dkv002. [DOI] [PubMed] [Google Scholar]
- 202.Meeuwes FO, Hukshorn CJ, Bloembergen P. 2015. Severe abdominal pain three weeks after a hemi-hepatectomy. Neth J Med 73:392–393. [PubMed] [Google Scholar]
- 203.Pascual J, Sureda A, Garcia-Hóz F, Erdozain JC, Perez-Hernandez F, Boixeda D. 1988. Spontaneous peritonitis due to Klebsiella oxytoca in a patient with cardiac ascites. Am J Gastroenterol 83:1313–1314. [PubMed] [Google Scholar]
- 204.Sarihan I, Demir E, Basaran S, Caliskan Y, Bozfakioglu S. 2017. Serratia marcescens, Morganella morganii, Klebsiella oxytoca related peritonitis attacks in a patient on automated peritoneal dialysis: a case report. Nefrologia 37:350–351. 10.1016/j.nefro.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 205.Surani A, Slama EM, Thomas S, Ross RW, Cunningham SC. 2020. Raoultella ornithinolytica and Klebsiella oxytoca pyogenic liver abscess presenting as chronic cough. IDCases 20:e00736. 10.1016/j.idcr.2020.e00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hanson B, Roat J, Pocha C. 2014. Cholecystitis and gallbladder perforation in cirrhotic patients: a clinical dilemma. Dig Liver Dis 46:960–961. 10.1016/j.dld.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 207.Harsch IA, Benninger J, Niedobitek G, Schindler G, Schneider HT, Hahn EG, Nusko G. 2001. Abdominal actinomycosis: complication of endoscopic stenting in chronic pancreatitis? Endoscopy 33:1065–1069. 10.1055/s-2001-18930. [DOI] [PubMed] [Google Scholar]
- 208.Kanjanauthai S, Kanluen T. 2008. Community-acquired Klebsiella oxytoca causing splenic abscess. Int J Infect Dis 12:448. 10.1016/j.ijid.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 209.Youssef D, Shams W, Kareem Abu Malouh A, Al-Abbadi MA. 2012. Chronic organizing retroperitoneal abscess caused by Klebsiella oxytoca masquerading as sarcoma: recognition by Diff-Quik stain on FNA material. Diagn Cytopathol 40:747–750. 10.1002/dc.21701. [DOI] [PubMed] [Google Scholar]
- 210.Sekowska A, Gospodarek E. 2010. Susceptibility of Klebsiella spp. to tigecycline and other selected antibiotics. Med Sci Monit 16:BR193–BR196. [PubMed] [Google Scholar]
- 211.Jayol A, Poirel L, Villegas MV, Nordmann P. 2015. Modulation of mgrB gene expression as a source of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents 46:108–110. 10.1016/j.ijantimicag.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 212.Cascio A, Cannavo SP, Guarneri C, Iaria C, Guarneri B. 2005. Klebsiella oxitoca folliculitis mimicking tinea barbae in a diabetic man. Int J Dermatol 44:588–589. 10.1111/j.1365-4632.2004.02193.x. [DOI] [PubMed] [Google Scholar]
- 213.Pérez-Llarena FJ, Fernández A, Zamorano L, Kerff F, Beceiro A, Aracil B, Cercenado E, Miro E, Oliver A, Oteo J, Navarro F, Bou G. 2012. Characterization of a novel IMP-28 metallo-β-lactamase from a Spanish Klebsiella oxytoca clinical isolate. Antimicrob Agents Chemother 56:4540–4543. 10.1128/AAC.00776-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Oishi H, Kagawa Y, Mitsumizo S, Tashiro Y, Kobayashi G, Udo K, Aoki S, Takayanagi M, Nagasawa Z, Araki K, Ohza N, Eguchi Y, Nakashima M. 2008. A fatal case of necrotizing fasciitis due to bacterial translocation of Klebsiella oxytoca. J Infect Chemother 14:62–65. 10.1007/s10156-007-0571-2. [DOI] [PubMed] [Google Scholar]
- 215.Lorenzini G, Picciotti M, Di Vece L, Pepponi E, Brindisi L, Vessio V, Maffei M, Viviano M. 2011. Cervical necrotizing fasciitis of odontogenic origin involving the temporal region—a case report. J Craniomaxillofac Surg 39:570–573. 10.1016/j.jcms.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 216.Prindaville B, Nopper AJ, Lawrence H, Horii KA. 2014. Chronic granulomatous disease presenting with ecthyma gangrenosum in a neonate. J Am Acad Dermatol 71:e44-5–e45. 10.1016/j.jaad.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 217.Sohn WI, Seo BF, Jung SN. 2012. Forehead abscess caused by Klebsiella oxytoca with undiagnosed type 2 diabetes. J Craniofac Surg 23:e247-9–e249. 10.1097/SCS.0b013e31824e6a5b. [DOI] [PubMed] [Google Scholar]
- 218.Sudy E. 2002. Pyoderma faciale: gram-negative recovery by means of needle aspiration. Cutis 69:261–264. [PubMed] [Google Scholar]
- 219.Shukla PC. 1994. Plantar cellulitis. Pediatr Emerg Care 10:23–25. 10.1097/00006565-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 220.Vali L, Dashti AA, El-Shazly S, Jadaon MM. 2015. Klebsiella oxytoca with reduced sensitivity to chlorhexidine isolated from a diabetic foot ulcer. Int J Infect Dis 34:112–116. 10.1016/j.ijid.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 221.Tang LM, Chen ST. 1995. Klebsiella oxytoca meningitis: frequent association with neurosurgical procedures. Infection 23:163–167. 10.1007/BF01793857. [DOI] [PubMed] [Google Scholar]
- 222.Carrie C, Walewski V, Levy C, Alexandre C, Baleine J, Charreton C, Coche-Monier B, Caeymaex L, Lageix F, Lorrot M, Klosowski S, Hess L, Zafer O, Gaudelus J, Pinquier D, Carbonnelle E, Cohen R, de Pontual L. 2019. Klebsiella pneumoniae and Klebsiella oxytoca meningitis in infants. Epidemiological and clinical features. Arch Pediatr 26:12–15. 10.1016/j.arcped.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 223.Chang WN, Lu CH, Huang CR, Chuang YC. 2000. Mixed infection in adult bacterial meningitis. Infection 28:8–12. 10.1007/s150100050003. [DOI] [PubMed] [Google Scholar]
- 224.Soto-Hernandez JL, Soto-Ramirez A, Perez-Neri I, Angeles-Morales V, Cardenas G, Barradas VA. 2020. Multidrug-resistant Klebsiella oxytoca ventriculitis, successfully treated with intraventricular tigecycline: a case report. Clin Neurol Neurosurg 188:105592. 10.1016/j.clineuro.2019.105592. [DOI] [PubMed] [Google Scholar]
- 225.Liliang PC, Lin YC, Su TM, Rau CS, Lu CH, Chang WN, Lee TC, Chen HJ. 2001. Klebsiella brain abscess in adults. Infection 29:81–86. 10.1007/s15010-001-0069-2. [DOI] [PubMed] [Google Scholar]
- 226.Rau CS, Chang WN, Lin YC, Lu CH, Liliang PC, Su TM, Tsai YD, Chang CJ, Lee PY, Lin MW, Cheng BC. 2002. Brain abscess caused by aerobic Gram-negative bacilli: clinical features and therapeutic outcomes. Clin Neurol Neurosurg 105:60–65. 10.1016/s0303-8467(02)00103-8. [DOI] [PubMed] [Google Scholar]
- 227.Chen JY, Chen PS, Chen YP, Lee WT, Lin LJ. 2006. Community-acquired Klebsiella oxytoca endocarditis: a case report. J Infect 52:e129–e131. 10.1016/j.jinf.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 228.Memon W, Miller M, Shabbir Z. 2018. Klebsiella oxytoca tricuspid valve endocarditis in an elderly patient without known predisposing factors. BMJ Case Rep 2018:bcr2018225352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hauser N, Tanner E, Keuroghlian M, Koduri L. 2017. A case of Klebsiella oxytoca endocarditis in an intravenous drug user. IDCases 9:77–78. 10.1016/j.idcr.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Aissaoui H, Hdidou Y, Bougrine R, Ismaili N, Ouafi NE. 2020. Infective endocarditis caused by Klebsiella oxytoca in a patient with hemodialysis: a care-compliant case report and review of the literature. J Saudi Heart Assoc 32:307–310. 10.37616/2212-5043.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mohamed A, Hall C, Hatch M, Ayan M, Winn R. 2016. Infective endocarditis caused by Klebsiella oxytoca in an intravenous drug user with cancer. Proc (Bayl Univ Med Cent) 29:181–182. 10.1080/08998280.2016.11929408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dago TR, Zewudie A, Mamo Y, Feyissa D, Geleta S. 2020. Multi-drug resistant post corneal repair Klebsiella oxytoca's keratitis. Int Med Case Rep J 13:537–541. 10.2147/IMCRJ.S278625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dursun Ö, Dinç E, Özer Ö, Kıroğlu Ş, Vatansever M, Adıgüzel U. 2021. A case of late-onset Klebsiella oxytoca keratitis treated with topical imipenem after deep anterior lamellar keratoplasty. Arq Bras Oftalmol 84:279–281. 10.5935/0004-2749.20210043. [DOI] [PubMed] [Google Scholar]
- 234.Chou TY, Adyanthaya R. 2012. Infectious crystalline keratopathy associated with Klebsiella oxytoca. J Ophthalmic Inflamm Infect 2:211–213. 10.1007/s12348-012-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. 2011. Late-onset Klebsiella oxytoca flap-margin-related corneal ulcer following laser in situ keratomileusis. J Cataract Refract Surg 37:1551–1554. 10.1016/j.jcrs.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 236.Joseph J, Sharma S, Dave VP. 2018. Filamentous Gram-negative bacteria masquerading as actinomycetes in infectious endophthalmitis: a review of three cases. J Ophthalmic Inflamm Infect 8:15. 10.1186/s12348-018-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Leahey AB, Avery RL, Gottsch JD, Mallette RA, Stark WJ. 1993. Suture abscesses after penetrating keratoplasty. Cornea 12:489–492. 10.1097/00003226-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 238.Silvagni-Gutierrez H, Rodriguez-Fernandez A, Toribio-Calvo B. 2017. Septic arthritis due to Klebsiella oxytoca. Med Clin (Barc) 149:132–133. 10.1016/j.medcli.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 239.Hertting O, Gremark O, Kallman O, Guler L, Bergstrom J. 2018. An infant with Klebsiella oxytoca septic arthritis. J Microbiol Immunol Infect 51:153–154. 10.1016/j.jmii.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 240.Menard A, Harambat J, Pereyre S, Pontailler JR, Megraud F, Richer O. 2010. First report of septic arthritis caused by Klebsiella oxytoca. J Clin Microbiol 48:3021–3023. 10.1128/JCM.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pang F, Jia XQ, Wang B, Li YH, Zhao QG. 2014. Control of an outbreak due to orthopedic infections caused by Enterobacteriaceae producing IMP-4 or IMP-8 carbapenemases. Pathol Biol (Paris) 62:152–155. 10.1016/j.patbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 242.Suthers E, Rosenstengel A, Hart J, Lewis JR, Kay I, Waterer G, Lee YC, Brims F. 2015. Pleural empyema caused by Klebsiella oxytoca: a case series. Respirology 20:507–509. 10.1111/resp.12476. [DOI] [PubMed] [Google Scholar]
- 243.Mammeri H, Poirel L, Nordmann P. 2003. In vivo selection of a chromosomally encoded β-lactamase variant conferring ceftazidime resistance in Klebsiella oxytoca. Antimicrob Agents Chemother 47:3739–3742. 10.1128/AAC.47.12.3739-3742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zheng B, Xu H, Yu X, Lv T, Jiang X, Cheng H, Zhang J, Chen Y, Huang C, Xiao Y. 2018. Identification and genomic characterization of a KPC-2-, NDM-1- and NDM-5-producing Klebsiella michiganensis isolate. J Antimicrob Chemother 73:536–538. 10.1093/jac/dkx415. [DOI] [PubMed] [Google Scholar]
- 245.Chen J, Cachay ER, Hunt GC. 2004. Klebsiella oxytoca: a rare cause of severe infectious colitis: first North American case report. Gastrointest Endosc 60:142–145. 10.1016/s0016-5107(04)01537-8. [DOI] [PubMed] [Google Scholar]
- 246.Garcia Rodriguez JA, Montes Martinez I, Gomez GJ, Ramos MA, Lopez AT. 1992. A case of malignant external otitis involving Klebsiella oxytoca. Eur J Clin Microbiol Infect Dis 11:75–77. 10.1007/BF01971280. [DOI] [PubMed] [Google Scholar]
- 247.Momodu II, Savaliya V. 2021. Septic arthritis. StatPearls Publishing LLC, Treasure Island, FL, USA. [PubMed] [Google Scholar]
- 248.Grau PM, Aguilar Mulet JM, Santiago Poveda C. 2018. Left hydropneumothorax in a patient with acute epigastric pain: an important clue! Intern Emerg Med 13:133–134. 10.1007/s11739-017-1727-4. [DOI] [PubMed] [Google Scholar]
- 249.Guevara N, Guzmán M, Merentes A, Rizzi A, Papaptzikos J, Rivero N, Oranges C, Vlllarroel H, Limas Y. 2015. Antimicrobial susceptibility patterns of Gram-negative bacteria isolated in urinary tract infections in Venezuela: results of the SMART study 2009–2012. Rev Chilena Infectol 32:639–648. 10.4067/S0716-10182015000700005. [DOI] [PubMed] [Google Scholar]
- 250.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P aeruginosa). Clin Infect Dis 72:e169–e183. 10.1093/cid/ciab295. [DOI] [PubMed] [Google Scholar]
- 251.De Waele JJ, Akova M, Antonelli M, Canton R, Carlet J, De Backer D, Dimopoulos G, Garnacho-Montero J, Kesecioglu J, Lipman J, Mer M, Paiva JA, Poljak M, Roberts JA, Rodriguez Bano J, Timsit JF, Zahar JR, Bassetti M. 2018. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med 44:189–196. 10.1007/s00134-017-5036-1. [DOI] [PubMed] [Google Scholar]
- 252.Bassetti M, De Waele JJ, Eggimann P, Garnacho-Montero J, Kahlmeter G, Menichetti F, Nicolau DP, Paiva JA, Tumbarello M, Welte T, Wilcox M, Zahar JR, Poulakou G. 2015. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med 41:776–795. 10.1007/s00134-015-3719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Berthelot P, Grattard F, Patural H, Ros A, Jelassi-Saoudin H, Pozzetto B, Teyssier G, Lucht F. 2001. Nosocomial colonization of premature babies with Klebsiella oxytoca: probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect Control Hosp Epidemiol 22:148–151. 10.1086/501881. [DOI] [PubMed] [Google Scholar]
- 254.Herzog KA, Schneditz G, Leitner E, Feierl G, Hoffmann KM, Zollner-Schwetz I, Krause R, Gorkiewicz G, Zechner EL, Hogenauer C. 2014. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J Clin Microbiol 52:1607–1616. 10.1128/JCM.03373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen Y, Brook TC, Soe CZ, O'Neill I, Alcon-Giner C, Leelastwattanagul O, Phillips S, Caim S, Clarke P, Hall LJ, Hoyles L. 2020. Preterm infants harbour diverse Klebsiella populations, including atypical species that encode and produce an array of antimicrobial resistance- and virulence-associated factors. Microb Genom 6:e000377. 10.1099/mgen.0.000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Karlowsky JA, Hackel MA, Bouchillon SLK, Lowman W, Kotb REM, Mohamed N, Stone GG, Sahm DF. 2021. In vitro activity of ceftaroline against bacterial pathogens isolated from patients with skin and soft tissue and respiratory tract infections in the middle East and Africa: AWARE global surveillance programme 2015–2018. J Glob Antimicrob Resist 24:249–256. 10.1016/j.jgar.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 257.Bernat BA, Laughlin LT, Armstrong RN. 1997. Fosfomycin resistance protein (FosA) is a manganese metalloglutathione transferase related to glyoxalase I and the extradiol dioxygenases. Biochemistry 36:3050–3055. 10.1021/bi963172a. [DOI] [PubMed] [Google Scholar]
- 258.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 259.Ambler RP. 1980. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331. 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 260.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bush K. 2013. The ABCD's of β-lactamase nomenclature. J Infect Chemother 19:549–559. 10.1007/s10156-013-0640-7. [DOI] [PubMed] [Google Scholar]
- 262.Bush K, Bradford PA. 2020. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 33:e00047-19. 10.1128/CMR.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bradford PA. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14:933–951. 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gheorghiu R, Yuan M, Hall LM, Livermore DM. 1997. Bases of variation in resistance to β-lactams in Klebsiella oxytoca isolates hyperproducing K1 β-lactamase. J Antimicrob Chemother 40:533–541. 10.1093/jac/40.4.533. [DOI] [PubMed] [Google Scholar]
- 266.Livermore DM. 1995. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev 8:557–584. 10.1128/CMR.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fournier B, Lagrange PH, Philippon A. 1996. β-Lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca. Antimicrob Agents Chemother 40:460–463. 10.1128/AAC.40.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fournier B, Gravel A, Hooper DC, Roy PH. 1999. Strength and regulation of the different promoters for chromosomal β-lactamases of Klebsiella oxytoca. Antimicrob Agents Chemother 43:850–855. 10.1128/AAC.43.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fournier B, Arlet G, Lagrange PH, Philippon A. 1994. Klebsiella oxytoca: resistance to aztreonam by overproduction of the chromosomally encoded β-lactamase. FEMS Microbiol Lett 116:31–36. 10.1111/j.1574-6968.1994.tb06671.x. [DOI] [PubMed] [Google Scholar]
- 270.Granier SA, Nicolas-Chanoine MH, Nguyen Van JC, Leflon-Guibout V, Kitzis MD, Goldstein FW. 2002. False susceptibility of Klebsiella oxytoca to some extended-spectrum cephalosporins. J Antimicrob Chemother 50:303–304. 10.1093/jac/dkf123. [DOI] [PubMed] [Google Scholar]
- 271.Fournier B, Lu CY, Lagrange PH, Krishnamoorthy R, Philippon A. 1995. Point mutation in the Pribnow box, the molecular basis of β-lactamase overproduction in Klebsiella oxytoca. Antimicrob Agents Chemother 39:1365–1368. 10.1128/AAC.39.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Walkty A, Adam HJ, Karlowsky JA, Alexander DC. 2020. Cefotaxime susceptibility should not be used to predict ceftriaxone susceptibility among Klebsiella oxytoca clinical isolates. J Glob Antimicrob Resist 21:270–271. 10.1016/j.jgar.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 273.Younes A, Hamouda A, Amyes SG. 2011. First report of a novel extended-spectrum β-lactamase KOXY-2 producing Klebsiella oxytoca that hydrolyses cefotaxime and ceftazidime. J Chemother 23:127–130. 10.1179/joc.2011.23.3.127. [DOI] [PubMed] [Google Scholar]
- 274.Fournier B, Roy PH. 1997. Variability of chromosomally encoded β-lactamases from Klebsiella oxytoca. Antimicrob Agents Chemother 41:1641–1648. 10.1128/AAC.41.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Decre D, Burghoffer B, Gautier V, Petit JC, Arlet G. 2004. Outbreak of multi-resistant Klebsiella oxytoca involving strains with extended-spectrum β-lactamases and strains with extended-spectrum activity of the chromosomal β-lactamase. J Antimicrob Chemother 54:881–888. 10.1093/jac/dkh440. [DOI] [PubMed] [Google Scholar]
- 276.Sirot D, Labia R, Pouedras P, Chanal-Claris C, Cerceau C, Sirot J. 1998. Inhibitor-resistant OXY-2-derived β-lactamase produced by Klebsiella oxytoca. Antimicrob Agents Chemother 42:2184–2187. 10.1128/AAC.42.9.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jeong SH, Bae IK, Lee JH, Sohn SG, Kang GH, Jeon GJ, Kim YH, Jeong BC, Lee SH. 2004. Molecular characterization of extended-spectrum β-lactamases produced by clinical isolates of Klebsiella pneumoniae and Escherichia coli from a Korean nationwide survey. J Clin Microbiol 42:2902–2906. 10.1128/JCM.42.7.2902-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Canica M, Ferreira M, Ferreira E, Cabral L. 2002. Phenotype and molecular characterization of the first inhibitor-resistant TEM-derived β-lactamase identified in Portugal. Antimicrob Agents Chemother 46:3688–3689. 10.1128/AAC.46.11.3688-3689.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Barthelemy M, Peduzzi J, Labia R. 1988. Complete amino acid sequence of p453-plasmid-mediated PIT-2 β-lactamase (SHV-1). Biochem J 251:73–79. 10.1042/bj2510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Szabo D, Melan MA, Hujer AM, Bonomo RA, Hujer KM, Bethel CR, Kristof K, Paterson DL. 2005. Molecular analysis of the simultaneous production of two SHV-type extended-spectrum β-lactamases in a clinical isolate of Enterobacter cloacae by using single-nucleotide polymorphism genotyping. Antimicrob Agents Chemother 49:4716–4720. 10.1128/AAC.49.11.4716-4720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yigit H, Queenan AM, Rasheed JK, Biddle JW, Domenech-Sanchez A, Alberti S, Bush K, Tenover FC. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing β-lactamase KPC-2. Antimicrob Agents Chemother 47:3881–3889. 10.1128/AAC.47.12.3881-3889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nuesch-Inderbinen MT, Kayser FH, Hachler H. 1997. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob Agents Chemother 41:943–949. 10.1128/AAC.41.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 284.Ortiz de la Rosa JM, Demord A, Poirel L, Greub G, Blanc D, Nordmann P. 2021. False immunological detection of CTX-M enzymes in Klebsiella oxytoca. J Clin Microbiol 59:e00609-21. 10.1128/JCM.00609-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nazik H, Aydin S, Albayrak R, Bilgi EA, Yildiz I, Kuvat N, Kelesoglu FM, Kelesoglu FM, Pakaştiçali N, Yilmaz F, Ongen B. 2014. Detection and spread of OXA-48-producing Klebsiella oxytoca isolates in Istanbul, Turkey. Southeast Asian J Trop Med Public Health 45:123–129. [PubMed] [Google Scholar]
- 286.Khan FA, Hellmark B, Ehricht R, Soderquist B, Jass J. 2018. Related carbapenemase-producing Klebsiella isolates detected in both a hospital and associated aquatic environment in Sweden. Eur J Clin Microbiol Infect Dis 37:2241–2251. 10.1007/s10096-018-3365-9. [DOI] [PubMed] [Google Scholar]
- 287.Ben Yahia H, Chairat S, Gharsa H, Alonso CA, Ben Sallem R, Porres-Osante N, Hamdi N, Torres C, Ben Slama K. 2020. First report of KPC-2 and KPC-3-producing Enterobacteriaceae in wild birds in Africa. Microb Ecol 79:30–37. 10.1007/s00248-019-01375-x. [DOI] [PubMed] [Google Scholar]
- 288.Navarro F, Perez-Trallero E, Marimon JM, Aliaga R, Gomariz M, Mirelis B. 2001. CMY-2-producing Salmonella enterica, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis and Escherichia coli strains isolated in Spain (October 1999-December 2000). J Antimicrob Chemother 48:383–389. 10.1093/jac/48.3.383. [DOI] [PubMed] [Google Scholar]
- 289.Yong D, Lim Y, Song W, Choi YS, Park DY, Lee H, Yum JH, Lee K, Kim JM, Chong Y. 2005. Plasmid-mediated, inducible AmpC β-lactamase (DHA-1)-producing Enterobacteriaceae at a Korean hospital: wide dissemination in Klebsiella pneumoniae and Klebsiella oxytoca and emergence in Proteus mirabilis. Diagn Microbiol Infect Dis 53:65–70. 10.1016/j.diagmicrobio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 290.Verdet C, Benzerara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G. 2006. Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating from Morganella morganii. Antimicrob Agents Chemother 50:607–617. 10.1128/AAC.50.2.607-617.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Marchese A, Arlet G, Schito GC, Lagrange PH, Philippon A. 1998. Characterization of FOX-3, an AmpC-type plasmid-mediated β-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob Agents Chemother 42:464–467. 10.1128/AAC.42.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hazen TH, Robinson GL, Harris AD, Rasko DA, Johnson JK. 2012. Genome sequence of Klebsiella oxytoca 11492-1, a nosocomial isolate possessing a FOX-5 AmpC β-lactamase. J Bacteriol 194:3028–3029. 10.1128/JB.00391-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bojorquez D, Belei M, Delira SF, Sholly S, Mead J, Tolmasky ME. 1998. Characterization of OXA-9, a β-lactamase encoded by the multiresistance transposon Tn1331. Cell Mol Biol 44:483–491. [PubMed] [Google Scholar]
- 294.Boyd DA, Mulvey MR. 2006. OXA-1 is OXA-30 is OXA-1. J Antimicrob Chemother 58:224–225. 10.1093/jac/dkl154. [DOI] [PubMed] [Google Scholar]
- 295.Yoon EJ, Jeong SH. 2021. Class D β-lactamases. J Antimicrob Chemother 76:836–864. 10.1093/jac/dkaa513. [DOI] [PubMed] [Google Scholar]
- 296.Hammerum AM, Hansen F, Nielsen HL, Jakobsen L, Stegger M, Andersen PS, Jensen P, Nielsen TK, Hansen LH, Hasman H, Fuglsang-Damgaard D. 2016. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother 71:3117–3124. 10.1093/jac/dkw289. [DOI] [PubMed] [Google Scholar]
- 297.Ruiz E, Rezusta A, Saenz Y, Rocha-Gracia R, Vinue L, Vindel A, Villuendas C, Azanedo ML, Monforte ML, Revillo MJ, Torres C. 2011. New genetic environments of aac(6')-Ib-cr gene in a multiresistant Klebsiella oxytoca strain causing an outbreak in a pediatric intensive care unit. Diagn Microbiol Infect Dis 69:236–238. 10.1016/j.diagmicrobio.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 298.Ruiz E, Saenz Y, Zarazaga M, Rocha-Gracia R, Martinez-Martinez L, Arlet G, Torres C. 2012. qnr, aac(6')-Ib-cr and qepA genes in Escherichia coli and Klebsiella spp.: genetic environments and plasmid and chromosomal location. J Antimicrob Chemother 67:886–897. 10.1093/jac/dkr548. [DOI] [PubMed] [Google Scholar]
- 299.Piedra-Carrasco N, Fabrega A, Calero-Caceres W, Cornejo-Sanchez T, Brown-Jaque M, Mir-Cros A, Muniesa M, Gonzalez-Lopez JJ. 2017. Carbapenemase-producing Enterobacteriaceae recovered from a Spanish river ecosystem. PLoS One 12:e0175246. 10.1371/journal.pone.0175246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Caltagirone M, Nucleo E, Spalla M, Zara F, Novazzi F, Marchetti VM, Piazza A, Bitar I, De Cicco M, Paolucci S, Pilla G, Migliavacca R, Pagani L. 2017. Occurrence of extended spectrum β-lactamases, KPC-type, and MCR-1.2-producing enterobacteriaceae from wells, river water, and wastewater treatment plants in Oltrepo Pavese area, northern Italy. Front Microbiol 8:2232. 10.3389/fmicb.2017.02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ahmad N, Khalid S, Ali SM, Khan AU. 2018. Occurrence of blaNDM variants among Enterobacteriaceae from a neonatal intensive care unit in a northern India hospital. Front Microbiol 9:407. 10.3389/fmicb.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schweizer C, Bischoff P, Bender J, Kola A, Gastmeier P, Hummel M, Klefisch FR, Schoenrath F, Fruhauf A, Pfeifer Y. 2019. Plasmid-mediated transmission of KPC-2 carbapenemase in Enterobacteriaceae in critically ill patients. Front Microbiol 10:276. 10.3389/fmicb.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Krishnaraju M, Kamatchi C, Jha AK, Devasena N, Vennila R, Sumathi G, Vaidyanathan R. 2015. Complete sequencing of an IncX3 plasmid carrying blaNDM-5 allele reveals an early stage in the dissemination of the blaNDM gene. Indian J Med Microbiol 33:30–38. 10.4103/0255-0857.148373. [DOI] [PubMed] [Google Scholar]
- 304.Antunes NT, Lamoureaux TL, Toth M, Stewart NK, Frase H, Vakulenko SB. 2014. Class D β-lactamases: are they all carbapenemases? Antimicrob Agents Chemother 58:2119–2125. 10.1128/AAC.02522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hu L, Zhong Q, Shang Y, Wang H, Ning C, Li Y, Hang Y, Xiong J, Wang X, Xu Y, Qin Z, Parsons C, Wang L, Yu F. 2014. The prevalence of carbapenemase genes and plasmid-mediated quinolone resistance determinants in carbapenem-resistant Enterobacteriaceae from five teaching hospitals in central China. Epidemiol Infect 142:1972–1977. 10.1017/S0950268813002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Luo Y, Yang J, Ye L, Guo L, Zhao Q, Chen R, Chen Y, Han X, Zhao J, Tian S, Han L. 2014. Characterization of KPC-2-producing Escherichia coli, Citrobacter freundii, Enterobacter cloacae, Enterobacter aerogenes, and Klebsiella oxytoca isolates from a Chinese hospital. Microb Drug Resist 20:264–269. 10.1089/mdr.2013.0150. [DOI] [PubMed] [Google Scholar]
- 307.Huang TD, Berhin C, Bogaerts P, Glupczynski Y, Multicentre Study Group . 2013. Prevalence and mechanisms of resistance to carbapenems in Enterobacteriaceae isolates from 24 hospitals in Belgium. J Antimicrob Chemother 68:1832–1837. 10.1093/jac/dkt096. [DOI] [PubMed] [Google Scholar]
- 308.Zarfel G, Hoenigl M, Wurstl B, Leitner E, Salzer HJ, Valentin T, Posch J, Krause R, Grisold AJ. 2011. Emergence of carbapenem-resistant Enterobacteriaceae in Austria, 2001–2010. Clin Microbiol Infect 17:E5–E8. 10.1111/j.1469-0691.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 309.Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob Agents Chemother 53:1998–2004. 10.1128/AAC.01355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. 2011. First report of Klebsiella oxytoca strain coproducing KPC-2 and IMP-8 carbapenemases. Antimicrob Agents Chemother 55:2937–2941. 10.1128/AAC.01670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Baraniak A, Grabowska A, Izdebski R, Fiett J, Herda M, Bojarska K, Zabicka D, Kania-Pudlo M, Mlynarczyk G, Zak-Pulawska Z, Hryniewicz W, Gniadkowski M, the KPC-PL Study Group . 2011. Molecular characteristics of KPC-producing Enterobacteriaceae at the early stage of their dissemination in Poland, 2008–2009. Antimicrob Agents Chemother 55:5493–5499. 10.1128/AAC.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rasheed JK, Biddle JW, Anderson KF, Washer L, Chenoweth C, Perrin J, Newton DW, Patel JB. 2008. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J Clin Microbiol 46:2066–2069. 10.1128/JCM.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Leung V, Loo VG, Frenette C, Domingo MC, Bourgault AM, Mulvey MR, Robson HG. 2012. First Canadian outbreak of Enterobacteriaceae-expressing Klebsiella pneumoniae carbapenemase type 3. Can J Infect Dis Med Microbiol 23:117–120. 10.1155/2012/725151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.He Q, Chen W, Huang L, Lin Q, Zhang J, Liu R, Li B. 2016. Performance evaluation of three automated identification systems in detecting carbapenem-resistant Enterobacteriaceae. Ann Clin Microbiol Antimicrob 15:40. 10.1186/s12941-016-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Conte D, Palmeiro JK, da Silva Nogueira K, de Lima TM, Cardoso MA, Pontarolo R, Degaut Pontes FL, Dalla-Costa LM. 2017. Characterization of CTX-M enzymes, quinolone resistance determinants, and antimicrobial residues from hospital sewage, wastewater treatment plant, and river water. Ecotoxicol Environ Saf 136:62–69. 10.1016/j.ecoenv.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 317.White L, Hopkins KL, Meunier D, Perry CL, Pike R, Wilkinson P, Pickup RW, Cheesbrough J, Woodford N. 2016. Carbapenemase-producing Enterobacteriaceae in hospital wastewater: a reservoir that may be unrelated to clinical isolates. J Hosp Infect 93:145–151. 10.1016/j.jhin.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 318.Tato M, Coque TM, Ruiz-Garbajosa P, Pintado V, Cobo J, Sader HS, Jones RN, Baquero F, Canton R. 2007. Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 metallo-β-lactamase in Spain: toward endemicity? Clin Infect Dis 45:1171–1178. 10.1086/522288. [DOI] [PubMed] [Google Scholar]
- 319.Carattoli A, Aschbacher R, March A, Larcher C, Livermore DM, Woodford N. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the USA. J Antimicrob Chemother 65:2070–2075. 10.1093/jac/dkq269. [DOI] [PubMed] [Google Scholar]
- 320.Cendejas E, Gomez-Gil R, Gomez-Sanchez P, Mingorance J. 2010. Detection and characterization of Enterobacteriaceae producing metallo-β-lactamases in a tertiary-care hospital in Spain. Clin Microbiol Infect 16:181–183. 10.1111/j.1469-0691.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 321.Ghaith DM, Mohamed ZK, Farahat MG, Aboulkasem Shahin W, Mohamed HO. 2019. Colonization of intestinal microbiota with carbapenemase-producing Enterobacteriaceae in paediatric intensive care units in Cairo, Egypt. Arab J Gastroenterol 20:19–22. 10.1016/j.ajg.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 322.Wang X, Chen G, Wu X, Wang L, Cai J, Chan EW, Chen S, Zhang R. 2015. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the blaNDM-1 element and clonal spread of progenitor resistant strains. Front Microbiol 6:595. 10.3389/fmicb.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Huang TW, Wang JT, Lauderdale TL, Liao TL, Lai JF, Tan MC, Lin AC, Chen YT, Tsai SF, Chang SC. 2013. Complete sequences of two plasmids in a blaNDM-1-positive Klebsiella oxytoca isolate from Taiwan. Antimicrob Agents Chemother 57:4072–4076. 10.1128/AAC.02266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Qin S, Fu Y, Zhang Q, Qi H, Wen JG, Xu H, Xu L, Zeng L, Tian H, Rong L, Li Y, Shan L, Xu H, Yu Y, Feng X, Liu HM. 2014. High incidence and endemic spread of NDM-1-positive Enterobacteriaceae in Henan province, China. Antimicrob Agents Chemother 58:4275–4282. 10.1128/AAC.02813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pedersen T, Sekyere JO, Govinden U, Moodley K, Sivertsen A, Samuelsen O, Essack SY, Sundsfjord A. 2018. Spread of plasmid-encoded NDM-1 and GES-5 carbapenemases among extensively drug-resistant and pandrug-resistant clinical Enterobacteriaceae in Durban, South Africa. Antimicrob Agents Chemother 62:e02178-17. 10.1128/AAC.02178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.An J, Guo L, Zhou L, Ma Y, Luo Y, Tao C, Yang J. 2016. NDM-producing Enterobacteriaceae in a Chinese hospital, 2014-2015: identification of NDM-producing Citrobacter werkmanii and acquisition of blaNDM-1-carrying plasmid in vivo in a clinical Escherichia coli isolate. J Med Microbiol 65:1253–1259. 10.1099/jmm.0.000357. [DOI] [PubMed] [Google Scholar]
- 327.Wendel AF, Brodner AH, Wydra S, Ressina S, Henrich B, Pfeffer K, Toleman MA, Mackenzie CR. 2013. Genetic characterization and emergence of the metallo-β-lactamase GIM-1 in Pseudomonas spp. and Enterobacteriaceae during a long-term outbreak. Antimicrob Agents Chemother 57:5162–5165. 10.1128/AAC.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, Edgar R, Solter E, Benenson S, Masarwa S, Carmeli Y. 2011. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J Antimicrob Chemother 66:2763–2766. 10.1093/jac/dkr382. [DOI] [PubMed] [Google Scholar]
- 329.Aquino-Andrade A, Merida-Vieyra J, Arias de la Garza E, Arzate-Barbosa P, De Colsa Ranero A. 2018. Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol 18:38. 10.1186/s12866-018-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Vanegas JM, Ospina WP, Felipe Higuita-Gutierrez L, Natalia JimenezJ. 2016. First reported case of an OXA-48-producing isolate from a Colombian patient. J Glob Antimicrob Resist 6:67–68. 10.1016/j.jgar.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 331.Pulss S, Stolle I, Stamm I, Leidner U, Heydel C, Semmler T, Prenger-Berninghoff E, Ewers C. 2018. Multispecies and clonal dissemination of OXA-48 carbapenemase in Enterobacteriaceae from companion animals in Germany, 2009-2016. Front Microbiol 9:1265. 10.3389/fmicb.2018.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.King TL, Schmidt S, Thakur S, Fedorka-Cray P, Keelara S, Harden L, Essack SY. 2021. Resistome of a carbapenemase-producing novel ST232 Klebsiella michiganensis isolate from urban hospital effluent in South Africa. J Glob Antimicrob Resist 24:321–324. 10.1016/j.jgar.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Olaitan AO, Rolain JM. 2015. Interruption of mgrB in the mediation of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents 46:354–355. 10.1016/j.ijantimicag.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 334.Mhaya A, Begu D, Tounsi S, Arpin C. 2020. MgrB inactivation is responsible for acquired resistance to colistin in Enterobacter hormaechei subsp. steigerwaltii. Antimicrob Agents Chemother 64:e00128-20. 10.1128/AAC.00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Newton-Foot M, Snyman Y, Maloba MRB, Whitelaw AC. 2017. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. clinical isolates from the Western Cape region of South Africa. Antimicrob Resist Infect Control 6:78. 10.1186/s13756-017-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhou HW, Zhang T, Ma JH, Fang Y, Wang HY, Huang ZX, Wang Y, Wu C, Chen GX. 2017. Occurrence of plasmid- and chromosome-carried mcr-1 in waterborne Enterobacteriaceae in China. Antimicrob Agents Chemother 61:e00017-17. 10.1128/AAC.00017-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tsui CKM, Sundararaju S, Al Mana H, Hasan MR, Tang P, Perez-Lopez A. 2020. Draft genome sequence of an extended-spectrum β-lactamase-producing Klebsiella oxytoca strain bearing mcr-9 from Qatar. Microbiol Resour Announc 9:e00429-20. 10.1128/MRA.00429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Borjesson S, Greko C, Myrenas M, Landen A, Nilsson O, Pedersen K. 2020. A link between the newly described colistin resistance gene mcr-9 and clinical Enterobacteriaceae isolates carrying blaSHV-12 from horses in Sweden. J Glob Antimicrob Resist 20:285–289. 10.1016/j.jgar.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 339.Shaw KJ, Rather PN, Hare RS, Miller GH. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57:138–163. 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis 45:88–94. 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 341.Wachino J, Yamane K, Shibayama K, Kurokawa H, Shibata N, Suzuki S, Doi Y, Kimura K, Ike Y, Arakawa Y. 2006. Novel plasmid-mediated 16S rRNA methylase, RmtC, found in a proteus mirabilis isolate demonstrating extraordinary high-level resistance against various aminoglycosides. Antimicrob Agents Chemother 50:178–184. 10.1128/AAC.50.1.178-184.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother 50:2872–2874. 10.1128/AAC.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Huovinen P, Sundstrom L, Swedberg G, Skold O. 1995. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother 39:279–289. 10.1128/AAC.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. 2004. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev 28:519–542. 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 345.Baysarowich J, Koteva K, Hughes DW, Ejim L, Griffiths E, Zhang K, Junop M, Wright GD. 2008. Rifamycin antibiotic resistance by ADP-ribosylation: structure and diversity of Arr. Proc Natl Acad Sci USA 105:4886–4891. 10.1073/pnas.0711939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Roberts MC. 2005. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett 245:195–203. 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 347.Golkar T, Zielinski M, Berghuis AM. 2018. Look and outlook on enzyme-mediated macrolide resistance. Front Microbiol 9:1942. 10.3389/fmicb.2018.01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Peirano G, Chen L, Kreiswirth BN, Pitout JDD. 2020. Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147. Antimicrob Agents Chemother 64:e01148-20. 10.1128/AAC.01148-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Fu Y, Zhang W, Wang H, Zhao S, Chen Y, Meng F, Zhang Y, Xu H, Chen X, Zhang F. 2013. Specific patterns of gyrA mutations determine the resistance difference to ciprofloxacin and levofloxacin in Klebsiella pneumoniae and Escherichia coli. BMC Infect Dis 13:8. 10.1186/1471-2334-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hooper DC, Jacoby GA. 2016. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med 6:a025320. 10.1101/cshperspect.a025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Anssour L, Messai Y, Estepa V, Torres C, Bakour R. 2016. Characteristics of ciprofloxacin-resistant Enterobacteriaceae isolates recovered from wastewater of an Algerian hospital. J Infect Dev Ctries 10:728–734. 10.3855/jidc.6727. [DOI] [PubMed] [Google Scholar]
- 352.Weigel LM, Steward CD, Tenover FC. 1998. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob Agents Chemother 42:2661–2667. 10.1128/AAC.42.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Brisse S, Milatovic D, Fluit AC, Verhoef J, Martin N, Scheuring S, Köhrer K, Schmitz FJ. 1999. Comparative in vitro activities of ciprofloxacin, clinafloxacin, gatifloxacin, levofloxacin, moxifloxacin, and trovafloxacin against Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, and Enterobacter aerogenes clinical isolates with alterations in GyrA and ParC proteins. Antimicrob Agents Chemother 43:2051–2055. 10.1128/AAC.43.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhang Y, Zhou H, Shen XQ, Shen P, Yu YS, Li LJ. 2008. Plasmid-borne armA methylase gene, together with blaCTX-M-15 and blaTEM-1, in a Klebsiella oxytoca isolate from China. J Med Microbiol 57:1273–1276. 10.1099/jmm.0.2008/001271-0. [DOI] [PubMed] [Google Scholar]
- 355.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, Gupta SK, Thongmalayvong B, Akkhavong K, Somphavong S, Paboriboune P, Chaisiri K, Komalamisra C, Adelowo OO, Fagade OE, Banjo OA, Oke AJ, Adler A, Assous MV, Morand S, Raoult D, Rolain JM. 2014. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents 44:500–507. 10.1016/j.ijantimicag.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 356.Minami J, Okabe A, Shiode J, Hayashi H. 1989. Production of a unique cytotoxin by Klebsiella oxytoca. Microb Pathog 7:203–211. 10.1016/0882-4010(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 357.Minami J, Katayama S, Matsushita O, Sakamoto H, Okabe A. 1994. Enterotoxic activity of Klebsiella oxytoca cytotoxin in rabbit intestinal loops. Infect Immun 62:172–177. 10.1128/iai.62.1.172-177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Dornisch E, Pletz J, Glabonjat RA, Martin F, Lembacher-Fadum C, Neger M, Högenauer C, Francesconi K, Kroutil W, Zangger K, Breinbauer R, Zechner EL. 2017. Biosynthesis of the enterotoxic pyrrolobenzodiazepine natural product tilivalline. Angew Chem Int Ed Engl 56:14753–14757. 10.1002/anie.201707737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Tse H, Gu Q, Sze KH, Chu IK, Kao RY, Lee KC, Lam CW, Yang D, Tai SS, Ke Y, Chan E, Chan WM, Dai J, Leung SP, Leung SY, Yuen KY. 2017. A tricyclic pyrrolobenzodiazepine produced by Klebsiella oxytoca is associated with cytotoxicity in antibiotic-associated hemorrhagic colitis. J Biol Chem 292:19503–19520. 10.1074/jbc.M117.791558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Darby A, Lertpiriyapong K, Sarkar U, Seneviratne U, Park DS, Gamazon ER, Batchelder C, Cheung C, Buckley EM, Taylor NS, Shen Z, Tannenbaum SR, Wishnok JS, Fox JG. 2014. Cytotoxic and pathogenic properties of Klebsiella oxytoca isolated from laboratory animals. PLoS One 9:e100542. 10.1371/journal.pone.0100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Unterhauser K, Poltl L, Schneditz G, Kienesberger S, Glabonjat RA, Kitsera M, Pletz J, Josa-Prado F, Dornisch E, Lembacher-Fadum C, Roier S, Gorkiewicz G, Lucena D, Barasoain I, Kroutil W, Wiedner M, Loizou JI, Breinbauer R, Diaz JF, Schild S, Hogenauer C, Zechner EL. 2019. Klebsiella oxytoca enterotoxins tilimycin and tilivalline have distinct host DNA-damaging and microtubule-stabilizing activities. Proc Natl Acad Sci USA 116:3774–3783. 10.1073/pnas.1819154116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.von Tesmar A, Hoffmann M, Abou Fayad A, Hüttel S, Schmitt V, Herrmann J, Müller R. 2018. Biosynthesis of the Klebsiella oxytoca pathogenicity factor tilivalline: heterologous expression, in vitro biosynthesis, and inhibitor development. ACS Chem Biol 13:812–819. 10.1021/acschembio.7b00990. [DOI] [PubMed] [Google Scholar]
- 363.Schneditz G, Rentner J, Roier S, Pletz J, Herzog KA, Bucker R, Troeger H, Schild S, Weber H, Breinbauer R, Gorkiewicz G, Hogenauer C, Zechner EL. 2014. Enterotoxicity of a nonribosomal peptide causes antibiotic-associated colitis. Proc Natl Acad Sci USA 111:13181–13186. 10.1073/pnas.1403274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Shibu P, McCuaig F, McCartney AL, Kujawska M, Hall LJ, Hoyles L. 2021. Improved molecular characterization of the Klebsiella oxytoca complex reveals the prevalence of the kleboxymycin biosynthetic gene cluster. Microb Genom 7:e000592. 10.1099/mgen.0.000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hering NA, Fromm A, Bucker R, Gorkiewicz G, Zechner E, Hogenauer C, Fromm M, Schulzke JD, Troeger H. 2019. Tilivalline- and tilimycin-independent effects of Klebsiella oxytoca on tight junction-mediated intestinal barrier impairment. Int J Mol Sci 20:5595. 10.3390/ijms20225595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bengoechea JA, Sa Pessoa J. 2019. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev 43:123–144. 10.1093/femsre/fuy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Wyres KL, Lam MMC, Holt KE. 2020. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol 18:344–359. 10.1038/s41579-019-0315-1. [DOI] [PubMed] [Google Scholar]
- 368.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Choi M, Hegerle N, Nkeze J, Sen S, Jamindar S, Nasrin S, Sen S, Permala-Booth J, Sinclair J, Tapia MD, Johnson JK, Mamadou S, Thaden JT, Fowler VG, Jr., Aguilar A, Terán E, Decre D, Morel F, Krogfelt KA, Brauner A, Protonotariou E, Christaki E, Shindo Y, Lin YT, Kwa AL, Shakoor S, Singh-Moodley A, Perovic O, Jacobs J, Lunguya O, Simon R, Cross AS, Tennant SM. 2020. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front Microbiol 11:1249. 10.3389/fmicb.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Benge GR. 1988. Bactericidal activity of human serum against strains of Klebsiella from different sources. J Med Microbiol 27:11–15. 10.1099/00222615-27-1-11. [DOI] [PubMed] [Google Scholar]
- 371.Riser E, Noone P, Howard FM. 1980. Epidemiological study of Klebsiella infection in the special care baby unit of a London hospital. J Clin Pathol 33:400–407. 10.1136/jcp.33.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Davis JK, Gaertner DJ, Cox NR, Lindsey JR, Cassell GH, Davidson MK, Kervin KC, Rao GN. 1987. The role of Klebsiella oxytoca in utero-ovarian infection of B6C3F1 mice. Lab Anim Sci 37:159–166. [PubMed] [Google Scholar]
- 373.Tarkkanen AM, Allen BL, Williams PH, Kauppi M, Haahtela K, Siitonen A, Orskov I, Orskov F, Clegg S, Korhonen TK. 1992. Fimbriation, capsulation, and iron-scavenging systems of Klebsiella strains associated with human urinary tract infection. Infect Immun 60:1187–1192. 10.1128/iai.60.3.1187-1192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Podschun R. 1990. Phenotypic properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentralbl Hyg Umweltmed 189:527–535. [PubMed] [Google Scholar]
- 375.Süsskind M, Lindner B, Weimar T, Brade H, Holst O. 1998. The structure of the lipopolysaccharide from Klebsiella oxytoca rough mutant R29 (O1-/K29-). Carbohydr Res 312:91–95. 10.1016/s0008-6215(98)00230-4. [DOI] [PubMed] [Google Scholar]
- 376.Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, Thomson NR, Holt KE. 2016. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom 2:e000102. 10.1099/mgen.0.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Abbas AF, Al-Saadi AGM, Alkhudhairy MK. 2020. Biofilm formation and virulence determinants of Klebsiella oxytoca clinical isolates from patients with colorectal cancer. J Gastrointest Cancer 51:855–860. 10.1007/s12029-019-00317-7. [DOI] [PubMed] [Google Scholar]
- 378.Alkhudhairy MK, Alshadeedi SMJ, Mahmood SS, Al-Bustan SA, Ghasemian A. 2019. Comparison of adhesin genes expression among Klebsiella oxytoca ESBL-non-producers in planktonic and biofilm mode of growth, and imipenem sublethal exposure. Microb Pathog 134:103558. 10.1016/j.micpath.2019.103558. [DOI] [PubMed] [Google Scholar]
- 379.Ishihara Y, Yagi T, Mochizuki M, Ohta M. 2012. Capsular types, virulence factors and DNA types of Klebsiella oxytoca strains isolated from blood and bile. Kansenshogaku Zasshi 86:121–126. 10.11150/kansenshogakuzasshi.86.121. [DOI] [PubMed] [Google Scholar]
- 380.Ong CL, Ulett GC, Mabbett AN, Beatson SA, Webb RI, Monaghan W, Nimmo GR, Looke DF, McEwan AG, Schembri MA. 2008. Identification of type 3 fimbriae in uropathogenic Escherichia coli reveals a role in biofilm formation. J Bacteriol 190:1054–1063. 10.1128/JB.01523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ong CL, Beatson SA, Totsika M, Forestier C, McEwan AG, Schembri MA. 2010. Molecular analysis of type 3 fimbrial genes from Escherichia coli, Klebsiella and Citrobacter species. BMC Microbiol 10:183. 10.1186/1471-2180-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Allen BL, Gerlach GF, Clegg S. 1991. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol 173:916–920. 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4:e4982. 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Duguid JP. 1959. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol 21:271–286. 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- 385.Huang YJ, Liao HW, Wu CC, Peng HL. 2009. MrkF is a component of type 3 fimbriae in Klebsiella pneumoniae. Res Microbiol 160:71–79. 10.1016/j.resmic.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 386.Wilksch JJ, Yang J, Clements A, Gabbe JL, Short KR, Cao H, Cavaliere R, James CE, Whitchurch CB, Schembri MA, Chuah ML, Liang ZX, Wijburg OL, Jenney AW, Lithgow T, Strugnell RA. 2011. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog 7:e1002204. 10.1371/journal.ppat.1002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Frost LS, Leplae R, Summers AO, Toussaint A. 2005. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 3:722–732. 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 388.Hubbard ATM, Newire E, Botelho J, Reine J, Wright E, Murphy EA, Hutton W, Roberts AP. 2020. Isolation of an antimicrobial-resistant, biofilm-forming, Klebsiella grimontii isolate from a reusable water bottle. Microbiologyopen 9:1128–1134. 10.1002/mbo3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Holcik M, Iyer VN. 1996. Structure and mode of action of kikA, a genetic region lethal to Klebsiella oxytoca and associated with conjugative antibiotic-resistance plasmids of the IncN group. Plasmid 35:189–203. 10.1006/plas.1996.0021. [DOI] [PubMed] [Google Scholar]
- 390.Yao Y, Lazaro-Perona F, Falgenhauer L, Valverde A, Imirzalioglu C, Dominguez L, Canton R, Mingorance J, Chakraborty T. 2017. Insights into a novel blaKPC-2-encoding IncP-6 plasmid reveal carbapenem-resistance circulation in several Enterobacteriaceae species from wastewater and a hospital source in Spain. Front Microbiol 8:1143. 10.3389/fmicb.2017.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ellington MJ, Davies F, Jauneikaite E, Hopkins KL, Turton JF, Adams G, Pavlu J, Innes AJ, Eades C, Brannigan ET, Findlay J, White L, Bolt F, Kadhani T, Chow Y, Patel B, Mookerjee S, Otter JA, Sriskandan S, Woodford N, Holmes A. 2020. A multispecies cluster of GES-5 carbapenemase-producing Enterobacterales Linked by a geographically disseminated plasmid. Clin Infect Dis 71:2553–2560. 10.1093/cid/ciz1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Liao TL, Lin AC, Chen E, Huang TW, Liu YM, Chang YH, Lai JF, Lauderdale TL, Wang JT, Chang SC, Tsai SF, Chen YT. 2012. Complete genome sequence of Klebsiella oxytoca E718, a New Delhi metallo-β-lactamase-1-producing nosocomial strain. J Bacteriol 194:5454. 10.1128/JB.01216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Shimada N, Kayama S, Shigemoto N, Hisatsune J, Kuwahara R, Nishio H, Yamasaki K, Wada Y, Sueda T, Ohge H, Sugai M. 2016. Complete nucleotide sequence of pKOI-34, an IncL/M plasmid carrying blaIMP-34 in Klebsiella oxytoca isolated in Japan. Antimicrob Agents Chemother 60:3156–3162. 10.1128/AAC.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Jiang X, Yin Z, Yin X, Fang H, Sun Q, Tong Y, Xu Y, Zhang D, Feng J, Chen W, Song Y, Wang J, Chen S, Zhou D. 2017. Sequencing of blaIMP-carrying IncN2 plasmids, and comparative genomics of IncN2 Plasmids harboring class 1 integrons. Front Cell Infect Microbiol 7:102. 10.3389/fcimb.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Yamagishi T, Matsui M, Sekizuka T, Ito H, Fukusumi M, Uehira T, Tsubokura M, Ogawa Y, Miyamoto A, Nakamori S, Tawa A, Yoshimura T, Yoshida H, Hirokawa H, Suzuki S, Matsui T, Shibayama K, Kuroda M, Oishi K. 2020. A prolonged multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales due to horizontal transmission of the IncN plasmid. Sci Rep 10:4139. 10.1038/s41598-020-60659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Arcari G, Di Lella FM, Bibbolino G, Mengoni F, Beccaccioli M, Antonelli G, Faino L, Carattoli A. 2020. A multispecies cluster of VIM-1 carbapenemase-producing Enterobacterales Linked by a novel, highly conjugative, and broad-host-range IncA plasmid forebodes the reemergence of VIM-1. Antimicrob Agents Chemother 64:e02435-19. 10.1128/AAC.02435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Miriagou V, Douzinas EE, Papagiannitsis CC, Piperaki E, Legakis NJ, Tzouvelekis LS. 2008. Emergence of Serratia liquefaciens and Klebsiella oxytoca with metallo-β-lactamase-encoding IncW plasmids: further spread of the blaVIM-1-carrying integron In-e541. Int J Antimicrob Agents 32:540–541. 10.1016/j.ijantimicag.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 399.Drancourt M, Bollet C, Carta A, Rousselier P. 2001. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int J Syst Evol Microbiol 51:925–932. 10.1099/00207713-51-3-925. [DOI] [PubMed] [Google Scholar]
- 400.Liu L, Feng Y, Wei L, Qiao F, Zong Z. 2020. Precise species identification and taxonomy update for the genus Kluyvera with reporting Kluyvera sichuanensis sp. nov. Front Microbiol 11:579306. 10.3389/fmicb.2020.579306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Bayliss SC, Thorpe HA, Coyle NM, Sheppard SK, Feil EJ. 2019. PIRATE: a fast and scalable pangenomics toolbox for clustering diverged orthologues in bacteria. Gigascience 8:giz119. 10.1093/gigascience/giz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37:1530–1534. 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Huang J, Zhu S, Zhao L, Chen L, Du M, Zhang C, Yang ST. 2020. A novel β-galactosidase from Klebsiella oxytoca ZJUH1705 for efficient production of galacto-oligosaccharides from lactose. Appl Microbiol Biotechnol 104:6161–6172. 10.1007/s00253-020-10679-9. [DOI] [PubMed] [Google Scholar]
- 404.Lee CM, Liao CH, Lee WS, Liu YC, Mu JJ, Lee MC, Hsueh PR. 2012. Outbreak of Klebsiella pneumoniae carbapenemase-2-producing K. pneumoniae sequence type 11 in Taiwan in 2011. Antimicrob Agents Chemother 56:5016–5022. 10.1128/AAC.00878-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Pang F, Jia XQ, Song ZZ, Li YH, Wang B, Zhao QG, Wang CX, Zhang Y, Wang LX. 2016. Characteristics and management of Enterobacteriaceae harboring IMP-4 or IMP-8 carbapenemase in a tertiary hospital. Afr Health Sci 16:153–161. 10.4314/ahs.v16i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ode T, Saito R, Kumita W, Sato K, Okugawa S, Moriya K, Koike K, Okamura N. 2009. Analysis of plasmid-mediated multidrug resistance in Escherichia coli and Klebsiella oxytoca isolates from clinical specimens in Japan. Int J Antimicrob Agents 34:347–350. 10.1016/j.ijantimicag.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 407.Koyano S, Saito R, Nagai R, Tatsuno K, Okugawa S, Okamura N, Moriya K. 2013. Molecular characterization of carbapenemase-producing clinical isolates of Enterobacteriaceae in a teaching hospital, Japan. J Med Microbiol 62:446–450. 10.1099/jmm.0.050708-0. [DOI] [PubMed] [Google Scholar]
- 408.Sho T, Muratani T, Mizokami Y, Hamasuna R, Fujimoto N, Matsumoto T. 2012. Mechanism of resistance of a highly carbapenem-resistant Klebsiella oxytoca isolate and comparison of susceptibility to five carbapenems. Int J Antimicrob Agents 39:268–269. 10.1016/j.ijantimicag.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 409.Chen LR, Zhou HW, Cai JC, Zhang R, Chen GX. 2009. Combination of IMP-4 metallo-β-lactamase production and porin deficiency causes carbapenem resistance in a Klebsiella oxytoca clinical isolate. Diagn Microbiol Infect Dis 65:163–167. 10.1016/j.diagmicrobio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 410.Aschbacher R, Giani T, Corda D, Conte V, Arena F, Pasquetto V, Scalzo K, Nicoletti M, Rossolini GM, Pagani E. 2013. Carbapenemase-producing Enterobacteriaceae during 2011-12 in the Bolzano area (northern Italy): increasing diversity in a low-endemicity setting. Diagn Microbiol Infect Dis 77:354–356. 10.1016/j.diagmicrobio.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 411.Manageiro V, Ferreira E, Almeida J, Barbosa S, Simoes C, Antibiotic Resistance Surveillance Program in Portugal (ARSIP), Bonomo RA, Canica M. 2015. Predominance of KPC-3 in a survey for carbapenemase-producing Enterobacteriaceae in Portugal. Antimicrob Agents Chemother 59:3588–3592. 10.1128/AAC.05065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sorli L, Miro E, Segura C, Navarro F, Grau S, Salvado M, Horcajada JP. 2011. Intra- and inter-species spread of carbapenemase genes in a non-hospitalized patient. Eur J Clin Microbiol Infect Dis 30:1551–1555. 10.1007/s10096-011-1259-1. [DOI] [PubMed] [Google Scholar]
- 413.Rodriguez-Martinez JM, Conejo MC, Diaz de Alba P, Lopez-Cerero L, Fernandez-Echauri P, Pascual A. 2012. Combination of blaVIM-1 and qnrS2 on the same plasmid in Klebsiella oxytoca and Klebsiella pneumoniae isolates in Seville. Enferm Infecc Microbiol Clin 30:246–248. 10.1016/j.eimc.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 414.Miro E, Segura C, Navarro F, Sorli L, Coll P, Horcajada JP, Alvarez-Lerma F, Salvado M. 2010. Spread of plasmids containing the blaVIM-1 and blaCTX-M genes and the qnr determinant in Enterobacter cloacae, Klebsiella pneumoniae and Klebsiella oxytoca isolates. J Antimicrob Chemother 65:661–665. 10.1093/jac/dkp504. [DOI] [PubMed] [Google Scholar]
- 415.Jamal W, Rotimi VO, Albert MJ, Khodakhast F, Nordmann P, Poirel L. 2013. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol 62:1239–1244. 10.1099/jmm.0.059915-0. [DOI] [PubMed] [Google Scholar]
- 416.Kristof K, Toth A, Damjanova I, Janvari L, Konkoly-Thege M, Kocsis B, Koncan R, Cornaglia G, Szego E, Nagy K, Szabo D. 2010. Identification of a blaVIM-4 gene in the internationally successful Klebsiella pneumoniae ST11 clone and in a Klebsiella oxytoca strain in Hungary. J Antimicrob Chemother 65:1303–1305. 10.1093/jac/dkq133. [DOI] [PubMed] [Google Scholar]
- 417.Minarini LA, Climaco EC, Guimaraes DB, Ferreira JC, Palazzo IC, Martinez R, Darini AL. 2008. Clonal transmission of ESBL-producing Klebsiella spp. at a university hospital in Brazil. Curr Microbiol 56:587–591. 10.1007/s00284-008-9129-5. [DOI] [PubMed] [Google Scholar]
- 418.Donati V, Feltrin F, Hendriksen RS, Svendsen CA, Cordaro G, Garcia-Fernandez A, Lorenzetti S, Lorenzetti R, Battisti A, Franco A. 2014. Extended-spectrum-β-lactamases, AmpC β-lactamases and plasmid mediated quinolone resistance in Klebsiella spp. from companion animals in Italy. PLoS One 9:e90564. 10.1371/journal.pone.0090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Dolejska M, Frolkova P, Florek M, Jamborova I, Purgertova M, Kutilova I, Cizek A, Guenther S, Literak I. 2011. CTX-M-15-producing Escherichia coli clone B2-O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J Antimicrob Chemother 66:2784–2790. 10.1093/jac/dkr363. [DOI] [PubMed] [Google Scholar]
- 420.Preston KE, Kacica MA, Limberger RJ, Archinal WA, Venezia RA. 1997. The resistance and integrase genes of pACM1, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid 37:105–118. 10.1006/plas.1997.1284. [DOI] [PubMed] [Google Scholar]
- 421.Schmitt J, Jacobs E, Schmidt H. 2007. Molecular characterization of extended-spectrum β-lactamases in Enterobacteriaceae from patients of two hospitals in Saxony, Germany. J Med Microbiol 56:241–249. 10.1099/jmm.0.46670-0. [DOI] [PubMed] [Google Scholar]
- 422.Uechi K, Tada T, Shimada K, Nakasone I, Sonozaki T, Kirikae T, Fujita J. 2018. Emergence of ArmA, a 16S rRNA methylase in highly aminoglycoside-resistant clinical isolates of Klebsiella pneumoniae and Klebsiella oxytoca in Okinawa, Japan. J Infect Chemother 24:68–70. 10.1016/j.jiac.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 423.Granier SA, Van Nguyen JC, Kitzis MD, Goldstein FW, Leflon-Guibout V, Nicolas-Chanoine MH. 2002. First description of a TEM-30 (IRT-2)-producing Klebsiella oxytoca isolate. Antimicrob Agents Chemother 46:1158–1159. 10.1128/AAC.46.4.1158-1159.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zarfel G, Lipp M, Gurtl E, Folli B, Baumert R, Kittinger C. 2017. Troubled water under the bridge: screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci Total Environ 593-594:399–405. 10.1016/j.scitotenv.2017.03.138. [DOI] [PubMed] [Google Scholar]
- 425.Shin SH, Roh H, Kim J, Cho S, Um Y, Lee J, Ryu YW, Chong H, Yang KS. 2015. Complete genome sequence of Klebsiella oxytoca M1, isolated from Manripo area of South Korea. J Biotechnol 198:1–2. 10.1016/j.jbiotec.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 426.Cai Z, Guo Q, Yao Z, Zheng W, Xie J, Bai S, Zhang H. 2020. Comparative genomics of Klebsiella michiganensis BD177 and related members of Klebsiella sp. reveal the symbiotic relationship with Bactrocera dorsalis. BMC Genet 21:138. 10.1186/s12863-020-00945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kang M, Chmara J, Duceppe MO, Phipps-Todd B, Huang H. 2020. Complete genome sequence of a Canadian Klebsiella michiganensis strain, obtained using oxford nanopore technologies sequencing. Microbiol Resour Announc 9:e00960-20. 10.1128/MRA.00960-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Iwase T, Ogura Y, Hayashi T, Mizunoe Y. 2016. Complete genome sequence of Klebsiella oxytoca strain JKo3. Genome Announc 4:e01221-16. 10.1128/genomeA.01221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Dantur KI, Chalfoun NR, Claps MP, Tortora ML, Silva C, Jure A, Porcel N, Bianco MI, Vojnov A, Castagnaro AP, Welin B. 2018. The endophytic strain Klebsiella michiganensis Kd70 lacks pathogenic island-like regions in its genome and is incapable of infecting the urinary tract in mice. Front Microbiol 9:1548. 10.3389/fmicb.2018.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Hossain S, De Silva BCJ, Dahanayake PS, Heo GJ. 2020. Phylogenetic relationships, virulence and antimicrobial resistance properties of Klebsiella sp. isolated from pet turtles in Korea. Lett Appl Microbiol 70:71–78. 10.1111/lam.13245. [DOI] [PubMed] [Google Scholar]