Critically ill patients are cared for by groups of clinicians and professionals with differing training, experiences, and skillsets who work in diverse clinical settings. It is therefore not surprising that the type of care delivered to critically ill patients varies widely between these settings and sometimes even within a single institution. In part, this variability may be explained by the lack of strong evidence supporting many common critical care interventions, and in the absence of compelling data, bedside clinicians may defer to previous training and experience to guide delivery of care. Even foundational critical care therapies, such as prescribing IV fluids (type and amount), the blood pressure titration target for vasopressor support, and the setting of individual parameters for mechanical ventilation have demonstrated substantial variation in practice (1, 2). In this viewpoint, we will make the case that, for the majority of critically ill patients, providing similar and reproducible care is superior to delivering individualized and variable care that substantially changes between institutions or different clinicians.
Many patients, unfortunately, do not receive optimal or evidence-based care during their ICU stay. Even interventions which have demonstrable benefits such as early antibiotics in sepsis, lung protective mechanical ventilation in acute respiratory distress syndrome (ARDS), early mobility, and avoidance of high dose sedative infusions in mechanically ventilated patients are not consistently delivered in the ICU. While there are likely many reasons patients do not receive desired care, the competing demands of time and the lack of sufficient resources are likely at least partly responsible. Heterogeneous training and experience may also be a contributor. In addition, even for interventions with established benefits, some clinicians may not agree with specific facets of bundled clinical care pathways.
Protocolized care and explicit clinical pathways provide a clear mechanism by which institutions can ensure desirable treatments are systematically considered, and, when appropriate, implemented in a timely manner (3). Protocols have demonstrated efficacy in ensuring desired care is delivered to patients with sepsis, ARDS, and shock among others (4-6). We will use the terms protocols and pathways interchangeably to refer to explicit mechanisms aimed at providing standard and desired care for a patient with a specific disease or syndrome.
PROTOCOLS AND PATHWAYS TO STANDARDIZE TREATMENT
A protocol provides a regimented treatment pathway allowing for consistent decision-making in patients with a specific diagnosis or syndrome. Protocols are often codified into clinical order sets that allow a clinician to select desired care from a series of prespecified options. In the optimal situation, protocols should encapsulate evidence from large-scale randomized clinical trials (RCTs) and focus on interventions or treatments that demonstrate clear benefit and improve patient-important outcomes. Protocols are best developed locally to ensure they are easily implemented and customized to meet the needs of the specific patient population. Robust evidence suggests that the use of a protocol is more likely to lead to a patient receiving desired care (7), and the presence of a treatment protocol has been shown to be the strongest predictor that a patient will receive desired care (5).
EVIDENCE SUPPORTING PROTOCOLS IN SPECIFIC SYNDROMES AND DISEASES
Sepsis
Protocolized care in sepsis has been shown to improve adherence to important process measures, including time to first antibiotics and the use of appropriate diagnostic tests (while avoiding overuse). The implementation of a standard sepsis protocol has also been associated with a decrease in hospital mortality in 18 ICUs in Utah and Idaho (from 20% to 10%) compared with a control period prior to protocol implementation (4). Similarly, introduction of a mandated sepsis treatment algorithm across New York State was associated with higher levels of desired care, including shorter time to first antibiotics, and decreased hospital mortality in patients treated after its implementation (6, 8). Finally, implementation of an educational platform to increase the use of a standardized protocol for sepsis in 25% of Spain’s ICUs was associated with an increase in completion of important patient-focused process measures and decreased mortality (9). Of note, the observed outcome improvements occurred despite the inclusion of controversial, and in some cases ineffective or harmful, measures which were included these protocols (e.g., resuscitation guided by central venous pressure, tight glycemic control, and use of activated protein C) (10, 11).
Mechanical Ventilation
Perhaps the strongest evidence supporting routine protocolization of care is in the realm of invasive mechanical ventilation. Standardized ventilator weaning protocols have led to more rapid and higher rates of successful removal from mechanical ventilation compared with usual care (12). By standardizing coordinated cessation of sedation paired with spontaneous breathing trials (i.e., “wake up and breathe”), patients were liberated more rapidly from ventilatory support compared with routine ventilator weaning alone (13). Importantly, a large prospective cohort study of 15,226 critically ill adults across 68 ICUs demonstrated that the use of standardized approaches to weaning, sedation, mobilization, and family/caregiver engagement (i.e., ABCDEF bundle) was associated with lower likelihood of ongoing need for invasive ventilation, delirium, and mortality (14). Implementing protocolized limitation of sedation infusions (15) and early mobilization (16) have shown similar impacts on liberation from mechanical ventilation.
Organ or Procedure Specific Protocols
For patients requiring a specific procedure, or those with a clearly identified organ pathology, there is evidence that following a defined pathway or protocol improves patient outcomes. For example, prescriptive perioperative analgesia and extubation pathways for low-risk coronary artery bypass surgical patients decrease time to extubation and decrease ICU length of stay with mortality rates equivalent to usual care (17). These postoperative protocols have even been shown to reduce costs in some circumstances, likely owing to reductions in resource utilization (18). Similarly, protocolized treatment of patients with ST elevation myocardial infarction has been associated with improved survival compared with standard care (19). Apart from cardiac care, treatment at a hospital participating in “Get with the Guidelines” protocol for stroke identification and management was associated with improved functional outcomes at the time of hospital discharge and reduced post discharge mortality (20).
WHEN NOT TO STANDARDIZE CARE
While we believe that the evidence supports standardizing care for most critically ill patients, there are clearly subsets of patients for whom one should not standardize care. These include patients who are so critically ill that we must consider rarely used rescue therapies that lack sufficient evidence to incorporate into routine protocols. Such interventions may include, for example, inhaled nitric oxide, extracorporeal membrane oxygenation, and airway pressure release ventilation. In high-utilization settings, it may however be feasible and prudent to standardize certain elements of their conduct. For extremely rare diseases (e.g., neutrophil chemotaxis disorders or pulmonary arterial hypertension), it may not be practical to develop protocols given the time and expense required. Further, there are some treatments for which the balance between desirable effects and harmful effects is less clear, therefore not allowing for clear guidance or protocolization. Certain interventions may have less robust evidence that propagates the variability in practice, and in these circumstances, it may not make sense to standardize care but rather allow for variability while encouraging further research. Further, it is possible that a patient may be inappropriately misclassified as having a syndrome that they, in fact, may not have, which could lead to inappropriate treatment. Finally, it is important to consider an individual patient’s values and preferences—which might outweigh any potential benefit to standardizing care in specific situations.
PRECISION MEDICINE AND INDIVIDUALIZED CARE
The development of precision medicine has revolutionized the field of oncology, with novel treatments available for both solid and liquid tumors. The presence of specific genetic markers in malignancy has allowed for targeted therapy that in many instances has proved much more successful than standardized care. Recent attempts to use clinical and laboratory features to better understand specific critical illness phenotypes offers the possibility of similarly personalizing treatment in the ICU setting. For example, Seymour et al (21) were able to characterize specific sepsis phenotypes that were associated with both host defense patterns and in-hospital mortality. In addition, an analysis of the Hydroxymethylglutaryl-CoA reductase inhibition in Acute Lung Injury to Reduce Pulmonary Dysfunction-2 trial of simvastatin in ARDS that divided patients into two subphenotypes demonstrated different outcomes between the two subphenotypes (22). Importantly, the treatment response to simvastatin also differed between the two subphenotypes, with those patients in the hyperinflammatory subphenotypes having statistically significant decrease in mortality compared with placebo-treated patients (22). There was no difference in outcome between treatment and placebo in the hypo inflammatory subphenotype (22). These examples suggest that in the future, we may have mechanisms to identify subsets of patients with critical illness that may respond to targeted therapy. Unfortunately, to date, attempts to individualize or stratify care of critically ill adults enrolled in RCTs by personalized physiologic measures have not been successful. For example, titration of positive end-expiratory pressure according to esophageal pressure or transpulmonary driving pressure (22, 23), or stratifying inflammation by interleukin-6 levels (24), have not led to improvements in patient outcomes.
WHAT LEVEL OF EVIDENCE SHOULD SUPPORT STANDARDIZED CARE?
How certain should we be in the benefit of an intervention or treatment pathway before it gets protocolized into the standard of care? To some degree, this depends on the intended implementation. To create a local protocol, the clinician should have relatively robust data that supports its use, consensus from local stakeholders, and some mechanism to determine whether standardizing care improves patient outcomes locally (e.g., quality improvement metrics). To implement standardized care more widely, evidence for benefit should be more definitive, usually data from RCTs enrolling a more diverse patient population allowing for generalizability. In Grading of Recommendations Assessment, Development and Evaluation language, it would most commonly be strong recommendations that allow for translation into protocols and standardized care (Fig. 1). Factors incorporated into decisions around strength of randomization include as follows: balance of benefits and harms, certainty of evidence, cost and resources, individual patient values and preferences, impact on health equity, and feasibility and acceptability. Similar to locally implemented guidelines, clinicians should strive for evidence that following the protocol or standardized care improves patient outcomes, even when done on a larger scale (25).
Figure 1.
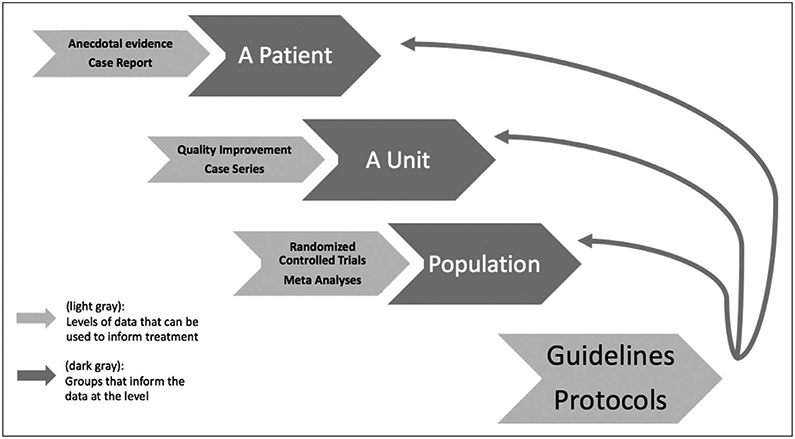
Type of evidence needed to change practice.
SUMMARY
Protocolization of therapy allows clinicians to systematically consider important treatment and diagnostic strategies in all patients. Protocols remain a key tool in the clinician’s hands aimed at minimizing unnecessary treatment variation and facilitating the delivery of desired care to patients. Personalized care based on individual biomarkers and physiology remains an intellectually attractive, but for now unproven and often inferior, method for delivering optimal care.
Footnotes
Dr. Sevransky’s institution has received funding from the Marcus Foundation for a sepsis clinical trial and from the Centers for Disease Control and Prevention Foundation. Dr. Rochwerg is supported by the Hamilton Health Sciences Early Career Award. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800 [DOI] [PubMed] [Google Scholar]
- 2.Sevransky JE, Nour S, Susla GM, et al. : Hemodynamic goals in randomized clinical trials in patients with sepsis: A systematic review of the literature. Crit Care 2007; 11:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris AH: Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 2000; 132:373–383 [DOI] [PubMed] [Google Scholar]
- 4.Miller RR 3rd, Dong L, Nelson NC, et al. ; Intermountain Healthcare Intensive Medicine Clinical Program: Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013; 188:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umoh NJ, Fan E, Mendez-Tellez PA, et al. : Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med 2008; 36:1463–1468 [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Gesten FC, Phillips GS, et al. : Mortality changes associated with mandated public reporting for sepsis. The results of the New York State Initiative. Am J Respir Crit Care Med 2018; 198:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SY, Sevransky J, Martin GS: Protocols in the management of critical illness. Crit Care 2012; 16:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Gesten F, Prescott HC, et al. : Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer R, Artigas A, Levy MM, et al. ; Edusepsis Study Group: Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 2008; 299:2294–2303 [DOI] [PubMed] [Google Scholar]
- 10.Klompas M, Osborn TM, Rhee C: Who owns sepsis? Ann Intern Med 2020; 172:210–211 [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, Chittock DR, Su SY-S, et al. : Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Baker AM, Dunagan DP, et al. : Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869 [DOI] [PubMed] [Google Scholar]
- 13.Girard TD, Kress JP, Fuchs BD, et al. : Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet 2008; 371:126–134 [DOI] [PubMed] [Google Scholar]
- 14.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strøm T, Martinussen T, Toft P: A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet 2010; 375:475–480 [DOI] [PubMed] [Google Scholar]
- 16.Schweickert WD, Pohlman MC, Pohlman AS, et al. : Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009; 373:1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong WT, Lai VK, Chee YE, et al. : Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev 2016; 9:CD003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton P: Fast-track improves CABG outcomes. JAMA 1993; 270:2023. [PubMed] [Google Scholar]
- 19.Lambert L, Brown K, Segal E, et al. : Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155 [DOI] [PubMed] [Google Scholar]
- 20.Song S, Fonarow GC, Olson DM, et al. : Association of Get With The Guidelines-Stroke program participation and clinical outcomes for Medicare beneficiaries with ischemic stroke. Stroke 2016; 47:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour CW, Kennedy JN, Wang S, et al. : Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019; 321:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. : Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 2017; 318:1335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. ; EPVent-2 Study Group: Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 2019; 321:846–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panacek EA, Marshall JC, Albertson TE, et al. ; Monoclonal Anti-TNF: a Randomized Controlled Sepsis Study Investigators: Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 2004; 32:2173–2182 [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi M, Shime N, Iguchi N, et al. : Effects of adherence to ventilator-associated pneumonia treatment guidelines on clinical outcomes. J Infect Chemother 2013; 19:599–606 [DOI] [PubMed] [Google Scholar]


