Drurey et al. show that excretory/secretory products from the parasitic helminth Heligmosomoides polygyrus suppress the host-protective small intestinal epithelial response.
Abstract
Drurey et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20211140) show that excretory/secretory products from the parasitic helminth Heligmosomoides polygyrus suppress the host-protective small intestinal epithelial response. These findings establish that helminths directly modulate the tissue in which they live, shining new light on the host–parasite interaction.
In this issue, Maizels and colleagues provide an exciting new perspective on the host–pathogen interaction during intestinal helminth parasite infection (Drurey et al., 2021). It has long been appreciated that changes to the structure and function of the intestinal epithelial barrier are critical in mediating parasite expulsion and repairing worm-induced damage (Coakley and Harris, 2020). However, how these changes are regulated and how helminths might directly modulate these responses have not been fully described to date. The authors show that the parasitic helminth Heligmosomoides polygyrus releases excretory/secretory products (HpES) that act directly on murine host intestinal epithelial cells during infection. In vitro and in vivo, HpES limited the accumulation of tuft cells, key chemosensory cells in the intestinal epithelium that mediate host protection during infection (Gerbe et al., 2016; Howitt et al., 2016; von Moltke et al., 2016). This novel finding sheds light on the complex interplay between the parasite and the host epithelial barrier (Drurey et al., 2021).

Insights from Elia D. Tait Wojno.
The authors use complementary in vitro and in vivo approaches to demonstrate the effect of HpES on murine small intestinal epithelial cells. Exposing murine small intestinal epithelial organoids to HpES in the context of treatment with recombinant murine IL-4 and IL-13 suppressed the type 2 cytokine–elicited tuft cell gene signature. The number of tuft cells in organoids that were induced by type 2 cytokines was also decreased with the addition of HpES or H. polygyrus L3 larvae, the infective stage of the parasite. In vivo, infection with H. polygyrus suppressed the tuft cell hyperplasia that results following infection with another helminth parasite, Nippostrongylus brasiliensis, or treatment with the metabolite succinate (Nadjsombati et al., 2018; Schneider et al., 2018). Treatment of mice with HpES was also sufficient to limit succinate-induced tuft cell hyperplasia. Finally, returning to the organoid system, the authors found that HpES altered expression of specific genes and pathways involved in intestinal epithelial differentiation and lineage specification, including Atoh1 and Hes1, and caused a spheroid morphology to emerge in organoid cultures. These data suggest that HpES acts directly on the small intestinal epithelium to regulate certain aspects of the complex tissue response to parasitic helminth infection (Drurey et al., 2021).
These findings are exciting for both immunoparasitology afficionados and a broader audience of those interested in mucosal immunology, intestinal stem cell function in infection, and the evolution of host–pathogen relationships. For decades, studies of murine helminth infection have been used to reveal the amazing ways in which hosts and pathogens evolve together when intimately connected by the requisites of parasitism (Maizels et al., 2018). Previous studies from these authors and others have shown that H. polygyrus produces an array of immunomodulatory factors that suppress host type 2 immune responses. HpES contains a protein called H. polygyrus alarmin release inhibitor, which dampens host IL-33 responses that are critical in driving anti-parasite effector responses (Osbourn et al., 2017). A TGF-β mimic in HpES also strongly promotes the emergence of T regulatory cells that in turn suppress the host immune response (Johnston et al., 2017). These responses appear to largely benefit the parasite, allowing it to mature, grow, feed, and treproduce successfully in its environment. However, it is also possible that this modulation benefits the host by limiting immunopathology and overexuberant inflammation during chronic worm infection.
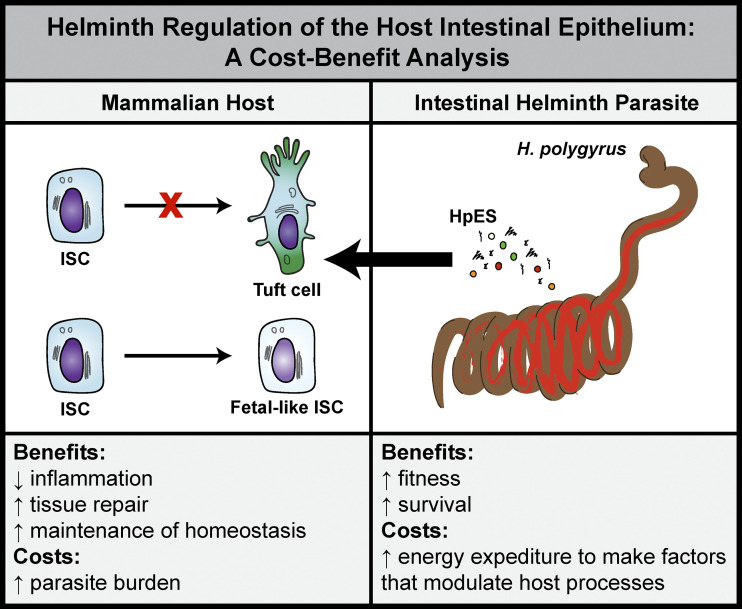
With these new findings, Drurey et al. establish that H. polygyus directly modulates the host intestinal epithelium, an important target of host-derived IL-13 that promotes wound healing and helminth expulsion (Coakley and Harris, 2020). By limiting the emergence of tuft cells, and also perhaps other secretory lineages such as goblet cells, the parasite promotes its own survival (see figure). As with parasite-mediated modulation of host immune responses, such suppression could also be highly beneficial for the host. In the context of chronic helminth infection, a persistent skew for the differentiation of secretory lineages could have negative consequences for normal host intestinal functions, including nutrient absorption and proper interactions with the microbiota. The fetal-like state that the authors describe in small intestinal organoids exposed to HpES could promote local tissue repair responses near the granulomas that form around encysted larvae in the intestinal submucosal layer or in areas where adult parasites feed on host tissues. Thus, it seems likely that H. polygyrus–mediated modulation of the epithelium is mutually beneficial to host and parasite (see figure). More research will be required to understand how parasite-directed regulation of the host immune and epithelial response dovetail to influence host fitness overall.
Helminth regulation of the host intestinal epithelium: A cost–benefit analysis. H. polygyrus produces HpES that modulates the host small intestinal epithelium in mice. HpES acts on intestinal stem cells (ISCs) to suppress tuft cell hyperplasia and cause the emergence of a fetal-like state. This modulation has potential costs and benefits for both host and parasite.
The fetal-like state observed in murine small intestinal organoids exposed to HpES is of particular interest. A previous study described a fetal-like state in intestinal epithelium associated with H. polygyrus granulomas (Nusse et al., 2018). This work showed that in epithelium that was associated with granulomas, Lgr5-expressing intestinal stem cells were depleted. Granuloma-associated epithelium also demonstrated an IFN target gene signature and IFNγ-dependent Sca-1 expression. Sca-1–expressing intestinal crypt cells sorted from H. polygyrus–infected mice formed spheroid organoids in culture and expressed genes typical of organoids formed from fetal tissue (Nusse et al., 2018). Drurey et al. observe a similar phenomenon, with HpES treatment resulting in the formation of large, spheroid-shaped organoids (Drurey et al., 2021). Neither study fully elucidated how such a fetal-like state benefits the host or the parasite, or both. However, as it appears that such a response can be elicited by both host- and parasite-derived products, it stands to reason that the fetal-like state is of benefit to one or both players (see figure).
The authors of both studies propose that a fetal-like state can promote wound healing and tissue repair (see figure), though whether a switch to a fetal-like state is required for efficient wound repair during infection is not clear. Outside the findings related to the parasite-elicited emergence of a fetal-like state in host epithelium, it has long been appreciated that the type 2 immune response mobilizes a clear wound-healing module in affected tissues (Gause et al., 2013). In this context, pro-repair immune cell types including alternatively activated macrophages and eosinophils are activated, extracellular matrix is deposited, myofibroblast differentiation occurs, and angiogenesis is observed (Gause et al., 2013). In this study, HpES exposure resulted in expression of molecules previously associated with host tissue repair, including genes involved in lipoxin synthesis (Drurey et al., 2021). These data suggest that parasite-derived products can modulate the production of potent bioactive lipid mediators, skewing host lipid responses toward production of the more pro-resolution lipoxins and away from production of more pro-inflammatory lipid mediators. However, our understanding of the role of host- and parasite-derived lipids in the regulation of inflammation and tissue repairs remains incomplete (Oyesola and Tait Wojno, 2021). Further exploration will be needed to decipher the role that lipids play in the H. polygyrus–mediated modulation of host tissue repair and how the parasite influences the ability of the host to repair worm-induced damage more generally.
These findings of Drurey et al. prompt several other questions and open new avenues for future inquiry. The most obvious question that results from this study is what factor or factors in HpES are responsible for modulating the host epithelium? With current technologies and biochemical approaches to analyze helminth products, identifying these compounds should be feasible. The discovery of such products will be exciting not only for our understanding of host–helminth interactions but could also be used to modulate the mammalian epithelium to combat pathology in other disease states that involve pathological type 2 inflammation, as in food allergy. Second, whether parasite-mediated modulation of host tuft and goblet cell responses occurs in infected humans is entirely unknown. Relatively little is understood about human small intestinal tuft cell function during helminth infection. New studies that leverage human small intestinal organoids could be used to answer such questions. Studies of the effect of helminth infection on human epithelial barriers also have clear implications for our understanding of the inverse relationship between helminth infection and allergic diseases. Third, whether other parasite species employ similar or related strategies to directly modulate the host epithelium remains an open question. It seems likely that this is indeed the case, but a better understanding of the helminth species–specific ability to modulate the host epithelium could reveal new aspects of both helminth and host biology.
Finally, this study prompts us to reconsider what the long-term and evolutionary consequences are for host–parasite interactions that modulate the host epithelium (see figure). The discovery of parasite-directed host epithelial responses strongly suggests that helminth infection has the potential to shape the function of the host intestine throughout the lifespan. More broadly, since hosts and parasites have evolved together over long periods of time, these finding also support the notion that parasites have been an important driving force in shaping the evolution of mammalian intestinal function (Gause et al., 2013), particularly interactions with commensal and pathogenic microbes and nutrient and fluid absorption. It follows then that parasites have also driven the evolution of mammalian processes that require maximal fitness and intense caloric investment, including higher reasoning functions and reproduction. The findings from Drurey et al. show us once again that helminth parasites have an impressive toolkit to modulate their tissue homes to evade the host anti-helminth response. More importantly, these findings inform our understanding of how the coevolution of hosts and parasites has been critical in shaping mammalian life as we know it.
References
- Coakley, G., and Harris N.L.. 2020. Trends Parasitol. 10.1016/j.pt.2020.07.002 [DOI] [PubMed] [Google Scholar]
- Drurey, C., et al. 2021. J. Exp. Med. 10.1084/jem.20211140 [DOI] [Google Scholar]
- Gause, W.C., et al. 2013. Nat. Rev. Immunol. 10.1038/nri3476 [DOI] [Google Scholar]
- Gerbe, F., et al. 2016. Nature. 10.1038/nature16527 [DOI] [Google Scholar]
- Howitt, M.R., et al. 2016. Science. 10.1126/science.aaf1648 [DOI] [Google Scholar]
- Johnston, C.J.C., et al. 2017. Nat. Commun. 10.1038/s41467-017-01886-6 [DOI] [Google Scholar]
- Maizels, R.M., et al. 2018. Immunity. 10.1016/j.immuni.2018.10.016 [DOI] [Google Scholar]
- Nadjsombati, M.S., et al. 2018. Immunity. 10.1016/j.immuni.2018.06.016 [DOI] [Google Scholar]
- Nusse, Y.M., et al. 2018. Nature. 10.1038/s41586-018-0257-1 [DOI] [Google Scholar]
- Osbourn, M., et al. 2017. Immunity. 10.1016/j.immuni.2017.09.015 [DOI] [Google Scholar]
- Oyesola, O.O., and Tait Wojno E.D.. 2021. Eur. J. Immunol. 10.1002/eji.202048909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C., et al. 2018. Cell. 10.1016/j.cell.2018.05.014 [DOI] [Google Scholar]
- von Moltke, J., et al. 2016. Nature. 10.1038/nature16161 [DOI] [Google Scholar]