Abstract
The whole-genome fingerprinting technique, fluorescent amplified-fragment length polymorphism (FAFLP) analysis, was applied to Mycobacterium tuberculosis. Sixty-five clinical isolates were analyzed to determine the value of FAFLP as a stand-alone genotyping technique and to compare it with the well-established IS6110 typing system. The genome sequence of M. tuberculosis strain H37Rv (S. T. Cole et al., Nature 393:537–544, 1998) was used to model computer-generated informative primer combination(s), and the precision and reproducibility of FAFLP were evaluated by comparing the results of in vitro and computer-generated experiments. Multiplex FAFLP was used to increase resolving power in a predictable and systematic fashion. FAFLP analysis was broadly congruent with IS6110 typing for those strains with multiple IS6110 copies. It was also able to resolve an epidemiologically unlinked group of strains with only one copy of IS6110; up to 10% of clinical isolates may fall into this category. For certain epidemiological investigations, it was concluded that a combination of FAFLP and IS6110 typing would give higher resolution than would either alone. FAFLP data were digital, precise, reproducible, and suitable for rapid electronic dissemination, manipulation, interlaboratory comparison, and storage in national or international epidemiological databases. Because FAFLP samples and analyzes base substitution across the genome as a whole, FAFLP could generate new information about the microevolution of the M. tuberculosis complex.
Control of the worldwide resurgence of tuberculosis requires mapping of routes of transmission of the causative agent, Mycobacterium tuberculosis. This pathogen displays marked genetic homogeneity, and isolates cannot be resolved by phenotypic strain typing. A mobile DNA element in the M. tuberculosis genome, insertion sequence IS6110, is the basis for a standard method for differentiating strains (12, 16). The number and location of IS6110 copies in the chromosome give rise to changes in restriction fragment length polymorphisms with PvuII or other restriction enzymes that cut within the element. This analysis, which requires genomic Southern blotting, has made possible studies of primary infections, reactivations of infection, exogenous reinfections, and chains of transmission (13).
When isolates of M. tuberculosis (8% of United Kingdom isolates) (10) possess a single copy of the insertion element, this yields an identical IS6110 type. For such isolates, recognition of related cases is impossible. In fact, it has been recognized that for any M. tuberculosis isolate with five or fewer IS6110 copies, a second genotyping technique is required to establish strain clonality (13). One such technique is spoligotyping (spacer oligotyping) (8). This amplifies the direct repeat locus, a series of 36-bp tandem elements interspersed with unique spacer sequences from 35 to 41 bp in size, and hybridizes it to a membrane-bound array of spacer sequence oligonucleotides. The pattern of hybridization signals then depends upon the strain-specific complement of spacer sequences. Spoligotyping is sometimes able to distinguish between strains which have few IS6110 copies and yield the same IS6110 type.
Amplified-fragment length polymorphism (14) uses PCR to selectively amplify defined subsets of DNA restriction fragments from across the whole genome. In its fluorescent form (fluorescent amplified-fragment length polymorphism [FAFLP]), one of the PCR primers are fluorophore labeled, making the amplified fragments visible to an automated DNA sequencer. Once the complete genome sequence for Escherichia coli was published (3), it was possible to predict the sizes of DNA fragments generated in FAFLP of that species and demonstrate the high experimental fidelity of the technique (2). Genome-sequence-based FAFLP analysis of E. coli showed accurate fragment sizing (±1 bp), reproducibility, high discriminatory power, and added phylogenetic value (2). Publication of the complete genome sequence of M. tuberculosis (3) now permits us to design genome-based FAFLP conditions to establish strain types for this pathogen and to determine genetic relationships between clinical isolates. In this study, we applied this analysis to 65 M. tuberculosis strains and isolates of known IS6110 type. Both epidemiologically related outbreak strains and sporadic isolates were analyzed in a blinded fashion.
MATERIALS AND METHODS
Strains.
Strains and isolates of M. tuberculosis were acquired during a 1993 study centered on three northwest London hospitals. Epidemiological links between patients carrying strains with similar IS6110 profiles were established subsequently in certain cases (see below).
IS6110 typing and spoligotyping.
Typing of these strains was carried out using standard methods (9, 12). Seven of the 65 strains had the same single-band IS6110 type but were epidemiologically unrelated (isolates 124, 131, 145, 157, 202, 1023, and 1026). These isolates were also subjected to spoligotyping (see below). Ten of the 65 strains constituted five pairs of epidemiologically related isolates, each pair having absolutely identical IS6110 types except 100 and 203. In this case, the IS6110 type differed by two fragments. These five pairs of isolates were 139 and 140 from the same patient, isolates 1053 and 1085 from hospital contacts, isolates 128 and 113 from the same patient, isolates 114 and 245 from community contacts, and isolates 100 and 203 from community contacts. Four isolates had identical IS6110 profiles (251, 252, 253, and 232): two of these were from the same patient (251 and 232), and two were from unrelated specimens (252 and 253), later confirmed as a case of laboratory contamination. A further three epidemiologically related isolates had identical IS6110 types (129, 158, and 264). Two were from the same patient (158 and 129), and one (264) was from a close contact of that patient.
Computer methods.
The complete genome sequence of M. tuberculosis strain H37Rv (3) was analyzed with Lasergene (DNAStar, Madison, Wis.) and MacVector (Oxford Molecular, Oxford, United Kingdom). Data concerning the size and predicted number of fragments following an MseI/EcoRI digest of the genome were imported into a spreadsheet. The fragment size data were then adjusted to allow for the addition of primer sequence during PCR.
FAFLP: digestion, ligation, and PCR steps.
Five hundred nanograms of genomic DNA was digested in a total volume of 20 μl, consisting of 5 U of MseI (New England Biolabs, Hitchin, Hertfordshire, United Kingdom), 2 μl of 10× MseI buffer, 0.2 μl of 10× bovine serum albumin, and 1.0 μl of DNase-free RNase A (10 μg/μl), for 1 h at 37°C. To this digest was added 5 U (1.0 μl) of EcoRI (Life Technologies), 1.68 μl of 0.5 M Tris HCl (pH 7.6), and 2.1 μl of 0.5 M NaCl (total volume, 25 μl), and the reaction mixtures were incubated for a further hour at 37°C. Endonucleases were inactivated at 65°C for 10 min prior to ligation. To the double-digested DNA was added 25 μl of a solution containing 40 U of T4 DNA ligase (New England Biolabs), 10 pmol of EcoRI adapter, 100 pmol of MseI adapter, and 5 μl of 10× T4 ligase buffer. The 1× ligase buffer contained 50 mM Tris-HCl, 10 mM MgCl2, 10 mM dithiothreitol, 1 mM ATP, and 25 μg of bovine serum albumin at pH 7.8 and 25°C. The reaction mixture was incubated at 12°C for 17 h, heated at 65°C for 10 min to inactivate ligase, and stored at −20°C.
The nonselective forward primer for the MseI adapter site was unlabeled. The reverse primer for the EcoRI adapter site, which contained the selective base A, G, C, or T and was labeled with the fluorescent dye FAM, JOE, NED, or TAMRA, was obtained from an amplified-fragment length polymorphism kit (PE Biosystems, Foster City, Calif.). PCRs were performed in 20-μl volumes containing 2 μl of ligated DNA, 0.1 μM labeled EcoRI primer, 0.25 μM MseI primer, 2 μl of 10× Taq polymerase buffer, 200 μM (each of the four) deoxynucleoside triphosphates, 1.5 mM MgCl2, and 0.5 U of Taq DNA polymerase. Touchdown PCR cycling conditions were used for amplifying the fragments: a 2-min denaturation step at 94°C (one cycle), followed by 30 cycles of denaturation at 94°C for 20 s, a 30-s annealing step (see below), and a 2-min extension step at 72°C. The annealing temperature for the first cycle was 66°C; for the next nine cycles, the temperature was decreased by 1 degree at each cycle. The annealing temperature for the remaining 20 cycles was 56°C. This was followed by a final extension at 60°C for 30 min. Isolate 98 was used as a positive control for each batch of reactions. PCR was performed in a PE-9600 thermocycler (Perkin-Elmer Corp., Norwalk, Conn.). Reaction products were stored at −20°C.
FAFLP: gel analysis.
The amplification products were separated on a 5% denaturing (sequencing) polyacrylamide gel on an ABI Prism 377 DNA automated sequencer (Perkin-Elmer Corp.). The gel was prepared by using 5% acrylamide (FMC SinGel)–6.0 M urea in 1× TBE (89 mM Tris-HCl [pH 7.4], 89 mM boric acid, 2 mM EDTA). Spacers and shark's-tooth combs were 0.2 mm in thickness. Gels were poured using a PE Biosystems 377 casting frame and gel pourer and allowed to polymerize at room temperature for at least 2 h. The sample (1.0 μl) was added to 2.5 μl of loading dye which was a mixture containing 5.0 μl of formamide, 1.0 μl of dextran blue–50 mM EDTA loading solution, and 0.5 μl of the internal lane standard, GeneScan 500, labeled with the fluorophore ROX (PE Biosystems). The sample mix was heated at 95°C for 2 min, cooled on ice, and immediately loaded onto the gel. Electrophoresis conditions were 3 kV, 51°C, for 2.5 to 3 h, using 1× TBE as buffer.
FAFLP: data capture and analysis.
GeneScan collection software (PE Biosystems) was used to automatically size individual fragments, with reference to internal lane standards. Results were viewed in the form of a gel image, an electropherogram, tabular data, or a combination of all three. Genotyper software (PE Biosystems) interpreted GeneScan data after the analysis parameters were set to medium smoothing. The presence or absence of precisely sized fragments was ascertained, and these digital data were transferred to spreadsheets for further analysis. Pairwise comparisons using the Dice coefficient were made between all strains. The distance matrix thus generated was used as input for the Fitch and Neighbor tree building program in PHYLIP (7). The digital data were also used to generate a tree using maximum likelihood (Restml, PHYLIP) and parsimony in PAUP 3.1.1 (Sinauer Associates, Sunderland, Mass.).
RESULTS
Modeling genome digestion and FAFLP using computer analysis.
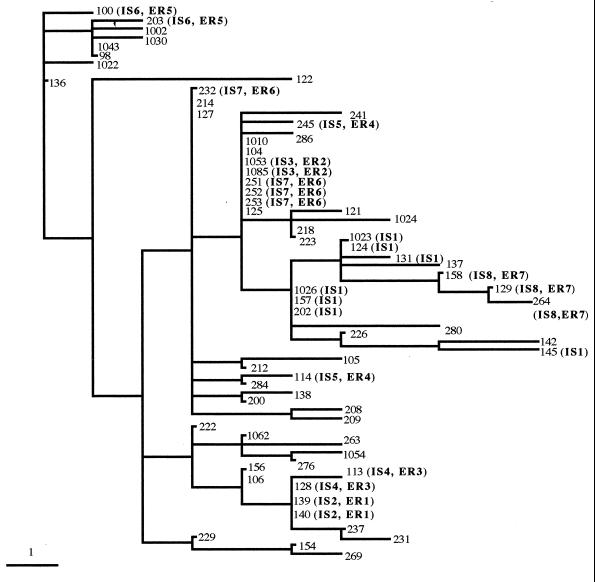
The optimum combination of enzymes and primers for analysis of M. tuberculosis had been predicted by modeling FAFLP using computer analysis of the sequenced genome of strain H37Rv. The four primer combinations used (EcoRI+A and MseI+0, EcoRI+G and MseI+0, EcoRI+C and MseI+0, and EcoRI+T and MseI+0) generated a total of 136 differently sized fragments ranging in size from 80 to 400 bp. Thirty-eight (28%) of these fragments were discriminatory. The A-selective primer combination (EcoRI+A and MseI+0) produced 40 of 136 fragments (29.4%), 13 of which were discriminatory (32.5% of the A-selective fragments, 9.6% of the total number of differently sized fragments produced). The C-selective primer combination (EcoRI+C and MseI+0) produced 30 of 136 fragments (22.1%), 8 of which were discriminatory (26.6% of the C-selective fragments, 5.9% of the total number of differently sized fragments produced). The G-selective primer combination (EcoRI+G and MseI+0) produced 48 of 136 fragments (35.3%), 10 of which were discriminatory (20.8% of the G-selective fragments, 7.4% of the total number of differently sized fragments produced). The T-selective primer combination (EcoRI+T and MseI+0), produced 18 of 136 fragments (13.2%), 7 of which were discriminatory (38.8% of the T-selective fragments, 5.1% of the total number of differently sized fragments produced). Table 1 shows the presence or absence of these precisely sized discriminating fragments generated in experimental FAFLP using nonselective MseI primer and each of the four EcoRI-selective primers containing A, C, T, or G at their 3′ terminus. Pairwise comparisons were made between all strains of the presence or absence of specifically sized fragments to generate a distance matrix. Figure 1 shows a tree produced from the distance matrix shown in Table 1, following a heuristic search using PAUP.
TABLE 1.
Sizes (base pairs) of discriminatory fragments generated by FAFLP analysis of 65 IS6110-typed M. tuberculosis strains with MseI+0 and either EcoRI+A, EcoRI+C, EcoRI+T, or EcoRI+G selective primers
FIG. 1.
FAFLP distance tree of 65 IS6110-typed strains of M. tuberculosis. The epidemiologically related (ER) and IS6110-type (IS) groups are as follows: ER1, isolates 139 and 140; ER2, isolates 1053 and 1085; ER3, isolates 113 and 128; ER4, isolates 114 and 245; ER5, isolates 100 and 203; ER6, isolates 251, 252, 253, and 232; ER7, isolates 158, 129, and 264; IS1, isolates 124, 131, 145, 157, 202, 1023, and 1026; IS2, isolates 139 and 140; IS3, isolates 1053 and 1085; IS4, isolates 113 and 128; IS5, isolates 114 and 245; IS6, isolates 100 and 203; IS7, isolates 251, 252, 253, and 232; and IS8, isolates 158, 129, and 264. Where there is no assigned type, the IS6110 pattern is unique. Note that FAFLP resolved the epidemiologically unlinked identical IS6110 group (isolates 124, 131, 145, 157, 202, 1023, and 1026).
By FAFLP analysis, all of the epidemiologically related groups clustered together in the same way as they did with IS6110 profiling, with two exceptions. These were isolate 232, which clustered slightly away from linked isolates 251, 252, and 253, and the pair of isolates 114 and 245, which were separated. Seven epidemiologically unrelated isolates with identical IS6110 profiles (124, 131, 145, 157, 202, 1023, and 1026) were split into four groups by FAFLP. Spoligotyping, by comparison, split these particular isolates into five groups. FAFLP trees produced using the Fitch and Restml programs in PHYLIP (7), tree-building programs with different algorithms to parsimony (used in PAUP), gave trees with the same topology (data not shown). Four trees generated from individual data produced by each primer combination had similar topology (data not shown).
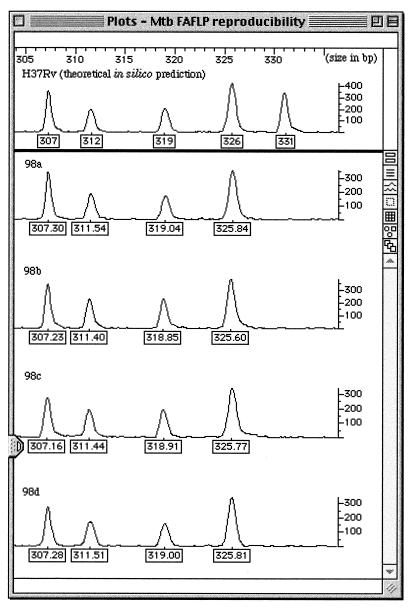
In Fig. 2, as an example of reproducibility for isolate 98, we show a section of data between 305 and 335 bp for the computer modeling of the H37Rv genome, compared with four different experimental FAFLP reactions. The five predicted H37Rv fragments for this size range (out of the 84 for the whole genome in the 50- to 500-bp size range) were located throughout the 4.4-Mbp H37Rv genome at positions 472246 to 472537 (319 bp), 1664355 to 1664653 (331 bp), 1679927 to 1680224 (326 bp), 1900190 to 1900469 (307 bp), and 2617269 to 2617551 (312 bp). (Mapping of the fragments takes account of the addition of primer sequences during their PCR amplification.) As shown in this example, each strain and clinical isolate gave an identical FAFLP profile throughout the study, with no more than ±1-bp variation in fragment sizing.
FIG. 2.
Computer modeling of H37Rv and FAFLP reproducibility study for clinical isolate 98. The top panel shows predicted fragment sizes for H37Rv in one size range (305 to 335 bp). A fragment of 331 bp predicted from the genome sequence for H37Rv is shown. The four lower panels show the parallel FAFLP readout for the same isolate. Four reactions were carried out separately and run on different gels. Numbered boxes show the size in base pairs of each fragment across one 30-bp window of polymorphic sequence (cf. Table 1). The vertical scale measures the efficiency of PCR amplification of each fragment. Mtb, M. tuberculosis.
DISCUSSION
The genomes of M. tuberculosis strains are remarkable for their lack of genetic heterogeneity (15), and the limited extent of variability revealed, even by FAFLP, among the 65 M. tuberculosis isolates in this study reflects the lack of genetic heterogeneity in this species. Epidemiological analysis has been greatly facilitated by IS6110 typing, since most isolates have multiple copies of this mobile element, giving rise to multiple bands on a genomic Southern blot (12). The sites of insertion of IS6110 in the M. tuberculosis chromosome are generally very variable, leading to extensive polymorphism. However, IS6110 typing cannot be used to reliably verify epidemiological links between isolates which contain five or fewer insertion elements (17), while the approximately 8% of (United Kingdom) isolates containing a single copy of IS6110 are refractory to IS6110 typing.
FAFLP analysis is based on sampling the whole genome rather than on the polymorphism associated with insertion sites of a single mobile element. In the present study, it splits a group of isolates with one IS6110 copy into four clusters (Fig. 1) which differ by one to six amplified fragments. Isolates in three of these clusters (which had between one and three fragment differences) might be assigned to the same strain if the epidemiological context so indicated, e.g., if they came from the same patient or a contact of that patient. In this case, however, there was no known epidemiological relationship, and the four clusters probably represent individual strains. Spoligotyping (8) resolved the single-copy IS6110 type into five clusters but yielded a significantly lower level of resolution than did FAFLP for strains with multiple IS6110 copies. FAFLP analysis would clearly be a useful adjunct to IS6110 typing when isolates with low IS6110 copy numbers are being analyzed. In general, for any M. tuberculosis isolate, a combination of FAFLP with IS6110 typing would give better resolution than would either technique alone.
FAFLP exhibits unique precision sizing of fragments and unique reproducibility of profiles, and this study indicates that it can discriminate between bacterial strains so closely related that the presence or absence of a single band is critical. Nevertheless, in certain cases (cf. Fig. 1), the chosen FAFLP conditions did not differentiate between strains from unlinked infections. This was also the case for IS6110 typing and spoligotyping. For example, the isolate pair 1053-1085 clustered on the same branch as the 251-252-253 group. In one anomalous case, a pair of FAFLP profiles with two fragment differences was found for isolates whose IS6110 type was the same (isolates 114 and 245). Although the difference was only two FAFLP fragments (Table 1), the various algorithms used to analyze these fragment data (including unweighted pair group method with averages, parsimony, and maximum likelihood) failed to demonstrate the relatively close nature of these two isolates in this particular case. This was due to the small number of data points being analyzed and also the background strains being used in the analysis. The apparent lack of association between these two epidemiologically related strains in Fig. 1 suggests that reversion to direct analysis of raw data (FAFLP profiles) is more appropriate for these isolates. The epidemiological context in this case would identify them as a pair of isolates of the same strain.
Three pairs of isolates (113 and 128, 232 and 251, and 158 and 129), belonging to the same multicopy IS6110 types, differed by one amplified fragment. We have no data on whether these pairs represent longitudinal sampling of the patients. There is as yet no published information on the rate of microevolution of M. tuberculosis (base substitutions) as it might influence FAFLP profiles, and this is a subject for further investigation. It is, however, known that longitudinal samples of a strain from a single patient show differences in IS6110 type (5), and perhaps our FAFLP data here simply reflect longitudinal microevolutionary change in a more sensitive manner. Again, the epidemiological context in these cases would identify them as a pair of isolates of the same strain.
Tenover has published criteria relating restriction site variation in pulsed-field gel electrophoresis (PFGE) to epidemiological evidence of clonality (11). These criteria state that two to three PFGE fragment differences compared with the outbreak pattern indicate close relationship and that the isolate is probably part of the outbreak (11). If these criteria are applied to our FAFLP data, all of the examples cited above would appear to be related. Nonetheless, the status of a single fragment difference in FAFLP is unlike that of a band shift in PFGE because an FAFLP fragment is very much smaller, is precisely sized (±1 bp), can be sequenced, and can be mapped to the genome. Therefore, if the context supports it, a single fragment difference in FAFLP may define a new strain. Even in PFGE, when applied on a large scale as in the PulseNet U.S. national surveillance of E. coli serotype O157, single-band differences are used to define certain epidemiological clones (E. Ribot, unpublished data). Clearly, the epidemiological context is essential to interpretation, and FAFLP, like any genotyping technique, cannot be considered in isolation from epidemiological data. In this way, the clinical microbiologist can guard against missing true epidemiological relationships or misinforming patients about superinfection with a separate strain. FAFLP is versatile, and its conditions can be varied systematically, so that different subsets of fragments can then be sampled from the genome. This may resolve ambiguous genetic relationships between isolates.
FAFLP analysis can be expected to yield insights about the evolution of M. tuberculosis as a species. The technique samples the whole genome sequence in a predictable and rigorous fashion, monitoring base substitutions accumulating throughout the genome, rather than being based on the footprints of a mobile genetic element. This could provide a valuable measure of microevolutionary change. As a genotyping methodology, FAFLP exhibits certain inherent advantages over existing techniques, since it is based directly on the genome sequence, sizes a much larger number of fragments, and does so with greater precision. It is also capable of predictable expansion or modification and can be automated. These features compare favorably with those of Southern-blotting-based typing.
In summary, genome-sequence-derived FAFLP is broadly congruent with IS6110 for typing M. tuberculosis. Its resolving power appears generally superior. This is particularly the case where strains contain few insertion elements, where FAFLP is capable of revealing chains of transmission not demonstrable by IS6110 typing. This cannot be overlooked, since up to 40% of M. tuberculosis isolates in certain significant countries like India have only one IS6110 copy (6). Also, most bovine M. tuberculosis isolates contain a single IS6110 copy (4). It must be said, however, that no technique can be used in isolation; attention must be paid to the epidemiological context. We do not propose that FAFLP should replace IS6110 typing; indeed, some laboratories might use it as an adjunct to IS6110 typing, much as spoligotyping is used at present. The conceptual basis of FAFLP is quite different from that of IS6110 typing. It samples approximately 0.1% of the genome in an unweighted manner, generating a predictable number of amplified fragments for a given enzyme-primer combination. These conditions can be varied to produce different subsets of DNA fingerprints for a given genome. By contrast, IS6110 typing is based simply on the replicative transposition of a mobile DNA element, which has population dynamics independent of the rest of the genome (1). IS6110 typing has provided a revolutionary advance for epidemiological study of tuberculosis. However, it does not make any use of the resource of the published complete genome sequence of M. tuberculosis and has no direct relevance to the microevolution of the M. tuberculosis genome. We consider that FAFLP analysis based on knowledge of the genome sequence could contribute to the study of the epidemiology and evolutionary genetics of M. tuberculosis.
ACKNOWLEDGMENTS
We thank Philip Mortimer and Jon Clewley for critical reading of the manuscript.
REFERENCES
- 1.Ajioka J W, Hartl D L. Population dynamics of transposable elements. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 939–958. [Google Scholar]
- 2.Arnold C, Metherell L, Willshaw G, Maggs A, Stanley J. Predictive fluorescent amplified-fragment length polymorphism analysis of Escherichia coli: high-resolution typing method with phylogenetic significance. J Clin Microbiol. 1999;37:1274–1279. doi: 10.1128/jcm.37.5.1274-1279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 4.Cousins D, Williams S, Liebana E, Aranaz A, Bunschoten A, van Embden J D, Ellis T. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J Clin Microbiol. 1998;36:168–178. doi: 10.1128/jcm.36.1.168-178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das S, Chan S L, Allen B W, Mitchison D A, Lowrie D B. Applications of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis patients in Hong Kong before, during and after short-course chemotherapy. Tubercle Lung Dis. 1993;74:47–51. doi: 10.1016/0962-8479(93)90068-9. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Paramasivan C N, Lowrie D B, Prabhakar R, Narayanan P R. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tubercle Lung Dis. 1995;76:550–554. doi: 10.1016/0962-8479(95)90533-2. [DOI] [PubMed] [Google Scholar]
- 7.Felsenstein J. PHYLIP—Phylogeny Inference Package. Cladistics. 1989;5:164–166. [Google Scholar]
- 8.Hermans P W M, van Soolingen D, Bik E M, De Haas P E W, Dale J W, van Emben J D A. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar, D., N. A. Saunders, J. M. Watson, A. M. Ridley, S. Nicholas, K. F. Barker, R. Wall, Q. N. Karim, S. Barrett, R. C. George, and A. C. McCartney. Clusters of new tuberculosis cases in North-west London: a survey from three hospitals based on IS6110 RFLP typing. J. Infect., in press. [DOI] [PubMed]
- 11.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenbach K D, Gicquel B, Harmans P, Martin C, McAdam R, Shinnick T M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Soolingen D, Hermans P W, de Haas P W, Soll D R, van Embden J D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kulper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler P R, Ratledge C. Metabolism of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 353–385. [Google Scholar]
- 16.Yang Z H, de Haas P E W, van Soolingen J D, van Embden J D A, Andersen A B. Restriction fragment length polymorphism of Mycobacterium tuberculosis strains isolated from Greenland during 1992: evidence of tuberculosis transmission between Greenland and Denmark. J Clin Microbiol. 1992;32:3018–3025. doi: 10.1128/jcm.32.12.3018-3025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen L, Ross B C, Jackson K M, Dwyer B. Characterization of Mycobacterium tuberculosis strains from Vietnamese patients by Southern blot hybridization. J Clin Microbiol. 1992;31:1615–1618. doi: 10.1128/jcm.31.6.1615-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]